Abstract
Objective
To comprehensively phenotype parents identified with nonclassic congenital adrenal hyperplasia (NCCAH) by family genetic studies, termed here as cryptic NCCAH and to define the incidence of cryptic NCCAH in the parents of a large cohort of patients with 21-hydroxylase deficiency.
Design
Genotyping was performed on 249 parents of 145 unrelated congenital adrenal hyperplasia CAH) patients. Parents with two CYP21A2 mutations underwent extensive evaluation.
Results
Of the 249 parents, ten (4%; seven females and three males) were identified as having cryptic NCCAH. The majority was of ethnicities previously reported to have a higher incidence of NCCAH. Cosyntropin stimulation performed in eight parents provided biochemical confirmation (17-hydroxyprogesterone range 56–364 nmol/l) and cortisol response was % 500 nmol/l in three parents (38%). Of the seven women (27–54 years) with cryptic NCCAH, four had prior infertility, two reported irregular menses, two had treatment for hirsutism, one had androgenic alopecia. Men were asymptomatic. All cryptic NCCAH parents reported normal puberty and had normal height. Adrenal hypertrophy and a small adrenal myelolipoma were observed in two parents; testicular adrenal rest tissue was not found.
Conclusions
Parents diagnosed with NCCAH by genetic testing are mostly asymptomatic. Temporary female infertility and suboptimal cortisol response were commonly observed. Ongoing glucocorticoid therapy is not indicated in adults with CAH identified by family genotype studies unless symptomatic, but glucocorticoid stress coverage should be considered in select cases. Parents of a child with CAH have a 1:25 risk of having NCCAH; if the mother of a child with CAH has infertility, evaluation for NCCAH is indicated.
Introduction
Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency is an autosomal recessive disorder accounting for ~95% of cases of CAH and is caused by mutations in the CYP21A2 gene. There is a wide range of clinical severity (1, 2), and in general a high level of genotype–phenotype correlation, such that particular CYP21A2 mutations lead to predictable degrees of impairment in enzyme activity (3, 4). Severe 21-hydroxylase impairment leads to classic CAH, with an estimated U.S. frequency of one in 16 000 live births (5). Classic CAH is characterized by genital ambiguity in females, and in the most severe cases, neonatal salt loss and adrenal crisis if not properly diagnosed and treated. Mild 21-hydroxylase impairment leads to nonclassic congenital adrenal hyperplasia (NCCAH), which is estimated to affect one of every 1000 Caucasians (6, 7). Patients with NCCAH do not suffer from clinically significant glucocorticoid deficiency, prenatal virilization of females does not occur, and patients may or may not have symptoms and signs of hyperandrogenism (8, 9). Genetic testing for CYP21A2 mutations is available and widely used (10, 11).
A wide range of clinical severity exists within the nonclassic subtype. Most studies on patients with NCCAH focus on reproductive outcomes (12, 13) and androgen excess (14) in symptomatic women. Although carriers of 21-hydroxylase deficiency may have mildly elevated adrenal hormones (15), in general, they are not at increased risk of hyperandrogenism (16).
NCCAH was discovered during studies on family members of patients with classic CAH (17–20) and helped to underscore the idea that CAH identified through family studies, termed ‘cryptic’, shares the same pathological basis as NCCAH, but the natural history of subjects identified solely by genotype remains to be delineated. In this study, we use the term ‘cryptic’ to describe an individual who is diagnosed with NCCAH based on family genetic studies, rather than symptomatology. To optimize genetic counseling of families, we sought, in our large population of patients with classic CAH and symptomatic NCCAH, to provide comprehensive phenotypic profiling of parents found to have cryptic NCCAH and to define the incidence of cryptic NCCAH in parents of patients with CAH.
Methods
Subjects
From 2006 to 2009, 198 probands with CAH due to 21-hydroxylase deficiency (153 classic and 45 nonclassic) were enrolled in a Natural History Study at the National Institutes of Health Clinical Center in Bethesda, MD, USA (Clinicaltrials.gov identifier NCT00250159). All patients and parents were invited to be genotyped. A total of 249 parents from 145 unrelated families underwent genetic analysis. Parents who did not participate were either deceased or unavailable. The study was approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. All subjects provided written informed consent.
Molecular analysis of the CYP21A2 gene
DNA was extracted and analyzed for CYP21 gene mutations according to standard methods (Esoterix, Inc., Calabasas, CA, USA), as described previously (3, 11). CYP21A2 duplications were excluded using Southern blot analysis and PCR-based methods (11).
Clinical evaluation
Of the total parents, eight found to have two CYP21A2 mutations (compound heterozygotes) underwent extensive evaluation, including history and physical examination, hormonal evaluation, and adrenal and testicular imaging. A 1 h cosyntropin stimulation test (i.v. administration of 250 µg cosyntropin) was performed, with measurement of basal and stimulated cortisol, and 17-hydroxyprogesterone (17-OHP). Baseline ACTH, androstenedione, free and total testosterone, DHEA, DHEA-S, and plasma renin activity were also assayed. A 60-min stimulated cortisol ≤500 nmol/l (≤ 18 µg/dl) (21, 22) was used to define biochemical adrenal insufficiency. Biochemical criterion for CAH due to 21-hydroxylase deficiency was defined as a 60 min 17-OHP concentration > 36 nmol/l (1200 ng/dl) (1, 23). Of the total parents, two with cryptic NCCAH refused to return for full evaluation but underwent a telephone interview.
