Abstract
Background/Aims
Dementia with Lewy Bodies (DLB) and Alzheimer's disease (AD) are the two most common neurodegenerative dementias. During the early stages, clinical distinction between them is often challenging. Our objective is to compare hippocampal atrophy patterns in mild AD and mild DLB. We hypothesized that DLB subjects have milder hippocampal atrophy relative to AD subjects.
Methods
We analyzed the T1-weighted magnetic resonance imaging data from 113 subjects: 55 AD, 16 DLB and 42 cognitively normal elderly (NC). Using the hippocampal radial distance technique and multiple linear regression, we analyzed the effect of clinical diagnosis on hippocampal radial distance, while adjusting for gender and age. 3D statistical maps were adjusted for multiple comparisons using permutation-based statistics with a threshold of p<0.01.
Results
Compared to NC, AD exhibited significantly greater atrophy in the Cornu Ammonis (CA) 1, CA2-3 and subicular regions bilaterally while DLB showed left-predominant atrophy in the CA1 region and subiculum. AD and DLB directly compared did not reveal statistically significant differences.
Conclusion
Hippocampal atrophy, while present in mildly impaired DLB subjects, is less severe than atrophy seen in mildly impaired AD subjects, when compared to NC. Both groups show predominant atrophy of the CA1 subfield and subiculum.
Keywords: Alzheimer's disease, Dementia with Lewy Bodies, hippocampus, MRI, atrophy
Introduction
Dementia with Lewy bodies (DLB) - the second most common neurodegenerative dementia after Alzheimer's disease (AD), has a complex clinical presentation including psychiatric, motor, sleep, and autonomic disturbances in addition to cognitive impairment 1. Prognosis and quality of life in DLB are generally even poorer than in AD 2, as are health-related costs 3 and mortality 4. DLB patients have a different response to drug treatment and are at a particularly high risk of developing severe hypersensitivity reactions to neuroleptic drugs 5.
Differentiating DLB from other dementias, including AD, is of particular clinical importance. Yet, this distinction can be difficult, especially early in the disease course when the classic DLB clinical profile may not yet be fully developed 6. Consequently, many pathologically confirmed cases of DLB were clinically misdiagnosed as AD pre mortem 7-10. While the clinical consensus criteria for DLB were recently revised 1 and preliminary evidence suggests that the newly revised criteria have greater sensitivity 11, a systematic evaluation of their sensitivity and specificity is not yet available.
The ability of a range of biomarkers to aid in the differential diagnosis of DLB is actively being explored. The most established method to date, dopamine transporter single photon emission computerized tomography (SPECT) 12, is relatively expensive and is not readily available at all centers. Structural neuroimaging using magnetic resonance imaging (MRI) is among the most established biomarkers for AD and is now used for the diagnosis of prodromal AD 13. Novel sensitive MRI analytic techniques have been recently developed and have enabled us to identify presymptomatic and early symptomatic structural changes in AD 14-16.
Few studies have compared the MRI changes in DLB and AD; most have used either visual rating, the region-of-interest (ROI) approach, whole brain imaging analysis methods such as voxel-based morphometry (VBM) or cortical thickness approaches to measure cortical or subcortical atrophy in DLB compared to normal controls (NC) or AD. These types of studies find that relative to AD, DLB subjects showed significantly less atrophy in the orbitofrontal and temporal lobes 17-19. In addition, temporal lobe and hippocampal atrophy are less pronounced in DLB than in AD 19-22 but the magnitude of the differences is small, precluding the ability of standard volumetric hippocampal assessments to readily distinguish between AD and DLB. Yet one recent study suggested that visual scoring of medial temporal atrophy on MRI obtained approximately 1.5 years prior to death might provide discriminatory power for distinguishing patients with moderately severe pathologically-confirmed AD (MMSE 13.8 ± 4.54) from those with moderately severe pathologically-confirmed DLB (MMSE 13.3 ± 7.83) and mild vascular cognitive impairment (MMSE 22.8 ± 4.36) 21. Another study applied a recently developed hippocampal partial subfield segmentation technique to the 3T MRI data of 16 AD (mean MMSE 21.5, range 16-27), 16 DLB (mean MMSE 18, range 15-27) and 16 NC (mean MMSE 29, range 26-30) 23. Their measurements were limited to the three most anterior contiguous coronal slices from the hippocampal body. Despite reported significant technical difficulties in ascertaining the CA1 subfield boundary, the authors were able to show significantly smaller CA1 area in AD compared to DLB.
