Abstract
Myocardial ischaemia–reperfusion injury can be significantly reduced by an episode(s) of ischaemia–reperfusion applied prior to or during myocardial ischaemia (MI) to peripheral tissue located at a distance from the heart; this phenomenon is called remote ischaemic conditioning (RIc). Here, we compared the efficacy of RIc in protecting the heart when the RIc stimulus is applied prior to, during and at different time points after MI. A rat model of myocardial ischaemia–reperfusion injury involved 30 min of left coronary artery occlusion followed by 120 min of reperfusion. Remote ischaemic conditioning was induced by 15 min occlusion of femoral arteries and conferred a similar degree of cardioprotection when applied 25 min prior to MI, 10 or 25 min after the onset of MI, or starting 10 min after the onset of reperfusion. These RIc stimuli reduced infarct size by 54, 56, 56 and 48% (all P < 0.001), respectively. Remote ischaemic conditioning applied 30 min into the reperfusion period was ineffective. Activation of sensory nerves by application of capsaicin was effective in establishing cardioprotection only when elicited prior to MI. Vagotomy or denervation of the peripheral ischaemic tissue both completely abolished cardioprotection induced by RIc applied prior to MI. Cardioprotection conferred by delayed remote postconditioning was not affected by either vagotomy or peripheral denervation. These results indicate that RIc confers potent cardioprotection even if applied with a significant delay after the onset of myocardial reperfusion. Cardioprotection by remote preconditioning is critically dependent on afferent innervation of the remote organ and intact parasympathetic activity, while delayed remote postconditioning appears to rely on a different signalling pathway(s).
Ischaemic heart disease is a major cause of morbidity and mortality in the western world (Lloyd-Jones et al. 2009). Reperfusion therapy, which ensures the rapid return of blood flow to the ischaemic myocardium, has been a key advance in the treatment of acute myocardial infarction (Braunwald & Kloner, 1985). However, restoration of blood flow also results in a cascade of harmful events leading to myocardial reperfusion injury (Yellon & Hausenloy, 2007; Prasad et al. 2009; Turer & Hill, 2010). Over the past two decades, the pathological mechanisms underlying reperfusion injury have been under intense scrutiny. The currently prevailing consensus states that lethal reperfusion injury – defined as the death of cardiomyocytes still viable at the end of the ischaemic period – is triggered within the first minute(s) of reperfusion (Piper & García-Dorado, 1999; Piper et al. 2004; Hausenloy et al. 2005; Ovize et al. 2010). Zhao et al. (2003) demonstrated significant reduction in infarct size in dogs when brief cycles of ischaemia–reperfusion were applied immediately at the onset of reperfusion following a prolonged ischaemic episode (ischaemic postconditioning; IPost). Likewise, in a rabbit model, IPost was effective in reducing infarct size only when initiated at the onset of reperfusion, but conferred no protection when applied 10 min into the reperfusion period (Yang et al. 2004). Kin et al. (2004), using a rat model of myocardial ischaemia–reperfusion, confirmed that IPost is only effective in protecting myocardium when applied not later than 1 min after the onset of reperfusion.
These results from in vivo experiments and numerous data obtained in studies conducted in isolated cardiomyocytes and whole heart preparations using a variety of techniques have led to a general consensus that any treatments of myocardial reperfusion injury can only be effective if applied either prior to or at the immediate onset of reperfusion (reviewed by Gomez et al. 2009; Ovize et al. 2010). However, Roubille et al. (2011) have recently demonstrated in a mouse model that IPost confers significant cardioprotection when applied as late as 30 min after reperfusion onset. These data directly challenged the prevailing concept that lethal myocardial injury occurs in the first minutes of reperfusion. The authors proposed the existence of a ‘dynamic wave-front of reperfusion-induced cell death’, rejecting the idea of an instantaneous reperfusion injury, and suggested that progressive myocardial damage develops over time during the reperfusion period.
Episode(s) of ischaemia–reperfusion in myocardial tissue remote from the index ischaemic myocardium, referred to as remote ischaemic conditioning (RIc), protect against ischaemia–reperfusion injury (Przyklenk et al. 1993). Ischaemic myocardium can also be protected by brief episodes of ischaemia–reperfusion applied either before or during ischaemia to peripheral tissue (Gho et al. 1996; Kerendi et al. 2005), and promising results of recent trials in patients with acute myocardial infarction (Bøtker et al. 2010) may facilitate the introduction of RIc procedure(s) into clinical practice.
