Abstract
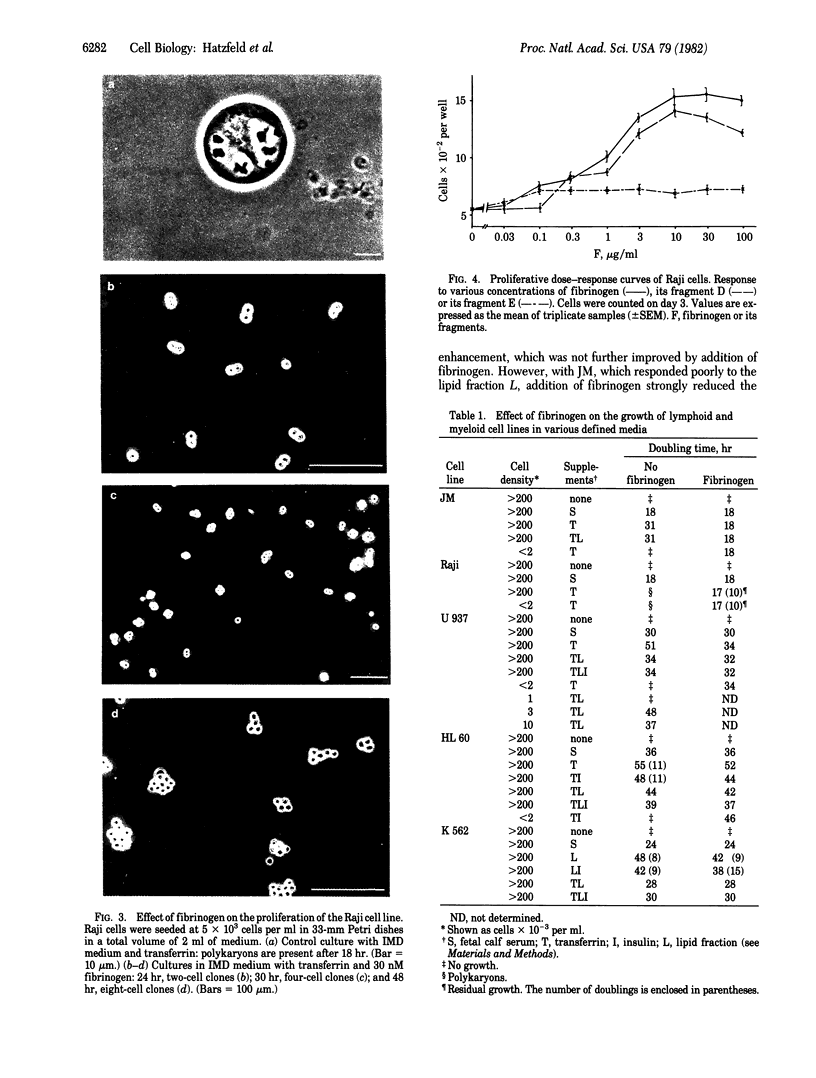
Purified fibrinogen at concentrations of 3-30 nM has been found to stimulate continuous growth of human lymphoid and myeloid cell lines under serum-free conditions. A strong proliferative response resulted from the synergism elicited by the addition of fibrinogen to transferrin-supplemented medium. This effect was observed with the pre-B-cell line Raji, the T lymphoma-derived JM, and the monocytic cell line U 937, either at high or low cell densities. With the promyelocytic cell line HL 60, fibrinogen did not shorten the doubling time of cultures seeded at high cell densities (2 x 10(5) cells per ml). However, at cell densities lower by 2 orders of magnitude and in the same medium, it promoted growth with a doubling time similar to that obtained at high cell concentrations. Fibrinogen also was found to increase the plating efficiency and colony size when human bone marrow cells were cultured in semisolid medium containing serum. In long-term bone marrow liquid cultures without fibrinogen, colony-forming cells were no longer detected after 6 weeks. In those cultured with fibrinogen, approximately equal to 50 granulocyte-macrophage colonies per 10(5) cells were obtained after 6 weeks, and 10, after 12 weeks. Purified fibrinogen fragment D possessed a stimulating activity similar to that of the intact fibrinogen molecule. This fragment cannot form fibrin, thus eliminating fibrin as a source of the mitogenic effect.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D., Sato G. Serum-free cell culture: a unifying approach. Cell. 1980 Dec;22(3):649–655. doi: 10.1016/0092-8674(80)90540-1. [DOI] [PubMed] [Google Scholar]
- Boswell H. S., Sharrow S. O., Singer A. Role of accessory cells in B cell activation. I. Macrophage presentation of TNP-Ficoll: evidence for macrophage-B cell interaction. J Immunol. 1980 Feb;124(2):989–996. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Gartner S., Kaplan H. S. Long-term culture of human bone marrow cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4756–4759. doi: 10.1073/pnas.77.8.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Watson J. Biochemical and biological characterization of lymphocyte regulatory molecules. V. Identification of an interleukin 2-producing human leukemia T cell line. J Exp Med. 1980 Dec 1;152(6):1709–1719. doi: 10.1084/jem.152.6.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmi P., Preud'homme J. L. Immunoglobulin expression in human lymphoblastoid cell lines with early B cell features. Scand J Immunol. 