Abstract
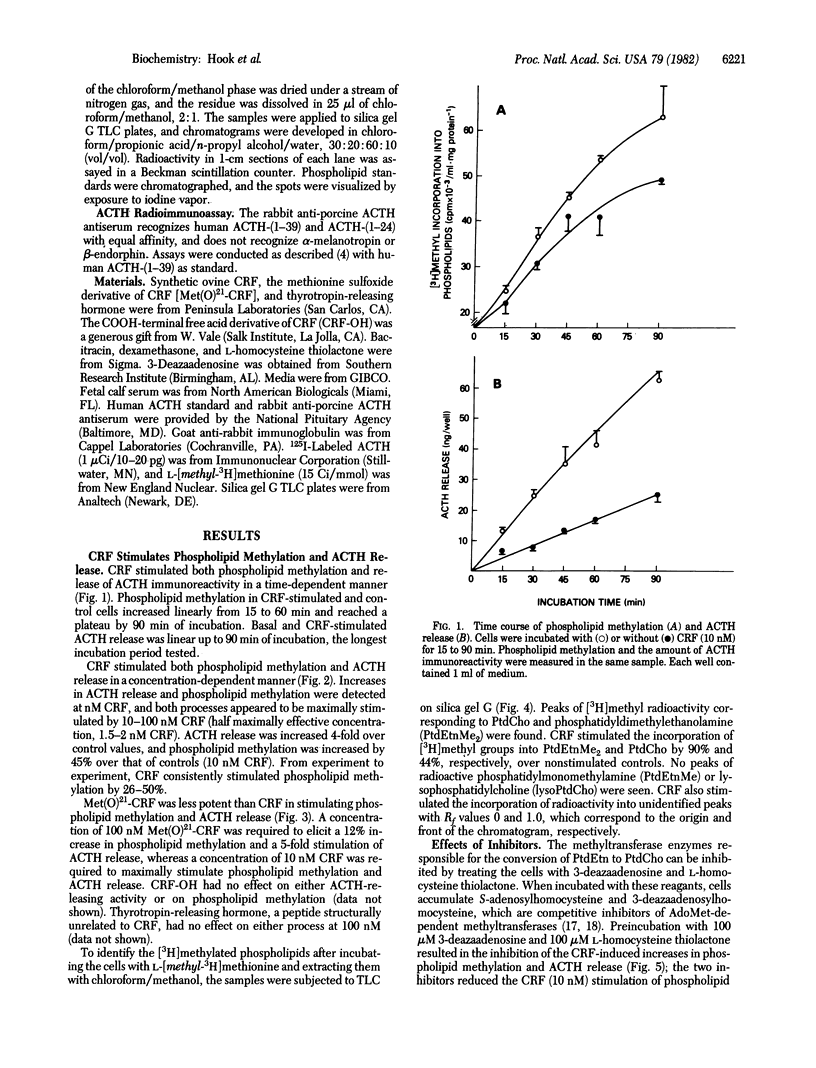
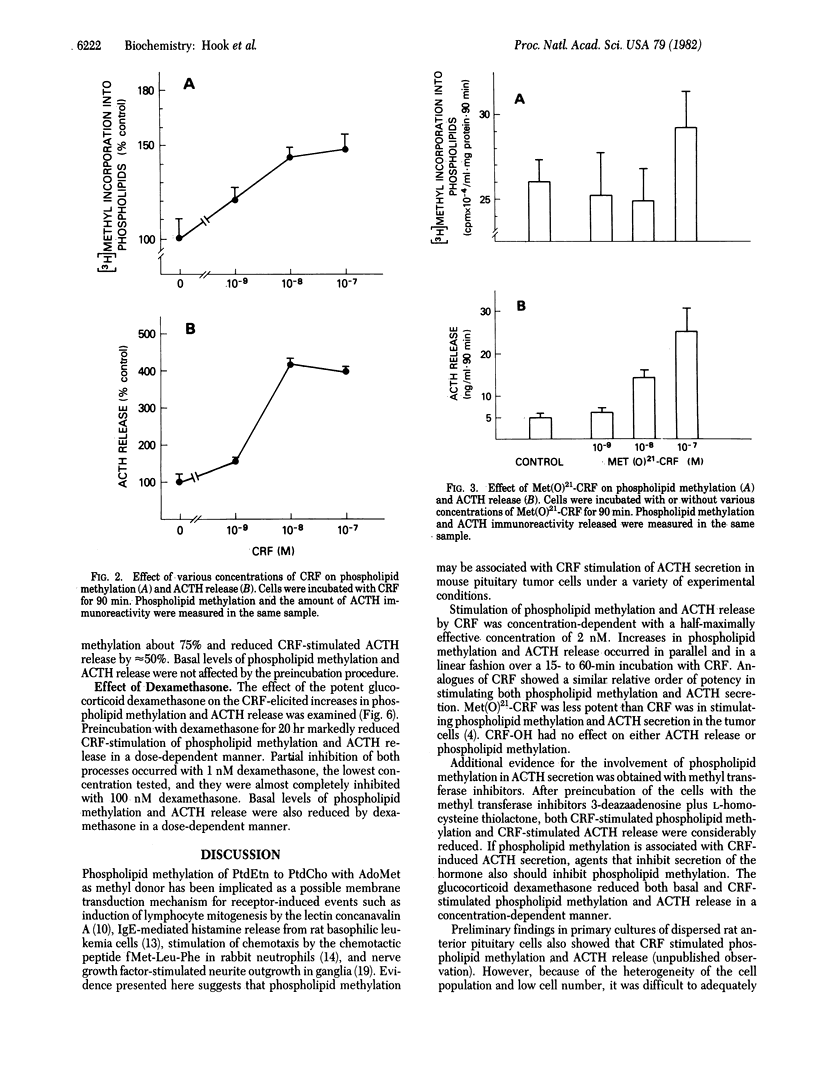
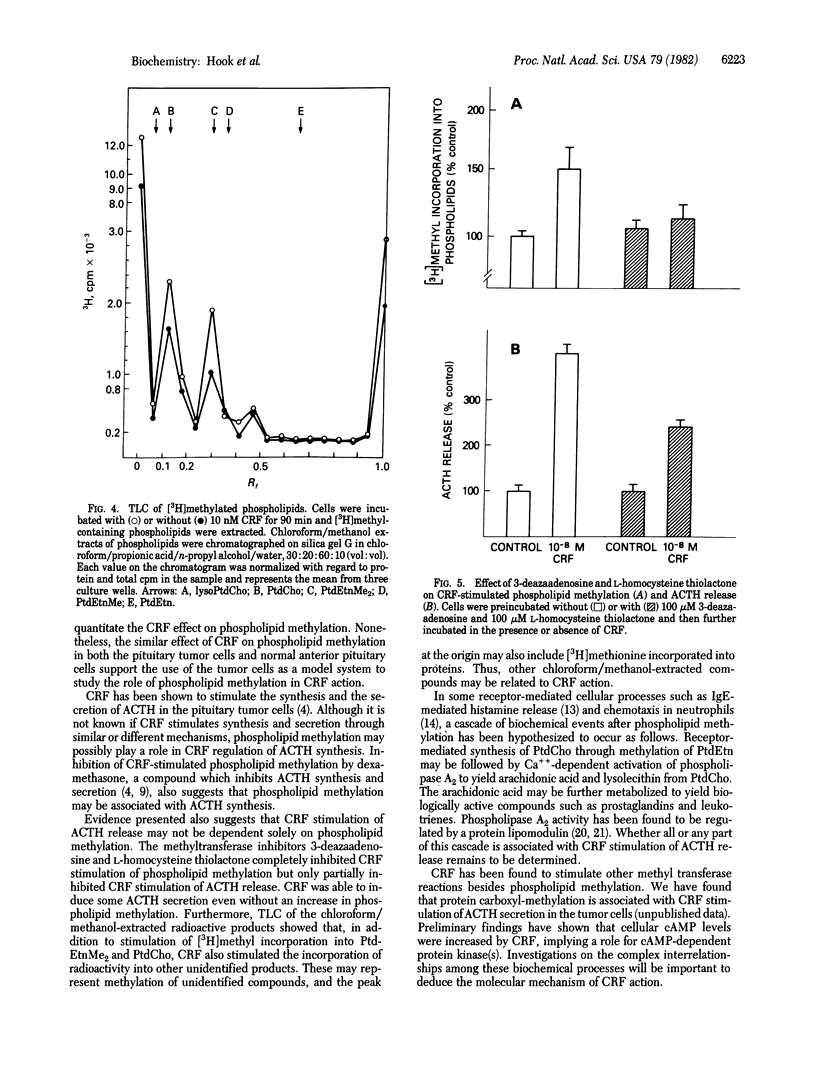
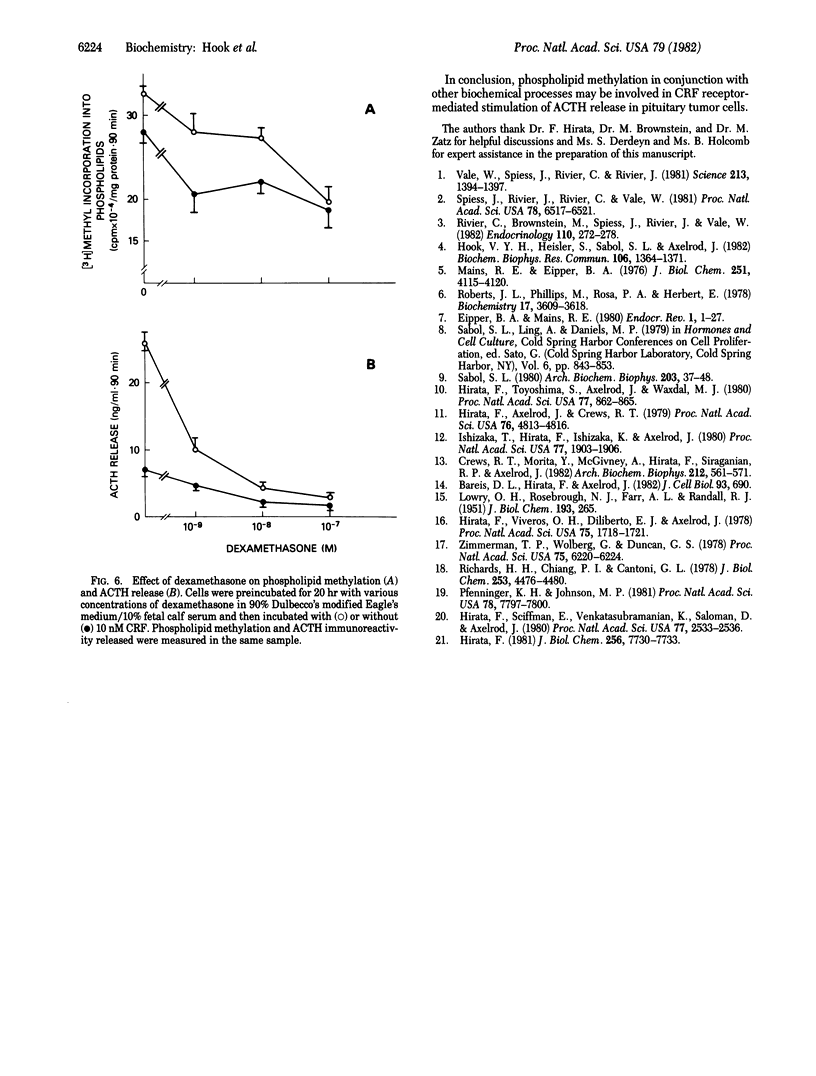
The 41-residue synthetic ovine corticotropin-releasing factor (CRF; corticoliberin) has been shown to stimulate release of corticotropin (adrenocorticotropic hormone; ACTH) and beta-endorphin from AtT-20/D16-16 mouse pituitary tumor cells. Phospholipid methylation of phosphatidylethanolamine to phosphatidylcholine with S-adenosylmethionine as methyl donor has been suggested as a possible membrane transduction mechanism for some receptor-induced events. CRF increased phospholipid methylation in pituitary tumor cells at concentrations that also stimulated immunoreactive ACTH secretion, and both processes increased linearly and in parallel with time. The methionine sulfoxide derivative of CRF was less potent than CRF was in stimulating both phospholipid methylation and hormone secretion, and the COOH-terminal free acid analogue of CRF had no effect on either process. CRF-induced increases in phospholipid methylation and ACTH secretion were reduced when cells were treated with the phospholipid methyltransferase inhibitors 3-deazaadenosine and L-homocysteine thiolactone. These CRF-stimulated effects were also blocked by the glucocorticoid dexamethasone. It is suggested that phospholipid methylation may be a CRF receptor-mediated event associated with ACTH release in pituitary tumor cells.