The present study tested the hypothesis that cardioprotection can be induced by a RIc stimulus applied with a significant delay after the onset of the myocardial reperfusion. We used a rat model of myocardial ischaemia–reperfusion and compared the efficacy of RIc in establishing cardioprotection when RIc is applied prior to, during and at different time points after myocardial ischaemia. Considering that both neural (autonomic) and humoral mechanisms appear to be important for remote ischaemic preconditioning (RPrec; Hausenloy & Yellon, 2008; Lim et al. 2010), we also evaluated the relative significance of these pathways in mediating remote ischaemic pre- and delayed postconditioning.
Methods
All the experiments were performed in accordance with the European Commission Directive 86/609/EEC (European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes) and the UK Home Office (Scientific Procedures) Act (1986) with project approval from the respective Institutional Animal Care and Use Committees.
Animal preparation
Adult male Wistar rats (280–320 g) were anaesthetized with pentobarbital sodium (induction 60 mg kg−1i.p.; maintenance 10 mg kg−1 h−1i.v.). Adequate anaesthesia was ensured by maintaining stable levels of arterial blood pressure and heart rate and monitored by the absence of a withdrawal response to a paw pinch. The right carotid artery and left jugular vein were cannulated for measurement of arterial blood pressure and administration of anaesthetic, respectively. The trachea was cannulated, and the animal was artificially ventilated with room air using a positive pressure ventilator with a tidal volume of ∼8–10 ml kg−1 and a ventilator frequency of ∼60 strokes min−1. Partial pressures of O2 and CO2 as well as pH of the arterial blood were measured regularly and, if required, ventilation was adjusted accordingly to maintain these values within the physiological ranges. A standard lead II ECG was recorded throughout the experiment. The body temperature was maintained at 37.0 ± 0.2°C with a servo-controlled heating pad.
Myocardial ischaemia–reperfusion
The heart was exposed via a left thoracotomy. A 5–0 monofilament polypropylene suture was passed around the left anterior descending coronary (LAD) artery to induce a temporary occlusion. The animal was subjected to 30 min of myocardial ischaemia induced by LAD artery ligation, followed by 120 min of reperfusion. Successful coronary artery occlusion was confirmed by elevation of the ST segment and an immediate fall in blood pressure by 15–30 mmHg.
Induction of RIc
The protocol described by Shahid et al. (2008) was used. Blood supply to the limbs was interrupted for 15 min by placing vessel clamps on both femoral arteries at the proximal level ∼1 cm below the inguinal ligament. The sham-RIc procedure involved dissection of both femoral arteries, but no occlusion was performed.
Measurements of infarct size
At the end of the reperfusion period, the LAD artery was re-occluded, and 1 ml of 1.5% Evans Blue dye was injected into the jugular vein for assessment of the area at risk. The animal was then given an anaesthetic overdose (pentobarbital 250 mg kg−1i.v.), the heart was excised, and the left ventricle (LV) was isolated, frozen and sectioned into five or six transverse slices from the apex to the base. The slices were weighed and photographed. The area at risk was demarcated by the absence of Evans Blue staining. Left ventricular slices were then incubated with 1% 2,3,5-triphenyltetrazolium chloride in Tris buffer (pH 7.4) for 15 min at 37°C, fixed in 4% formalin for 24 h, and photographed again. Viable myocardium is stained red by 2,3,5-triphenyltetrazolium chloride, whereas necrotic myocardium appears pale yellow. The area at risk and the necrotic area were determined by computerized planimetry, normalized to the weight of each slice, with the degree of necrosis (i.e. infarct size) expressed as the percentage of area at risk.
Experimental protocols
Experimental protocols are illustrated in Figs 1–4.
Figure 1. Cardioprotection conferred by remote ischaemic conditioning applied prior to, during and at different time points after myocardial ischaemia (MI).
