Abstract
Presently, orthopedic and oral/maxillofacial implants represent a combined $2.8 billion market, a figure expected to experience significant and continued growth. Although traditional permanent implants have been proved clinically efficacious, they are also associated with several drawbacks, including secondary revision and removal surgeries. Non-permanent, biodegradable implants offer a promising alternative for patients, as they provide temporary support and degrade at a rate matching tissue formation, and thus, eliminate the need for secondary surgeries. These implants have been in clinical use for nearly 25 years, competing directly with, or maybe even exceeding, the performance of permanent implants. The initial implantation of biodegradable materials, as with permanent materials, mounts an acute host inflammatory response. Over time, the implant degradation profile and possible degradation product toxicity mediate long-term biodegradable implant-induced inflammation. However, unlike permanent implants, this inflammation is likely to cease once the material disappears. Implant-mediated inflammation is a critical determinant for implant success. Thus, for the development of a proactive biodegradable implant that has the ability to promote optimal bone regeneration and minimal detrimental inflammation, a thorough understanding of short- and long-term inflammatory events is required. Here, we discuss an array of biodegradable orthopedic implants, their associated short- and long- term inflammatory effects, and methods to mediate these inflammatory events.
Keywords: biodegradable, orthopedic biomaterials, foreign body reaction, inflammation, fibrosis
I. INTRODUCTION
The search for a promising alternative for the replacement or functional restoration of damaged bone tissues has largely pointed to biodegradable implants.1 The concept of biodegradable implants was introduced by Kulkarni et al. in the late 1960s. Subsequently, the first usage of these materials in orthopedic surgery was completed by Rokkanento to treat ankle fractures in 1984 with internal fixation devices.2–4 Over the past three decades, the number of biodegradable implants in clinical use has significantly increased, which includes cylinder rods, plates, screws, tacks, suture anchors, mesh, plugs, wires, arrows, and drug delivery devices.2
Biodegradable implants offer a number of financial, psychological, and clinical advantages over permanent metal implants.5 They provide the appropriate amount of mechanical strength when necessary, and degrade at a rate similar to new tissue formation, thereby transferring the load safely to the healed bone and eliminating the need for an additional revision and removal operation.6 Of note, the added expense of metallic implant removal, which occurs 19 to 54% of the time depending on the fracture type, makes resorbable implants more cost-effective by approximately seven hundred dollars less per metallic implant.7 Permanent implants, which include metallic (ie, stainless steel, cobalt-chromium, and titanium alloys) and non-degradable plastic (ie, polyethylene and polyurethane) implants, are often cited as being too strong or stiff for optimal bone healing as well as creating stress shielding.8 In addition, metal implants are often removed, either after the healing process is complete, or due to complications such as the accumulation of metals in tissues,9,10 growth restriction,11 pain,12 and/or implant migration.13
An astonishing number, over 90%, of patients in a perception prospective study conducted by Mittal et al. considered revision and removal surgeries to be the largest drawback of metal implants.2 The avoidance of removal surgeries, as seen with biodegradable implants, is highly appreciated by patients, since it results in decreased patient costs, recovery time, and time off from work. Biodegradable implants may also present less long-term inflammation since their stay in the body is only temporary, as compared to permanent implants. Additionally, the patient will not have to experience the added trauma from these surgeries, therefore reducing the physiological impact and stress on the patient and their family. For health-care providers, reduced financial costs via more efficient use of healthcare resources and surgeon time results in an increased number of possible patients that may be treated.7 From a clinical point of view, biodegradable implants result in decreased stress shielding,14 and a lack of interference with imaging techniques. There have even been positive results reported on the use of biodegradable screws and pins in ankles.15–19
In addition to serving as fracture fixations and stabilization devices, biodegradable matrices with appropriate pore size and interconnectivity are used to promote the regeneration of engineered bone tissue.. Bone defect repair utilizing tissue engineering strategies has been a rapidly growing field of research.20,21 Biodegradable implants and porous matrices may also be used as drug releasing devices. For instance, these implants encapsulated with growth factors and antibiotics have potential to accelerate healing processes and prevent chronic bone infections, respectively.22
Although biodegradable implants overcome inherent disadvantages of permanent metal implants, they are also associated with various shortcomings. Long-term performance of the biodegradable implant is largely determined by the inflammatory events at the implant-host tissue interface,23 and their degradation profiles. The following is a review of the advances that have been made in understanding the acute and chronic inflammatory events at the implant-host tissue interface and the developing methods for controlling these events. A thorough understanding of the body’s response at the bone-implant interface is crucial for the development of proactive implants that promote optimal outcomes for patients.24
II. BIODEGRADABLE ORTHOPEDIC IMPLANTS
The concept of biodegradable implants came into existence through the discovery of sutures that resorb in the body.25,26 Based on the success of biodegradable sutures, many biodegradable implants were designed for various clinical needs. For centuries, metals, metal alloys, and non-degradable plastics have dominated the field of orthopedic implants. However, due to the problems associated with permanent implants, the quest for biodegradable implants has been intensified resulting in a number of biodegradable implants for orthopedic use.27,28 In the last few years, the number of FDA-approved biodegradable orthopedic implants and the market for these products have shown tremendous growth.29
II.A. Types of Biodegradable Materials
Biodegradable polymers are also known as bioresorbable or bioabsorbable polymers. These polymers can be synthetic or natural and degrade through hydrolysis/enzymatic degradation. According to the literature, polyglycolide (PGA) was the first biodegradable polymer known to the device community.26 Since then, researchers have developed a wide variety of biodegradable polymer groups such as poly(α-esters), polyphosphazenes, and polyurethanes.30,31 Among them, the poly(α-esters) group, which includes polyglycolide (PGA), polylactide (PLLA), and their co-polymer poly(lactide-co-glycolide) (PLGA), have attracted much attention by the orthopedic device community, due to their excellent biocompatibility and tunable physical and degradation properties. Polymers such as polydioxanone (PDO), polycaprolactone (PCL), polyhydroxybutyrate (PHB), and polyhydroxyvalerate (PHV) have also been accepted for use in orthopedic devices. In addition to synthetic polymers, researchers are also developing implants utilizing biopolymers such as collagen, elastin, and hyaluronic acid, as well as natural polymers such as chitosan, silk, and starch. Further, the synthetic and natural polymers have been combined with nano-hydroxyapatite (nano-HAP) or tri-calcium phosphate (TCP) to design orthopedic implants that have bone-like composition. A list of synthetic and natural polymers and their composites that have been developed into orthopedic implants is presented in Table 1.
TABLE 1.
Summary of Biodegradable Orthopedic Implants
| Biodegradable Material | Type of Implant | Properties that Influence FBR | Adverse Event |
|---|---|---|---|
| In Clinical Use
| |||
| Poly(α-esters): PGA PLLA, PDLLA, PLLAHA, PLLA-TCP PLGA PDO PGA-TMC |
Pins, Screws, Plates, Rods, Tacks, Suture anchors, Spine cages, and Scaffolds | Acidic degradation (Lactic/Glycolic Acids) Hydrophobic Implant fragmentation & particle generation |
Fluid accumulation Sinus formation Swelling Osteolysis Synovitis Cyst formation (human studies) |
| Collagen Collagen-HA Collagen-TCP |
Injectable gels and Foams for BTE | Biological | None |
| Calcium Phosphate TCP, HA |
Bone cements Scaffolds |
Slow degradation Particle generation |
None (rabbit study) |
|
| |||
| Under Development
| |||
| Poly(α-esters): PCL, PHB, PHV, PHBV | Scaffolds | Acidic degradation Hydrophobic Slow degradation |
None (human study) |
| Poly(propylene fumerate) | Scaffolds, Injectable gels | Acidic degradation (Fumaric acid, Propylene glycol) | Minimal inflammatory response (rabbit study) |
| Poly(ester urethanes) | Injectable gels and Scaffolds | Neutral degradation (Ammonia, Carboxylic acids) Enzymatic degradation |
No acute inflammation (mice study) |
| Polyphosphazenes PNEG, PNEA, PNEPhA | Scaffolds | Neutral degradation (Ammonia + Phosphate) Tunable hydrophobicity |
Less inflammatory response compared to PLGA (rat study) |
| Natural Polymers: Chitin & Chitosan, Starch | Injectable gels Scaffolds |
Natural and biological enzymatic degradation | Chronic inflammation (rabbit study) |
PGA – polyglycolide; PLLA – poly(L)lactide; PDLLA – poly(DL)lactide; TCP – tri-calcium phosphate; HA – hydroxyapatite; PLGA – poly(lactide-co-glycolide); PDO – polydioxanone; PGA-TMC - poly(glycolide-co-trimethylene carbonate); PCL – poly(caprolactone); PHB – poly(hydroxybutyrate); PHV - poly(hydroxyvalerate); PHBV – poly(hydroxybutyrate-co-hydroxyvalerate); PNEG – poly[bis(ethyl glycinato)phosphazene]; PNEA – poly[bis(ethyl alanato)phosphazene]; PNEPhA – poly[bis(ethyl phenylalaninato) phosphazene].
II.B. Biodegradable Implants
A number of polymers, mainly poly(α-esters), have been fabricated into various orthopedic devices for fracture fixation, interference fixation, and meniscal repair. Devices such as screws, plates, rods, staples, and tacks, are currently being used in orthopedic, maxillofacial, and spine surgery applications (Fig. 1).28,29,32 In addition, scaffolds for bone tissue engineering, and cements and gels for bone defect filling are currently under development.33 A large amount of clinical data is now available on biodegradable implants, mainly screws. The data suggests that in a majority of the cases the implants fulfill the clinical need, however, the long-term follow-up studies have shown some adverse effects (Table 1). Biodegradable implants and the possible short-term and long-term foreign body reactions are reviewed in the following sections.
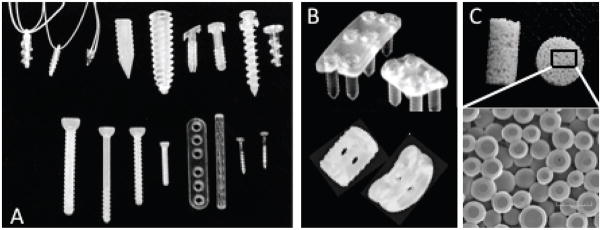
FIGURE 1.
Biodegradable orthopedic implants. (A) Screws, plates, rods and tacks, commonly utilized for fracture fixation and meniscal repair (reprinted from Ciccone et al. 200128). (B) Plates and cages for spinal repair. (C) Porous scaffolds for bone tissue engineering [reprinted from Journal of the American Academy of Orthopaedic Surgeons, vol. 9, no. 5, pp. 280–8, Ciccone WJ, Motz C, Bentley C, Tasto JP. Bioabsorbable implants in orthopaedics: new developments and clinical applications. Copyright (2001), with permission from Elsevier].
III. SHORT-TERM EVENTS OF BIODEGRADABLE IMPLANT-ASSOCIATED FOREIGN BODY REACTIONS
III.A. Onset of Foreign Body Reaction
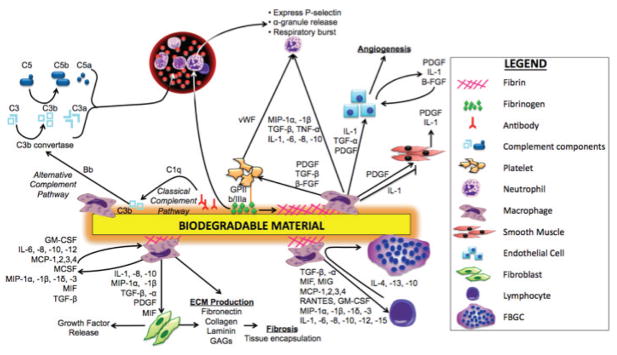
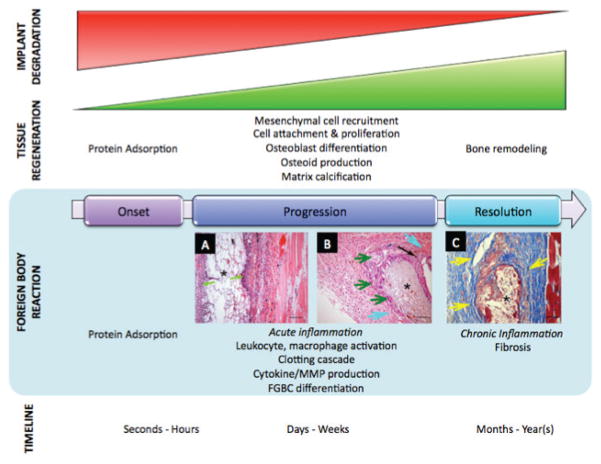
Surgical implantation of a biomaterial, regardless of its being inert and nontoxic, will initiate the on-set of a foreign body reaction (FBR).34 It has been established that the combination of two events is the key player in triggering an inflammatory cascade by the host -- the immediate adsorption and binding of blood plasma proteins and other molecules onto the biomaterial’s surface, as well as the injury of the micro-vasculature and tissue during implantation and the associated histamine release by local mast cells. The mechanisms by which surface- absorbed proteins modulate the progression of FBR, including leukocyte recruitment, differentiation into foreign body giant cells (FBGCs), and cytokine and matrix metalloproteinases (MMPs) release, have been largely investigated and are illustrated in Fig. 2.
FIGURE 2.
Schematic illustration of biodegradable material-induced inflammation.39,47,63,91,92
1. Biomaterial Surface Protein Adsorption
The protein layer that immediately forms on the biomaterial upon implantation has been referred to as a ‘conditioning film.’35 The ‘Vroman effect,’ first described about half a century ago, explains the competition, rearrangements, and displacement of absorbed proteins that occurs at the biomaterial surface.36,37 Small proteins adsorb first since they can be rapidly transported to the surface. Then, gradually, they are replaced with larger proteins with higher affinity.23 After complex processes of adsorption and desorption, an equilibrium is finally reached at the interface.38 This ‘conditioning film’ of proteins on the biomaterial has been proven to mediate host tissue-implant interactions and initiate FBRs.35 Specifically, this protein film is recognized by the integrin receptors present on neutrophils and macrophages, and thus, converts the implant into a biologically recognizable material.39 It is important to note that the onset and progression of the FBR is independent of whether the implant is permanent or biodegradable, but dependent on the protein layer bound to the biomaterial’s surface.