1981;13(4):303–311. doi: 10.1111/j.1365-3083.1981.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Hatzfeld J., Miskin R., Reich E. Acetylcholine receptor: effects of proteolysis on receptor metabolism. J Cell Biol. 1982 Jan;92(1):176–182. doi: 10.1083/jcb.92.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking W. G., Golde D. W. Long-term human bone marrow cultures. Blood. 1980 Jul;56(1):118–124. [PubMed] [Google Scholar]
- Iscove N. N., Guilbert L. J., Weyman C. Complete replacement of serum in primary cultures of erythropoietin-dependent red cell precursors (CFU-E) by albumin, transferrin, iron, unsaturated fatty acid, lecithin and cholesterol. Exp Cell Res. 1980 Mar;126(1):121–126. doi: 10.1016/0014-4827(80)90476-0. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Melchers F. Complete replacement of serum by albumin, transferrin, and soybean lipid in cultures of lipopolysaccharide-reactive B lymphocytes. J Exp Med. 1978 Mar 1;147(3):923–933. doi: 10.1084/jem.147.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F., Edgington T. S. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979 Jun 25;254(12):5357–5363. [PubMed] [Google Scholar]
- Messner H. A., Jamal N., Izaguirre C. The growth of large megakaryocyte colonies from human bone marrow. J Cell Physiol Suppl. 1982;1:45–51. doi: 10.1002/jcp.1041130410. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Sheridan A. P. Pluripotential stem cell replication in continuous human, prosimian, and murine bone marrow culture. Blood Cells. 1979 Jun 15;5(2):297–311. [PubMed] [Google Scholar]
- Orly J., Sato G. Fibronectin mediates cytokinesis and growth of rat follicular cells in serum-free medium. Cell. 1979 Jun;17(2):295–305. doi: 10.1016/0092-8674(79)90155-7. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Iscove N. N., Tees R., Aarden L., von Boehmer H. Clones of killer and helper T cells: growth requirements, specificity and retention of function in long-term culture. Immunol Rev. 1980;51:315–336. doi: 10.1111/j.1600-065x.1980.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Toogood I. R., Dexter T. M., Allen T. D., Suda T., Lajtha L. G. The development of a liquid culture system for the growth of human bone marrow. Leuk Res. 1980;4(5):449–461. doi: 10.1016/0145-2126(80)90027-2. [DOI] [PubMed] [Google Scholar]
- Williams J. E., Hantgan R. R., Hermans J., McDonagh J. Characterization of the inhibition of fibrin assembly by fibrinogen fragment D. Biochem J. 1981 Sep 1;197(3):661–668. doi: 10.1042/bj1970661. [DOI] [PMC free article] [PubMed] [Google Scholar]