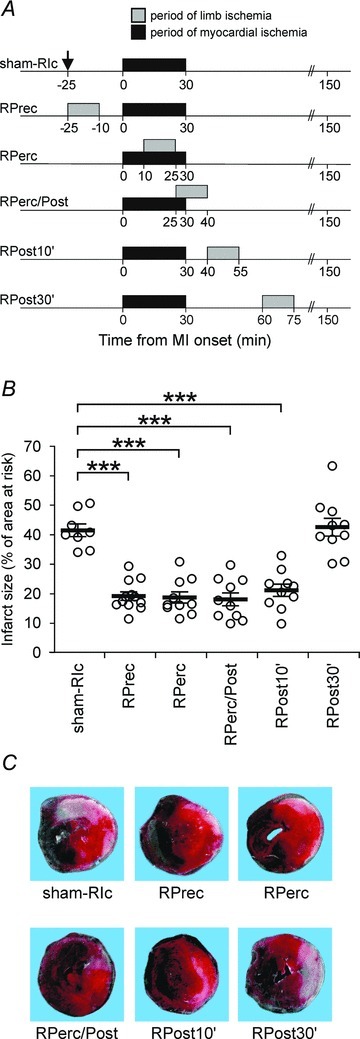
A, illustration of the experimental protocols. In all protocols, the model of myocardial ischaemia–reperfusion injury involved 30 min of left coronary artery occlusion followed by 120 min of reperfusion. Remote ischaemic conditioning (RIc) was induced by 15 min occlusion of both femoral arteries. The sham-RIc procedure involved dissection of both femoral arteries without occlusion (arrow). Abbreviations: RPrec, remote preconditioning; RPerc, remote perconditioning; RPerc/Post, remote per-postconditioning; RPost10′, delayed remote postconditioning applied 10 min into reperfusion; and RPost30′, delayed remote postconditioning applied 30 min into reperfusion. B, infarct size is presented as a percentage of the area at risk. Remote ischaemic conditioning confers significant cardioprotection when applied 25 min prior to myocardial ischaemia, 10 or 25 min after the onset of myocardial ischaemia or starting 10 min after the onset of reperfusion. Individual data and means ± SEM are shown. ***P < 0.001. C, images illustrate representative sections of triphenyltetrazolium chloride-stained hearts from all the experimental groups following 30 min ischaemia and 120 min reperfusion.
Figure 4. Intact parasympathetic activity is essential for cardioprotection induced by remote preconditioning but not by delayed remote postconditioning or direct myocardial preconditioning.
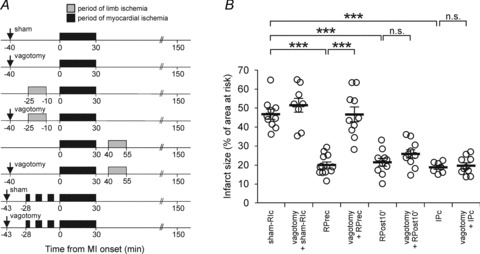
A, illustration of the experimental protocols. Arrow indicates time of bilateral vagotomy or sham surgery. B, infarct size is presented as a percentage of the area at risk. Vagotomy abolishes cardioprotection induced by remote ischaemic conditioning (RIc) applied prior to myocardial ischaemia, whereas cardioprotection conferred by delayed remote postconditioning (RPost10′) or myocardial ischaemic preconditioning (IPc) is not affected. Individual data and means ± SEM are shown. ***P < 0.001; n.s., not significant.
Efficacy of RIc cardioprotection depending upon timing of application of the RIc stimulus (Fig. 1A)
Remote ischaemic conditioning was induced by 15 min occlusion of both femoral arteries, followed by reperfusion of the limbs starting at the following time points: (1) 25 min prior to myocardial ischaemia [remote preconditioning (RPrec) group, n= 12]; (2) 10 min after the onset of myocardial ischaemia [remote perconditioning (RPrec) group, n= 10]; (3) 25 min after the onset of myocardial ischaemia and continuing 10 min into the myocardial reperfusion period [remote per-postconditioning (RPerc/Post) group, n= 10]; (4) 10 min after the onset of the reperfusion period [delayed remote postconditioning (RPost10′) group, n= 10]; and (5) 30 min after the onset of the reperfusion period [delayed remote postconditioning (RPost30′) group, n= 10]. Control animals were subjected to myocardial ischaemia–reperfusion only (n= 8).
Efficacy of capsaicin-induced cardioprotection depending upon timing of capsaicin application (Fig. 2A)
Figure 2. Activation of sensory nerves supplying peripheral tissue by capsaicin is effective in establishing cardioprotection only when applied prior to myocardial ischaemia.
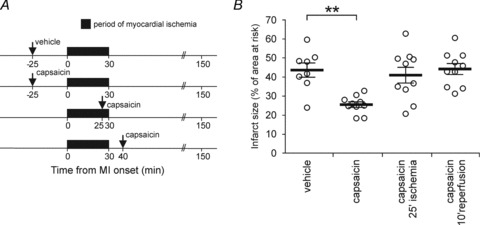
A, illustration of the experimental protocols. Arrow indicates time of subcutaneous administration of capsaicin (3 μg in 10 μl) or vehicle (10% ethanol and 10% Tween 80 in saline; 10 μl) into both hindpaws. B, infarct size is presented as a percentage of the area at risk. Capsaicin application confers significant cardioprotection only when applied prior to myocardial ischaemia. Individual data and means ± SEM are shown. **P < 0.01.
