Abstract
Juvenile common carp (Cyprinus carpio) were used as a model to investigate acute toxicity and oxidative stress caused by silver nanoparticles (Ag-NPs). The fish were exposed to different concentrations of Ag-NPs for 48 h and 96 h. After exposure, antioxidant enzyme levels were measured, including glutathione-S-transferase (GST), superoxidase dismutase, and catalase (CAT). Other biochemical parameters and histological abnormalities in different tissues (i.e., the liver, gills, and brain) were also examined. The results showed that Ag-NPs agglomerated in freshwater used during the exposure experiments, with particle size remaining <100 nm. Ag-NPs had no lethal effect on fish after 4 days of exposure. Biochemical analysis showed that enzymatic activities in the brain of the fish exposed to 200 μg/L of Ag-NPs were significantly reduced. Varied antioxidant enzyme activity was recorded in the liver and gills. Varied antioxidant enzyme activity was recorded for CAT in the liver and GST in the gills of the fish. However, the recovery rate of fish exposed to 200 μg/L of Ag-NPs was slower than when lower particle concentrations were used. Other biochemical indices showed no significant difference, except for NH3 and blood urea nitrogen concentrations in fish exposed to 50 μg/L of Ag-NPs. This study provides new evidence about the effects of nanoparticles on aquatic organisms.
1. Introduction
Nanomaterials occur in nature via various geobiophysicochemical processes, such as volcanic eruptions, bacterial metabolism, and other erosion and reduction mechanisms. In recent decades, nanoparticles have been increasingly manufactured and used in daily consumer products, such as textiles, paints, pharmaceuticals, and cosmetic products, as well as in pollution treatment and remediation processes [1, 2]. However, it is widely known that nanoparticles may cause potential adverse effects in organisms, including human and mammals [3–7]. Consequently, the adverse effects of incompletely retrieved nanoparticles pose an increasing threat to the public [7–9]. Therefore, it is important to develop an understanding of the effects and active mechanisms of nanoparticles.
Silver (Ag) is among the most used metals in human life and is well known for its antimicrobial effect. During the recent rapid development of nanotechnology, silver nanoparticles (Ag-NPs) have been used for various types of consumer products, such as cosmetics, heath care products, and textiles. Consequently, information about the toxicity and carcinogenic effects of Ag is available, with it being considered nontoxic and noncarcinogenic when used in appropriate amounts [10]. However, at relatively high concentrations, Ag-NPs cause cellular toxicity and other adverse impacts, such as the inhibition of mitochondrial function in rat liver cells, mouse germline stem cells, and human fibroblasts [11–13]. Furthermore, an increasing number of recent reports have provided evidence of the cytotoxicity of Ag-NPs at doses of low exposure [14, 15]. Ag-NPs also have an adverse impact on aquatic organisms, such as zebrafish and medaka fish, and cause oxidative stress, cellular apoptosis, chromosomal aberrations [16], and other developmental toxicity effects during early life stages and adulthood [17]. Nanoparticles combined with other substances may also threaten organisms in other ways. This is because changes in the functional groups on the nanoparticle surface may facilitate substances to penetrate deep into the cells of the body, causing unexpected impacts on organisms. However, it is unclear whether the effects of coating materials increase the adverse effects of nanomaterials. In this study, we investigated the effects of Ag-NPs in the presence of citric acid as a capping material. Citric acid is an organic chemical commonly found in the natural environment and human body. Therefore, the interaction of citric acid with exposed materials is a common mechanism in the natural environment.
Common carp (Cyprinus carpio) is an abundant species in the freshwater environment. Due to their large size, carp have a better capacity for resistance to pollutants than other laboratory fish, such as zebrafish and Japanese medaka. Hence, carp are not usually used in experiments to examine the fatal effects of exposure to low doses of pollutants. However, because of the dominance of this species in the natural environment, carp is considered as one of the most suitable models to assess the non-fatal effects of pollutants by evaluating fluctuations in antioxidant enzyme levels or other changes in fish histology and physiology.
The objectives of this study were to investigate the adverse effects of Ag-NPs capped with citric acid (Ag-NPs) by measuring antioxidant enzymatic activities, including those of catalase (CAT), superoxide dismutase (SOD), and glutathione-S-transferase (GST). Target organs included the brain, liver, and gills of the common carp (C. carpio). In addition, other biochemical parameters in the blood and histological changes in the skin, liver, and gills of the fish were examined.
2. Materials and Methods
2.1. Chemicals and Reagents
In the current study, we developed on the work of our previous study [18]. For this reason, we used the same biological samples as those used in the previous study to present detailed data about the toxicity of nanoparticles.
All chemicals were purchased from Sigma-Aldrich and were of analytical grade or better. Citrate-capped Ag-NPs were purchased from ABCNanotech (Daejeon, Korea) in a suspension form. The average particle size stipulated by the manufacturer was 15 nm. Particles were dispersed in deionized (DI) water as black colloids. The average pH of the original solution was 6.5, with a particle content of 20% by weight. The dispersion of the particles was confirmed using a transmission electron microscope (TEM; JEOL, Japan) and the dynamic Light scattering method (DLS; ELS-8000, Otsuka, Japan). A stock solution of Ag-NPs was prepared at 10 mg/L. Working solutions (25, 50, 100, and 200 μg/L) were obtained by diluting the stock solution with respective amounts of DI water.
The concentration of elemental Ag was measured using an inductive coupled plasma mass spectrometer (ICP-MS, Perkin Elmer, USA). Mean concentrations were obtained by using the data to plot a standard curve with Ag standard solution (SCP Science, QC, Canada) and 5% HNO3 solution (electronic grade, SCP science, QC, Canada). The ranges of the standard were 0.0, 1.0, 5.0, 10.0, 20.0, 40.0, 60.0, 80.0, and 100.0 μg/L, and the r2 value was 0.9997.
2.2. Carp Breeding and Maintenance
Juvenile carp were obtained from the National Academy of Agricultural Science, Korea. As shown in Table 1, the body weight and length of the fish were 18.6~33.8 g and 10.9~13.5 cm, respectively. The freshwater used to culture the fish was dechlorinated and continuously aerated. Water temperature was kept at 24 ± 1°C, hardness was maintained at 40~60 mg CaCO3/L, pH of the water was 7.2, and oxygen saturation was more than 85%. All the fish were acclimatized for 2 weeks prior to the exposure test.
Table 1.
Measurements of the common carp used in the experiments.
| Ag-NP concentration | Fish length (cm) | Fish weight (g) | Liver (g) | Gill (g) | Brain (g) |
|---|---|---|---|---|---|
| Control | 12.1 | 25.1 | 0.694 | 0.653 | 0.232 |
| 25 μg/L | 12.7 | 28.2 | 0.698 | 0.736 | 0.298 |
| 50 μg/L | 12.1 | 24.4 | 0.631 | 0.573 | 0.285 |
| 100 μg/L | 11.5 | 21.5 | 0.624 | 0.507 | 0.269 |
| 200 μg/L | 12.6 | 25.9 | 0.790 | 0.704 | 0.306 |
| Minimum | 10.9 | 18.6 | 0.386 | 0.492 | 0.219 |
| Maximum | 13.5 | 33.8 | 0.977 | 0.840 | 0.350 |
| Average | 12.2 | 25.0 | 0.687 | 0.635 | 0.278 |
2.3. Acute Exposure Test
Fish were exposed to Ag-NPs for a period of 48 h and 96 h, according to the OECD Test Guideline 203: Fish Acute Toxicity Test [19]. Freshwater conditions were as described in Section 2.2. The carp were exposed to Ag-NP concentrations of 25, 50, 100, and 200 μg/L. A blank control test (without Ag-NPs) was performed at the same time and under the same exposure conditions. All the experiments were conducted in an 80-L glass tank containing 60 L of water, with 10 fish in each exposure tank. The water was not changed during the test.
2.4. Measurement of Enzymatic Activity and Other Biochemical Parameters
After exposure, the gills, liver, and brain of the fish were collected, frozen, and stored separately at −20°C for further treatment. The samples were rinsed with 0.15 mM KCl solution and homogenized on ice with 50 mM phosphate buffer (pH 7.0). The suspension was sonicated in an ultrasonic bath for 30 min and then centrifuged at 10,000 ×g at 4°C for 10 min. The supernatant was then removed and stored at −80°C, prior to analysis.
GST activity was measured using a GST Tag assay kit (Novagen, Germany), in which a sample was combined with 1-chloro-2,4-dinitrobenzene substrate in the reaction buffer. The absorbance of the reaction was monitored at 340 nm by using a UV spectrophotometer (Tecan Infinite F200, Austria).
CAT activity was measured using the Abei method, in which 50 mM H2O2 was used as a substrate [20]. A solution of 50 mM peroxide was prepared in 50 mM potassium phosphate buffer. The decomposition of H2O2 catalyzed by catalase can be followed using UV spectroscopy on the basis of the absorbance of H2O2 at a wavelength of 240 nm. The optimum pH for catalase activity is about 7.0.