Most prior MRI studies comparing DLB and AD have included patients with moderately severe dementia. Yet, recent advances in biomarker development and structural neuroimaging in particular now allow us the unprecedented opportunity to detect structural changes in the presymptomatic stages for other dementing disorders such as AD 15, 24. In this study, we analyzed the imaging data of DLB and AD subjects in the mild dementia stages. Our objective was to compare hippocampal atrophy patterns in mild AD and mild DLB. We hypothesized that DLB subjects would have milder hippocampal atrophy relative to subjects diagnosed with AD, with potentially greater involvement of the CA2-3 subfields, as was recently suggested by one post mortem study 21.
Methods
Subjects
The Dementia study in Western Norway (DemWest) is a multisite longitudinal study of the natural history and biological correlates of dementia. Details of inclusion and assessment procedures have been published previously 25. In brief, 196 subjects with mild dementia defined as Mini-Mental State Examination (MMSE) 26 score of 20 or higher, were recruited from referrals to all geriatric medicine, old age psychiatry and neurology outpatient clinics in Rogaland and Hordaland counties in Western Norway between March 2005 and March 2007. The study was later enriched with additional DLB subjects, which included some in the moderate dementia stages (MMSE range 18-20). Standardized clinical instruments were employed to detect and rate cognition, psychiatric and motor symptoms as previously described 25. Diagnosis of AD was made according to the National Institute of Neurologic and Communicate Disorders and Stroke and the AD and Related Disorders Association (NINCDS-ADRDA) criteria 27 and diagnosis of DLB according to the revised DLB consensus criteria 1. Two research psychiatrists independently applied the diagnostic criteria twice – at baseline and after one year. In cases of disagreement, and whenever more than one set of operationalized diagnostic criteria was met, final diagnostic ascertainment was made based on consensus between the two physicians after careful review of all available information. All DemWest patients are actively recruited to autopsy. To date, seven patients have undergone post mortem exams using standard methods and diagnostic criteria, as previously described 28, 29. In all seven cases, the pathological diagnosis agreed with the pre mortem clinical diagnosis. Our study analyzed the imaging data of 55 AD and 16 DLB DemWest subjects who provided a baseline structural MRI scan of sufficient quality for imaging analyses.
Our control group consisted of 42 cognitively normal elderly subjects who were enrolled in the ParkWest study - another longitudinal project in Western Norway. These NC subjects were scanned during the same time period on the same scanners and with the same imaging protocol as our DemWest subjects. ParkWest NC were free from parkinsonism, dementia, major depression, and psychosis and scored within the cognitively normal range on the detailed ParkWest neuropsychological battery which has been previously described 30. Both studies were approved by The Regional Norwegian Committee for Medical Research Ethics, Western Norway. All subjects gave written informed consent for the participation in the study after procedures had been explained in detail in accordance with the Declaration of Helsinki.
Imaging data collection and analysis
Subjects were scanned at five different sites located in Stavanger, Haugesund, Haraldsplass, Bergen and Arendal. The following protocols were used:
- Stavanger: 1.5 T Phillips Intera (Best, The Netherlands), repetition time (TR)/echo time (TE) 10.0/4.6 msec, flip angle 30 degrees, 2 mm slices, 1 mm gap, number of excitations (NEX) 2, Matrix 256x256. Nominal resolution is 1×1×1.28 millimeter.
- Haugesund: 1.5 T Phillips Intera (Best, The Netherlands), TR/TE 20.0/4.6 msec, flip angle 30 degrees, 1 mm slice thickness with no gap, NEX 1, Matrix 256×256. Nominal resolution is 1×1×1.46 millimeter.
