Abstract
Screening complex biological specimens such as exhaled air, tissue, blood and urine to identify biomarkers in different forms of cancer has become increasingly popular over the last decade, mainly due to new instruments and improved bioinformatics. However, despite some progress, the identification of biomarkers has shown to be a difficult task with few new biomarkers (excluding recent genetic markers) being considered for introduction to clinical analysis. This review describes recent advances in gas chromatographic methods for the identification of biomarkers in the detection, diagnosis and treatment of cancer. It presents a general overview of cancer metabolism, the current biomarkers used for cancer diagnosis and treatment, a background to metabolic changes in tumors, an overview of current GC methods, and collectively presents the scope and outlook of GC methods in oncology.
Keywords: cancer, biomarkers, gas chromatography, mass spectrometry, metabolomics, exhaled air, blood, tissue, urine, extracellular fluid.
Overview of cancer metabolism
Cancer is a disease of DNA deregulation where endogenous as well as exogenous factors are linked to its development 1. Different forms of cancer (mainly lung-, prostate-, colon- and breast cancer), are now responsible for a quarter of all deaths among males and females in the United States 2. The transformation of normal cells to malignant cells is associated with important metabolic disturbances, in which the starting points and paths that a cell can take in becoming malignant are extremely variable. The resulting changes in cellular function are known as the 'hallmarks of cancer' 3 and may be described as: self-sufficient growth signals, insensitive anti-growth signals, evading apoptosis (process of programmed cell death), unlimited replication and sustained angiogenesis (growth of new blood vessels from pre-existing ones), tissue invasion and metastasis (spread of cancer), unstable genome, infection and inflammation and cancer metabolic phenotype.
The last two hallmarks - infection and inflammation 4 and cancer metabolic phenotype 5 - have recently been added to the list of the hallmarks of cancer 3. Specifically, the 'cancer metabolic phenotype' considers that mutations present are predominantly clustered in a few pathways across different cancers, therefore the ultimate effects on metabolism are similar 6. It is uniquely explained due to: high glucose uptake, increase in glycolytic activity, decrease in mitochondrial activity for the production of energy, low bioenergetic expenditure, increase in phospholipid turnover and change in the lipid profile, increase in amino acid, protein transport and DNA synthesis, increase in hypoxia (deprivation of necessary oxygen supply) and an increase in the tolerance of reactive oxygen species (ROS).
The above effects may be characterized by specific chemical signal(s) that evidence the onset or proliferation of the cancer, and so analytical chemistry methods should be able to monitor these chemical changes.
Technologies for metabolic profiling in oncology
The detection of cancer, when localized and early treatment applied, usually ends in better clinical outcomes 7. Many different methods are currently available for tumor identification, including: positron emission tomography (PET), magnetic resonance imaging (MRI), computerized tomography (CT), ultrasonography and endoscopy 8-10, which are collectively physical 'visualization/detection' methods for the presence of cancer. They do not focus on the discrete metabolic products of the cancer.
Chemical analytical methods offer an alternative approach to identification of the presence of a cancerous condition by analyzing diagnostic biomarkers, and this should constitute either a valuable confirmatory tool or a method of first choice if the link between cancer and its chemical characteristics or signature can be proven. These methods may be used for the detection of different biomarkers beside diagnostic markers. Prognostic biomarkers harbouring information on outcome regardless of treatment as well as predictive biomarkers containing information on outcome for a specific treatment may be found. In addition, the methods offer the possibility of easily repeated assessments, which allows them to be used for monitoring treatment effect.
Other technologies (in addition to the above) which have become increasingly available and are being applied by medical researchers recently for early detection and diagnosis of cancer, including: magnetic resonance spectroscopy (MRS), nuclear magnetic resonance (NMR) (mostly 1H NMR), Fourier-transform ion cyclotron resonance mass spectrometry (FT-MS) and other (MS) methods, which may often be in combination with separation methods (liquid chromatography (LC)-MS and gas chromatography (GC)-MS) 11. Whilst NMR may be a popular choice of technique employed in cancer studies ex vivo 11, each technique has its own advantages and disadvantages 12. NMR may be less sensitive, less readily automated and a more expensive approach, but it is also nonselective for metabolite detection and can simultaneously quantify multiple metabolites 13. Table 1 illustrates PET approaches, compared to NMR approaches, used for analysis of different biochemical processes linked to various cancers 6. MRS can be used as a complement to MRI, and allows for the detection of specific metabolites in vivo 14. The main advantage associated with MRS is its ability to detect and quantify certain tissue-specific metabolites (including N-acetyl-asparate, creatine/phosphocreatine, choline compounds, myo-inositol, glutamine-glutamate-GABA complex, lactate and certain free lipids). Disadvantages of MRS include its low sensitivity and spectral resolution.
Table 1.
PET tracers and NMR observed metabolites used for the diagnosis of various cancers (reproduced from 6)
| Biochemical Process | PET Tracer | NMR Observed Metabolites |
|---|---|---|
| Energy metabolism: Glycolysis; incomplete TCA | [19F]fluorodehydrogenase | citrate, glucose, acetate, glutamine, creatine, lactate, pyruvate, succinate |
| Membrane and lipid synthesis | [11C]choline [18F]fluorocholine [18F]fluoroacetate |
choline containing metabolites, glycerol, lipids, triglycerides, creatine |
| DNA synthesis | [11C]thymidine [18F]fluorothymidine |
|
| Amino acid transport and protein synthesis | 3,4-dihydroxy-6-[18F]fluoro-l-phenylalanine [11C]-L-methionine [18F]-1-m tyrosine |
Alanine, phenylalanine, threonine, tryptophan, valine, glycine, asparagines, aspartate, leucine, glutamate, glutamine, tyrosine, histidine |
| Hypoxia | [18F]fluoromisonidazole |
In comparison, MS is very sensitive for specific molecules, and it is selective and readily automated, with lower costs 15. MS is therefore emerging as a promising technique in clinical diagnosis 12. In the last decade, GC-MS in cancer detection has gained attention e.g. through the work of Phillips and co-workers, who developed a 'breathanalyzer' for automated breath analysis and detection of volatile organic compounds (VOC) from different types of cancer 16-24.
The profiling of metabolites (such as VOCs in breath analysis described above) has led to the coinage of the terms 'global metabolomics' or 'metabolomics' (which is concerned with the analysis of as many metabolites as possible) and 'targeted metabolomics' or metabolic profiling (which concentrates on specific individual, or groups of, metabolites). The term 'metabolomics' was first defined in 2002 as the unbiased quantification of all or a large number of metabolites of low molecular weight in a biological sample 25. This approach is particularly helpful in identifying metabolic network changes in pathologies, as measurements can be made in vivo, ex vivo and in vitro, in body fluids (blood, urine, liquor cerebrospinalis etc.) as well as in tissue samples and cell lines.
The challenge for any chemical instrumental method lies in its specificity towards the chemical measurement at hand, and the ability to quantify the chemical amounts and/or their changes over and above background matrix or natural levels. For potential cancer markers, this should also be at a biologically-relevant concentration. Additionally, a better understanding of cancer cell molecular routes should also offer the way to effectively classify patients in various differentially responding classes.
Biomarkers for cancer diagnosis
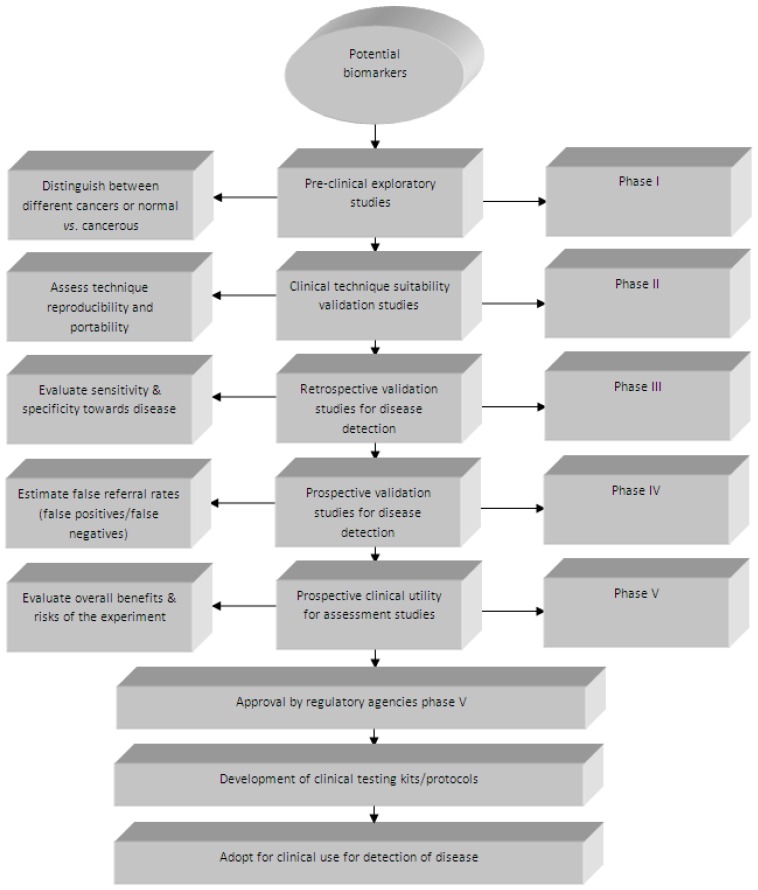
The separation of specific biomarkers from the many other genes, proteins or metabolites present in biological samples, allows their unique measurement, assessment and evaluation as an indicator of normal biological or pathologic processes (or pharmacologic responses to therapeutic interventions). These genes, proteins or metabolites, if identified and correlated with the presence of a specific cancer, can be referred to as 'biomarkers' ('cancer biomarkers' or 'onco-markers'). A biomarker has been defined as ''a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic/pharmacodynamic responses to a therapeutic intervention'' 26. They can be divided into the following three types: 1) diagnostic markers (such as tumour markers), 2) prognostic markers and 3) predictive markers. More attention should be given when determining whether a certain biomarker is a prognostic or predictive marker (even though many markers harbour both predictive and prognostic information). Currently, no biomarker is available which obtains 100% sensitivity (persons having disease) and 100% specificity (persons without disease) 27. Prostate specific antigen (PSA), which is currently the most effective serum (protein) biomarker for prostate cancer, has high sensitivity (more than 90%), but low specificity (~ 25%), which leads to many unnecessary biopsies (as the cancer is not detectable) 28. Currently, there are a few commonly used biological markers in oncology, and some biomarkers have been identified, including: epidermal growth factor receptor (EGFRmut) 29, V-Ki-ras2 kirsten rat sarcoma viral oncogene homolog (KRAS) 30, echinoderm microtubule-associated protein-like 4/ anaplastic lymphoma kinase (EML4/ALK) 31 and serine/threonine-protein kinase B-Raf (BRAF) 32. Regrettably, over the past two decades fewer than 12 biomarkers have been approved by the US Food and Drug Administration (FDA) for cancer detection, including only five 'major' biomarkers, namely: carcinoembryonic antigen (CEA) for gastrointestinal malignancies, prostate-specific antigen (PSA) for prostate cancer, cancer antigen 125 (CA125) for ovarian cancer, cancer antigen 19-9 (CA19-9) for pancreatic cancer, and carcinoma antigen 15-3 (CA 15-3) for breast cancer. Cancer, being a heterogeneous disease (comprising different biological phenotypes), results in complications for the development of biomarkers, because a marker which is initially used for one cell type might not be compatible with several different cell types. If two markers therefore originate from the same pathways, factors which contribute to their increase or decrease in cancerous cells will also (most likely) be the same. The challenge then remains to identify onco-markers that have very few, or no metabolites, in common across all cancers. The National Cancer Institute's Early Detection Research Network (NCI-EDRN) has developed a five-stage process towards discovery and evaluation of onco-markers 33 (Figure 1). Recent commentary by Diamandis also addresses some of the important current issues 34. For instance, Diamandis observes that many initially promising biomarkers have not been validated for clinical use in cancer biomarker development, and thus there is a need for rigorous validation and confirmation of the causal link to the cancer 34. Design of experiment (DOE) in cancer biomarker validation research must include the use of high quality tumor samples from patients enrolled in controlled clinical studies, as well as sufficient sample number in order to assess the specificity, sensitivity and predictive values (positive and negative) 35. In addition, the relationship between absence/presence or lower/higher levels of biomarkers and the survival times or response rates should also be scrutinized.
