Abstract
Depletion of glutathione by inhibition of its synthesis by buthionine sulfoximine, an irreversible inhibitor of gamma-glutamylcysteine synthetase, leads to increased sensitivity to (i) irradiation and (ii) oxidative stress. In the present work, an intracellular cysteine delivery system was used to promote glutathione synthesis, and this was found to protect against toxicity. Thus, administration of L-2-oxothiazolidine-4-carboxylate protected against acetaminophen toxicity in mice; the thiazolidine, which is converted to L-cysteine by the enzyme 5-oxo-L-prolinase (present in many animal tissues and in plants) promotes the synthesis of glutathione, which is the actual protectant. The effect of this thiazolidine in increasing the level of glutathione is prevented by administration of buthionine sulfoximine. This thiazolidine may be useful in the treatment of other toxicities and in the treatment of certain diseases. It may also be valuable as a component of amino acid mixtures used in therapy and as a safener in agriculture.
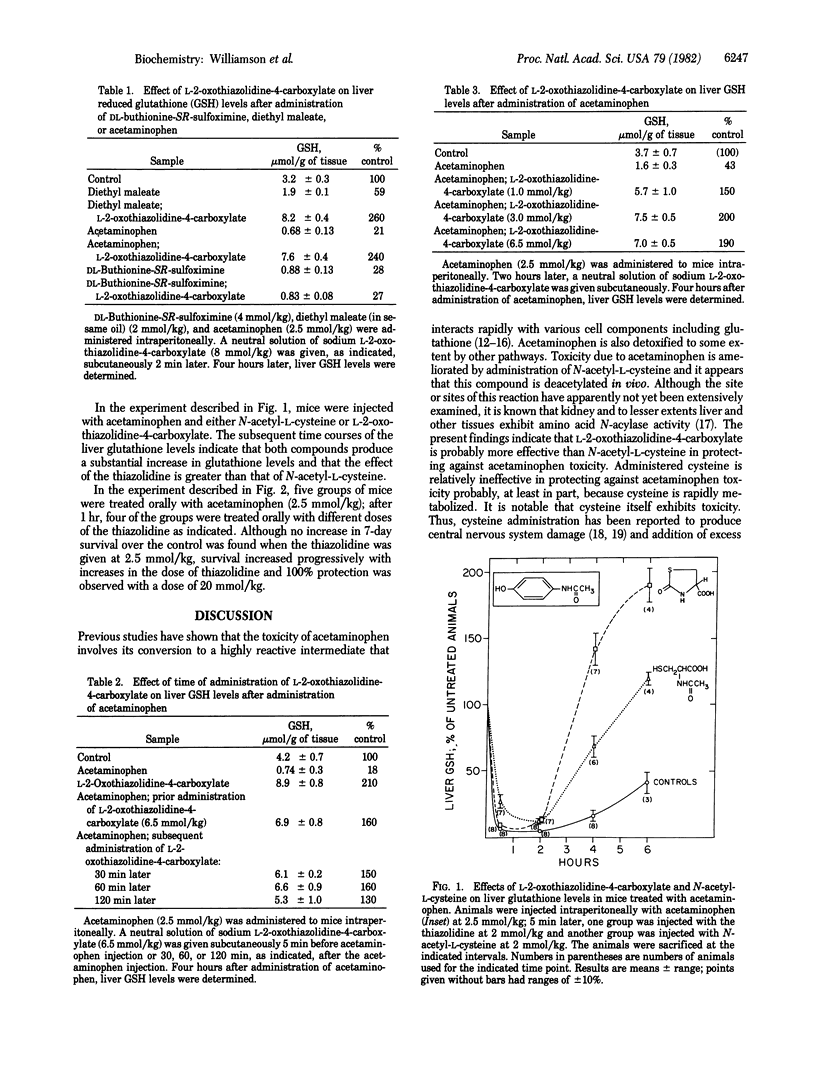
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrick B. A., Griffith O. W., Cerami A. Inhibition of glutathione synthesis as a chemotherapeutic strategy for trypanosomiasis. J Exp Med. 1981 Mar 1;153(3):720–725. doi: 10.1084/jem.153.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrick B. A., Nathan C. F., Griffith O. W., Cohn Z. A. Glutathione depletion sensitizes tumor cells to oxidative cytolysis. J Biol Chem. 1982 Feb 10;257(3):1231–1237. [PubMed] [Google Scholar]
- BIRNBAUM S. M., LEVINTOW L., KINGSLEY R. B., GREENSTEIN J. P. Specificity of amino acid acylases. J Biol Chem. 1952 Jan;194(1):455–470. [PubMed] [Google Scholar]
- BIRNBAUM S. M., WINITZ M., GREENSTEIN J. P. Quantitative nutritional studies with water-soluble, chemically defined diets. III. Individual amino acids as sources of non-essential nitrogen. Arch Biochem Biophys. 1957 Dec;72(2):428–436. doi: 10.1016/0003-9861(57)90218-7. [DOI] [PubMed] [Google Scholar]
- Burns R. A., Milner J. A. Sulfur amino acid requirements of immature Beagle dogs. J Nutr. 1981 Dec;111(12):2117–2124. doi: 10.1093/jn/111.12.2117. [DOI] [PubMed] [Google Scholar]
- DEBEY H. J., MACKENZIE J. B., MACKENZIE C. G. The replacement by thiazolidinecarboxylic acid of exogenous cystine and cysteine. J Nutr. 1958 Dec 10;66(4):607–619. doi: 10.1093/jn/66.4.607. [DOI] [PubMed] [Google Scholar]
- Dethmers J. K., Meister A. Glutathione export by human lymphoid cells: depletion of glutathione by inhibition of its synthesis decreases export and increases sensitivity to irradiation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7492–7496. doi: 10.1073/pnas.78.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Horowitz J. H., Rypins E. B., Henderson J. M., Heymsfield S. B., Moffitt S. D., Bain R. P., Chawla R. K., Bleier J. C., Rudman D. Evidence for impairment of transsulfuration pathway in cirrhosis. Gastroenterology. 1981 Oct;81(4):668–675. [PubMed] [Google Scholar]
- Jollow D. J., Mitchell J. R., Potter W. Z., Davis D. C., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973 Oct;187(1):195–202. [PubMed] [Google Scholar]
- Karlsen R. L., Grofova I., Malthe-Sørenssen D., Fonnum F. Morphological changes in rat brain induced by L-cysteine injection in newborn animals. Brain Res. 1981 Mar 9;208(1):167–180. doi: 10.1016/0006-8993(81)90628-4. [DOI] [PubMed] [Google Scholar]