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bareis D. L., Hirata F., Schiffmann E., Axelrod J. Phospholipid metabolism, calcium flux, and the receptor-mediated induction of chemotaxis in rabbit neutrophils. J Cell Biol. 1982 Jun;93(3):690–697. doi: 10.1083/jcb.93.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F. T., Morita Y., McGivney A., Hirata F., Siraganian R. P., Axelrod J. IgE-mediated histamine release in rat basophilic leukemia cells: receptor activation, phospholipid methylation, Ca2+ flux, and release of arachidonic acid. Arch Biochem Biophys. 1981 Dec;212(2):561–571. doi: 10.1016/0003-9861(81)90399-4. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980 Winter;1(1):1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J., Crews F. T. Concanavalin A stimulates phospholipid methylation and phosphatidylserine decarboxylation in rat mast cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4813–4816. doi: 10.1073/pnas.76.10.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Schiffmann E., Venkatasubramanian K., Salomon D., Axelrod J. A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci U S A. 1980 May;77(5):2533–2536. doi: 10.1073/pnas.77.5.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F. The regulation of lipomodulin, a phospholipase inhibitory protein, in rabbit neutrophils by phosphorylation. J Biol Chem. 1981 Aug 10;256(15):7730–7733. [PubMed] [Google Scholar]