Anthropometric data and information about gravidity, parity, and the presence of signs of androgen excess (acne, hirsutism with Ferriman-Gallwey score, clitoromegaly, infertility, and menstrual dysfunction) were collected. Height SDS was determined using anthropometric reference data (http://www.cdc.gov/growthcharts/, CDC Growth Charts 2010). Parental height of the affected parents was obtained by report and midparental height in centimeters was estimated using the following formula: male midparental height = (maternal height + paternal height + 13)/2; female midparental height = (maternal height + paternal height – 13)/2.
Parents with cryptic NCCAH underwent computed tomography imaging of the adrenal glands. The size of the adrenal glands was evaluated and the glands were classified as normal or hypertrophied. If adrenal nodules were present, their size was measured and attenuation measurements were obtained. If the attenuation of the nodules measured < − 10 Hounsfield units, they were considered to contain fat and represent adrenal myelolipoma. Male parents underwent testicular imaging to evaluate for the presence of testicular adrenal rest tissue. All images were evaluated by a single radiologist (NA).
Hormonal assays
Serum cortisol, testosterone, ACTH, DHEA, DHEA-S, and androstenedione were measured by chemiluminescence immunoassay (Mayo Medical Labs, Rochester, MN, USA for androstenedione and DHEA; NIH Clinical Center Laboratory for all others). Free testosterone was calculated based on the previously-derived constants (24). Plasma 17-OHP was measured by liquid chromatography–tandem mass spectrometry (Mayo Medical Labs). Plasma renin activity was measured by RIA (Mayo Medical Labs). Resting catecholamines and metanephrines were measured by HPLC (NIH Clinical Center Laboratory, Bethesda, MD, USA).
Statistical analyses
Hormonal values are expressed as mean and range. The frequencies of reproductive and androgen excess-related outcomes are expressed as absolute numbers and percentages. Height was evaluated by height SDS and as the difference between adult height achieved and the calculated midparental height.
The incidence of cryptic NCCAH in the parents was calculated as a percent of the total population of parents of patients with CAH who underwent molecular testing. We calculated the probability that a parent (known carrier) would have CAH. To determine this likelihood, we used Bayes’ theorem to express the conditional probability of a parent being affected, given an affected child (Supplementary data, see section on supplementary data given at the end of this article). The prior probabilities were based on the assumptions of having one affected child, that both alleles act independently, and that the disease is recessively inherited. The population prevalence of NCCAH was assumed to be one in 1000 (7).
Results
Of the 249 parents studied, ten (4%) were identified as having NCCAH by genotype (Table 1). Of the ten parents, eight returned for hormonal and radiological evaluation (Table 2).
Table 1.
Genotype and clinical characteristics of parents with cryptic 21-hydroxylase deficiency.
| Patient | Age (years) |
Sex | Ethnicity | Genotype | Proband’s genotype/ phenotype |
Clinical history | Adrenal imaging | Hospitalizations and surgeries |
|---|---|---|---|---|---|---|---|---|
| 1 | 54 | F | Ashkenazi Jewish | p.V281L/deletion | p.V281L/p.V281L NC | Androgenic alopecia, two term pregnancies | Not performed | None |
| 2 | 49 | F | Italian | Deletion/p.V281L | Deletion/deletion SV | Two term pregnancies, one following clomiphene | Not performed | Tonsillectomy Appendectomy Cholecystectomy C-sections×2 |
| 3 | 46 | F | Anglo-Saxon Russian | p.I172N/p.P482Sa | p.I172N/p.V281L NC | Cystic acne requiring antibiotics and irregular menses as adolescent, two term pregnancies, one following clomiphene | Normal | C-section Arthroscopic knee surgery Bladder suspension surgery Jaw surgery |
| 4 | 45 | F | Anglo-Saxon Italian | p.I172N/p.P453S | p.I172N/p.I172N SV | Four term pregnancies, one following infertility for 4 years, no therapy, topical eflornithine for hirsutism | Normal | Knee surgeries×5 |
| 5 | 41 | F | Hispanic Italian | IVS2-13A/C> G/p.V281L | IVS2-13A/C> G/p.V281L NC | Irregular menses until age 27, two term pregnancies, electrolysis for hirsutism | Bilateral adrenal hypertrophy; right 1.5-mm myelolipoma | None |
| 6 | 33 | F | Italian | Deletion/p.V281L | Deletion/deletion SW | Two term pregnancies, both following clomiphene | Normal | None |
| 7 | 27 | F | Greek German Anglo-Saxon Swedish | 30 kb Deletion/p.P453S | Deletion/30 kb deletion SW | Acne as teenager, one term pregnancy | Normal | Bilateral ankle surgery D&C following miscarriage |
| 8 | 74 | M | Italian | p.I172N/p.V281L | p.I172N/p.I172N NC | Normal puberty, four children | Not performed | None |
| 9 | 66 | M | Latvian | p.I172N/p.P453S | IVS2–13A/C> G/p.I172N SV | Normal puberty, two children | Normal | None |
| 10 | 55 | M | Anglo-Saxon | IVS2–13A/C> G/p.V281L | IVS2–13A/C> G/deletion SW | Normal puberty, three children | Moderate bilateral adrenal hypertrophy | Multiple traumatic injuries after motorcycle accident with prolonged ICU stay |
Rare mutation detected by sequencing CYP21A2; mutations in bold are those not detected in proband. SW, salt wasting CAH; SV, simple virilizing CAH; NC, nonclassic CAH.
