Abstract
Pulsed lasers can evoke neural activity from motor as well as sensory neurons in vivo. Lasers allow more selective spatial resolution of stimulation than the conventional electrical stimulation. To date, few studies have examined pulsed, mid-infrared laser stimulation of nerves and very little of the available optical parameter space has been studied. In this study, a pulsed diode laser, with wavelength between 1.844–1.873 μm, was used to elicit compound action potentials (CAPs) from the auditory system of the gerbil. We found that pulse durations as short as 35 μs elicit a CAP from the cochlea. In addition, repetition rates up to 13 Hz can continually stimulate cochlear spiral ganglion cells for extended periods of time. Varying the wavelength and, therefore, the optical penetration depth, allowed different populations of neurons to be stimulated. The technology of optical stimulation could significantly improve cochlear implants, which are hampered by a lack of spatial selectivity.
Index Terms: Auditory nerve, cochlear implant, spiral ganglion cell
I. Introduction
Recently, pulsed mid-infrared lasers have been used to evoke neural activity in motor systems and sensory systems, as an alternative to electrical stimulation [1]–[3]. The use of lasers to evoke neural responses has several appealing features compared to electrical stimulation: no direct contact is necessary between the stimulating source and the tissue, spatial resolution of stimulation is improved, and no stimulation artifact is generated hindering simultaneous data acquisition. However, very little of the available optical parameter space has been investigated in optical stimulation of nerves [4], [5]. In addition, parameters that are optimized for one application, such as motor nerve stimulation, are not necessarily the ideal parameters to be used for stimulation of a sensory system, such as the cochlea.
The use of optical stimulation in the auditory system could be beneficial for cochlear implants. In the mammalian cochlea, high frequency tones activate spiral ganglion neurons in the base of the cochlea and low frequency tones activate neurons in the apex, known as tonotopicity [6]–[11]. In individuals who are profoundly deaf, multiple-electrode cochlear implants are designed to directly electrically stimulate discrete spiral ganglion cell populations along the cochlea, attempting to restore the tonotopic responses of the normal acoustically stimulated cochlea. A successful multichannel cochlear implant should, therefore, transfer a maximum of information to discrete, spatially selected groups of auditory neurons. Stimulation at one electrode should not affect the neural response to stimulation resulting from neighboring electrodes.
However, the assumption that discrete neural populations can be electrically activated is not always true. Although it is widely assumed that stimuli applied between closely spaced bipolar electrodes can locally stimulate spiral ganglion cells, [12], [13], it has been shown that closely spaced electrode pairs at high current levels will activate a broad region of auditory neurons [12], [14]. If two electrodes stimulate the same neural population, sound sensation encoded via these two electrode contacts might be confused or even be indistinguishable. The electrode interaction reduces the number of independent channels that can be used by a cochlear implant user to parallel process acoustic information. Psychoacoustic experiments [15]–[18] and electrophysiological studies, [12], [19]–[21] showed that current injected into the cochlea spreads via the scala tympani and, consequently, stimulates large populations of spiral ganglion cells. In other words, the electric fields of neighboring electrodes overlap. This limitation is based on fundamental physical principles of electrical stimulation that even the best electrode design has not yet overcome. By substituting optical sources for electrodes in cochlear implants, it may be possible to confine neural activation to spiral ganglion cells immediately adjacent to the optical source. Spatial confinement of neural activation could lead to improved performance by implant users. We have shown that optical stimulation of gerbil cochleae is more selective that electric stimulation using an immunohistochemical staining method [4]. Currently, we are conducting further experiments to compare the selectivity of optical versus electrical stimulation using more sensitive methods than the c-FOS immunohistochemical staining method. With tone-on-light masking/tone-on-electric masking and with single fiber experiments, we compare optical stimulation with bipolar electric stimulation.
This paper details the effect of laser pulse duration, wavelength, and repetition rate on the evoked neural responses from the gerbil cochlea. It is necessary to examine these optical parameters to determine the optimal subset of laser parameters for use in a cochlear implant.
II. Materials and Methods
All measurements were made in vivo using adult gerbils Meriones unguiculatus. The care and use of the animals in this study were in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Northwestern University.
A. Animal Surgery and Preparation
Animal surgery was made as described previously [22]. Gerbils were anesthetized by an initial intraperitoneal injection of sodium pentobarbital (80 mg/kg body weight). Maintenance doses were 17 mg/kg body weight and were given throughout an experiment whenever the animal showed signs of increasing arousal, which was assessed every 30 min by a paw withdrawal reflex. After the animal was fully anesthetized, breathing was facilitated by performing a tracheotomy and securing a length of PE90 tubing into the opening in the trachea. The animal was then positioned, belly up, on a heating pad used to maintain body temperature at 38 °C, and its head was stabilized in a heated head holder. A dermal incision was made from the lower right jaw to the right shoulder in order to expose the right submandibular gland, which was subsequently ligated and removed. The muscles attached to the bulla and to the styloid bone were carefully dissected. Next, the bulla was opened to allow access to the cochlea. A silver electrode was hooked onto the bony rim of the round window of the cochlea, and a ground electrode was placed under the skin at the left jaw. After cutting the cartilaginous outer ear canal, a speculum (to connect the sound delivery system) was cemented with dental acrylic to the bony part of the outer ear canal. The surgical platform containing the animal was then moved onto a vibration isolation table in a soundproof booth. Two chest electrodes were attached to monitor heart rate. A headphone (Beyer 770Pro) was coupled to the speculum at the ear canal to acoustically stimulate the cochlea.
