Abstract
Vaccinia virus (VACV) enters cells by a low pH endosomal route or by direct fusion with the plasma membrane. We previously found differences in entry properties of several VACV strains: entry of WR was enhanced by low pH, reduced by bafilomycin A1 and relatively unaffected by heparin, whereas entry of IHD-J, Copenhagen and Elstree were oppositely affected. Since binding and entry modes may have been selected by specific conditions of in vitro propagation, we now examined the properties of three distinct, recently isolated cowpox viruses and a monkeypox virus as well as additional VACV and cowpox virus strains. The recent isolates were more similar to WR than to other VACV strains, underscoring the biological importance of endosomal entry by orthopoxviruses. Sequence comparisons, gene deletions and gene swapping experiments indicated that viral determinants, other than or in addition to the A26 and A25 “fusion-suppressor” proteins, impact entry properties.
Keywords: Vaccinia virus, Cowpox virus, Monkeypox virus, Endocytosis, Fusion, Bafilomycin A1
Highlights
► Recently isolated cowpox viruses enter cells via a low pH endocytic route. ► Monkeypox virus also enters cells through a low pH endocytic pathway. ► Some strains of vaccinia virus enter cells by a low pH route. ► Other vaccinia virus strains enter via a neutral pH, heparin-sensitive pathway. ► Deletion or swapping of A26 proteins did not alter route of vaccinia virus entry.
Introduction
Entry of enveloped viruses into cells involves virion attachment, merging of the viral and cellular membranes and release of the core or nucleocapsid into the cytoplasm (White et al., 2008). Our understanding of the mechanisms used by poxviruses to enter cells has come primarily from investigations with vaccinia virus (VACV), the prototype of the Orthopoxvirus (OPXV) genus of the Poxviridae (Moss, 2006, Moss, 2007, Schmidt et al., 2012). The basic infectious particle is the mature virion (MV), which consists of a nucleoprotein core surrounded by a lipoprotein membrane (Condit et al., 2006). MVs can enter cells by fusing with the plasma or endosomal membrane representing neutral and low pH pathways, respectively (Armstrong et al., 1973, Carter et al., 2005, Townsley et al., 2006). Endocytosis may occur by macropinocytosis or dynamin-mediated fluid phase uptake consistent with a role for actin dynamics and cell signaling (Huang et al., 2008, Mercer and Helenius, 2008, Mercer et al., 2010a, Moser et al., 2010, Moss, 2006, Sandgren et al., 2010, Villa et al., 2010). A second infectious form of VACV known as the extracellular enveloped virus (EV) departs the cell by exocytosis and contains an additional membrane that is ultimately ruptured prior to or during the next round of infection to allow fusion of the enclosed MV with the plasma membrane or endocytic vesicle (Ichihashi, 1996, Law et al., 2006, Sandgren et al., 2010, Schmidt et al., 2011).
Four VACV proteins (A26, A27, D8 and H3) are involved in attachment of MVs via interactions with cell surface glycosaminoglycans (GAGs) or laminin (Chiu et al., 2007, Chung et al., 1998, Hsiao et al., 1998, Lin et al., 2000). Twelve highly conserved, non-glycosylated transmembrane proteins participate in subsequent steps leading to entry of the core into the cytoplasm (Bisht et al., 2008, Brown et al., 2006, Izmailyan et al., 2006, Nichols et al., 2008, Ojeda et al., 2006a, Ojeda et al., 2006b, Satheshkumar and Moss, 2009, Senkevich and Moss, 2005, Senkevich et al., 2005, Senkevich et al., 2004, Townsley et al., 2005a, Townsley et al., 2005b, Wolfe et al., 2012). Nine of the 12 proteins (A16, A21, A28, G3, G9, H2, J5, L5, and O3) have been designated as integral components of the entry fusion complex (EFC), two (L1 and F9) as EFC-associated, and one (I2) has not been assessed in this regard. The phenotypes of the EFC and EFC-associated proteins are similar except for their role in the stability of the complex, which may depend on their location and subunit interactions. Both EFC and EFC-associated proteins are required for the initial fusion of viral and cellular membranes (Laliberte et al., 2011). Absence of the I2 protein results in a diminution of other EFC proteins in the MV raising the possibility that it has an additional role or an indirect effect on entry (Nichols et al., 2008).
Despite the high conservation of the membrane fusion apparatus among poxviruses, differences in the mode of entry exist between VACV strains (Bengali et al., 2009, Chang et al., 2010, Mercer et al., 2010a). Notable are the different degrees of low pH enhancement and inhibition by bafilomycin A1 and heparin suggesting strain-specific preferences in attachment to cells and the use of neutral and low pH pathways (Bengali et al., 2009). Chang et al. (2010) reported that differences in the mode of entry of VACV strains are related to the expression of the A25 and A26 proteins, which they refer to as “fusion suppressors”. Specifically, loss of function of either of these proteins resulted in bafilomycin-insensitivity and enhanced fusion of MVs with the plasma membrane of HeLa cells at neutral pH. A26 apparently suppresses fusion at neutral pH by binding to the A16 and G9 EFC proteins (Chang et al., 2012).
We suspected that VACV strains might have adapted to particular entry pathways during their extensive passage in cultured cells. Therefore, we were interested in examining other OPXVs particularly those that were recently isolated from nature including strains of cowpox virus (CPXV) and monkeypox virus (MPXV). We found that the entry characteristic of these recent isolates were more similar to the Western Reserve (WR) strain of VACV, which preferentially uses a low pH-dependent endocytic pathway, than to other VACV strains that do not. In addition, bafilomycin-insensitivity of some VACV strains could not be attributed to differences in their A26 protein, suggesting that other factors are involved.
Results
Construction of OPXV recombinants that express firefly luciferase (LUC)
In a previous study (Bengali et al., 2009), we compared the entry of the following strains of VACV: WR, IHD-J, Copenhagen, New York City Board of Health (Dryvax, Wyeth), and Elstree (Lister). The WR strain differed from the others with regard to a several-fold enhancement of entry by brief low pH treatment prior to or after virus attachment to cells. WR also differed from IHD-J, Copenhagen and Lister with respect to greater inhibition of entry by bafilomycin A1 and lower inhibition of attachment by heparin. For the present study, we selected an additional VACV strain, Modified VACV Ankara (MVA), because of its deployment as a safe smallpox vaccine and as a vector for a variety of infectious diseases and the Brighton strain of CPXV (CPXV-BR) because of its use for evaluation of poxvirus therapeutics and pathogenesis studies. We were particularly interested, however, in three CPXV strains with distinct genome sequence differences isolated in Germany from humans (GER_1990_2 and GER_1991_3) and a monkey (GER_2002_MKY) (Carroll et al., 2011, Meyer et al., 1999), and the MPXV strain ZAI-1979-005 isolated from a fatal human infection (Breman et al., 1980) because of their better provenance and fewer in vitro passages compared to VACV strains. VACV WR and VACV IHD-J were used for comparison as they represented prototypes of the two entry modes found in our previous VACV studies.
