Abstract
The development of viral nanoparticles (VNP) displaying multiple copies of the buckyball (C60) and their photodynamic activity is described. VNP-C60 conjugates were assembled using click chemistry. Cell uptake and cell killing using white light therapy and a prostate cancer cell line is demonstrated.
Photodynamic therapy (PDT) is a minimally invasive focal therapy, less invasive than surgery and resulting in fewer adverse effects compared to systemic chemotherapy.1 In PDT, a photosensitizer is delivered systemically to the tumor and locally activated by a probe containing a low-power light source and optical fibers. Management of localized cancers using PDT holds great promise and is undergoing clinical tests. A wide array of photosensitizing drugs has been developed, such as porphyrins2 and phthalocyanine dyes.3 Novel materials currently being explored include the buckyball (C60) and its derivatives.4 Upon irradiation of C60 with UV or white light, the molecule is excited to a triplet state, which can directly interact with oxygen to generate reactive singlet oxygen. The fullerene triplet can also be reduced by biological reductants to give the fullerene radical anion; electron transfer from the latter to oxygen generates the superoxide radical and ultimately produces highly reactive hydroxyl radicals through dissociation of hydrogen peroxide.5 Either event leads to cell death. C60 is a highly hydrophobic material, and it has been reported that colloidal aggregates induce toxicity in human cells.6 A delivery vehicle is therefore required to further develop this material for in vivo PDT. Several approaches toward the solubilisation and stabilization of C60 in aqueous media have been developed; these include C60-micelle and liposome nanocomposites.7
Nevertheless, tissue-specific delivery of C60-conjugates to cancerous cells in vivo remains challenging. Therefore we turned toward the development of a bio-inspired nanotechnology, specifically viral nanoparticles (VNPs) loaded with C60 drug. Although the use of VNPs as carriers for the delivery of therapies is widely discussed, only two reports have been published describing the use of VNPs in PDT. Staphylococcus-targeted ruthenium-CCMV conjugates have been developed to treat bacterial infections,8 and MS2 particles loaded with porphyrins have been targeted to T-cells using receptor-specific aptamers as candidates for PDT in the treatment of leukemia.9 VNPs can be produced in large scale using molecular farming or fermentation. VNPs are highly multivalent and amendable through genetic engineering and chemical modification.10 Hundreds of copies of drugs, imaging moieties, or targeting ligands can be displayed on the exterior or interior VNP surfaces. We have previously shown that VNPs modified with targeting ligands can be effectively targeted to prostate tumors.11 VNPs can be used as carriers for delivery of chemotherapies12 and photosensitizers.13 In this study, we turned toward the development of bacteriophage Qβ-C60 conjugates as PDT agents for treatment of prostate cancer. The 30 nm-sized icosahedral capsid has T = 3 symmetry and is formed by 180 copies of a single coat protein. Qβ displays 720 reactive Lys side chains, 4 each per 180 identical coat protein units.
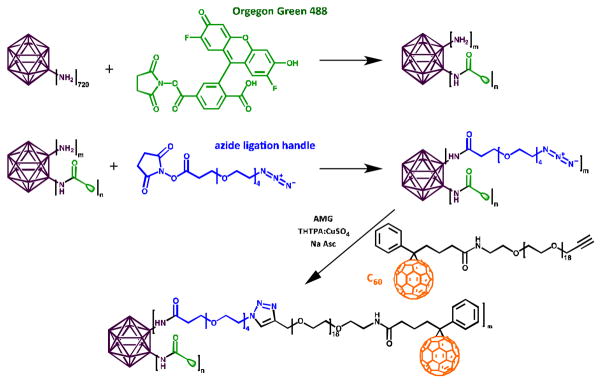
Qβ was modified with Oregon Green 488 (O488, Invitrogen) and C60 at solvent-exposed surface lysines. N-hydroxysuccinimide (NHS) ester chemistry was used to conjugate O488 and an azide ligation handle (azido (PEO)4 propionic acid succinimidyl ester, Invitrogen) to Qβ (Scheme 1).
Scheme 1.
Bioconjugation of O488 and C60 to Qβ.
