Abstract
Studies of sensory-motor performance, including those concerned with changes due to age, disease or therapeutic intervention, often use measures based on jerk, the time-derivative of acceleration, to quantify smoothness and coordination. However, results have been mixed, some studies reporting sensitive discrimination of subtle differences, others failing to find significant differences, even when they are obviously present. One reason for this state of affairs is that different measures have been used with different scaling factors. These measures are sensitive to movement amplitude and/or duration to different degrees. We show that jerk-based measures with dimensions vary counter-intuitively with movement smoothness, whereas a dimensionless jerk-based measure properly quantifies common deviations from smooth, coordinated movement.
Introduction
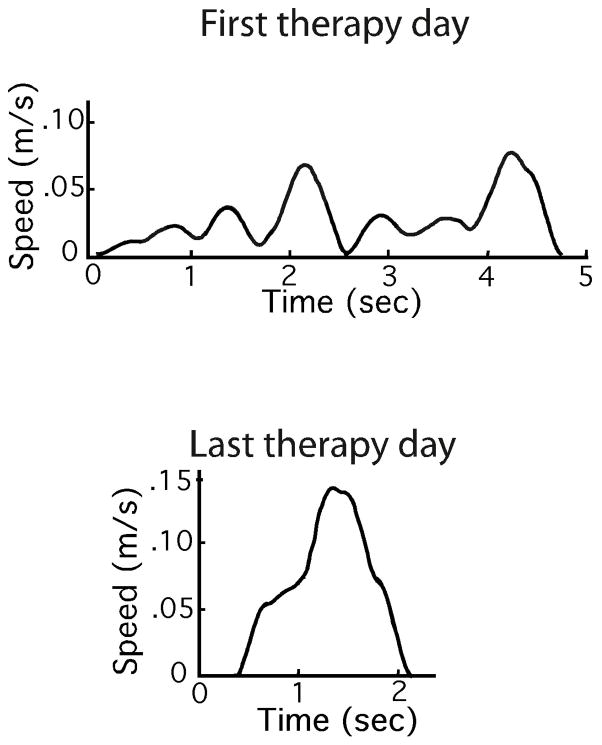
Smoothness is widely regarded as a hallmark of skilled, coordinated movement. Jerk, the time-derivative of acceleration, has been used as an empirical measure of this quality. However, mixed results have been reported. Studies of patients with Parkinson’s disease (Teulings, Contreras-Vidal, Stelmach, & Adler, 1997) and Huntington’s disease (Smith, Brandt, & Shadmehr, 2000) reported statistically significant abnormalities of jerk measures, as expected. Less clear was a comparison of stroke patients with age-matched control subjects by (Platz, Denzler, Kaden, & Mauritz, 1994) who reported that intra-subject variability of jerk was statistically different for patients and controls but mean jerk was not. In a study of patients with cerebellar dysfunction (Goldvasser, McGibbon, & Krebs, 2001) were unable to detect significant abnormalities on the basis of jerk measures. Similarly, (Wininger, Kim, & Craelius, 2009) were unable to detect significant differences between stroke patients and healthy control subjects using jerk measures. This is surprising given that, with recovery, movements made by stroke patients become progressively less fragmented and more coordinated, as illustrated in Figure 1 (Rohrer, et al., 2002), (Rohrer, et al., 2004).
Figure 1.
Tangential speed profiles of reaching movements made by a chronic-phase stroke survivor at the beginning (top panel) and end (bottom panel) of sensory-motor therapy. For further details see (Rohrer, et al., 2004).
One confound which may account for these mixed results is that the different studies applied different jerk measures, each of which scaled differently with movement amplitude and duration and intervals of arrest. This is an important consideration as movements made by patients with sensory-motor dysfunction are often slower than those of unimpaired subjects. Furthermore, they often undershoot or overshoot their target as well as have short intervals of no movement. Several different ways to normalize jerk-based measures have been used to reduce dependency on those variables. The purpose of this paper is to demonstrate how these different jerk-based measures vary with movement amplitude and especially duration; further, we aim to clarify how jerk-based measures may be independent of duration and amplitude, thereby quantifying what they are intended to quantify—smoothness.