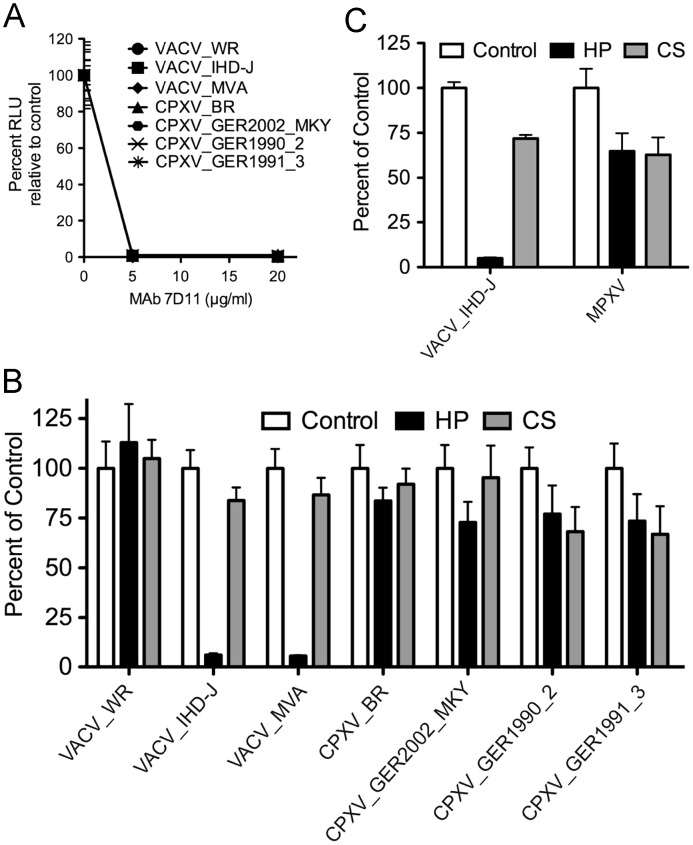
Poxviruses package a complete system for transcription of early genes, which occurs almost immediately following entry of cores into the cytoplasm. Based on this attribute, we previously constructed recombinant VACVs that express firefly LUC at early times and analyzed enzyme activity as a sensitive and quantitative measure of virus entry (Bengali et al., 2009, Townsley et al., 2006). We inserted the same LUC expression cassette into the homologous F12-F13 intergenic regions of other VACVs as well as the CPXV strains and MPXV in order to analyze their entry into cells. Neutralization of each of the OPXVs with the monoclonal antibody (MAb) 7D11 to the conserved VACV L1 entry protein prevented LUC expression, demonstrating the specificity of the assay ( Fig. 1A). L1 MAb inhibits at a post-hemifusion step prior to entry of the core into the cytoplasm (Laliberte et al., 2011).
Fig. 1.
Effects of neutralizing MAb, heparin and chondroitin sulfate on entry of recombinant LUC-expressing MVs. Purified MVs were incubated on ice with (A) 0, 5 or 20 μg/ml of MAb 7D11 for 30 min or (B and C) 0 or 50 μg/ml of heparin (HP) or chondroitin sulfate (CS) for 30 min. The treated MVs were adsorbed to BS-C-1 cells at 4 °C for 1 h in the presence of inhibitors and then incubated at 37 °C for 1 h. Cells were lysed and LUC activity determined. (C) MPXV experiments were conducted as described in the above panels except in a BSL-3 laboratory. Abbreviation: RLU, relative light units.
Effects of soluble GAGs on entry of OPXVs
Heparin was previously shown to exert a differential effect on the entry of VACV strains. At a concentration of 50 μg/ml, entry of VACV WR was inhibited by approximately 20% whereas VACV IHD-J was inhibited by 90% (Bengali et al., 2009). Further analysis indicated that heparin prevented the binding of IHD-J to cells. We found that MVA was as sensitive to heparin as the IHD-J strain; in comparison, the other OPXVs were much less sensitive to heparin than IHD-J and in this regard were similar to WR (Fig. 1B and C). Chondroitin sulfate had a much less inhibitory effect than heparin on MVA and IHD-J and did not show a strong strain-specific effect (Fig. 1B and C).
Effects of low pH and inhibition of endosomal acidification
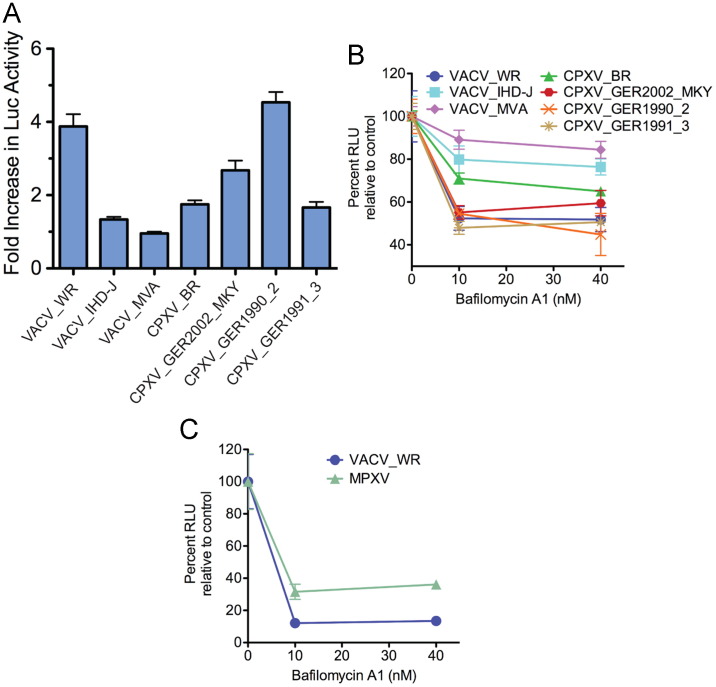
VACV WR is stimulated several fold by brief low pH treatment immediately following adsorption and inhibited by 50% or more by bafilomycin A1, whereas IHD-J is relatively unaffected by low pH or bafilomycin A1 (Bengali et al., 2009). MVA was also unaffected by the low pH pulse ( Fig. 2A), resembling IHD-J. Three of the recent CPXV strains exhibited a modest stimulation of 1.5- to 2.5-fold and one (GER_1990_2) was stimulated more than 4-fold, similar to VACV WR (Fig. 2A).
