Abstract
Purpose
Soy isoflavones, structurally similar to endogenous estrogens, may affect breast cancer through both hormonally-mediated and non-hormonally related mechanisms. Although the effects of soy are not well understood, some breast cancer survivors increase their soy intake post-diagnosis in attempt to improve their prognosis. Therefore, we examined the role of soy isoflavone intake and the risk of breast cancer recurrence by hormone receptor status, menopausal status, and tamoxifen therapy.
Materials and methods
A cohort of 1954 female breast cancer survivors, diagnosed during 1997–2000, was prospective followed for 6.31 years and 282 breast cancer recurrences were ascertained. Isoflavone intake was assessed by mailing modified Block and supplemental soy food frequency questionnaires to participants, on average 23 months post-diagnosis. Risk of breast cancer recurrence, measured by hazard ratios (HR) and 95% confidence intervals (CI), was estimated using multivariable delayed-entry Cox proportional hazards models.
Results
Suggestive trends for a reduced risk of cancer recurrence were observed with increasing quintiles of daidzein and glycetin intake compared to no intake among postmenopausal women (P for trend: P = .08 for daidzein, P = .06 for glycetin) and among tamoxifen users (P = .10 for daidzein, P = .05 for glycetin). Among postmenopausal women treated with tamoxifen, there was an approximately 60% reduction in breast cancer recurrence comparing the highest to the lowest daidzein intakes (>1453 micrograms (µg)/day versus < 7.7 µg/day) (HR, 0.48; 95% CI, 0.21–0.79, P = .008).
Conclusion
Soy isoflavones consumed at levels comparable to those in Asian populations may reduce the risk of cancer recurrence in women receiving tamoxifen therapy and moreover, appears not to interfere with tamoxifen efficacy. Further confirmation is required in other large prospective studies before recommendations regarding soy intake can be issued to breast cancer survivors.
Keywords: Breast Neoplasms, Daidzein, Genistein, Isoflavones, Neoplasm Recurrence, Local, Postmenopause, Receptors, Estrogen, Soy Foods, Tamoxifen
Introduction
Found mainly in soy foods, isoflavones are part of a larger class of flavonoid compounds that have been demonstrated to be potent dietary anti-cancer agents [1]. The primary isoflavones daidzein, genistein, and glycetin comprise 40%, 50%, and 10% of the soybean, respectively [2]. Soy intake is high in many Asian countries where breast cancer incidence is lower compared to Western countries [3–5]. Migration studies have shown that after successive generations, breast cancer incidence in Asian women becomes similar to that of Western women [6–9]. Furthermore, an increasing incidence of breast cancer among Japanese women parallels the Westernization of the Japanese diet [10, 11]. Collectively, these observations suggest that the difference in breast cancer incidence rates between Western and Eastern women are largely influenced by changes in lifestyle and diet rather than genetics [12].
Though the association between soy and incident breast cancer has been studied in Asian and Western populations, the results are inconclusive. Some epidemiological studies have demonstrated an inverse relationship between soy consumption and breast cancer risk, while other studies have shown an increased risk or no effect [13]. The chemopreventive effects of soy appear to vary by menopausal status; therefore, the risk benefits afforded by soy consumption may depend heavily on a woman’s endogenous environment [13].
Isoflavones are structurally similar to 17β-estradiol, the primary endogenous estrogen, and therefore can compete for binding to estrogen receptors (ER) in the breast and stimulate cell proliferation. The isoflavones, however, exhibit only weak estrogenic activity: daidzein has 10−4 the activity per mole as 17β-estradiol [14, 15]. Depending on the estrogen environment, isoflavones could act as ER-antagonists by attenuating the estrogenic response of 17β-estradiol or as ER-agonists when there is a greater chance of binding to ERs.
However, isoflavones also act independently of the ER: they exhibit anti-proliferative, anti-oxidant, and anti-inflammatory properties in vivo and in vitro [16, 17]. Furthermore, there is large inter-individual variability in isoflavone metabolism, which primarily depends on intestinal flora [16] and genetic polymorphisms (e.g. the cytochrome p450 genes, NQO1) [18]. Thus, the mechanism and role of soy in breast carcinogenesis remain unresolved.
Little is known about the effects of soy consumption begun post-diagnosis among breast cancer survivors. Menopause is often prematurely induced during the course of breast cancer treatment with tamoxifen, an anti-estrogen widely prescribed to women with ER positive (ER+) tumors as a long-term adjuvant therapy to prevent recurrences [19]. Postmenopausal women may take soy-based supplements as an alternative to hormone replacement therapy because they are a natural source of exogenous estrogen [20, 21]. Although there is no clear understanding of how soy affects tumor growth when combined with tamoxifen, concerns exist that high soy intake may increase the risk of recurrence or stimulate the growth of existing ER+ tumors [22]. Experimental studies have demonstrated that genistein may reduce the efficacy of tamoxifen by competing for binding to estrogen receptors [23]. Therefore, physicians in the United States caution women who have received tamoxifen therapy against consuming soy foods and supplements [19].
Although further research is warranted before recommendations regarding soy intake can safely be made to breast cancer survivors, soy protein isolates and isoflavone capsules containing high doses of purified soy isoflavones are being directly marketed and recommended to breast cancer survivors to prevent recurrence [13]. This practice is of significant public health interest since after a breast cancer diagnosis, it is common for women to change their diet and lifestyle and use alternative therapies [24–26]. Therefore, we investigated whether soy intake is related to risk of recurrence in a cohort of breast cancer survivors in the Life After Cancer Epidemiology (LACE) Study using a supplemental questionnaire specifically designed to address this research question.
Materials and Methods
LACE cohort recruitment
Details of the LACE study have been previously described elsewhere [27]. Briefly, women diagnosed with breast cancer from 1997 to 2000 were recruited from the Kaiser Permanente Northern California (KPNC) Cancer Registry (82%), the Utah Cancer Registry (12%), and a subset of women who declined participation in the Women’s Healthy Eating and Living (WHEL) Trial, a dietary intervention trial to reduce risk for breast cancer recurrence among survivors (6%) [28]. The study was approved by the Institutional Review Board of the Kaiser Permanente Medical Program of Northern California and the University of Utah.
To be eligible for LACE, women had to 1) be 18–79 years of age at diagnosis, 2) receive a diagnosis of primary breast cancer (Stage I > 1 cm, II or IIIA) within 39 months of enrollment, 3) have no other cancers within five years of enrollment, 4) complete cancer treatment aside from adjuvant hormonal therapy, and 5) be free of any recurrence by self-report. Medical chart review was used to further verify that women were free of breast cancer recurrence.
Between January 2000 and April 2002, 5,656 women who initially met the LACE eligibility criteria were sent a recruitment package. Of these, 2,614 (46%) agreed to participate and completed the questionnaires. The LACE cohort was constituted by 2,280 women after 334 were confirmed ineligible after a medical record review. Reasons for exclusion were breast cancer recurrence, new primary breast cancer, or death between diagnosis and three months after study enrollment (37%), incorrect stage (34%), another cancer diagnosis within five years before enrollment (10%), prior breast cancer (6%), more than 39 months since diagnosis (6%), incomplete demographic and medical data (3%), currently receiving treatment (2%), and language difficulty (2%).