There is evidence that activation of C fibre afferents by topical application of capsaicin to the peripheral tissue prior to myocardial ischaemia–reperfusion limits myocardial injury, mimicking the effect of remote preconditioning (Jones et al. 2009). Capsaicin (3 μg in 10 μl) or vehicle (10% ethanol and 10% Tween 80 in saline; 10 μl) were injected subcutaneously into both hindpaws at the following time points: (1) 25 min prior to myocardial ischaemia (n= 10); (2) 25 min after the onset of myocardial ischaemia (n= 10); or (3) 10 min after the onset of reperfusion (n= 10).
Efficacy of RIc cardioprotection depending upon intact innervation of the remote ischaemic tissue (Fig. 3A)
Figure 3. Intact innervation of the remote ischaemic tissue is essential for cardioprotection induced by remote preconditioning but not for delayed remote postconditioning.
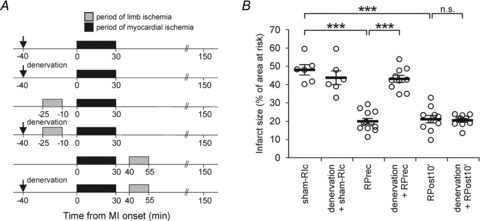
A, illustration of the experimental protocols. Arrow indicates time of limb denervation by sectioning sciatic and femoral nerves or sham surgery. B, infarct size is presented as a percentage of the area at risk. Denervation of the peripheral ischaemic tissue abolishes cardioprotection induced by remote ischaemic conditioning (RIc) applied prior to myocardial ischaemia, whereas cardioprotection conferred by delayed remote postconditioning (RPost10′) is not affected. Individual data and means ± SEM are shown. ***P < 0.001; n.s., not significant.
The femoral nerves were exposed after separation from the femoral artery and vein ∼1 cm below the inguinal ligament. The sciatic nerves were exposed following blunt dissection of the biceps femoris. Femoral arteries were occluded for 15 min commencing 25 min prior to myocardial ischaemia (RPrec group, n= 12) or 10 min after the onset of myocardial reperfusion (RPost10′ group, n= 10). Femoral and sciatic nerves were sectioned bilaterally 40 min prior to myocardial ischaemia in animals that were treated as follows: (1) subjected to sham-RIc (denervation/sham-RIc group, n= 6); (2) subjected to occlusion of the femoral arteries commencing 25 min prior to myocardial ischaemia (denervation/RPrec group, n= 10); and (3) subjected to occlusion of the femoral arteries commencing 10 min after the onset of myocardial reperfusion (denervation/RPost10′ group, n= 10). In sham-operated animals, dissection of the respective nerves was performed without sectioning (control, n= 6).
Efficacy of RIc cardioprotection depending upon intact parasympathetic activity (Fig. 4A)
Activity of the autonomic nervous system is essential for remote preconditioning, because this phenomenon is abolished under ganglionic blockade following systemic administration of hexamethonium (Loukogeorgakis et al. 2005), which blocks transmission in sympathetic and parasympathetic ganglia. Given the protective effect of the vagal tone against myocardial injury (Mioni et al. 2005; Katare et al. 2009), we assessed whether RIc applied prior to or after myocardial ischaemia requires an intact parasympathetic supply to the heart. Both vagi were exposed at the level of the neck via the ventral approach and sectioned bilaterally 40 min prior to myocardial ischaemia in animals that were treated as follows: (1) subjected to sham-RIc (vagotomy/sham-RIc group, n= 8); (2) subjected to occlusion of the femoral arteries commencing 25 min prior to myocardial ischaemia (vagotomy/RPrec group, n= 10); and (3) subjected to occlusion of the femoral arteries commencing 10 min after the onset of reperfusion (vagotomy/RPost10′ group, n= 10). In sham-operated animals, dissection of vagi was performed without sectioning (control, n= 10).
Efficacy of myocardial ischaemic preconditioning (IPc) depending upon intact parasympathetic activity (Fig. 4A)
Vagi were sectioned bilaterally 15 min prior to the IPc procedure. Ischaemic preconditioning was elicited by three brief periods of ischaemia (first 3 min, followed by two episodes lasting 5 min each) separated by 5 min periods of reperfusion before myocardial ischaemia (n= 7). In sham-operated animals, dissection of vagi was performed without sectioning (control, n= 10).
Statistical analysis
Data are reported as means ± SEM. Data were compared by ANOVA followed by the Tukey–Kramer post hoc test to determine the main group effect. Values of P < 0.05 were considered to be significant.
Results
There were no differences in the areas at risk between groups of animals recruited into any of the protocols. Figures 1–4 illustrate infarct size data displayed as percentages of the area at risk.
