Abstract
Rationale
Gamma-hydroxybutyrate (GHB) is a gamma-aminobutyric acid (GABA) analog that is used to treat narcolepsy but that is also abused. GHB has many actions in common with the GABAB receptor agonist baclofen, but their underlying GABAB receptor mechanisms may be different.
Objective
The aim of this study is to further investigate a possible differential role of glutamate in GABAB receptor-mediated effects of GHB and baclofen.
Materials and methods
The experiments examined the effects of non-competitive antagonists at the N-methyl-d-asparate (NMDA) subtype of glutamate receptors on GHB-induced catalepsy and compared these effects with those on baclofen-induced catalepsy.
Results
In C57BL/6J mice, ketamine, phencyclidine (PCP), and dizocilpine (MK-801) all enhanced GHB-induced catalepsy. They did so with a potency order (i.e., MK-801 > PCP > ketamine) consistent with their relative potencies as NMDA antagonists but not as inhibitors of dopamine or organic cation transporters. Ketamine, PCP, and MK-801 enhanced catalepsy along inverted U-shaped dose–response curves likely because higher doses affected motor coordination, which limited their catalepsy-enhancing effects. Doses that were maximally effective to enhance GHB-induced catalepsy did not affect the cataleptic effects of baclofen.
Conclusions
The finding that NMDA receptor antagonists enhance the cataleptic effects of GHB but not those of baclofen is further evidence that the GABAB receptor mechanisms mediating the effects of GHB and GABAB agonists are not identical. Differential interactions of glutamate with the GABAB receptor mechanisms mediating the effects of GHB and baclofen may explain why GHB is effective for treating narcolepsy and is abused, whereas baclofen is not.
Keywords: Catalepsy, Mice, GHB, Baclofen, GABA-B, MK-801, PCP, Ketamine, NMDA, Glutamate
Introduction
γ-Hydroxybutyrate (GHB) is a putative neuromodulator (Maitre 1997) involved in the regulation of sleep (Mamelak et al. 1977) and used clinically to treat narcolepsy in the USA (Tunnicliff and Raess 2002; Fuller and Hornfeldt 2003) and alcoholism in Europe (Poldrugo and Addolorato 1999). GHB is also used recreationally (Rodgers et al. 2004; Gonzalez and Nutt 2005). Its precise mechanism of action is unknown.
GHB binds to GABAB receptors (Mathivet et al. 1997; Lingenhoehl et al. 1999) and specific GHB receptors (Benavides et al. 1982; Snead and Liu 1984) in brain that are thought to be involved in the behavioral effects of GHB. At present, there appears to be little evidence for a role for GHB receptors in the in vivo effects of GHB (Wong et al. 2004). Instead, many studies suggest that GABAB receptors are particularly important for various behavioral effects of GHB, including discriminative stimulus effects (e.g., Winter 1981, Colombo et al. 1998, Carter et al. 2003, and Koek et al. 2004, 2006), decreased operant responding (Goodwin et al. 2005), and directly observable effects such as hypolocomotion (Kaupmann et al. 2003), catalepsy (Carter et al. 2005), ataxia (Goodwin et al. 2005), and loss of righting (Carai et al. 2001). Consistent with the involvement of GABAB receptors, all of these effects of GHB are produced also by the prototypical GABAB receptor agonist baclofen (Carter et al. 2003, 2004, 2005).
Although GABAB receptors likely mediate behavioral effects that GHB has in common with baclofen, there is evidence that the underlying GABAB receptor mechanisms may not be identical. The GABAB receptor antagonist CGP35348 antagonizes the discriminative stimulus effects of GHB and baclofen, consistent with the involvement of GABAB receptors, but is less potent to antagonize these effects of GHB than those of baclofen (Koek et al. 2004; Carter et al. 2006). Recently, we reported that CGP35348 was also less potent to antagonize the cataleptic effects of GHB and its precursor GBL than those of the GABAB receptor agonists baclofen and SKF97541 (Koek et al. 2007b). Together, these findings suggest a possible role for different GABAB receptor subtypes or different interactions with the same GABAB receptor in the behavioral effects of GHB and baclofen.
Additional evidence that the GABAB receptor mechanisms underlying the effects of GHB and baclofen are not identical has recently been obtained in interaction studies with N-methyl-d-aspartate (NMDA) receptor antagonists. The NMDA antagonist dizocilpine (MK-801) enhances GHB-induced catalepsy in rats (Sevak et al. 2004, 2005), and GHB and the NMDA antagonist phencyclidine (PCP) enhance each other’s discriminative stimulus effects in rats (Koek et al. 2007a). Interestingly, the latter interaction may be specific for GHB because it did not occur with PCP and baclofen (Koek et al. 2007a). The present study is part of an effort to examine the generality of the differential enhancement of behavioral effects of GHB and baclofen by NMDA antagonists. Here, instead of discriminative stimulus effects, the cataleptic effects of GHB and baclofen, which typically occur at higher doses, were examined. GHB- and baclofen-induced catalepsy was studied in animals pretreated with dizocilpine, PCP, or ketamine. Although dizocilpine, PCP, and ketamine are potent antagonists at the NMDA subtype of glutamate receptor, they not only share NMDA antagonist properties but can also produce other effects, such as inhibition of dopamine uptake (e.g., Snell et al. 1988) and, discovered recently, inhibition of organic cation transporters (Amphoux et al. 2006). Thus, to test the hypothesis that NMDA antagonism enhances GHB, the present study examined if PCP, dizocilpine, and ketamine enhance the cataleptic effects of GHB with relative potencies that correlate with their NMDA antagonist properties and not with their ability to inhibit dopamine uptake or organic cation transporters.