Table 2.
Laboratory data of parents with cryptic 21-hydroxylase deficiency.
| Cosyntropin stimulation | Early morning basal levels | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | 17-OHPa (nmol/l) | Cortisol (nmol/l) | ACTH (pmol/l) |
Androstene- dione (nmol/l) |
Test. free (pmol/l) |
Test. total (nmol/l) |
DHEA (nmol/l) |
DHEAS (µmol/l) |
Plasma renin activity (µg/l per hour) |
Epinephrine (pmol/l) |
Metanephrine (pmol/l) |
||
| Basal | 60 min | Basal | 60 min | ||||||||||
| 2 | 22.7 | 125 | 395 | 497b | 5.3 | 6.0 | 10 | 1.1 | 27.1 | 4.4 | 2.4 | 49 | 161 |
| 3 | 14.8 | 56 | 497 | 582 | 3.3 | 2.8 | 4 | 0.5 | 4.2 | <0.4 | 2.9 | 44 | 120 |
| 4 | 15.1 | 132 | 312 | 582 | 3.2 | 3.9 | 24 | 1.0 | 34c | 3.4 | 1.1 | 55 | 120 |
| 5 | 49.2 | 211 | 417 | 566 | 8.1 | 13.0c | 56 | 2.5 | 7.6c | 1.2 | 251 | 146 | |
| 6 | 13.7 | 157 | 246b | 574 | 5.0 | 6.6 | 316c | 2.0 | 16.3 | 4.1 | 1.2 | 60 | |
| 7 | 6.3 | 110 | 160b | 370b | 1.7 | 3.9 | 21 | 1.1 | 2.7 | 0.7 | 142 | 120 | |
| 9 | 12.3 | 117 | 259b | 491b | 6.4 | 4.5 | 552 | 22.1 | 10.8 | 4.4c | 0.6 | 262 | 244 |
| 10 | 81.0 | 363 | 403 | 676 | 5.8 | 10.5c | 2290c | 15.3 | 3.3 | 1.7 | |||
To convert from SI units to metric: 17-OHP×33.1 ng/dl; cortisol×0.0362 g/dl; ACTH×4.54 pg/ml; androstenedione×28.65 ng/dl; free testosterone×0.288 pg/ml; testosterone×28.82 ng/dl; DHEA×28.82 ng/dl; DHEA-S×36.9 g/dl; epinephrine×0.183 pg/ml; and metanephrine 0.192 pg/ml.
Elevated for age and sex.
Phenotypic profiling
Hormonal evaluation
Cosyntropin stimulation testing was performed in eight of the ten identified parents. All met biochemical criteria for CAH (Table 2). Mean stimulated 17-OHP was 159 nmol/l (range 56–364 nmol/l) (5198 ng/dl; range 1860–12 000 ng/dl). Suboptimal cortisol response to cosyntropin was observed in three subjects (38%). These three subjects did not report illnesses consistent with adrenal insufficiency and had undergone a variety of surgeries (Table 1). Eight had basal plasma 17-OHP > 6 nmol/l (200 ng/dl). None had elevated ACTH. All subjects had normal plasma renin activity, as well as normal epinephrine and metanephrine concentrations (Table 2).
Pubertal history, hyperandrogenism, and infertility
Of the seven women evaluated, two reported irregular menses and had been treated with oral contraceptives to achieve regular cycling (Table 1), four women reported infertility, but three of these women also had pregnancies that were easily conceived. Of the seven women, three women had ovulatory induction therapy; one reported infertility for 4 years, during which she was not treated; one woman had androgenic alopecia; two had treatment for hirsutism. It was reported that two female subjects had a history of acne during adolescence and one had tetracycline therapy. None of the subjects had hirsutism or acne at the time of evaluation. All reported normal timing of puberty. Mean age at menarche was 12.9 years.