Efficacy of RIc cardioprotection depending upon timing of application of the RIc stimulus
In this protocol, the mean infarct size of the control group was 41 ± 2% (Fig. 1B and C). Remote ischaemic preconditioning induced by 15 min occlusion of both femoral arteries, followed by 10 min of reperfusion applied prior to myocardial ischaemia conferred significant cardioprotection, as evident from a marked reduction in infarct size (19 ± 1%, P < 0.001; Fig. 1B and C). A similar degree of cardioprotection was achieved when a 15 min period of limb ischaemia was applied either 10 or 25 min after the onset of myocardial ischaemia and continuing 10 min into reperfusion or starting 10 min after the onset of the reperfusion period (all differences are significant with P < 0.001; Fig. 1B). Remote ischaemic conditioning applied 30 min after the onset of reperfusion had no effect on infarct size. There were no differences in mean arterial pressure and heart rate between groups of animals during ischaemia and reperfusion (Table S1). Thus, the peripheral RIc stimulus is equally efficient in protecting the heart when applied before myocardial ischaemia, during ischaemia and as late as 10 min after restoration of the blood flow through the compromised myocardium.
Efficacy of capsaicin-induced cardioprotection depending upon timing of capsaicin application
Administration of capsaicin into the hindpaws 25 min prior to myocardial ischaemia markedly reduced the infarct size (by 43%, P < 0.01; Fig. 2B). Injection of capsaicin 25 min after an onset of myocardial ischaemia or 10 min into the reperfusion period had no effect on infarct size (Fig. 2B). There were no differences in systemic haemodynamic variables between groups of animals during ischaemia and reperfusion (Table S1). These data suggest that activation of sensory nerves and recruitment of the neural pathway(s) is effective in establishing cardioprotection only when applied prior to myocardial ischaemia.
Efficacy of RIc cardioprotection depending upon intact innervation of the remote ischaemic tissue
Complete denervation of the limbs by sectioning the sciatic and femoral nerves was found to abolish cardioprotection induced by RPrec (43 ± 1 versus control 44 ± 3%; Fig. 3B). Interestingly, denervation of the limbs had no effect on cardioprotection conferred by the RIc stimulus applied 10 min after the onset of reperfusion (Fig. 3B). These data are in agreement with the results of the capsaicin experiment and imply that the neural pathway of cardioprotection, which involves sensory innervation of remote tissue, is recruited if the RIc stimulus is applied prior to myocardial ischaemia.
Efficacy of RIc and myocardial IPc cardioprotection depending on intact parasympathetic activity
Bilateral vagotomy completely abolished cardioprotection induced by RIc applied 25 min prior to myocardial ischaemia (Fig. 4B). Vagotomy had no effect on cardioprotection conferred by RIc applied 10 min after the onset of reperfusion or cardioprotection established by myocardial IPc (Fig. 4B). In rats anaesthetized with pentobarbital, chronotropic vagal tone is significantly reduced (O’Leary & Jones, 2003); therefore, vagotomy had no significant effect on heart rate throughout the experimental protocol (Table S1). These observations indicate that the neural pathway of cardioprotection, which involves parasympathetic innervation of the heart, operates only when activated prior to myocardial ischaemia.
Discussion
The results of the present study demonstrate, for the first time, that a similar degree of cardioprotection could be achieved by ischaemia–reperfusion of the remote tissue applied prior to, during or as late as 10 min after the onset of myocardial reperfusion. Pathways that confer cardioprotection in response to pre- and delayed postconditioning appear to be distinct. Remote preconditioning cardioprotection is critically dependent on afferent innervation of the remote organ and intact parasympathetic activity, while delayed remote postconditioning appears to rely on a different mechanism(s).
Time-dependent postischaemic cardioprotection
Here we show that the time window for cardioprotection conferred by ischaemia–reperfusion of the remote tissue is significantly wider than was previously thought. By extension, a proportion of cardiomyocytes in the area at risk remains viable well into the reperfusion period and can be rescued by application of an appropriate therapeutic procedure, such as delayed RPost, as shown in the present study. Moreover, the degree of cardioprotection established by the RIc stimulus applied prior to, during and as late as 10 min after the onset of myocardial reperfusion was found to be remarkably similar. These results are supported by recent observations of Roubille et al. (2011) and indicate that the death of cardiomyocytes is not triggered shortly after restoration of the blood flow (Griffiths & Halestrap, 1995; Griffiths et al. 1998; Piper & García-Dorado, 1999; Di Lisa et al. 2001; Hausenloy et al. 2004, 2005; Piper et al. 2004; Argaud et al. 2005; Ovize et al. 2010) but is an ongoing process, which also occurs during the later stages of reperfusion.