Fibrinogen, albumin, complement fragments, and non-specific antibodies are among the proteins that are absorbed on the biomaterial’s surface.40–42 It has been proved that fibrinogen, unlike complement and immunoglobulin G, is essential for the host to mount an inflammatory response to the implanted biomaterial. Animals unable to synthesize complement and immunoglobulin G still experience an acute inflammatory response to biomaterial implantation, with neutrophils and macrophages surrounding the biomaterial. On the other hand, animals with low levels of fibrinogen require injections of fibrinogen to mount an inflammatory response to the implanted biomaterial.43
Implant-bound fibrinogen interacts with macrophages via a macrophage integrin, Mac-1/CD11b/CD18, and P1, a short sequence within the fibrinogen D domain.43,44 It has been demonstrated that the absorption of fibrinogen to implant surfaces leads to the unmasking of the P1 sequence and a second sequence, P2. Also, on the biomaterial surface, the P1 and P2 sites may be exposed via the coagulation cascade and undergo thrombin-mediated conversion of fibrinogen to fibrin. Fibrin(ogen) P1 and P2 sites together interact with Mac-1 and allow for the binding of macrophages; and thus, they are directly related to the recruitment of macrophages, and inflammatory response severity.41 Activated macrophages release cytokines and chemokines, including IL-1, TNF-α, VEGF, monocyte chemoattractant protein (MCP)-1, and IL-8, which in turn activate the proximal vasculature and attract leukocytes and fibroblasts (Fig. 2).45 Soluble fibrinogen, unlike implant-absorbed fibrinogen, does not have the P1 sequence exposed, and thus, is not pro-inflammatory.44 Implant-bound fibrinogen, via the GPIIb/IIIa integrin, may also bind to platelets. These cell fragments contain α-granules, which are composed of several growth factors, such as insulin-like growth factor-1 (IGF-1), platelet-derived growth factor (PDGF), and transforming growth factor-β (TGF-β), and clotting proteins including thrombospondin, fibronectin, and von Willebrand factor (vWF). The α-granules also express the adhesion molecule P-selectin, which plays an essential role in the initial recruitment of leukocytes to the site of injury during FBR.
The complement cascade may be triggered by two different pathways during the implantation of a biomaterial, namely, via the alternative and classical complement pathways (Fig. 2).46 The alternative complement pathway is activated with the spontaneous adsorption of complement protein, C3b, onto the biomaterial’s surface. C3b may further complex with complement factor Bb, resulting in the formation of C3b convertase (C3bBb), which is responsible for the splicing of C3 into many C3a’s and C3b’s. Subsequently, this leads to the release of C5a, a potent leukocyte chemoattractant. C3a and C5a are anaphylatoxic peptides, and have been shown to increase the permeability of the capillary bed, induce the release of histamine from mast cells, and increase monocyte/macrophage activity.47 Furthermore, macrophages can recognize and bind to the absorbed C3b via CR1/CD35 or CD11b/CD18 and CD11c/CD18 cell-membrane receptors. 40,48 On the other hand, with a specific binding of antibodies to the implant surface, activation of the classical complement pathway may ensue. The Fc domain of these antibodies is recognized by complement factor C1q, causing deposition and activation of C3b on the biomaterial, resulting in a similar response as seen with spontaneously adsorbed C3b. Macrophages can also recognize the Fc domain via Fc receptors, which upon stimulation cause the macrophages to release pro-inflammatory cytokines, such as IL-1, IL-6, and TNFa, furthering the FBR progression.40,45
2. Histamine Release by Local Mast Cells
Microvasculature and tissue damage during implantation results in a histaminic response in the surrounding tissue.43 Tang et al. demonstrated that histamine release by mast cells triggers the rapid migration of inflammatory cells, such as neutrophils and monocytes, towards the implanted biomaterial, and progression of a FBR.49 Specifically, mast cell degranulation and histamine release causes hyperemia, enhanced expression of endothelial adhesion molecules (ie, P-selectin), and subsequent migration of phagocytic cells through the endothelial barrier. Chemokines, including macrophage inflammatory protein-1a (MIP-1a) and monocyte chemoattractant protein-1 (MCP-1), direct phagocytic cells toward the biomaterial. Phagocytic cells adhere to the implant surface through the binding sites on the adsorbed fibrinogen and Mac-1. The importance of histamine in the progression of FBRs has been demonstrated as histamine release is largely observed adjacent to subcutaneously implanted biomaterial. Furthermore, the importance of histamine in the progression of FBRs has been demonstrated through the inhibition of the H1 and H2 histamine receptors (ie, pyrilamine and famotidine, respectively), which results in significantly lower numbers of neutrophils and macrophages recruited to the site of implantation.41 Also, a congenital deficiency of mast cells results in a decrease in inflammatory cells at intraperitoneal and subcutaneous implants; however, after replenishing these animals with mast cells, a normal inflammatory response may be observed upon implantation.49
III.B. Progression of Foreign Body Reaction
1. Leukocyte Extravasation
The homing of polymorphonuclear leukocytes (ie, neutrophils) around the implanted biomaterial physically marks the beginning of an acute inflammatory reaction.34,50 Neutrophils are stimulated by the histamine and pro-inflammatory cytokines, such as IL-1β and TNF-α, released from mast cells. These signals also stimulate the expression of vascular cell adhesion molecule (VCAM)-1, intercellular adhesion molecule (ICAM)-1, and E-selectin on vascular endothelial cells.40 Increased neutrophil-endothelial cell interaction, via the binding of endothelial cell adhesion molecules and neutrophil ligands, results in the extravasation of neutrophils from the bloodstream. Chemokines direct the neutrophils to the implantation site once they have left the bloodstream, at which time they will degranulate and release pro-inflammatory mediators to further enhance the recruitment of additional inflammatory cells. In addition to enhancing the inflammatory response, neutrophils induce angiogenesis by the production of several angiogenic factors, such as vascular endothelial growth factor (VEGF), also known as vascular permeability factor (VPF). VEFG-induced neovascularization is believed to be critical in the development of granulation tissue and successful engraftment of implanted scaffolds into the host.50
2. Monocyte Recruitment and Differentiation into Macrophages
Inflammatory monocytes and macrophages replace short-lived neutrophils by way of chemotaxis.34,40,51 Monocytes are recruited by the pro-inflammatory cytokines, such as monocyte chemotactic protein-1 (MCP-1) and IL-6, which are released from the neutrophils and mast cells surrounding the implant. 52 After adhering to the biomaterial, monocytes differentiate into macrophages. Macrophages differ morphologically from monocytes as they have a greater cytoplasmic-to-nuclear area ratio and are more spread; however, they express both F4/80 and CD11b, demonstrating their monocytic lineage (Fig. 3a, b).52
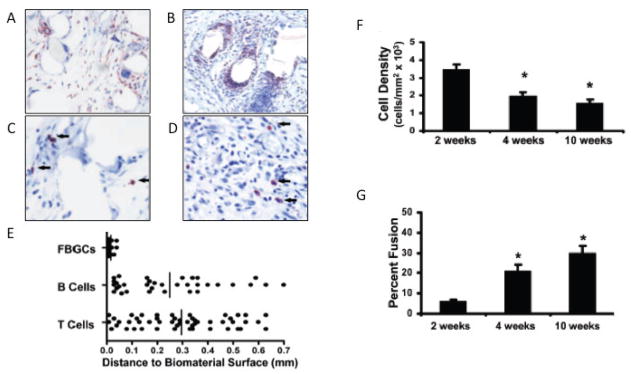
FIGURE 3.
Histological analysis of a subcutaneous mouse model of nylon mesh biomaterial implantation was performed by analysis of tissue sections of the implant and lesion area at 4 weeks post-implantation (reprinted from Higgins et al.200952). (A, B) Immunohistochemical staining of macrophages and FBGCs in implant tissue sections. Tissue sections were stained for (A) F4/80 and (B) CD11b. (C, D) Immunohistochemical staining of (C) B220 for B cells and (D) CD3 for T cells in implant tissue sections. Arrows indicate stained lymphocytes. (E) The distances between stained CD3, B220 cells or FBGCs and the nearest implant surface. (F) Adhesion cell density. (G) Percentage of macrophage fusion [reprinted from the American Journal of Pathology, vol. 175, no. 1, pp. 161–70, Higgins D, Basaraba R, Hohnbaum A, Lee E, Grainger D, Gonzalez-Juarrero M. Localized immunosuppressive environment in the foreign body response to implanted biomaterials. Copyright (2009), with permission from Elsevier].
The accumulation of macrophages is a phenomenon that has been specifically associated with biodegradable implants.39 A dense layer of macrophages is commonly observed in the first few weeks of implantation, as well as the end stage of the resorption process, which depends on the degradation time of the specific biomaterial used.50 Changes in the biomechanical and morphological properties of the degrading materials may significantly intensify the FBR.
Numerous studies have identified macrophages to be the key cellular mediator of implant-associated FBRs.52–60 Being ‘professional’ phagocytic cells, activated macrophages secrete a large array of products, including proteolytic enzymes, free radicals, and reactive oxygen species, in their attempt to degrade the implant. Also, macrophages secrete MMPs and tissue inhibitor of metalloproteinases (TIMPs), which allow for wound healing and tissue regeneration.53,54,61 As seen in Fig. 2, macrophages play a central role in a very complex interaction involving a wide array of inflammatory cytokines, chemokines, growth factors, and complement components, as well as numerous cell types, including other macrophages, neutrophils, lymphocytes, endothelial cells, and fibroblasts. Though T and B lymphocytes were originally thought to be the source of many cytokines during the FBRs, this notion has been largely diminished as a result of several recent studies. For instance, T-cell-deficient mice are able to develop a normal FBR with normal levels of IL-13 and IL-4.62 In addition, Higgins et al. found that B- and T- cells are not in close proximity to the FBGCs and thus, may not have significant direct cell-to-cell interactive effects (Fig. 3c, d, e). The presence of only a few lymphocytes in the lesion area also suggests that the high levels of T-cell-associated cytokines found in the lesion are from another source, most likely macrophage- or neutrophil-derived.52
Macrophages can recognize biomaterials via several different pathways, as illustrated in Fig. 2. Upon implantation, adsorbed proteins, such as fibrinogen and fibronectin, may trigger macrophage responses.48 Also, macrophages may bind with implant surface-adsorbed immunoglobins and/or complement components.47 Lastly, the presence of active cytokines and growth factors may allow for macrophage adhesion via ligand-receptor complexes. In any event, macrophages bind to foreign materials via integrin-mediated adhesive interactions. Integrins are heterodimeric transmembrane proteins and primary receptors, which regulate cell adhesion to extracellular matrix (ECM) proteins, as well as intercellular adhesive interactions. These integrin interactions are essential in regulating the functions of macrophages, including rearrangement of the actin cytoskeleton, cell movement, activation of specific cellular functions, gene transcription, cell proliferation and survival.53
Macrophages interact with many cell types that mediate the progression of the FBR via cytokines (Fig. 2). For example, tumor necrosis factor-α (TNF-α) release is a potent inflammatory cytokine that can activate virtually all cells, and is strongly implicated during inflammation and tissue destruction.63 In addition, macrophages have been shown to interact with neutrophils via interleukin-1β (IL-1β), IL-6, IL-8, IL-10, transforming growth factor-β (TGF-β), TNF-α, macrophage inflammatory protein-1α (MIP-1α), and MIP-1β, which results in neutrophil granulation and further cytokine release. Additionally, macrophages have been linked to the clotting cascade through their interaction with platelets via platelet-derived growth factor (PDGF), TGF-β, and β-fibroblast growth factor (β-FGF). Not only do macrophages interact with the coagulation cascade, they also play a crucial role in promoting angiogenesis via releasing IL-1, TGF-β, and PDGF to activate endothelial cells. The release of PDGF also stimulates smooth muscle cells and fibroblasts, leading to the formation of more mature vessels, and less ‘leaky’ vessels. Also, macrophages secrete numerous mediators that influence their own actions, such as IL-6, IL-10, IL-12, MCP -1, -2, -3, -4, macrophage colony-stimulating factor (MCSF), MIP-1α, -1β, -1δ, -3, macrophage migration inhibitory factor (MIF), and TGF-β. In respect to progression of the FBR, macrophages have also been shown to secrete high levels of pro-inflammatory cytokines, including IL-1β and IL-6, early upon biomaterial implantation.43 However, as time progresses, the levels of pro-inflammatory cytokines diminish, and macrophages express higher levels of anti-inflammatory IL-10, which may have a role in macrophage fusion, and in the suppression of inflammatory responses during the FBR.52
Macrophages have been recently associated with fibrotic matrix formation via interacting with fibroblasts that function in ECM production. A subpopulation of macrophages, referred to as ‘matrix forming macrophages,’ may play an important role in synthesizing the important component of the fibrotic capsule often found surrounding implants. 64 Muldashev et al. identified macrophages with an unusual structure not commonly seen in macrophages --large vacuoles, Hale-positive cytoplasm, presence of the endo- and exocytosis signs, and evidence of active metabolism. Staining positive in the Hale reaction indicates these macrophages are degrading and fragmenting deposited collagen fiber, and then subsequently releasing glycosaminoglycans. Thus, a possible mechanism explaining the formation of the collagen matrix around implants involves fibroblasts synthesizing collagen fibers, and matrix forming macrophages synthesizing the proteoglycan component.
3. Macrophages Differentiate into FBGCs
FBGCs are a morphologic variant of macrophages, and form when the macrophages undergo “frustrated phagocytosis” and are not effective at removing the biomaterial implant (Fig. 3a, b).54 Therefore, as the FBR progresses, the percent of macrophage fusion into FBGCs increases, while cell number surrounding the implants subsequently decreases (Fig. 3f, g). Encapsulation of the biomaterial in a continuous sheet of large, highly multinucleated FBGCs has been proposed as a protective mechanism to isolate the foreign object from the host. The walling-off process by FBGC formation represents an acute phase, which will eventually result in a relatively avascular, highly fibrous capsule that further limits the interaction of the foreign material with the body.
FBGC formation occurs through IL-4 and IL-13 – induced fusion of macrophages,52 and requires the expression of vitronectin,43,45 P2X7, CD98, SiRPaD44, and dendritic cell-specific transmembrane protein (DC-STAMP).40 FBGCs are considered to be at the terminal stage in the monocyte-macrophage differentiation system.53 FBGCs can contain anywhere from three to a hundred nuclei, with their size depending on the intensity of the inflammatory response. Fittingly, the number of FBGCs progressively decreases with time as the biomaterial is degraded and the FBR is resolved.39
Unlike macrophages, FBGCs are poorly phagocytic; however, they exhibit higher extracellular degradation capacity, and express high levels of lysosomes and respiratory enzyme activities. 53,65–67 FBGCs can also secrete chemokines and cytokines to direct and/or activate additional inflammatory and wound healing cells.63 Unlike macrophages, which have been shown to be strongly linked to the fibrotic process and angiogenesis, Luttikhuizen et al. demonstrated that FBGCs are not strongly involved in these two processes as they exhibit only low levels of TGF-β, VEGF, and FGF expression.