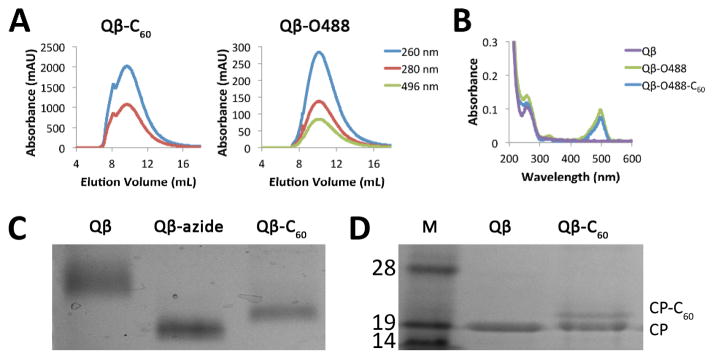
Copper-catalyzed azide-alkyne cycloaddition (CuAAC) was used to conjugate a propargyl-O-PEG-C60 derivative14 (Scheme 1 and Supporting Information). The reaction was purified and analysed by size exclusion chromatography (SEC) (Fig. 1A). Fractions corresponding to labeled, intact particles (9–15 mL) were collected and concentrated with 10 kDa cut-off centrifugal filters. SEC indicated covalent attachment of C60 and O488 to Qβ particles (Fig. 1A). Native particles elute at 10.2 mL from the column, compared to 9.7 mL for Qβ-C60 and 10.1 mL for Qβ-O488. The shift suggests that Qβ-C60 were larger in size; this is as expected, since C60 measures approximately 1 nm in diameter. Attachment of O488 was validated based on the co-elution of O488 (Abs at 496 nm) with the Qβ particles (Abs at 260 nm and 280 nm). O488 conjugation was determined using a Nanodrop spectrophotometer (Fig. 1B) and the characteristic extinction coefficient of O488 at 496 nm of 70,000 M−1 cm−1. Qβ and Qβ-C60 were labeled with 110±10 dyes each. C60 conjugation was monitored using native and denaturing SDS gel electrophoresis. After separation, the gels were photographed using AlphaImager (Biosciences) imaging system after staining with Coomassie Blue (Fig. s 1C+D). In native gels, Qβ particles change mobility based on charge and size. Mobility toward the anode increased upon azide conjugation; converting the amine group of Lys side chains into an azide results in decreased positive charge of the particles, thus enhancing the electrophoretic mobility toward the anode. Electrophoretic mobility is slowed down upon C60-conjugation due to increased size of the complex; this is in good agreement with SEC data. In denaturing gels, heating of the sample and presence of the anionic detergent would likely separate any non-covalent complex formation between Qβ and C60. Thus, the lower mobility band indicates successful covalent conjugation. Quantification of C60 moieties per Qβ particle was determined based on density analysis and ImageJ band analysis tool (http://imagej.nih.gov/ij). It was estimated that 60±10% C60 drugs were attached to Qβ (Fig. S1). Non-specific binding of C60 to Qβ was not observed; this was tested by mixing Qβ and C60, while omitting the coupling reagents, followed by analysis on native and denaturing gels.
Fig. 1.
(A) SEC of Qβ-C60 and Qβ-O488. Intact particles elute at approximately 10 mL. The co-elution of O488 (496 nm) validates attachment. (B) UV/visible spectra of Qβ, Qβ-O488, and Qβ-O488-C60. (C) Native agarose gel. (D) Qβ and Qβ-C60 proteins separated on a denaturing 4–12% SDS-PAGE. M=SeeBlue Plus2 molecular weight marker. Approximately 33% of the Qβ coat proteins (CPs) are labeled with C60 as estimated by densitometric analysis using ImageJ software.
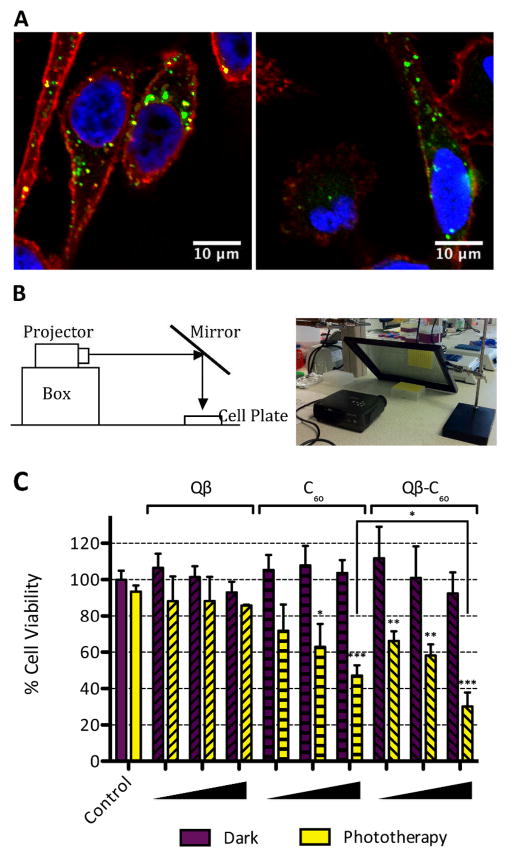
Fluorescently-labeled Qβ-C60 formulations were evaluated in tissue culture using human prostate cancer cells. Since invasive surgery at the prostate can be associated with various complications and chemotherapy induces many adverse effects, PDT is a desired alternative for the treatment of prostate cancer. We chose PC-3 cells, a highly metastatic cell line, because it is a well-characterized model for prostate cancer, and we have already developed methods to target and image PC-3 derived tumor xenografts in animal models.11 First cell interactions were evaluated using confocal microscopy to assess whether conjugation of C60 affected cellular uptake. Live PC-3 cells were incubated with 5 μg of Qβ-O488 and Qβ-O488-C60 at 37°C, 5% CO2 for 3 h. Post incubation, cells were washed thoroughly, fixed, cell membranes were stained using wheat germ agglutinin (WGA) conjugated with Alexa Fluor 555 (WGA-A555) (Invitrogen), and cell nuclei were stained using 4′,6-diamidino-2-phenylindole (DAPI) (MP Biomedicals). Confocal images were obtained using Olympus FluoView™ FV1000 LSCM and data processed using ImageJ software. We