The present study was conducted in mice to examine the generality of our previous findings in rats. The results show that differential enhancement of GHB and baclofen by NMDA antagonists is not limited to discriminative stimulus effects. Taken together, our studies suggest the possibility that GABAB receptors on glutamatergic neurons mediate behavioral effects of GHB, whereas GABAB receptors on GABAergic neurons mediate behavioral effects of baclofen. This difference may have implications for their different profiles of preclinical and clinical activities.
Materials and methods
Animals
Fifty-seven adult male C57BL/6J mice (The Jackson laboratory, Bar Harbor, ME, USA), weighing 23–34 g at the beginning of the experiments, were housed in groups of four in an environmentally controlled room (temperature, 24°C; relative humidity, 45%), under a 14:10 h light/dark cycle (light on at 0700 hours), with food (rodent sterilizable diet; Harlan Teklad) and water continuously available. The animals were maintained, and the experiments were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the 1966 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences).
Catalepsy
As described elsewhere (Carter et al. 2005; Koek et al. 2007b), catalepsy was examined using a horizontal cylindrical metal bar (diameter, 1 cm) supported 4 cm above the floor by two 8×8-cm square pieces of Plexiglas (Instrumentation Services, University of Texas Health Science Center). Mice were tested for catalepsy by placing their forepaws on the bar while the hind paws remain on the floor and then recording the time that both forepaws remained on the bar up to a maximum of 30 s. Each animal received an i.p. injection of saline or of a particular dose of an NMDA antagonist and was tested for catalepsy 15 min later by an observer unaware of the pretreatment condition. Immediately after this test, animals received i.p. injections at 15-min intervals with cumulative doses of GHB (32–320 mg/kg) and were tested for catalepsy at the end of each interval. Each pretreatment was tested in eight mice, except saline control pretreatment, which was tested in 13 mice. Mice were tested repeatedly but not more than 16 times and with at least a week between tests. In an effort to control for repeated testing, pretreatment conditions were tested in a non-systematic order. To examine the selectivity of the effects on GHB-induced catalepsy, doses of the NMDA antagonists that were maximally effective to enhance GHB were also studied as a pretreatment to cumulative doses of baclofen (1.78–10 mg/kg; n=8).
Ataxia
Ataxia was studied by means of an inverted screen apparatus (Instrumentation Services, University of Texas Health Science Center) based on that described previously (Coughenour et al. 1977) and consisting of four 13×13-cm wire screens (no. 4 mesh) located 23 cm above four Plexiglas containers. The four screens were connected to a rod and handle that could be rotated 180° to simultaneously invert the four screens. Failure to remain on the apparatus for 60 s after it was rotated was scored as exhibiting ataxia. Animals received an i.p. injection of saline or a particular dose of an NMDA antagonist (n=4) and, 15 min later, were examined for ataxia.
Data analysis
For each pretreatment drug and dose, the area under the treatment drug dose–effect curve (AUC) was calculated for each animal by means of GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego, CA, USA). Because the same treatment drug doses were used throughout, the AUC offered a measure of the effectiveness of the treatment drug under various pretreatment conditions. Pretreatment drug effects on the individual AUC values for GHB and, on the time the forepaws remained on the bar when the pretreatment was given alone, were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s test (GraphPad Prism). Effects of pretreatment drugs on AUC values for baclofen were analyzed by Student’s t test (GraphPad Prism). Relations between the potency of pretreatment drugs to affect AUC values and to produce other previously reported effects were examined by calculating Pearson’s correlation coefficient using log-transformed potency values.
Daily administration of a relatively high (200 mg/kg) dose of GHB during 14 days has been reported to produce tolerance to its cataleptic effects in mice (Itzhak and Ali 2002). In the present study, drug doses were tested in a non-systematic order, with at least 1 week between tests. In an effort to examine possible effects of repeated testing under the conditions of the present study, the dose–response data of the GHB experiments were analyzed by dividing the data at each dose into two groups based on whether they were obtained earlier or later in the study. Two-way ANOVA followed by Bonferroni post-tests (GraphPad Prism) were used to compare dose–response data obtained earlier and later in the study.
Drug effects on ataxia were analyzed by comparing the percentage of drug-treated animals showing ataxia with the percentage of saline-treated controls showing ataxia by means of Fisher’s exact test (GraphPad Prism). Correlations were quantified by Pearson’s correlation coefficient.