Adult height
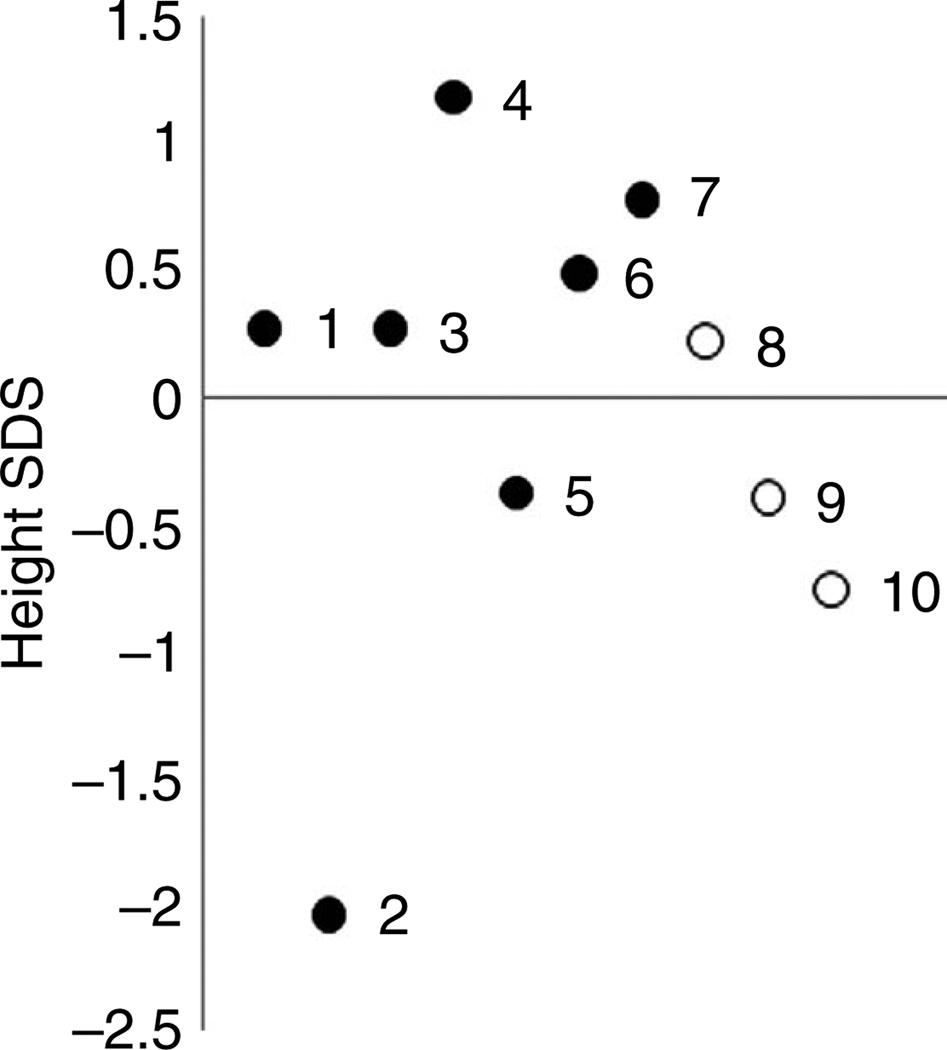
A wide range of adult height was observed ranging from − 2.0 to 1.2 standard deviation units (SDU) (Fig. 1). Similarly, the difference between adult height and midparental target height ranged from − 1.9 to 1.1 SDU, and the majority was within 1 SDU.
Figure 1.
Height SDS of parents with cryptic NCCAH. Numbers correspond to patient number in tables. Open circle, female; filled circle, male.
Adrenal and testicular imaging
Of the total parents, seven parents with cryptic NCCAH underwent computed tomography of the adrenal glands; five had normal-sized adrenal glands; two had bilateral adrenal hypertrophy; and one had a 0.15 cm right adrenal myelolipoma (Table 1). In the two subjects with adrenal abnormalities, early morning ACTH concentrations were normal: 8.1 pmol/l (36.8 pg/ml) and 5.8 pmol/l (26.5 pg/ml), and stimulated 17-OHP concentrations were 211 nmol/l (6970 ng/dl) and 364 nmol/l (12 000 ng/dl) respectively.
Testicular adrenal rest tissue was not detected in the two males who underwent testicular imaging.
Incidence of cryptic NCCAH among parents
In our population of 249 parents who underwent CYP21A2 genetic analysis, ten parents, or 4% of parents genotyped, were found to have CAH. Thus, in our cohort, the risk that the parent of a child with CAH will have a diagnosis of CAH is one in 25. The majority (nine of ten) of mutations that were present in a parent but not in the child was associated with NCCAH: five had p.V281L, three had p.P453S, and one had a rare mutation, p.P482S (25); one parent of a child with NCCAH carried a deletion not found in the child. In addition, the majority of parents identified with cryptic NCCAH was of ethnicities previously reported to have a higher incidence of NCCAH (Ashkenazi Jewish, Italian, Hispanic, Eastern European) (7). There was no reported consanguinity. All of the parents identified with cryptic NCCAH were compound heterozygous for a severe mutation: six carried a salt-wasting mutation and four carried a simple-virilizing mutation.
Based on Bayes’ theorem and the estimated population prevalence of one in 1000, the conditional probability of a parent having CAH was determined to be 3.2%, not significantly different from our observed prevalence of 4% (Supplementary data, see section on supplementary data given at the end of this article).
Discussion
In our large cohort of patients with 21-hydroxylase deficiency, we found that 4% of parents of children with either classic CAH or symptomatic NCCAH had a previously unknown diagnosis themselves of ‘cryptic’ NCCAH. Our study offers a systematic evaluation of parents identified with NCCAH by genetic studies, thus expanding our understanding of the natural history and disease progression of this entity, and providing useful information for genetic counseling. We found that parents diagnosed with CAH by family genetic testing are mostly asymptomatic. However, female infertility was reported in approximately one-half. Suboptimal cortisol response was observed in a subset, as was the presence of small adrenal myelolipoma, though the clinical significance of these findings is unknown. Males did not have testicular adrenal rest tissue.