SOD activity was measured by using an SOD assay kit (Dojindo Laboratories, Kumamoto, Japan). Total protein concentration was measured using the Bradford method, with bovine serum albumin as the standard protein [21].
Other biochemical parameters were examined in this study, namely, ammonia (NH3), glucose (GLU), total cholesterol (TCHO), alkaline phosphates (ALP), glutamic oxaloacetic transaminase/aspartate aminotransferase (GOT/AST), alanine aminotransferase (GPT/ALT), ν-glutamyltransferase (GGT), albumin (ALB), blood urea nitrogen (BUN), creatinine (CRE), and total bilirubin (TBIL).
2.5. Histopathology
After exposure, the skin, gills, and liver of the carp were dissected and fixed in Bouin's solution for 12 h or overnight. Subsequently, these samples were washed with water and then dehydrated using a graded ethanol series (70%, 75%, 80%, 90%, and 100%). The samples were embedded in paraffin at 62°C and sectioned at a thickness of 5 μm by using a precision microtome (MT-990; RMC Products, USA). The sections were stained using the hematoxylin and eosin (H&E) staining method with Alcian blue and the periodic acid-Schiff (AB-PAS) reaction. An Olympus BX-51 light microscope (Olympus Corp., Japan) coupled with an ARTCAM-150 PIII digital camera was used to examine any abnormalities in the samples.
2.6. Statistical Analysis
Triplicates of all samples were exposed to Ag-NPs for statistical purposes. Data were calculated and analyzed using Excel (Microsoft Corporation, WA, USA) software and plotted using Sigma Plot (SPSS Inc., CA, USA). The differences between the samples and blank controls were evaluated using one-way analysis of variance and the Student's t-test. The difference was considered significant when P < 0.05.
2.7. Data Analysis
RNA extraction and DNA microarray analysis were performed according to the protocol previously described by Lee et al. [18]. Subio platform ver. 1.6 was used for the expression analysis. The sequences of each clone ID were aligned on the basis of sequence homology by using the basic local alignment search tool (BLAST) in the National Center for Biotechnology Information database [22]. The translated nucleotide sequences were compared with the protein databases by using the BLASTX analysis software.
3. Results
3.1. Ag-NPs Properties
The size distribution and morphology of Ag-NPs were examined using TEM and DLS. As shown in Figure 1, Ag-NPs became agglomerated in the solution, and the nominal size measured using TEM was ~12 nm. However, the mean size of the agglomerated particles varied as much as several hundred nanometers. The DLS results showed that the mean diameter of Ag-NPs at 5 mg/L and 10 mg/L was ~70 nm and ~90 nm, respectively (Table 2). However, particles with a diameter of less than 20 nm were frequently observed in the solution. Elemental Ag was measured using ICP-MS, and the results are shown in Table 3, with relative standard deviations ranging around 10%. In general, Ag-NPs were well dispersed in DI water solution, with some agglomeration and aggregation.
Figure 1.
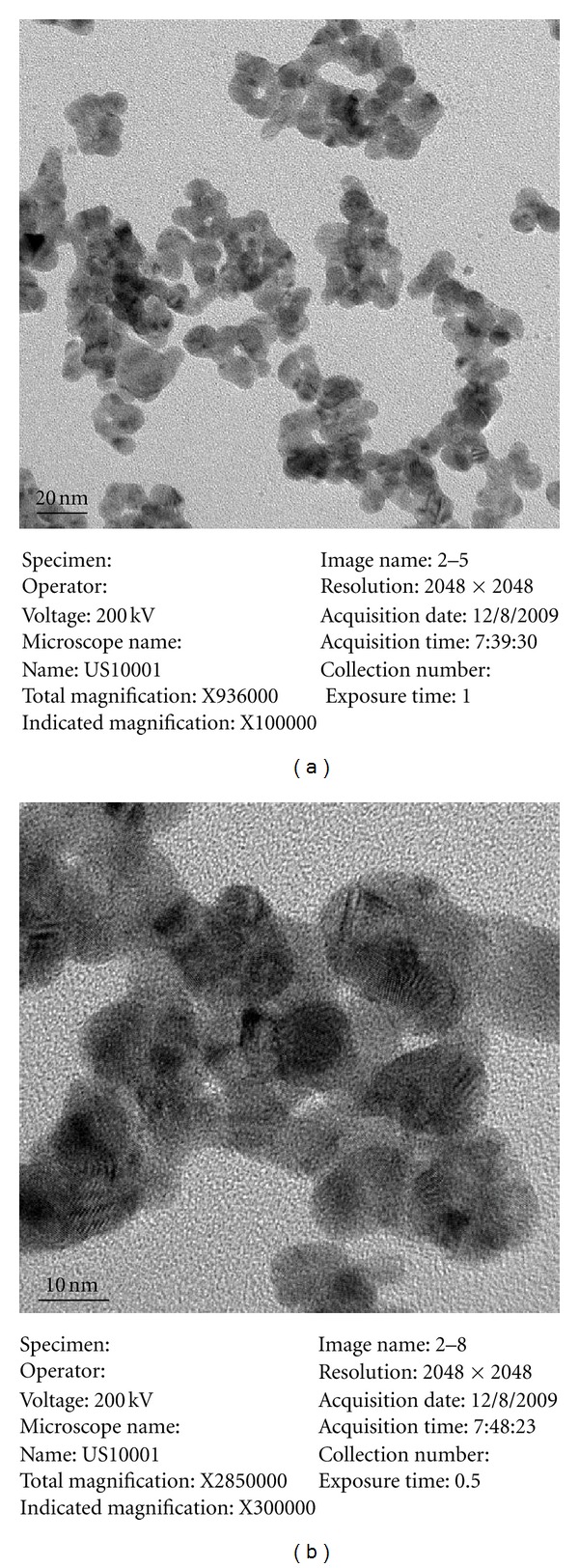
TEM images of Ag-NPs used in the experiments.
Table 2.
Size distribution of Ag-NPs measured using DLS.
| Concentration | Mean diameter (nm) | Coefficient of variation | Standard deviation (nm) | Normal standard deviation | Variance |
|---|---|---|---|---|---|
| Nominal | 12.2 ± 10.5 | — | — | — | — |
| 5 mg/L | 71.7 | 0.767 | 54.966 | 0.767 | 0.876 |
| 10 mg/L | 91.2 | 0.800 | 72.979 | 0.800 | 0.894 |
Table 3.
Concentration of Ag-NPs (μg/L) measured using ICP-MS.
| Nominal concentration | Measured concentration | RSD (%) | Element |
|---|---|---|---|
| 1.56 | 0.87 | 12.62 | Ag |
| 3.13 | 1.33 | 6.52 | Ag |
| 6.25 | 3.09 | 6.26 | Ag |
| 12.5 | 7.70 | 8.94 | Ag |
| 25.0 | 14.65 | 4.18 | Ag |
| 50.0 | 34.94 | 10.57 | Ag |
3.2. Antioxidant Enzymatic Activities
The antioxidant enzymes, including those of GST, CAT, and SOD, were measured, and the results are shown in Figures 2, 3, and 4. Generally, enzymatic activities changed with different Ag-NP concentrations. After 48 h of exposure, GST activities in the liver, gills, and brain were not significantly different than that observed when the fish were exposed to 25 μg/L and 50 μg/L of Ag-NPs (Figure 2(a)). When the concentration of Ag-NPs increased to 100 μg/L and 200 μg/L, GST activity was more significantly reduced in the gills than in the liver and brain. After 96 h of exposure, GST activity was elevated in the liver; this was correlated with an increase in Ag-NP concentration in the media (Figure 2(b)). GST activity in liver samples exposed to 100 μg/L and 200 μg/L was about 190% higher than that observed in the control fish. In comparison, GST activity in the brain and gills declined at 200 μg/L and was significantly lower than that in the control group. GST activity in the liver of fish after 96 h of exposure was also higher than that in fish subjected to 48 h of exposure, especially at 100 μg/L of Ag-NPs. After 96 h of exposure, GST activity was also higher in the liver than in the brain and gills.
Figure 2.