Dimensions of jerk-based measures
Part of the appeal of a jerk-based measure is that maximizing smoothness has been shown to provide a competent (albeit coarse-grained) mathematical model of coordination (Flash & Hogan, 1985). To minimize mean-squared jerk, a motion profile must be a simple fifth-order polynomial function relating displacement in each degree of freedom to time. The algebraic simplicity of this form yields straightforward qualitative and quantitative characteristics of maximally-smooth movements such as a straight path and a specific ratio of peak to mean speed.
One important detail of this application of optimization theory is that the scaling factor of the jerk measure is irrelevant. The function x(t)1 that minimizes the functional is the same for any value of the constant C. This constant may be chosen for analytical convenience. The reason is because, in this application, the value of the jerk measure is unimportant. What matters is that the functional based on jerk has an unambiguous minimum and that is ensured by the quadratic form of the integrand.
The situation is quite different if a jerk-based measure is to be used to quantify the smoothness of experimental data. In this case, the constant C plays a key role in establishing the dimensions of the measure and, hence, how it scales with movement amplitude and duration. Table 1 lists several different smoothness measures that have been used in the literature, the latter two using the absolute value (rather than square) as a measure of jerk magnitude. This list is probably incomplete; there are more ways to combine or rearrange the basic operations measuring jerk magnitude (e.g., squaring, taking absolute value) and accumulating this measure over a movement (e.g., integrating, averaging). Importantly, all of these measures, even those including a normalization factor, depend on movement extent and/or duration as reflected in their dimensions.
TABLE 1.
Several Jerk-Based Measures of Movement Smoothness and Their Dimensions
| Measure | Formula | Dimensions | Study | ||
|---|---|---|---|---|---|
| Integrated squared jerk |
|
|
Platz, Denzler, Kaden, & Mauritz (1994) | ||
| Mean squared jerk |
|
|
Wininger, Kim, & Craelius (2009) | ||
| Cumulative squared jerk |
|
|
Smith, Brandt, & Shadmehr (2000) | ||
| Root mean squared jerk |
|
|
Young & Marteniuk (1997) | ||
| Mean squared jerk normalized by peak speed |
|
|
Hester et al. (2006) | ||
| Integrated absolute jerk |
|
|
Goldvasser, McGibbon, & Krebs (2001) | ||
| Mean absolute jerk normalized by peak speed |
|
|
Rohrer et al. (2002) |
Note. L = length; T = time; tk = discrete samples.
To be pragmatic, if movement extent and duration vary little, this dependency is of little consequence. Even if movement extent or duration varies, for some purposes this dependency may be advantageous and be part of an intended characterization. For example, (Rohrer, et al., 2002) used mean-absolute-jerk normalized by peak speed in a study of sub-acute phase and chronic-phase stroke survivors. This measure is independent of movement extent but depends inversely on the second power of movement duration, yielding smaller values for longer movements even when shape is invariant. However, it also exhibits a distinctive non-monotonic variation with periods of arrest, which provided insight about the process underlying recovery after neurological injury and its response to sensory-motor therapy.
Nevertheless, if a measure of the shape of movement is desired—without any dependency on duration and amplitude—that measure must be dimensionless. The integrated squared jerk has dimensions of length squared divided by the 5th power of time, L2/T5 (see Table 1). Hence, a dimensionless-squared-jerk measure is where A is movement amplitude or extent and D = t2 − t1 is duration. This is by no means the only way to obtain a dimensionless measure of smoothness. Because mean speed is the ratio of movement amplitude to duration, vmean = A/D, the jerk measure could be re-written as . Alternatively, any other representative speed could be used to define a dimensionless smoothness measure, e.g. peak speed . In the following, for clarity we confine our attention to the former measure, which has been used in the literature (Takada, Yashiro, & Takagi, 2006; Yashiro, Nakamura, Mizumori, Yatani, & Takada, 2004).