Drugs
γ-Hydroxybutyrate sodium (GHB) and (±)baclofen were purchased from Sigma-Aldrich (USA), ketamine hydrochloride from Fort Dodge Laboratories (Fort Dodge, IA, USA), and dizocilpine from Research Biochemicals International (Natick, MA, USA). Phencyclidine was obtained from NIDA (Research Technology Branch, Rockville, MD, USA). All compounds were dissolved in physiological saline (0.9% NaCl), except GHB, which was dissolved in sterile water. All compounds were injected i.p. in a volume of 5 to 20 ml/kg. Doses are expressed as the form of the compound listed above.
Results
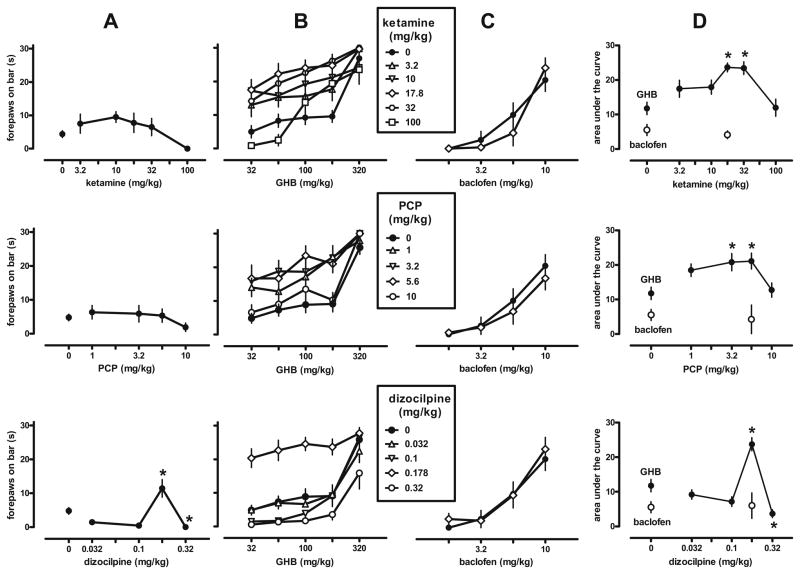
Neither ketamine nor PCP produced catalepsy in C57BL/6J mice when given alone (Fig. 1a). Pretreatment with either drug did not significantly affect the mean time that both forepaws remained on the bar (ketamine, F[5,47]=2.28, P> 0.05; PCP, F[4,44]=1.02, P>0.20). However, dizocilpine significantly (F[4,44]=14.63, P<0.001) affected this measure in a biphasic manner, increasing the time the forepaws remained on the bar at a dose of 0.178 mg/kg and decreasing it at 0.32 mg/kg.
Fig. 1.
Cataleptic effects of cumulative doses of GHB and the GABAB receptor agonist baclofen after administration of saline or different doses of the NMDA antagonists ketamine, phencyclidine (PCP), or dizocilpine (MK-801) in C57BL/6J mice (n=8–13 per dose, all injections i.p.). a Effects of the NMDA antagonists before the administration of GHB. b and c Effects of the NMDA antagonists on the dose–response curves of GHB and baclofen, respectively. d Effects of the NMDA antagonists on the area under the dose–response curves of GHB (filled symbols) and baclofen (open circles) shown in b and c, respectively. Symbols represent mean ± SEM; if not shown, SEM values are contained by the symbol. Asterisks P<0.05 (Dunnett’s test vs control)
In animals pretreated with saline, GHB and baclofen dose-dependently produced catalepsy (Fig. 1b, c; solid circles). Ketamine, PCP, and dizocilpine enhanced the cataleptic effects of GHB (Fig. 1b) and significantly increased the AUC values for GHB (Fig. 1d, solid circles; F[5,47]=7.0, P<0.001, F[4,44]=4.78, P<0.005, and F [4,44]=20.71, P<0.001, respectively; AUC values obtained earlier and later in the study were not significantly different, assessed by ANOVA and post-tests). Ketamine, PCP, and dizocilpine significantly and maximally increased the AUC values for GHB at 17.8, 5.6, and 0.178 mg/kg, respectively. At these doses, none of the NMDA antagonists significantly altered the cataleptic effects of baclofen (Fig. 1c) measured as area under the curve (Fig. 1d; open circles; t≤0.64, df=18, P≥0.50). At higher doses that no longer enhanced GHB-induced catalepsy (i.e., 100 mg/kg ketamine, 10 mg/kg PCP, 0.32 mg/kg dizocilpine), all three NMDA antagonists produced ataxia in 100% of the animals tested, compared with 0% ataxia in saline-treated animals (P<0.05; data not shown).