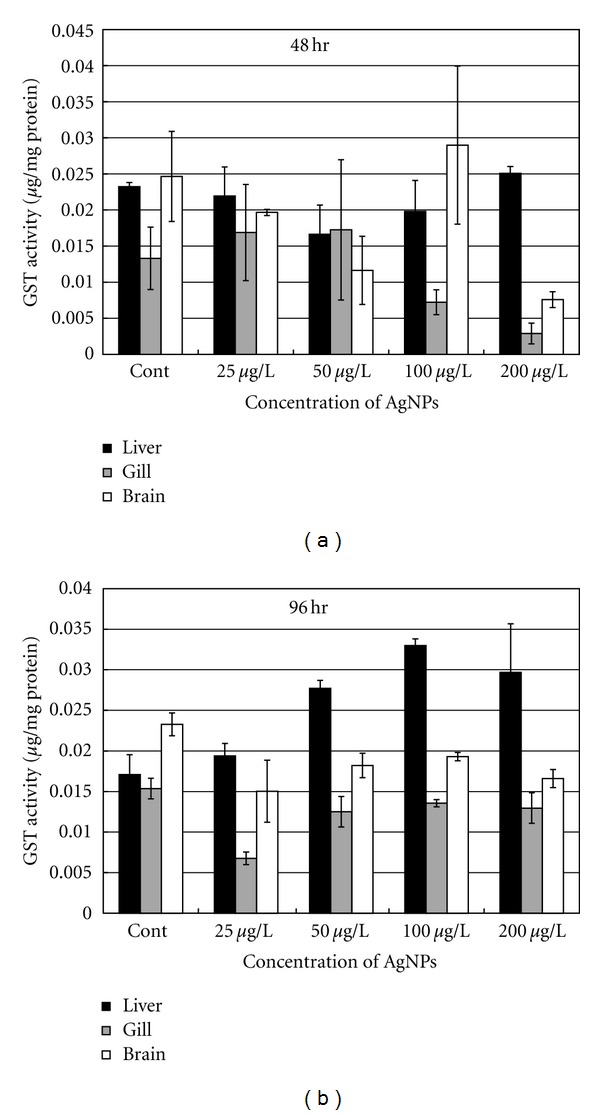
GST activity in different organs of the common carp exposed to various concentrations of Ag-NPs at (a) 48 h and (b) 96 h.
Figure 3.
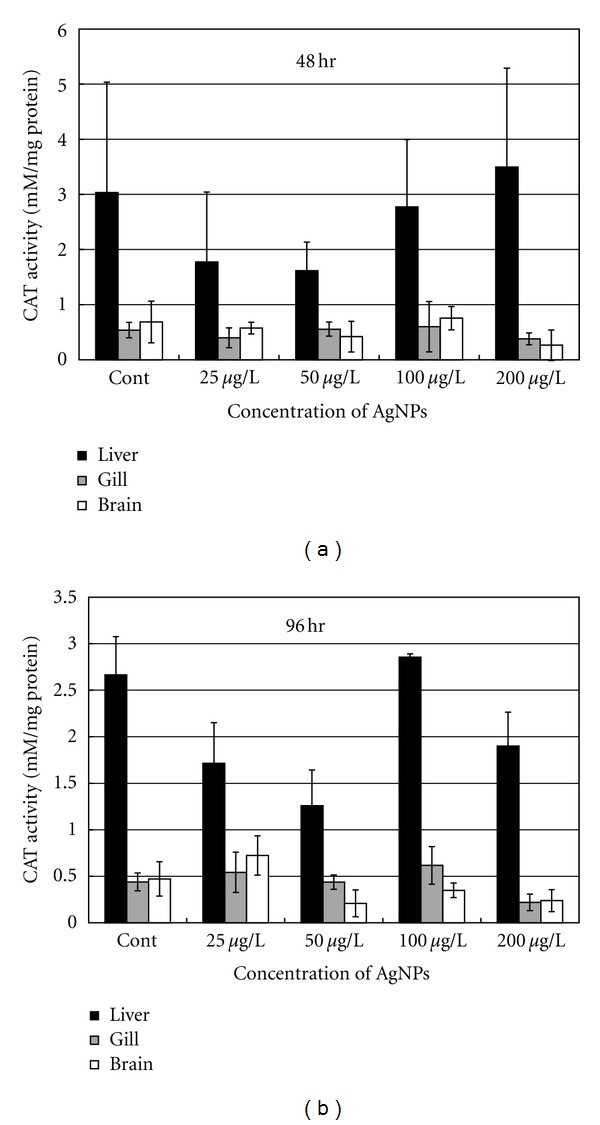
CAT activity in different organs of the common carp exposed to various concentrations of Ag-NPs at (a) 48 h and (b) 96 h.
Figure 4.
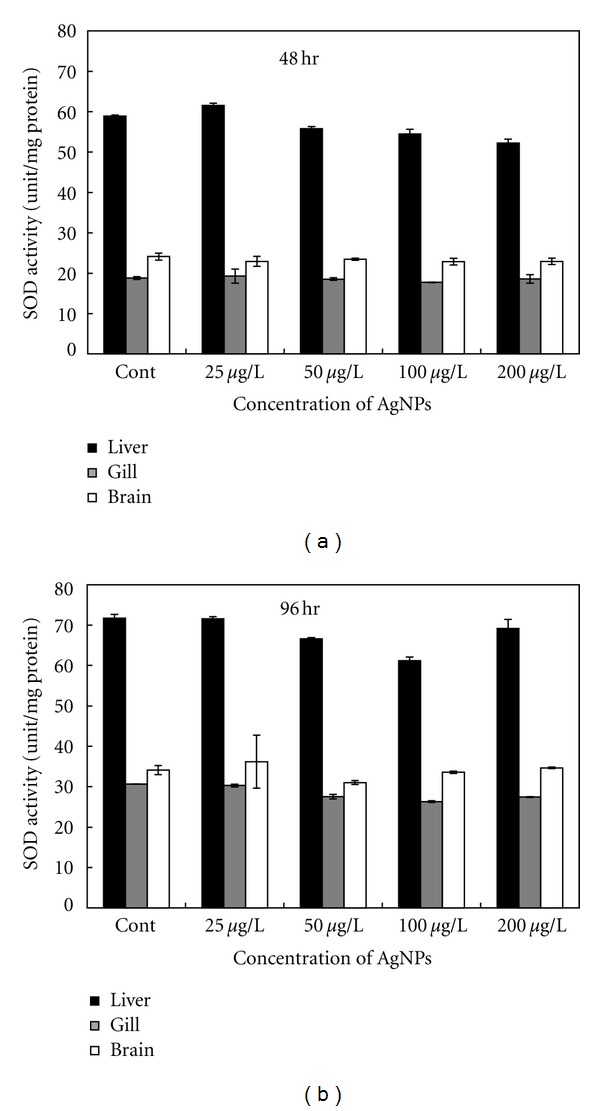
SOD activity in different organs of the common carp exposed to various concentrations of Ag-NPs at (a) 48 h and (b) 96 h.
As shown in Figure 3, higher levels of CAT activity were detected in the liver than in the brain and gills of the experimental fish. Similar to GST activity, CAT activity in the liver changed at different Ag-NP concentrations, but it remained relatively stable in the brain and gills. No significant difference in CAT activity was recorded between the brain and gills. CAT concentrations in the liver of the fish after 48 h of exposure showed no significant difference to that in the control group (Figure 3(a)). However, CAT activity declined at lower Ag-NP concentrations (i.e., 25 μg/L and 50 μg/L) and increased at higher Ag-NP concentrations (i.e., 100 μg/L and 200 μg/L). However, after 96 h of exposure, CAT activity was significantly reduced in the liver at 25 μg/L and 50 μg/L, recovered at 100 μg/L, and then decreased again at 200 μg/L of Ag-NPs (Figure 3(b)).
SOD activity differed from that of GST and CAT in that it was relatively stable in all examined tissues (Figure 4). However, similar to GST and CAT, higher levels of SOD activity were detected in the liver than in the brain and gills. There was no significant difference in SOD levels among the different exposure groups.
3.3. Biochemical Parameters in the Blood
In this study, the biochemical parameters in the blood of the exposed fish were also analyzed to understand the changes in metabolism and fish response to the stressor (i.e., Ag-NPs). Detailed information was published in our previous study [18], where the concentration of biochemical parameters such as ammonia (NH3), glutamic oxaloacetic transaminase (GOT), and total cholesterol (TCHO) declined at the beginning of exposure and gradually recovered with increase in Ag-NP concentration. Other parameters such as alanine aminotransferase (GPT), ν-glutamyl transferase (GGT), albumin (ALB), blood urea nitrogen (BUN), creatinine (CRE), and total bilirubin (TBIL) remained stable or fluctuated around the levels of the control groups, without any significant difference. In comparison, alkaline phosphatase (ALP) levels increased with increase in Ag-NPs concentration. After reaching the highest concentration when exposed to 50 μg/L of Ag-NPs, ALP concentration began to decline when the fish were exposed to 100 μ/L and 200 μg/L of Ag-NPs. However, these biochemical parameters in blood of common carp were not significantly changed.
3.4. Histopathology
Histopathology of the skin, gills, and liver of the fish was observed. The results are shown in Figures 5, 6, and 7. The histological structure of the skin (Figure 5) is composed of an epidermal layer and a dermal layer. The epidermis is the outermost layer of the skin, where the epithelial and secretory cells are distributed. Secretory cells comprise club and mucous cells. When dyed with H&E stain, the vacuolated mucous cells appeared as blank circles or elliptical in shape and were marked with a blue color (color index 313C) as a result of the AB-PAS (pH 2.5) reaction. Vacuolated club cells also appeared elliptical in shape when using the AB-PAS (pH 2.5) reaction.