Fig. 2.
Effects of low pH and bafilomycin A1 treatment on cell entry of LUC-expressing recombinant MVs. (A) BS-C-1 cells were incubated with MVs at a multiplicity of 1 plaque forming unit (PFU) per cell at 4 °C for 1 h, followed by washing to remove unbound virus and exposure to pH 5 or pH 7.4 buffer for 3 min at 37oC. Cells were then washed and incubated at 37 °C at neutral pH for 1 h. Cells were lysed and LUC activity measured. The ratios of LUC activity following low and neutral pH are plotted for each virus. Error bars are shown. (B and C) BS-C-1 cells were pretreated with 0, 10 or 40 nM bafilomycin A1 for 1 h at 37 °C. Pretreated cells were incubated with indicated recombinant MVs in the presence of bafilomycin A1 for 1 h at 4 °C, unattached virus was removed by washing, and the cells were incubated at 37 °C at for 1 h in the absence or presence of bafilomycin A1. The cells were lysed and LUC activity measured. Abbreviation: RLU, relative light units.
VACV IHD-J and MVA were relatively insensitive to bafilomycin A1, while VACV WR, the three recent isolates of CPXV and MPXV were strongly inhibited (Fig. 2B and C). CPXV-BR exhibited intermediate sensitivity to bafilomycin A1 (Fig. 2B).
Effects of actin and cell-signaling inhibitors
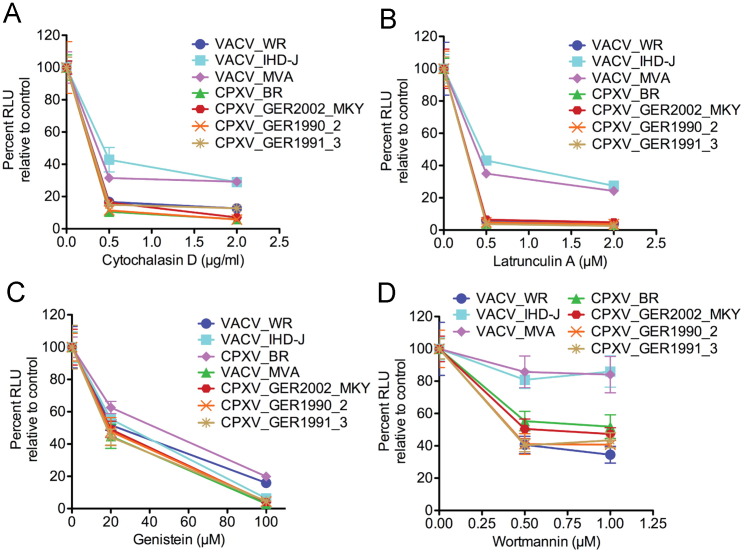
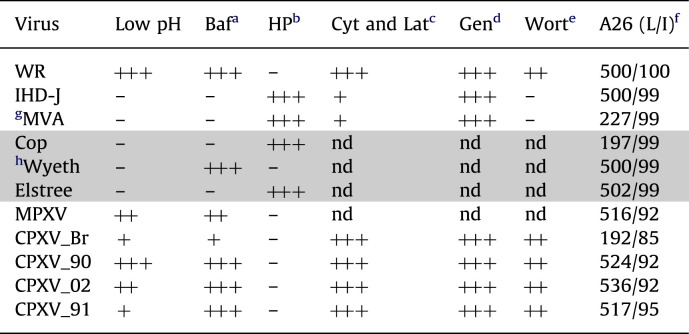
Mercer et al. (2010a) compared the effects of a number of inhibitors on entry of WR and IHD-J strains of VACV. We extended this analysis by comparing the effects of the actin inhibitors cytochalasin D and latrunculin, the protein kinase inhibitor genestein, and the phosphoinositide 3-kinase inhibitor wortmannin on three strains of VACV and four strains of CPXV. Inhibitors were tested at two concentrations in order to better evaluate sensitivities. Cytochalasin D inhibited entry of all of the OPXVs, although VACV IHD-J and MVA appeared less sensitive than others ( Fig. 3A). Similarly, IHD-J and MVA appeared less sensitive to latrunculin A, another actin inhibitor (Fig. 3B). Entry of each of the OPXVs was strongly inhibited by genestein (Fig. 3C). IHD-J and MVA were the least sensitive to wortmannin. With respect to WR and IHD-J, our data with cytochalasin D and wortmannin agreed with Mercer et al. (2010a); however IHD-J and WR appeared similarly sensitive to genestein in our hands. Overall, IHD-J and MVA were most similar to each other and VACV WR grouped with the other OPXVs that appear to prefer the endosomal route ( Table 1).
Fig. 3.
Effects of cytochalasin D, latrunculin A, genestein and wortmannin on entry of LUC-expressing recombinant MVs. BS-C-1 cells were pretreated with (A) 0, 0.5 or 2.0 μg/ml of cytochalasin D; (B) 0, 0.5 or 2 μM latrunculin; (C) 0, 20 or 100 μM genestein; (D) 0, 0.5 or 1.0 μM wortmannin. Cells were infected and incubated as in Fig. 2 and LUC activity determined. The percent activity relative to 0 drug was plotted with error bars.
Table 1.
Properties of Orthopoxviruses.
Number of plus marks indicates relative enhancement of entry with low pH or decrease in entry by inhibitors. Shaded rows refer to experimental data obtained previously (Bengali et al., 2009). nd, not done
abafilomycin A1.
bheparin.
ccytochalasin A and latrunculin.
dgenestein.
ewortmanin.
f(length in amino acids/% identity to WR).
gACAM3000.
hACAM2000.