Performance of jerk-based measures
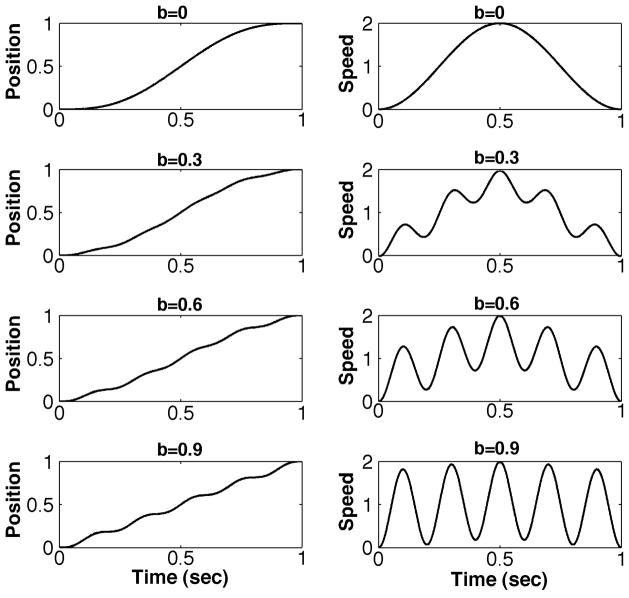
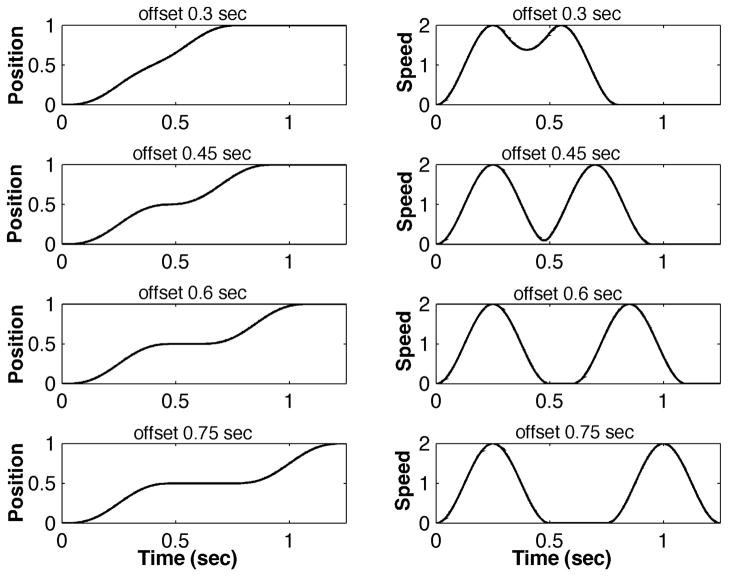
Given the plethora of jerk-based measures it is useful to understand how they vary with the characteristics of typical experimental movement data that they are intended to characterize. To that end, we constructed a series of idealized motion profiles mimicking a typical point-to-point movement. The function we used is loosely related to a cycloidal motion. Specifically, movements were constructed with displacement being the sum of (i) a terminated linear ramp and (ii) a suitably-scaled sinusoid with a period equal to the movement duration, and (iii) a suitably-scaled sinusoid with a period equal to an integer (n) divisor of the movement duration,
where the constant b determines the fractional deviation from a smooth cycloidal movement. With n = 1 the result is a symmetric movement with a smooth “bell-shaped” speed profile (an offset cosine function). With n > 1 the result is a symmetric movement with a series of “hesitations” (Figure 2).
Figure 2.
Examples of cycloidal point-to-point movements with various degrees of smoothness: n = 5, A = 1, D = 1, and b = [0.0, 0.3, 0.6, 0.9]. Left column: position vs. time. Right column: tangential speed vs. time.