Figure 1.
The five different phases of onco-marker development and validation as described by the NCI-EDRN (reproduced with permission from 33)
Metabolic changes in tumors
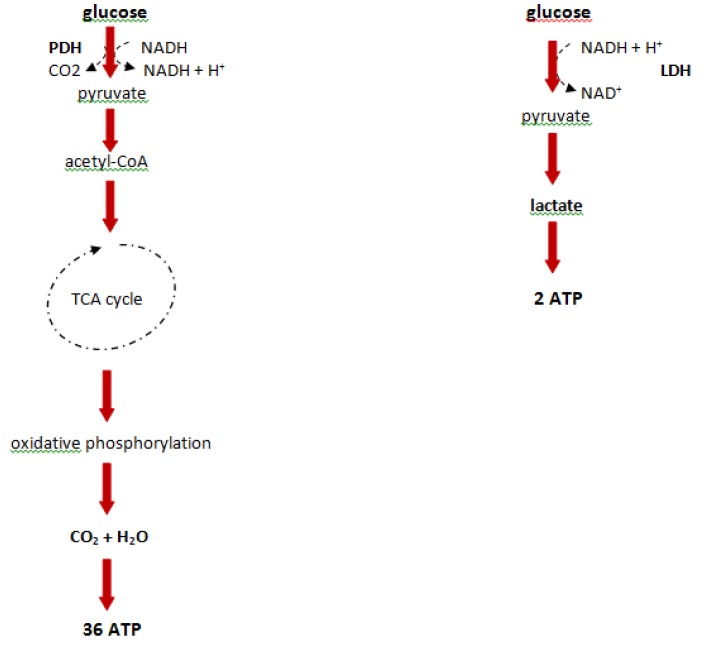
In the early 1920s, Warburg first reported an altered metabolism that is defined by increased levels of glucose and an elevated glycolytic rate, through the comparison of liver cancer cells to normal liver cells 36. This phenomenon which converts large amounts of glucose to lactate, even in the presence of oxygen, is known as the Warburg effect (aerobic glycolysis). In normal cells the presence of oxygen inhibits glycolysis as described by Pasteur (the Pasteur effect) 37. Glycolysis is the process by which glucose is metabolized to form two molecules of pyruvate with a gain of two ATP molecules from one glucose molecule. In 'normal' conditions, pyruvate converts to acetyl-coenzyme A (acetyl-CoA), which provides the basis for the citric acid cycle (TCA cycle) and oxidative phosphorylation, and this process nets about 38 ATP molecules from the glucose molecule. Although tumor cells do not use glucose efficiently, they often convert pyruvate to lactate (Figure 2). Warburg hypothesized that this inefficient use of energy in tumor cells was a defect in mitochondrial respiration, and as shown in the last few decades, mutations present in mitochondrial DNA and alterations in mitochondrial enzyme activities have been described 38, 39. However, other recent reports have also shown that in certain metabolic circumstances, tumor cells are able to switch back to mitochondrial respiration, thus mitochondrial respiration cannot be permanently defective in tumor cells 40. Tumor cells require energy and cell building blocks such as amino acids, nucleic acids and phospholipids, with cell proliferation only occurring when tumor metabolism is able to provide these 'budget metabolic intermediates', which has been defined as the 'metabolic budget system' 41.
Figure 2.
Degradation of glucose in differentiated tissues and tumor cells. PDH: pyruvate dehydrogenase, LDH: lactate dehydrogenase, CoA: co-enzyme A, NADH: nicotineamide adenine dinucleotide
The regulation of the metabolic system is very complex, but can generally be attributed to certain 'key players', such as pyruvate kinase type 2 (M2-PK), hypoxia inducible factor (HIF), serine/threonine protein kinase (Akt), tumor protein 53 (p53) and lactate dehydrogenase (LDH), which are discussed below (however, their influence on cell metabolism has yet to be deduced). Other less well-known influences include oncogenes such as phosphoinositide-3-kinase (PI3K) (ovarian and gastrointestinal cancer), human epidermal growth factor receptor 2 (Her2) (mammary carcinoma) and tumor suppressors including von Hippel-Lindau disease (VHL) (clear cell renal carcinoma), tuberous sclerosis complex -1 and -2 (TSC -1 and -2)and phosphatase and tensin homolog (PTEN) (prostate cancer) 42.
Pyruvate kinase is known to play a major role in nutrient supply and general cell proliferation. Differing isozymes of pyruvate kinase are expressed in different tissues; PK type L (L-PK) is expressed in the liver, kidney and intestine; PK type M1 in the brain and muscle; and PK type M2 in differentiated tissue such as fat tissue, lung, pancreatic islets, retina, and in all normal cells (proliferating, adult stem and embryonic) and more importantly in tumor cells 43. In normal proliferating cells M2-PK is found mainly in its tetrameric form, but tumor cells generally have large amounts of the dimeric form of M2-PK. The ratio of tetramer-to-dimer is maintained by energy production molecules such as adenosine triphosphate (ATP), as well as other oncoproteins such as HPV-16 E7 and pp60v-src 44. Several studies have also shown that M2-PK increases in the plasma of different types of tumors and in stool samples of patients with colorectal cancer 45.
Tumor hypoxia is also very important in the regulation of glycolytic metabolism, as no respiration is possible without the presence of oxygen. HIF activation plays a major role in angiogenesis and tumorgenesis as it targets the transcription of over 60 genes, and by the loss of the von Hippel-Lindau tumor suppressor. This normalizes HIF activity levels through the repression of c-Myc activity (c-Myc are cellular DNA-binding proteins encoded by the c-Myc genes, which are involved in nucleic acid metabolism and in mediating the cellular response to growth factors) or activation of receptor tyrosine kinase signaling 46. Over-expression of HIF-1 has been linked with breast, cervical, endometrial stomach and ovarian cancers 47.
The serine/threonine kinase Akt regulates glycolysis by affecting many molecules involved in the glycolytic process while maintaining the survival of the cell. Akt induces aerobic glycolysis without affecting respiration, through an activated mutant of Akt1 (Akt 1 - cell survival pathways, Akt2 - insulin signal pathway, Akt3 - expression mainly in the brain). Akt1 (namely Myr-Akt1) triggers the accumulation of nicotinamide adenine dinucleotide (NADH) and lactate, and accelerates the consumption of glucose (Figure 2) 48.
Tumor protein 53 (Tp53) is generally known as the 'protector' of the genome, due to its role in cell cycle regulation, and is often mutated in tumors 49. The role of Tp53 in creating a metabolic checkpoint for cell cycle progression in not as well-known 50. In addition, the loss of Tp53 results in increased glycolysis, through the loss of the protein SCO2 homolog (SCO2) expression, impairing respiration and leaving glycolysis as the main source of ATP production 49.
Lactate dehydrogenase (LDH) mediates the conversion of pyruvate to lactate, which usually occurs under circumstances where there is limited oxygen. In tumor cells, lactate accumulates which is indicative of a change in the balance between glycolysis and oxidative phosphorylation. The over-expression of LDH in tumor cells can be a vital part of the metabolic changes which occur during tumorgenesis, as previous studies have shown that the expression of LDH can be induced directly by oncogenes (c-Myc) 51 or indirectly through the activation of HIF-1 52.