Scientists have largely questioned whether the presence of FBGCs near an implant is just a sign of biodegradability and a part of normal bone healing after the biomaterial implantation, or actually a sign of insufficient biocompatibility, inflammation, and implant failure. Nuss et al. have nicely outlined the arguments for both sides of this debate.39 Briefly, one may argue that FBGCs are not a sign of pathology and just a normal part of bone healing after biomaterial implantation since (i) FBGCs mediate the resorption of biodegradable materials, (ii) their presence does not impair bone formation, and (iii) their presence indicates low degradability of the implant. On the other hand, FBGCs may be a sign of pathology (ie, insufficient biocompatibility and implant failure) as (i) the fusion of macrophages is induced by poorly tolerated foreign bodies, (ii) the presence of FBGCs is associated with structural and functional failure of the implant, and (iii) FBGCs seem to concentrate the phagocytic and degradative activities at the tissue-material interface and, therefore, are responsible for the damage and failure of the implant.
More likely, we believe FBGCs are not a sign of failure for biodegradable implants. Major differences between permanent and biodegradable implants are seen in the progression of the FBR. For instance, non-degradable permanent implants generally induce chronic inflammation that is characterized by an ongoing presence of macrophages, FBGCs, an extensive vascular network, and a low number of PMNs. In contrast, biodegradable implants are infiltrated by FBGCs that phagocytose and completely degrade the biomaterial. Since resorption of the biodegradable implant is the ultimate goal, and the regression of FBGCs is seen with the resorption of the biomaterial, the FBGCs are not indicative of insufficient biocompatibility and implant failure.
IV. STAGE-DEPENDENT IMPLANT-MEDIATED INFLAMMATION
The events in the FBR to a biodegradable implant are stage-dependent (Fig. 4). In other words, the onset and progression of FBR is chiefly dependent on the implant’s physical surface cues and attributes, 68 and is independent of the implant’s biodegradability. Specifically, the absorbed protein layer that coats the implant’s surface is responsible for much of the acute cellular events.69 However, over time, the protein coat is continuously changing and the implant begins to degrade via hydrolysis, or enzymatic attack, subsequently allowing for bone tissue regeneration.39 Thus, the resolution of the FBR is essentially dictated by the degradation profile and products of the biodegradable implant. The following section will discuss the events and factors involved in FBR resolution, as well as methods that have previously been utilized to mitigate factors that may possibly lead to the implant’s failure.
FIGURE 4.
Stage-dependent implant-mediated inflammation. Factors that mediate short-term inflammatory responses to biodegradable implants are independent of their biodegradability characteristics, and instead dependent on trauma-induced inflammation and implant surface characteristics. Long-term inflammation induced by these implants, however, is largely affected by their biodegradation.165
V. LONG-TERM EVENTS OF BIODEGRADABLE IMPLANT-ASSOCIATED FBR
The eagerness to utilize biodegradable implants has been constrained by early reports of FBRs and the resulting short-term complications. However, long-term FBR events, whether beneficial or detrimental, are important variables to elucidate. The focus of this section is on biodegradable implants, specifically, factors that contribute to FBRs, as well as the long-term consequences that a non-specific FBR has on the implant and surrounding tissue. Some noted outcomes include toxicity and chronic inflammation as a result of degradation products, fibrous capsule formation, and bone tissue osteolysis. Ultimately, there have been a series of documented long-term effects of biodegradable implants, but the principal finding in many studies is that there are limited or unsignificant associated long-term effects with biodegradable implants.16,70–74
V.A. Implant Degradation
The degradation process of biodegradable polymers generally occurs in two phases. During the first phase, polymers go through a chain scission process. Consequently, the molecular weight of the material is decreased, leading to a loss of mass and mechanical strength. The second phase is characterized by particulate formation through implant breakdown, which, subsequently, are engulfed by macrophages and removed.75 These phases appear to begin with the presence of FBGCs at the biomaterial’s surface. An unstirred microenvironment is developed at the tissue-implant interface, at which FBGCs release degradation enzymes and reactive oxygen species begin to biodegrade the polymer. The chemistry of the biomaterial surface also dictates the rate of degradation and determines its biological impact.76 Factors that affect the degradation of a polymeric implant include the implant material and its crystallinity, implant geometry, site implantation, and the sterilization method used prior to a surgical intervention. 75 In vivo degradation of PLGA-based polymers has been shown to depend strongly on the ratio of monomers found in the co-polymers. For instance, PLGA 50:50 has been shown to have a half-life of approximately 3 weeks, while PLGA 85:15 has a half-life of approximately 12 weeks.77
Despite numerous beneficial attributes, biodegradable polyesters (ie, poly(lactide) (PLA), poly(glycolide) (PGA), poly(lactide-co-glycolide) (PLGA)) have not yet been adopted globally in clinical settings. FBRs caused by acidic by-products of the implant and the variable tissue response to degradation rates have been well documented. For instance, as PLGA degrades, lactic acid and glycolic acid monomers are released in the surrounding tissue. These monomers are later oxidized to pyruvic acid and eventually metabolized in the Kreb cycle. When the release of organic acids over-whelms the system, bone can start to demineralize. Nair and Schug demonstrated this clinically with solid PLGA implants, as they observed a decline in the radiodensity of bone tissue surrounding solid PLGA implants in patients.78
The resulting acidic environment also has a profound effect on the cytokine profiles of inflammatory and endothelial cells surrounding the implants. It has been demonstrated that the decrease in pH alters the amount of vascularization incorporated in a biomaterial long after implantation. Further analysis has shown that osteoblasts, which are responsible for the release of VEGF during healing, generated significantly less VEGF under acidic conditions.79 In addition, polymeric implants with fast degradation times can also alter the amount of blood vessel formation and implant integration. In an in vivo study with mouse models, Sung et al. demonstrated that PLGA implants, with a fast degradation rate, display a significantly lower density of cells expressing CD31, an endothelial cell specific marker, two to four months post-implantation. This resulted in less angiogenesis and decreased bone cell recruitment when compared to the relatively slow degradation of poly e-caprolactone (PCL) implants.80
The rate of polymer degradation is directly related to the associated long-term FBR of a biodegradable implant. A fast rate of biomaterial breakdown can overwhelm the local scavenging mechanisms in the body and cause a more se vere FBR. In a large clinical study that included over 2000 patients in Finland, scientists observed a decrease in the rate of FBR from 5.3% of patients to 0.2% (1 out of 491 patients), when the fast degrading PGA implants were replaced by relatively slow degrading PLA implants.2 The response to degradation can vary from a mild reaction to a painful, erythematous papule over the implant. More severe cases result in osteolytic implant failure requiring surgical debridement and removal. Slower degradation provides an environment where the scavenging mechanisms can cope with the removal of breakdown products. In clinical trials that involve a myriad of polymeric orthopedic devices, the timing of adverse events can occur from two weeks to six months after implantation (Ambrose and Clanton 2004). Current efforts in biodegradable polymer synthesis aim to resolve the response caused by rapid degradation and particulate formation, by developing polymer types with longer degradation times, such as PCL, and less or no acidic degradation products, such as HAP composites.
V.B. Osteolysis
Osteolysis can be described as a change in the normal homeostatic state of bone that results in an increase in bone resorption and the inappropriate breakdown of bone matrix. Commonly associated with disease processes, osteolysis can appear secondary to a FBR. It is likely that an osteolytic FBR is due to non-specific activation of bone resorption by the hydrolyzed debris, as well as activation of macrophages through polymeric particles. The release of mediators (ie, interleukins, prostaglandins) activates osteoclasts that may then shift the balance of bone remodeling toward bone resorption.
In addition to particle-cell interactions between polymeric debris and osteoclasts, the degradation rate of the polymeric implant may also play a role in osteolysis. Compared to polylactides (PLAs), fast reabsorbable polyglycolides (PGAs) have a higher incidence of adverse tissue reactions that lead to osteolytic FBRs. Supporting this assertion, a clinical study involving PGA implants reported significant tissue reactions with sinus formation in 107 out of 2037 patients, while only one out of 491 patients treated with PLLA implants had an osteolytic FBR.81,82 An additional factor that contributes to osteolysis is the placement of the biodegradable implant in relation to bone. It is likely that intraosseous locations have different rates of debris clearance, neovascularization and tissue replacement than extraosseous locations, and therefore, a variable biological response is presented with various biodegradable implants.81 This may explain why the osteolytic changes associated with intraosseous PGA fixation implants can be characterized as an expected reaction, as opposed to a complication. These mild osteolytic changes probably have no effect on fracture healing or the static properties of the bone, unless a certain level is exceeded.83
Symptoms of osteolysis can range from no pain to joint swelling. A typical FBR with osteolysis presents as a painful, fluctuating papule, located over the implant. If left untreated, the papule usually bursts, and reveals a discharging sinus. More specifically, osteolytic FBRs to biodegradable polyglycolide fixation devices have been documented to cause sinuses that discharge polymeric debris (Fig. 5).82 The characteristic appearance of a nonbacterial inflammatory sinus that may occur after reduction and internal fixation of a bimalleolar fracture using PGA screws is shown in Fig. 5a, along with a radiograph of osteolytic lesions that resulted after a trimalleolar fracture repair using PGA screws in Fig. 5b. Histologically, FBGCs surrounding polymeric particles of various sizes are often observed in these cases. For instance, a histopathological slide taken 4.8 years after a bimalleolar fracture repair with PLLA screws shows FBGCs surrounding polymeric particles of various sizes is seen in Fig. 5c. The biopsy typically shows a uniform picture of a non-specific inflammatory FBR.
FIGURE 5.
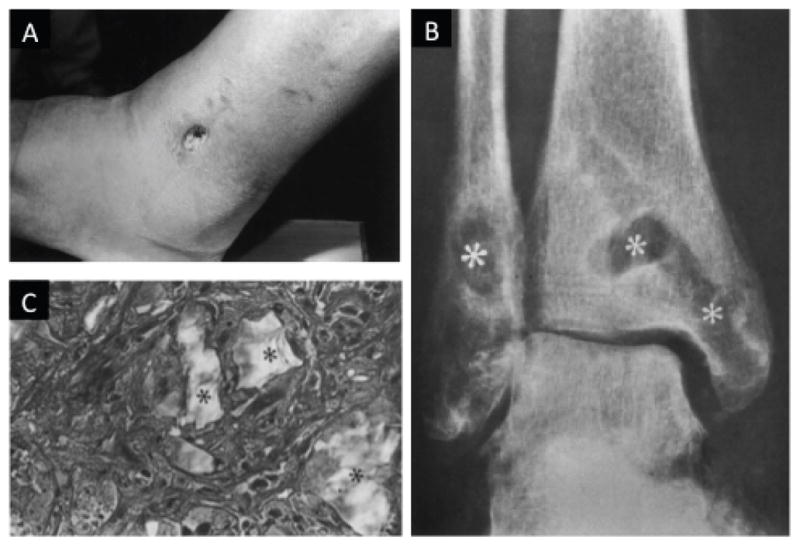
(A) A foreign-body reaction to biodegradable fixation devices made of polyglycolide resulted in a sinus discharging polymeric debris. Observed sinus 11 weeks postoperative on the lateral side of the left ankle of a 43-yr-old man with a bimalleolar fracture that was treated by open reduction and internal fixation using polyglycolide screws. (B) Anteroposterior radiograph of osteolytic lesions (asterisks) at the implant tracks in a 45-yr-old woman with a trimalleolar fracture of the right ankle that was treated by open reduction and internal fixation using polyglycolide screws, 9 weeks postoperative. (C) The characteristic histopathological picture of a non-specific foreign-body reaction to biodegradable implants. Polymeric particles of various sizes (asterisks) are seen surrounded by foreign-body giant cells. Masson-Goldner stain, original magnification 350x [reprinted from Biomaterials, vol. 21, no. 24, pp. 2615–21, Bostman O, PihlajamaÅnki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal f xation: a review. Copyright (2000), with permission from Elsevier].
To our knowledge, there has not been any literature comparing the prevalence of osteolysis between biodegradable and permanent implants. Osteolysis, however, may also occur as a result of permanent implants undergoing aseptic loosening and other impediments that reduce the longevity of the implant. Although there have been documented osteolytic FBRs for biodegradable implants, the number of cases are decreasing as more knowledge is acquired about polymeric biocompatibility. Currently, scientists are working to improve the biocompatibility of biodegradable implants to limit reactions that result in osteolysis.
V.C. Fibrotic Encapsulation
Fibrosis is essentially disorganized tissue regeneration. It originates from an increase in the production of collagen I and III, fibronectin, and proteoglycans due to TGF-β overproduction from the progression phase. These elements combine intra- and intermolecularly, leading to the formation of collagen bundles. In addition, a concurrent decrease in matrix degrading proteases, and an up-regulation of protease inhibitors by TGF-β, leads to an environment where ECM formation dominates. Under the influence of TGF-β and PDGF released from macrophages, fibroblast-like cells differentiate into myofibroblasts and proliferate (Fig. 2). Although myofibroblasts are important in wound healing, they are also active producers of fibrotic tissue and aid in capsule shrinking during the resolution of the FBR.40
Biodegradable polyester implants induce a fibrotic walling-off phenomenon after implantation. However, there have been conflicting reports as to whether the implantation of biodegradable implants leads to a host FBR similar to that of permanent implants with respect to fibrotic encapsulation of the implant. For example, in a rabbit study comparing the performance of PLA and PGA screws to metallic screws, the polyester screws were shown to be inert with no significant difference in long-term biocompatibility.16 In another study comparing the tissue response to PGA, polydioxanone (PDA), polylevolactide, and stainless steel pins, scientists determined that all exhibit non-specific walling-off in cancellous bone of distal rabbit femurs (Fig. 6).84 Some studies, however, have found fibrotic tissue surrounding the implant that may be associated with the implant’s degradation. Pihlajamäki et al. observed fibrotic tissue formation accompanying the degradation process of PLLA screws, while no fibrous tissue was visible at the tissue-implant interface of the metallic screws. However, as time elapsed and the screws degraded, the walling-off phenomenon terminated, demonstrating the fibrosis to be related to the PLLA screw implants’ degradability.85
FIGURE 6.