- Haraldsplass: 1.5 T General Electric Signa Excite (Milwaukee, Wisconsin), TR/TE 8.2 /3.1 msec, flip angle 7 degrees, 1 mm slice thickness, 1mm gap, NEX 1, Matrix 256×256. Nominal resolution is 1×1×1.29 millimeter.
- Bergen: 1.5 T General Electric Signa Excite (Erlangen, Germany), TR/TE 8.2/3.1 msec, flip angle 7 degrees, 1 mm slice thickness with no gap, NEX 1, Matrix 256×256. Nominal resolution is 1×1×1.33 millimeter.
- Arendal: 1.0 T Philips Intera (Best, The Netherlands), TR/TE 25/6.9 msec, flip angle 30 degrees, 2 mm slice thickness with no gap, NEX 1, Matrix 256×256. Nominal resolution is 1×1×1.28 millimeter.
T2-weighted and fluid attenuated inversed recovery (FLAIR) sequences were collected to evaluate subjects for strokes, and/or structural lesions. Subjects with these findings were excluded from our imaging analyses. Also excluded from the imaging analyses were subjects with baseline scan artifacts or scans of insufficient quality. Our final cohort of DemWest subjects consisted of 71 patients. There were no significant age, sex, education, disease duration and MMSE differences between DemWest subjects who underwent an MRI and those who did not.
Individual MRI scans were automatically registered to the International Consortium on Brain Mapping 53 (ICBM53) template, an average of 53 normal adult brains, using a 9-parameter transformation. This step orients each brain volume into the ICBM53 standardized coordinate system by rotating and globally scaling to correct for differences in head tilt and head size between subjects. Next, hippocampi were manually traced on coronal slices by one researcher (HH, intrarater reliability Cronbach's alpha=0.9) blinded to subjects’ age, sex, education, MMSE score and diagnosis, following our detailed hippocampal tracing protocol as previously described 31. The traces included the hippocampus proper, dentate gyrus and subiculum. Traces were converted into hippocampal contours and transformed into 3D parametric surface mesh models, which were then separated into top and bottom components 32. These mesh models assured normalization of the spatial frequency of the digitized surface points. Next, a medial core, threading down the center of the hippocampus, was computed. Radial distance was measured from the medial core to the surface of the hippocampus. Each radial distance value was recorded at the corresponding surface coordinate point. These resulting individual hippocampal radial distance maps were combined across subjects to create group average distance maps for quantitative comparisons of surface morphology between diagnostic groups 32.
Intra- and Inter-Scanner Reliability Analyses
Human phantom scanning of three cognitively normal individuals was performed to test scanner reliability. Each volunteer was scanned twice at all scanners included in this study. We performed inter-scanner reliability analyses. The hippocampi were manually traced (JHS, interrater reliability Cronbach's alpha=0.896) and the volumes obtained as previously described. Inter-site Cronbach's alpha for hippocampal volumes was 0.967.
Statistical methods
One-way analyses of variance (ANOVA) with post hoc Bonferroni correction for multiple comparisons were run to examine between-group diagnostic differences in continuous variables such age, education, and MMSE scores. A chi-squared test was used to determine differences in gender distribution. The effect of diagnosis on hippocampal radial distance was studied by means of linear regression while correcting for demographic variables that showed significant between group differences. Our 3D statistical maps were adjusted for multiple comparisons, using permutation-based statistics with a threshold of p<0.01.
Results
Mean demographic data for the diagnostic groups NC (n=42), AD (n=55), DLB (n=16) are shown in Table 1. The AD and DLB subjects were well matched for overall cognitive impairment as assessed by MMSE (AD: 23.9±2.2. DLB: 23±2.7, p=0.4), the Clinical Dementia Rating (CDR) global score (AD: 0.76±0.30, DLB: 0.80±0.25, p=0.671), and CDR Sum of Boxes (AD: 4.39±1.93, DLB: 5.03±1.99, p=0.260). Significant differences in age were found, with the DLB group being the oldest and the NC group being the youngest (p<0.0001). Gender distribution was also significantly different with the DLB group having more men than women, while the opposite was true for the AD and NC groups (p=0.026). As expected, NC subjects had a significantly higher mean MMSE score (28.81±1.1) than AD (23.9±2.2) and DLB subjects (23.0±2.7) (p<0.0001), but there was no significant difference between AD and DLB. Age and sex were included as covariates in our radial distance multiple regression models.