Figure 5.
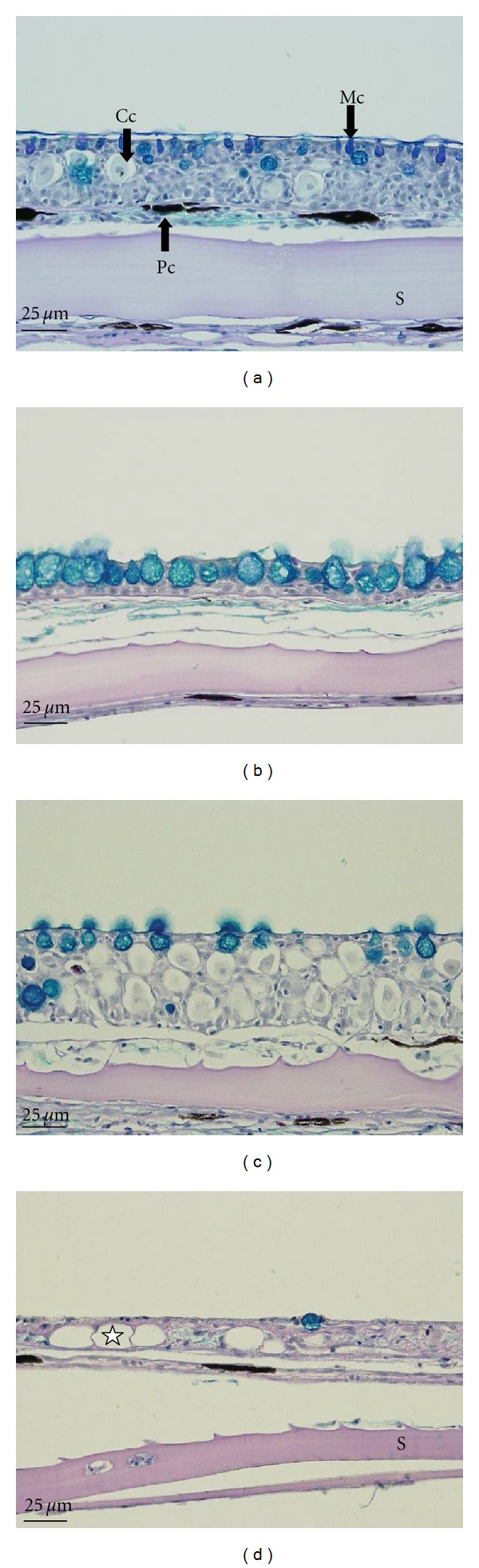
Histological changes in the skin of the common carp (Cyprinus carpio) exposed to Ag-NPs for 96 h. (a) Control. (b) 50 μg/L. Increase in mucous cells shown by staining with Alcian blue. (c) 100 μg/L. Increase in club cells. (d) 200 μg/L. Vacuolation of the epithelium (★). Cc: club cell, Mc: mucous cell, Pc: pigment cell, S: scale. AB-PAS (pH 2.5) reaction.
Figure 6.
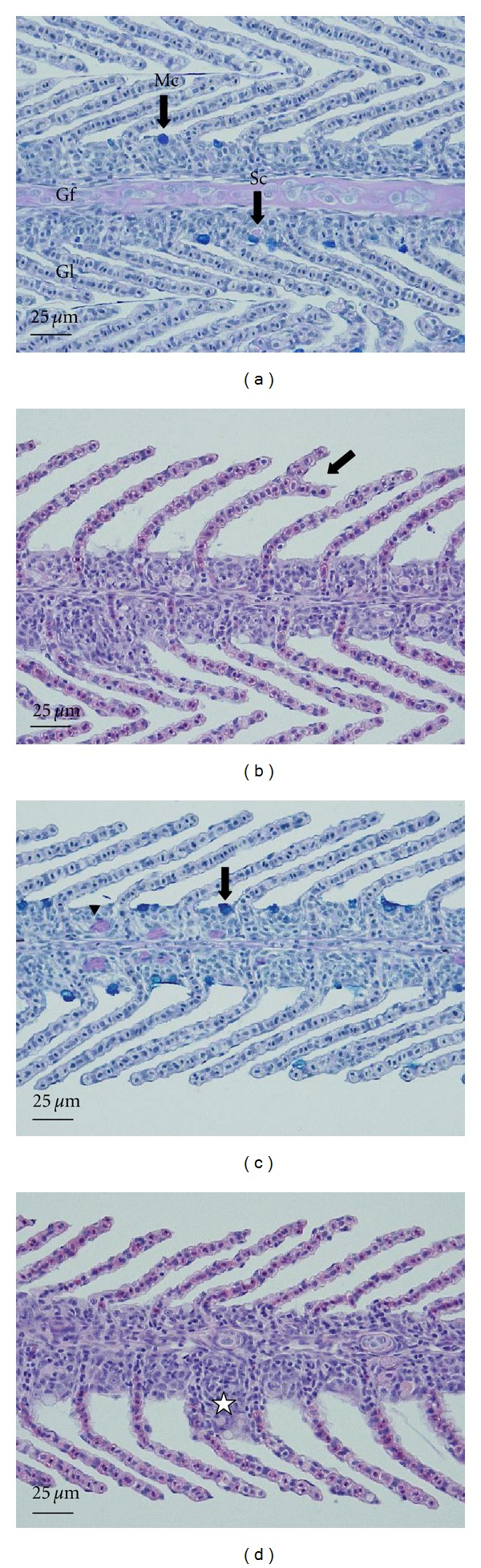
Histological changes in the gill of the common carp (Cyprinus carpio) exposed to Ag-NPs for 96 h. (a) Control. Mc: mucous cell, Gf: gill filament, Gl: gill lamella, Sc: secretion cell. AB-PAS (pH 2.5) reaction. (b) 50 μg/L. Bifurcation of the filament. H&E stain. (c) 100 μg/L. Increase in mucous cells. AB-PAS (pH 2.5) reaction. (d) 200 μg/L. Hyperplasia of the lamellar epithelium (★). H&E stain.
Figure 7.
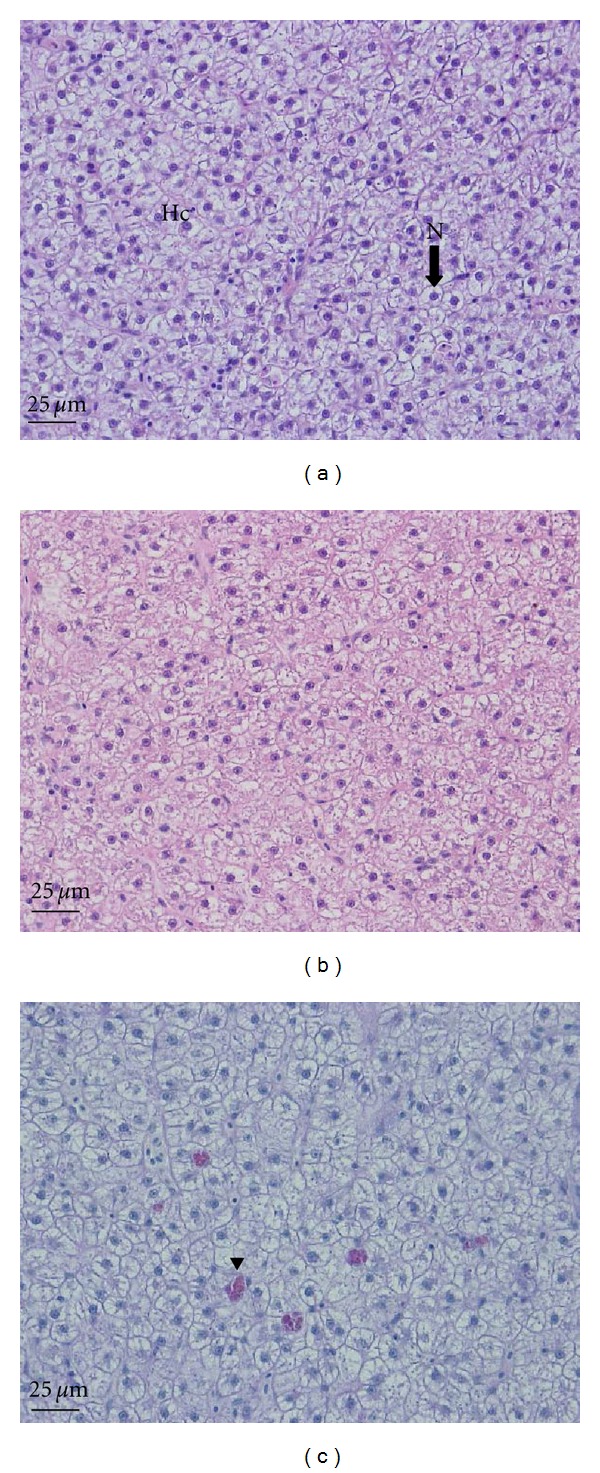
Histological changes in the liver of the common carp (Cyprinus carpio) exposed to Ag-NPs for 96 h. (a) Control. AB-PAS (pH 2.5). (b) 100 μg/L. Atrophy of the hepatocyte nucleus. H&E stain. (c) 200 μg/L. Accumulation of eosinophilic granules (arrow head). AB-PAS (pH 2.5). Hc: hepatocyte, N: nucleus.