- Hirata F., Toyoshima S., Axelrod J., Waxdal M. J. Phospholipid methylation: a biochemical signal modulating lymphocyte mitogenesis. Proc Natl Acad Sci U S A. 1980 Feb;77(2):862–865. doi: 10.1073/pnas.77.2.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Viveros O. H., Diliberto E. J., Jr, Axelrod J. Identification and properties of two methyltransferases in conversion of phosphatidylethanolamine to phosphatidylcholine. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1718–1721. doi: 10.1073/pnas.75.4.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook V. Y., Heisler S., Sabol S. L., Axelrod J. Corticotropin releasing factor stimulates adrenocorticotropin and beta-endorphin release from AtT-20 mouse pituitary tumor cells. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1364–1371. doi: 10.1016/0006-291x(82)91264-5. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Hirata F., Ishizaka K., Axelrod J. Stimulation of phospholipid methylation, Ca2+ influx, and histamine release by bridging of IgE receptors on rat mast cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1903–1906. doi: 10.1073/pnas.77.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Biosynthesis of adrenocorticotropic hormone in mouse pituitary tumor cells. J Biol Chem. 1976 Jul 10;251(13):4115–4120. [PubMed] [Google Scholar]
- Pfenninger K. H., Johnson M. P. Nerve growth factor stimulates phospholipid methylation in growing neurites. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7797–7800. doi: 10.1073/pnas.78.12.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards H. H., Chiang P. K., Cantoni G. L. Adenosylhomocysteine hydrolase. Crystallization of the purified enzyme and its properties. J Biol Chem. 1978 Jun 25;253(12):4476–4480. [PubMed] [Google Scholar]
- Rivier C., Brownstein M., Spiess J., Rivier J., Vale W. In vivo corticotropin-releasing factor-induced secretion of adrenocorticotropin, beta-endorphin, and corticosterone. Endocrinology. 1982 Jan;110(1):272–278. doi: 10.1210/endo-110-1-272. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Phillips M., Rosa P. A., Herbert E. Steps involved in the processing of common precursor forms of adrenocorticotropin and endorphin in cultures of mouse pituitary cells. Biochemistry. 1978 Aug 22;17(17):3609–3618. doi: 10.1021/bi00610a030. [DOI] [PubMed] [Google Scholar]
- Sabol S. L. Storage and secretion of beta-endorphin and related peptides by mouse pituitary tumor cells: regulation by glucocorticoids. Arch Biochem Biophys. 1980 Aug;203(1):37–48. doi: 10.1016/0003-9861(80)90151-4. [DOI] [PubMed] [Google Scholar]
- Spiess J., Rivier J., Rivier C., Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6517–6521. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. P., Wolberg G., Duncan G. S. Inhibition of lymphocyte-mediated cytolysis by 3-deazaadenosine: evidence for a methylation reaction essential to cytolysis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6220–6224. doi: 10.1073/pnas.75.12.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]