Table 1.
Demographic characteristics for diagnostic comparisons study
| Variable | NC (n=42) | AD (n=55) | DLB (n=16) | p-value |
|---|---|---|---|---|
| Age, years | 69.86±6.756 (50-82) | 74.76±7.371 (55-89) | 78.13±6.479 (69-88) | p<0.0001 |
| Gender (M:F) | 14:28 | 13:42 | 9:7 | p=0.046 |
| Education, years | 10.214±2.526 (7-17) | 9.482±2.453 (7-18) | 8.531±1.803 (7-12) | p=0.053 |
| MMSE* | 28.81±1.110 (27-30) | 23.85±2.155 (19-29) | 23.00±2.683 (18-26) | p<0.0001 (AD vs DLB, p=0.4) |
| CDR# | N/A | 0.76±0.302 (1-2) | 0.80±0.254 (1-1) | p=0.671 |
| CDR Sum of Boxes# | N/A | 4.39±1.931 (2-9) | 5.03±1.986 (3-10) | p=0.260 |
Note:
Two subjects (one AD and one DLB) had MMSE=19 and one DLB subject had MMSE=18.
CDR and CDR Sum of Boxes available for 15 DLB subjects.
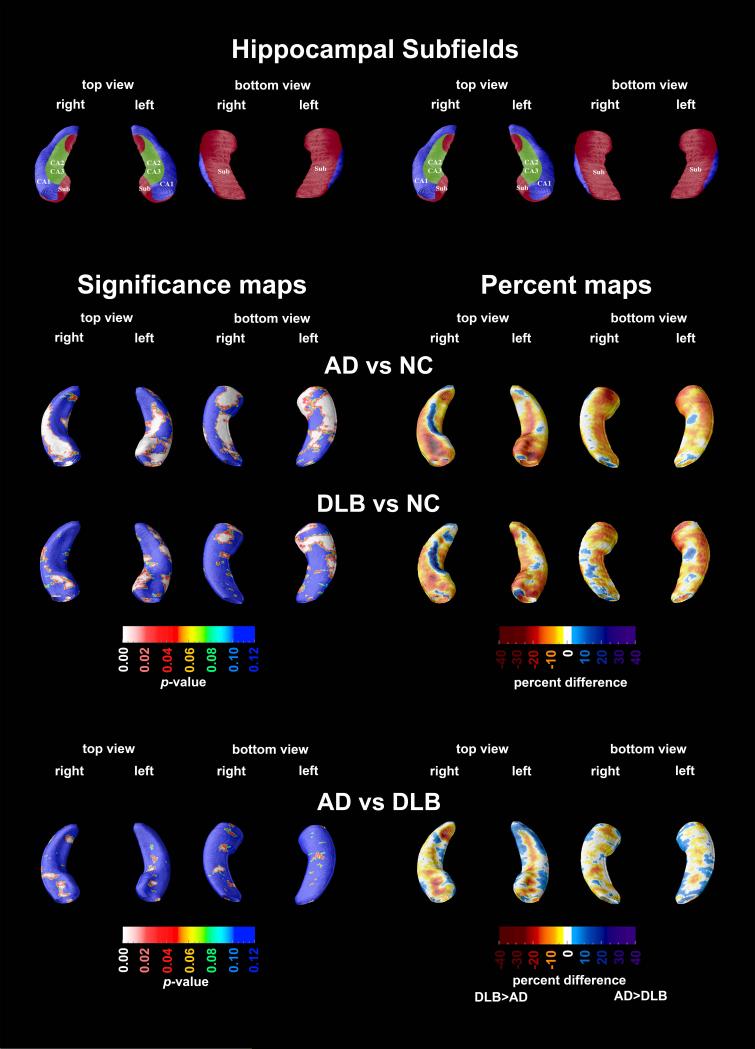
Compared to normal controls, the AD group exhibited significantly greater atrophy in the CA1, CA2-3 and subicular region bilaterally (Left: pcorrected<0.0001; Right: pcorrected=0.0001; Figure 1, second row), while the DLB group showed left greater than right atrophy localizing mainly to the CA1 region and subiculum (Left: pcorrected=0.0004; Right: pcorrected=0.056; Figure 1, third row). Quantitatively, both DLB and AD groups had between 10-40% smaller radial distance in the statistically significant areas relative to NC. The AD versus DLB comparison did not reveal statistically significant between-group differences (Figure 1, bottom row).