Effect of deletion and swapping of the A26 ORF on bafilomycin A1 sensitivity
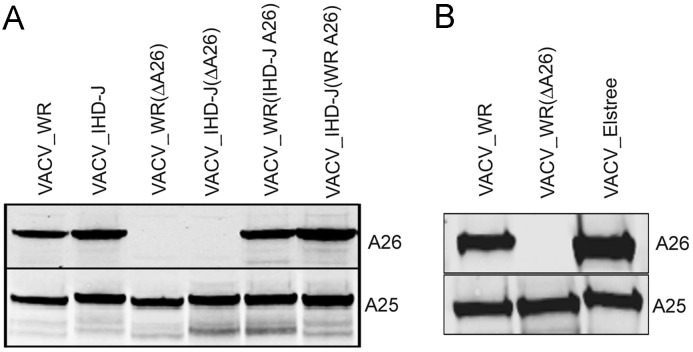
Chang et al. (2010) reported that bafilomycin A1-insensitivity of VACV correlated with loss of either the A26R or A25R gene, which act as fusion suppressors. The A26 open reading frame (ORF) sequences of six VACV strains, MPXV, CPXV-BR and the three recent CPXV isolates are shown in Fig. S1 and summarized in Table 1. MVA and Copenhagen have truncated A26 ORFs. However, the A26 ORFs of the other VACV strains were 500 to 502 amino acids long and 99% identical to that of WR. Except for CPXV-BR, the CPXV and MPXV A26 ORFs were longer than those of VACV mainly due to a longer run of aspartates in the CPXV isolates and had >90% identity with WR. The CPXV strains all had conserved full-length A-type inclusion protein sequences and made large ATI bodies; except for CPXV-BR the ATIs contained occluded MVAs (personal communication of R. Kastenmeyer and A. Weisberg). Neither MPXV nor the VACV strains make ATIs as the corresponding ORF is truncated at the C-terminus to form the A25 protein or largely missing in the case of MVA and Copenhagen. Although the bafilomycin A1 insensitivity of MVA and Copenhagen strains of VACV could be related to truncations of the A26 ORFs, the insensitivity of IHD-J and Elstree could not (Table 1). Furthermore, like WR, the A26 and A25 proteins of IHD-J and Elstree were synthesized and incorporated into MVs as shown by Western blotting with polyclonal antibodies that recognized the homologous proteins of the three VACV species ( Fig. 4A and B). To further investigate the relationship between A26 and the low pH entry mode, we constructed WR and IHD-J deletion mutants and also swapped A26 ORFs of the two VACV prototype strains. A27, which binds A26, was not swapped because the ORFs of these two strains are identical. Western blots showed the presence of the swapped A26 proteins in the MVs of the WR and IHD-J recombinant viruses and the absence of the A26 protein in the deletion mutants (Fig. 4A). Although the A25 and A26 proteins interact with each other (Howard et al., 2008), A25 still associates with MVs in the absence of A26 indicating an interaction with an additional protein.
Fig. 4.
Detection of A26 and A25 in purified recombinant MVs. (A) Purified Luc-recombinant MVs of VACV strains WR and IHD-J, A26 deletion mutants VACV WR(ΔA26) and VACV IHD-J(ΔA26), and swap mutants VACV WR(IHD-J A26) and VACV IHD-J(WR A26) were analyzed by Western blotting with antibody to the VACV A26 protein and the CPXV ATI protein, which recognizes VACV A25 because of high sequence conservation. (B) VACV WR, VACV WR(ΔA26) and VACV Elstree MVs were analyzed as in panel A.
Next, we measured entry of the wild type and mutant MVs using the LUC assay. Deletion of the A26 ORF from WR or IHD-J did not enhance bafilomycin A1 inhibition of MV entry in either BSC-1 ( Fig. 5A) or HeLa (Fig. 5B) cells. Furthermore, the swapping of WR and IHD-J A26 ORFs did not alter the bafilomycin A1 sensitivity of the recipient virus (Fig. 5A and B).
Fig. 5.
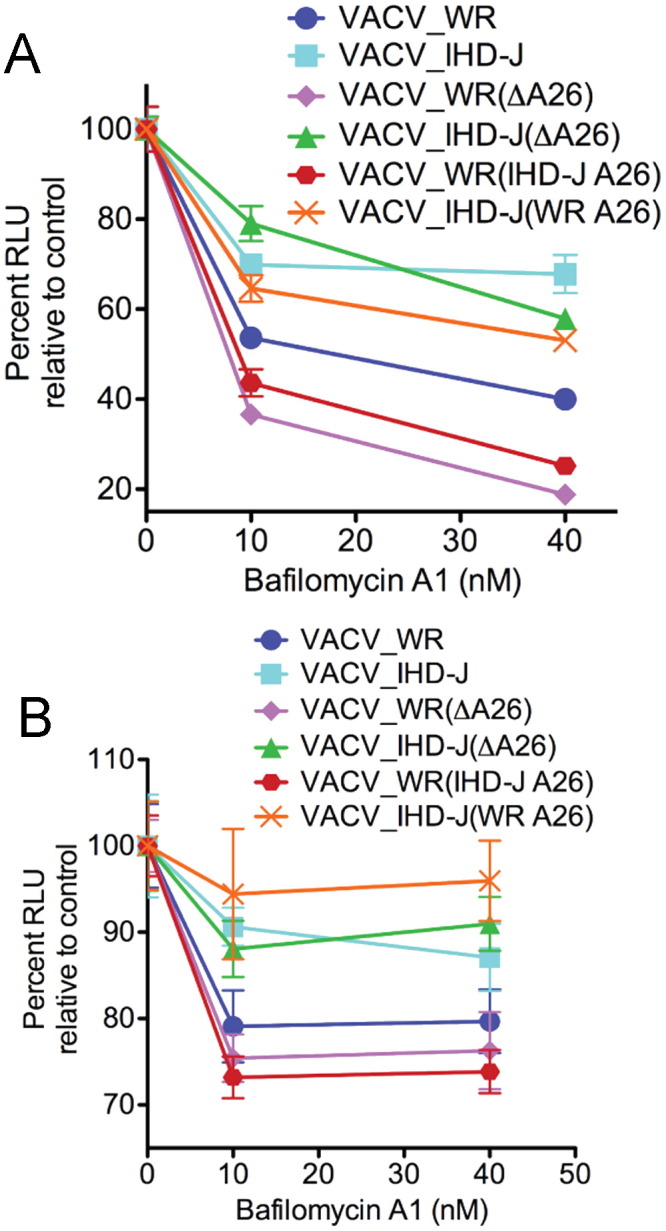
Effect of bafilomycin A1 on entry of LUC-expressing mutant viruses. BS-C-1 (A) and HeLa (B) cells were treated with bafilomycin A1, infected with LUC-expressing WR and IHD-J or A26 deletion mutants or swap mutants defined in the legend to Fig. 4. LUC activity was measured as described in the legend to Fig. 2.