had previously shown that Qβ and Qβ-C60 complexes are taken up by breast cancer cells,14 and we show here that the VNP delivery vehicle is also taken up by prostate cancer cells (Fig. 2A). Instead of being widely distributed throughout the cytoplasm, the punctate pattern indicates uptake via endocytosis and targeting to the endolyososmal compartment; the cell interaction is consistent with data reported in the literature.12e,15 Further, uptake is inhibited at 4°C thus further supporting internalization through an energy-dependent endocytosis mechanism. Our data indicate that the display of multiple copies of hydrophobic C60 drugs does not inhibit prostate cancer cell uptake.
Fig. 2.
(A) Confocal microscopy of PC-3 cells treated with Qβ-O488 (left) and Qβ-O488-C60 (right) show internalization (green). The nucleus was stained with DAPI (blue), and the cell membrane was stained with Alexa Fluor 555-labeled wheat germ agglutinin (red). (B) Experimental set-up for white light PDT. (C) Cell viability assay indicates cell killing after an hour of phototherapy. Three concentrations were tested: 1, 10, and 25 μM C60 (corresponding to 0.0167, 0.1667, and 0.4167 μM Qβ). Statistical comparisons were made to the dark control and significance is indicated by asterisks (* p<0.05, ** p<0.01, *** p<0.001). There was a significant difference between the levels of cell killing of Qβ-C60 compared to free C60 at 25 μM drug concentration (p<0.05).
Next, drug efficacy was tested using white light therapy. Triplicates of Qβ, C60, and Qβ-C60 in 100 μL of medium were added to live cells in increasing amounts, matching the concentrations of Qβ and C60 to Qβ-C60, respectively. Following incubation for 3 h, the medium was removed, the cells were washed with saline, and 100 μL of fresh medium was added. The cells were returned to the incubator for 24 h to allow bound particles to be internalized. A mirror was then used to reflect white light from a Proxima DP1000x projector onto the cells in a 6 by 8 in. rectangle at a dose of 2 mW/cm2 (Fig. 2B). The cells were irradiated for 1 h (6.4 J/cm2). The cell plate was then incubated at 37°C for 96 h. Cell viability was measured using an XTT cell proliferation assay kit (ATCC). Cell viability data are shown in Fig. 2C. Toxicity of Qβ was not apparent in the dark, indicating that the biological nanomaterial is not cytotoxic, even at high concentrations (0.42 μM, i.e. 1.67×109 particles/cell). Data indicate increased photodynamic activity of the Qβ-C60 versus free drug compound when used at 25 μM drug concentration. Up to 70% of PC-3 cells treated with the Qβ-C60 formulation were killed using white light therapy, while only 50% of cells treated with propargyl-O-PEG-C60 alone were found dead (p < 0.05), thus indicating an advantage of delivering C60 drugs to cancer cells using VNPs.
Besides the increased photodynamic activity of VNP-C60 compared to free drug, the nanoparticle carrier offers further advantages, i.e. multivalent formulations can be designed. Qβ-C60 displays 60 C60 moieties, i.e. 660 reactive Lys side chains remain available for further functionalization with, for example, targeting ligands such as the peptide bombesin,11 and chemotherapies.12e The synergistic effect from the multivalent display of targeting ligands, along with the increased activity seen here from drug delivery via a carrier suggest the development of targeted combination therapies has the potential to greatly enhance the effectiveness of PDT. Furthermore, it should be noted that propargyl-PEG-C60 had low solubility in buffered solutions (precipitation was noted at 100 μM concentration in 20% DMSO/buffer mixtures). By visual inspection, the 25 μM concentration used for the in vitro studies was the highest concentration without noticeable precipitation. Conjugation to Qβ increased the solubility such that there was no precipitation at a concentration of 4.2 mg/mL Qβ-C60, which corresponds to an concentration of 100 μM C60.