Differences between KPNC participants and non-participants were compared [27], and both groups were similar in terms of cancer severity (stage, number of positive nodes) and treatment (chemotherapy, type of surgery). The only significant differences were that women approached within 15 months of diagnosis were more likely to enroll than those approached later, and women younger than 50 years were less likely to enroll than older women. The analyses were restricted to 1954 women who completed a soy questionnaire and/or Block FFQ, as described below. Subjects entered the cohort on average 1.88 years after diagnosis (range: 0.92 to 3.24 years) and were subsequently followed on average 6.31 years (range: 0.11 to 8.65 years) post-enrollment.
Outcome assessment
The endpoint of interest was breast cancer recurrence including local, regional, and distant disease as well as new contralateral breast cancers. Recurrences were ascertained by a mailed semi-annual (until April 2005) or annual (after April 2005) health status update questionnaire that asked participants to report any events occurring in the preceding six or 12 months, respectively. All non-respondents to the health status questionnaire were called to complete the questionnaire by telephone. Participants receiving care outside of KPNC who reported any event were contacted to obtain permission to view their protected health information. All reported events were verified by medical record. For this analysis, a total of 282 breast cancer recurrences (144 distant, 36 local/regional, 32 opposite breast, 17 other/don’t know, 53 missing information) were confirmed as of October 21, 2008. The mean time from enrollment to recurrence was 3.31 years (range: 0.27–8.51 years).
General diet
Diet was assessed for the 12 months prior to entering the cohort using the Fred Hutchinson Cancer Research Center (FHCRC) Food Frequency Questionnaire (FFQ), a self-administered, semi-quantitative FFQ with over 100 food and beverage items adapted from the 95- item Health Habits and Lifestyle Questionnaire (HHLQ) developed by Block and colleagues at the National Cancer Institute [29]. For each food or beverage, participants marked a category representing frequency of consumption and indicated the corresponding serving size as small, medium, or large. Questionnaires were sent to the FHCRC for nutrient analyses. Questionnaires indicating extremes of total energy (less than 500 kcal or more than 4,000 kcal) were considered unreliable and were excluded from the analysis (n=56).
Soy intake from diet
Although the FHCRC FFQ included two food items (tofu and soy milk) that are isoflavone-rich sources based on their genistein and daidzein values, a separate soy FFQ was mailed to participants to better estimate their soy intake for the 12 month period prior to entering the cohort. This soy questionnaire included 14 items (soy yogurt, soy frozen yogurt, soy ice cream, soy cheese, soy hot dogs and cold cuts, other meat substitutes made from soy, tempeh, miso, soybeans, roasted soy nuts, soy sauce, soybean sprouts, alfalfa sprouts, and protein power supplements made from soy) selected from the 40-item soy questionnaire developed by Kirk and colleagues [30] based on their soy contribution to dietary intake of genistein, daidzein, or coumestrol [31, 32].
Soy intake from supplements
Information on soy supplement use since diagnosis was obtained by 1) asking respondents about the frequency and duration of use of specific herbal supplements using an extensive list of supplements and herbs compiled by the WHEL study [33] and 2) allowing participants to report their supplements not listed on the pre-determined list in an open-ended question. A yes/no use variable was created since the responses indicating the frequency and duration of soy supplement use were low with only 53 women reporting soy supplement use.
Covariates
Covariates were selected in three ways: 1) a priori knowledge based on a review of the literature, 2) a Pearson correlation matrix between the covariate (continuous variables) and isoflavone intakes, and 3) a forward stepwise regression approach. A variable was tested as a potential confounder if it was related to isoflavone intake, as identified through the Pearson correlation matrix (r2 ≥ .1, P < .05), and if the literature demonstrated it as a cause of breast cancer. Covariates identified from the first two approaches were added to the model in a forward stepwise manner and included in the final model if they improved goodness-of-fit (P < .05 for likelihood ratio tests comparing the full model to the more parsimonious model) and/or changed the hazard ratios or the coefficients of the main effect variables by more than 10% [34]. The final covariates in the fully adjusted models included soy supplement use (yes/no), BMI one year before diagnosis (kg/m2), menopausal status (pre-, post-, unknown) at diagnosis, tobacco pack-years, tumor stage (I, IIA, IIB, IIIA), ER and/or progesterone receptor (PR) status (ER− and PR−, ER− and PR+, ER+ and PR-, ER+ and PR+), age at diagnosis (years), race (Black, non-Hispanic White, Hispanic White, Asian, other), and total energy intake (kilocalories). The stratified models were adjusted for all these terms excluding the term used for stratification.
Statistical analysis
We modeled daidzein, genistein, and glycetin separately because their intakes were highly collinear. Daidzein, genistein, and glycetin were categorized into quintiles to capture the highest levels of intake. The 95th percentile was represented as a separate category, assuming that any beneficial effect of soy would be seen only at a level similar to that consumed in Asian populations [35–37]. The ‘no’, ‘low’, ‘medium’, and ‘high’ intake categories presented in Table 1 correspond to 0, 0.1–149.5, 149.6–9596.54, and ≥ 9596.55 µg of daidzein per day, respectively, based on quintiles of daidzein consumption.
TABLE 1.
Characteristics of the LACE cohort who completed a soy Food Frequency Questionnaire (n = 1954), stratified by levels of soy intakea
| No soy intakeb (n = 458) |
Low soy intakea (n = 723) |
Medium soy intake a (n = 677) |
High soy Intakea (n = 96) |
P valuec | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Age (years) | |||||||||
| < 50 | 75 | 17.1 | 137 | 31.3 | 196 | 44.7 | 30 | 6.8 | <0.0001 |
| 50–60 | 123 | 19.9 | 222 | 36.0 | 238 | 38.6 | 34 | 5.5 | |
| 60–70 | 149 | 26.8 | 223 | 40.2 | 158 | 28.5 | 25 | 4.5 | |
| > 70 | 111 | 32.3 | 141 | 41.0 | 85 | 24.7 | 7 | 2.0 | |
| Race | |||||||||
| Black | 32 | 38.6 | 27 | 32.5 | 20 | 24.1 | 4 | 4.8 | <0.0001 |
| NH-White | 373 | 23.4 | 635 | 39.9 | 515 | 32.4 | 68 | 4.3 | |