Effect of deletion and swapping of the A26 ORF on fusion from without
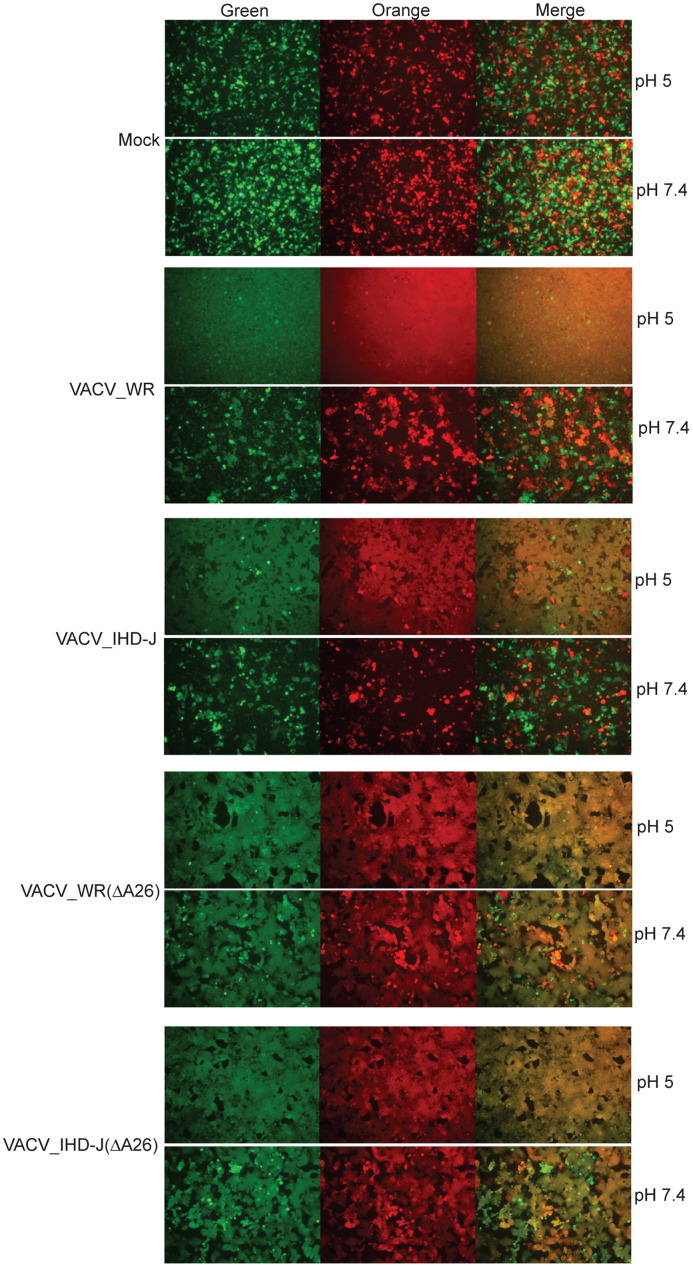
The ability of exogenous VACV to induce the formation of syncytia following adsorption has been referred to as fusion from without (Gong et al., 1990). As originally described, high virus multiplicities and a low pH pulse are required for cell–cell fusion. Recently, Chang et al. (2010) reported that VACV WR A25 and A26 deletion mutants, unlike wild type WR, could trigger syncytia formation at neutral pH. We followed the procedure of Chang et al. (2010) by mixing HeLa cells expressing orange fluorescent protein with HeLa cells expressing green fluorescent protein at a ratio of 1:1. The cells were either mock infected or infected with a high multiplicity of purified MVs. After adsorption, phosphate buffered saline at pH 7.4 or 5.0 was provided for 2 min. The buffer was then removed and the incubation continued with neutral pH medium for an additional 1 h. The mock-infected monolayer was composed of individual orange and green cells at the latter time after either pH 5.0 or pH 7.4 treatments ( Fig. 6). The WR- and IHD-J-infected monolayers that had brief pH 5.0 incubations were almost completely fused but there were only small numbers of fused cells in the absence of the low pH pulse (Fig. 6). The syncytia-forming abilities of the WR and IHD-J strains with swapped A26 ORFs were similar to their parents (not shown). In contrast, cells infected with WR or IHD-J A26 deletion mutants showed enhanced syncytia formation at neutral pH, which was increased further with a low pH pulse (Fig. 6). MVA showed extensive syncytia formation after either pH treatment (not shown).
Fig. 6.
Fusion from without. HeLa cells were separately transfected with plasmid expressing CMV-promoter controlled orange fluorescent protein or green fluorescent protein. After 24 h, the cells were harvested, mixed in 1:1 ratio and plated together in a 96-well plate. After forming a monolayer, the cells were infected with 100 PFU of purified virus per cell, treated with either pH 7.4 or 5.0 buffer for 2 min, and incubated in neutral pH medium for additional 1 h at 37 °C. Fluorescent cells were detected with a Leica DM IRB fluorescent microscope.
Discussion
We previously reported that VACV strains varied in their entry pathways as determined by low pH enhancement and inhibition by bafilomycin A1 and heparin (Bengali et al., 2009). Indeed, the low pH enhancement and bafilomycin A1 sensitivity of the WR strain appeared exceptional compared to several other VACV strains tested. However, the origin of VACV is unknown and most strains have been extensively propagated in cell culture. It seemed possible, therefore, that strain differences were selected by particular virus growth conditions. To extend these studies, we analyzed recently isolated OPXVs. There is evidence that CPXV is comprised of several diverse species (Carroll et al., 2011). We obtained three genetically distinct CPXVs isolated from documented human and monkey infections as well as a highly pathogenic MPXV isolate. The entry properties, determined using a LUC expression assay, are summarized in Table 1. The three recent CPXV isolates were similar to each other and to VACV WR with regard to sensitivity to bafilomycin A1, cytochalasin, latrunculin, genestein, and wortmannin and by their resistance to heparin. The degree of enhancement by low pH, however, varied. The latter result was not too surprising as low pH stimulation appears to be due to at least two factors of which one can be separated from bafilomycin A1 sensitivity (Townsley and Moss, 2007). Although analyzed less extensively, MPXV seemed similar to the CPXVs. Our finding that the WR strain of VACV resembled the recent CPXV isolates was gratifying, as WR is the prototype OPXV for laboratory studies. Based on the bafilomycin A1-sensitivity, we concluded that the low pH endocytic pathway is a major route of entry by OPXVs and that preferential neutral pH entry pathway of some VACV strains was likely acquired during laboratory passage. In support of this idea, IHD_J enters HeLa cells more rapidly than WR (Bengali et al., 2009). In cells with a dense cortical layer, however, endocytosis may have an advantage over fusion at the plasma membrane (Mercer et al., 2010b). In addition, the previously noted inverse relationship between bafilomycin A1 and heparin sensitivity (Bengali et al., 2009) held up with the additional viruses studied here. This feature suggests that adaptation to entry through the plasma membrane relies on binding to GAGs.