Since all the above processes involve exquisite changes to cell activity (and cells - by definition - are the progenitor of metabolites) then it is sensible to integrate the products of metabolites within cells for evidence of the overproduction or repression of specific metabolites to evaluate cancer activity. The assessment of volatile and semi-volatile compounds for their role in cancer identification using GC is reviewed below.
Overview of gas chromatographic methods in diagnosis, prognosis and monitoring treatment protocols of cancer
In this section the important role that GC can play in metabolic profiling, and recent developments in technology through the analysis of differing sample-types, including blood, urine, tissues, cell lines, saliva, tears and exhaled air, will be highlighted. Primarily key studies that have been highly cited will be included in this review, as indications of the primary types of cancer they are relevant to.
Exhaled air
The analysis of breath has recently generated considerable interest as a promising non-invasive approach, allowing for the detection of oxidative stress biomarkers, and in the pathogenesis of different cancers and other types of diseases 53-55. Different classes of VOCs can be measured in exhaled breath, including substances derived from fatty acid lipid peroxidation of cell membranes (saturated hydrocarbons and oxygen-containing compounds). The first study of this type, was performed by Linus Pauling in 1971, who detected about 250 VOCs in a sample of breath by gas-liquid partition chromatography 56.
Phillips and co-workers, brilliantly developed novel methods for the detection of volatile organic compounds (VOC) in breath of patients suffering from different types of cancer 16-24, 57. A recent example of their work is the detection of lung cancer through the use of weighted digital analysis (WDA) of breath biomarkers, in which breath samples were collected from 404 patients (193 with untreated primary lung cancer and 211 controls with no lung cancer), and a number of biomarkers were employed in a multivariate model which was able to predict disease with 84.5% sensitivity and 81.0% specificity 17. This model used 30 breath VOCs (Table 2) (mainly alkanes, alkane derivatives and benzene derivatives), and was an improvement on the multilinear regression analysis of the same data which yielded sensitivity of 68.4% and specificity of 73.5%. This logic was based on the work of Schneider et al. who showed that a new marker tumor M2-PK improved sensitivity to the detection of progression of lung cancer 58. The authors also noted the importance of compromising between the additions of more variables (with the possibility of obtaining improved accuracy) the inclusion of variables with poorer correlations can degrade predictive accuracy.
Table 2.
Major VOC identifiers of primary lung cancer in breath (reproduced from 17)
| Metabolite number | Breath VOC |
|---|---|
| 1 | Isopropyl alcohol |
| 2 | 4-penten-ol |
| 3 | Ethane, 1,1,2-trichloro-1,2,2-trifluoro |
| 4 | Propane, 2-methoxy-2-methyl |
| 5 | 1-propene, 1-(methylthio)-, (E)- |
| 6 | 2,3-hexanedione |
| 7 | 5,5-dimethyl-1,3-hexadiene |
| 8 | 3-hexanone, 2-methyl- |
| 9 | 1H-indene, 2,3-dihydro-4-methyl |
| 10 | Camphor |
| 11 | Bicyclo[2,2,1]heptan-2-one, 1,7,7-trimethyl-,(IS)- |
| 12 | 3-cyclohexane-1-methanol, 4-trimethyl- |
| 13 | p-menth-1-en-8-ol |
| 14 | 5-isopropenyl-2-methyl-7-oxabicyclo[4,1,0]heptan-2-ol |
| 15 | Isomethyl ionone |
| 16 | 2,2,7,7-tetramethyltricyclo[6,2,1,0(1,6)]undec-4-en-3-one |
| 17 | 2,2,4-trimethyl-1,3-pentanediol diisobutyrate |
| 18 | Benzoic acid, 4-ethoxy-, ethyl ester |
| 19 | Bicyclo[3,2,2]nonane-1,5-dicarboxylic acid, 5-ethyl ester |
| 20 | Pentanoic acid, 2,2,4-trimethyl-3-carboxyisopropyl, isobutyl easter |
| 21 | Propanoic acid, 2-methyl-, 1-(1,1-dimethylethyl)-2-methyl-1,3-propanediyl ester |
| 22 | 1,2,4,5-tetroxane, 3,3,6,6-tetraphenyl- |
| 23 | Benzophenone |
| 24 | 2,5-cyclohexadien-1-one, 2,6-bis(1,1-dimethylethyl)-4-ethyllidene |
| 25 | Furan, 2-[(2-ethoxy-3,4-dimethyl-2-cyclohexen-1-ylidene)methyl]- |
| 26 | Benzene, 1,1-(1,2-cyclobutanediyl)bis-, cis- |
| 27 | Benzene, 1,1-[1-(ethylthio)propylidene]bis- |
| 28 | Anthracene, 1,2,3,4-tetrahydro-9-propyl- |
| 29 | 9,10-anthracenediol, 2-ethyl- |
| 30 | Benzene, 1,1-ethylidenebis[4-ethyl- |
* metabolites listed exactly as they appear in the original paper
A recent study by Poli and co-workers dealt with the determination of aldehydes in breath of patients with lung cancer by on-fiber derivatization solid-phase microextraction (SPME)-GC-MS 59. Lung cancer is the leading cause of cancer death in the world, and the 5-year survival rate is less than 20%. On-fiber SPME offers many advantages over 'conventional' breath sampling methods, including sample extraction and concentration in one step and the stated improvement of aldehyde stability 59. The method was then applied to 40 patients with non-small cell lung cancer (NSCLC) and a 38 cohort 'normal' control group who showed no symptoms, with 90% of NSCLC and 92.1% of controls being correctly identified. Discriminant (multivariate) analysis was performed to confirm initial univariate results, with a good discriminant capability (90%) being confirmed.
Song and co-workers also performed quantitative breath analysis of VOCs on lung cancer patients using GC-MS. They identified 1-butanol and 3-hydroxy-2-butanone as possible markers, since these compounds were found at significantly higher concentrations in the breath of lung cancer patients 60. Exhaled breath was collected from 43 patients with NSCLC and 41 healthy controls, with the number of compounds detected in samples ranging from 68 to 114. The quantitative values of the VOCs detected were evaluated typically by receiver operating characteristic (ROC) curve. Points on the ROC curve represent a sensitivity/specificity pair corresponding to a particular decision threshold, with the area under the ROC curve being a measure of how well a parameter can distinguish between diseased vs. normal states 61. Tests which predict disease with area under curve (AUC) > 0.9 are usually considered accurate, with the AUC for 1-butanol and 3-hydroxy-2-butanone being 0.940 and 0.964 respectively 60.
Another recent study by Kischkel et al. highlighted the need for potential breath onco-markers to undergo more rigorous standardized methods of sampling, analysis and data processing, and to also take into account the effects of environmental contaminants 62. Endogenous and exogenous breath biomarkers were determined in exhaled breath of 31 lung cancer patients, 31 smokers and 31 controls by SPME-GC-MS. Compounds such as 2,5-dimethylfuran, toluene and o-xylene were detected in smokers but not in non-smokers or ex-smokers 62. The results obtained showed that concentrations of VOCs may depend on a range of parameters, including environmental conditions or patient medical history, and that all statistical algorithms will fail if these variables are not taken into account.
The automated needle trap heart-cut GC-MS and needle trap GCxGC-ToFMS methods reported for breath gas analysis in the clinical environment was another significant study, and needle trap devices (NTD) were shown to be a promising alternative to SPME 63. The main advantages found were sensitivity, stability of sorbed substance, speed, small sample volumes, and potential on-site applicability. The sample study included 11 patients undergoing cardiac surgery, in which propofol, 1,2-dichloroethane and 2,2,4,6,6-pentamethylheptane were shown to be at higher concentrations, however 1,2-dichloroethane and 2-propanol could be due to environmental contaminants.
VOCs secreted to the headspace from cancer tissues and bacterial cultures were analyzed by SPME-GC-MS by Buszewski and co-workers, who found that 18 compounds were identified in cancerous stomach tissue, with 7 of them present in both cancerous and normal tissue 55. A similar approach was used by the same group 64 to monitor expired air arising from the presence of Helicobacter pylori in the stomach. Previous attempts have been made to identify compounds secreted from cancer tissue by Ligor et al. 65. The acetone ratio (AR) (ratio between acetone peak area and peak area of other compounds) was calculated for ethanol, butane, carbon disulfide, 1-propanol, 2-butanone and 2-pentanone, in order to characterize the physiological meaning and the diagnostic potential of the compounds. Acetone has previously been identified as an important biomarker in diabetes and ketoacidosis 66. Concentrations of aliphatic hydrocarbons ranged between 4.5-136.0 ppb and 3.0-97.3 ppb for oxygen-containing molecules. The method proposed might be used as a rapid screening method for the detection of early carcinogenic processes in the stomach.
Tissue
Careful sample preparation is needed for the analysis of tissue, as tumor tissue can also be contaminated by cells on the periphery of the tissue and stroma. Sample microdissection or fine needle aspirate is able to limit the contamination; however this requires expert sample collection and more expensive resources. Important work in the identification of biomarkers in cancer from tissue by GC is discussed below.
Wu and co-workers identified possible tissue onco-markers for oesophageal cancer by the use of GC-MS 67. Biopsied specimens of matched tumor and normal mucosae were obtained from each of 20 patients with oesophageal cancer, comprising 18 with esophageal squamous cell carcinoma (ESCC) and 2 with adenocarcinoma. A two-sample t-test was followed by a diagnostic model (principal components analysis (PCA) and ROC curves) and was used to discriminate normal from cancerous samples, and to detect 84 metabolites with identification of 20 potential onco-markers. The 20 possible biomarkers were found to be different, with a statistical significance of P<0.05, and tumors could be differentiated from normal mucosae with an AUC value of 1 67. Possible biomarkers included the chemical classes amino acids (L-valine, isoleucine, serine), carbohydrates (L-altrose, D-galactofuranoside, arabinose), nucleosides (purine, pyrimidine), fatty acids (tetradecanoic acid), inorganic acids (phosphoric acid) and others.