Figure 1.
3D statistical and percent difference maps. Red and white areas in the significance maps show statistical significance (p<0.05). A schematic representation of the hippocampal subfields mapped onto the hippocampal surface is presented in the top row (CA1 in blue, CA2 and 3 in green, subiculum in red; definitions based on Mai 38, Duvernoy 39, and West and Gunderson 40)
Discussion
Differentiating DLB from AD early in the disease course can be challenging, as the full clinical profile of DLB may not yet be fully developed. Here, we used an advanced surface based technique to determine if characteristic structural changes are present in the mild dementia stages of DLB and AD that might aid clinicians in their differential diagnosis. We observed significant hippocampal atrophy in both diagnostic groups compared to NC. Our mild AD subjects showed greater atrophy in all hippocampal subfields as previously demonstrated 33. Our mild DLB subjects showed significant atrophy of the CA1 and subiculum relative to NC. Another group, Sabatoli et al., using the same hippocampal radial distance technique, investigated the atrophy pattern in 14 mild to moderate DLB subjects (MMSE range 13-29) relative to NC and reported atrophy restricted to the anterior portions of CA1 22. Despite enrolling a cognitively milder DLB cohort (MMSE range 18-26), our study was able to detect more widespread atrophy of the CA1 and subiculum.
Our DLB and AD groups were well matched for overall cognitive impairment, allowing a fair diagnostic comparison. However, with the current sample size of the DLB group (n=16), we failed to show statistically significant differences between the groups. To further analyze the failure to detect significant differences between AD and DLB, we constructed a confidence interval (CI) of hippocampal volumes to determine the range of likely values for which we would expect to find a difference between the groups. The difference between the left volumes of AD and DLB had a 95% CI of 59.0 ± 340.2 mm3 and the right volume difference had a 95% CI of -69.2 ± 349.4 mm3. These intervals indicate that at most the difference between groups is 10%. Using the average volume for each subject, the confidence interval for the difference in means is -5.1 ± 315 mm3. With this interval, the difference between groups is 7.5%. Therefore, in our study the AD and DLB groups are indeed very similar in terms of hippocampal volume. The calculated confidence intervals can exclude the possibility that these groups are substantially different from one another. While these findings seem to disagree with those reported by others 23, there are several plausible explanations for this. Firstly, DLB is a heterogeneous disorder with the majority of cases showing, in addition to Lewy bodies, pathologic characteristics of AD such as amyloid plaques and neurofibrillary tangles. Mixed DLB/AD cases frequently show hippocampal atrophy and neurofibrillary tangle pathology of severity similar to that seen in AD 21. As such, studies that have relatively small sample sizes could have a study population skewed towards either pure or mixed DLB. Neither our study nor the study by Firbank et al. had post mortem diagnostic verification to ascertain the pathological diagnoses of our subjects. Secondly, the study by Firbank et al. used a recently developed method for subfield tracing that involves CA1, CA2 and CA3/4 subfield differentiation on only three contiguous coronal sections from the hippocampal structure with tracing beginning on the first slice where the hippocampal head is no longer visible. Such sparse sampling of the hippocampal structure potentially driven by the tediousness and substantial technical difficulties behind subfield tracing, even at 4T 23, 34, 35, may or may not generalize well to the whole subfield or the whole hippocampus. Upon close inspection of the AD versus DLB comparison (Figure 1, bottom row), one can appreciate that there are significant between-group differences in the superolateral CA1 area adjacent to the right hippocampal head. This is likely a very similar area where Firbank et al. reported greater atrophy in AD versus DLB. Furthermore, post mortem subjects with DLB show mild neuronal loss in CA1 and minimal neuritic and neurofibrillary tangle pathology in the CA2-3 regions 36. Similarly to AD 37, neurofibrillary tangles have been linked with hippocampal atrophy in DLB 21.