As illustrated in Figure 5(a), the skin histology of the control fish showed that normal-sized mucous cells (Mcs) were present, with appropriate amounts of club cells (Ccs) in the epidermal layer. Exposure to 50 μg/L of Ag-NPs (Figure 5(b)) increased the size and number of mucous cells marked with Alcian blue by using the AB-PAS (pH 2.5) positive reaction. On increasing the level of exposure to 100 μg/L of Ag-NPs, the amount of club cells in the epidermal layer significantly increased. The size of the mucous cells was also larger than those of the control fish (Figure 5(c)). At 200 μg/L of Ag-NPs, hyperplasia (★) was frequently recorded in the epidermal layer of the exposed samples (Figure 5(d)).
Carp have one pair of gills on each side of the body, like other teleosts. The gill raker is lined up at the front of the gill arch. As shown in Figure 6(a), numerous gill lamellae are lined up along either side of the gill filament. The gill filament consists of a cartilaginous support, which is a multilayered epithelium. In comparison, the gill lamella is surrounded by simple squamous cells from the outer layer. Pillar cells are present between the capillaries of the lamella. Secretory cells consist of chloride and mucous cells. After staining with H&E, vacuolated mucous cells appeared as blank circles or elliptical in shape but were marked with a blue color (color index 313C) as a result of the AB-PAS (pH 2.5) reaction.
As shown in Figure 6(b), exposure to 50 μg/L of Ag-NPs caused the bifurcation of the filament (black arrow). Figure 6(c) shows an increased number of mucosa cells (black arrow) in the gills of the fish exposed to 100 μg/L of Ag-NPs. The exposure of the fish to 200 μg/L of Ag-NPs caused hyperplasia (★) of the epithelium in the lamella; this finding was not observed in the control group (Figure 6(d)). This phenomenon was also recorded at other concentrations of Ag-NPs, but it was most clearly observed at a concentration of 200 μg/L.
The liver of carp consists of numerous lobules, along with various bile ducts, capillaries, and the pancreas. Hepatocytes from the control group were clearly observed to have a round polygon shape with a nucleus (Figure 7(a)). Exposure to 100 μg/L of Ag-NPs caused extreme atrophy of the hepatocyte nucleus in the liver of the fish (Figure 7(b)). Fish exposed to 200 μg/L of Ag-NPs showed atrophy of the hepatocyte nucleus and an accumulation of eosinophilic granules (color index 2415C, black arrowhead) in the liver (Figure 7(c)).
3.5. TEM Analysis of Tissues
As shown in Figure 8, Ag-NPs penetrated the skin of common carp after 96 h of exposure, regardless of particle concentrations. Ag-NPs were present in every layer of the skin, including the epithelium (Figure 8(b)), epidermis (Figure 8(a)), and mitochondrion (Figure 8(c)). Ag-NPs penetration occurred with single and agglomerated particles. The particles stayed either in the junctions of the cells, on the cell membrane, or inside the cell. Penetration by a large number of Ag-NPs in the skin would result in damaging the cell junction, membrane, or other cell structures, depending on the size and concentration of the particles. The penetration of large agglomerations of particles into the skin (as shown in Figure 8(b)) may destroy the structure and connection between the skin layers, while small amounts of single particles (as shown in Figure 8(c)) may stick on the membrane of cells, causing them to malfunction.
Figure 8.
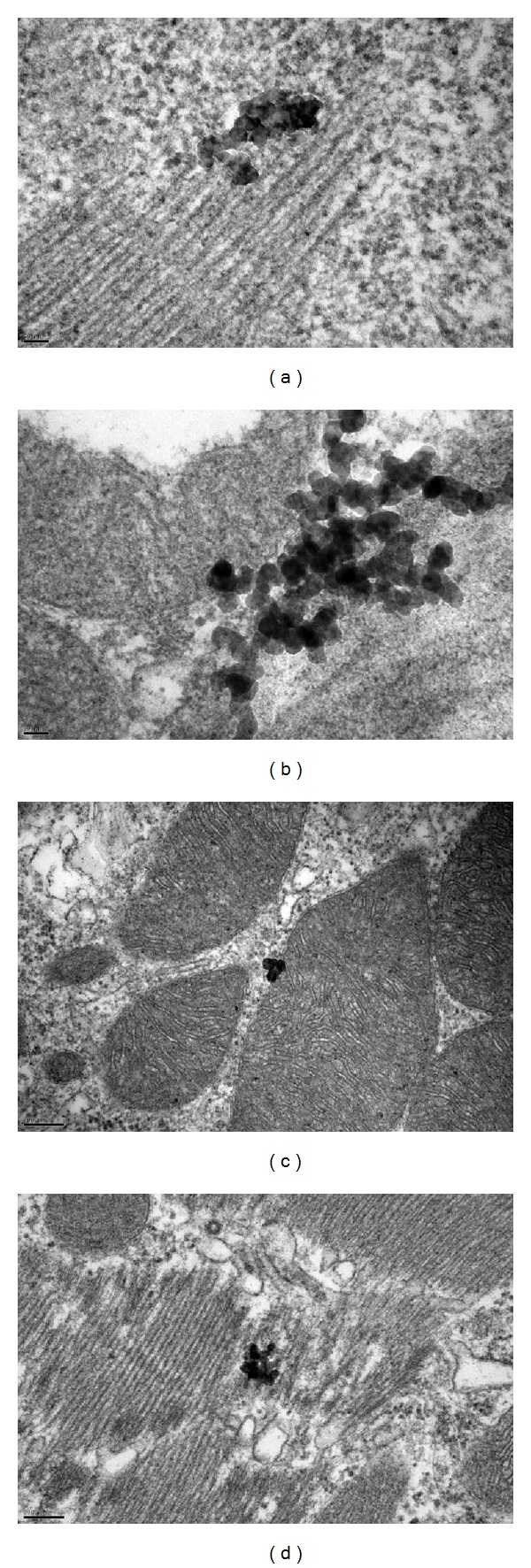
TEM image of the skin of the common carp (Cyprinus carpio) exposed to Ag-NPs for 96 h. (a) 25 μg/L. Epidermis. (b) 50 μg/L. Epithelium. (c) 100 μg/L. Mitochondrion in epithelium. (d) 200 μg/L. Epithelium.
In this study, the penetration of Ag-NPs in the gills of common carp after exposure was evaluated. Figure 9 shows the presence of nanoparticles in the gill structures, including the gill lamella, the nucleus of gill cells, and blood corpuscles. If the skin is the first defensive structure of the fish body, the gill is an important organ that allows gaseous transfer and filtration of foreign materials. Ag-NPs were detected in every part of the gill at varying levels of penetration, such as between blood corpuscles and inside cell nuclei. The effects of nanoparticles on cell function might be more severe because of deep penetration. The presence of nanoparticles in different parts of the gills indicated that common carp is significantly affected by exposure to Ag-NPs. The effects may be directly expressed by damage to the tissue structure or indirectly expressed through abnormal levels of other biochemical markers.
Figure 9.

TEM image of the gill of the common carp (Cyprinus carpio) exposed to Ag-NPs for 96 h. (a) 25 μg/L. Gill lamella. (b) 50 μg/L. Nucleus. (c) 100 μg/L. Blood corpuscle. (d) 100 μg/L. Nucleus.
In this study, we also investigated the penetration of Ag-NPs into other important organs of the fish, including the brain and liver. The results are shown in Figure 10, indicating that particles entered the brain and liver of common carp exposed to 200 μg/L of Ag-NPs for 96 h. As shown in Figure 10, the particles were able to penetrate deep into the brain cells. This result indicated that the particles were carried through the body of the fish by the circulation of blood, subsequently accumulating in different important organs. Liver is an important organ of the body that facilitates the detoxification of substances, whereas the brain controls the activity of the whole body. Damage to these organs may result in the malfunction of fish activities and, more seriously, may cause fish mortality. The findings of this study are comparable to those of a previous report by Park et al. [23, 24], who observed that titanium dioxide nanoparticles caused chronic inflammatory disease and oxidative stress by intratracheal instillation in mice.
Figure 10.