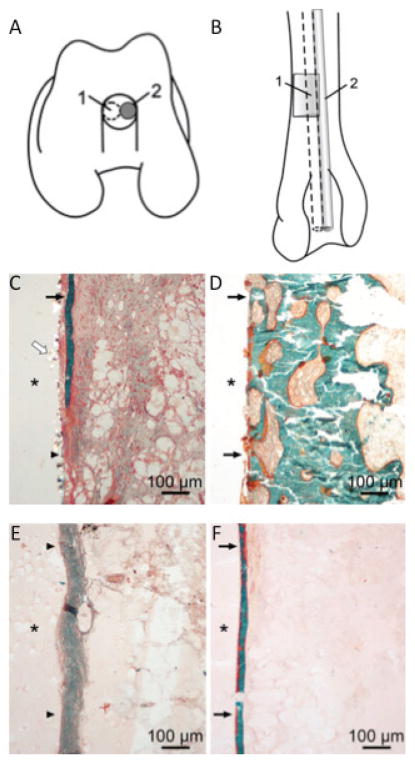
Comparison of non-specific walling-off phenomenon resulting from permanent and biodegradable implants. Schematic drawing of the operated femur (A,B). The distal end of the femur shows the polymer implant (1) and metallic implant (2) in the drill hole (A), and the distal half of the femur shows the bioabsorbable (1) and metallic pin (2) placement in the intramedullary canal in the anteroposterior view (B). In the histologic specimens, a walling-off response was observed for both bioabsorbable and metallic pins (C–F). Fibrous tissue and bone trabecula outlining the PDS pin at 3 weeks (C). A bone rim is seen outlining the metallic wire at 12 weeks (D). A fibrous tissue zone surrounds the PGA pin at 24 weeks (E). Thin bone trabecula outlining the PLLA pin at 52 weeks (F). * Implant channel; black arrows, bone; arrowheads, fibrous tissue; white arrow, PDS particles. Masson-Goldner trichrome staining. (reprinted from Pihlajamäki et al. 2010.16)
The walling-off phenomenon has been a major concern to the success of implants. While the fibrotic encapsulation serves to reduce the inflammatory process, it also restricts angiogenesis and nutrient supply that may result in implant failure during osteointegrative processes.39 Neovascularization, which is essential to wound healing and bone regeneration during polymeric degradation, may be inhibited by the fibrotic encapsulation. Also, the fibrous cap confines the implant and prevents it from reacting with the surrounding tissue.86 Ultimately, this can alter the length of polymer degradation and functional tissue formation.
V.D. Sarcoma
A foreign-body sarcoma is a tumor that developed without a chemical carcinogenic substance acting as a main cause or an adjuvant. Sarcomas have been a documented event following the implantation of biodegradable implants. Based on increasing evidence, it appears that the cause of these sarcomas is not the chemical content of the biodegradable polymer, but instead is related to the implant’s form and size, length of implantation, and degree of inflammation. For example, incidence of foreign body-induced sarcomas is higher with implants with little tissue reaction, while tumor formation is inhibited with chronic inflammation. These reactions have been seen with PLA implants, which have slow degradation rates and a sarcoma incidence level as high as 80% in rats. It is important to note, however, the incidence of sarcomas in the clinical setting has been extremely scarce. For instance, it has been reported that foreign body-induced sarcomas have been observed in only 50 cases out of millions,87 while it was also reported that no sarcoma cases have been observed in association with craniofacial implants made with biodegradable polymers in humans.88 Thus, implant-induced sarcomas may be species-dependent, and not related to the actual implant.
VI. IMPLANT-ASSOCIATED FACTORS THAT INFLUENCE FBR
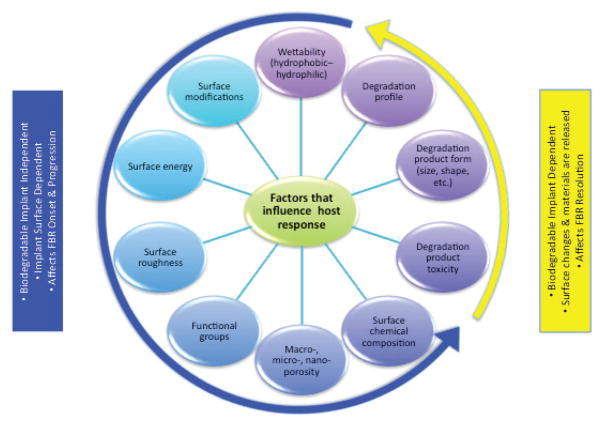
It is well established that the biomaterial’s physical properties and degradation profile modulate the host FBR and inflammation, and ultimately, determine the fate of the implant and bone regeneration (Fig. 7). Ideally, as the implant degrades, bone formation and resolution of the FBR will occur. A clear understanding of how various biomaterial factors influence FBR and interactions with host cells is critical for the fabrication of a host-friendly biomaterial that will elicit the least detrimental FBR, and optimal bone tissue regeneration and integration.
FIGURE 7.
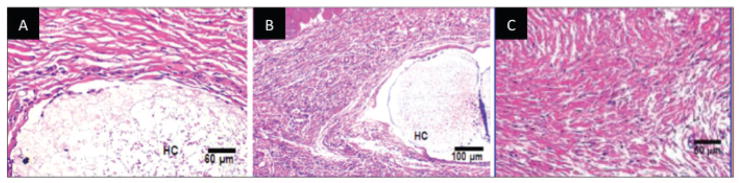
Biodegradable orthopedic implant-induced inflammation, and the parallel tissue regeneration and wound-healing paradigm. Characteristic histopathological picture of a non-specific foreign-body reaction to biodegradable implants include (A) acute inflammation marked by neutrophils (green arrows); (B) chronic inflammation marked by multinucleated giant cells (dark green arrows), fibrosis (light blue arrows), and mixed inflammatory cells (black arrow); and (C) fibrosis around the implant (yellow arrows) stained with Masson’s trichrome stain (collagen is stained blue). These images were obtained from samples generated by implanting PVA hydrogel/PLGA microsphere composites (marked by *) into the subcutaneous tissue of rats after (A) 3, (B) 30, and (C) 60 days. Bar = 100 μm134 [reprinted from the Journal of Diabetes Science and Technology, vol. 2, no. 6, pp. 1003–15, Onuki Y, Bhardwaj U, Papadimitrakopoulos F, Burgess D. A review of the biocompatibility of implantable devices: current challenges to overcome foreign body response. Copyright (20089), with permission from Diabetes Technology Society].
VI.A. Implant Geometry
Implant geometry plays a crucial role in the acute stages of the FBR. Implant geometry includes the gross geometry (ie, macrostructure), surface geometry (ie, microstructure or microtopography), and internal geometry (ie, porosity and mean pore size). These factors are perhaps the most important in determining the implant surface-bone tissue interface and, thus, biocompatibility in vivo.39
The overall geometry of the implant may significantly affect the implant’s biocompatibility if it causes irritation of the surrounding tissue. Implant structures with sharp angles provoke the greatest inflammatory response, with high numbers of inflammatory cells and cellular enzyme activity around implant.89 If too much irritation is caused, premature degradation may occur and lead to implant failure. Furthermore, implant microtopography influences the FBR in several aspects. For example, it directs the degree of the initial fibrin peri-implant matrix upon which osteogenic and inflammatory cells must migrate. Microtopography also affects the actual cell behavior, including adhesion, morphology, migration, orientation, and differentiation.38,69 It has been suggested that topographical changes result in alterations in the distribution of forces on a cell, resulting in a change in cell behavior.69 In addition, implant surfaces of greater microtopography have increased surface area and, thus, experience increased fibrinogen absorption and platelet adhesion.37,38,90,91 As mentioned previously, platelets play an important role in the activation and progression of the FBR. Platelets activated on microtextured candidate implant surfaces will upregulate neutrophils to a greater extent than platelets activated on smoother implant surfaces.91 Increased inflammatory responses have also been observed on implants with roughened surfaces, in comparison to smooth surfaces, which involved increased macrophage and FBGC numbers.92–95 The ability of roughened surfaces to increase platelet activation results in heightened density gradients of cytokines and growth factors through which inflammatory and osteogenic cells enter the transient fibrin matrix. As a result, microtopography also directly influences osteoconduction and osteointegration. Increased osteogenic cells are attracted to roughened implant surfaces, at which they have the ability to begin synthesizing de novo bone. Thus, there is a direct correlation between implant surface roughness and amount of bone formation at the bone-implant interface.96–101 For instance, titanium implants with surfaces that have undergone a large-grit sandblast or acid etching demonstrated a significantly higher extent of boneimplant interface than titanium implants that had decreased roughness in vivo (ie, electropolished, medium-grit sandblasted and plasma sprayed).96 Thus, the transient fibrin matrix, which is related to the surface roughness, is critical for osteogenic and inflammatory cell migration to the implant surface, and acts as a prelude to contact osteogenesis. The importance of this is observed as the process of implant wetting prior to implantation is not practiced often, since this is expected to dilute the implantcontacting fibrin and compromise osteogenic cell migration to the implant surface.91
Recent bone tissue engineering efforts have also demonstrated that internal structure of the biodegradable porous implant (ie, scaffold) influences the FBR and tissue regeneration. Pore structure is determined by average pore size, distribution, geometry, and interconnectedness within the implant.102 Generally, porous, in comparison to solid, implants result in a more loosely packed and more vascularized surrounding capsule and, thus, improved permeability for increased nutrient infiltration.40 Therefore, recent efforts were to identify the optimal pore-sized scaffolds that will allow for the highest bone ingrowth and neovascularization. It was concluded that three-dimensional scaffolds with higher porosity and pore sizes are associated with greater the bone ingrowth in vivo.103 This notion is well supported by the lack of reports demonstrating enhanced osteogenesis in scaffolds with low pore sizes. Furthermore, scaffolds must have direct and extensive surface contact between the implant and host bone for osteoconduction, which can be achieved through increased porosity and pore size that would allow for bone tissue ingrowth.39 A porosity of at least 80 to 90% and a pore size of at least 300 μm are recommended for improved bone formation and neovascularization. 39,103 Coincidently, 300 μm is also the average diameter of a human osteon, which is the fundamental structural unit of bone. Deviation from this optimal range of 300 μm pore diameter result in decreased vascularization, osteoblast attachment, differentiation and proliferation, and ultimately, bone formation.104 The significance of vascularization in constructs for bone regeneration is observed as scaffolds with small pores limit oxygen transport (ie, hypoxia) and induce osteochondral formation before osteogenesis, while large pores that are well-vascularized lead to direct osteogenesis.105 Thus, fulfillment of geometric specifications is critical to obtain optimal bone regeneration.
VI.B. Implant Surface Chemistry: Wettability
Surface wettability is a crucial implant surface characteristic to examine since it may be the initial parameter affecting protein absorption at the implant surface. Water is commonly believed to be the first molecule to contact biomaterials upon implantation, since water is abundant and is also a small and agile molecule, only about 0.25 nm in the longest dimension. A widely accepted definition of wettability states that hydrophobic surfaces have contact angles greater than 65° (pure water adhesiontension (τ°< 30 dyn/cm), whereas, hydrophilic (ie, more wettable) surfaces have contact angles less than 65°(τ°> 30 dyn/cm).106
Although it is generally accepted that hydrophobic surfaces thermodynamically favor the adsorption of proteins from the surrounding aqueous environment upon implantation, a mechanism of enhanced cell response on hydrophilic surfaces may be due to the difference in the array of absorbed proteins.107 Hydrophilic surfaces have been shown to preferentially adsorb vitronectin, prothrombin, factors VII, IX and X, and activate complement via covalent bonding of C3b to free OH and NH3 on the implant surface.36,37,47 On the other hand, when hydrophobic surfaces are exposed to blood plasma, they bind certain coagulation factors, such as factor V, fibrinogen, and fibronectin.36,37 Hydrophobic surfaces induce strongly irreversible adsorption, thereby leading to the denaturation of the protein’s native conformation and bioactivity, and reduced cell-adhesive function.37,107–110 Cell functions, including proliferation, require reorganization of the surface-absorbed proteins, which is largely impaired on more hydrophobic surfaces due to the tight and irreversible binding.111 Thus, the ability of absorbed proteins to retain their functionality on hydrophilic surfaces may contribute to the improved cell responses.
The previously accepted dogma states that hydrophobic surfaces inhibit cellular adhesion, while hydrophilic surfaces promote cellular adhesion. This notion has been used to rationalize why materials, such as titanium and HAP, which are hydrophilic, are able to integrate with bone. However, numerous studies have reported discrepancies when detailing whether hydrophobic or hydrophilic surfaces promote cell adhesion. For example, in one study, surface energy appeared to be of more importance initially, with hydrophobic surfaces producing faster cell activation and differentiation than hydrophilic.112 Also, very hydrophobic materials such as PTFE (contact angles of 105°–116°) inhibit cellular adhesion.63 This observed cellular inhibition might have resulted from conformational changes of the adsorbed proteins, such that ligand moieties needed for integrin binding and cellular adhesion became inaccessible. In contrast, hydrophilic materials such as tissue culture polystyrene (TCPS) are known to support cellular adhesion. Furthermore, in a study comparing a PLGA/PVA scaffold with hydrophilic properties, to another PLGA scaffold with hydrophobic properties, cell adhesion and growth faired better on the PLGA/PVA scaffolds. 113 Other hydrophilic materials that present highly hydrated surfaces (ie, hydrogels, polyethylene oxide, pHEMA, PEO) create a substantial barrier to protein adsorption and inhibit cellular adhesion.37
Interestingly, this feature of hydrophilic surfaces to display decreased cell adhesion has led to the suggestion that hydrophilic surfaces have superior biocompatibility, and could potentially be used to reduce the FBR. Specifically, hydrophilic surfaces have been associated with limited adhesion of monocytes and macrophages, an increased level of apoptosis in adhered monocytes and macrophages, and a reduced amount of macrophage fusion into FBGCs.65,114–116 Hydrophilic surfaces have also been associated with adherent cells producing statistically greater amounts of cytokines and chemokines, such as IL-10, IL-1β, IL-6, IL-8, and MIP-1β, than their hydrophobic counterparts.117,118 Also, with increasing time, production of the antiinflammatory cytokine IL-10 increased, whereas IL-1β, IL-6, and IL-8 decreased and MIP-1β was relatively constant. The inverse trend of decreased cell adherence and increased cell activation/activity can be attributed to the increased release of certain cytokines in cells undergoing apoptosis.119
There are many conflicting reports on the affect of surface wettability on protein absorption, as well as cell adhesion and activity. Even when comparing single cell types, there is inconsistent data, indicating that there is definitely interplay between various surface characteristics, including wettability, charge, topography, and porosity. In addition, it is difficult to accurately study the effect of changing one factor, such as wettability, since there is a strong inter-relationship between the various characteristics of a surface (ie, changing one property almost inevitably changes others as well).68 For example, increasing hydophobicity of a sample may be associated with changes in the chemical groups expressed on the implant surface, and thus the surface free energy. It is also important to note that cell-material interactions are governed by the many various surface properties, and one property is not single-handedly responsible for the resulting FBR.