In our calculation, we used the knowledge that each parent who underwent genotyping had a child affected with CAH. Interestingly, when carrier status was assumed and considered in the probability calculations, our 4% prevalence of NCCAH in the parents of children with CAH, although seemingly high, was not higher than that expected (10). Our calculations support the prior general population prevalence estimate of one in 1000 for NCCAH (7, 26). However, we did not consider other information that could potentially affect risk, such as ethnicity, number of children with CAH, or prior history of infertility or menstrual irregularities. It must be recognized that these important details may confer increased risk in particular individuals and should be considered in genetic counseling sessions.
It has previously been suggested that individuals with cryptic NCCAH later became symptomatic (19, 27) and would benefit from glucocorticoid treatment given the cost of and psychological effects of hyperandrogenism (27). Our findings do not support this and demonstrate that adults diagnosed with NCCAH based on family genetic studies appear to be mostly asymptomatic. They are of normal adult height and report normal pubertal progression and do not suffer from significant virilization. However, female infertility was common, suggesting that evaluation for NCCAH should be initiated in women with infertility, especially in those with a family history of CAH or ethnicity that confers higher risk. Given the benign clinical histories of our identified parents with cryptic NCCAH, there is no indication for ongoing glucocorticoid therapy in these adults, who may be seen as compound heterozygous with subclinical symptoms. Our findings support the recent Endocrine Society Clinical Practice Guidelines (28), which recommend against treatment in asymptomatic individuals with NCCAH. Moreover, genotyping is sometimes used as a confirmatory test for newborns who test positive to neonatal screening or when the neonatal biochemical scenario requires further testing (29). Our data provides further evidence that clinical symptomatology should dictate the management of those diagnosed with CAH based on laboratory findings consistent with NCCAH; hormonal and molecular findings do not necessarily progress to significant clinical symptomatology.
Our finding of low cortisol responses to cosyntropin stimulation in 38% of our cryptic parents is consistent with recent studies showing suboptimal cortisol response in a subset of patients with NCCAH (10, 30). Bidet et al. (10) reported weak (cortisol < 414 nmol/l) response to cosyntropin stimulation in approximately one-third of patients with NCCAH; Verma et al. (30) reported that five out of eight NCCAH patients studied have suboptimal cortisol response. Our subjects with low cortisol levels denied history of severe illnesses or dehydration as well as history of decompensation or signs/symptoms that would be consistent with adrenal insufficiency, despite having undergone a variety of surgical procedures. Thus, it is difficult to know how to interpret abnormal biochemical data in the setting of unremarkable clinical histories. Although the need for stress dosing in these asymptomatic cases is unknown, we chose to caution these subjects that should they be involved in extreme trauma or need to undergo major surgery in the future, stress-dose glucocorticoids should be considered, but routine stress dosing would not be warranted.
Adults with CAH have an increased incidence of adrenal tumors (31). It has previously been reported that carriers of classic CAH mutations have a higher frequency of adrenal adenomas than that found in the general population (32). Theoretically, subjects with cryptic NCCAH would be expected to have a higher incidence of adrenal adenomas than carriers due to chronically elevated ACTH levels, predisposing to tumor formation. However, only two out of seven cryptic parents who underwent imaging were found to have adrenal abnormalities (hypertrophy in one patient, hypertrophy and a 1.5-mm myelolipoma in another patient), despite the older age of these individuals (up to age 66 years) and the generous stimulated 17-OHP concentrations. Interestingly, the adrenal tumor observed in our parent with cryptic NCCAH was a myelolipoma, which has been associated with CAH (33, 34). Adrenal myelolipomas are generally benign, asymptomatic, and do not require resection, but large tumors may suggest the diagnosis of CAH (35–37). Similarly, no testicular adrenal rest tissue was found in males. To our knowledge, testicular adrenal rest tissue has not been reported in patients with NCCAH. The lack of adrenal and testicular tumors or clinically significant adrenal hypertrophy supports the notion that individuals incidentally diagnosed by family genetic studies do not require ongoing glucocorticoid treatment, and clinical symptoms, rather than hormone concentrations, should dictate management.
Early morning basal 17-OHP has been proposed as an effective screen for NCCAH (6, 10, 38). Armengaud et al. (38) used a threshold value of 6 nmol/l (200 ng/dl) to diagnose NCCAH on the basis of a basal plasma 17-OHP and reported 100% sensitivity and 99% specificity. By this criterion, all of our parents would have been identified. Our subjects’ 17-OHP concentrations following cosyntropin stimulation had a wide range. Bidet et al. (10) reported that only 17.5% of their cohort of NCCAH patients had abnormal basal plasma 17-OHP but that the range of 60-min stimulated values was very broad. Azziz et al. (6) used a criterion of post-stimulation 17-OHP > 30.3 nmol/l (1000 ng/dl) for diagnosis of 21-hydroxylase deficiency; by this definition, all of our subjects fit the biochemical criteria for NCCAH. Interestingly, few of our subjects had other biochemical parameters outside the normal range for sex and age, and elevations were not consistent for all androgens. All had normal resting epinephrine and metanephrine concentrations, consistent with a recent report (30) demonstrating that untreated NCCAH patients have normal adrenomedullary function.