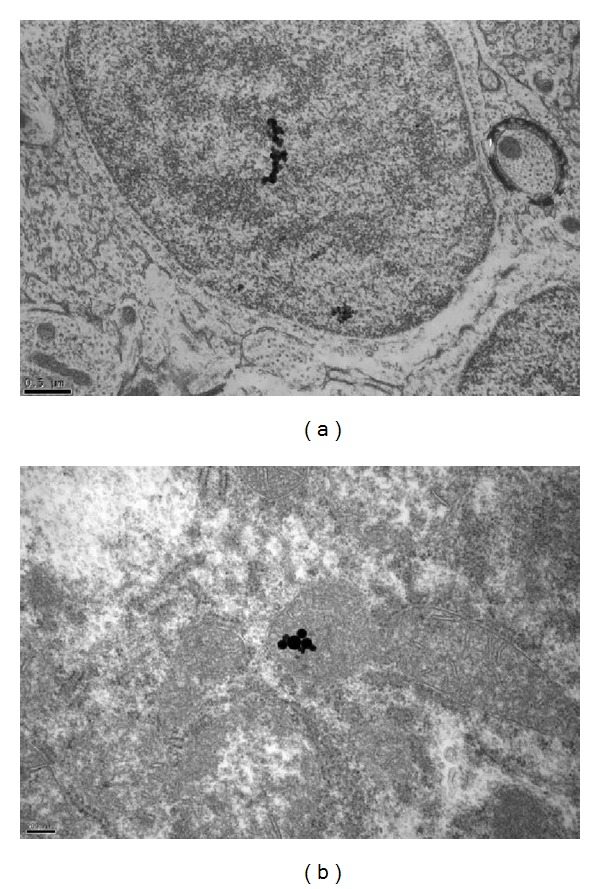
TEM image of the brain (a) and liver (b) of the common carp (Cyprinus carpio) exposed to 200 μg/L of Ag-NPs for 96 h.
3.6. Gene Expression Profiling of Common Carp Exposed to Ag-NPs
Alteration of the level of genomic expression towards 100 μg/L of Ag-NPs exposure for 96 h in the liver of the common carp was analyzed using the DNA microarray method. In response to Ag-NP exposure, there was a 2-fold upregulation and downregulation of 502 genes and 1,850 genes, respectively. Differentially expressed genes (>2-fold, P < 0.05) with defined functions are summarized in Table 4. Among the upregulated genes, 12 genes, including that for the myc associate protein X (MAX), were shown to be involved in cell apoptosis, proliferation, protein synthesis, and energy production (Table 4). Generally, MAX has been known to participate in cell death and cell proliferation, with the interaction of myc [25]. Among the downregulated genes, 11 genes, including that for glutathione peroxidase 4a (GPx), GST, and the retinol binding protein (RBP), were shown to play a role in oxidative stress response, cellular defense, cell migration, detoxification, and fibrinolysis (Table 4). Both GPx and GST have protective effects against oxidative stress (caused by the reactive oxygen species ROS), while RBP has a major function in retinol metabolism [26, 27]. Our findings indicate that Ag-NPs might cause biological malfunctions or dysregulation in cellular processes, including cell death, proliferation, and resistance to oxidative stress. Ag-NPs also contribute towards changing the gene expression of particular sets of genes, which might be considered as plausible biomarkers of Ag-NPs toxicity.
Table 4.
Genes were altered 2-fold in response to Ag-NPs in the common carp, which were modified from our previous study [18] (P < 0.05).
| GenBank accession no. |
Fold change | P Value | Description | Species | E value |
|---|---|---|---|---|---|
| Upregulated | |||||
| ADO27831.1 | 2.93 | 0.00 | NADH dehydrogenase 1 subunit c2 | Ictalurus furcatus | 7.00E−62 |
| ACI66030.1 | 2.15 | 0.00 | ATP synthase subunit O, mitochondrial precursor | Salmo salar | 4.00E−77 |
| BAA99395.1 | 2.03 | 0.00 | myc associate protein X | Cyprinus carpio | 3.00E−87 |
| BAE78447.1 | 2.32 | 0.01 | Cytochrome b | Cyprinus carpio | 5.00E−77 |
| CAB60196.1 | 3.77 | 0.00 | Putative natural resistance-associated macrophage protein | Cyprinus carpio | 0 |
| AAR10978.1 | 2.78 | 0.00 | Mitochondrial uncoupling protein 2 | Squalius cephalus | 0 |
| AAD24542.1 | 4.65 | 0.00 | MHC class II-associated invariant chain | Danio rerio | 3.00E−104 |
| CAA62498.1 | 2.02 | 0.00 | MHC class I protein | Cyprinus carpio | 0 |
| BAA32797.1 | 2.64 | 0.00 | CXCR4 | Cyprinus carpio | 0 |
| CAM56519.1 | 2.96 | 0.00 | Ribosomal protein S9 | Danio rerio | 2.00E−88 |
| NP_001018426.1 | 2.32 | 0.00 | 60S ribosome subunit biogenesis protein NIP7 homolog | Danio rerio | 1.00E−93 |
| AEM37694.1 | 2.20 | 0.00 | Ribosomal protein L3 | Epinephelus bruneus | 1.00E−24 |
| Downregulated | |||||
| ACR33821.1 | 0.49 | 0.00 | Glutathione peroxidase 4a | Cyprinus carpio | 4.00E−116 |
| ABF57552.1 | 0.25 | 0.00 | Pi-class glutathione-S-transferase | Cyprinus carpio | 4.00E−149 |
| ACN11531.1 | 0.49 | 0.01 | Cytochrome oxidase subunit II | Cyprinus carpio | 5.00E−123 |
| BAB88920.1 | 0.43 | 0.00 | Complement control protein factor I-A | Cyprinus carpio | 0 |
| ACA64701.1 | 0.31 | 0.00 | Liver-basic fatty acid-binding protein b | Cyprinus carpio | 7.00E−84 |
| BAA36700.1 | 0.16 | 0.00 | Serum amyloid A protein | Cyprinus carpio | 2.00E−46 |
| CAC34942.1 | 0.27 | 0.00 | Apolipoprotein A-I | Cyprinus carpio | 9.00E−114 |
| CAC12738.1 | 0.42 | 0.01 | Retinol-binding protein | Cyprinus carpio | 4.00E−160 |
| BAA36618.1 | 0.45 | 0.00 | Complement C3-H1 | Cyprinus carpio | 0 |
| AEM37715.1 | 0.28 | 0.01 | Plasminogen | Epinephelus bruneus | 4.00E−16 |
| ACD99642.1 | 0.27 | 0.01 | Transferrin variant G | Cyprinus carpio | 1.00E−151 |
4. Discussion
Because of its strong antibacterial activity, Ag is regularly used in preservative and hygiene products. Ag-NPs, categorized at a nanoscale, are expected to possess many physical and chemical properties that are different from those of their bulk particles. However, the novel properties of these particles require study, particularly because of their potential effects when exposed to organisms, including human beings. One of the novel properties of nanoparticles in general (Ag-NPs in particular) is their minute diameter, in comparison with that of their bulky counterparts [28, 29]. Because of the ultrafine particle-size, Ag-NPs may penetrate deep into the organs and, therefore, alter normal metabolic and bodily functions. Fortunately, naturally occurring nanoparticles usually form aggregations, agglomerations, or complexes with other substances, such as natural organic materials. These complexes and/or aggregations/agglomerations reduce the size advantage of nanoparticles, thus reducing their toxicity or other adverse effects on aquatic organisms. To evaluate the impact of other materials on the toxicity of nanoparticles, synthesized particles with coated materials have been used in various experiments. Stabilizers of nanoparticles include ligands, surfactants, and polymers with different functional groups, such as –COOH and –NH2. Several studies on the toxicity of stabilized Ag-NPs have been performed [17]. However, the toxicity and adverse effects of Ag-NPs capped with natural organic substances remain unclear.
In this study, we used citric acid as a capping agent to stabilize Ag-NPs in solution. As shown in Table 1, this stabilizer keeps the particles well separated, with a nominal size of 10~20 nm. In addition, citric acid is a common organic substance in the natural environment and our daily life (e.g., fruit juice). Hence, developing an understanding of the mechanisms and effects of citrate-Ag-NPs would contribute towards evaluating the fate and impacts of Ag-NPs in environmental matrixes.
The antioxidant enzyme system is responsible for the elimination of oxidative stress during the early stage of the body's defensive mechanism. This system comprises several enzyme classes, including GST, CAT, and SOD. Abnormal changes in enzymes reflect the level of oxidative stress encountered by the body. In the current study, higher levels of GST, CAT, and SOD activities were detected in the liver compared to the brain and gills. This is because the liver is the major detoxification organ of the body. In this study, after 48 h of exposure, GST and CAT activities in the liver were observed to vary, but they generally declined at lower Ag-NP concentrations and recovered at higher Ag-NP concentrations. GST is known for its catalysis function in the reduction process of glutathione (GSH), in which endogenous and xenobiotic chemicals are detoxified [30]. The reduction of GST occurs as a result of the overutilization of existing enzymes to overcome oxidative stress caused by Ag-NPs, which causes an increase in GSH concentration in specific organs [31]. In this study, the carp showed a significant reduction in GST activity in the gills after 48 h of exposure to 200 μg/L of Ag-NPs, but recovered after 96 h of exposure (Figure 2).