Although the effect of hydrophilicity/hydrophobicity has been quite controversial and inconsistent in experimental data, it is now well accepted that very hydrophilic and hydrophobic surfaces are not good for cell attachment. Rather, surfaces with moderate wettability are able to adsorb a proper amount of proteins, and at the same time preserve their natural conformation, resulting in positive cell response. Because most synthetic biodegradable polymers are hydrophobic, including the polyesters, extensive efforts have thus been devoted towards increasing the biomaterial’s hydrophilicity, such as surface modifications before cell seeding.37 This concept of moderately hydrophilic material surface being able to induce “good” cell interaction, although widely accepted, requires further detailed studies to gain a better understanding of the optimal hydrophilicity/hydrophobicity equilibrium for a specific cell’s behavior on a specific material.
VII. EFFORTS TO MITIGATE FBR
Much effort has been devoted to altering the surface of biomaterials in order to overcome or reduce the FBRs associated with implantation, while also enhancing integration and biomechanical properties of bone.23 With increased understanding of these properties, we can improve the tolerance of implants. In the following section, we will review how these surface changes can mitigate the FBR, as well as provide a beneficial component to bone tissue integration.
VII.A. Hydrophilicity/Hydrophobicity of Implant Surface
Biomaterial surfaces display distinct surface wettability characteristics that play a significant role in biological host responses, namely cellular adhesion and FBGC formation. As previously mentioned, studies suggest that hydrophilic implant surfaces may decrease FBR via various mechanisms. Namely, implants with hydrophilic surfaces are associated with limited macrophage adhesion, increased apoptosis in adhered macrophages, and reduced macrophage fusion to FBGCs. This associated decrease in FBR may be attributed to the promotion of apoptosis, which, unlike necrosis, does not provoke cell inflammation, as the sealed bodies do not release inflammation-inducing intracellular fragments. Also, hydrophilic implant surfaces result in a decreased production of pro-inflamma tory cytokines (ie, IL-1 and IL-6) and increased anti-inflammatory cytokines (ie, IL-10) by adherent macrophages.117 Hydrophilic implant surfaces are well tolerated by the immune system, and result in reduced fibrinogen adsorption and interaction with fibrotic cells, thereby decreasing implant-induced fibrosis.120 Thus, hydrophilic, apoptosis-inducing biomaterials can decrease the number of adhered macrophages and formation of FBGCs, and thereby effectively reduce or alter the release of cytokines, reactive oxygen species, degradative enzymes, and fibrosis that compromise the biological response.117
As a result of these observations, efforts have been made to increase the hydrophilicity of biomaterials, since many biodegradable polymers are hydrophobic. Various proposed methods include (i) plasma treatments that introduce polarized groups (ie, hydroxyl, carboxyl, amino, and sulfate groups) to material surfaces by using different reaction gases (ie, air, carbon dioxide, sulfur dioxide, ammonia, organic compounds), (ii) graft copolymerization by introducing radicals or peroxide groups onto hydrophilic polymers via ozone oxidation, x-rays, electron beam and laser treatments, and (iii) photo-oxidation to universally introduce peroxide groups by immersing the materials in hydrogen peroxide while under UV radiation.107 Also, in order to create more protein adsorption-resistant surfaces, the implants were coated with polyethylene glycol,121–124 and other hydrophilic polymers, including poly(2-hydroxyethyl methacrylate), poly(N-isopropyl acrylamide), poly(acrylamide), and phosphoryl choline-based polymers.34,125
VII.B. Biomimetic Surface Modifications
Optimal bone regeneration occurs when the implant mimics the native bone ECM as closely as possible. The structure of the biodegradable scaffold should serve as a synthetic replica and act as a temporary ECM, in order to support cell attachment and guide three-dimensional bone tissue formation.126 Since the two main components of bone ECM are Type I collagen fibrils and HAP crystals, each of which are less than 50 nanometers in diameter, nanostructures for bone regeneration have gained much attention. 127–129 Nano-featured scaffolds include nanofibrous scaffolds, and scaffolds with nano-modified surface topography and composition. For example, nanofibrous scaffolds composed of chitosan, a biodegradable and nontoxic natural polymer, have been demonstrated to achieve complete bone regeneration with no inflammatory signs. It has been observed that macrophages and lymphocytes enhance bone regeneration in rabbit calvarial defect model after four weeks post-implantation.130
Surface modifications used to improve implant osteocompatibility and tissue integration include HAP and calcium phosphate (CaP) coatings, which are heavily investigated due to their similarity to bone mineral, as well as cell adhesion molecules with the Arg-Gly-Asp sequence, which promote cell attachment (Table 2).35 Appleford et al. demonstrated enhanced osetocompatibility of HAP scaffolds coated with micro-size and nano-size HAP with significant bone ingrowth and blood vessel infiltration compared to the control at 12 weeks.131 In addition, Hunt et al. demonstrated significantly more new bone formation and decreased inflammatory response when comparing composite HAP/PLLA interference screws to PLLA screws in a large animal ACL reconstruction model.132 Likewise, PLGA scaffolds coated with CaP resulted in a significantly lower inflammatory response, as determined by histomorphometrical analysis of FBGCs, in comparison to non-coated PLGA scaffolds at 8-weeks post-implantation.133 Thus, through the incorporation or coating of implants with biomimetic agents, increased bone regeneration and decreased FBR may be attained.
TABLE 2.
Agents Reported in the Literature to Alleviate the Host FBR
| Agent | Implant Type | Mechanism of Delivery | Selected References |
|---|---|---|---|
| Dexamethasone (Dex) | Injectable PLGA based colloidal gel | Dex-loaded gels | Wang et al. 2010135 |
| Dex/PLGA microspheres | Dex-loaded microspheres | Zolnik and Burgess 2008, Hickey et al. 2002, Patil et al. 2004, Bhardwaj et al. 2007, Kim et al. 2003 136–139,141 | |
| PLGA nanosphere-coated microspheres | Dex-loaded nanospheres | Park et al. 2011160 | |
| Porous PLGA scaffolds | Dex incorporated into PLGA | Kim and Martin 2006140 | |
| Prednisolone | PLGA microspheres | Prednisolone-loaded microspheres | Khaled et al. 2010142 |
| α-Melanocyte Stimulating Hormone (α-MSH) | Poly-l-glutamic acid (PGA) | Covalently coupled to PGA | Fioretti et al. 2010161 |
| PAR1 peptide thrombin receptor agonist (PAR1- AP) | Lactic and glycolic acid copolymer scaffold | PAR1-AP encapsulation | Dugina et al. 2004144 |
| Tetracycline | Composite PLA/PLGA dispersed in a bioceramic matrix (Osteosynt) | Tetracycline-loaded composites | Pataro et al. 2007145 |
| VEGF | PLGA microsphere/PVA hydrogel composites loaded with VEGF & Dex | VEGF-loaded microspheres | Patil, Papadimitrakopoulos and Burgess 2007162 |
| Arg-Gly-Asp (RGD) peptide | PLGA microspheres | Surface modification | Park et al. 2010163 |
| Hydroxyapatite (HA) | Bioactive glass | HA-coating | Xie et al. 2010164 |
| PLLA-HA screws | HA incorporation | Hunt and Callaghan 2008132 | |
| HA scaffolds coated with nano-sized HA or microsized HA | HA-coating | Appleford et al. 2009131 | |
| Calcium phosphate (CaP) | Macroporous PLGA/CaP composite scaffolds coated with CaP | CaP coated | Lickorish et al. 2004133 |
VII.C. Incorporation of Anti-Inflammatory Agents and Growth Factors
The inclusion of anti-inflammatory agents in the implants provides a direct approach to controlling the FBR upon implantation. Though many of the described approaches have mostly been applied to permanent implant surfaces, they can be further adopted for use with biodegradable implants as well.
Anti-inflammatory glucocorticoids, such as dexamethasone (DEX), have been used due to their ability to stop the formation of pro-inflammatory mediators (prostaglandins, leukotrienes). In a study involving DEX-loaded PLGA microspheres/PVA hydrogel composites, significantly lower quantities of neutrophils and macrophages, and fibrosis were observed when compared to control samples loaded with no DEX, after 4 weeks of implantation (Fig. 8).134 DEX-loaded composites were similar to the normal host tissue, with only a few neutrophils, and no evidence of a fibrous cap until day 21. However, once the drug was depleted, neutrophil levels increased. This study, along with numerous others, demonstrated the effect of DEX release on FBR.86,135–141
FIGURE 8.
Pharmacodynamic changes in representative tissue sections after subcutaneous implantation of PLGA microsphere/PVA hydrogel composites (HC) containing dexamethasone at (A) day 7 and (B) day 30 post-implantation. (C) Untreated normal tissue. Hematoxylin and eosin stain, HC = hydrogel composite [reprinted from the Journal of Diabetes Science and Technology, vol. 2, no. 6, pp. 1003–15, Onuki Y, Bhardwaj U, Papadimitrakopoulos F, Burgess D. A review of the biocompatibility of implantable devices: current challenges to overcome foreign body response. Copyright (20089), with permission from Diabetes Technology Society].
Other anti-inflammatory agents previously utilized to modulate macrophage behavior and reduce levels of pro-inflammatory cytokines upon implantation of biodegradable implants include prednisolone,142 α-melanocyte stimulating hormone (α-MSH),143 PAR1 peptide thrombin receptor agonist (PAR1-AP),144 and tetracycline.145 Scientists have also coated non-degradable implants with heparin and synthetic thrombin receptor agonist to shorten the inflammatory FBR, since they functions to bind to regulators of coagulation and complement activation.80,146–153 Also, anti-inflammatory coatings previously reported to reduce neutrophil recruitment and FBR upon implantation of non-biodegradable implants include superoxide dismutase mimetics,154 interleukin-1 receptor antagonist,155 curcumin,156–158 and vitamin E.159
VIII. CONCLUSIONS & FUTURE DIRECTIONS
Biodegradable implants are emerging as an effective alternative to permanent implants because of their capacity to mimic the physical properties of metals, while reducing the need of a secondary surgical intervention for implant removal. Advancement, however, of these novel implants requires a thorough consideration of the biological response to the implant material, as well as their degradation characteristics. Upon implantation of biodegradable implants, the short-term reactions of the surrounding tissue are similar to the acute interactions seen with permanent implants. A series of events occur at the tissue-implant interface that proceeds from protein adsorption and mast cell histamine release, to monocyte recruitment and foreign body giant cell formation. As for long-term effects, biodegradable implants, ideally, should show excellent biocompatibility and be able to exist in the body while evoking minimal to no immune responses. However, long-term inflammatory events of biodegradable implants are initiated by the degradation of the polymer, and heighten especially when the host’s by-product and particle clearing mechanisms are overwhelmed.
The main challenge for development of biodegradable implants is to moderate the long-term FBR. Although a tolerable level of inflammation is vital to the healing process, chronic stimulation of the immune system can negatively impact the function of the implant and its degradation profile. Because the surface chemistry can impact the behavior of immune cells, surface modifications have been studied to lessen the host’s response. This has been accomplished by engineering ways to reduce protein adsorption, alter the wettability of the biomaterial, and even provide a template for regulated delivery of anti-inflammatory agents. Future work should continue to enhance our understanding of the cell-implant interaction, and seek to further ameliorate the FBR by manipulating various biodegradable implant characteristics.