The data obtained in the present study are largely in agreement with the results reported by Roubille et al. (2011), who observed a significant reduction in infarct size by delayed myocardial IPost applied 30 min after the onset of reperfusion. The key finding was the similar degree of cardioprotection conferred by delayed myocardial IPost (Roubille et al. 2011) and delayed RPost (present study). The difference in the time window for delayed cardioprotection between the two studies may be related to the fact that different animal species (rats versus mice) were used. Also, as we found no evidence that a neural pathway confers cardioprotection elicited by delayed RPost, a humoral mechanism appears to mediate this phenomenon (discussed in the following subsection). As RPost10′ in the present study involved occlusion of both femoral arteries for 15 min, only a minimal amount of humoral factor(s), if any, would be expected to be released from the ischaemic peripheral tissue into the systemic circulation for up to 25 min after restoration of the blood flow through the compromised myocardium. Thus, the time windows for direct and remote postconditioning cardioprotection may well be very similar.
Neural and humoral mechanisms of cardioprotection
Data obtained in the present study also contribute in a significant manner to an ongoing debate regarding the role of humoral versus neural mechanisms in establishing cardioprotection conferred by a remote preconditioning stimulus. The mechanism of RPrec-induced cardioprotection was suggested to involve humoral factor(s) produced during ischaemia–reperfusion of the remote tissue and released into the systemic circulation (Konstantinov et al. 2005; Hausenloy & Yellon, 2008; Shimizu et al. 2009; Kingma et al. 2011) or a neural component (Gho et al. 1996; Dong et al. 2004; Loukogeorgakis et al. 2005; Hausenloy & Yellon, 2008; Jones et al. 2009; Steensrud et al. 2010), or both (Lim et al. 2010).
Our results obtained in the experiments involving denervation of the remote organ, capsaicin application and bilateral vagotomy suggest that both neural and humoral mechanisms may be equally potent in establishing RIc cardioprotection. Their relative significance appears to be critically dependent upon the timing of application of the RIc stimulus. Indeed, in accord with the existing evidence (Dong et al. 2004; Lim et al. 2010) denervation of the remote organ abolished cardioprotection elicited by RIc applied prior to myocardial ischaemia. However, such denervation had no effect on cardioprotection conferred by RIc applied during myocardial reperfusion. Likewise, RPrec failed to establish cardioprotection following bilateral vagotomy, whereas the cardioprotective effects of delayed RPost or myocardial IPc were not affected. Finally, activation of limb C fibre afferents by topical application of capsaicin (Jones et al. 2009) was found to reduce myocardial injury dramatically, but only when capsaicin was applied before myocardial ischaemia. These observations suggest that the neural pathway of cardioprotection, which involves afferent innervation of the remote tissue and parasympathetic activity, is effective only when activated prior to myocardial ischaemia. Cardioprotection established by the RIc stimulus applied during the reperfusion period appears to recruit a completely different mechanism, which is likely to be humoral in nature. Identification of the humoral factor(s) is currently being tackled by many research groups and lies beyond the scope of the present investigation. However, our results may help to reconcile the data reported in the above-mentioned studies, because both neural and humoral pathways of cardioprotection can be recruited, depending on certain conditions (e.g. timing of RIc stimulus application).
Lethal reperfusion injury
There is a general consensus that lethal reperfusion injury is triggered within the first few minutes following reopening of the occluded coronary artery (Piper & García-Dorado, 1999; Piper et al. 2004; Hausenloy et al. 2005; Ovize et al. 2010). In addition to the evidence mentioned in the Introduction, this idea is supported by experimental studies that implicate opening of the mitochondrial permeability transition pore (mPTP) within the first minutes (∼5 min) of reperfusion (Griffiths & Halestrap, 1995; Griffiths et al. 1998; Di Lisa et al. 2001) as the key event responsible for cardiomyocyte death (Crompton et al. 1987; Borutaite et al. 2003; Green & Kroemer, 2004; Baines, 2009). Evidence supporting a key role of mPTP opening in lethal reperfusion injury was also obtained in transgenic animals (Lim et al. 2007). Signalling pathways and mechanisms implicated in cardioprotection during myocardial ischaemia–reperfusion, including KATP channels, reactive oxygen species, nitric oxide and others, were suggested to be active upstream of mPTP. Inhibition of mPTP is also thought to be the main mechanism of the beneficial action of prosurvival kinases, such as reperfusion injury salvage (RISK) kinase (Juhaszova et al. 2004; Hausenloy et al. 2005) and survivor activating factor enhancement (SAFE) kinase (Boengler et al. 2010). The data obtained in the present study suggest that reperfusion cell death is not entirely mediated via mPTP opening or that not all cardiomyocytes in the area at risk display the same dynamics of mPTP opening or that cardiomyocytes remain viable and can be rescued after (partial) mPTP opening.