Oxidative stress increased with a reduction in GST concentration; this may cause the development of toxic effects or even carcinogenic effects. However, higher Ag-NP concentrations may trigger the production of enzymes to counteract the severe effects of the particles. Hence, an increase in GST concentration may be used as an indicator for the depletion of GSH in detoxification processes. However, the correlation between GSH and GST requires further study.
The main function of CAT is to catalyze the decomposition of H2O2 [32]. In this study, the activity of CAT detected in the liver was significantly higher than that in the brain and gills (Figure 3). CAT concentration in the liver also declined when the fish were exposed to lower Ag-NP concentrations and then recovered with an increase in Ag-NP concentrations. However, after 96 h of exposure at 200 μg/L of Ag-NPs, CAT levels in all the examined organs (i.e., brain, liver, and gills) were significantly lower than those in the control group, as well as for fish exposed to 100 μg/L of Ag-NPs (Figure 3(b)). This phenomenon was not observed during the 48 h test under the same concentration. These results indicate that the impact of Ag-NPs on the fish was more severe under longer periods of exposure. The results of CAT and GST activities also indicated that when the fish were exposed to short periods (48 h) of Ag-NPs at a concentration of <100 μg/L, oxidative stress mainly occurred in the liver.
SOD is an enzyme that primarily catalyzes the dismutation of superoxide radicals. Thus, the depletion of this enzyme may indicate that the antioxidant defense system is overwhelmed [33]. In the present study, SOD activity in the brain and gills was not significantly different between the control and exposed fish. However, SOD activity was significantly lower in the liver of fish exposed to 200 μg/L of Ag-NPs compared to the liver of fish in the control group (Figure 4(a)). It has been suggested that the antioxidant defense system of the liver is severely affected at this concentration, as illustrated by the reduction of other enzymes (i.e., CAT and GST) in the liver of fish exposed to lower Ag-NPs concentrations. However, after 96 h of exposure, SOD activity returned to the control level (Figure 4(b)). Hence, we hypothesize that GST and CAT were produced in sufficient quantities to counteract the oxidative stress caused by Ag-NPs. However, the correlation and combined effects of GST, CAT, and SOD, as well as other antioxidant enzymes, require further study.
As shown in the results of the biochemical analysis, the concentrations of NH3 and GOT declined and recovered, similar to that observed for other antioxidant enzymes (e.g., CAT and GST). It is known that NH3 in the blood is associated with hepatic detoxification (i.e., the urea cycle). Therefore, fluctuations in blood NH3 concentrations may be related to the antioxidant defense system. Our previous study showed that BUN concentration also decreased at 200 μg/L of Ag-NPs; hence, changes in the enzyme system may alter the capability of urine transformation. However, other biochemical parameters were not significantly altered. Along with slight fluctuations in antioxidant enzymes, this result indicates that <200 μg/L of Ag-NPs exposure did not have a severe effect on the fish.
The histological analysis of the tissues also showed that Ag-NPs have potential adverse effects on exposed fish. As shown in Figure 5, the histopathology of the skin samples showed an increase in the number and size of mucous and club cells, as well as vacuolation of the epithelium. Mucous and club cells located in the epidermal layer of the skin are responsible for the excretion of waste, respiration, ionic and osmotic regulation, disease resistance, communication, and other protection functions [34]. An increase in the number and size of these cells may be a necessary response of the body to counteract the effects of exogenous chemicals, such as nanoparticles. However, if nanoparticle concentrations exceed the resistance level of the skin and excretory systems, they may have a lethal effect on cells. For example, when the fish were exposed to 200 μg/L of Ag-NPs (as shown in Figure 5(d)), vacuolation of the epidermal epithelium was observed. Malfunctioning or injured skin may also cause nanoparticles to have a fatal effect on the fish. Therefore, in this study, stresses caused by the exposure of common carp to Ag-NPs may have potentially toxic effects.
In this study, fish exposed to Ag-NPs were not subjected to any lethal effects. However, chronic exposure to Ag-NPs may cause other types of damage that kill fish. One impact of Ag-NPs is the inhibition of the oxygen/carbon dioxide exchange process because of injury to the respiratory organ (gills). As shown in Figure 6, the exposure of fish to Ag-NPs initially caused the bifurcation of the filament (Figure 6(b)), an increase in the number and size of mucous cells (Figure 6(c)), and hyperplasia of the lamellar epithelium (Figure 6(d)). Similar to the effects on the skin, these slight changes to the gills did have a severe effect on the fish, but it could result in potentially fatal effects if the carp were exposed to higher concentrations of Ag-NPs over an extended period. The adverse effects observed in the liver of the exposed carp (Figure 7), such as atrophy of the hepatocyte nucleus and eosinophilic granule formation, may therefore be used as indicators.
We used genomic analysis to evaluate changes in gene expression towards Ag-NP exposure in the liver of common carp, and we subsequently selected several biomarkers of Ag-NP toxicity. In our microarray data, the MAX gene was upregulated in response to Ag-NP treatment. MAX is a transcription factor that belongs to the myc protein family [35]. Repression of MAX has a lethal effect on the early embryonic blastocyst [36]. However, the upregulation of MAX blocks apoptosis in endothelial cells [37] and is involved in the maintenance of healthy morphology in the retinal ganglion cells of rats [38], indicating the presence of protective cellular functions. In addition, this observation might indicate that programmed cell death and cell morphology are mediated and orientated by MAX, leading to cellular defense against Ag-NP toxicity.
In our microarray data, the RBP gene was downregulated on Ag-NP exposure. Generally, RBP has been reported to solubilize transport proteins and protect their ligands. This protein regulates the disposition, metabolism, and activity of retinoids [27]. Repression of RBP in the epidermis might cause a reduction in the amount of retinol in keratinocytes, indicating cornification of the epidermis [39]. Another study documented that the underexpression of RBP is associated with inhibiting differentiation and the formation of large tumors in hepatocellular carcinoma [40]. These observations might indicate that Ag-NPs cause abnormal retinoid metabolism, resulting in tumorigenesis.
In our microarray data, GST was downregulated after 96 h Ag-NP treatment. GST has been reported to play a critical role in the detoxification of various types of toxicants (such as carcinogens) and has a protective effect on ROS-mediated DNA damage [41–45]. A recent assessment indicated that GST activity might be useful towards evaluating oxidative stress [46, 47]. Hence, Ag-NPs might induce the accumulation of intracellular oxidative stress, giving rise to abnormal biological functions of responsive components, including GST. Gene expression level of GST obtained from microarray analysis was found to be downregulated while the GST activity was induced in presence of Ag-NPs, particularly after 96 h exposure to 100 μg/L in common carp liver tissue. Possible explanation is that the inhibitory gene expression at mRNA level manifests at initial stage due to the Ag-NPs toxic effect whereas the induction of GST activity would play an important role as defensive mechanism in response to the Ag-NPs-induced oxidative stress at later stage.
At present, while the functions of many common carp genes remain under investigation, the inferred functions of unknown genes might be used as putative bioindicators of Ag-NP toxicity. The results of the current study indicated that MAX, GST, and RBP might be viable candidates as Ag-NP biomarkers. However, detailed study of the toxicity-mediated molecular mechanisms of Ag-NPs and further functional analysis of common carp genes are necessary to elucidate cellular responses to Ag-NPs and the detoxification processes.
5. Conclusion
Citrate-capped Ag nanoparticles dispersed well in freshwater, with an estimated average particle size of 7–21 nm (TEM) and 72–91 nm (DLS analysis). Despite the formation of aggregations/agglomerations in the solution, nanosized particles of citrate-Ag-NPs were dominant. Among the examined tissues, liver was the most susceptible to changes in particle concentration. The antioxidant enzyme system was mostly active in the liver. According to the analysis of the antioxidant enzyme system and other biochemical parameters, liver was the most severely affected organ when the carp were exposed to Ag-NPs. The activities of antioxidant enzymes, such as GST and CAT, fluctuated with different Ag-NP concentrations, whereas SOD activity remained stable. The histopathology showed the following: (1) in the skin: an increase in the number and size of club cells and mucous cells and vacuolation of the epithelium; (2) in the gill: bifurcation of the filament, an increase in the number of mucous cells, and hyperplasia of the lamellar epithelium; (3) in the liver: atrophy of the hepatocyte nucleus and accumulation of eosinophilic granules. The adverse effects recorded in the current study were not severe enough to cause carp mortality, but they might potentially lead to lethal effects if the fish were exposed to higher concentrations of Ag-NPs over a longer period.
Acknowledgments
The authors would like to thank Seok-Won Jeong, Kyung Hee University, for assistance with microarray analysis and paper preparation.