Metabolite profiling of human colon carcinoma by using GC-ToFMS was reported by Denkert and co-workers, who detected a total of 206 metabolites by performing a liquid-liquid extraction procedure 68. Of this number, 107 could be identified, with 84 being registered in the Kyoto encyclopedia of genes and genomes (KEGG) database and 71 being main reaction partners in at least one of the reactions annotated in KEGG reaction 69.The identified metabolites were believed to be related to abnormalities in biochemical pathways, according to a new method that calculates the distance of each pair of metabolites in the KEGG database interaction lattice. Paired samples of normal colon tissue and colorectal cancer tissue were differentiated at a bonferroni corrected significance level of p = 0.00170 and p = 0.00005 in unsupervised PCA analysis (for the first two components). Supervised analysis was performed thereafter, and found 82 metabolites to be significantly different at values of p<0.01.
Chen et al. identified metabolomic markers of gastric cancer metastasis using 100 mg tissue sample with GC-MS 70. Gastric tumors of both metastatic and non-metastatic origin were studied. PCA analysis and the AUC of ROC curves (AUC value of 1) were used to confirm the differentiation performance, with 29 different metabolites being differentially expressed (20 were up-regulated and 9 down-regulated in the metastasis group compared to the non-metastasis group). These metabolites were involved in many biochemical pathways, including glycolysis (lactic acid, alanine), serine metabolism (serine, phosphoserine), proline metabolism (proline), glutamic acid metabolism, tricarboxylic acid cycle (succinate, malic acid), nucleotide metabolism (pyrimidine), fatty acid metabolism (docosanoic acid, octadecanoic acid) and methylation (glycine), with serine and proline metabolisms being highlighted during the progression of metastasis.
Reichenbach and co-workers recently developed an important approach which avoids the problem of comprehensive peak matching, through the use of some reliable peaks for alignment and peak-based retention-plane windows to define important features which can then be appropriately matched for cross-sample analysis 71. A cohort of 18 samples from breast-cancer tumors (from different individuals) was analysed by GCxGC-HRMS. The features defined allowed classification that was useful in discriminating between samples of different grades (as labelled by a cancer pathologist) and can provide information to identify potential biomarkers. In addition, the approach described could benefit by using soft ionization with GCxGC, which would provide more direct characterization of the molecular composition.
Mal et al. used GCxGC-ToFMS to perform tissue-based global metabolic profiling of 63 samples from both normal and colorectal cancer (CRC) 72. PCA was performed on all samples, and showed that the QC samples clustered in greater proximity when compared to the control and cancer groups. The OPLS-DA (orthogonal partial least-squares discriminate analysis) model was also used, which concluded to 44 marker metabolites being selected using two latent variables (R2Y 0.979, Q2 (cum) 0.932) and a corresponding ROC curve value of AUC 1.000. The 44 significantly altered metabolites were involved in carbohydrate, lipid, amino acid and nucleotide metabolism and were linked to their respective biochemical pathways and biological relevance in CRC (colorectal cancer).
Buckendahl and co-workers investigated AMPK expression in 70 ovarian carcinomas, 14 'borderline' tumors and 5 normal ovaries by brilliantly linking protein expression data to GC-ToFMS metabolomic data 73. Overall, a higher expression was observed in ovarian carcinomas, compared to borderline tumours and normal ovaries. In addition, a decrease in AMPK expression correlated significantly with higher tumour grade and was of adverse prognosis in subjects with late tumour stages. A higher concentration of glucose was seen in AMPK-negative carcinomas, as well as overexpression of other metabolites involved in carbohydrate metabolism. The results of this study show a role of AMPK in the progression of ovarian tumours, and a prognostic impact of AMPK expression for overall patient survival.
Recently, Lv et al. analysed fatty acid profiles determined by GC-MS and multivariate analysis for the identification of possible biomarkers 74. A total of 114 patients (40 breast cancer patients, 40 benign patients and 34 normal patients) were studied by using a specific extraction method which yielded free fatty acids profiles. Three saturated fatty acids (C14:0, C16:0 and C18:0) and three unsaturated fatty acids (C18:2, C18:3 and C20:5) in breast cancer were different than the controls, with C16:0, C18:0, C18:2 and total fatty acid having the greatest potential to act as biomarkers of breast cancer. However, the results of this study should be validated in another study with a larger sample size, as tertiary 'training sets'.
An excellent example of the use of metabolic flux analysis (MFA) on human breast cancer cells was provided by Yang et al. who used 2D NMR and GC-MS after in vivo labelling with [U-13C] glucose 75. Using this method, each metabolite deriving from glucose catabolism was present as a variety of isotopomers, differing by percentage and position of 13C atoms. They were able to view changes in fluxes through: [i] the pentose phosphate pathway (PPP) and amino acid biosynthesis in MCF10 cell lines, [ii] the TCA cycle and anaplerotic pathway in hypoxia-resistant MCF-7 mammary carcinoma cells and [iii] the fatty acid metabolism in MDA-MB-435 breast cancer cells 75 treated with Orlistat (an inhibitor of FAS) 76.
Blood
There are certain advantages in choosing biofluid (blood, urine) for analyses, mainly the simpler sample preparation involved and the ease of sample acquisition 77. However, sample handling is critical as temperature, changes in pH, metabolite pK, and ionic content can all alter the metabolic profile obtained. There are established pre-collection guidelines for diet, medication and exertion, sample extraction methods and post-collection regulation of temperature and handling.
Li et al. developed a GC-MS approach for the analysis of volatiles including hexanal and heptanal in human blood using headspace single-drop microextraction (HS-SDME) with droplet derivatization 78. Hexanal and heptanal have been regarded as likely possible biomarkers for lung cancer detection, and have been found at elevated levels both in breath 21, 79 and blood analysis 66. Aldehydes were headspace extracted, concentrated and derivatized by using O-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine hydrochloride (PFBHA) reagent with aldehyde oximes formed and then analyzed by GC-MS. Optimal HS-SDME with droplet derivatization parameters included a sample temperature of 40 oC, an extraction time of 6 min, a stirring rate of 1100 rpm and a solvent volume of 2.0 μL. Hexanal and heptanal concentrations were found to be approximately 100 times higher than the concentration levels observed in the controls.
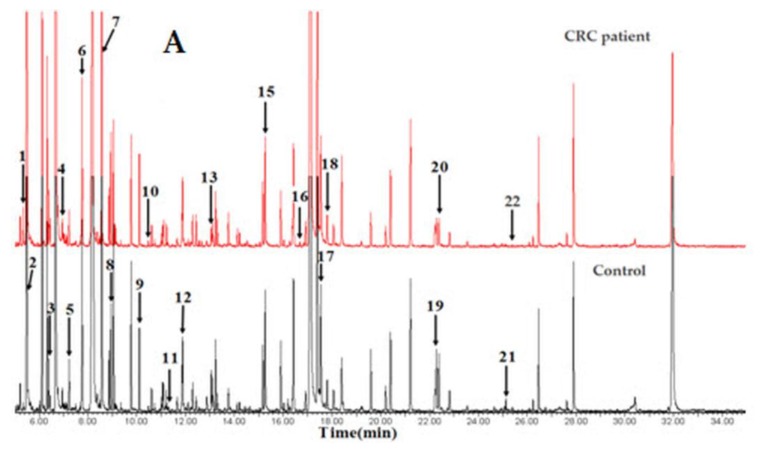
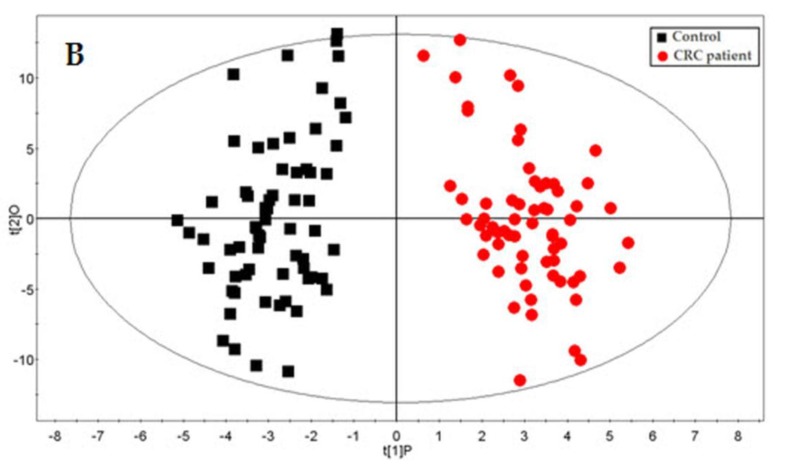
Qiu and co-workers competently demonstrated the applicability of GC-ToFMS and UPLC/QToFMS for serum metabolite profiling of human colorectal cancer, in which 64 CRC patients and 65 healthy controls were analyzed 80. OPLS-DA was used to clearly discriminate healthy controls from CRC patients. Overall, 33 different metabolites were identified using both analytical technologies (22 by GC-ToFMS and 16 by UPLC/QToFMS), with five (pyruvate, lactate, tryptophan, tyrosine and uridine) being common to both platforms. The metabolites were linked to their biochemical pathways, affecting glycolysis, arginine and proline metabolism and fatty acid metabolism, which are associated with CRC morbidity. Table 3 shows the 22 different metabolites identified by GC-ToFMS, and Figure 3 presents the total ion chromatogram (TIC) from a CRC patient and a healthy control (A) with the corresponding OPLS-DA score plot (B).
Table 3.