Several strengths and limitations of our study should be recognized. Major strengths of the study include its design, the well-characterized patient cohort using standardized instruments to validate core and suggestive DLB features, and its focus on the mild dementia stages. The state-of-the-art imaging analysis is another strength, as it allows the identification of focal regionally specific disease-associated differences. One limitation of our study is the lack of pathologic validation for all cases. However, DemWest subjects are routinely approached for post mortem diagnostic assessment. Post mortem diagnostic confirmation has been completed on the first seven cases coming to autopsy and in all cases the clinical and pathologic diagnosis were in agreement. Finally, we should also recognize that despite our best efforts to control for between-group variability by entering age and gender as potential confounders in the linear regression analyses some residual variance due to these and other demographic imbalances might still be present.
Figure 2.
Average radial distance maps and standard deviation maps of each diagnostic group
Acknowledgment
We would like to acknowledge the Research Council of Norway (grant number 177966), the Western Norway Regional Health Authority (grant number 911218), the Norwegian Parkinson's disease Association and research grants from Stavanger University Hospital and the Western Norway Regional Health Authority (grant number 911464) for financial support in the design and conduct of the study as well as collection and management of obtained data. Our work is also supported by NIH (EB008281, AG020098, RC2 AG036535) and NIA P50 A16570, which provided funding for the analysis and interpretation of data. All sources of support were involved in the preparation, review and approval of the manuscript. Finally, we would like to acknowledge Ketil Oppedal and Turi Olene Dalaker for their contribution to the human phantom scans.
Financial Disclosure The current study is supported by the Research Council of Norway (grant number 177966), the Western Norway Regional Health Authority (grant number 911218), the Norwegian Parkinson's disease Association and research grants from Stavanger University Hospital and the Western Norway Regional Health Authority (grant number 911464), NIH (EB008281, AG020098, RC2 AG036535) and NIA P50 A16570.
Footnotes
Research conducted at: Department of Neurology, David Geffen School of Medicine, UCLA, Los Angeles, CA, USA
Conflict of Interest: Authors have no conflicts of interest or financial interests pertaining to this submission.
References
- 1.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 2.Bostrom F, Jonsson L, Minthon L, Londos E. Patients with dementia with lewy bodies have more impaired quality of life than patients with Alzheimer disease. Alzheimer disease and associated disorders. 2007;21(2):150–154. doi: 10.1097/WAD.0b013e318065c4a9. [DOI] [PubMed] [Google Scholar]
- 3.Bostrom F, Jonsson L, Minthon L, Londos E. Patients with Lewy body dementia use more resources than those with Alzheimer's disease. International journal of geriatric psychiatry. 2007;22(8):713–719. doi: 10.1002/gps.1738. [DOI] [PubMed] [Google Scholar]
- 4.Williams MM, Xiong C, Morris JC, Galvin JE. Survival and mortality differences between dementia with Lewy bodies vs Alzheimer disease. Neurology. 2006;67(11):1935–1941. doi: 10.1212/01.wnl.0000247041.63081.98. [DOI] [PubMed] [Google Scholar]
- 5.Aarsland D, Perry R, Larsen JP, et al. Neuroleptic sensitivity in Parkinson's disease and parkinsonian dementias. The Journal of clinical psychiatry. 2005;66(5):633–637. [PubMed] [Google Scholar]