References
- 1.Masciangioli T, Zhang W-X. Environmental technologies at the nanoscale. Environmental Science and Technology. 2003;37(5):102A–108A. doi: 10.1021/es0323998. [DOI] [PubMed] [Google Scholar]
- 2.Nohynek GJ, Lademann J, Ribaud C, Roberts MS. Grey Goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Critical Reviews in Toxicology. 2007;37(3):251–277. doi: 10.1080/10408440601177780. [DOI] [PubMed] [Google Scholar]
- 3.Oberdörster G, Maynard A, Donaldson K, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Particle and Fibre Toxicology. 2005;2, article 8 doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duong CN, Schlenk D, Chang NI, Kim SD. The effect of particle size on the bioavailability of estrogenic chemicals from sediments. Chemosphere. 2009;76(3):395–401. doi: 10.1016/j.chemosphere.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 5.HAO L, WANG Z, XING B. Effect of sub-acute exposure to TiO2 nanoparticles on oxidative stress and histopathological changes in Juvenile Carp (Cyprinus carpio) Journal of Environmental Sciences. 2009;21(10):1459–1466. doi: 10.1016/s1001-0742(08)62440-7. [DOI] [PubMed] [Google Scholar]
- 6.Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environmental Health Perspectives. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore MN. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environment International. 2006;32(8):967–976. doi: 10.1016/j.envint.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311(5761):622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 9.Nowack B, Bucheli TD. Occurrence, behavior and effects of nanoparticles in the environment. Environmental Pollution. 2007;150(1):5–22. doi: 10.1016/j.envpol.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 10. EPA, U. S. Integrated Risk Information System (IRIS), http://www.epa.gov/IRIS/subst/0099.htm.
- 11.Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicology in Vitro. 2005;19(7):975–983. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 12.Braydich-Stolle L, Hussain S, Schlager JJ, Hofmann MC. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicological Sciences. 2005;88(2):412–419. doi: 10.1093/toxsci/kfi256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen HC, Lin YN, Jian SR, et al. Observation of growth of human fibroblasts on silver nanoparticles. Journal of Physics. 2007;61(1):445–449. [Google Scholar]
- 14.Kawata K, Osawa M, Okabe S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells. Environmental Science and Technology. 2009;43(15):6046–6051. doi: 10.1021/es900754q. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Choi JE, Choi J, et al. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicology in Vitro. 2009;23(6):1076–1084. doi: 10.1016/j.tiv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Wise JP, Goodale BC, Wise SS, et al. Silver nanospheres are cytotoxic and genotoxic to fish cells. Aquatic Toxicology. 2010;97(1):34–41. doi: 10.1016/j.aquatox.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y, Zhou Q, Li H, Liu W, Wang T, Jiang G. Effects of silver nanoparticles on the development and histopathology biomarkers of Japanese medaka (Oryzias latipes) using the partial-life test. Aquatic Toxicology. 2010;100(2):160–167. doi: 10.1016/j.aquatox.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Lee B, Kim K, Jeong S, et al. Gene expression profiling of silver nanoparticles-exprosed Cyprinus carpio using DNA microarray. Cancer Prevention Research. 2011;16(2):147–154. [Google Scholar]
- 19. OECD Test No. 203: fish, Acute toxicity test; Organisation for Economic Co-operation and Development: Paris, France, 1992.
- 20.Abei H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York, NY, USA: Academic Press; 1974. pp. 673–684. [Google Scholar]
- 21.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 22.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 23.Park EJ, Yi J, Chung KH, Ryu DY, Choi J, Park K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicology Letters. 2008;180(3):222–229. doi: 10.1016/j.toxlet.2008.06.869. [DOI] [PubMed] [Google Scholar]
- 24.Park EJ, Yoon J, Choi K, Yi J, Park K. Induction of chronic inflammation in mice treated with titanium dioxide nanoparticles by intratracheal instillation. Toxicology. 2009;260(1–3):37–46. doi: 10.1016/j.tox.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Skouteris GG, Schröder CH. c-myc and max interactions in quiescent and mitogen-stimulated primary hepatocytes. Experimental Cell Research. 1996;225(2):237–244. doi: 10.1006/excr.1996.0173. [DOI] [PubMed] [Google Scholar]
- 26.Ketterer B, Meyer DJ. Glutathione transferases: a possible role in the detoxication and repair of DNA and lipid hydroperoxides. Mutation Research. 1989;214(1):33–40. doi: 10.1016/0027-5107(89)90195-4. [DOI] [PubMed] [Google Scholar]
- 27.Noy N. Retinoid-binding proteins: mediators of retinoid action. Biochemical Journal. 2000;348(3):481–495. [PMC free article] [PubMed] [Google Scholar]
- 28.Colvin VL. The potential environmental impact of engineered nanomaterials. Nature Biotechnology. 2003;21(10):1166–1170. doi: 10.1038/nbt875. [DOI] [PubMed] [Google Scholar]
- 29.Warheit DB. How meaningful are the results of nanotoxicity studies in the absence of adequate material characterization? Toxicological Sciences. 2008;101(2):183–185. doi: 10.1093/toxsci/kfm279. [DOI] [PubMed] [Google Scholar]
- 30.Douglas KT. Mechanism of action of glutathione-dependent enzymes. Advances in enzymology and related areas of molecular biology. 1987;59:103–167. doi: 10.1002/9780470123058.ch3. [DOI] [PubMed] [Google Scholar]
- 31.Federici G, Shaw BJ, Handy RD. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): gill injury, oxidative stress, and other physiological effects. Aquatic Toxicology. 2007;84(4):415–430. doi: 10.1016/j.aquatox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cellular and Molecular Life Sciences. 2004;61(2):192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van der Oost R, Beyer J, Vermeulen NPE. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental Toxicology and Pharmacology. 2003;13(2):57–149. doi: 10.1016/s1382-6689(02)00126-6. [DOI] [PubMed] [Google Scholar]
- 34.Shephard KL. Functions for fish mucus. Reviews in Fish Biology and Fisheries. 1994;4(4):401–429. [Google Scholar]
- 35.Blackwood EM, Eisenman RN. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991;251(4998):1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- 36.Shen-Li H, O’Hagan RC, Hou H, Horner JW, Lee HW, DePinho RA. Essential role for Max in early embryonic growth and development. Genes and Development. 2000;14(1):17–22. [PMC free article] [PubMed] [Google Scholar]
- 37.Shichiri M, Kato H, Doi M, Marumo F, Hirata Y. Induction of Max by adrenomedullin and calcitonin gene-related peptide antagonizes endothelial apoptosis. Molecular Endocrinology. 1999;13(8):1353–1363. doi: 10.1210/mend.13.8.0324. [DOI] [PubMed] [Google Scholar]
- 38.Petrs-Silva H, Chiodo V, Chiarini LB, Hauswirth WW, Linden R. Modulation of the expression of the transcription factor Max in rat retinal ganglion cells by a recombinant adeno-associated viral vector. Brazilian Journal of Medical and Biological Research. 2005;38(3):375–379. doi: 10.1590/s0100-879x2005000300008. [DOI] [PubMed] [Google Scholar]
- 39.Siegenthaler G, Saurat JH. Loss of retinol-binding properties for plasma retinol-binding protein in normal human epidermis. Journal of Investigative Dermatology. 1987;88(4):403–408. doi: 10.1111/1523-1747.ep12469731. [DOI] [PubMed] [Google Scholar]
- 40.Ho JCY, Cheung ST, Poon WS, Lee YT, Ng IOL, Fan ST. Down-regulation of retinol binding protein 5 is associated with aggressive tumor features in hepatocellular carcinoma. Journal of Cancer Research and Clinical Oncology. 2007;133(12):929–936. doi: 10.1007/s00432-007-0230-0. [DOI] [PubMed] [Google Scholar]
- 41.Salinas AE, Wong MG. Glutathione S-transferases—a review. Current Medicinal Chemistry. 1999;6(4):279–309. [PubMed] [Google Scholar]
- 42.Whalen R, Boyer TD. Human glutathione S-transferases. Seminars in Liver Disease. 1998;18(4):345–358. doi: 10.1055/s-2007-1007169. [DOI] [PubMed] [Google Scholar]
- 43.Wilce MCJ, Parker MW. Structure and function of glutathione S-transferasese. Biochimica et Biophysica Acta. 1994;1205(1):1–18. doi: 10.1016/0167-4838(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 44.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Critical Reviews in Biochemistry and Molecular Biology. 1995;30(6):445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 45.Hayes JD, Strange RC. Potential contribution of the glutathione S-transferase supergene family to resistance to oxidative stress. Free Radical Research. 1995;22(3):193–207. doi: 10.3109/10715769509147539. [DOI] [PubMed] [Google Scholar]
- 46.Neefjes VME, Evelo CTA, Baars LGM, Blanco CE. Erythrocyte glutathione S transferase as a marker of oxidative stress at birth. Archives of Disease in Childhood. 1999;81(2):F130–F133. doi: 10.1136/fn.81.2.f130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeo M-K, Kim H-E. Gene expression in zebrafish embryos following exposure to Cu-doped TiO2 and pure TiO2 nanometer-sized photocatalysts. Molecular and Cellular Toxicology. 2012;8:127–137. [Google Scholar]