References
- 1.Reichert WM, Ratner BD, Anderson J, Coury A, Hoffman AS, Laurencin CT, Tirrell D. 2010 Panel on the Biomaterials Grand Challenges. J Biomed Mater Res A. 2011 Feb;96(2):275–87. doi: 10.1002/jbm.a.32969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittal R, Morley J, Dinopoulos H, Drakoulakis E, Vermani E, Giannoudis P. Use of bioresorbable implants for stabilisation of distal radius fractures: the United Kingdom patients’ perspective. Injury. 2005 Feb;36(2):333–8. doi: 10.1016/j.injury.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Kulkarni RK, Pani KC, Neuman C, Leonard F. Polylactic acid for surgical implants. Arch Surg. 1966 Nov;93(5):839–43. doi: 10.1001/archsurg.1966.01330050143023. [DOI] [PubMed] [Google Scholar]
- 4.Rokkanen P, Böstman O, Vainionpää S, Makela EA, Hirvensalo E, Partio EK, Vihtonen K, Pätiälä H, Törmälä P. Absorbable devices in the fixation of fractures. J Trauma. 1996 Mar;40(3 Suppl):S123–7. doi: 10.1097/00005373-199603001-00027. [DOI] [PubMed] [Google Scholar]
- 5.Rokkanen P, Böstman O, Hirvensalo E, Mäkelä E, Partio E, Pätiälä H, Vainionpää SI, Vihtonen K, Törmälä P. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000 Dec;21(24):2607–13. doi: 10.1016/s0142-9612(00)00128-9. [DOI] [PubMed] [Google Scholar]
- 6.Böstman O. Economic considerations on avoiding implant removals after fracture fixation by using absorbable devices. Scand J Soc Med. 1994 Mar;22(1):41–5. doi: 10.1177/140349489402200107. [DOI] [PubMed] [Google Scholar]
- 7.Böstman O, Pihlajamäki H. Routine implant removal after fracture surgery: a potentially reducible consumer of hospital resources in trauma units. J Trauma. 1996 Nov;41(5):846–9. doi: 10.1097/00005373-199611000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Huiskes R, Weinans H, van Rietbergen B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin Orthop Relat Res. 1992 Jan;(274):124–34. [PubMed] [Google Scholar]
- 9.Kim YK, Yeo HH, Lim SC. Tissue response to titanium plates: a transmitted electron microscopic study. J Oral Maxillofac Surg. 1997 Apr;55(4):322–6. doi: 10.1016/s0278-2391(97)90115-4. [DOI] [PubMed] [Google Scholar]
- 10.Matthew IR, Frame JW. Ultrastructural analysis of metal particles released from stainless steel and titanium miniplate components in an animal model. J Oral Maxillofac Surg. 1998 Jan;56(1):45–50. doi: 10.1016/s0278-2391(98)90915-6. [DOI] [PubMed] [Google Scholar]
- 11.Wong L, Dufresne CR, Richtsmeier JT, Manson PN. The effect of rigid fixation on growth of the neurocranium. Plast Reconstr Surg. 1991 Sep;88(3):395–403. doi: 10.1097/00006534-199109000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Alpert B, Seligson D. Removal of asymptomatic bone plates used for orthognathic surgery and facial fractures. J Oral Maxillofac Surg. 1996 May;54(5):618–21. doi: 10.1016/s0278-2391(96)90645-x. [DOI] [PubMed] [Google Scholar]
- 13.Taylor M, Tanner KE. Fatigue failure of cancellous bone: a possible cause of implant migration and loosening. J Bone Joint Surg Br. 1997 Mar;79(2):181–2. doi: 10.1302/0301-620x.79b2.7461. [DOI] [PubMed] [Google Scholar]
- 14.Juutilainen T, Pätiälä H, Ruuskanen M, Rokkanen P. Comparison of costs in ankle fractures treated with absorbable or metallic fixation devices. Arch Orthop Trauma Surg. 1997;116(4):204–8. doi: 10.1007/BF00393710. [DOI] [PubMed] [Google Scholar]
- 15.Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, Zichner L, Langer R, Vunjak-Novakovic G, Kaplan DL. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005 Jan;26(2):147–55. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 16.Pihlajamäki H, Salminen S, Tynninen O, Böstman O, Laitinen O. Tissue restoration after implantation of polyglycolide, polydioxanone, polylevolactide, and metallic pins in cortical bone: an experimental study in rabbits. Calcif Tissue Int. 2010 Jul;87(1):90–8. doi: 10.1007/s00223-010-9374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witte F, Ulrich H, Rudert M, Willbold E. Biodegradable magnesium scaffolds: Part 1: appropriate inflammatory response. J Biomed Mater Res A. 2007 Jun;81(3):748–56. doi: 10.1002/jbm.a.31170. [DOI] [PubMed] [Google Scholar]
- 18.Zhang E, Xu L, Yu G, Pan F, Yang K. In vivo evaluation of biodegradable magnesium alloy bone implant in the first 6 months implantation. J Biomed Mater Res A. 2009 Sep;90(3):882–93. doi: 10.1002/jbm.a.32132. [DOI] [PubMed] [Google Scholar]
- 19.Laschke M, Strohe A, Scheuer C, Eglin D, Verrier S, Alini M, Pohlemann T, Menger MD. In vivo biocompatibility and vascularization of biodegradable porous polyurethane scaffolds for tissue engineering. Acta Biomater. 2009 Jul;5(6):1991–2001. doi: 10.1016/j.actbio.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Nukavarapu S, Kumbar S, Brown J, Krogman N, Weikel A, Hindenlang M, Nair LS, Allcock HR, Laurencin CT. Polyphosphazene/nano-hydroxyapatite composite microsphere scaffolds for bone tissue engineering. Biomacromolecules. 2008 Jul;9(7):1818–25. doi: 10.1021/bm800031t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mistry AS, Mikos AG. Tissue engineering strategies for bone regeneration. Adv Biochem Eng Biotechnol. 2005;94:1–22. doi: 10.1007/b99997. [DOI] [PubMed] [Google Scholar]
- 22.Garvin K, Feschuk C. Polylactide-polyglycolide antibiotic implants. Clin Orthop Relat Res. 2005 Aug;(437):105–10. doi: 10.1097/01.blo.0000175720.99118.fe. [DOI] [PubMed] [Google Scholar]
- 23.Puleo D, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999 Dec;20(23–24):2311–21. doi: 10.1016/s0142-9612(99)00160-x. [DOI] [PubMed] [Google Scholar]
- 24.Bonnema H, Popa E, van Timmeren M, van Wachem P, de Leij L, van Luyn M. Distribution patterns of the membrane glycoprotein CD44 during the foreign-body reaction to a degradable biomaterial in rats and mice. J Biomed Mater Res A. 2003 Mar;64(3):502–8. doi: 10.1002/jbm.a.10404. [DOI] [PubMed] [Google Scholar]
- 25.Reed AM, Gilding DK. Biodegradable polymers for use in surgery - poly(glycolic)/poly(lactic acid) homo- and copolymers: 2. In vitro degradation. Polymer. 1981;22(4):494–8. [Google Scholar]
- 26.Gilding DK, Reed AM. Biodegradable polymers for use in surgery - polyglycolic/poly(lactic acid) homo- and copolymers: 1. Polymer. 1979;20(12):1459–64. [Google Scholar]
- 27.Ambrose CG, Clanton TO. Bioabsorbable implants: review of clinical experience in orthopedic surgery. Ann Biomed Eng. 2004 Jan;32(1):171–7. doi: 10.1023/b:abme.0000007802.59936.fc. [DOI] [PubMed] [Google Scholar]
- 28.Ciccone WJ, Motz C, Bentley C, Tasto JP. Bioabsorbable implants in orthopaedics: new developments and clinical applications. J Am Acad Orthop Surg. 2001 Sep-Oct;9(5):280–8. doi: 10.5435/00124635-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Pietrzak W, Verstynen M, Sarver D. Bioabsorbable fixation devices: status for the craniomaxillofacial surgeon. J Craniofac Surg. 1997;8(2):92–6. [PubMed] [Google Scholar]
- 30.Nukavarapu SP, Kumbar SG, Allcock HR, Laurencin CT. Biodegradable polyphosphazene scaffolds for tissue engineering. In: Andrianov AK, editor. Polyphosphazenes for Biomedical Applications. Hoboken (NJ): Wiley; 2009. pp. 117–38. [Google Scholar]
- 31.Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 2000 Dec;21(23):2335–46. doi: 10.1016/s0142-9612(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 32.Vaccaro AR. The role of the osteoconductive scaffold in synthetic bone graft. Orthopedics. 2002 May;25(5 Suppl):S571–8. doi: 10.3928/0147-7447-20020502-05. [DOI] [PubMed] [Google Scholar]
- 33.Nukavarapu S, Wallace J, Elgendy H, Lieberman J, Laurencin C. An Introduction to Biomaterials and their Applications. Taylor & Francis Group; Bone and Biomaterials. in press. [Google Scholar]
- 34.Bridges AW, García AJ. Anti-inflammatory polymeric coatings for implantable biomaterials and devices. J Diabetes Sci Technol. 2008 Nov;2(6):984–94. doi: 10.1177/193229680800200628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunski J, Puleo D, Nanci A. Biomaterials and biomechanics of oral and maxillofacial implants: current status and future developments. Int J Oral Maxillofac Implants. 2000 Jan-Feb;15(1):15–46. [PubMed] [Google Scholar]
- 36.Vroman L. Effect of absorbed proteins on the wettability of hydrophilic and hydrophobic solids. Nature. 1962 Nov;196:476–7. doi: 10.1038/196476a0. [DOI] [PubMed] [Google Scholar]
- 37.Wilson C, Clegg R, Leavesley D, Pearcy M. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005 Jan-Feb;11(1–2):1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 38.Roach P, Eglin D, Rohde K, Perry C. Modern biomaterials: a review - bulk properties and implications of surface modifications. J Mater Sci Mater Med. 2007 Jul;18(7):1263–77. doi: 10.1007/s10856-006-0064-3. [DOI] [PubMed] [Google Scholar]
- 39.Nuss KM, von Rechenberg B. Biocompatibility issues with modern implants in bone - a review for clinical orthopedics. Open Orthop J. 2008;2:66–78. doi: 10.2174/1874325000802010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luttikhuizen D, Harmsen M, Van Luyn M. Cellular and molecular dynamics in the foreign body reaction. Tissue Eng. 2006 Jul;12(7):1955–70. doi: 10.1089/ten.2006.12.1955. [DOI] [PubMed] [Google Scholar]
- 41.Zdolsek J, Eaton JW, Tang L. Histamine release and fibrinogen adsorption mediate acute inflammatory responses to biomaterial implants in humans. J Transl Med. 2007;5:31. doi: 10.1186/1479-5876-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kasemo B, Gold J. Implant surfaces and interface processes. Adv Dent Res. 1999 Jun;13:8–20. doi: 10.1177/08959374990130011901. [DOI] [PubMed] [Google Scholar]
- 43.Ward W, Li A, Siddiqui Y, Federiuk I, Wang X. Increased expression of interleukin-13 and connective tissue growth factor, and their potential roles during foreign body encapsulation of subcutaneous implants. J Biomater Sci Polym Ed. 2008;19(8):1065–72. doi: 10.1163/156856208784909408. [DOI] [PubMed] [Google Scholar]
- 44.Hu W, Eaton J, Ugarova T, Tang L. Molecular basis of biomaterial-mediated foreign body reactions. Blood. 2001 Aug;98(4):1231–8. doi: 10.1182/blood.v98.4.1231. [DOI] [PubMed] [Google Scholar]
- 45.Luttikhuizen D, van Amerongen M, de Feijter P, Petersen A, Harmsen M, van Luyn M. The correlation between difference in foreign body reaction between implant locations and cytokine and MMP expression. Biomaterials. 2006 Dec;27(34):5763–70. doi: 10.1016/j.biomaterials.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Müller-Eberhard H. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–47. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- 47.Nilsson B, Ekdahl K, Mollnes T, Lambris J. The role of complement in biomaterial-induced inflammation. Mol Immunol. 2007 Jan;44(1–3):82–94. doi: 10.1016/j.molimm.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 48.Kao W, Hubbell J, Anderson J. Protein-mediated macrophage adhesion and activation on biomaterials: a model for modulating cell behavior. J Mater Sci Mater Med. 1999 Oct-Nov;10(10/11):601–5. doi: 10.1023/a:1008971222932. [DOI] [PubMed] [Google Scholar]
- 49.Tang L, Jennings T, Eaton J. Mast cells mediate acute inflammatory responses to implanted biomaterials. Proc Natl Acad Sci U S A. 1998 Jul;95(15):8841–6. doi: 10.1073/pnas.95.15.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rücker M, Laschke M, Junker D, Carvalho C, Schramm A, Mülhaupt R, Gellrich NC, Menger MD. Angiogenic and inflammatory response to biodegradable scaffolds in dorsal skinfold chambers of mice. Biomaterials. 2006 Oct;27(29):5027–38. doi: 10.1016/j.biomaterials.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 51.Santavirta S, Konttinen Y, Saito T, Grönblad M, Partio E, Kemppinen P, Rokkanen P. Immune response to polyglycolic acid implants. J Bone Joint Surg Br. 1990 Jul;72(4):597–600. doi: 10.1302/0301-620X.72B4.2166048. [DOI] [PubMed] [Google Scholar]
- 52.Higgins D, Basaraba R, Hohnbaum A, Lee E, Grainger D, Gonzalez-Juarrero M. Localized immunosuppressive environment in the foreign body response to implanted biomaterials. Am J Pathol. 2009 Jul;175(1):161–70. doi: 10.2353/ajpath.2009.080962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia Z, Triffitt J. A review on macrophage responses to biomaterials. Biomed Mater. 2006 Mar;1(1):R1–9. doi: 10.1088/1748-6041/1/1/R01. [DOI] [PubMed] [Google Scholar]
- 54.Shen M, Horbett T. The effects of surface chemistry and adsorbed proteins on monocyte/macrophage adhesion to chemically modified polystyrene surfaces. J Biomed Mater Res. 2001 Dec;57(3):336–45. doi: 10.1002/1097-4636(20011205)57:3<336::aid-jbm1176>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 55.Xia Z, Zhu T, Du J, Zheng Q, Wang L, Li S, Chang CY, Fang SY. Macrophages in degradation of collagen/hydroxylapatite (CHA), beta-tricalcium phosphate ceramics (TCP) artificial bone graft. An in vivo study. Chin Med J (Engl) 1994 Nov;107(11):845–9. [PubMed] [Google Scholar]
- 56.Takebe J, Champagne C, Offenbacher S, Ishibashi K, Cooper L. Titanium surface topography alters cell shape and modulates bone morphogenetic protein 2 expression in the J774A.1 macrophage cell line. J Biomed Mater Res A. 2003 Feb;64(2):207–16. doi: 10.1002/jbm.a.10275. [DOI] [PubMed] [Google Scholar]
- 57.Lu J, Descamps M, Dejou J, Koubi G, Hardouin P, Lemaitre J, Proust JP. The biodegradation mechanism of calcium phosphate biomaterials in bone. J Biomed Mater Res. 2002;63(4):408–12. doi: 10.1002/jbm.10259. [DOI] [PubMed] [Google Scholar]
- 58.Labow R, Sa D, Matheson L, Santerre J. Polycarbonate- urethane hard segment type influences esterase substrate specificity for human- macrophage-mediated biodegradation. J Biomater Sci Polym Ed. 2005;16(9):1167–77. doi: 10.1163/1568562054798563. [DOI] [PubMed] [Google Scholar]