This mathematical form was chosen primarily for analytical convenience rather than any biological significance. Nevertheless, the motion profiles generated are qualitatively similar to a substantial body of the published experimental movement data.
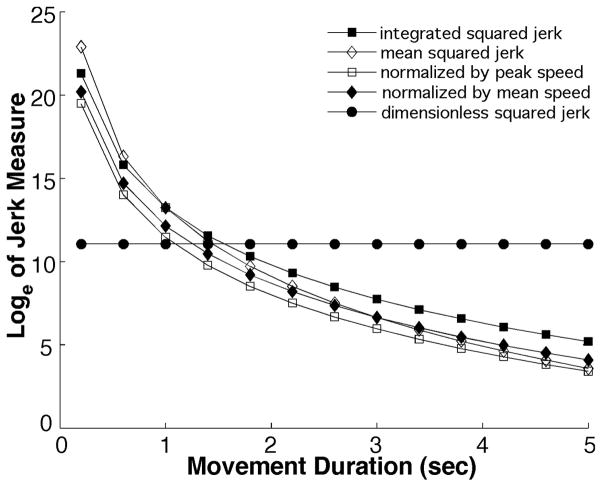
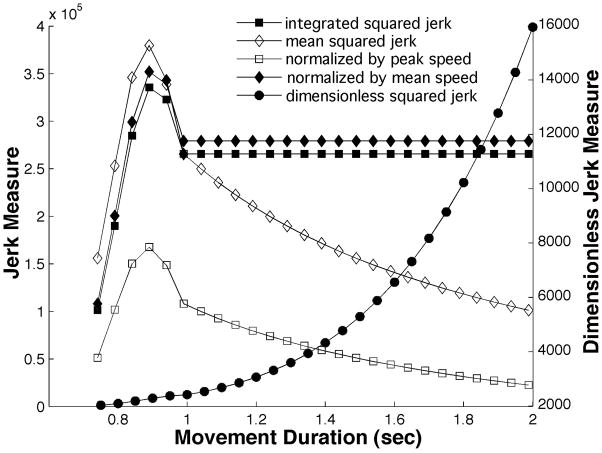
Figure 3 shows how several squared-jerk measures of smoothness vary with movement duration D while movement amplitude A and the number of “hesitations” n remain constant. The main point to note is that, while the several jerk measures with units (integrated squared jerk, mean squared jerk, mean squared jerk normalized by peak speed, mean squared jerk normalized by mean speed) differ in their precise details, all exhibit an essentially similar variation with movement duration: as movement duration increases, all of these measures become dramatically smaller (note that the ordinate is plotted on a logarithmic scale). In contrast, the dimensionless squared jerk measure is completely independent of movement duration—as intended.
Figure 3.
Variation of several measures of smoothness based on squared jerk with movement duration while movement amplitude and shape (number of speed peaks, deviation of speed profile from a cycloidal movement) remain constant. For graphical presentation the ordinate is plotted on a logarithmic scale.
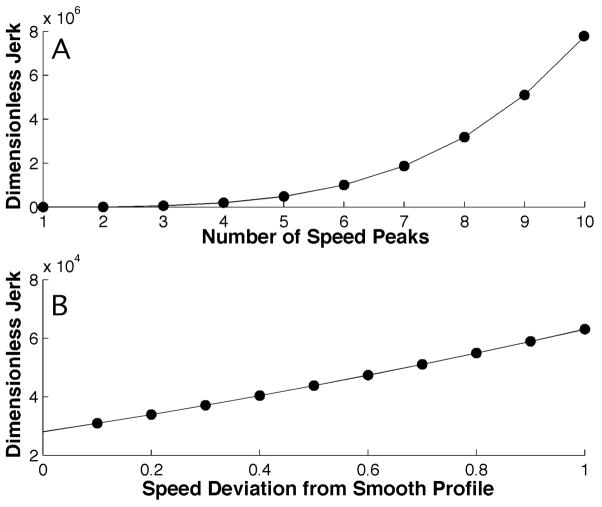
How well does the dimensionless squared jerk measure reflect smoothness? Figure 4A shows the variation of dimensionless squared jerk with the number of speed peaks in a movement n, while movement amplitude A, duration D and the magnitude of speed fluctuations b remain constant. As expected, the dimensionless squared jerk measure grows with the number of peaks n. Figure 4B shows the variation of dimensionless squared jerk with the magnitude of speed fluctuations, while movement amplitude, duration and the number of speed peaks remain constant. Again, as expected, the dimensionless squared jerk measure grows with the magnitude of speed fluctuations–as it should do intuitively.