- MACKENZIE C. G., HARRIS J. N-formylcysteine synthesis in mitochondria from formaldehyde and L-cysteine via thiazolidinecarboxylic acid. J Biol Chem. 1957 Jul;227(1):393–406. [PubMed] [Google Scholar]
- Mazelis M., Creveling R. K. 5-Oxoprolinase (l-Pyroglutamate Hydrolase) in Higher Plants: Partial Purification and Characterization of the Wheat Germ Enzyme. Plant Physiol. 1978 Nov;62(5):798–801. doi: 10.1104/pp.62.5.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A., Griffith O. W. Effects of methionine sulfoximine analogs on the synthesis of glutamine and glutathione: possible chemotherapeutic implications. Cancer Treat Rep. 1979 Jun;63(6):1115–1121. [PubMed] [Google Scholar]
- Meister A. On the cycles of glutathione metabolism and transport. Curr Top Cell Regul. 1981;18:21–58. doi: 10.1016/b978-0-12-152818-8.50009-8. [DOI] [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Davis D. C., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973 Oct;187(1):185–194. [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973 Oct;187(1):211–217. [PubMed] [Google Scholar]
- Moldéus P., Jones D. P., Ormstad K., Orrenius S. Formation and metabolism of a glutathione-S-conjugate in isolated rat liver and kidney cells. Biochem Biophys Res Commun. 1978 Jul 14;83(1):195–200. doi: 10.1016/0006-291x(78)90416-3. [DOI] [PubMed] [Google Scholar]
- Nagasawa H. T., Goon D. J., Zera R. T., Yuzon D. L. Prodrugs of L-cysteine as liver-protective agents. 2(RS)-Methylthiazolidine-4(R)-carboxylic acid, a latent cysteine. J Med Chem. 1982 May;25(5):489–491. doi: 10.1021/jm00347a001. [DOI] [PubMed] [Google Scholar]
- Nishiuch Y., Sasaki M., Nakayasu M., Oikawa A. Cytotoxicity of cysteine in culture media. In Vitro. 1976 Sep;12(9):635–638. doi: 10.1007/BF02797462. [DOI] [PubMed] [Google Scholar]
- Olney J. W., Ho O. L., Rhee V. Cytotoxic effects of acidic and sulphur containing amino acids on the infant mouse central nervous system. Exp Brain Res. 1971;14(1):61–76. doi: 10.1007/BF00234911. [DOI] [PubMed] [Google Scholar]
- Potter W. Z., Davis D. C., Mitchell J. R., Jollow D. J., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. 3. Cytochrome P-450-mediated covalent binding in vitro. J Pharmacol Exp Ther. 1973 Oct;187(1):203–210. [PubMed] [Google Scholar]
- Richman P. G., Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem. 1975 Feb 25;250(4):1422–1426. [PubMed] [Google Scholar]
- Rouzer C. A., Scott W. A., Griffith O. W., Hamill A. L., Cohn Z. A. Depletion of glutathione selectively inhibits synthesis of leukotriene C by macrophages. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2532–2536. doi: 10.1073/pnas.78.4.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Wellner D., Meister A. A survey of inborn errors of amino acid metabolism and transport in man. Annu Rev Biochem. 1981;50:911–968. doi: 10.1146/annurev.bi.50.070181.004403. [DOI] [PubMed] [Google Scholar]
- Williamson J. M., Meister A. Stimulation of hepatic glutathione formation by administration of L-2-oxothiazolidine-4-carboxylate, a 5-oxo-L-prolinase substrate. Proc Natl Acad Sci U S A. 1981 Feb;78(2):936–939. doi: 10.1073/pnas.78.2.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf P., Meister A. The metabolic formation and utilization of 5-oxo-L-proline (L-pyroglutamate, L-pyrrolidone carboxylate). Adv Enzymol Relat Areas Mol Biol. 1975;43:519–556. doi: 10.1002/9780470122884.ch7. [DOI] [PubMed] [Google Scholar]