- 6.Tiraboschi P, Salmon DP, Hansen LA, Hofstetter RC, Thal LJ, Corey-Bloom J. What best differentiates Lewy body from Alzheimer's disease in early-stage dementia? Brain. 2006;129(Pt 3):729–735. doi: 10.1093/brain/awh725. [DOI] [PubMed] [Google Scholar]
- 7.Merdes AR, Hansen LA, Jeste DV, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003;60(10):1586–1590. doi: 10.1212/01.wnl.0000065889.42856.f2. [DOI] [PubMed] [Google Scholar]
- 8.Hohl U, Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. Diagnostic accuracy of dementia with Lewy bodies. Arch Neurol. 2000;57(3):347–351. doi: 10.1001/archneur.57.3.347. [DOI] [PubMed] [Google Scholar]
- 9.Lopez OL, Becker JT, Kaufer DI, et al. Research evaluation and prospective diagnosis of dementia with Lewy bodies. Arch Neurol. 2002;59(1):43–46. doi: 10.1001/archneur.59.1.43. [DOI] [PubMed] [Google Scholar]
- 10.Nelson PT, Jicha GA, Kryscio RJ, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. 2010;257(3):359–366. doi: 10.1007/s00415-009-5324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rongve A, Bronnick K, Ballard C, Aarsland D. Core and suggestive symptoms of dementia with lewy bodies cluster in persons with mild dementia. Dement Geriatr Cogn Disord. 2010;29(4):317–324. doi: 10.1159/000295111. [DOI] [PubMed] [Google Scholar]
- 12.Aarsland D, Kurz M, Beyer M, Bronnick K, Piepenstock Nore S, Ballard C. Early discriminatory diagnosis of dementia with Lewy bodies. The emerging role of CSF and imaging biomarkers. Dement Geriatr Cogn Disord. 2008;25(3):195–205. doi: 10.1159/000113417. [DOI] [PubMed] [Google Scholar]
- 13.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 14.Apostolova LG, Dutton RA, Dinov ID, et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63(5):693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- 15.Apostolova LG, Mosconi L, Thompson PM, et al. Subregional hippocampal atrophy predicts Alzheimer's dementia in the cognitively normal. Neurobiol Aging. 2010;31(7):1077–1088. doi: 10.1016/j.neurobiolaging.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Apostolova LG, Thompson PM, Green AE, et al. 3D comparison of low, intermediate, and advanced hippocampal atrophy in MCI. Hum Brain Mapp. 2010;31(5):786–797. doi: 10.1002/hbm.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballmaier M, O'Brien JT, Burton EJ, et al. Comparing gray matter loss profiles between dementia with Lewy bodies and Alzheimer's disease using cortical pattern matching: diagnosis and gender effects. Neuroimage. 2004;23(1):325–335. doi: 10.1016/j.neuroimage.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Beyer MK, Larsen JP, Aarsland D. Gray matter atrophy in Parkinson disease with dementia and dementia with Lewy bodies. Neurology. 2007;69(8):747–754. doi: 10.1212/01.wnl.0000269666.62598.1c. [DOI] [PubMed] [Google Scholar]
- 19.Whitwell JL, Weigand SD, Shiung MM, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer's disease. Brain. 2007;130(Pt 3):708–719. doi: 10.1093/brain/awl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber R, McKeith IG, Ballard C, Gholkar A, O'Brien JT. A comparison of medial and lateral temporal lobe atrophy in dementia with Lewy bodies and Alzheimer's disease: magnetic resonance imaging volumetric study. Dement Geriatr Cogn Disord. 2001;12(3):198–205. doi: 10.1159/000051258. [DOI] [PubMed] [Google Scholar]
- 21.Burton EJ, Barber R, Mukaetova-Ladinska EB, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer's disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain. 2009;132(Pt 1):195–203. doi: 10.1093/brain/awn298. [DOI] [PubMed] [Google Scholar]
- 22.Sabattoli F, Boccardi M, Galluzzi S, Treves A, Thompson PM, Frisoni GB. Hippocampal shape differences in dementia with Lewy bodies. Neuroimage. 2008;41(3):699–705. doi: 10.1016/j.neuroimage.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 23.Firbank MJ, Blamire AM, Teodorczuk A, et al. High resolution imaging of the medial temporal lobe in Alzheimer's disease and dementia with Lewy bodies. J Alzheimers Dis. 2010;21(4):1129–1140. doi: 10.3233/jad-2010-100138. [DOI] [PubMed] [Google Scholar]