While the present work was proceeding, Chang and coworkers reported that the A25 and A26 proteins served as fusion suppressors and that fusion with the plasma membrane and bafilomycin A1-resistance were enhanced in the absence of either (Chang et al., 2010). They further reported that the absence of A26 correlated with VACV strain specificity (although their IHD-J strain and ours appear to differ in bafilomycin sensitivity) and that A26 interacts with components of the EFC (Chang et al., 2012). The A25 protein is a truncated form of the A-type inclusion protein of CPXV but is conserved in many OPXVs that do not form A-type inclusions. The A26 protein is required for the occlusion of MVs within A-type inclusions of CPXV (McKelvey et al., 2002) and contributes to cell attachment by binding to laminin (Chiu et al., 2007). A26 is complexed with both A25 and A27 and is tethered via the latter to the A17 transmembrane protein on the MV (Ching et al., 2009, Howard et al., 2008). A25 presumably interacts directly with either A27 or A17 since it still associates with MVs lacking A26. Neither A25 nor A26 is a component of EVs (Ulaeto et al., 1996) and may be missing from a small subset of MVs that are precursors to EVs.
We had not originally considered a specific role for A25 or A26 with regard OPXV entry pathways because we knew that both of these ORFs are highly conserved in the bafilomycin A1 insensitive IHD-J and Elstree strains and the bafilomycin A1 sensitive WR and Wyeth strains. Moreover, neither full-length nor truncated ATI protein is present in purified CPXV MVs (Patel et al., 1986). Nevertheless, upon learning of the data from the Chang laboratory (Chang et al., 2010), we compared the A26 sequences and the A25 or ATI protein sequences of all of the OPXVs used in our present and previous study. The recently isolated CPXVs all had conserved A26 ORFs and full-length ATI proteins, whereas CPXV-BR has a truncated A26 and full-length ATI. Since CPXV-BR is less sensitive to bafilomycin A1 than the more recent CPXV strains, this difference would be consistent with a role for A26 in regulating the entry pathway. As Chang et al. (2010) previously noted, the MVA and Copenhagen strains of VACV have truncated A26 proteins and are missing A25 and we also find that they are bafilomycin A1 insensitive. However, this leaves us with the conundrum as to why the IHD-J and Elstree strains were bafilomycin A1 resistant, though they have conserved A26 and A25 ORFs. One possibility was that the A26 and A25 proteins were not expressed or expressed but not present in MVs. However, we showed that purified IHD-J and Elstree MVs, like WR MVs, have both proteins. Moreover, deleting A26 from WR and IHD-J did not alter their relative sensitivities to bafilomycin A1, nor did swapping the A26 ORFs. We did find, however, that deleting the A26 ORFs from WR and IHD-J enhanced their ability to induce HeLa cells to undergo fusion from without at neutral pH. While syncytium formation is related to entry and requires the entry-fusion complex, there are obvious differences including the very high virus multiplicity (100 PFU per cell or more) used for the latter, whereas our entry studies were carried out at a multiplicity of 1 PFU per cell.
There are some experimental differences between our studies and those of Chang et al. The majority of our entry experiments were carried out with BS-C-1 cells, whereas the Chang laboratory used mainly HeLa cells. They reported (Chang et al., 2010) that WR A25 and A26 deletion mutants remained sensitive to bafilomycin A1 in BS-C-40 (derived from BS-C-1) cells and human umbilical vein cells in contrast to HeLa, L and CHO cells. Thus, data from our laboratory as well as the Chang laboratory are consistent with additional viral and cell factors controlling OPXV entry pathways.
Materials and methods
Cells and viruses
African green monkey kidney BS-C-1 and human HeLa S3 cells were maintained in minimum essential medium with Earle's salts (EMEM, Quality Biological, Gaithersburg, MD). The medium was supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. The following strains of VACV were used: WR (ATCC VR-1354; GenBank accession number NC_006998), IHD-J from S. Dales, and MVA (ATCC VR-1508). CPXV-Br was obtained from ATCC (VR-302) and CPXV GER_1990_2, CPXV GER_1991_3 and CPXV GER_2002_MKY from H. Meyer (Carroll et al., 2011, Meyer et al., 1999). MPXV-ZAI-1979-005 was provided by I. Damon. Recombinant WRvFire and IHD-JvFire expressing firefly LUC via a synthetic early-late promoter were previously described (Bengali et al., 2009, Townsley et al., 2006). Similar vFire recombinants were made using the CPXV and MPXV strains. MVs were purified by sedimentation through two 36% (w/v) sucrose cushions and banding once on a 25–40% (w/v) sucrose gradient for all experiments. Purified stocks were stored at −80 °C and sonicated on ice for 1 min prior to infection. Studies with VACV and CPXV strains were carried out under BSL-2 conditions. MPXV studies were carried out in a BSL-3 laboratory with approval by NIH and the Centers for Disease Control and Prevention.
Luc entry assay
The assay was carried out essentially as described (Townsley et al., 2006). Virus was allowed to adsorb for 1 h at 4 °C at neutral pH. Unattached virus was removed by washing. For pH activation, the cells were incubated for 3 min at 37 °C with Dulbecco's phosphate buffered saline with Ca2+ and Mg2+ at pH 7.4 or adjusted with HCl and 1 mM 2-morpholinoethane-sulfonice acid to pH 5. The pH was then neutralized and washed with EMEM containing 2.5% FBS, 2 mM L-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin (EMEM-2.5) and incubated with 1 ml EMEM-2.5 at 37 °C for 1 h. Cells were then harvested by washing with phosphate buffered saline (pH 7.4) and incubation with 300 μl of Cell Culture Lysis Reagent (Promega, Madison, WI) for 30 min at room temperature on an orbital shaker. Luc assay was performed by adding 20 μl of cell lysate to 100 μl of luc activity assay substrate (Promega), mixed, and chemiluminescence was measured using a luminometer (Berthold Sirius, Bad Wilbad, Germany).
Inhibition of endosomal acidification
Cells were treated with bafilomycin A1 (Sigma, St. Louis, MO) at 10 or 40 nm for 1 h at 37 °C, which remained present throughout the adsorption and subsequent incubations.
Effects of other inhibitors on virus entry
Virus was treated with heparin (50 μg/ml) and chondroitin sulfate (50 μg/ml) (Sigma) in EMEM-2.5 for 30 min on ice. Without removing the inhibitors, virus was added to cells for adsorption at neutral pH as described above. Additional drugs were obtained from Sigma and stock solution made in DMSO. Drug was diluted in tissue culture media at the indicated concentrations and incubated with cells for 1 h at 37 °C and remained present throughout the adsorption and subsequent incubations.