| Hisp-White | 33 | 30.8 | 33 | 30.8 | 34 | 31.8 | 7 | 6.5 | |
| Asian | 3 | 4.1 | 3 | 4.1 | 55 | 75.3 | 12 | 16.4 | |
| Other | 17 | 17.3 | 24 | 24.5 | 53 | 54.1 | 4 | 4.1 | |
| Years education completed | |||||||||
| ≤ High school | 180 | 34.3 | 211 | 40.2 | 117 | 22.3 | 17 | 3.2 | <0.0001 |
| Some college | 179 | 24.7 | 277 | 38.3 | 238 | 32.9 | 30 | 4.1 | |
| ≥ College grad | 99 | 14.1 | 233 | 33.2 | 321 | 45.8 | 48 | 6.8 | |
| ER/PR status | |||||||||
| ER−/PR− | 72 | 23.9 | 110 | 36.5 | 99 | 32.9 | 20 | 6.6 | 0.51 |
| ER−/PR+ | 7 | 19.4 | 15 | 41.7 | 12 | 33.3 | 2 | 5.6 | |
| ER+/PR− | 78 | 27.9 | 93 | 33.2 | 99 | 35.4 | 10 | 3.6 | |
| ER+/PR+ | 296 | 22.5 | 500 | 38.1 | 457 | 34.8 | 61 | 4.6 | |
| Tamoxifen use at baseline | |||||||||
| Never | 103 | 23.7 | 154 | 35.5 | 146 | 33.6 | 31 | 7.1 | 0.005 |
| Past | 43 | 31.9 | 44 | 32.6 | 37 | 27.4 | 11 | 8.1 | |
| Current | 311 | 22.5 | 525 | 38.0 | 492 | 35.6 | 54 | 3.9 | |
| Tumor Stage | |||||||||
| I | 221 | 23.9 | 351 | 37.9 | 312 | 33.7 | 41 | 4.4 | 0.87 |
| IIA | 142 | 22.0 | 240 | 37.2 | 231 | 35.8 | 33 | 5.1 | |
| IIB | 79 | 24.6 | 113 | 35.2 | 110 | 34.3 | 19 | 5.9 | |
| IIIA | 16 | 27.6 | 17 | 29.3 | 22 | 37.9 | 3 | 5.2 | |
| Menopausal status at diagnosis | |||||||||
| Postmenopausal | 340 | 26.8 | 504 | 39.7 | 371 | 29.3 | 53 | 4.2 | <0.0001 |
| Premenopausal | 70 | 16.8 | 125 | 30.0 | 192 | 46.2 | 29 | 7.0 | |
| Unclear | 48 | 17.9 | 93 | 34.7 | 114 | 42.5 | 13 | 4.9 | |
| Smoking | |||||||||
| Never | 254 | 24.3 | 369 | 35.3 | 366 | 35.0 | 56 | 5.4 | 0.0003 |
| Past | 156 | 20.3 | 293 | 38.2 | 281 | 36.6 | 38 | 4.9 | |
| Current | 47 | 34.1 | 60 | 43.5 | 30 | 21.7 | 1 | 0.7 | |
| Alcohol use (servings/week) | |||||||||
| Mean ± SD | 2.1 ± 5.1 | 2.9 ± 7.1 | 2.8 ± 5.9 | 2.5 ± 5.1 | 0.15 | ||||
| Range | 0–45.0 | 0.0–66.1 | 0.0–63.2 | 0.0–30.0 | |||||
| Daidzein intake (mcg/day) | |||||||||
| Mean ± SD | 0.0 ± 0.0 | 22.7 ± 30.4 | 2096.8 ± 2256.4 | 19170.4 ± 10920.5 | <0.0001 | ||||
| Range | 0.0–0.0 | 0.3–149.6 | 152.0–9531.8 | 9596.6–73252.8 | |||||
| Genistein intake (mcg/day) | |||||||||
| Mean ± SD | 0.0 ± 0.0 | 43.5 ± 81.7 | 3027.3 ± 3179.9 | 27364.5 ± 15764.0 | <0.0001 | ||||
| Range | 0.0–0.0 | 0.5–630.0 | 215.2–13908.2 | 12278.9–104308.8 | |||||
| Glycetin intake (mcg/day) | |||||||||
| Mean ± SD | 9.0 ± 9.0 | 10.1 ± 10.1 | 209.4 ± 387.7 | 1022.9 ± 1301.8 | <0.0001 | ||||
| Range | 0.0–74.2 | 0.0–103.4 | 0.0–5835.4 | 0.0–7447.8 | |||||
Soy intake categories based on daidzein distribution: 'no' = 0, 'low' = 0.1–149.5, 'medium' = 149.6–9596.54, 'high' = ≥9596.55 mcg/day
No soy intake includes women who consumed soy sauce only and no other soy foods (n = 273)
Two-sided p-values were obtained from chi-square tests for categorical variables and F-tests used for continuous variables
Descriptive statistics include frequency distributions for ordinal and nominal variables, and as means, standard deviations (SD) and ranges for continuous variables, stratified by level of soy consumption (‘no’, ‘low’, ‘medium’, and ‘high’ intake). Crude associations between covariates and levels of soy consumption were assessed via chi-square tests for categorical variables and one-way analysis of variance for continuous variables.
Multivariable delayed-entry Cox proportional hazards models, with years since diagnosis as the time scale, were used to calculate hazard ratios (HR) and 95% confidence intervals (CI), accounting for the varying time of enrollment into the cohort relative to the year of diagnosis. Effect modification by hormonal factors was assessed by stratifying on menopausal status (premenopausal, postmenopausal), hormone receptor status (ER− and PR− versus ER+ or PR+), ever/never tamoxifen use, or tamoxifen use in postmenopausal women (in order to examine effects by tamoxifen use in a low endogenous estrogen environment) versus all others. If stratified analyses suggested heterogeneity in effect across levels of soy intake, we tested for interaction by including appropriate interaction terms in the model and used a likelihood ratio test to compare the additive and non-additive models (including interaction terms). Tests for trend in HRs across categories of soy intake were performed by treating the variables as continuous, using the midpoint of each category as the covariate value. P values less than .05 were considered significant for main effects (no interaction terms in model) while less than .20 was considered significant for non-additive models. Using a larger level of significance increases the probability of detecting interactions that may be present [34]. Estimates of the cumulative distribution of time to recurrence stratified by level of soy intake, adjusted for covariates (at the mean for all covariates), were obtained via the Cox proportional hazards regression models and presented as a survival curve. All analyses were performed using SAS version 9.0 (SAS Institute, Inc., Cary, North Carolina).
Results
Table 1 describes the characteristics of 1954 female breast cancer survivors enrolled in the LACE Study who completed a soy FFQ, stratified by levels of daidzein intake. Levels of soy intake differed significantly by age at diagnosis (P < .0001), ethnicity (P < .0001), education (P < .0001), tamoxifen use at baseline (P = .005), menopausal status at diagnosis (P < .0001) and smoking status (P = .0003). There appeared to be a clear inverse relationship between soy consumption and age within each category of soy intake. Women in the oldest age category consumed the least amount of soy: 32% of women aged 70 years and older had no soy intake compared to 17% of women less than 50 years old. There were more Asian women in the ‘medium’ and ‘high’ soy intake category than women of any other ethnicity: 75% and 16% of Asians had ‘medium’ and ‘high’ soy intake compared to 32% and 4% in non-Hispanic White women. About 7% of the college graduates had high soy intake compared to only 3% women with less than or equal to a high school education. Premenopausal women were more likely to have medium or high soy intakes (53%) compared to postmenopausal women (33 %). About 10% of the non-smokers (never and past smokers) had ‘high’ soy intake compared to less than 1% of current smokers. The mean (SD) of daidzein and genistein intakes in the LACE cohort were 1.7 (4.9) and 2.4 (7.0) milligrams per day, respectively, and were highly collinear (r = 0.997, data not shown). The most frequently consumed soy foods in the LACE cohort were soy sauce, breakfast and diet shakes and drinks, tofu, diet bars, and soy protein isolate powder (Table 2).
TABLE 2.