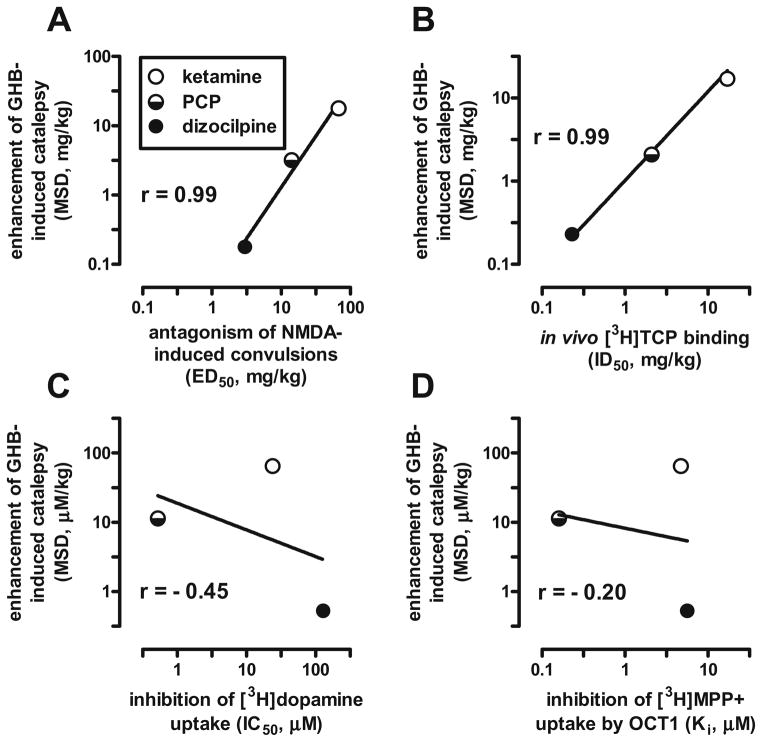
The relative potencies with which ketamine, PCP, and dizocipline enhanced the cataleptic effects of GHB correlated positively and strongly (r=0.99) with their potency to antagonize NMDA-induced convulsions (Fig. 2a) and with their potency to inhibit binding of the PCP derivative thienylcyclohexyl piperidine (TCP) at the NMDA receptor-associated ion channel (Fig. 2b). In contrast, the catalepsy-enhancing effects of ketamine, PCP, and dizocilpine correlated only weakly and negatively with their potency to inhibit dopamine and organic cation transporters (Fig. 2c, d; r=−0.45 and −0.20, respectively). The doses at which the NMDA antagonists enhanced GHB-induced catalepsy were 4- to 16-fold lower than those reported (Koek and Colpaert 1990) to block NMDA-induced convulsions (Fig. 2a) and were very similar to those reported (Maurice and Vignon 1990) to inhibit TCP-binding in vivo (Fig. 2b).
Fig. 2.
Relation between the potencies of the NMDA antagonists ketamine, phencyclidine (PCP), and dizocilpine (MK-801) to enhance GHB-induced catalepsy in mice and their potencies to a antagonize NMDA-induced convulsions in mice (Koek and Colpaert 1990), b inhibit in vivo labeling of the NMDA receptor-associated ion channel with the phencyclidine derivative [3H]TCP in mouse brain, which correlates strongly (r=0.93) with in vitro [3H]TCP binding in rat brain (Maurice and Vignon 1990), c inhibit [3H]dopamine uptake into rat striatal synaptosomes (Johnson and Snell 1985; Snell et al. 1988), and d inhibit [3H]MPP+ uptake by rat organic cation transporter 1 (OCT1) in transfected HEK293 cells (Amphoux et al. 2006). MSD minimum significant dose, r Pearson’s correlation coefficient
Discussion
The main finding of this study is that GHB-induced catalepsy was selectively enhanced by dizocilpine, PCP, and ketamine, with a potency order (i.e., dizocilpine > PCP > ketamine, based on their minimum effective dose: 0.178, 3.2, and 17.8 mg/kg, respectively) similar to their relative potencies to antagonize effects of NMDA in vivo (e.g., Koek et al. 1990) and consistent with their relative affinities at binding sites in the ion channel of the NMDA receptor complex labeled with PCP (e.g., Wong et al. 1988) or the PCP derivative, TCP (e.g., Maurice and Vignon 1990). Dizocilpine significantly increased catalepsy when given alone. It is unlikely that NMDA antagonism is involved in these effects of dizocilpine because neither PCP nor ketamine produced catalepsy when given alone. Whichever the mechanism, this dose of dizocilpine did not generally enhance the cataleptic effects of other drugs but selectively enhanced GHB-induced catalepsy as did the other NMDA antagonists PCP and ketamine. The present results in mice (1) are consistent with previous findings that dizocilpine enhances GHB-induced catalepsy in rats (Sevak et al. 2004, 2005), (2) extend them to other channel-blocking NMDA antagonists, (3) show that their catalepsy-enhancing effects correlate positively with their NMDA antagonist properties instead of with their dopamine or organic cation transporter blocking effects, and (4) suggest that their catalepsy-enhancing effects are selective for GHB. The latter finding is consistent with evidence that PCP and GHB enhance each other’s discriminative stimulus effects, but PCP and baclofen do not (Koek et al. 2007a). Taken together, these findings are further evidence that the GABAB receptor mechanisms mediating the effects of GHB and baclofen are not identical (e.g., Koek et al. 2007b), and suggest that these GABAB receptor mechanisms are differentially modulated by glutamatergic systems.