Clinical relevance
Identification of possible targets and development of therapeutic strategies to protect the heart following acute myocardial infarction has been a challenging task. Demonstration that IPc of the heart confers significant cardioprotection against lethal ischaemia–reperfusion injury by recruitment of innate protective mechanisms (Murry et al. 1986) triggered significant interest in identification of these mechanisms. Subsequently, Zhao et al. (2003) described a similar cardioprotective effect of IPost, elicited by cycles of coronary artery occlusion–reperfusion applied at the immediate onset of reperfusion.
However, widespread routine clinical application of myocardial IPc and IPost in patients with acute myocardial infarction is limited by the unpredictable onset of the ischaemic event or by technical difficulties requiring complex invasive procedures to apply direct myocardial IPost. Remote ischaemic conditioning represents an effective and safe strategy for limiting myocardial injury (Bøtker et al. 2010) and may be easily applied via a limb cuff to a majority of patients in different clinical settings. The timing of treatment(s) is an important issue, and results of the present study demonstrate that the RIc stimulus is highly effective in reducing myocardial injury even if applied with a significant delay after restoration of blood flow through the compromised myocardium.
Conclusion
Remote ischaemic conditioning effectively protects the heart against ischaemia–reperfusion injury even if applied with a significant delay after the onset of myocardial reperfusion. Remote preconditioning cardioprotection is critically dependent on afferent innervation of the remote organ and intact parasympathetic activity, while delayed remote postconditioning recruits a different signalling pathway(s). These results support data recently reported by Roubille et al. (2011) and together challenge the current theory that lethal reperfusion injury is triggered within the first minutes of reperfusion.
Acknowledgments
This study was supported by the British Council (S.M.), Academy of Medical Sciences/Health Foundation (G.L.A.), The Wellcome Trust (A.V.G.), Swedish Research Council (10857) and Swedish Heart-Lung Foundation.
Supporting Information
The following supporting information is available in the online version of this article:
Table S1.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supplememtarymaterials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Argafud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M. Postconditioning inhibits mitochondrial permeability transition. Circulation. 2005;111:194–197. doi: 10.1161/01.CIR.0000151290.04952.3B. [DOI] [PubMed] [Google Scholar]
- Baines CP. The mitochondrial permeability transition pore and ischemia/reperfusion injury. Basic Res Cardiol. 2009;104:181–188. doi: 10.1007/s00395-009-0004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borutaite V, Jekabsone A, Morkuniene R, Brown GC. Inhibition of mitochondrial permeability transition prevents mitochondrial dysfunction, cytochrome c release and apoptosis induced by heart ischemia. J Mol Cell Cardiol. 2003;35:357–366. doi: 10.1016/s0022-2828(03)00005-1. [DOI] [PubMed] [Google Scholar]
- Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sørensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76:1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M, Costi A, Hayat L. Evidence for the presence of a reversible Ca2+-dependent pore activated by oxidative stress in heart mitochondria. Biochem J. 1987;245:915–918. doi: 10.1042/bj2450915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lisa F, Menabò R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- Dong JH, Liu YX, Ji ES, He RR. Limb ischemic preconditioning reduces infarct size following myocardial ischemia-reperfusion in rats. Sheng Li Xue Bao. 2004;56:41–46. [PubMed] [Google Scholar]
- Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193–2000. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- Gomez L, Li B, Mewton N, Sanchez I, Piot C, Elbaz M, Ovize M. Inhibition of mitochondrial permeability transition pore opening: translation to patients. Cardiovasc Res. 2009;83:226–233. doi: 10.1093/cvr/cvp063. [DOI] [PubMed] [Google Scholar]
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307:93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths EJ, Ocampo CJ, Savage JS, Rutter GA, Hansford RG, Stern MD, Silverman HS. Mitochondrial calcium transporting pathways during hypoxia and reoxygenation in single rat cardiomyocytes. Cardiovasc Res. 1998;39:423–433. doi: 10.1016/s0008-6363(98)00104-7. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–386. doi: 10.1093/cvr/cvn114. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM, Mani-Babu S, Duchen MR. Preconditioning protects by inhibiting the mitochondrial permeability transition. Am J Physiol Heart Circ Physiol. 2004;287:H841–H849. doi: 10.1152/ajpheart.00678.2003. [DOI] [PubMed] [Google Scholar]