- 24.Apostolova LG, Thompson PM. Mapping progressive brain structural changes in early Alzheimer's disease and mild cognitive impairment. Neuropsychologia. 2008;46(6):1597–1612. doi: 10.1016/j.neuropsychologia.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aarsland D, Rongve A, Nore SP, et al. Frequency and case identification of dementia with Lewy bodies using the revised consensus criteria. Dement Geriatr Cogn Disord. 2008;26(5):445–452. doi: 10.1159/000165917. [DOI] [PubMed] [Google Scholar]
- 26.Folstein M, Folstein S, McHugh PR. Clinical predictors of improvement after electroconvulsive therapy of patients with schizophrenia, neurotic reactions, and affective disorders. Biol Psychiatry. 1973;7(2):147–152. [PubMed] [Google Scholar]
- 27.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 28.Kirvell SL, Elliott MS, Kalaria RN, Hortobagyi T, Ballard CG, Francis PT. Vesicular glutamate transporter and cognition in stroke: a case-control autopsy study. Neurology. 2010;75(20):1803–1809. doi: 10.1212/WNL.0b013e3181fd6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hortobagyi T, Troakes C, Nishimura AL, et al. Optineurin inclusions occur in a minority of TDP-43 positive ALS and FTLD-TDP cases and are rarely observed in other neurodegenerative disorders. Acta Neuropathol. 2011;121(4):519–527. doi: 10.1007/s00401-011-0813-3. [DOI] [PubMed] [Google Scholar]
- 30.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72(13):1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- 31.Narr KL, van Erp TG, Cannon TD, et al. A twin study of genetic contributions to hippocampal morphology in schizophrenia. Neurobiol Dis. 2002;11(1):83–95. doi: 10.1006/nbdi.2002.0548. [DOI] [PubMed] [Google Scholar]
- 32.Thompson PM, Hayashi KM, De Zubicaray GI, et al. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22(4):1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 33.Apostolova LG, Dinov ID, Dutton RA, et al. 3D comparison of hippocampal atrophy in amnestic mild cognitive impairment and Alzheimer's disease. Brain. 2006;129(Pt 11):2867–2873. doi: 10.1093/brain/awl274. [DOI] [PubMed] [Google Scholar]
- 34.Mueller SG, Stables L, Du AT, et al. Measurement of hippocampal subfields and age-related changes with high resolution MRI at 4T. Neurobiol Aging. 2007;28(5):719–726. doi: 10.1016/j.neurobiolaging.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mueller SG, Schuff N, Raptentsetsang S, Elman J, Weiner MW. Selective effect of Apo e4 on CA3 and dentate in normal aging and Alzheimer's disease using high resolution MRI at 4 T. Neuroimage. 2008;42(1):42–48. doi: 10.1016/j.neuroimage.2008.04.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lippa CF, Pulaski-Salo D, Dickson DW, Smith TW. Alzheimer's disease, Lewy body disease and aging: a comparative study of the perforant pathway. J Neurol Sci. 1997;147(2):161–166. doi: 10.1016/s0022-510x(96)05321-x. [DOI] [PubMed] [Google Scholar]
- 37.Zarow C, Vinters HV, Ellis WG, et al. Correlates of hippocampal neuron number in Alzheimer's disease and ischemic vascular dementia. Ann Neurol. 2005;57(6):896–903. doi: 10.1002/ana.20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mai JK, Assheuer J, Paxinos G. Atlas of the human brain. 2nd ed. Elsevier Academic Press; Amsterdam ; Boston: 2004. [Google Scholar]
- 39.Duvernoy HM. The human hippocampus : an atlas of applied anatomy. J.F. Bergmann; München: 1988. [Google Scholar]
- 40.West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296(1):1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]