Different metabolites obtained by OPLS-DA using GC/ToFMS (reproduced from 66)
| Metabolite number | Retention time | Metabolite |
|---|---|---|
| 1 | 5.343 | pyruvate |
| 2 | 5.497 | lactate |
| 3 | 6.452 | 2-hydroxybutanoic acid |
| 4 | 6.956 | 3-hydroxybutanoic acid |
| 5 | 7.231 | urea |
| 6 | 7.762 | valine |
| 7 | 8.563 | leucine |
| 8 | 8.948 | proline |
| 9 | 10.110 | threonine |
| 10 | 10.727 | threonic acid |
| 11 | 11.442 | malic acid |
| 12 | 11.928 | 4-hydroxyproline |
| 13 | 13.059 | citrulline |
| 14 | 15.261 | 2-piperdinecarcarboxylic acid |
| 15 | 16.028 | ornithine |
| 16 | 16.32 | hippurate |
| 17 | 17.551 | lysine |
| 18 | 17.807 | tyrosine |
| 19 | 22.225 | tryptophan |
| 20 | 22.495 | oleic acid |
| 21 | 25.135 | oleamide |
| 22 | 25.392 | uridine |
Figure 3.
GC-ToFMS analysis of colorectal cancer (CRC) patient and healthy control: (A) TIC of CRC patient (top) and healthy control (bottom) and (B) OPLS-DA scores plot differentiating CRC patients and healthy controls (reproduced with permission from 80)
Urine
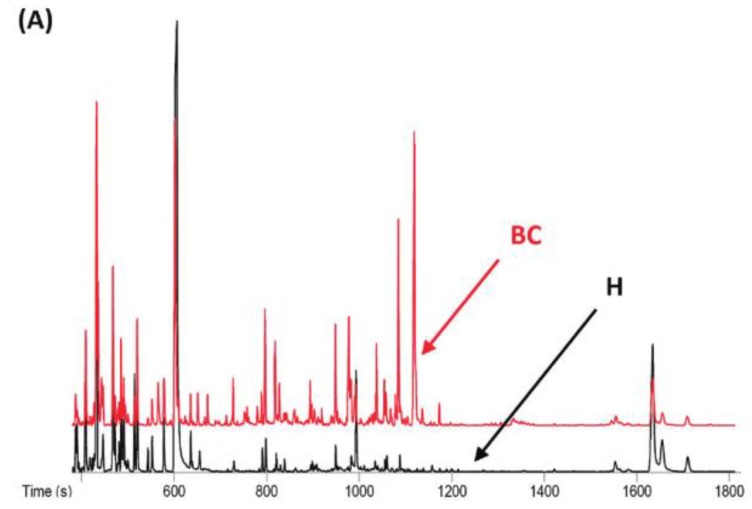
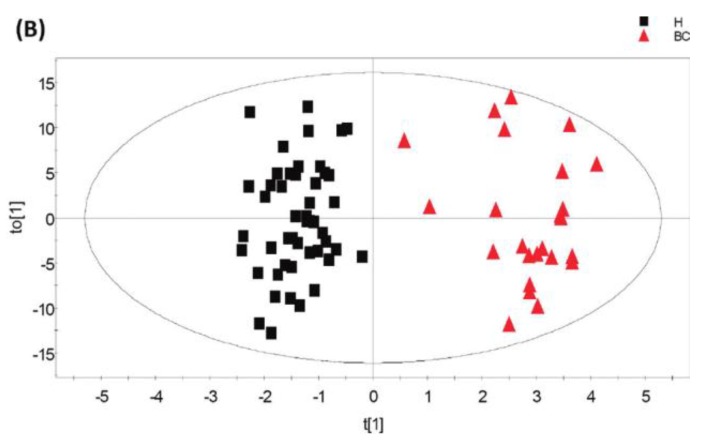
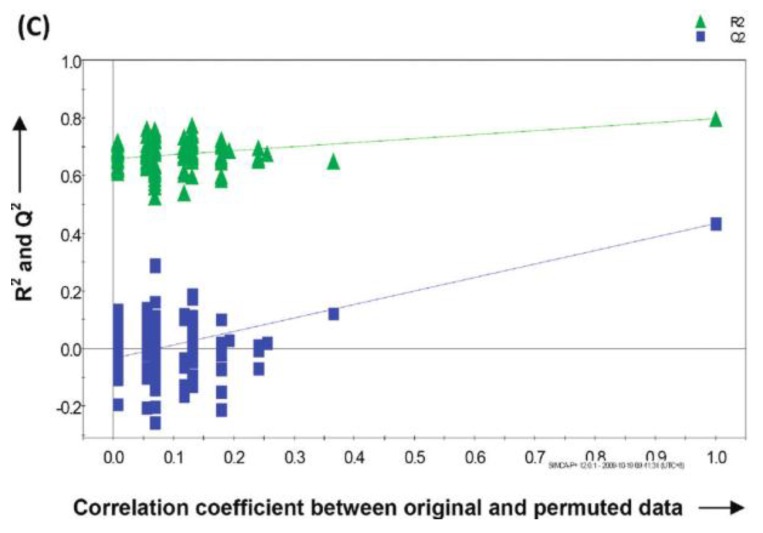
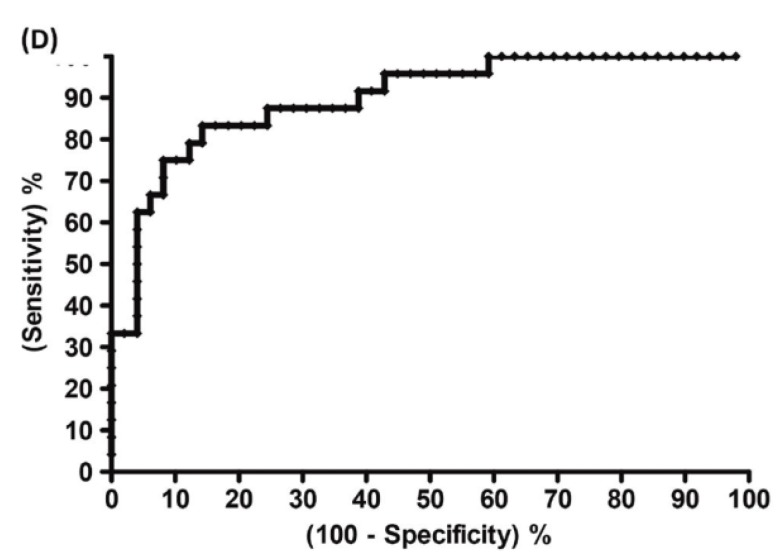
A recent study showed that well-trained dogs can detect the presence of cancer in a cancer patient's urine samples based on urine odour 81. The causative link between odour and the dog's response must clearly be due to small volatile molecules arising from the cancer. Using this knowledge, Pasikanti and co-workers undertook a noninvasive urinary metabonomic diagnosis of human bladder cancer (BC) using GC-ToFMS, by profiling the urinary metabolites of 24 BC subjects and 51 healthy controls 82. The data obtained were analyzed by multivariate principal component analysis followed by OPLS-DA, in which PC patients were clearly differentiated from non-BC subjects (OPLS-DA, 4 latent variables, R2X = 0.420, R2Y = 0.912 and Q2 = (cumulative) = 0.245, ROC AUC of 0.90) (Figure 4). Fifteen biomarkers were identified according to their ability to differentiate BC and non-BC subjects (Table 4). The OPLS-DA model showed 100% specificity and sensitivity in distinguishing BC from non-BC regardless of stage and grade, and when applied to an independent set of samples, 92% sensitivity and 80% specificity were obtained.
Figure 4.
GC-ToFMS analysis of bladder cancer (BC) patient and healthy control (H): (A) TIC of BC patient (top) and healthy control (bottom), (B) OPLS-DA scores plot differentiating BC patients and healthy controls, (C) validation plot from 100 permutation tests and (D) receiver operating characteristic (ROC) calculated from validated Y-predicted values of PLS-DA model (reproduced with permission from 82)
Table 4.
Biomarkers differentiating bladder cancer (BC) from non-BC subjects by OPLS-DA using GC/ToFMS (reproduced from 81)
| Metabolite number | Metabolite identity | Ratio of change in concentration (BC compared to non-BC) |
|---|---|---|
| 1 | senecioic acid | 0.39 |
| 2 | 2-butenedioic acid | 0.37 |
| 3 | ribonic acid | 0.54 |
| 4 | 2,5-furandicarboxylic acid | 0.28 |
| 5 | melibiose | 1.70 |
| 6 | sumiki's acid | 0.22 |
| 7 | uridine | 1.81 |
| 8 | 2-propenoic acid | 0.79 |
| 9 | glycerol | 0.75 |
| 10 | gluconic acid | 0.65 |
| 11 | valerate | 0.72 |
| 12 | fructose | 0.67 |
| 13 | L-valine | 1.52 |
| 14 | citric acid | 0.77 |
| 15 | ribitol | 0.74 |
Woo et al. used metabolomic approaches for biomarker identification across three cancers specific to women's health for metabolites expressed in urine 15. This included 10 breast, 9 ovarian and 12 cervical cancer patients and 22 healthy controls, with analysis of completed by using a combination of GC-MS and LC-MS technologies. Two previously known biomarkers (5-hydroxymethyl-2-deoxyurdine and 8-hydroxy-2-deoxyguanosine) in breast cancer were confirmed, and an additional three potential markers for ovarian cancer (1-methyladenosine, 3-methyluridine and 4-androstene-3,17-dione) were significantly increased in the sample. Among the three potential biomarkers however, 1-methyladenosine and 3-methyluridine were not specific to ovarian cancer.
Kind and co-workers reported a comprehensive urinary metabolomic approach in the identification of kidney cancer (renal cell carcinoma (RCC)) by hydrophilic interaction chromatography (HILIC-LC-MS), UPLC-MS and GC-ToFMS 83. A very large part of the urine metabolome was covered by using the three techniques mentioned, with more than 2000 “mass spectral features” detected in the urine. ANOVA and PLS approaches were used to discriminate between RCC patients and healthy controls, regardless of the smaller sample cohort (6 RCC and 6 controls), with the important features being reduced to less than 30 metabolites in each data set. The analyses by GC-ToFMS using two different alignment procedures (optimized MZmine and XCMS) yielded a total number of identified components of 326 by MZmine (28 significant p<0.05) and 417 by XCMS (29 significant p<0.05).