Baclofen produces catalepsy in rats after peripheral (i.p.; Mehta and Ticku 1987) and central (ventromedial thalamic nucleus; Wullner et al. 1987) administration, likely related to its effects on striatal dopamine synthesis, which are similar to those of the neuroleptic haloperidol (Waldmeier 1991). However, these neurochemical effects of baclofen are mediated by GABAB receptors, unlike those of haloperidol (Waldmeier 1991). Consistent with the involvement of GABAB receptors, baclofen-induced catalepsy is blocked by the GABAB receptor antagonist δ-aminovalericacid and not by bicuculline, bromocriptine, or scopolamine (Mehta and Ticku 1987). In the present study, catalepsy was produced by cumulative i.p. doses of baclofen and also by a cumulative i.p. dose of 320 mg/kg GHB, consistent with previous reports of catalepsy following 560 mg/kg GHB i.p. in Spraque–Dawley rats (Sevak et al. 2004), 200 mg/kg GHB i.p. in OF.1 mice (Navarro et al. 1998), 300 mg/kg GHB i.p. in Swiss–Webster mice (Itzhak and Ali 2002), and 320 mg/kg GHB i.p. in C57BL/6J mice (Carter et al. 2005; Koek et al. 2007b), the same strain as used in the present study. The lowest dose of GHB and baclofen that produced near-maximal catalepsy in the cumulative dosing procedure used here (i.e., 320 and 10 mg/kg i.p., respectively) was the same as in the single dosing procedure used previously (Carter et al. 2005; Koek et al. 2007b), suggesting that, under the conditions of these experiments, the potency of GHB and baclofen to produce catalepsy in C57BL/6J mice is not markedly affected by the use of single or cumulative dosing. The observation that GHB, which has agonist activity at GABAB receptors (Lingenhoehl et al. 1999), has cataleptic effects in common with a well-characterized GABAB receptor agonist and the finding that these effects can be blocked by the GABAB receptor antagonist CGP35348 (e.g., Koek et al. 2007b) are further evidence that GABAB receptors are involved in GHB-induced catalepsy.
Although GABAB receptors appear to be involved, the GABAB receptor mechanisms underlying the effects of GHB and baclofen may not be identical. For example, the GABAB receptor antagonist CGP35348 attenuated GHB-and baclofen-induced catalepsy, demonstrating a role for GABAB receptors, but was threefold less potent to antagonize the effects of GHB than those of baclofen, suggesting differential involvement of GABAB receptors in the cataleptic effects of GHB and baclofen (Koek et al. 2007b). Furthermore, PCP and GHB enhance each other’s discriminative stimulus effects, but PCP and baclofen do not (Koek et al. 2007a), suggesting that the mechanisms underlying these effects of GHB and baclofen are differentially modulated by the glutamatergic systems with which PCP interacts (Koek et al. 2007a). The present finding that PCP and other antagonists at the NMDA subtype of glutamate receptors enhance the cataleptic effects of GHB but not those of baclofen suggests that differential modulation by glutamate of the GABAB systems underlying the effects of GHB and baclofen may have considerable generality.
Recent electrophysiological studies offer additional evidence of differing effects of GHB and baclofen. When applied at clinically relevant concentrations, GHB disinhibits and baclofen inhibits ventral tegmental dopamine neurons (Cruz et al. 2004). This suggests that GHB would be more likely than baclofen to activate the dopamine system implicated in addiction and that baclofen, which may be useful to reduce relapse to cocaine-taking (e.g., Weerts et al. 2007), may have more pronounced anti-craving effects than GHB (Cruz et al. 2004). GHB and baclofen also differ in their effects on excitatory neuro-transmission at NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) subtypes of glutamate receptors in neocortex (Li et al. 2007). Both GHB and baclofen inhibited excitatory postsynaptic currents elicited by NMDA and AMPA, and these effects could be reversed by the selective GABAB receptor antagonist CGP62349. Interestingly, GHB more potently depressed NMDA receptor-mediated postsynaptic currents, whereas baclofen more potently depressed responses mediated by the AMPA subtype of glutamate receptors (Li et al. 2007). These latter findings, together with the present results, suggest a special role for NMDA receptors in the GABAB receptor-mediated effects of GHB. Thus, there is emerging evidence that the effects of GHB and baclofen, although in many respects similar, may be mediated by different GABAB systems.
There is evidence for functional GABAB receptor subtypes (Seabrook et al. 1990; Bonanno and Raiteri 1992; Lanza et al. 1993; Fassio et al. 1994; Yamada et al. 1999). Conceivably, differential activity of GHB and baclofen at GABAB autoreceptors and heteroreceptors could account for the differential ability of CGP35348 to antagonize GHB and baclofen. Alternatively, GHB and baclofen may interact differently with the same GABAB receptor (e.g., GHB may induce conformational changes in the GABAB receptor that differ from those induced by baclofen). Differential activity of GHB and baclofen at auto- and hetero-receptors could also account for the differential enhancement of their effects by antagonists at the NMDA subtype of glutamate receptor observed in the present study, with effects of GHB mediated by glutamatergic GABAB heteroreceptors and effects of baclofen by GABAB autoreceptors. Further studies examining the different GABAB mechanisms underlying effects of GHB and baclofen may help to explain why GHB is effective to treat narcolepsy and is abused, whereas there is no evidence that baclofen is effective in any sleep disorder and no evidence that baclofen is abused.