Conclusions
We have successfully demonstrated conjugation of C60 to Qβ using click chemistry as a highly efficient method for the development of biocompatible therapeutic agents for PDT. We have shown that using Qβ as a scaffold enhances cellular internalization of the C60 by prostate cancer cells, resulting in greater therapeutic efficacy. In addition, we found that the propargyl-O-PEG-C60 derivative alone in the absence of light therapy also did not result in cell toxicity. However, its insolubility in water detracts from its potential as a PDT drug by itself. Overall, Qβ-C60 is a promising platform for PDT, with additional sites for functionalization with additional biomedically relevant moieties, such as targeting ligands or additional drugs. Future work will explore in vivo applications of Qβ-C60 for targeted and combination therapies.
Supplementary Material
Acknowledgments
This work was supported by NIH/NIBIB grants R00 EB009105 and P30 EB011317 (NFS); T32 EB007509 training fellowship (AMW), U. Dayton Honors Thesis Research Fellowship (MJR); Alcoa undergraduate student funding (ACY). We thank Prof. Comfort (U. Dayton) for co-mentorship to MJR and Prof. Finn and Dr. Preslovski (TSRI) for providing Qβ plasmids and THTPA ligand.
References
- 1.Moore CM, Pendse D, Emberton M. Nat Clin Pract Urol. 2009;6:18. doi: 10.1038/ncpuro1274. [DOI] [PubMed] [Google Scholar]
- 2.(a) Wolun-Cholewa M, Piedel B. Ginekol Pol. 2011;82:503. [PubMed] [Google Scholar]; (b) Evans CL, Abu-Yousif AO, Park YJ, Klein OJ, Celli JP, Rizvi I, Zheng X, Hasan T. PLoS One. 2011;6:e23434. doi: 10.1371/journal.pone.0023434. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Smith GS, Therrien B. Dalton Trans. 2011;40:10793. doi: 10.1039/c1dt11007a. [DOI] [PubMed] [Google Scholar]; (d) Zhu X, Lu W, Zhang Y, Reed A, Newton B, Fan Z, Yu H, Ray PC, Gao R. Chem Commun (Camb) 2011;47:10311. doi: 10.1039/c1cc13328d. [DOI] [PubMed] [Google Scholar]
- 3.(a) Zhao B, Yin JJ, Bilski PJ, Chignell CF, Roberts JE, He YY. Toxicol Appl Pharmacol. 2009;241:163. doi: 10.1016/j.taap.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Samia AC, Chen X, Burda C. J Am Chem Soc. 2003;125:15736. doi: 10.1021/ja0386905. [DOI] [PubMed] [Google Scholar]; (c) Anderson CY, Freye K, Tubesing KA, Li YS, Kenney ME, Mukhtar H, Elmets CA. Photochem Photobiol. 1998;67:332. [PubMed] [Google Scholar]; (d) Cheng Y, Meyers JD, Broome AM, Kenney ME, Basilion JP, Burda C. J Am Chem Soc. 2011;133:2583. doi: 10.1021/ja108846h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakry R, Vallant RM, Najamul-Haq M, Rainer M, Szabo Z, Huck CW, Bonn GK. Int J Nanomedicine. 2007;2:639. [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Arbogast JW, Darmanyan AP, Foote CS, Rubin Y, Diedricj FN, Alvarez MM, Anz SJ, Whetten RL. J Phys Chem. 1991;95:11. [Google Scholar]; (b) Nakanishi I, Ohkubo K, Fujita S, Fukuzumi S, Konishi T, Fujitsuka M, Ito O, Miyata N. J Chem Soc, Perkin Trans 2. 2002;2:1829. [Google Scholar]