Recently, the metabolic profiling of human urine was applied in hepatocellular carcinoma (HCC) patients using GC-MS 67. A total of 20 male HCC patients and 20 healthy male subjects were used in the study, and 103 metabolites were detected with 66 being identified as known metabolites. A two-sample t-test was performed with p<0.05, and 18 metabolites were shown to be significantly different between the HCC and 'normal' male patients. Key metabolites identified which are known to contribute to the process of urine metabolism associated with HCC include glycine (higher levels in HCC), hypoxanthine, primidine, xylitol, glucose and glycogen (all lower levels in HCC). PCA and ROC curves yielded a separation between the two groups with an AUC value of 0.9275.
Extracellular fluid
The extracellular compartment (EC) contains low molecular metabolites as well as proteins and peptides in the extracellular fluid (ECF). Chemical analytical methods may be used to detect specific biomarkers as well as metabolomic or proteomic profiles harbouring prognostic or predictive information. The ECF may be assessed using microdialysis, a method developed for the assessment of ECF in various compartments. In microdialysis, a catheter with a semi-permeable membrane at the tip is inserted into the tissue of interest and the catheter is perfused with dialysis fluid. Due to the equilibration of metabolites of the membrane the dialysis fluid may be collected and analysed. The method thereby provides a way to analyse metabolites in the ECF - not only in absolute values - but to also follow temporal changes such as changes of metabolites. The methods have gained clinical interest in the monitoring of brain-injuries in the intensive care unit setting 84, 85. The dialysate may be analysed with various methods including basic photometric methods as well as MS or NMR. In the neuro-oncological setting, the method has been developed to monitor metabolic changes in the ECF on ambulatory glioma patients 86. In this study, lower glucose levels were detected intratumourally compared to in the brain adjacent to tumour (BAT) region. The microdialysis method may also be utilised for pharmacokinetic studies to monitor drug uptake and changes in the metabolic profile during treatment. In a study of boron-phenylalanin distribution during boron neutron capture therapy (BNCT) changes in glycerol levels were detected indicating an acute cytotoxic effect of BNCT on glioma cells 87. Microdialysis may be used to monitor temporal changes of metabolic profiles during treatments such as radiotherapy and may also be utilized to deliver cytotoxic compounds and monitor metabolic treatment effect concomitantly 88. The development of modern metabolomics methods and multivariate statistics offers a new way to analyse changes in microdialysis samples. Distinct metabolic profiles were detected in brain tumour tissue using GC-ToFMS, as compared to the BAT region and interestingly the metabolite patterns changed during radiotherapy treatment of ambulatory glioma patients 89.
The scope and outlook - the future of gas chromatographic methods in oncology
The use of GC methods for the detection, diagnosis, and to monitor treatment of cancer have over the past decade become more common through the use of a variety of sample matrices, as described above (mainly exhaled air, tissue, blood and urine but also extracellular fluid). Future studies can also concentrate on different types of bodily fluids such as ascitic fluid in ovarian cancer, secretions in pancreatic cancer, or pleural fluid in lung cancer. Thus far, two multidimensional (MD) analytical tools have been predominantly used in cancer studies i.e. GC-MS including GC-ToFMS. Continuing advances in HRGC (high-resolution GC) with MS developments now allow a range of newer techniques such as GC-MS-MS (GC-Tandem MS), MDGC-MS (multi-dimensional GC with MS) and GC×GC-ToFMS (comprehensive two-dimensional GC-ToFMS) to be used to monitor changes in metabolite screening of complex biochemical processes that occur in the presence of cancerous cells. The use of these MDGC separation techniques, which are based on improvements in the separation domain (coupled GC column methods) should logically allow for the detection and identification of more metabolites, due to the improved separation offered by the coupled column technology, as discussed by Mitrevski et al. 90 and Almstetter et al. 91. This should therefore contribute to discovery of new cancer bio-markers. It is apparent that these three-dimensional instrumental methods have not been widely applied to cancer bio-marker studies, but are increasingly used for plant metabolite studies, and for an increasing array of similarly complex samples.
Kouremenos and co-workers recently presented a newly developed GC microwave derivatization method for the analysis of compounds of relevance to metabolite profiling, with GC-MS and GCxGC-ToFMS techniques used to monitor the derivatized products 92. This was subsequently applied to the screening of in-born errors of metabolism (IEMs) in infant urine 93. The method significantly decreased the time taken for derivatization, was more sensitive, and produced fewer side reactions (a more complete derivatization reaction) when compared to conventional derivatization techniques. Based on GCxGC-ToFMS data, a potentially new short-chain acid bio-marker for the IEM screen was reported. By extension, this rapid derivatization method could also be applied to the analysis of cancerous urine, blood and tissue samples, with GCxGC an obvious instrumental finish for these studies.
By the use of such technology, an improved ability to diagnose cancer earlier in the progression of the disease may become possible. By finding new biomarkers chemical analytical methods may contribute to clinical decision-making by providing new prognostic- as well as predictive information. This however, depends on several factors, such as the creation of spectral databases of metabolites, correlating identities with biochemical pathways and cross-validation of MS-identified metabolites with other quantitative assays. It is reported that 60-90% of the total metabolites which are detected in a biological matrix are unidentifiable, even with the use of fast-scanning mass spectrometers such as ToFMS with deconvolution 94. The challenge remains to develop approaches which allow the rapid identification of 'general unknowns' are needed - currently this low rate of compound identification is attributed to the lack of absolute structural information offered by mass spectrometers (i.e. isomers cannot be fully characterized; MS spectral matching is imprecise). GC×GC may assist in this through the 3-dimensional correlation of 1tR, 2tR and MS data, along the lines of present retention index/MS spectral matching in 1DGC-MS.
The studies outlined in this review demonstrate that the sufficiently robust and non-invasive metabolic profiling approaches described can be promising screening tools in the early diagnosis of cancer (complementary to existing clinical procedures). A better understanding of molecular routes correlated with metabolism in cancer cells should offer the effective classification of patients in various differentially responding classes, and provide personalized effective drug treatments.
Abbreviations
- NMR
nuclear magnetic resonance
- FT-MS
fourier transform-mass spectrometry
- GC-MS
gas chromatography-mass spectrometry
- LC-MS
liquid chromatography-mass spectrometry
- HILIC-LC-MS
hydrophilic interaction chromatography
- ToFMS
time-of-flight mass spectrometry
- HRMS
high resolution mass spectrometry
- UPLC
ultra performance liquid chromatography
- QToFMS
quadruple - time-of-flight tandem mass spectrometry
- GCxGC
comprehensive two-dimensional gas chromatography
- MDGC
multidimensional gas chromatography
- SPME
solid phase microextraction
- HS-SDME
headspace-single drop microextraction
- DOE
design of experiment
- OPLS-DA
orthogonal projections to latent structures-discriminant analysis
- MZmine
open source software for mass spectrometry data processing for LC-MS applications
- XCMS
mass spectrometry data processing for LC-MS metabolomics applications
- ROC
receiver operating characteristic
- AUC
area under curve.
Biographies
 Dr. Konstantinos A. Kouremenos obtained his Ph.D. degree in Analytical Chemistry from RMIT University, Melbourne, Australia, where he developed GCxGC methods for the metabolic profiling of infant urine (inborn errors of metabolism). He is currently a postdoctoral fellow at the Department of Molecular Biology, Umea University, Umea, Sweden and is working on the use of metabolic profiling as a tool for the early diagnosis of staphylococcus aureus infection.
Dr. Konstantinos A. Kouremenos obtained his Ph.D. degree in Analytical Chemistry from RMIT University, Melbourne, Australia, where he developed GCxGC methods for the metabolic profiling of infant urine (inborn errors of metabolism). He is currently a postdoctoral fellow at the Department of Molecular Biology, Umea University, Umea, Sweden and is working on the use of metabolic profiling as a tool for the early diagnosis of staphylococcus aureus infection.
 Dr. Mikael Johansson is a consultant in Oncology and works clinically mainly with lung cancer and brain tumors. His current field of research is biomarker discovery and signal transduction inhibitors in lung cancer and malignant glioma. He holds a position as lecturer at the Department of Oncology, Umea University, Umea, Sweden.
Dr. Mikael Johansson is a consultant in Oncology and works clinically mainly with lung cancer and brain tumors. His current field of research is biomarker discovery and signal transduction inhibitors in lung cancer and malignant glioma. He holds a position as lecturer at the Department of Oncology, Umea University, Umea, Sweden.
 Prof. Philip J. Marriott joined the School of Chemistry, Monash University, Australia, in 2010. He has been active in high resolution separations since his Ph.D. More recently, he has led a successful research program in GC×GC and MDGC, and was the first to introduce cryogenic modulation in GC×GC. He has been credited with a number of innovations in developing technologies associated with GC×GC and MDGC. In 2012 he received the Scientific Achievement Award for his work on GC×GC, and has published about 280 research papers and book chapters.
Prof. Philip J. Marriott joined the School of Chemistry, Monash University, Australia, in 2010. He has been active in high resolution separations since his Ph.D. More recently, he has led a successful research program in GC×GC and MDGC, and was the first to introduce cryogenic modulation in GC×GC. He has been credited with a number of innovations in developing technologies associated with GC×GC and MDGC. In 2012 he received the Scientific Achievement Award for his work on GC×GC, and has published about 280 research papers and book chapters.