Soy intake among members of the LACE cohort who reported consumptiona (n = 1255)
| Isoflavone Distribution | n | Mean | SD | Range |
|---|---|---|---|---|
| Daidzein (mcg/day) | 1255 | 2608.2 | 5756.5 | 0–73,252.8 |
| Genistein (mcg/day) | 1255 | 3749.9 | 8513.7 | 0 – 104,308.9 |
| Glycetin (mcg/day) | 1231 | 196.6 | 526.4 | 0 – 7,447.8 |
| Soy Foods (servings/month)b | ||||
| Soy Sauce | 1253 | 2.6 | 5.3 | 0 – 91.3 |
| Breakfast and diet shakes and drinks | 1253 | 2.0 | 7.3 | 0 – 91.3 |
| Tofu | 1231 | 1.9 | 5.5 | 0 – 60.8 |
| Diet bars | 1255 | 1.6 | 4.6 | 0 – 30.4 |
| Soy protein isolate powder | 1252 | 1.4 | 6.7 | 0 – 91.3 |
| Soy Milk | 1172 | 1.0 | 5.2 | 0 – 91.3 |
| Alfalfa Sprouts | 1253 | 0.7 | 2.4 | 0 – 45.6 |
| Liquid nutrition (e.g. Ensure) | 1251 | 0.7 | 4.4 | 0 – 60.8 |
| Cooked soybeans or edamame | 1255 | 0.5 | 2.3 | 0 – 33.0 |
| Miso soup | 1250 | 0.5 | 2.3 | 0 – 60.8 |
| Milk/casein protein powder | 1253 | 0.5 | 4.3 | 0 – 60.8 |
| Soy Cheese | 1252 | 0.5 | 3.2 | 0 – 60.8 |
| Soy Yogurt | 1252 | 0.4 | 2.2 | 0 – 30.4 |
| Soy Meat Substitute | 1252 | 0.4 | 1.9 | 0 – 22.5 |
| Roasted Soy Nuts | 1253 | 0.3 | 1.8 | 0 – 33.0 |
| Soybean sprouts | 1245 | 0.3 | 1.3 | 0 – 30.4 |
| Soy Frozen Yogurt | 1253 | 0.2 | 1.4 | 0 – 30.4 |
| Soy franks/cold cuts | 1250 | 0.2 | 1.0 | 0 – 22.0 |
| Tempeh | 1250 | 0.1 | 0.8 | 0 – 15.0 |
| Soy Ice Cream | 1254 | 0.1 | 1.0 | 0 – 15.0 |
Women who consumed only soy sauce but no other soy foods were excluded (n = 273)
servings/month = frequency × portion
Table 3 describes the effect of soy intake on breast cancer recurrence among all women and also stratified by menopausal status. Overall, there was a non-significant decreasing risk of breast cancer recurrence with increasing intakes of both daidzein (P = .20 for trend) and glycetin (P = .10 for trend). The relationship was most apparent among postmenopausal women where tests for linear trend were borderline significant for daidzein (P = .08) and glycetin (P = .06).
Table 3.
Multivariable delayed entry cox-proportional hazards models describing soy isoflavone intake and breast cancer recurrence, stratified by menopausal status
| All womena | Premenopausalb | Postmenopausalb | P valuec | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 1838, 266 events) | (n= 387, 54 events) | (n = 1203, 171 events) | |||||||||||
| n | HR | 95% CI | n | HR | 95% CI | n | HR | 95% CI | |||||
| Daidzein Intake (mcg/day) | |||||||||||||
| 0 | 416 | 1.00 | Reference | 65 | 1.00 | Reference | 310 | 1.00 | Reference | ||||
| 0.10–7.77 | 311 | 1.16 | 0.81 | 1.68 | 49 | 0.94 | 0.33 | 2.66 | 228 | 1.15 | 0.75 | 1.76 | |
| 7.78–149.59 | 374 | 0.87 | 0.60 | 1.26 | 69 | 1.26 | 0.52 | 3.10 | 255 | 0.77 | 0.49 | 1.20 | |
| 149.60–1,453.00 | 363 | 0.97 | 0.68 | 1.41 | 96 | 0.78 | 0.31 | 1.93 | 202 | 1.08 | 0.70 | 1.69 | |
| 1,453.10–9,596.54 | 284 | 0.71 | 0.45 | 1.11 | 81 | 0.85 | 0.34 | 2.14 | 157 | 0.48 | 0.24 | 0.93 | ** |
| ≥ 9,596.55 | 90 | 0.96 | 0.52 | 1.76 | 27 | 1.74 | 0.63 | 4.76 | 51 | 0.70 | 0.27 | 1.77 | |
| p for trend | 0.20 | 0.79 | 0.08 | 0.29 | |||||||||
| Genistein Intake (mcg/day) | |||||||||||||
| 0 | 416 | 1.00 | Reference | 65 | 1.00 | Reference | 310 | 1.00 | Reference | ||||
| 0.10–6.99 | 338 | 1.09 | 0.76 | 1.58 | 56 | 0.93 | 0.34 | 2.55 | 245 | 1.02 | 0.66 | 1.58 | |
| 7.00–220.61 | 346 | 0.92 | 0.64 | 1.34 | 68 | 1.32 | 0.54 | 3.21 | 231 | 0.86 | 0.55 | 1.34 | |
| 220.62–2,184.8 | 364 | 0.97 | 0.67 | 1.40 | 89 | 0.75 | 0.30 | 1.90 | 209 | 1.03 | 0.66 | 1.61 | |
| 2,199.82–13,025.87 | 283 | 0.72 | 0.46 | 1.13 | 82 | 0.83 | 0.33 | 2.08 | 156 | 0.57 | 0.31 | 1.08 | * |
| ≥ 13,025.88 | 91 | 0.95 | 0.52 | 1.75 | 27 | 1.75 | 0.65 | 4.76 | 52 | 0.69 | 0.27 | 1.75 | |
| p for trend | 0.24 | 0.82 | 0.16 | 0.59 | |||||||||
| Glycetin Intake (mcg/day) | |||||||||||||
| 0–3.61 | 378 | 1.00 | Reference | 74 | 1.0 | Reference | 240 | 1.00 | Reference | ||||
| 3.62–8.16 | 357 | 1.01 | 0.71 | 1.43 | 54 | 1.35 | 0.56 | 3.30 | 265 | 1.08 | 0.70 | 1.66 | |
| 8.17–14.99 | 368 | 0.68 | 0.46 | 1.01* | 72 | 0.86 | 0.33 | 2.25 | 250 | 0.70 | 0.43 | 1.12 | |
| 15.00–78.53 | 365 | 0.75 | 0.51 | 1.12 | 75 | 0.98 | 0.39 | 2.47 | 244 | 0.68 | 0.41 | 1.14 | |
| 78.54–795.39 | 277 | 0.78 | 0.50 | 1.22 | 86 | 0.72 | 0.27 | 1.93 | 150 | 0.84 | 0.47 | 1.52 | |
| ≥ 795.40 | 92 | 0.80 | 0.42 | 1.50 | 26 | 1.60 | 0.54 | 4.72 | 53 | 0.51 | 0.18 | 1.38 | |
| p for trend | 0.10 | 0.89 | 0.06 | 0.85 | |||||||||
Unstratified models were adjusted for soy supplement use, BMI 1 year before diagnosis, menopausal status, tobacco pack-years, tumor stage, ER status, age, race and kilocalories.
Stratified models were adjusted for soy supplement use, BMI 1 year before diagnosis, tobacco pack-years, tumor stage, ER status, age, race, and kilocalories.
P-value for interaction.
p ≤ 0.10
p ≤ 0.05
Table 4 presents the effect of isoflavone intake on breast cancer recurrence, stratified by hormone receptor status and by tamoxifen use. The risk of recurrence according to intake of glycetin (P = .04 for interaction) varied significantly by hormone receptor type (ER− and PR− vs. ER+ or PR+), yet no evidence of interaction was apparent for intakes of daidzein and genistein (P > 0.20). Similarly, when stratified by tamoxifen use, a significant interaction between tamoxifen use and the effect of glycetin on breast cancer recurrence was observed (P = .03 for interaction). Women who used tamoxifen had a significant decreased risk of breast cancer recurrence with increasing intake of glycetin (P = .05 for trend) and a suggestive, but non-significant relationship for intakes of daidzein (P = .10 for trend) and genistein (P = .13 for trend). Women in the highest categories of daidzein and genistein intakes had an approximately 50% reduction in breast cancer recurrence that was not significant (HR for the 95th percentile of daidzein intake, 0.48; 95% CI, 0.19–1.21; HR for the 95th percentile of genistein intake, 0.48; 95% CI, 0.19–1.22). No apparent potential benefit for women who had never used tamoxifen was observed. In contrast, while there was no evidence of a trend across levels of soy intake, women who had never used tamoxifen and were in the 95th percentile of isoflavone intake had a borderline significant increased risk of breast cancer recurrence (HR for the 95th percentile of daidzein intake, 2.40; 95% CI, 0.93–6.18; HR for the 95th percentile of genistein intake, 2.42; 95% CI, 0.95–6.21).