Figure 4.
Variation of dimensionless squared jerk with movement smoothness. In the top panel (A) the number of speed peaks, n, is varied while movement amplitude, duration and deviation of the smooth cycloidal movement remain constant. In the bottom panel (B) the deviation from a smooth cycloidal movement, b, is varied while movement amplitude, duration and number of peaks remain constant.
Sensitivity to arrest periods
One concern raised about any of the jerk-based measures is that, as they are computed by integrating across an entire movement profile, they may appear to be insensitive to brief periods of arrest. For example, pathological movements often exhibit a distinctive fragmentation, even exhibiting intervals of complete rest (Krebs, Aisen, Volpe, & Hogan, 1999; Rohrer, et al., 2002). During those intervals, all derivatives are (approximately) zero; hence they contribute little or nothing to an integrated measure. However, for movements of the same extent and duration, periods of arrest are necessarily accompanied by intervals in which the time-derivatives of movement are exaggerated—pronounced speed fluctuations are a signature of movement fragmentation—and the jerk-based measures are especially sensitive to those derivatives.
To investigate how arrest periods influence jerk-based measures of smoothness, we constructed a series of idealized motion profiles composed of two identical cycloidal movements: terminated ramp plus scaled sinusoid with period equal to ramp duration, or b = 0. Compared to the examples above, each sub-movement had half of the movement amplitude but a different onset time. Specifically, the second submovement was initiated after a delay of W that varied from less than the duration of one submovement to greater than that duration so that total movement duration was Ttotal = D+W (Figure 5 shows examples).
Figure 5.
Examples of point-to-point movements composed of two identical cycloidal movement fragments with various amounts of delay between the onset of the first and second submovements. Left column: position vs. time. Right column: tangential speed vs. time.
Figure 6 shows how several squared-jerk measures vary with movement duration as induced by the temporal separation of the two 500 ms submovements. When the two submovements overlap in time, all four of the measures with units (integrated squared jerk, mean squared jerk, mean squared jerk normalized by peak speed, mean squared jerk normalized by mean speed) exhibit a non-monotonic variation with duration. When the two submovements are separated in time, as exemplified in the two bottom rows of Figure 5, integrated squared jerk remains constant—the rest periods contribute nothing to the integral—while mean squared jerk declines with total movement duration. Similarly, mean squared jerk normalized by mean speed does not vary as separation increases while mean squared jerk normalized by peak speed declines with total movement duration.
Figure 6.
Variation of several measures of smoothness based on squared jerk with the total duration of a movement composed of two identical cycloidal submovements. The four measures with units are plotted at a common scale displayed on the left ordinate. The dimensionless measure is plotted at a different scale displayed on the right ordinate.
In contrast, the dimensionless squared jerk measure increases monotonically with the temporal separation of the two submovements. Unlike the four measures with units, this measure increases monotonically even when the two submovements overlap. It also continues to increase when the two submovements are separated in time. This properly reflects the change of movement shape with duration; as the separation of submovements grows longer, the movement profile becomes progressively less smooth.