Each of the parents we studied demonstrated the presence of a mutation not found in the child who was originally found to have CAH. These mutations included p.V281L (n = 5), p.P453S (n = 3), deletion (n = 1), and p.P482S (n = 1). All of our parents with cryptic NCCAH carried one severe mutation and one nonclassic mutation. Hormonal concentrations, adrenal size, and presence of symptoms were not associated with a particular genotype. Thus, carrying a more severe mutation was not associated with a more severe phenotype in our population, as had previously been reported (3).
We sought to determine when the issue of genetically identified NCCAH might be clinically important. The majority of the mothers reported a history of infertility, and glucocorticoid therapy is an effective treatment for infertility and recurrent miscarriages in NCCAH (39). Thus, if the mother of a child with CAH has subsequent infertility or a history of multiple miscarriages requiring medical attention, an evaluation for NCCAH is indicated. Given the implications for reduced female fertility and the apparent lack of abnormalities in males, it is surprising that the majority of parents identified as having cryptic NCCAH were female. Our sample is too small to provide any robust statistical assessment of this imbalance. Thus, further studies on large cohorts of CAH patients are needed to evaluate this finding.
As genetic testing becomes more widely available, we expect that more family members of patients with CAH will be identified in whom there is only biochemical and molecular evidence of disease, in the absence of striking clinical features. Assessing how these individuals should be counseled and the natural history of their condition is of critical importance to avoid glucocorticoid over-treatment. However, the incidence of one in 25 parents in our population suggests that NCCAH should be considered in the mother of a child with CAH if she has infertility. The underlying reasons for the relatively asymptomatic nature of this condition warrant further study.
Supplementary Material
Acknowledgements
We gratefully acknowledge the enthusiastic participation of our patients and their relatives, without whom this work would not have been possible. We also thank the research assistant Annie Sullivan for her assistance with data management and manuscript preparation.
Funding
This work was supported (in part) by the Intramural Research Programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institutes of Health Clinical Center, and (in part) by The Congenital Adrenal Hyperplasia Research, Education and Support (CARES) Foundation. Dr Nandagopal received fellowship support from the Inter-Institute Pediatric Endocrinology Training Program, National Institute of Child Health and Human Development; both Dr Nandagopal and Dr Merke are Commissioned Officers in the United States Public Health Service.
Footnotes
Supplementary data
This is linked to the online version of the paper at http://dx.doi.org/10.1530/EJE-11-0019.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365:2125–2136. doi: 10.1016/S0140-6736(05)66736-0. [DOI] [PubMed] [Google Scholar]
- 2.Moran C. Nonclassic adrenal hyperplasia. Fertility and Sterility. 2006;86(Supplement 1):S3. doi: 10.1016/j.fertnstert.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP. Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. Journal of Clinical Endocrinology and Metabolism. 2000;85:1059–1065. doi: 10.1210/jcem.85.3.6441. [DOI] [PubMed] [Google Scholar]
- 4.Jaaskelainen J, Levo A, Voutilainen R, Partanen J. Population-wide evaluation of disease manifestation in relation to molecular genotype in steroid 21-hydroxylase (CYP21) deficiency: good correlation in a well defined population. Journal of Clinical Endocrinology and Metabolism. 1997;82:3293–3297. doi: 10.1210/jcem.82.10.4271. [DOI] [PubMed] [Google Scholar]
- 5.Therrell BL, Jr, Berenbaum SA, Manter-Kapanke V, Simmank J, Korman K, Prentice L, Gonzalez J, Gunn S. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics. 1998;101:583–590. doi: 10.1542/peds.101.4.583. [DOI] [PubMed] [Google Scholar]
- 6.Azziz R, Hincapie LA, Knochenhauer ES, Dewailly D, Fox L, Boots LR. Screening for 21-hydroxylase-deficient nonclassic adrenal hyperplasia among hyperandrogenic women: a prospective study. Fertility and Sterility. 1999;72:915–925. doi: 10.1016/s0015-0282(99)00383-0. [DOI] [PubMed] [Google Scholar]
- 7.Speiser PW, Dupont B, Rubinstein P, Piazza A, Kastelan A, New MI. High frequency of nonclassical steroid 21-hydroxylase deficiency. American Journal of Human Genetics. 1985;37:650–667. [PMC free article] [PubMed] [Google Scholar]