Table 4.
Multivariable delayed entry cox-proportional hazards models describing soy isoflavone intake and breast cancer recurrence, stratified by hormone receptor status and tamoxifen usea
| ER− and PR − | ER+ or PR+ | P valueb | Never Tamoxifen | Ever Tamoxifen | P valueb | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n=287, 43 events) | (n=1551, 223 events) | (n=410, 61 events) | (n=1443, 209 events) | |||||||||||||||
| n | HR | 95% CI | n | HR | 95% CI | n | HR | 95% CI | n | HR | 95% CI | |||||||
| Daidzein Intake (mcg/day) | ||||||||||||||||||
| 0 | 67 | 1.00 | Reference | 349 | 1.00 | Reference | 92 | 1.00 | Reference | 327 | 1.00 | Reference | ||||||
| 0.10–7.77 | 54 | 1.69 | 0.73 | 3.91 | 257 | 1.08 | 0.72 | 1.63 | 68 | 1.63 | 0.72 | 3.68 | 244 | 1.12 | 0.75 | 1.69 | ||
| 7.78–149.59 | 54 | 0.96 | 0.36 | 2.53 | 320 | 0.86 | 0.57 | 1.28 | 80 | 1.31 | 0.58 | 2.99 | 296 | 0.78 | 0.52 | 1.19 | ||
| 149.60–1453.00 | 55 | 0.73 | 0.26 | 2.03 | 308 | 1.01 | 0.68 | 1.50 | 79 | 0.93 | 0.39 | 2.22 | 290 | 1.00 | 0.67 | 1.50 | ||
| 1453.10–9596.54 | 37 | 0.36 | 0.08 | 1.69 | 247 | 0.76 | 0.48 | 1.23 | 60 | 0.69 | 0.23 | 2.08 | 224 | 0.73 | 0.45 | 1.20 | ||
| ≥ 9596.55 | 20 | 1.45 | 0.43 | 4.95 | 70 | 0.82 | 0.40 | 1.68 | 31 | 2.40 | 0.93 | 6.18* | 62 | 0.48 | 0.19 | 1.21 | ||
| p for trend | 0.38 | 0.29 | 0.56 | 0.69 | 0.10 | 0.24 | ||||||||||||
| Genistein Intake (mcg/day) | ||||||||||||||||||
| 0 | 67 | 1.00 | Reference | 349 | 1.00 | Reference | 92 | 1.00 | Reference | 327 | 1.00 | Reference | ||||||
| 0.10–6.99 | 60 | 1.72 | 0.75 | 3.94 | 278 | 1.00 | 0.66 | 1.51 | 73 | 1.70 | 0.76 | 3.79 | 267 | 1.02 | 0.68 | 1.54 | ||
| 7.00–220.61 | 45 | 0.81 | 0.27 | 2.38 | 301 | 0.93 | 0.62 | 1.39 | 69 | 1.25 | 0.53 | 2.95 | 277 | 0.86 | 0.57 | 1.31 | ||
| 220.62–2,184.8 | 58 | 0.83 | 0.31 | 2.21 | 306 | 0.99 | 0.66 | 1.47 | 86 | 0.96 | 0.41 | 2.22 | 285 | 0.99 | 0.66 | 1.48 | ||
| 2,199.82–13, 025.87 | 36 | 0.38 | 0.08 | 1.79 | 247 | 0.77 | 0.48 | 1.24 | 59 | 0.70 | 0.23 | 2.10 | 224 | 0.74 | 0.45 | 1.21 | ||
| ≥ 13,025.88 | 21 | 1.34 | 0.39 | 4.57 | 70 | 0.83 | 0.40 | 1.69 | 31 | 2.42 | 0.95 | 6.21 | 63 | 0.48 | 0.19 | 1.22 | ||
| p for trend | 0.38 | 0.35 | 0.52 | 0.72 | 0.13 | 0.25 | ||||||||||||
| Glycetin Intake (mcg/day) | ||||||||||||||||||
| 0–3.61 | 63 | 1.00 | Reference | 315 | 1.00 | Reference | 83 | 1.00 | Reference | 299 | 1.00 | Reference | ||||||
| 3.62–8.16 | 57 | 0.31 | 0.11 | 0.81 | 300 | 1.22 | 0.83 | 1.81 | 79 | 0.32 | 0.13 | 0.78** | 279 | 1.26 | 0.85 | 1.87 | ||
| 8.17–14.99 | 57 | 0.23 | 0.07 | 0.73 | 311 | 0.81 | 0.53 | 1.25 | 77 | 0.26 | 0.10 | 0.73** | 295 | 0.82 | 0.53 | 1.26 | ||
| 15.00–78.53 | 57 | 0.89 | 0.38 | 2.09 | 308 | 0.73 | 0.46 | 1.15 | 84 | 0.83 | 0.40 | 1.74 | 286 | 0.69 | 0.43 | 1.10 | ||
| 78.54–795.39 | 34 | 0.32 | 0.09 | 1.19* | 243 | 0.87 | 0.54 | 1.41 | 58 | 0.87 | 0.37 | 2.01 | 219 | 0.77 | 0.46 | 1.28 | ||
| ≥ 795.40 | 19 | 0.38 | 0.08 | 1.79 | 73 | 0.94 | 0.47 | 1.89 | 29 | 0.68 | 0.22 | 2.12 | 64 | 0.85 | 0.40 | 1.80 | ||
| p for trend | 0.29 | 0.17 | 0.04 | 0.82 | 0.05 | 0.03 | ||||||||||||
All models were adjusted for soy supplement use, BMI 1 year before diagnosis, tobacco pack-years, tumor stage, menopausal status, age, race, and kilocalories.
P-value for interaction.