- 6.Kovochich M, Espinasse B, Auffan M, Hotze EM, Wessel L, Xia T, Nel AE, Wiesner MR. Environ Sci Technol. 2009;43:6378. doi: 10.1021/es900990d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Hu Z, Zhang C, Huang Y, Sun S, Guan W, Yao Y. Chem Biol Interact. 2012;195:86. doi: 10.1016/j.cbi.2011.11.003. [DOI] [PubMed] [Google Scholar]; (b) Liao F, Saitoh Y, Miwa N. Oncol Res. 2011;19:203. doi: 10.3727/096504011x12970940207805. [DOI] [PubMed] [Google Scholar]; (c) Metanawin T, Tang T, Chen R, Vernon D, Wang X. Nanotechnology. 2011;22:235604. doi: 10.1088/0957-4484/22/23/235604. [DOI] [PubMed] [Google Scholar]; (d) Otake E, Sakuma S, Torii K, Maeda A, Ohi H, Yano S, Morita A. Photochem Photobiol. 2010;86:1356. doi: 10.1111/j.1751-1097.2010.00790.x. [DOI] [PubMed] [Google Scholar]; (e) Lee I, Mackeyev Y, Cho M, Li D, Kim JH, Wilson LJ, Alvarez PJ. Environ Sci Technol. 2009;43:6604. doi: 10.1021/es901501k. [DOI] [PubMed] [Google Scholar]; (f) Akiyama M, Ikeda A, Shintani T, Doi Y, Kikuchi J, Ogawa T, Yogo K, Takeya T, Yamamoto N. Org Biomol Chem. 2008;6:1015. doi: 10.1039/b719671g. [DOI] [PubMed] [Google Scholar]; (g) Ikeda A, Doi Y, Nishiguchi K, Kitamura K, Hashizume M, Kikuchi J, Yogo K, Ogawa T, Takeya T. Org Biomol Chem. 2007;5:1158. doi: 10.1039/b701767g. [DOI] [PubMed] [Google Scholar]; (h) Iwamoto Y, Yamakoshi Y. Chem Commun (Camb) 2006:4805. doi: 10.1039/b614305a. [DOI] [PubMed] [Google Scholar]
- 8.Suci PA, Varpness Z, Gillitzer E, Douglas T, Young M. Langmuir. 2007;23:12280. doi: 10.1021/la7021424. [DOI] [PubMed] [Google Scholar]
- 9.(a) Stephanopoulos N, Tong GJ, Hsiao SC, Francis MB. Acs Nano. 2010;4:6014. doi: 10.1021/nn1014769. [DOI] [PubMed] [Google Scholar]; (b) Tong GJ, Hsiao SC, Carrico ZM, Francis MB. Journal of the American Chemical Society. 2009;131:11174. doi: 10.1021/ja903857f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pokorski JK, Steinmetz NF. Mol Pharm. 2011;8:29. doi: 10.1021/mp100225y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinmetz NF, Ablack AL, Hickey JL, Ablack J, Manocha B, Mymryk JS, Luyt LG, Lewis JD. Small. 2011;7:1664. doi: 10.1002/smll.201000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Ashley CE, Carnes EC, Phillips GK, Durfee PN, Buley MD, Lino CA, Padilla DP, Phillips B, Carter MB, Willman CL, Brinker CJ, Caldeira J, do C, Chackerian B, Wharton W, Peabody DS. ACS Nano. 2011;5:5729. doi: 10.1021/nn201397z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wu W, Hsiao SC, Carrico ZM, Francis MB. Angew Chem Int Ed Engl. 2009;48:9493. doi: 10.1002/anie.200902426. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lockney DM, Guenther RN, Loo L, Overton W, Antonelli R, Clark J, Hu M, Luft C, Lommel SA, Franzen S. Bioconjug Chem. 2011;22:67. doi: 10.1021/bc100361z. [DOI] [PubMed] [Google Scholar]; (d) Zhao Q, Chen W, Chen Y, Zhang L, Zhang J, Zhang Z. Bioconjug Chem. 2011;22:346. doi: 10.1021/bc1002532. [DOI] [PubMed] [Google Scholar]; (e) Pokorski JK, Breitenkamp K, Liepold LO, Qazi S, Finn MG. J Am Chem Soc. 2011;133:9242. doi: 10.1021/ja203286n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Stephanopoulos N, Tong GJ, Hsiao SC, Francis MB. ACS Nano. 2010;4:6014. doi: 10.1021/nn1014769. [DOI] [PubMed] [Google Scholar]; (b) Suci PA, Varpness Z, Gillitzer E, Douglas T, Young M. Langmuir. 2007;23:12280. doi: 10.1021/la7021424. [DOI] [PubMed] [Google Scholar]
- 14.Steinmetz NF, Hong V, Spoerke ED, Lu P, Breitenkamp K, Finn MG, Manchester M. J Am Chem Soc. 2009;131:17093. doi: 10.1021/ja902293w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pokorski JK, Hovlid ML, Finn MG. Chembiochem. 2011;12:2441. doi: 10.1002/cbic.201100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.