- 59.Solheim E, Sudmann B, Bang G, Sudmann E. Biocompatibility and effect on osteogenesis of poly(ortho ester) compared to poly(DL-lactic acid) J Biomed Mater Res. 2000 Feb;49(2):257–63. doi: 10.1002/(sici)1097-4636(200002)49:2<257::aid-jbm15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 60.Khouw I, van Wachem P, de Leij L, van Luyn M. Inhibition of the tissue reaction to a biodegradable biomaterial by monoclonal antibodies to IFN-gamma. J Biomed Mater Res. 1998 Aug;41(2):202–10. doi: 10.1002/(sici)1097-4636(199808)41:2<202::aid-jbm4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 61.Jones J, McNally A, Chang D, Qin L, Meyerson H, Colton E, Kwon IL, Matsuda T, An derson JM. Matrix metalloproteinases and their inhibitors in the foreign body reaction on biomaterials. J Biomed Mater Res A. 2008 Jan;84(1):158–66. doi: 10.1002/jbm.a.31220. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez A, Macewan S, Meyerson H, Kirk J, Anderson J. The foreign body reaction in Tcell- deficient mice. J Biomed Mater Res A. 2009 Jul;90(1):106–13. doi: 10.1002/jbm.a.32050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones KS. Effects of biomaterial-induced inflammation on fibrosis and rejection. Semin Immunol. 2008 Apr;20(2):130–6. doi: 10.1016/j.smim.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 64.Muldashev E, Muslimov S, Musina L, Nigmatullin R, Lebedeva A, Shangina O, Khasanov RA. The role of macrophages in the tissues regeneration stimulated by the biomaterials. Cell Tissue Bank. 2005;6(2):99–107. doi: 10.1007/s10561-004-5805-2. [DOI] [PubMed] [Google Scholar]
- 65.Brodbeck W, Patel J, Voskerician G, Christenson E, Shive M, Nakayama Y, Matsuda T, Ziats NP, Anderson JM. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proc Natl Acad Sci U S A. 2002 Aug;99(16):10287–92. doi: 10.1073/pnas.162124199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Piattelli A, Franco M, Ferronato G, Santello M, Martinetti R, Scarano A. Resorption of composite polymer-hydroxyapatite membranes: a time-course study in rabbit. Biomaterials. 1997 Apr;18(8):629–33. doi: 10.1016/s0142-9612(96)00163-9. [DOI] [PubMed] [Google Scholar]
- 67.Sack U, Kuhn H, Kämpfer I, Genest M, Arnold S, Pfeiffer G, Emmrich F. Orthotopic implantation of inflamed synovial tissue from RA patients induces a characteristic arthritis in immunodeficient (SCID) mice. J Autoimmun. 1996 Feb;9(1):51–8. doi: 10.1006/jaut.1996.0007. [DOI] [PubMed] [Google Scholar]
- 68.Gallagher W, Lynch I, Allen L, Miller I, Penney S, O’Connor D, Pennington S, Keenan AK, Dawson KA. Molecular basis of cellbiomaterial interaction: insights gained from transcriptomic and proteomic studies. Biomaterials. 2006 Dec;27(35):5871–82. doi: 10.1016/j.biomaterials.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 69.Siebers M, ter Brugge P, Walboomers X, Jansen J. Integrins as linker proteins between osteoblasts and bone replacing materials. A critical review. Biomaterials. 2005 Jan;26(2):137–46. doi: 10.1016/j.biomaterials.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 70.Pihlajamäki H, Visuri T. Long-term outcome after surgical treatment of unresolved Osgood-Schlatter disease in young men: surgical technique. J Bone Joint Surg Am. 2010 Sep;92(Suppl 1 Pt 2):258–64. doi: 10.2106/JBJS.J.00450. [DOI] [PubMed] [Google Scholar]
- 71.Sitharaman B, Shi X, Walboomers X, Liao H, Cuijpers V, Wilson L, Mikos AG, Jansen JA. In vivo biocompatibility of ultra-short single-walled carbon nanotube/biodegradable polymer nanocomposites for bone tissue engineering. Bone. 2008 Aug;43(2):362–70. doi: 10.1016/j.bone.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 72.Staiger M, Pietak A, Huadmai J, Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006 Mar;27(9):1728–34. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Usui Y, Aoki K, Narita N, Murakami N, Nakamura I, Nakamura K, Ishigaki N, Yamazaki H, Horiuchi H, Kato H, Taruta S, Kim YA, Endo M, Saito N. Carbon nanotubes with high bone-tissue compatibility and boneformation acceleration effects. Small. 2008 Feb;4(2):240–6. doi: 10.1002/smll.200700670. [DOI] [PubMed] [Google Scholar]
- 74.Witte F, Ulrich H, Palm C, Willbold E. Biodegradable magnesium scaffolds: Part II: peri-implant bone remodeling. J Biomed Mater Res A. 2007 Jun;81(3):757–65. doi: 10.1002/jbm.a.31293. [DOI] [PubMed] [Google Scholar]
- 75.Ambrose C, Clanton T. Bioabsorbable implants: review of clinical experience in orthopedic surgery. Ann Biomed Eng. 2004 Jan;32(1):171–7. doi: 10.1023/b:abme.0000007802.59936.fc. [DOI] [PubMed] [Google Scholar]
- 76.Anderson J, Rodriguez A, Chang D. Foreign body reaction to biomaterials. Semin Immunol. 2008 Apr;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu L, Peter S, Lyman M, Lai H, Leite S, Tamada J, Uyama S, Vacanti JP, Langer R, Mikos AG. In vitro and in vivo degradation of porous poly(DL-lactic-co-glycolic acid) foams. Biomaterials. 2000 Sep;21(18):1837–45. doi: 10.1016/s0142-9612(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 78.Nair Pn PR, Schug J. Observations on healing of human tooth extraction sockets implanted with bioabsorbable polylacticpolyglycolic acids (PLGA) copolymer root replicas: a clinical, radiographic, and histologic follow-up report of 8 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004 May;97(5):559–69. doi: 10.1016/S1079210403006334. [DOI] [PubMed] [Google Scholar]
- 79.Spector JA, Mehrara BJ, Greenwald JA, Saadeh PB, Steinbrech DS, Bouletreau PJ, Smith LP, Longaker MT. Osteoblast expression of vascular endothelial growth factor is modulated by the extracellular microenvironment. Am J Physiol Cell Physiol. 2001 Jan;280(1):C72–80. doi: 10.1152/ajpcell.2001.280.1.C72. [DOI] [PubMed] [Google Scholar]
- 80.Sung H, Meredith C, Johnson C, Galis Z. The effect of scaffold degradation rate on threedimensional cell growth and angiogenesis. Biomaterials. 2004 Nov;25(26):5735–42. doi: 10.1016/j.biomaterials.2004.01.066. [DOI] [PubMed] [Google Scholar]
- 81.Böstman O, Pihlajamäki H. Late foreignbody reaction to an intraosseous bioabsorbable polylactic acid screw. A case report. J Bone Joint Surg Am. 1998 Dec;80(12):1791–4. doi: 10.2106/00004623-199812000-00010. [DOI] [PubMed] [Google Scholar]
- 82.Böstman O, Pihlajamäki H. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials. 2000 Dec;21(24):2615–21. doi: 10.1016/s0142-9612(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 83.Weiler A, Helling H, Kirch U, Zirbes T, Rehm K. Foreign-body reaction and the course of osteolysis after polyglycolide implants for fracture fixation: experimental study in sheep. J Bone Joint Surg Br. 1996 May;78(3):369–76. [PubMed] [Google Scholar]
- 84.Pihlajamäki H, Salminen S, Laitinen O, Tynninen O, Böstman O. Tissue response to polyglycolide, polydioxanone, polylevolactide, and metallic pins in cancellous bone: An experimental study on rabbits. J Orthop Res. 2006 Aug;24(8):1597–606. doi: 10.1002/jor.20191. [DOI] [PubMed] [Google Scholar]
- 85.Pihlajamäki H, Böstman O, Tynninen O, Laitinen O. Long-term tissue response to bioabsorbable poly-L-lactide and metallic screws: an experimental study. Bone. 2006 Oct;39(4):932–7. doi: 10.1016/j.bone.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 86.Morais J, Papadimitrakopoulos F, Burgess D. Biomaterials/tissue interactions: possible solutions to overcome foreign body response. AAPS J. 2010 Jun;12(2):188–96. doi: 10.1208/s12248-010-9175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pistner H, Stallforth H, Gutwald R, Mühling J, Reuther J, Michel C. Poly(L-lactide): a long-term degradation study in vivo. Part II: Physico-mechanical behaviour of implants. Biomaterials. 1994 May;15(6):439–50. doi: 10.1016/0142-9612(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 88.Rubin JP, Yaremchuk MJ. Complications and toxicities of implantable biomaterials used in facial reconstructive and aesthetic surgery: a comprehensive review of the literature. Plast Reconstr Surg. 1997 Oct;100(5):1336–53. doi: 10.1097/00006534-199710000-00043. [DOI] [PubMed] [Google Scholar]
- 89.Matlaga B, Yasenchak L, Salthouse T. Tissue response to implanted polymers: the significance of sample shape. J Biomed Mater Res. 1976 May;10(3):391–7. doi: 10.1002/jbm.820100308. [DOI] [PubMed] [Google Scholar]
- 90.Park J, Davies J. Red blood cell and platelet interactions with titanium implant surfaces. Clin Oral Implants Res. 2000 Dec;11(6):530–9. doi: 10.1034/j.1600-0501.2000.011006530.x. [DOI] [PubMed] [Google Scholar]
- 91.Davies J. Understanding peri-implant endosseous healing. J Dent Educ. 2003 Aug;67(8):932–49. [PubMed] [Google Scholar]
- 92.Ziats N, Miller K, Anderson J. In vitro and in vivo interactions of cells with biomaterials. Biomaterials. 1988 Jan;9(1):5–13. doi: 10.1016/0142-9612(88)90063-4. [DOI] [PubMed] [Google Scholar]
- 93.Behling C, Spector M. Quantitative characterization of cells at the interface of longterm implants of selected polymers. J Biomed Mater Res. 1986 May-Jun;20(5):653–66. doi: 10.1002/jbm.820200509. [DOI] [PubMed] [Google Scholar]
- 94.Salthouse T. Some aspects of macrophage behavior at the implant interface. J Biomed Mater Res. 1984 Apr;18(4):395–401. doi: 10.1002/jbm.820180407. [DOI] [PubMed] [Google Scholar]
- 95.Taylor S, Gibbons D. Effect of surface texture on the soft tissue response to polymer implants. J Biomed Mater Res. 1983 Mar;17(2):205–27. doi: 10.1002/jbm.820170202. [DOI] [PubMed] [Google Scholar]
- 96.Buser D, Schenk R, Steinemann S, Fiorellini J, Fox C, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991 Jul;25(7):889–902. doi: 10.1002/jbm.820250708. [DOI] [PubMed] [Google Scholar]
- 97.Lossdörfer S, Schwartz Z, Wang L, Lohmann C, Turner J, Wieland M, Cochran DL, Boyan BD. Microrough implant surface topographies increase osteogenesis by reducing osteoclast formation and activity. J Biomed Mater Res A. 2004 Sep;70(3):361–9. doi: 10.1002/jbm.a.30025. [DOI] [PubMed] [Google Scholar]
- 98.Schwartz Z, Boyan B. Underlying mechanisms at the bone-biomaterial interface. J Cell Biochem. 1994 Nov;56(3):340–7. doi: 10.1002/jcb.240560310. [DOI] [PubMed] [Google Scholar]
- 99.Schroeder A, van der Zypen E, Stich H, Sutter F. The reactions of bone, connective tissue, and epithelium to endosteal implants with titanium-sprayed surfaces. J Maxillofac Surg. 1981 Feb;9(1):15–25. doi: 10.1016/s0301-0503(81)80007-0. [DOI] [PubMed] [Google Scholar]
- 100.Rich A, Harris A. Anomalous preferences of cultured macrophages for hydrophobic and roughened substrata. J Cell Sci. 1981 Aug;50:1–7. doi: 10.1242/jcs.50.1.1. [DOI] [PubMed] [Google Scholar]
- 101.Thomas K, Cook S. An evaluation of variables influencing implant fixation by direct bone apposition. J Biomed Mater Res. 1985 Oct;19(8):875–901. doi: 10.1002/jbm.820190802. [DOI] [PubMed] [Google Scholar]
- 102.Rumpler M, Woesz A, Dunlop J, van Dongen J, Fratzl P. The effect of geometry on threedimensional tissue growth. J R Soc Interface. 2008 Oct;5(27):1173–80. doi: 10.1098/rsif.2008.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005 Sep;26(27):5474–91. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 104.Tsuruga E, Takita H, Itoh H, Wakisaka Y, Kuboki Y. Pore size of porous hydroxyapatite as the cell-substratum controls BMPinduced osteogenesis. J Biochem. 1997 Feb;121(2):317–24. doi: 10.1093/oxfordjournals.jbchem.a021589. [DOI] [PubMed] [Google Scholar]
- 105.Kuboki Y, Jin Q, Takita H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J Bone Joint Surg Am. 2001;83-A(Suppl 1 Pt 2):S105–15. [PubMed] [Google Scholar]
- 106.Vogler EA. Structure and reactivity of water at biomaterial surfaces. Adv Colloid Interface Sci. 1998 Feb;74:69–117. doi: 10.1016/s0001-8686(97)00040-7. [DOI] [PubMed] [Google Scholar]
- 107.Ma Z, Mao Z, Gao C. Surface modification and property analysis of biomedical polymers used for tissue engineering. Colloids Surf B Biointerfaces. 2007 Nov;60(2):137–57. doi: 10.1016/j.colsurfb.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 108.Lewandowska K, Balachander N, Sukenik C, Culp L. Modulation of fibronectin adhesive functions for fibroblasts and neural cells by chemically derivatized substrata. J Cell Physiol. 1989 Nov;141(2):334–45. doi: 10.1002/jcp.1041410215. [DOI] [PubMed] [Google Scholar]
- 109.Absolom D, Zingg W, Neumann A. Protein adsorption to polymer particles: role of surface properties. J Biomed Mater Res. 1987 Feb;21(2):161–71. doi: 10.1002/jbm.820210202. [DOI] [PubMed] [Google Scholar]
- 110.Grinnell F, Feld M. Fibronectin adsorption on hydrophilic and hydrophobic surfaces detected by antibody binding and analyzed during cell adhesion in serum-containing medium. J Biol Chem. 1982 May;257(9):4888–93. [PubMed] [Google Scholar]
- 111.Altankov G, Grinnell F, Groth T. Studies on the biocompatibility of materials: fibroblast reorganization of substratum-bound fibronectin on surfaces varying in wettability. J Biomed Mater Res. 1996 Mar;30(3):385–91. doi: 10.1002/(SICI)1097-4636(199603)30:3<385::AID-JBM13>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 112.Eriksson C, Nygren H, Ohlson K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials. 2004 Aug;25(19):4759–66. doi: 10.1016/j.biomaterials.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 113.Oh SH, Kang SG, Lee JH. Degradation behavior of hydrophilized PLGA scaffolds prepared by melt-molding particulate-leaching method: comparison with control hydrophobic one. J Mater Sci Mater Med. 2006 Feb;17(2):131–7. doi: 10.1007/s10856-006-6816-2. [DOI] [PubMed] [Google Scholar]
- 114.Brodbeck W, Nakayama Y, Matsuda T, Colton E, Ziats N, Anderson J. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine. 2002 Jun;18(6):311–9. doi: 10.1006/cyto.2002.1048. [DOI] [PubMed] [Google Scholar]