p ≤ 0.10
p ≤ 0.05
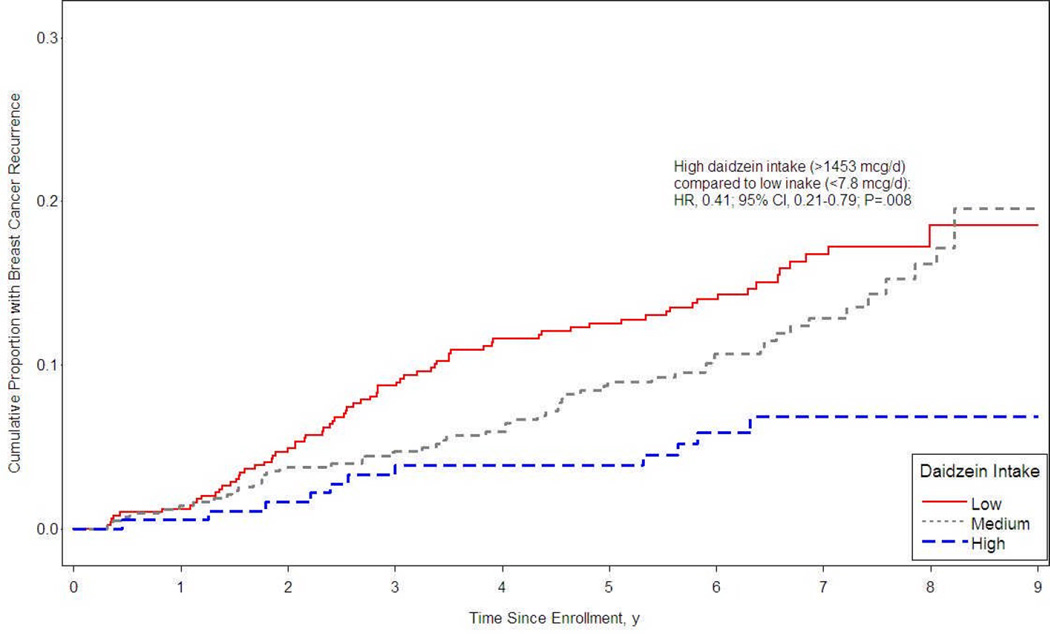
Since increasing daidzein intake was associated with a marginally decreased risk of breast cancer recurrence in both postmenopausal women and in women who had ever used tamoxifen, we examined postmenopausal women who were ever treated with tamoxifen (n = 970) and estimated the distribution of time to breast cancer recurrence, by levels of daidzein intake (‘low’, ‘medium’, and ‘high’), adjusted for soy supplement use, BMI one year prior to diagnosis, tobacco pack-years, tumor stage, age at diagnosis, race, and total energy intake (Figure 1). The ‘low’, ‘medium’, and ‘high’ categories correspond to 0–7.6, 7.7–1452.9, and ≥1453 µg/day daidzein intake. After a mean follow-up time of 6.3 years from entry into the cohort, the proportion of women who recurred was inversely related to daidzein intake and was significantly lower in the highest category of daidzein intake compared to those in the lowest category (HR, 0.41; 95% CI, 0.21–0.79, P = .008 for highest versus lowest intake; P = .006 for trend).
Figure 1.
Breast cancer recurrence among postmenopausal women using Tamoxifen, according to daidzein intake
Discussion
The LACE Study is one of the first prospective studies among breast cancer survivors to examine the relationship between soy intake and breast cancer prognosis. Another prospective study of breast cancer survivors in Shanghai, China demonstrated no effect of soy on breast cancer survival by menopausal or hormone receptor status [38] but concluded that their study was limited by the inability to examine the interaction between soy and tamoxifen, since in vitro studies have previously demonstrated an interaction. For the first time, we report that among women treated with tamoxifen, consumption of soy products after a breast cancer diagnosis may be associated with a reduced risk of recurrence. .
We also examined soy effects stratified by hormone receptor status (ER− and PR− versus ER+ or PR+ tumors), separating luminal from non-luminal tumors [39], since soy may bind to the ER [14, 15]. This approach is supported by a recent study demonstrating that soy affects breast cancer risk differently by hormone receptor status [40]. We found a decreasing risk of recurrence with increasing isoflavone intake among tumors that were ER+ or PR+, but not ER− and PR−. The magnitude of benefit for breast cancer recurrence associated with soy consumption was similar for the strata of hormone receptor positive tumors and for the strata of tamoxifen users but slightly greater and more significant when stratified by tamoxifen use. Since all women who receive tamoxifen therapy are hormone receptor positive, and most (90%) women who had ER+ or PR+ tumors received tamoxifen, it is difficult to disentangle whether it is the hormone receptor positivity, the tamoxifen therapy, or the combination of the two that is driving the observed effects. It is also possible that the effects seen with the highest intakes of these soy components are due to other confounding factors (i.e. a healthy lifestyle) associated with high soy consumption that were not accounted for in our analyses
There is experimental and epidemiological evidence to support the possibility that soy components may have no negative impact on tamoxifen use or may in fact act synergistically with tamoxifen to provide a stronger antitumor effect than tamoxifen alone. A cross-sectional study among Asian-American women with breast cancer found no evidence that soy intake adversely affected levels of circulating tamoxifen [41], implying that isoflavones presumably do not interfere with tamoxifen binding to estrogen receptors. Furthermore, soy protein isolates or soy-based miso in combination with tamoxifen were found to be more effective than tamoxifen alone in preventing chemically-induced rat mammary cancers [42, 43]. Isoflavones may also prevent the formation of carcinogenic metabolites of tamoxifen via inhibition of the cytochrome P450 enzymes, thereby making this synergistic interaction a more effective therapy for breast cancer survivors [44]. It is important to note that our results cannot be generalized to women diagnosed with advanced stage of breast cancer and apply only to women who have survived on average two years since diagnosis
Our demonstration that the protective effects of soy were limited to postmenopausal women and strongest for postmenopausal tamoxifen users also suggests that a woman’s hormonal milieu is an important factor to consider in the relationship between soy and breast cancer recurrence. These results are consistent with previous studies in Asian populations that have shown a stronger effect of isoflavones in reducing breast cancer incidence in postmenopausal rather than in premenopausal women [45, 46]. It is also conceivable that the observed interactive effects between soy and tamoxifen may be most apparent in postmenopausal women who typically have low circulating estradiol levels, since blood levels of tamoxifen have been shown to be higher in postmenopausal women age 65 or older compared with premenopausal women age 45 or younger [41].
Of note is that we observed a possible increased risk of recurrence associated with the highest levels of daidzein and genistein intake among non-users of tamoxifen. However, this relationship was somewhat attenuated when the analyses was limited to non-users of tamoxifen who were ER– and PR− (HR for daidzein, 1.77; 95% CI, 0.51–6.06; HR for genistein, 1.64; 95% CI, 0.48–5.64). Perhaps for those women whose tumors have some hormone receptor positivity (a tumor is classified receptor positive if >5% of the cells express the receptor) and who do not use tamoxifen, their tumors are not benefiting from the synergistic effect of soy and tamoxifen binding to the tumor. It may be possible that soy could then contribute more to the total estrogen pool, thereby putting women at an increased risk for recurrence. Finally, we must acknowledge that our results must be considered in the context of multiple tests. As recommended by Rothman [47, 48] and Savitz [49], we chose not to adjust for multiple comparisons, but we acknowledge an inflation of the overall Type 1 error rate for the family of tests conducted in this study.
A limitation of this study is that the cohort is largely comprised of Caucasian females with low consumption of soy foods. The isoflavone intakes reported in our population are similar to those reported in other Western populations [50, 51] but lower than the average intake reported in Asian populations, where daidzein and genistein intakes ranged from 9.5 to 18.3 mg per day and 14.9 to 31.4 mg per day, respectively [35–37]. Although the lowest levels of intake in the Asian studies were comparable to our highest levels of intake in our cohort (≥ 9.6 mg per day for daidzein and ≥13.0 mg per day for genistein) and we observed the strongest protective effects at that level, we also observed protective effects in women who were both postmenopausal and used tamoxifen at much lower intake levels (> 1.5 mg/day daidzein intake). In addition, we had incomplete reporting of specific supplement brand names such that we were unable to quantitatively account for the amount of soy isoflavones received from supplements in the analysis which may have led to some misclassification. However, since so few women reported using soy supplements (n=53), this was unlikely to substantially alter our results. Finally, although we considered the possibility of including other dietary factors (e.g. fat, protein) in our analysis, we ultimately decided not to adjust for these covariates in our models since we recently found no association between dietary patterns representing high fat/red meat-based protein (Western) and low fat/vegetable-based protein (prudent) and risk of recurrence in the cohort [52]. Therefore, our observed effects of soy on risk of recurrence in the present analysis are most likely independent of other macro- and/or micronutrients.