Acknowledgments
The work was supported by the US Public Health Service Grants DA15692 (W.K.) and DA17918 (C.P.F.)
The authors thank Adela Garza and Jason Persyn for technical assistance. The work was supported by the US Public Health Service Grants DA15692 (W.K.) and DA17918 (C.P.F.).
Contributor Information
Wouter Koek, Email: koek@uthscsa.edu, Department of Psychiatry, The University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900, USA. Department of Pharmacology, The University of Texas Health Science Center at San Antonio, San Antonio, TX, USA.
Charles P. France, Department of Psychiatry, The University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900, USA. Department of Pharmacology, The University of Texas Health Science Center at San Antonio, San Antonio, TX, USA
References
- Amphoux A, Vialou V, Drescher E, Brüss M, Mannoury La Cour C, Rochat C, Millan MJ, Giros B, Bönisch H, Gautron S. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–952. doi: 10.1016/j.neuropharm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Benavides J, Rumigny JF, Bourguignon JJ, Cash C, Wermuth CG, Mandel P, Vincendon G, Maitre M. High affinity binding sites for gamma-hydroxybutyric acid in rat brain. Life Sci. 1982;30:953–961. doi: 10.1016/0024-3205(82)90624-5. [DOI] [PubMed] [Google Scholar]
- Bonanno G, Raiteri M. Functional evidence for multiple gamma-aminobutyric acid B receptor subtypes in the rat cerebral cortex. J Pharmacol Exp Ther. 1992;262:114–118. [PubMed] [Google Scholar]
- Carai MA, Colombo G, Brunetti G, Melis S, Serra S, Vacca G, Mastinu S, Pistuddi AM, Solinas C, Cignarella G, Minardi G, Gessa GL. Role of GABA(B) receptors in the sedative/hypnotic effect of gamma-hydroxybutyric acid. Eur J Pharmacol. 2001;428:315–321. doi: 10.1016/s0014-2999(01)01334-6. [DOI] [PubMed] [Google Scholar]
- Carter LP, Flores LR, Wu H, Chen W, Unzeitig AW, Coop A, France CP. The role of GABAB receptors in the discriminative stimulus effects of gamma-hydroxybutyrate in rats: time course and antagonism studies. J Pharmacol Exp Ther. 2003;305:668–674. doi: 10.1124/jpet.102.047860. [DOI] [PubMed] [Google Scholar]
- Carter LP, Wu H, Chen W, Cruz CM, Lamb RJ, Koek W, Coop A, France CP. Effects of gamma-hydroxybutyrate (GHB) on schedule-controlled responding in rats: role of GHB and GABAB receptors. J Pharmacol Exp Ther. 2004;308:182–188. doi: 10.1124/jpet.103.058909. [DOI] [PubMed] [Google Scholar]
- Carter LP, Wu H, Chen W, Matthews MM, Mehta AK, Hernandez RJ, Thomson JA, Ticku MK, Coop A, Koek W, France CP. Novel {gamma}-hydroxybutyric acid (GHB) analogs share some, but not all, of the behavioral effects of GHB and GABAB receptor agonists. J Pharmacol Exp Ther. 2005;313:1314–1323. doi: 10.1124/jpet.104.077578. [DOI] [PubMed] [Google Scholar]
- Carter LP, Chen W, Coop A, Koek W, France CP. Discriminative stimulus effects of GHB and GABA(B) agonists are differentially attenuated by CGP35348. Eur J Pharmacol. 2006;538:85–93. doi: 10.1016/j.ejphar.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Gessa GL. Involvement of GABA(A) and GABA(B) receptors in the mediation of discriminative stimulus effects of gamma-hydroxybutyric acid. Physiol Behav. 1998;64:293–302. doi: 10.1016/s0031-9384(98)00062-6. [DOI] [PubMed] [Google Scholar]
- Coughenour LL, Mclean JR, Parker RB. A new device for the rapid measurement of impaired motor function in mice. Pharmacol Biochem Behav. 1977;6:351–353. doi: 10.1016/0091-3057(77)90036-3. [DOI] [PubMed] [Google Scholar]
- Cruz HG, Ivanova T, Lunn ML, Stoffel M, Slesinger PA, Lüscher C. Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- Fassio A, Bonanno G, Cavazzani P, Raiteri M. Characterization of the GABA autoreceptor in human neocortex as a pharmacological subtype of the GABAB receptor. Eur J Pharmacol. 1994;263:311–314. doi: 10.1016/0014-2999(94)90727-7. [DOI] [PubMed] [Google Scholar]