- 115.Brodbeck W, Shive M, Colton E, Nakayama Y, Matsuda T, Anderson J. Influence of biomaterial surface chemistry on the apoptosis of adherent cells. J Biomed Mater Res. 2001 Jun;55(4):661–8. doi: 10.1002/1097-4636(20010615)55:4<661::aid-jbm1061>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 116.Collier T, Anderson J, Brodbeck W, Barber T, Healy K. Inhibition of macrophage development and foreign body giant cell formation by hydrophilic interpenetrating polymer network. J Biomed Mater Res A. 2004 Jun;69(4):644–50. doi: 10.1002/jbm.a.30030. [DOI] [PubMed] [Google Scholar]
- 117.Jones J, Chang D, Meyerson H, Colton E, Kwon I, Matsuda T, Anderson JM. Proteomic analysis and quantification of cytokines and chemokines from biomaterial surface-adherent macrophages and foreign body giant cells. J Biomed Mater Res A. 2007 Dec;83(3):585–96. doi: 10.1002/jbm.a.31221. [DOI] [PubMed] [Google Scholar]
- 118.Anderson J, Jones J. Phenotypic dichotomies in the foreign body reaction. Biomaterials. 2007 Dec;28(34):5114–20. doi: 10.1016/j.biomaterials.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bzowska M, Guzik K, Barczyk K, Ernst M, Flad H, Pryjma J. Increased IL-10 production during spontaneous apoptosis of monocytes. Eur J Immunol. 2002 Jul;32(7):2011–20. doi: 10.1002/1521-4141(200207)32:7<2011::AID-IMMU2011>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 120.Nair A, Zou L, Bhattacharyya D, Timmons R, Tang L. Species and density of implant surface chemistry affect the extent of foreign body reactions. Langmuir. 2008 Mar;24(5):2015–24. doi: 10.1021/la7025973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tziampazis E, Kohn J, Moghe PV. PEGvariant biomaterials as selectively adhesive protein templates: model surfaces for controlled cell adhesion and migration. Biomaterials. 2000 Mar;21(5):511–20. doi: 10.1016/s0142-9612(99)00212-4. [DOI] [PubMed] [Google Scholar]
- 122.Unsworth LD, Sheardown H, Brash JL. Protein- resistant poly(ethylene oxide)-grafted surfaces: chain density-dependent multi ple mechanisms of action. Langmuir. 2008 Mar;24(5):1924–9. doi: 10.1021/la702310t. [DOI] [PubMed] [Google Scholar]
- 123.Unsworth LD, Sheardown H, Brash JL. Polyethylene oxide surfaces of variable chain density by chemisorption of PEO-thiol on gold: adsorption of proteins from plasma studied by radiolabelling and immunoblotting. Biomaterials. 2005 Oct;26(30):5927–33. doi: 10.1016/j.biomaterials.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 124.Michel R, Pasche S, Textor M, Castner DG. Influence of PEG architecture on protein adsorption and conformation. Langmuir. 2005 Dec;21(26):12327–32. doi: 10.1021/la051726h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vadgama P. Modifying surfaces and interfaces for improved biomaterial performance. Med Device Technol. 2007 Jan-Feb;18(1):22–5. [PubMed] [Google Scholar]
- 126.Liu X, Smith L, Hu J, Ma P. Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials. 2009 Apr;30(12):2252–8. doi: 10.1016/j.biomaterials.2008.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nukavarapu S, Kumbar S, Merrell J, Laurencin C. Electrospun polymeric nanofiber scaffolds for tissue regeneration. In: Laurencin CT, Nair LS, editors. Nanotechnology and Tissue Engineering: The Scaffold. New York: CRC Presss; 2008. pp. 199–219. [Google Scholar]
- 128.Nukavarapu S, Kumbar S, Nair L, Laurencin C. Nanostructures for Tissue Engineering/Regenerative Medicine. In: Gonsalves K, Halberstadt C, Laurencin CT, Nair L, editors. Biomedical Nanostructures. Hoboken (NJ): John Wiley & Sons, Inc; 2008. pp. 371–401. [Google Scholar]
- 129.Igwe J, Amini A, Mickael P, Laurencin C, Nukavarapu S. Nanostructured Scaffolds for Bone Tissue Engineering. In: Zilberman M, editor. Studies in Mechanobiology, Tissue Engineering and Biomaterials. New York: Springer; 2011. [Google Scholar]
- 130.Shin S, Park H, Kim K, Lee M, Choi Y, Park Y, Lee YM, Ku Y, Rhyu IC, Han SB, Lee SJ, Chung CP. Biological evaluation of chitosan nanofiber membrane for guided bone regeneration. J Periodontol. 2005 Oct;76(10):1778–84. doi: 10.1902/jop.2005.76.10.1778. [DOI] [PubMed] [Google Scholar]
- 131.Appleford MR, Oh S, Oh N, Ong JL. In vivo study on hydroxyapatite scaffolds with trabecular architecture for bone repair. J Biomed Mater Res A. 2009 Jun;89(4):1019–27. doi: 10.1002/jbm.a.32049. [DOI] [PubMed] [Google Scholar]
- 132.Hunt JA, Callaghan JT. Polymer-hydroxyapatite composite versus polymer interference screws in anterior cruciate ligament reconstruction in a large animal model. Knee Surg Sports Traumatol Arthrosc. 2008 Jul;16(7):655–60. doi: 10.1007/s00167-008-0528-8. [DOI] [PubMed] [Google Scholar]
- 133.Lickorish D, Chan J, Song J, Davies JE. An in-vivo model to interrogate the transition from acute to chronic inflammation. Eur Cell Mater. 2004;8:12–9. doi: 10.22203/ecm.v008a02. discussion 20. [DOI] [PubMed] [Google Scholar]
- 134.Onuki Y, Bhardwaj U, Papadimitrakopoulos F, Burgess D. A review of the biocompatibility of implantable devices: current challenges to overcome foreign body response. J Diabetes Sci Technol. 2008 Nov;2(6):1003–15. doi: 10.1177/193229680800200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Q, Wang J, Lu Q, Detamore MS, Berkland C. Injectable PLGA based colloidal gels for zero-order dexamethasone release in cranial defects. Biomaterials. 2010 Jun;31(18):4980–6. doi: 10.1016/j.biomaterials.2010.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zolnik BS, Burgess DJ. Evaluation of in vivo-in vitro release of dexamethasone from PLGA microspheres. J Control Release. 2008 Apr;127(2):137–45. doi: 10.1016/j.jconrel.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 137.Hickey T, Kreutzer D, Burgess DJ, Moussy F. In vivo evaluation of a dexamethasone/PLGA microsphere system designed to suppress the inflammatory tissue response to implantable medical devices. J Biomed Mater Res. 2002 Aug;61(2):180–7. doi: 10.1002/jbm.10016. [DOI] [PubMed] [Google Scholar]
- 138.Patil SD, Papadimitrakopoulos F, Burgess DJ. Dexamethasone-loaded poly(lactic-coglycolic) acid microspheres/poly(vinyl alcohol) hydrogel composite coatings for inflammation control. Diabetes Technol Ther. 2004 Dec;6(6):887–97. doi: 10.1089/dia.2004.6.887. [DOI] [PubMed] [Google Scholar]
- 139.Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ. Controlling acute inflammation with fast releasing dexamethasone-PLGA microsphere/pva hydrogel composites for implantable devices. J Diabetes Sci Technol. 2007 Jan;1(1):8–17. doi: 10.1177/193229680700100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim DH, Martin DC. Sustained release of dexamethasone from hydrophilic matrices using PLGA nanoparticles for neural drug delivery. Biomaterials. 2006 May;27(15):3031–7. doi: 10.1016/j.biomaterials.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 141.Kim H, Kim HW, Suh H. Sustained release of ascorbate-2-phosphate and dexametha sone from porous PLGA scaffolds for bone tissue engineering using mesenchymal stem cells. Biomaterials. 2003 Nov;24(25):4671–9. doi: 10.1016/s0142-9612(03)00358-2. [DOI] [PubMed] [Google Scholar]
- 142.Khaled KA, Sarhan HA, Ibrahim MA, Ali AH, Naguib YW. Prednisolone-loaded PLGA microspheres. in vitro characterization and in vivo application in adjuvant-induced arthritis in mice. AAPS PharmSci- Tech. 2010 Jun;11(2):859–69. doi: 10.1208/s12249-010-9445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhong Y, Bellamkonda RV. Controlled release of anti-inflammatory agent alpha-MSH from neural implants. J Control Release. 2005 Sep;106(3):309–18. doi: 10.1016/j.jconrel.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 144.Dugina TN, Kiseleva EV, Lange MA, Vasil’eva TV, Grandfils C, Markvicheva EA, Bespalova ZhD, Pal’keeva ME, Strukova SM. Effect of synthetic peptide thrombin receptor agonist encapsulated in microparticles based on lactic and glycolic acid copolymer on healing of experimental skin wounds in mice. Bull Exp Biol Med. 2004 Nov;138(5):463–6. doi: 10.1007/s10517-005-0071-2. [DOI] [PubMed] [Google Scholar]
- 145.Pataro AL, Oliveira MF, Teixeira KI, Turchetti- Maia RM, Lopes MT, Wykrota FH, Sinisterra RD, Cortés ME. Polymer: bioceramic composites optimization by tetracycline addition. Int J Pharm. 2007 May;336(1):75–81. doi: 10.1016/j.ijpharm.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 146.Gerritsen M, Kros A, Sprakel V, Lutterman JA, Nolte RJ, Jansen JA. Biocompatibility evaluation of sol-gel coatings for subcutaneously implantable glucose sensors. Biomaterials. 2000 Jan;21(1):71–8. doi: 10.1016/s0142-9612(99)00136-2. [DOI] [PubMed] [Google Scholar]
- 147.Wang XH, Li DP, Wang WJ, Feng QL, Cui FZ, Xu YX, Song XH. Covalent immobilization of chitosan and heparin on PLGA surface. Int J Biol Macromol. 2003 Nov;33(1–3):95–100. doi: 10.1016/s0141-8130(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 148.van Bilsen PH, Popa ER, Brouwer LA, Vincent J, Taylor CE, de Leij LF, Hendriks M, van Luyn MJ. Ongoing foreign body reaction to subcutaneous implanted (heparin) modified Dacron in rats. J Biomed Mater Res A. 2004 Mar;68(3):423–7. doi: 10.1002/jbm.a.20069. [DOI] [PubMed] [Google Scholar]
- 149.Fu J, Ji J, Yuan W, Shen J. Construction of anti-adhesive and antibacterial multilayer films via layer-by-layer assembly of heparin and chitosan. Biomaterials. 2005 Nov;26(33):6684–92. doi: 10.1016/j.biomaterials.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 150.Rele SM, Cui W, Wang L, Hou S, Barr-Zarse G, Tatton D, Gnanou Y, Esko JD, Chaikof EL. Dendrimer-like PEO glycopolymers exhibit anti-inflammatory properties. J Am Chem Soc. 2005 Jul;127(29):10132–3. doi: 10.1021/ja0511974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tseng PY, Rele SS, Sun XL, Chaikof EL. Membrane-mimetic films containing thrombomodulin and heparin inhibit tissue factorinduced thrombin generation in a flow model. Biomaterials. 2006 Apr;27(12):2637–50. doi: 10.1016/j.biomaterials.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 152.Du YJ, Brash JL, McClung G, Berry LR, Klement P, Chan AK. Protein adsorption on polyurethane catheters modified with a novel antithrombin-heparin covalent complex. J Biomed Mater Res A. 2007 Jan;80(1):216–25. doi: 10.1002/jbm.a.30977. [DOI] [PubMed] [Google Scholar]
- 153.Rusanova AV, Makarova AM, Strukova SM, Markvicheva EA, Gorbachyova LR, Stashevskaya KS, Vasil’eva TV, Sidorova EI, Bespalova ZhD, Grandfils Ch. Thrombin receptor agonist peptide immobilized in microspheres stimulates reparative processes in rats with gastric ulcer. Bull Exp Biol Med. 2006 Jul;142(1):35–8. doi: 10.1007/s10517-006-0285-y. [DOI] [PubMed] [Google Scholar]
- 154.Udipi K, Ornberg RL, Thurmond KB, Settle SL, Forster D, Riley D. Modification of inflammatory response to implanted biomedical materials in vivo by surface bound superoxide dismutase mimics. J Biomed Mater Res. 2000 Sep;51(4):549–60. doi: 10.1002/1097-4636(20000915)51:4<549::aid-jbm2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 155.Kim DH, Smith JT, Chilkoti A, Reichert WM. The effect of covalently immobilized rhIL- 1ra-ELP fusion protein on the inflammatory profile of LPS-stimulated human monocytes. Biomaterials. 2007 Aug;28(23):3369–77. doi: 10.1016/j.biomaterials.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nguyen KT, Shaikh N, Shukla KP, Su SH, Eberhart RC, Tang L. Molecular responses of vascular smooth muscle cells and phagocytes to curcumin-eluting bioresorbable stent materials. Biomaterials. 2004 Oct;25(23):5333–46. doi: 10.1016/j.biomaterials.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 157.Su SH, Nguyen KT, Satasiya P, Greilich PE, Tang L, Eberhart RC. Curcumin impregnation improves the mechanical properties and reduces the inflammatory response associated with poly(L-lactic acid) fiber. J Biomater Sci Polym Ed. 2005;16(3):353–70. doi: 10.1163/1568562053654077. [DOI] [PubMed] [Google Scholar]
- 158.Pan CJ, Tang JJ, Weng YJ, Wang J, Huang N. Preparation, characterization and anticoagulation of curcumin-eluting controlled biodegradable coating stents. J Control Release. 2006 Nov;116(1):42–9. doi: 10.1016/j.jconrel.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 159.Hahn SK, Jelacic S, Maier RV, Stayton PS, Hoffman AS. Anti-inflammatory drug delivery from hyaluronic acid hydrogels. J Biomater Sci Polym Ed. 2004;15(9):1111–9. doi: 10.1163/1568562041753115. [DOI] [PubMed] [Google Scholar]
- 160.Park JS, Yang HN, Woo DG, Jeon SY, Park KH. The promotion of chondrogenesis, osteogenesis, and adipogenesis of human mesenchymal stem cells by multiple growth factors incorporated into nanospherecoated microspheres. Biomaterials. 2011 Jan;32(1):28–38. doi: 10.1016/j.biomaterials.2010.08.088. [DOI] [PubMed] [Google Scholar]
- 161.Fioretti F, Mendoza-Palomares C, Helms M, Al Alam D, Richert L, Arntz Y, Rinckenbach S, Garnier F, Haïkel Y, Gangloff SC, Benkirane-Jessel N. Nanostructured assemblies for dental application. ACS Nano. 2010 Jun;4(6):3277–87. doi: 10.1021/nn100713m. [DOI] [PubMed] [Google Scholar]
- 162.Patil SD, Papadimitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control Release. 2007 Jan;117(1):68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 163.Park JS, Yang HN, Jeon SY, Woo DG, Na K, Park KH. Osteogenic differentiation of human mesenchymal stem cells using RGDmodified BMP-2 coated microspheres. Biomaterials. 2010 Aug;31(24):6239–48. doi: 10.1016/j.biomaterials.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 164.Xie XH, Yu XW, Zeng SX, Du RL, Hu YH, Yuan Z, Lu EY, Dai KR, Tang TT. Enhanced osteointegration of orthopaedic implant gradient coating composed of bioactive glass and nanohydroxyapatite. J Mater Sci Mater Med. 2010 Jul;21(7):2165–73. doi: 10.1007/s10856-010-4077-6. [DOI] [PubMed] [Google Scholar]
- 165.Williams D. On the mechanisms of biocompatibility. Biomaterials. 2008 Jul;29(20):2941–53. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]