Measures of shape should be dimensionless
These examples show that a dimensionless jerk measure may readily be formulated to reflect changes of movement shape, independent of amplitude and duration, yet reflective of common departures from smoothness, including multiple speed peaks or periods of arrest. The dimensionless jerk measure plotted in Figure 6 was used by (Yashiro, et al., 2004) to distinguish the effects of different mouth-guard designs on jaw movements during speech articulation. It was also used by (Takada, et al., 2006) to discriminate between irregularities of chewing movements induced by occlusal interference at different tooth sites. A similar measure was successfully used by (Teulings, et al., 1997) to detect statistically significant differences between movements of patients with Parkinson’s disease and age-matched unimpaired subjects. (Ketcham, Seidler, Gemmert, & Stelmach, 2002) used the same measure to quantify significant degradation of movement quality in older adults.
Dimensionless numbers are used extensively in engineering and physics. They are especially useful to characterize essential features of physical phenomena and how they vary with size and scale. For example, Reynolds’ number (the ratio of inertial to viscous forces) provides a universal characterization of fluid flow, enabling identification of turbulent and laminar flow and the transitions between these two regimes. The Froude number (the ratio of inertial to gravitational forces) has been used to predict optimal walking speeds in different gravitational environments (Minetti, 2001). Intuitively, smoothness is an aspect of movement quality distinct from speed and distance and should therefore be independent of them, i.e. should be dimensionless.
Of course, jerk-based measures are not the only way to quantify smoothness. Numerous alternatives may be defined and many of them may be superior. For example, robust and statistically sensitive methods to identify a sequence of submovements underlying continuous movements have been developed (Rohrer & Hogan, 2003), (Rohrer & Hogan, 2006). However, care is required. One simple alternative is to count peaks in the speed profile. While this may have intuitive appeal, it suffers some intrinsic weaknesses: Firstly, the sum of two or more submovements, each with single-peaked speed profiles, may generate spurious peaks in the composite speed profile (Rohrer & Hogan, 2003). Secondly, this measure is completely insensitive to the presence or extent of periods of arrest. The Movement Arrest Period Ratio (Beppu, Suda, & Tanaka, 1984) addresses the latter weakness, but it is insensitive to fluctuations in speed that do not result in a detectable period of arrest, even though they clearly signal a departure from the smoothness of normal, coordinated movements.
A weakness of both of these measures is that they essentially discard much of the available data. Measures that depend on all of a data stream are more likely to yield statistically reliable results and quantify what they are meant to quantify: departures from smoothness. Quantities based on the integrated magnitude of derivatives such as jerk are simple to implement and provide an intuitively meaningful measure of shape and smoothness—provided they are appropriately scaled to be dimensionless.
Acknowledgments
We would like to thank Tjeerd Dijkstra for his helpful comments. Neville Hogan was supported in part by Toyota Motor Corporation’s Partner Robot Division; by the New York State Center of Research Excellence, contract CO19772; and by the Eric P. and Evelyn E. Newman Fund. Dagmar Sternad was supported by grants from the National Science Foundation, BCS-0450218, the National Institutes of Health, R01 HD045639, and the Office of Naval Research, N00014-05-1-0844.