Fusion from without
HeLa cells were transfected with plasmid expressing CMV-promoter controlled orange fluorescent protein or green fluorescent protein. At 24 h post-transfection, cells were harvested by trypsinization, mixed in 1:1 ratio and plated together in a 96-well plate. After forming a monolayer (12–16 h post-plating), the cells were infected with 100 PFU of purified virus per cell for 1 h at 4 °C. Cells were washed to remove unbound viruses, treated with either pH 7.4 or 5.0 buffer for 2 min, washed and incubated in neutral pH E-MEM for additional 1 h at 37 °C. Cells were fixed with 4% paraformaldehyde for 15 min, fluorescent cells were visualized with a Leica DM IRB fluorescent microscope and images were processed by Adobe Photoshop software to determine cell–cell fusion.
Acknowledgments
We thank Scott Sammons of the Centers for Disease Control and Prevention for providing IHD-J genome sequences, David Pickup of Duke University for antibody to the CPXV ATI protein, Catherine Cotter for help with cell culture, Jeffery Americo for conducting the MPXV experiments under BSL-3 conditions, and Robin Kastenmeyer and Andrea Weisberg for unpublished information regarding ATI formation by CPXV isolates. Research funds were provided by the DIR, NIAID, NIH.
Footnotes
Supplementary data associated with this article can be found in the online version at 10.1016/j.virol.2012.08.044.
Appendix A. Supplementary materials
References
- Armstrong J.A., Metz D.H., Young M.R. The mode of entry of vaccinia virus into L cells. J. Gen. Virol. 1973;21:533–537. doi: 10.1099/0022-1317-21-3-533. [DOI] [PubMed] [Google Scholar]
- Bengali Z., Townsley A.C., Moss B. Vaccinia virus strain differences in cell attachment and entry. Virology. 2009;389:132–140. doi: 10.1016/j.virol.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht H., Weisberg A.S., Moss B. Vaccinia virus L1 protein is required for cell entry and membrane fusion. J. Virol. 2008;82:8687–8694. doi: 10.1128/JVI.00852-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breman J.G., Kalisa R., Steniowski M.V., Zanotto E., Gromyko A.I., Arita I. Human monkeypox, 1970–79. Bull. WHO. 1980;58:165–182. [PMC free article] [PubMed] [Google Scholar]
- Brown E., Senkevich T.G., Moss B. Vaccinia virus F9 virion membrane protein is required for entry but not virus assembly, in contrast to the related l1 protein. J. Virol. 2006;80:9455–9464. doi: 10.1128/JVI.01149-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D.S., Emerson G.L., Li Y., Sammons S., Olson V., Frace M., Nakazawa Y., Czerny C.P., Tryland M., Kolodziejek J., Nowotny N., Olsen-Rasmussen M., Khristova M., Govil D., Karem K., Damon I.K., Meyer H. Chasing Jenner's vaccine: revisiting cowpox virus classification. Plos One. 2011;6:e23086. doi: 10.1371/journal.pone.0023086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter G.C., Law M., Hollinshead M., Smith G.L. Entry of the vaccinia virus intracellular mature virion and its interactions with glycosaminoglycans. J. Gen. Virol. 2005;86:1279–1290. doi: 10.1099/vir.0.80831-0. [DOI] [PubMed] [Google Scholar]
- Chang S.J., Chang Y.X., Izmailyan R., Tang Y.L., Chang W. Vaccinia virus A25 and A26 proteins are fusion suppressors for mature virions and determine strain-specific virus entry pathways into HeLa, CHO-K1, and L cells. J. Virol. 2010;84:8422–8432. doi: 10.1128/JVI.00599-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.J., Shih A.C., Tang Y.L., Chang W. Vaccinia mature virus fusion regulator A26 protein binds to A16 and G9 proteins of the viral entry fusion complex and dissociates from mature virions at low pH. J. Virol. 2012;86:3809–3818. doi: 10.1128/JVI.06081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching Y.C., Chung C.S., Huang C.Y., Hsia Y., Tang Y.L., Chang W. Disulfide bond formation at the C termini of vaccinia virus A26 and A27 proteins does not require viral redox enzymes and suppresses glycosaminoglycan-mediated cell fusion. J. Virol. 2009;83:6464–6476. doi: 10.1128/JVI.02295-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W.L., Lin C.L., Yang M.H., Tzou D.L.M., Chang W. Vaccinia virus 4c (A26L) protein on intracellular mature virus binds to the extracellular cellular matrix laminin. J. Virol. 2007;81:2149–2157. doi: 10.1128/JVI.02302-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C.-S., Hsiao J.-C., Chang Y.-S., Chang W. A27L protein mediates vaccinia virus interaction with cell surface heparin sulfate. J. Virol. 1998;72:1577–1585. doi: 10.1128/jvi.72.2.1577-1585.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R.C., Moussatche N., Traktman P. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus Res. 2006;66:31–124. doi: 10.1016/S0065-3527(06)66002-8. [DOI] [PubMed] [Google Scholar]
- Gong S.C., Lai C.F., Esteban M. Vaccinia virus induces cell fusion at acid pH and this activity is mediated by the N-terminus of the 14-kDa virus envelope protein. Virology. 1990;178:81–91. doi: 10.1016/0042-6822(90)90381-z. [DOI] [PubMed] [Google Scholar]
- Howard A.R., Senkevich T.G., Moss B. Vaccinia virus A26 and A27 proteins form a stable complex tethered to mature virions by association with the A17 transmembrane protein. J. Virol. 2008;82:12384–12391. doi: 10.1128/JVI.01524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao J.C., Chung C.S., Chang W. Cell surface proteoglycans are necessary for A27L protein- mediated cell fusion: identification of the N-terminal region of A27L protein as the glycosaminoglycan-binding domain. J. Virol. 1998;72:8374–8379. doi: 10.1128/jvi.72.10.8374-8379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.Y., Lu T.Y., Bair C.H., Chang Y.S., Jwo J.K., Chang W. A novel cellular protein, VPEF, facilitates vaccinia virus penetration into HeLa cells through fluid phase endocytosis. J. Virol. 2008;82:7988–7999. doi: 10.1128/JVI.00894-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihashi Y. Extracellular enveloped vaccinia virus escapes neutralization. Virology. 1996;217:478–485. doi: 10.1006/viro.1996.0142. [DOI] [PubMed] [Google Scholar]