The LACE Study had several strengths. The large sample size and access to complete treatment data allowed us to stratify on different factors that could affect a woman’s estrogen levels. The prospective design reduces errors in dietary recall among study participants that could be problematic in case-control studies. Another major strength includes the use of a supplemental validated and comprehensive FFQ specifically targeting soy products.
Conclusion
In summary, soy intake at levels comparable to those consumed in Asian populations, may reduce the risk of recurrence in women who have been treated with tamoxifen and furthermore does not appear to negate effects of tamoxifen. The reduction in risk observed among tamoxifen users may be especially beneficial for postmenopausal women. Since this is the first study to examine the effect of soy consumption after a breast cancer diagnosis by tamoxifen status, the results demonstrating benefit in tamoxifen users should be replicated and the effect in non-tamoxifen users should be clarified before practical guidelines on soy consumption can be issued. Future studies should continue to examine interactive effects of soy with adjuvant therapies and incorporate biomarkers that account for individual metabolism as well as intake.
Acknowledgements
The LACE Study was funded by the National Cancer Institute (CA80027 and PC67000). Neela Guha was a 2007 DOR/CHR Fellow, and her research was supported, in part, by a grant from the Division of Research, Kaiser Permanente Northern California, to the Center for Health Research at the University of California, Berkeley. We thank all participants and study study staff.
Footnotes
The authors report no conflicts of interest.
References
- 1.Reinli K, Block G. Phytoestrogen content of foods--a compendium of literature values. Nutr Cancer. 1996;26(2):123–148. doi: 10.1080/01635589609514470. [DOI] [PubMed] [Google Scholar]
- 2.Murphy PA, Song T, Buseman G, Barua K, Beecher GR, Trainer D, Holden J. Isoflavones in retail and institutional soy foods. J Agric.Food Chem. 1999;47(7):2697–2704. doi: 10.1021/jf981144o. [DOI] [PubMed] [Google Scholar]
- 3.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, Boyle P, editors. Cancer Incidence in Five Continents No. 160. Lyon, France: IARC Scientific Publications; 2007. [Google Scholar]
- 4.Parkin DM. Cancers of the breast, endometrium and ovary: geographic correlations. Eur J Cancer Clin Oncol. 1989;25(12):1917–1925. doi: 10.1016/0277-5379(89)90373-8. [DOI] [PubMed] [Google Scholar]
- 5.Rose DP, Boyar AP, Wynder EL. International comparisons of mortality rates for cancer of the breast, ovary, prostate, and colon, and per capita food consumption. Cancer. 1986;58(11):2363–2371. doi: 10.1002/1097-0142(19861201)58:11<2363::aid-cncr2820581102>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Kaur JS. Migration patterns and breast carcinoma. Cancer. 2000;88(5 Suppl):1203–1206. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1203::aid-cncr4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Stanford JL, Herrinton LJ, Schwartz SM, Weiss NS. Breast cancer incidence in Asian migrants to the United States and their descendants. Epidemiology. 1995;6(2):181–183. doi: 10.1097/00001648-199503000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Trichopoulos D, Yen S, Brown J, Cole P, MacMahon B. The effect of westernization on urine estrogens, frequency of ovulation, and breast cancer risk. A study of ethnic Chinese women in the Orient and the USA. Cancer. 1984;53(1):187–192. doi: 10.1002/1097-0142(19840101)53:1<187::aid-cncr2820530133>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, Wu-Williams AH, Kolonel LN, Horn-Ross PL, Rosenthal JF, Hyer MB. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85(22):1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 10.Adlercreutz H. Epidemiology of phytoestrogens. Baillieres. Clin Endocrinol Metab. 1998;12(4):605–623. doi: 10.1016/s0950-351x(98)80007-4. [DOI] [PubMed] [Google Scholar]
- 11.Kato I, Tominaga S, Kuroishi T. Relationship between westernization of dietary habits and mortality from breast and ovarian cancers in Japan. Jpn J Cancer Res. 1987;78(4):349–357. [PubMed] [Google Scholar]
- 12.Parkin DM, Khlat M. Studies of cancer in migrants: rationale and methodology. Eur J Cancer. 1996;32A(5):761–771. doi: 10.1016/0959-8049(96)00062-7. [DOI] [PubMed] [Google Scholar]
- 13.Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98(7):459–471. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- 14.Santell RC, Chang YC, Nair MG, Helferich WG. Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic/pituitary axis in rats. J.Nutr. 1997;127(2):263–269. doi: 10.1093/jn/127.2.263. [DOI] [PubMed] [Google Scholar]
- 15.Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17(2):271–275. doi: 10.1093/carcin/17.2.271. [DOI] [PubMed] [Google Scholar]
- 16.Phytoestrogens In Functional Foods. Boca Raton, Florida: CRC Press; 2005. [Google Scholar]
- 17.Magee PJ, Rowland IR. Phyto-oestrogens, their mechanism of action: current evidence for a role in breast and prostate cancer. Br J Nutr. 2004;91(4):513–531. doi: 10.1079/BJN20031075. [DOI] [PubMed] [Google Scholar]
- 18.Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20(2):187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 19.Constantinou AI, White BE, Tonetti D, Yang Y, Liang W, Li W, van Breemen RB. The soy isoflavone daidzein improves the capacity of tamoxifen to prevent mammary tumours. Eur. J. Cancer. 2005;41(4):647–654. doi: 10.1016/j.ejca.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Harris PF, Remington PL, Trentham-Dietz A, Allen CI, Newcomb PA. Prevalence and treatment of menopausal symptoms among breast cancer survivors. J Pain Symptom Manage. 2002;23(6):501–509. doi: 10.1016/s0885-3924(02)00395-0. [DOI] [PubMed] [Google Scholar]
- 21.Morris KT, Johnson N, Homer L, Walts D. A comparison of complementary therapy use between breast cancer patients and patients with other primary tumor sites. Am J Surg. 2000;179(5):407–411. doi: 10.1016/s0002-9610(00)00358-5. [DOI] [PubMed] [Google Scholar]
- 22.Messina M, McCaskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006;20;98(18):1275–1284. doi: 10.1093/jnci/djj356. [DOI] [PubMed] [Google Scholar]
- 23.Jones JL, Daley BJ, Enderson BL, Zhou JR, Karlstad MD. Genistein inhibits tamoxifen effects on cell proliferation and cell cycle arrest in T47D breast cancer cells. Am. Surg. 2002;68(6):575–577. [PubMed] [Google Scholar]
- 24.Lee MM, Lin SS, Wrensch MR, Adler SR, Eisenberg D. Alternative therapies used by women with breast cancer in four ethnic populations. J Natl Cancer Inst. 2000;92(1):42–47. doi: 10.1093/jnci/92.1.42. [DOI] [PubMed] [Google Scholar]