Footnotes
Notation: x is position, t is time, the overdot denotes time differentiation and subscripts 1 and 2 identify the beginning and end of movement respectively
References
- Beppu H, Suda M, Tanaka R. Analysis of cerebellar motor disorders by visually guided elbow tracking movement. Brain. 1984;107:787–809. doi: 10.1093/brain/107.3.787. [DOI] [PubMed] [Google Scholar]
- Flash T, Hogan N. The Coordination Of Arm Movements - An Experimentally Confirmed Mathematical-Model. Journal Of Neuroscience. 1985;5(7):1688–1703. doi: 10.1523/JNEUROSCI.05-07-01688.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldvasser D, McGibbon CA, Krebs DE. High curvature and jerk analysis of arm ataxia. Biological Cybernetics. 2001;84:85–90. doi: 10.1007/s004220000201. [DOI] [PubMed] [Google Scholar]
- Hester T, Hughes R, Sherrill DM, Knorr B, Akay M, Stein J, et al. Using wearable sensors to measure motor abilities following stroke. Paper presented at the International Workshop on Wearable and Implantable Body Sensor Networks; Cambridge, MA. 2006. Apr 3–5, [Google Scholar]
- Ketcham CJ, Seidler RD, Gemmert AWAV, Stelmach GE. Age-Related Kinematic Differences as Influenced by Task Difficulty, Target Size, and Movement Amplitude. Journal of Gerontology: Psychological Sciences. 2002;57B(1):P54–P64. doi: 10.1093/geronb/57.1.p54. [DOI] [PubMed] [Google Scholar]
- Krebs HI, Aisen ML, Volpe BT, Hogan N. Quantization of continuous arm movements in humans with brain injury. Proceedings Of The National Academy Of Sciences Of The United States Of America. 1999;96(8):4645–4649. doi: 10.1073/pnas.96.8.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minetti AE. INVARIANT ASPECTS OF HUMAN LOCOMOTION IN DIFFERENT GRAVITATIONAL ENVIRONMENTS. Acta Astronautica. 2001;49(3–10):191–198. doi: 10.1016/s0094-5765(01)00098-4. [DOI] [PubMed] [Google Scholar]
- Platz T, Denzler P, Kaden B, Mauritz KH. Motor Learning after Recovery from Hemiparesis. Neuropsychologia. 1994;32(10):1209–1223. doi: 10.1016/0028-3932(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Fasoli S, Krebs HI, Hughes R, Volpe B, Frontera WR, et al. Movement smoothness changes during stroke recovery. Journal Of Neuroscience. 2002;22(18):8297–8304. doi: 10.1523/JNEUROSCI.22-18-08297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer B, Fasoli S, Krebs HI, Volpe B, Frontera WR, Stein J, et al. Submovements grow larger, fewer, and more blended during stroke recovery. Motor Control. 2004;8(4):472–483. doi: 10.1123/mcj.8.4.472. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Hogan N. Avoiding spurious submovement decompositions: a globally optimal algorithm. Biological Cybernetics. 2003;89(3):190–199. doi: 10.1007/s00422-003-0428-4. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Hogan N. Avoiding spurious submovement decompositions II: a scattershot algorithm. Biological Cybernetics. 2006;94:409–414. doi: 10.1007/s00422-006-0055-y. [DOI] [PubMed] [Google Scholar]
- Smith MA, Brandt J, Shadmehr R. Motor disorder in Huntington’s disease begins as a dysfunction in error feedback control. Nature. 2000;403:544–549. doi: 10.1038/35000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K, Yashiro K, Takagi M. Reliability and sensitivity of jerk-cost measurement for evaluating irregularity of chewing jaw movements. Physiological Measurement. 2006;27:609–622. doi: 10.1088/0967-3334/27/7/005. [DOI] [PubMed] [Google Scholar]
- Teulings HL, Contreras-Vidal JL, Stelmach GE, Adler CH. Parkinsonism Reduces Coordination of Fingers, Wrist, and Arm in Fine Motor Control. Experimental Neurology. 1997;146:159–170. doi: 10.1006/exnr.1997.6507. [DOI] [PubMed] [Google Scholar]
- Wininger M, Kim NH, Craelius W. Spatial resolution of spontaneous accelerations in reaching tasks. Journal of Biomechanics. 2009;42:29–34. doi: 10.1016/j.jbiomech.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Nakamura T, Mizumori T, Yatani H, Takada K. Clinical Validity of Measuring Jerk-cost of Jaw Movement during Speech: Effect of Mouthguard Design on Smoothness of Jaw Movements. Paper presented at the SICE Annual Conference; Sapporo, Hokkaido Institute of Technology, Japan. 2004. Aug 4–6, [Google Scholar]
- Young RP, Marteniuk RG. Acquisition of a multi-articular kicking task: Jerk analysis demonstrates movements do not become smoother with learning. Human Movement Science. 1997;16(5):677–701. [Google Scholar]