- Fuller DE, Hornfeldt CS. From club drug to orphan drug: sodium oxybate (Xyrem) for the treatment of cataplexy. Pharmacotherapy. 2003;23:1205–1209. doi: 10.1592/phco.23.10.1205.32756. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Nutt DJ. Gamma hydroxy butyrate abuse and dependency. J Psychopharmacol. 2005;19:195–204. doi: 10.1177/0269881105049041. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Froestl W, Weerts EM. Involvement of gamma-hydroxybutyrate (GHB) and GABA-B receptors in the acute behavioral effects of GHB in baboons. Psychopharmacology (Berl) 2005;180:342–351. doi: 10.1007/s00213-005-2165-y. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Ali SF. Repeated administration of gamma-hydroxybutyric acid (GHB) to mice: assessment of the sedative and rewarding effects of GHB. Ann N Y Acad Sci. 2002;965:451–460. doi: 10.1111/j.1749-6632.2002.tb04186.x. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Snell LD. Effects of phencyclidine (PCP)-like drugs on turning behavior, 3H-dopamine uptake, and 3H-PCP binding. Pharmacol Biochem Behav. 1985;22:731–735. doi: 10.1016/0091-3057(85)90521-0. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Cryan JF, Wellendorph P, Mombereau C, Sansig G, Klebs K, Schmutz M, Froestl W, van der PH, Mosbacher J, Brauner-Osborne H, Waldmeier P, Bettler B. Specific gamma-hydroxybutyrate-binding sites but loss of pharmacological effects of gamma-hydroxybutyrate in GABA(B)(1)-deficient mice. Eur J Neurosci. 2003;18:2722–2730. doi: 10.1111/j.1460-9568.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- Koek W, Colpaert FC. Selective blockade of N-methyl-d-aspartate (NMDA)-induced convulsions by NMDA antagonists and putative glycine antagonists: relationship with phencyclidine-like behavioral effects. J Pharmacol Exp Ther. 1990;252:349–357. [PubMed] [Google Scholar]
- Koek W, Woods JH, Colpaert FC. N-Methyl-d-aspartate antagonism and phencyclidine-like activity: a drug discrimination analysis. J Pharmacol Exp Ther. 1990;253:1017–1025. [PubMed] [Google Scholar]
- Koek W, Flores LR, Carter LP, Lamb RJ, Chen W, Wu H, Coop A, France CP. Discriminative stimulus effects of gamma-hydroxybutyrate in pigeons: role of diazepam-sensitive and -insensitive GABA(A) and GABA(B) receptors. J Pharmacol Exp Ther. 2004;308:904–911. doi: 10.1124/jpet.103.056093. [DOI] [PubMed] [Google Scholar]
- Koek W, Wu H, Coop A, France CP. Discriminative stimulus effects of gamma-hydroxybutyrate: role of training dose. J Pharmacol Exp Ther. 2006;317:1–9. doi: 10.1124/jpet.105.096909. [DOI] [PubMed] [Google Scholar]
- Koek W, Khanal M, France CP. Synergistic interactions between ‘club drugs’: gamma-hydroxybutyrate and phencyclidine enhance each other’s discriminative stimulus effects. Behav Pharmacol. 2007a;18:807–810. doi: 10.1097/FBP.0b013e3282f18d45. [DOI] [PubMed] [Google Scholar]
- Koek W, Mercer SL, Coop A. Cataleptic effects of g-hydroxybutyrate (GHB), its precursor g-butyrolactone (GBL), and GABAB receptor agonists in mice: differential antagonism by the GABAB receptor antagonist CGP35348. Psychopharmacology. 2007b;192:407–414. doi: 10.1007/s00213-007-0718-y. [DOI] [PubMed] [Google Scholar]
- Lanza M, Fassio A, Gemignani A, Bonanno G, Raiteri M. CGP 52432: a novel potent and selective GABAB autoreceptor antagonist in rat cerebral cortex. Eur J Pharmacol. 1993;237:191–195. doi: 10.1016/0014-2999(93)90268-m. [DOI] [PubMed] [Google Scholar]
- Li Q, Kuhn CM, Wilson WA, Lewis DV. Effects of gamma hydroxybutyric acid on inhibition and excitation in rat neocortex. Neuroscience. 2007;150:82–92. doi: 10.1016/j.neuroscience.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingenhoehl K, Brom R, Heid J, Beck P, Froestl W, Kaupmann K, Bettler B, Mosbacher J. Gamma-hydroxybutyrate is a weak agonist at recombinant GABA(B) receptors. Neuropharmacology. 1999;38:1667–1673. doi: 10.1016/s0028-3908(99)00131-8. [DOI] [PubMed] [Google Scholar]
- Maitre M. The gamma-hydroxybutyrate signalling system in brain: organization and functional implications. Prog Neurobiol. 1997;51:337–361. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Mamelak M, Escriu JM, Stokan O. The effects of gamma-hydroxybutyrate on sleep. Biol Psychiatry. 1977;12:273–288. [PubMed] [Google Scholar]
- Mathivet P, Bernasconi R, De BJ, Marescaux C, Bittiger H. Binding characteristics of gamma-hydroxybutyric acid as a weak but selective GABAB receptor agonist. Eur J Pharmacol. 1997;321:67–75. doi: 10.1016/s0014-2999(96)00916-8. [DOI] [PubMed] [Google Scholar]