- Izmailyan R.A., Huang C.Y., Mohammad S., Isaacs S.N., Chang W. The envelope G3L protein is essential for entry of vaccinia virus into host cells. J. Virol. 2006;80:8402–8410. doi: 10.1128/JVI.00624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laliberte J.P., Weisberg A.S., Moss B. The membrane fusion step of vaccinia virus entry is cooperatively mediated by multiple viral proteins and host cell components. PLoS Pathog. 2011;7:e1002446. doi: 10.1371/journal.ppat.1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M., Carter G.C., Roberts K.L., Hollinshead M., Smith G.L. Ligand-induced and non-fusogenic dissolution of a viral membrane. Proc. Natl. Acad. Sci. USA. 2006;103:5989–5994. doi: 10.1073/pnas.0601025103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C.L., Chung C.S., Heine H.G., Chang W. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 2000;74:3353–3365. doi: 10.1128/jvi.74.7.3353-3365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey T.A., Andrews S.C., Miller S.E., Ray C.A., Pickup D.J. Identification of the orthopoxvirus p4c gene, which encodes a structural protein that directs intracellular mature virus particles into A-type inclusions. J. Virol. 2002;76:11216–11225. doi: 10.1128/JVI.76.22.11216-11225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J., Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- Mercer J., Knebel S., Schmidt F.I., Crouse J., Burkard C., Helenius A. Vaccinia virus strains use distinct forms of macropinocytosis for host-cell entry. Proc. Natl. Acad. Sci. USA. 2010;107:9346–9351. doi: 10.1073/pnas.1004618107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J., Schelhaas M., Helenius A. Virus entry by endocytosis. Annu. Rev. Biochem. 2010;79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- Meyer H., Schay C., Mahnel H., Pfeffer M. Characterization of orthopoxviruses isolated from man and animals in Germany. Arch. Virol. 1999;144:491–501. doi: 10.1007/s007050050520. [DOI] [PubMed] [Google Scholar]
- Moser T.S., Jones R.G., Thompson C.B., Coyne C.B., Cherry S. A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS Pathog. 2010;6:e1000954. doi: 10.1371/journal.ppat.1000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Poxvirus entry and membrane fusion. Virology. 2006;344:48–54. doi: 10.1016/j.virol.2005.09.037. [DOI] [PubMed] [Google Scholar]
- Moss B. In: Knipe D.M., Howley P.M., editors. Vol. 2. Lippincott Williams and Wilkins; Philadelphia: 2007. Poxviridae: the viruses and their replication; pp. 2905–2946. (Fields Virology). (2 vols.) [Google Scholar]
- Nichols R.J., Stanitsa E., Unger B., Traktman P. The vaccinia I2L gene encodes a membrane protein with an essential role in virion entry. J. Virol. 2008;82:10247–10261. doi: 10.1128/JVI.01035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda S., Domi A., Moss B. Vaccinia virus G9 protein is an essential component of the poxvirus entry-fusion complex. J. Virol. 2006;80:9822–9830. doi: 10.1128/JVI.00987-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda S., Senkevich T.G., Moss B. Entry of vaccinia virus and cell–cell fusion require a highly conserved cysteine-rich membrane protein encoded by the A16L gene. J. Virol. 2006;80:51–61. doi: 10.1128/JVI.80.1.51-61.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D.D., Pickup D.J., Joklik W.K. Isolation of cowpox virus A-type inclusions and characterization of their major protein component. Virology. 1986;149:174–189. doi: 10.1016/0042-6822(86)90119-4. [DOI] [PubMed] [Google Scholar]
- Sandgren K.J., Wilkinson J., Miranda-Saksena M., McInerney G.M., Byth-Wilson K., Robinson P.J., Cunningham A.L. A differential role for macropinocytosis in mediating entry of the two forms of vaccinia virus into dendritic cells. Plos Pathog. 2010;6:e1000866. doi: 10.1371/journal.ppat.1000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satheshkumar P.S., Moss B. Characterization of a newly Identified 35 amino acid component of the vaccinia virus entry/fusion complex conserved in all chordopoxviruses. J. Virol. 2009;83:12822–12832. doi: 10.1128/JVI.01744-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F.I., Bleck C.K., Helenius A., Mercer J. Vaccinia extracellular virions enter cells by macropinocytosis and acid-activated membrane rupture. EMBO J. 2011;30:3647–3661. doi: 10.1038/emboj.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F.I., Bleck C.K., Mercer J. Poxvirus host cell entry. Curr. Opinion Virol. 2012;2:20–27. doi: 10.1016/j.coviro.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Senkevich T.G., Moss B. Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell–cell fusion. J. Virol. 2005;79:4744–4754. doi: 10.1128/JVI.79.8.4744-4754.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich T.G., Ojeda S., Townsley A., Nelson G.E., Moss B. Poxvirus multiprotein entry-fusion complex. Proc. Natl. Acad. Sci. USA. 2005;102:18572–18577. doi: 10.1073/pnas.0509239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich T.G., Ward B.M., Moss B. Vaccinia virus A28L gene encodes an essential protein component of the virion membrane with intramolecular disulfide bonds formed by the viral cytoplasmic redox pathway. J. Virol. 2004;78:2348–2356. doi: 10.1128/JVI.78.5.2348-2356.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley A., Senkevich T.G., Moss B. The product of the vaccinia virus L5R gene is a fourth membrane protein encoded by all poxviruses that is requried for cell entry and cell–cell fusion. J. Virol. 2005;79:10988–10998. doi: 10.1128/JVI.79.17.10988-10998.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley A., Senkevich T.G., Moss B. Vaccinia virus A21 virion membrane protein is required for cell entry and fusion. J. Virol. 2005;79:9458–9469. doi: 10.1128/JVI.79.15.9458-9469.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley A.C., Moss B. Two distinct low-pH steps promote entry of vaccinia virus. J. Virol. 2007;81:8613–8620. doi: 10.1128/JVI.00606-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley A.C., Weisberg A.S., Wagenaar T.R., Moss B. Vaccinia virus entry into cells via a low pH-dependent-endosomal pathway. J. Virol. 2006;80:8899–8908. doi: 10.1128/JVI.01053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulaeto D., Grosenbach D., Hruby D.E. The vaccinia virus 4c and A-type inclusion proteins are specific markers for the intracellular mature virus particle. J. Virol. 1996;70:3372–3375. doi: 10.1128/jvi.70.6.3372-3377.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa N.Y., Bartee E., Mohamed M.R., Rahman M.M., Barrett J.W., McFadden G. Myxoma and vaccinia viruses exploit different mechanisms to enter and infect human cancer cells. Virology. 2010;401:266–279. doi: 10.1016/j.virol.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.M., Delos S.E., Brecher M., Schornberg K. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit. Rev. Biochem. Mol. Biol. 2008;43:189–219. doi: 10.1080/10409230802058320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe C.L., Ojeda S., Moss B. Transcriptional repression and RNA silencing act synergistically to demonstrate the function of the eleventh component of the vaccinia virus entry-fusion complex. J. Virol. 2012;86:293–301. doi: 10.1128/JVI.05935-11. [DOI] [PMC free article] [PubMed] [Google Scholar]