- Jones WK, Fan GC, Liao S, Zhang JM, Wang Y, Weintraub NL, Kranias EG, Schultz JE, Lorenz J, Ren X. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation. 2009;120:S1–S9. doi: 10.1161/CIRCULATIONAHA.108.843938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113:1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katare RG, Ando M, Kakinuma Y, Arikawa M, Handa T, Yamasaki F, Sato T. Vagal nerve stimulation prevents reperfusion injury through inhibition of opening of mitochondrial permeability transition pore independent of the bradycardiac effect. J Thorac Cardiovasc Surg. 2009;137:223–231. doi: 10.1016/j.jtcvs.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Kerendi F, Kin H, Halkos ME, Jiang R, Zatta AJ, Zhao ZQ, Guyton RA, Vinten-Johansen J. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100:404–412. doi: 10.1007/s00395-005-0539-2. [DOI] [PubMed] [Google Scholar]
- Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, Kerendi F, Guyton RA, Vinten-Johansen J. Postconditioning attenuates myocardial ischemia–reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74–85. doi: 10.1016/j.cardiores.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Kingma JG, Jr, Simard D, Voisine P, Rouleau JR. Role of the autonomic nervous system in cardioprotection by remote preconditioning in isoflurane-anaesthetized dogs. Cardiovasc Res. 2011;89:384–391. doi: 10.1093/cvr/cvq306. [DOI] [PubMed] [Google Scholar]
- Konstantinov IE, Li J, Cheung MM, Shimizu M, Stokoe J, Kharbanda RK, Redington AN. Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation. 2005;79:1691–1695. doi: 10.1097/01.tp.0000159137.76400.5d. [DOI] [PubMed] [Google Scholar]
- Lim SY, Davidson SM, Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: the essential role of the mitochondrial permeability transition pore. Cardiovasc Res. 2007;75:530–535. doi: 10.1016/j.cardiores.2007.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol. 2010;105:651–655. doi: 10.1007/s00395-010-0099-y. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 2005;46:450–456. doi: 10.1016/j.jacc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Mioni C, Bazzani C, Giuliani D, Altavilla D, Leone S, Ferrari A, Minutoli L, Bitto A, Marini H, Zaffe D, Botticelli AR, Iannone A, Tomasi A, Bigiani A, Bertolini A, Squadrito F, Guarini S. Activation of an efferent cholinergic pathway produces strong protection against myocardial ischemia/reperfusion injury in rats. Crit Care Med. 2005;33:2621–2628. doi: 10.1097/01.ccm.0000186762.05301.13. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- O’Leary DM, Jones JF. Discharge patterns of preganglionic neurones with axons in a cardiac vagal branch in the rat. Exp Physiol. 2003;88:711–723. doi: 10.1113/eph8802590. [DOI] [PubMed] [Google Scholar]
- Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, Heusch G, Vinten-Johansen J, Yellon DM, Schulz R, Working Group of Cellular Biology of Heart of European Society of Cardiology Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2010;87:406–423. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- Piper HM, Abdallah Y, Schäfer C. The first minutes of reperfusion: a window of opportunity for cardioprotection. Cardiovasc Res. 2004;61:365–371. doi: 10.1016/j.cardiores.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Piper HM, García-Dorado D. Prime causes of rapid cardiomyocyte death during reperfusion. Ann Thorac Surg. 1999;68:1913–1919. doi: 10.1016/s0003-4975(99)01025-5. [DOI] [PubMed] [Google Scholar]
- Prasad A, Stone GW, Holmes DR, Gersh B. Reperfusion injury, microvascular dysfunction, and cardioprotection: the ‘dark side’ of reperfusion. Circulation. 2009;120:2105–2112. doi: 10.1161/CIRCULATIONAHA.108.814640. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- Roubille F, Franck-Miclo A, Covinhes A, Lafont C, Cransac F, Combes S, Vincent A, Fontanaud P, Sportouch-Dukhan C, Redt-Clouet C, Nargeot J, Piot C, Barrère-Lemaire S. Delayed postconditioning in the mouse heart in vivo. Circulation. 2011;124:1330–1336. doi: 10.1161/CIRCULATIONAHA.111.031864. [DOI] [PubMed] [Google Scholar]
- Shahid M, Tauseef M, Sharma KK, Fahim M. Brief femoral artery ischaemia provides protection against myocardial ischaemia–reperfusion injury in rats: the possible mechanisms. Exp Physiol. 2008;93:954–968. doi: 10.1113/expphysiol.2007.041442. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, Li J, Gross G, Wilson GJ, Callahan J, Redington AN. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 2009;117:191–200. doi: 10.1042/CS20080523. [DOI] [PubMed] [Google Scholar]
- Steensrud T, Li J, Dai X, Manlhiot C, Kharbanda RK, Tropak M, Redington A. Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am J Physiol Heart Circ Physiol. 2010;299:H1598–H1603. doi: 10.1152/ajpheart.00396.2010. [DOI] [PubMed] [Google Scholar]
- Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106:360–368. doi: 10.1016/j.amjcard.2010.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–1110. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