References
- 1.Oliveira PA, Colaco A, Chaves HR. et al. Chemical carcinogenesis. An Acad Bras Cienc. 2007;79:593–616. doi: 10.1590/s0001-37652007000400004. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457:36–7. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 5.Young CD, Anderson SM. Sugar and fat - that's where it's at: metabolic changes in tumors. Breast Cancer Res. 2008;10:202. doi: 10.1186/bcr1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cuperlovic-Culf M. Wang, E, editor. Cancer Systems Biology: CRC Press, USA; 2010. Cancer Metabolic Networks. [Google Scholar]
- 7.Etzioni R, Urban N, Ramsey S. et al. The case for early detection. Nat Rev Cancer. 2003;3:243–52. doi: 10.1038/nrc1041. [DOI] [PubMed] [Google Scholar]
- 8.Bhunchet E, Hatakawa H, Sakai Y. et al. Fluorescein electronic endoscopy: a novel method for detection of early stage gastric cancer not evident to routine endoscopy. GastrointestEndosc. 2002;55:562–71. doi: 10.1067/mge.2002.122031. [DOI] [PubMed] [Google Scholar]
- 9.Lemiere C, Peretti-Viton P, Thomas P. et al. Spreading evaluation in primitive bronchogenic carcinoma: benefit of cerebral MRI compared to CT scan. Eur J Cancer. 1995;31A:1715. doi: 10.1016/0959-8049(95)00269-o. [DOI] [PubMed] [Google Scholar]
- 10.Kopans DB. Lancet 1999; 354: 2096. [DOI] [PubMed]
- 11.Serkova NJ, Glunde K. Metabolomics of cancer. Methods Mol Biol. 2009;520:273–95. doi: 10.1007/978-1-60327-811-9_20. [DOI] [PubMed] [Google Scholar]
- 12.Lindon JC, Holmes E, Bollard ME. et al. Metabolomics technologies and their applications in physiological monitoring, drug safety assessment and disease diagnosis. Biomarkers. 2004;9:1–31. doi: 10.1080/13547500410001668379. [DOI] [PubMed] [Google Scholar]
- 13.Zuppi C, Messana I, Forni F. et al. 1H NMR spectra of normal urines: Reference ranges of the major metabolites. Clin Chim Acta. 1997;265:85–97. doi: 10.1016/s0009-8981(97)00110-1. [DOI] [PubMed] [Google Scholar]
- 14.Gujar SK, Maheshwari S, Björkman-Burtscher I. et al. Magnetic resonance spectroscopy. J Neuroophthalmol. 2005;25:217–26. doi: 10.1097/01.wno.0000177307.21081.81. [DOI] [PubMed] [Google Scholar]
- 15.Woo HM, Kim KM, Choi MH. et al. Mass spectrometry based metabolomic approaches in urinary biomarker study of women's cancers. Clin Chim Acta. 2009;400:63–9. doi: 10.1016/j.cca.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Phillips M, Altorki N, Austin JHM. et al. Prediction of lung cancer using volatile biomarkers in breath. Cancer Biomarkers. 2007;3:95–109. doi: 10.3233/cbm-2007-3204. [DOI] [PubMed] [Google Scholar]
- 17.Phillips M, Altorki N, Austin JHM. et al. Detection of lung cancer using weighted digital analysis of breath biomarkers. Clin Chim Acta. 2008;393:76–84. doi: 10.1016/j.cca.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips M, Cataneo R, Cummin A. et al. Detection of lung cancer with volatile markers in the breath. Chest. 2003;123:2115– 23. doi: 10.1378/chest.123.6.2115. [DOI] [PubMed] [Google Scholar]
- 19.Phillips M, Cataneo RN, Condos R. et al. Volatile biomarkers of pulmonary tuberculosis in the breath. Tuberculosis. 2007;87:44–52. doi: 10.1016/j.tube.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Phillips M, Cataneo RN, Greenberg J. et al. Effect of age on the breath methylated alkane contour, a display of apparent new markers of oxidative stress. J Lab Clin Med. 2000;136:243–9. doi: 10.1067/mlc.2000.108943. [DOI] [PubMed] [Google Scholar]
- 21.Phillips M, Gleeson K, Hughes J. et al. Volatile organic compounds in breath as markers of lung cancer: a cross-sectional study. Lancet. 1999;353:1930– 3. doi: 10.1016/S0140-6736(98)07552-7. [DOI] [PubMed] [Google Scholar]
- 22.Phillips M, Cataneo R, Ditkoff B. et al. Prediction of breast cancer using volatile biomarkers in the breath. Breast Cancer Res Treat. 2006;99:19–21. doi: 10.1007/s10549-006-9176-1. [DOI] [PubMed] [Google Scholar]
- 23.Phillips M, Boehmer JP, Cataneo RN. et al. Prediction of heart transplant rejection with a breath test for markers of oxidative stress. Am J Cardiol. 2004;94:1593–4. doi: 10.1016/j.amjcard.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 24.Phillips M, Cataneo R, Greenberg J. et al. Effect of oxygen on breath markers of oxidative stress. Eur Respir J. 2003;21:48– 51. doi: 10.1183/09031936.02.00053402. [DOI] [PubMed] [Google Scholar]
- 25.Fiehn O. Metabolomics - the link between genotypes and phenotypes. Potsdam: Kluwer Academic Publishers; 2002. [PubMed] [Google Scholar]
- 26.Biomarkers definitions working group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 27.Manne U, Srivastava RG, Srivastava S. Recent advances in biomarkers for cancer diagnosis and treatment. Drug Discov Today. 2005;10:965–76. doi: 10.1016/S1359-6446(05)03487-2. [DOI] [PubMed] [Google Scholar]
- 28.Gillatt D, Reynard JM. What is the 'normal range' for prostate-specific antigen? Use of a receiver operating characteristic curve to evaluate a serum marker. Br J Urol. 1995;75:341–6. doi: 10.1111/j.1464-410x.1995.tb07346.x. [DOI] [PubMed] [Google Scholar]
- 29.William P, Juliann C. Rational, Biologically Based Treatment of EGFR-mutant Non-small-cell Lung Cancer. Nat Rev Cancer. 2010;10:760–74. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jančík S, Drábek J, Radzioch D. et al. Clinical Relevance of KRAS in Human Cancers. J Biomed Biotechnol. 2010:150960. doi: 10.1155/2010/150960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horn L, Pao W. EML4-ALK: honing in on a new target in non-small-cell lung cancer. J Clin Oncol. 2009;27:4232–5. doi: 10.1200/JCO.2009.23.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi M, Sonobe M, Takahashi T. et al. Clinical significance of BRAF gene mutations in patients with non-small cell lung cancer. Anticancer Res. 2011;31:4619–23. [PubMed] [Google Scholar]
- 33.Sullivan PM, Etzioni R, Feng Z. et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–61. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 34.Diamandis EP. Cancer Biomarkers: Can We Turn Recent Failures into Success? J Natl Cancer Inst. 2010;102:1–6. doi: 10.1093/jnci/djq306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon R. Roadmap for developing and validating therapeutically relevant genomic classifiers. J Clin Oncol. 2005;23:7332–41. doi: 10.1200/JCO.2005.02.8712. [DOI] [PubMed] [Google Scholar]
- 36.Warburg O. The Metabolism of Tumors. London: Constable; 1930. [Google Scholar]
- 37.Pasteur L. Experiences et vues nouvelles sur la nature des fermentations. Comp Rend Acad Sci. 1861;52:1260–4. [Google Scholar]
- 38.Cuezva JM, Krajewski M, Lopez de Heredia M. et al. The bioenergetic signature of cancer: a marker in tumor progression. Cancer Res. 2002;62:6674–81. [PubMed] [Google Scholar]
- 39.Rossingol R, Gilkerson R, Aggeler R. et al. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res. 2004;64:985–93. doi: 10.1158/0008-5472.can-03-1101. [DOI] [PubMed] [Google Scholar]
- 40.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425– 34. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 41.Eigenbrodt E, Glossmann H. Glycolysis - one of the keys to cancer? Trends Pharmacol Sci. 1980;1:240–5. [Google Scholar]
- 42.Kroemer G, Pouyssegur J. Tumor Cell Metabolism: Cancer's Achilles' Heel. Cancer Cell. 2008;13:472–82. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Reinacher M, Eigenbrodt E. Immunohistological demonstration of the same type of pyruvate kinase isoenzyme (M2-PK) in tumors of chicken and rat. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;37:79–88. doi: 10.1007/BF02892557. [DOI] [PubMed] [Google Scholar]
- 44.Mazurek S, Boschek CB, Hugo F. et al. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300– 8. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Tonus C, Neupert G, Sellinger M. Colorectal cancer screening by non-invasive metabolic biomarker fecal tumor. World J Gastroenterol. 2006;12:7007–11. doi: 10.3748/wjg.v12.i43.7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, Gao P, Fukuda R. et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of c-myc activity. Cancer Cell. 2007;11:407– 20. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Quintero M, Mackenzie N, Brennan PA. Hypoxia-inducible factor 1 (HIF-1) in cancer. Eur J Surg Oncol. 2004;30:465–8. doi: 10.1016/j.ejso.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Elstrom RL, Bauer DE, Buzzai M. et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892– 9. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 49.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307– 10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 50.Jones RG, Plas DR, Kubek S. et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283– 93. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 51.Shim H, Dolde C, Lewis BC. et al. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci USA. 1997;94:6658– 63. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68– 72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 53.Chan HP, Lewis C, Thomas PS. Exhaled breath analysis: Novel approach for early detection of lung cancer. Lung Cancer. 2009;63:164–8. doi: 10.1016/j.lungcan.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 54.Miekisch W, Schubert J, Noeldge-Schomburg G. Diagnostic potential of breath analysis - focus on volatile organic compounds. Clin Chim Acta. 2004;347:25– 39. doi: 10.1016/j.cccn.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 55.Buszewski B, Ulanowska A, Ligor T. et al. Identification of volatile organic compounds secreted from cancer tissues and bacterial cultures. J Chromatogr B. 2008;868:88–94. doi: 10.1016/j.jchromb.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 56.Pauling L, Robinson AB, Teranishi R. et al. Quantitative Analysis of Urine Vapor and Breath by Gas-Liquid Partition Chromatography. Proc Nat Acad SciUSA. 1971;68:2374–6. doi: 10.1073/pnas.68.10.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phillips M. Method for the collection and assay of volatile organic compounds in breath. Anal Biochem. 1997;247:272– 8. doi: 10.1006/abio.1997.2069. [DOI] [PubMed] [Google Scholar]