- 25.Pinto BM, Maruyama NC, Clark MM, Cruess DG, Park E, Roberts M. Motivation to modify lifestyle risk behaviors in women treated for breast cancer. Mayo Clin Proc. 2002;77(2):122–129. doi: 10.4065/77.2.122. [DOI] [PubMed] [Google Scholar]
- 26.Salminen E, Heikkila S, Poussa T, Lagstrom H, Saario R, Salminen S. Female patients tend to alter their diet following the diagnosis of rheumatoid arthritis and breast cancer. Prev Med. 2002;34(5):529–535. doi: 10.1006/pmed.2002.1015. [DOI] [PubMed] [Google Scholar]
- 27.Caan B, Sternfeld B, Gunderson E, Coates A, Quesenberry C, Slattery ML. Life After Cancer Epidemiology (LACE) Study: a cohort of early stage breast cancer survivors (United States) Cancer Causes Control. 2005;16(5):545–556. doi: 10.1007/s10552-004-8340-3. [DOI] [PubMed] [Google Scholar]
- 28.Pierce JP, Faerber S, Wright FA, Rock CL, Newman V, Flatt SW, Kealey S, Jones VE, Caan BJ, Gold EB, Haan M, Hollenbach KA, Jones L, Marshall JR, Ritenbaugh C, Stefanick ML, Thomson C, Wasserman L, Natarajan L, Thomas RG, Gilpin EA. A randomized trial of the effect of a plant-based dietary pattern on additional breast cancer events and survival: the Women's Healthy Eating and Living (WHEL) Study. Control Clin Trials. 2002;23(6):728–756. doi: 10.1016/s0197-2456(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 29.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am. J. Epidemiol. 1986;124(3):453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 30.Kirk P, Patterson RE, Lampe J. Development of a soy food frequency questionnaire to estimate isoflavone consumption in US adults. J. Am. Diet. Assoc. 1999;99(5):558–563. doi: 10.1016/S0002-8223(99)00139-X. [DOI] [PubMed] [Google Scholar]
- 31.Frankenfeld CL, Patterson RE, Horner NK, Neuhouser ML, Skor HE, Kalhorn TF, Howald WN, Lampe JW. Validation of a soy food-frequency questionnaire and evaluation of correlates of plasma isoflavone concentrations in postmenopausal women. Am. J. Clin. Nutr. 2003;77(3):674–680. doi: 10.1093/ajcn/77.3.674. [DOI] [PubMed] [Google Scholar]
- 32.Pillow PC, Duphorne CM, Chang S, Contois JH, Strom SS, Spitz MR, Hursting SD. Development of a database for assessing dietary phytoestrogen intake. Nutr. Cancer. 1999;33(1):3–19. doi: 10.1080/01635589909514742. [DOI] [PubMed] [Google Scholar]
- 33.Newman V, Rock CL, Faerber S, Flatt SW, Wright FA, Pierce JP. Dietary supplement use by women at risk for breast cancer recurrence. The Women's Healthy Eating and Living Study Group. J. Am. Diet. Assoc. 1998;98(3):285–292. doi: 10.1016/s0002-8223(98)00068-6. [DOI] [PubMed] [Google Scholar]
- 34.Jewell NP. Statistics for Epidemiology. 1st edn. Boca Raton, Florida: Chapman & Hall/CRC Press; 2003. [Google Scholar]
- 35.Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000;130(9):2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 36.Wakai K, Egami I, Kato K, Kawamura T, Tamakoshi A, Lin Y, Nakayama T, Wada M, Ohno Y. Dietary intake and sources of isoflavones among Japanese. Nutr. Cancer. 1999;33(2):139–145. doi: 10.1207/S15327914NC330204. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto S, Sobue T, Sasaki S, Kobayashi M, Arai Y, Uehara M, Adlercreutz H, Watanabe S, Takahashi T, Iitoi Y, Iwase Y, Akabane M, Tsugane S. Validity and reproducibility of a self-administered food-frequency questionnaire to assess isoflavone intake in a japanese population in comparison with dietary records and blood and urine isoflavones. J. Nutr. 2001;131(10):2741–2747. doi: 10.1093/jn/131.10.2741. [DOI] [PubMed] [Google Scholar]
- 38.Boyapati SM, Shu XO, Ruan ZX, Dai Q, Cai Q, Gao YT, Zheng W. Soyfood intake and breast cancer survival: a followup of the Shanghai Breast Cancer Study. Breast Cancer Res Treat. 2005;92(1):11–17. doi: 10.1007/s10549-004-6019-9. [DOI] [PubMed] [Google Scholar]
- 39.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki T, Matsuo K, Tsunoda N, Hirose K, Hiraki A, Kawase T, Yamashita T, Iwata H, Tanaka H, Tajima K. Effect of soybean on breast cancer according to receptor status: a case-control study in Japan. Int J Cancer. 2008;123(7):1674–1680. doi: 10.1002/ijc.23644. [DOI] [PubMed] [Google Scholar]
- 41.Wu AH, Pike MC, Williams LD, Spicer D, Tseng CC, Churchwell MI, Doerge DR. Tamoxifen, soy, and lifestyle factors in Asian American women with breast cancer. J Clin Oncol. 2007;20;25(21):3024–3030. doi: 10.1200/JCO.2006.10.5023. [DOI] [PubMed] [Google Scholar]
- 42.Gotoh T, Yamada K, Ito A, Yin H, Kataoka T, Dohi K. Chemoprevention of N-nitroso-N-methylurea-induced rat mammary cancer by miso and tamoxifen, alone and in combination. Jpn. J. Cancer Res. 1998;89(5):487–495. doi: 10.1111/j.1349-7006.1998.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mai Z, Blackburn GL, Zhou JR. Soy phytochemicals synergistically enhance the preventive effect of tamoxifen on the growth of estrogen-dependent human breast carcinoma in mice. Carcinogenesis. 2007;28(6):1217–1223. doi: 10.1093/carcin/bgm004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J, Halls SC, Alfaro JF, Zhou Z, Hu M. Potential beneficial metabolic interactions between tamoxifen and isoflavones via cytochrome P450-mediated pathways in female rat liver microsomes. Pharm. Res. 2004;21(11):2095–2104. doi: 10.1023/b:pham.0000048202.92930.61. [DOI] [PubMed] [Google Scholar]
- 45.Wu AH, Koh WP, Wang R, Lee HP, Yu MC. Soy intake and breast cancer risk in Singapore Chinese Health Study. Br J Cancer. 2008;99(1):196–200. doi: 10.1038/sj.bjc.6604448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamamoto S, Sobue T, Kobayashi M, Sasaki S, Tsugane S. Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst. 2003;95(12):906–913. doi: 10.1093/jnci/95.12.906. [DOI] [PubMed] [Google Scholar]
- 47.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 48.Rothman KJ, Greenland S. Modern Epidemiology. 2nd edn. Lippincott-Raven Publishers; 1998. [Google Scholar]
- 49.Savitz D. Interpreting Epidemiologic Evidence: Strategies for Study Design and Analysis. New York: Oxford University Press; 2003. [Google Scholar]
- 50.Horn-Ross PL, John EM, Lee M, Stewart SL, Koo J, Sakoda LC, Shiau AC, Goldstein J, Davis P, Perez-Stable EJ. Phytoestrogen consumption and breast cancer risk in a multiethnic population: the Bay Area Breast Cancer Study. Am J Epidemiol. 2001;154(5):434–441. doi: 10.1093/aje/154.5.434. [DOI] [PubMed] [Google Scholar]
- 51.Huang MH, Harrison GG, Mohamed MM, Gornbein JA, Henning SM, Go VL, Greendale GA. Assessing the accuracy of a food frequency questionnaire for estimating usual intake of phytoestrogens. Nutr. Cancer. 2000;37(2):145–154. doi: 10.1207/S15327914NC372_5. [DOI] [PubMed] [Google Scholar]
- 52.Kwan ML, Weltzien E, Kushi LH, Castillo A, Slattery ML, Caan B. Dietary patterns and breast cancer recurrence and survival among women with early stage breast cancer. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.19.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]