- Maurice T, Vignon J. In vivo labeling of phencyclidine (PCP) receptors with 3H-TCP in the mouse brain. J Neurosci Res. 1990;26:377–385. doi: 10.1002/jnr.490260315. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. Baclofen induces catatonia in rats. Neuropharmacology. 1987;26:1419–1423. doi: 10.1016/0028-3908(87)90108-0. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Pedraza C, Martin M, Manzaneque JM, Davila G, Maldonado E. Tiapride-induced catalepsy is potentiated by gamma-hydroxybutyric acid administration. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:835–844. doi: 10.1016/s0278-5846(98)00043-8. [DOI] [PubMed] [Google Scholar]
- Poldrugo F, Addolorato G. The role of gamma-hydroxybutyric acid in the treatment of alcoholism: from animal to clinical studies. Alcohol Alcohol. 1999;34:15–24. doi: 10.1093/alcalc/34.1.15. [DOI] [PubMed] [Google Scholar]
- Rodgers J, Ashton CH, Gilvarry E, Young AH. Liquid ecstasy: a new kid on the dance floor. Br J Psychiatry. 2004;184:104–106. doi: 10.1192/bjp.184.2.104. [DOI] [PubMed] [Google Scholar]
- Seabrook GR, Howson W, Lacey MG. Electrophysiological characterization of potent agonists and antagonists at pre- and postsynaptic GABAB receptors on neurones in rat brain slices. Br J Pharmacol. 1990;101:949–957. doi: 10.1111/j.1476-5381.1990.tb14186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevak RJ, France CP, Koek W. Neuroleptic-like effects of gamma-hydroxybutyrate: interactions with haloperidol and dizocilpine. Eur J Pharmacol. 2004;483:289–293. doi: 10.1016/j.ejphar.2003.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, France CP. Streptozotocin-induced diabetes differentially modifies haloperidol- and gamma-hydroxybutyric acid (GHB)-induced catalepsy. Eur J Pharmacol. 2005;517:64–67. doi: 10.1016/j.ejphar.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Snead OC, III, Liu CC. Gamma-hydroxybutyric acid binding sites in rat and human brain synaptosomal membranes. Biochem Pharmacol. 1984;33:2587–2590. doi: 10.1016/0006-2952(84)90629-4. [DOI] [PubMed] [Google Scholar]
- Snell LD, Yi SJ, Johnson KM. Comparison of the effects of MK-801 and phencyclidine on catecholamine uptake and NMDA-induced norepinephrine release. Eur J Pharmacol. 1988;145:223–226. doi: 10.1016/0014-2999(88)90235-x. [DOI] [PubMed] [Google Scholar]
- Tunnicliff G, Raess BU. Gamma-hydroxybutyrate (orphan medical) Curr Opin Investig Drugs. 2002;3:278–283. [PubMed] [Google Scholar]
- Waldmeier PC. The GABAB antagonist, CGP 35348, antagonizes the effects of baclofen, gamma-butyrolactone and HA 966 on rat striatal dopamine synthesis. Naunyn-Schmiedebergs Arch Pharmacol. 1991;343:173–178. doi: 10.1007/BF00168606. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Froestl W, Kaminski BJ, Griffiths RR. Attenuation of cocaine-seeking by GABA B receptor agonists baclofen and CGP44532 but not the GABA reuptake inhibitor tiagabine in baboons. Drug Alcohol Depend. 2007;89:206–13. doi: 10.1016/j.drugalcdep.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JC. The stimulus properties of gamma-hydroxybutyrate. Psychopharmacology (Berl) 1981;73:372–375. doi: 10.1007/BF00426468. [DOI] [PubMed] [Google Scholar]
- Wong EHF, Knight AR, Woodruff GN. (3H)MK-801 labels a site on the N-methyl-d-asparate receptor channel complex in rat brain membranes. J Neurochem. 1988;50:274–281. doi: 10.1111/j.1471-4159.1988.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Wong CG, Gibson KM, Snead OC., III From the street to the brain: neurobiology of the recreational drug gamma-hydroxybutyric acid. Trends Pharmacol Sci. 2004;25:29–34. doi: 10.1016/j.tips.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wullner U, Klockgether T, Schwarz M, Sontag KH. Behavioral actions of baclofen in the rat ventromedial thalamic nucleus: antagonism by delta-aminovalerate. Brain Res. 1987;422:129–136. doi: 10.1016/0006-8993(87)90547-6. [DOI] [PubMed] [Google Scholar]
- Yamada K, Yu B, Gallagher JP. Different subtypes of GABAB receptors are present at pre- and postsynaptic sites within the rat dorsolateral septal nucleus. J Neurophysiol. 1999;81:2875–2883. doi: 10.1152/jn.1999.81.6.2875. [DOI] [PubMed] [Google Scholar]