- 58.Schneider JPG, Bitterlich N, Neu K. et al. Fuzzy logic-based tumor marker profiles including a new marker tumor M2-PK improved sensitivity to the detection of progression in lung cancer patients. Anticancer Res. 2003;23:899–906. [PubMed] [Google Scholar]
- 59.Poli D, Goldoni M, Corradi M. et al. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber derivatization SPME-GC/MS. J Chromatogr B. 2010;878:2643–51. doi: 10.1016/j.jchromb.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 60.Song G, Qin T, Liu H. et al. Quantitative breath analysis of volatile organic compounds of lung cancer patients. Lung Cancer. 2010;67:227–31. doi: 10.1016/j.lungcan.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 61.Griner PF, Mayewski RJ, Mushlin AI. et al. Selection and interpretation of diagnostic tests and procedures. Principles and applications. Ann Intern Med. 1981;94(4 pt 2):557–92. [PubMed] [Google Scholar]
- 62.Kischkel S, Miekisch W, Sawacki A. et al. Breath biomarkers for lung cancer detection and assessment if smoking related effects. Clin Chim Acta. 2010;411:1637–44. doi: 10.1016/j.cca.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 63.Mieth M, Schubert JK, Groger T. et al. Automated Needle Trap Heart-Cut GC/MS and Needle Trap Comprehensive Two-Dimensional GC/TOF-MS for Breath Gas Analysis in the Clinical Environment. Anal Chem. 2010;82:2541–51. doi: 10.1021/ac100061k. [DOI] [PubMed] [Google Scholar]
- 64.Ulanowska A, Kowalkowski T, Hrynkiewicz K. et al. Determination of volatile organic compounds in human breath for Helicobacter pylori detection by SPME-GC/MS. Biomed Chromatogr. 2011;25:391–7. doi: 10.1002/bmc.1460. [DOI] [PubMed] [Google Scholar]
- 65.Ligor T, Szeliga J, Jackowski M. et al. The analysis of healthy volunteers' exhaled breath by the use of solid-phase microextraction and GC-MS. J Breath Res. 2007;2:046006. doi: 10.1088/1752-7155/2/4/046006. [DOI] [PubMed] [Google Scholar]
- 66.Deng C, Zhang J, Yu X. et al. Determination of acetone in human breath by gas chromatography-mass spectrometry and solid-phase microextraction with on-fiber derivatization. J Chromatogr B. 2004;810:269–75. doi: 10.1016/j.jchromb.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 67.Wu H, Xue R, Dong L. et al. Metabolomic profiling of human urine in hepatocellular carcinoma patients using gas chromatography/mass spectrometry. Anal Chim Acta. 2009;648:98–104. doi: 10.1016/j.aca.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 68.Denkert C, Budczies J, Weichert W. et al. Metabolite profiling of human colon carcinoma - deregulation of TCA cycle and amino acid turnover. Mol Cancer. 2008;7:72. doi: 10.1186/1476-4598-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perroud B, Lee J, Valkova N. et al. Pathway analysis of kidney cancer using proteomics and metabolic profiling. Molecular Cancer. 2006;5:64. doi: 10.1186/1476-4598-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen JL, Tang HQ, Hu JD. et al. Metabolomics of gastric cancer metastasis detected by gas chromatography and mass spectrometry. World J Gastroenterol. 2010;16:5874–80. doi: 10.3748/wjg.v16.i46.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reichenbach SE, Tian X, Tao Q. et al. Informatics for cross-sample analysis with comprehensive two-dimensional gas chromatography and high-resolution mass spectrometry (GCxGC-HRMS) Talanta. 2011;83:1279. doi: 10.1016/j.talanta.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 72.Mal M, Koh PK, Cheah PY. et al. Metabotyping of human colorectal cancer using two-dimensional gas chromatography mass spectrometry. Anal Bioanal Chem. 2012;403:483–93. doi: 10.1007/s00216-012-5870-5. [DOI] [PubMed] [Google Scholar]
- 73.Buckendahl AC, Budczies J, Fiehn O. et al. Prognostic impact of AMP-activated protein kinase expression in ovarian carcinoma: correlation of protein expression and GC/TOF-MS-based metabolomics. Oncol Rep. 2011;25:1005–12. doi: 10.3892/or.2011.1162. [DOI] [PubMed] [Google Scholar]
- 74.Lv W, Yang T. Identification of possible biomarkers for breast cancer from free fatty acid profiles determined by GC-MS and multivariate statistical analysis. Clin Biochem. 2012;45:127–33. doi: 10.1016/j.clinbiochem.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 75.Yang C, Richardson AD, Osterman A. et al. Profiling of central metabolism in human cancer cells by two-dimensional NMR, GC-MS analysis, and isotopomer modeling. Metabolomics. 2008;4:13–29. [Google Scholar]
- 76.Kridel SJ, Axelrod F, Rozenkrantz N. et al. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64:2070–5. doi: 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]
- 77.Zhang A, Sun H, Wang P. et al. Recent and potential developments of biofluid analyses in metabolomics. J Proteomics. 2012;75:1079–88. doi: 10.1016/j.jprot.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 78.Li N, Deng C, Yin X. et al. GC/MS analysis of hexanal and heptanal in human blood by HS-SDME. Anal Biochem. 2005;342:318–26. doi: 10.1016/j.ab.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 79.O'Neill HJ, Gordon SM, O'Neill MH. et al. Computerized classification technique for screening for the presence of breath biomarkers in lung cancer. ClinChem. 1988;34:1613–8. [PubMed] [Google Scholar]
- 80.Qiu Y, Cai G, Su M. et al. Serum Metabolite Profiling of Human Colorectal Cancer Using GC-TOFMS and UPLC-QTOFMS. J Proteome Res. 2009;8:4844–50. doi: 10.1021/pr9004162. [DOI] [PubMed] [Google Scholar]
- 81.Mitra AP, Datar RH, Cote RJ. Molecular pathways in invasive bladder cancer: new insights into mechanisms, progression, and target identification. J Clin Oncol. 2006;24:5552–64. doi: 10.1200/JCO.2006.08.2073. [DOI] [PubMed] [Google Scholar]
- 82.Pasikanti KK, Esuvaranathan K, Ho PC. et al. Noninvasive Urinary Metabonomic Diagnosis of Human Bladder Cancer. J Proteome Res. 2010;9:2988–95. doi: 10.1021/pr901173v. [DOI] [PubMed] [Google Scholar]
- 83.Kind T, Tolstikov V, Fiehn O. et al. A comprehensive urinary metabolomic approach for identifying kidney cancer. Anal Biochem. 2007;363:185–95. doi: 10.1016/j.ab.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 84.Persson L, Hillered L. Chemical monitoring of neurosurgical intensive care patients using intracerebral microdialysis. J Neurosurg. 1992;76:72–80. doi: 10.3171/jns.1992.76.1.0072. [DOI] [PubMed] [Google Scholar]
- 85.Persson L, Valtysson J, Enblad P. et al. Neurochemical monitoring using intra-cerebral microdialysis in patients with subarachnoid hemorrhage. J Neurosurg. 1996;84:606–16. doi: 10.3171/jns.1996.84.4.0606. [DOI] [PubMed] [Google Scholar]
- 86.Roslin M, Henriksson R, Bergstrom P. et al. Baseline levels of glucose metabolites, glutamate and glycerol in malignant glioma assessed by stereotactic microdialysis. J Neurooncol. 2003;61:151–60. doi: 10.1023/a:1022106910017. [DOI] [PubMed] [Google Scholar]
- 87.Bergenheim AT, Capala J, Roslin M. et al. Distribution of BPA and metabolic assessment in glioblastoma patients during BNCT treatment: a microdialysis study. J Neurooncol. 2005;71:287–93. doi: 10.1007/s11060-004-1724-0. [DOI] [PubMed] [Google Scholar]
- 88.Tabatabaei P, Bergstrom P, Henriksson R. et al. Glucose metabolites, glutamate and glycerol in malignant glioma tumours during radiotherapy. J Neurooncol. 2008;90:35–9. doi: 10.1007/s11060-008-9625-2. [DOI] [PubMed] [Google Scholar]
- 89.Wibom C, Surowiec I, Mörén L. et al. Metabolomic patterns in glioblastoma and changes during radiotherapy: a clinical microdialysis study. J Proteome Res. 2010;9:2909–19. doi: 10.1021/pr901088r. [DOI] [PubMed] [Google Scholar]
- 90.Mitrevski BS, Kouremenos KA, Marriott PJ. Accelerating analysis for metabolites, drugs and their metabolites in biological samples using multidimensional gas chromatography. Bioanalysis. 2009;1:367–91. doi: 10.4155/bio.09.28. [DOI] [PubMed] [Google Scholar]
- 91.Almstetter MF, Oefner PJ, Dettmer K. Comprehensive two-dimensional gas chromatography in metabolomics. Anal Bioanal Chem. 2012;402:1993–2013. doi: 10.1007/s00216-011-5630-y. [DOI] [PubMed] [Google Scholar]
- 92.Kouremenos KA, Harynuk J, Winniford WL. et al. One-pot microwave derivatization of target compounds relevant to metabolomics with comprehensive two-dimensional gas chromatography. J Chromatogr B. 2010;878:1761–70. doi: 10.1016/j.jchromb.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 93.Kouremenos KA, Pitt JJ, Marriott PJ. Metabolic profiling of infant urine using comprehensive two-dimensional gas chromatography: Application to the diagnosis of organic acidurias and biomarker. J Chromatogr A. 2010;1217:104–11. doi: 10.1016/j.chroma.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 94.Lu H, Liang Y, Dunn WB. et al. Comparative evaluation of software for deconvolution of metabolomics data based on GC-TOF-MS. Trends Anal Chem. 2008;27:215–27. [Google Scholar]