- 8.Kuttenn F, Couillin P, Girard F, Billaud L, Vincens M, Boucekkine C, Thalabard JC, Maudelonde T, Spritzer P, Mowszowicz I, Boue A, Mauvais-Jarvis P. Late-onset adrenal hyperplasia in hirsutism. New England Journal of Medicine. 1985;313:224–231. doi: 10.1056/NEJM198507253130404. [DOI] [PubMed] [Google Scholar]
- 9.Dewailly D, Vantyghem-Haudiquet MC, Sainsard C, Buvat J, Cappoen JP, Ardaens K, Racadot A, Lefebvre J, Fossati P. Clinical and biological phenotypes in late-onset 21-hydroxylase deficiency. Journal of Clinical Endocrinology and Metabolism. 1986;63:418–423. doi: 10.1210/jcem-63-2-418. [DOI] [PubMed] [Google Scholar]
- 10.Bidet M, Bellanne-Chantelot C, Galand-Portier MB, Tardy V, Billaud L, Laborde K, Coussieu C, Morel Y, Vaury C, Golmard JL, Claustre A, Mornet E, Chakhtoura Z, Mowszowicz I, Bachelot A, Touraine P, Kuttenn F. Clinical and molecular characterization of a cohort of 161 unrelated women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency and 330 family members. Journal of Clinical Endocrinology and Metabolism. 2009;94:1570–1578. doi: 10.1210/jc.2008-1582. [DOI] [PubMed] [Google Scholar]
- 11.Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, McDonnell NB, Merke DP. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Journal of Clinical Endocrinology and Metabolism. 2010;96:E161–E172. doi: 10.1210/jc.2010-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moran C, Azziz R, Carmina E, Dewailly D, Fruzzetti F, Ibanez L, Knochenhauer ES, Marcondes JA, Mendonca BB, Pignatelli D, Pugeat M, Rohmer V, Speiser PW, Witchel SF. 21-Hydroxylasedeficient nonclassic adrenal hyperplasia is a progressive disorder: a multicenter study. American Journal of Obstetrics and Gynecology. 2000;183:1468–1474. doi: 10.1067/mob.2000.108020. [DOI] [PubMed] [Google Scholar]
- 13.Moran C, Azziz R, Weintrob N, Witchel SF, Rohmer V, Dewailly D, Marcondes JA, Pugeat M, Speiser PW, Pignatelli D, Mendonca BB, Bachega TA, Escobar-Morreale HF, Carmina E, Fruzzetti F, Kelestimur F. Reproductive outcome of women with 21-hydroxylase-deficient nonclassic adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism. 2006;91:3451–3456. doi: 10.1210/jc.2006-0062. [DOI] [PubMed] [Google Scholar]
- 14.Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, Taylor K, Boots LR. Androgen excess in women: experience with over 1000 consecutive patients. Journal of Clinical Endocrinology and Metabolism. 2004;89:453–462. doi: 10.1210/jc.2003-031122. [DOI] [PubMed] [Google Scholar]
- 15.Charmandari E, Merke DP, Negro PJ, Keil MF, Martinez PE, Haim A, Gold PW, Chrousos GP. Endocrinologic and psychologic evaluation of 21-hydroxylase deficiency carriers and matched normal subjects: evidence for physical and/or psychologic vulnerability to stress. Journal of Clinical Endocrinology and Metabolism. 2004;89:2228–2236. doi: 10.1210/jc.2003-031322. [DOI] [PubMed] [Google Scholar]
- 16.Knochenhauer ES, Cortet-Rudelli C, Cunnigham RD, Conway-Myers BA, Dewailly D, Azziz R. Carriers of 21-hydroxylase deficiency are not at increased risk for hyperandrogenism. Journal of Clinical Endocrinology and Metabolism. 1997;82:479–485. doi: 10.1210/jcem.82.2.3759. [DOI] [PubMed] [Google Scholar]
- 17.New MI, Dupont B, Pollack MS, Levine LS. The biochemical basis for genotyping 21-hydroxylase deficiency. Human Genetics. 1981;58:123–127. doi: 10.1007/BF00284159. [DOI] [PubMed] [Google Scholar]
- 18.Levine LS, Dupont B, Lorenzen F, Pang S, Pollack M, Oberfield SE, Kohn B, Lerner A, Cacciari E, Mantero F, Cassio A, Scaroni C, Chiumello G, Rondanini GF, Gargantini L, Giovannelli G, Virdis R, Bartolotta E, Migliori C, Pintor C, Tato L, Barboni F, New MI. Genetic and hormonal characterization of cryptic 21-hydroxylase deficiency. Journal of Clinical Endocrinology and Metabolism. 1981;53:1193–1198. doi: 10.1210/jcem-53-6-1193. [DOI] [PubMed] [Google Scholar]
- 19.Levine LS, Dupont B, Lorenzen F, Pang S, Pollack M, Oberfield S, Kohn B, Lerner A, Cacciari E, Mantero F, Cassio A, Scaroni C, Chiumello G, Rondanini GF, Gargantini L, Giovannelli G, Virdis R, Bartolotta E, Migliori C, Pintor C, Tato L, Barboni F, New MI. Cryptic 21-hydroxylase deficiency in families of patients with classical congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism. 1980;51:1316–1324. doi: 10.1210/jcem-51-6-1316. [DOI] [PubMed] [Google Scholar]
- 20.Lee PA, Rosenwaks Z, Urban MD, Migeon CJ, Bias WD. Attenuated forms of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Journal of Clinical Endocrinology and Metabolism. 1982;55:866–871. doi: 10.1210/jcem-55-5-866. [DOI] [PubMed] [Google Scholar]
- 21.Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic–pituitary–adrenal axis by insulin hypoglycemia test. Journal of Clinical Endocrinology and Metabolism. 1998;83:2350–2354. doi: 10.1210/jcem.83.7.4980. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt IL, Lahner H, Mann K, Petersenn S. Diagnosis of adrenal insufficiency: evaluation of the corticotropin-releasing hormone test and basal serum cortisol in comparison to the insulin tolerance test in patients with hypothalamic–pituitary–adrenal disease. Journal of Clinical Endocrinology and Metabolism. 2003;88:4193–4198. doi: 10.1210/jc.2002-021897. [DOI] [PubMed] [Google Scholar]
- 23.Wilson RC, Mercado AB, Cheng KC, New MI. Steroid 21-hydroxylase deficiency: genotype may not predict phenotype. Journal of Clinical Endocrinology and Metabolism. 1995;80:2322–2329. doi: 10.1210/jcem.80.8.7629224. [DOI] [PubMed] [Google Scholar]
- 24.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. Journal of Clinical Endocrinology and Metabolism. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 25.Barbaro M, Lajic S, Baldazzi L, Balsamo A, Pirazzoli P, Cicognani A, Wedell A, Cacciari E. Functional analysis of two recurrent amino acid substitutions in the CYP21 gene from Italian patients with congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism. 2004;89:2402–2407. doi: 10.1210/jc.2003-031630. [DOI] [PubMed] [Google Scholar]
- 26.Zerah M, Ueshiba H, Wood E, Speiser PW, Crawford C, McDonald T, Pareira J, Gruen D, New MI. Prevalence of nonclassical steroid 21-hydroxylase deficiency based on a morning salivary 17-hydroxyprogesterone screening test: a small sample study. Journal of Clinical Endocrinology and Metabolism. 1990;70:1662–1667. doi: 10.1210/jcem-70-6-1662. [DOI] [PubMed] [Google Scholar]
- 27.New MI. Extensive clinical experience: nonclassical 21-hydroxylase deficiency. Journal of Clinical Endocrinology and Metabolism. 2006;91:4205–4214. doi: 10.1210/jc.2006-1645. [DOI] [PubMed] [Google Scholar]
- 28.Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism. 2010;95:4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White PC. Neonatal screening for congenital adrenal hyperplasia. Nature Reviews. Endocrinology. 2009;5:490–498. doi: 10.1038/nrendo.2009.148. [DOI] [PubMed] [Google Scholar]
- 30.Verma S, Green-Golan L, Vanryzin C, Drinkard B, Mehta SP, Weise M, Eisenhofer G, Merke DP. Adrenomedullary function in patients with nonclassic congenital adrenal hyperplasia. Hormone and Metabolic Research. 2010;42:607–612. doi: 10.1055/s-0030-1253385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaresch S, Kornely E, Kley HK, Schlaghecke R. Adrenal incidentaloma and patients with homozygous or heterozygous congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism. 1992;74:685–689. doi: 10.1210/jcem.74.3.1311000. [DOI] [PubMed] [Google Scholar]
- 32.Baumgartner-Parzer SM, Pauschenwein S, Waldhausl W, Polzler K, Nowotny P, Vierhapper H. Increased prevalence of heterozygous 21-OH germline mutations in patients with adrenal incidentalomas. Clinical Endocrinology. 2002;56:811–816. doi: 10.1046/j.1365-2265.2002.01299.x. [DOI] [PubMed] [Google Scholar]
- 33.Murakami C, Ishibashi M, Kondo M, Ohshiro S, Fujita M, Sato S, Kako M, Furue H, Mizuguchi K, Yamaji T. Adrenal myelolipoma associated with congenital adrenal 21-hydroxylase deficiency. Internal Medicine. 1992;31:803–806. doi: 10.2169/internalmedicine.31.803. [DOI] [PubMed] [Google Scholar]
- 34.Nermoen I, Folling I, Vegge K, Larmo A, Nedrebo BG, Husebye ES, Lovas K. Two adults with adrenal myelolipoma and 21-hydroxylase deficiency. Case Reports in Medicine. 2009;2009 doi: 10.1155/2009/916891. Article ID 916891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenney PJ, Wagner BJ, Rao P, Heffess CS. Myelolipoma: CT and pathologic features. Radiology. 1998;208:87–95. doi: 10.1148/radiology.208.1.9646797. [DOI] [PubMed] [Google Scholar]
- 36.Ravichandran R, Lafferty F, McGinniss MJ, Taylor HC. Congenital adrenal hyperplasia presenting as massive adrenal incidentalomas in the sixth decade of life: report of two patients with 21-hydroxylase deficiency. Journal of Clinical Endocrinology and Metabolism. 1996;81:1776–1779. doi: 10.1210/jcem.81.5.8626833. [DOI] [PubMed] [Google Scholar]
- 37.Oliva A, Duarte B, Hammadeh R, Ghosh L, Baker RJ. Myelolipoma and endocrine dysfunction. Surgery. 1988;103:711–715. [PubMed] [Google Scholar]
- 38.Armengaud JB, Charkaluk ML, Trivin C, Tardy V, Breart G, Brauner R, Chalumeau M. Precocious pubarche: distinguishing late-onset congenital adrenal hyperplasia from premature adrenarche. Journal of Clinical Endocrinology and Metabolism. 2009;94:2835–2840. doi: 10.1210/jc.2009-0314. [DOI] [PubMed] [Google Scholar]
- 39.Bidet M, Bellanne-Chantelot C, Galand-Portier MB, Golmard JL, Tardy V, Morel Y, Clauin S, Coussieu C, Boudou P, Mowzowicz I, Bachelot A, Touraine P, Kuttenn F. Fertility in women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Journal of Clinical Endocrinology and Metabolism. 2010;95:1182–1190. doi: 10.1210/jc.2009-1383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.