B. Acoustic Measurements
Acoustically evoked compound action potentials (CAPs) were measured using a modified tracking procedure [23], [24]. CAPs were measured between 50 kHz-2 kHz, with a resolution of 6 steps/octave. The acoustic stimuli were 12 ms tonebursts, (including 1 ms rise time and 1 ms fall time). CAP threshold was defined as 30 ± 3 μV (N1/P1 amplitude). Thirty-two waveforms, presented in opposite phase, were averaged for a single measurement.
C. Optical Stimulation
A diode laser (Aculight, RINS version alpha) was used for the optical stimulation of the auditory nerves. The laser operates between 1.844–1.873 μm, by varying the temperature of the diode. Pulse durations were selected between 35 μs and 1 ms. The laser is capable of operating between 1–13 Hz. The laser output was coupled to a low-OH 200-μm -diameter optical fiber (FIP series, Polymicro; Phoenix, AZ). The fiber was heated to 36 °C with a heating wire coil (NI60, Omega; Stamford, CT) to prevent hearing loss upon cooling the cochlea. The optical fiber was inserted through the same surgical access to the bulla as for the recording electrode described above. The optical fiber was approximated to the round window membrane, but did not penetrate it, and was visually oriented toward the spiral ganglion cells in Rosenthal’s canal in the upper basal turn [4] The fiber was fixed in place, approximately 0.5 mm from the spiral ganglion cells (as seen in Fig. 1), and was not in direct contact with cochlear structures. The spot size at the basal turn of the spiral ganglion cells was calculated to be 310 μm the optical fiber (index of refraction = 1.45) is immersed in cochlear fluid, which can be optically characterized as saline (index of refraction = 1.33). Radiant exposures, as measured at the tip of the fiber, ranged from 1–100 mJ/cm2.
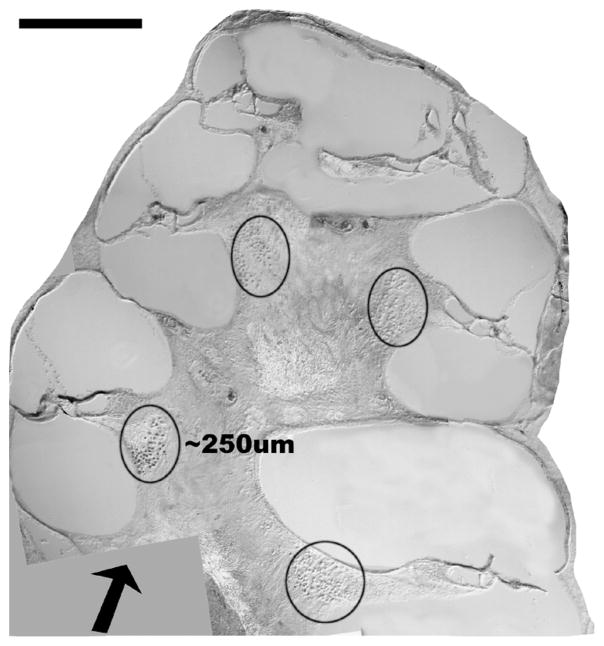
Fig. 1.
Orientation of the optical fiber in the gerbil cochlea. This is a mid-modiolar cross-section of the gerbil cochlea. The arrow indicates the orientation and placement of the optical fiber with respect to the cochlea. The optical path length through the stimulated spiral ganglion cell population (circled) is approximately 250 μm. The calibration bar at the top left of the figure equals 500 μm.
Optically evoked CAPs were recorded using the aforementioned silver electrode; responses to ten stimulus presentations were averaged for each measurement. The optical stimulation levels required to elicit a fixed amplitude CAP (50-μV CAP criterion) was determined at pulse durations of 35, 100, 300, 600 μs, and 1 ms, at 1.855 μm. In another set of experiments, the wavelength was varied between 1.844–1.873 μm and the CAPs were recorded, with a pulse duration of 35 μs. All experiments were conducted at a repetition rate of 2 Hz, with the exception of the extended stimulation experiment, which was conducted at 13 Hz. To document effects caused by longer periods of stimulation of the auditory nerve, optically evoked CAPs were measured every 5 min for up to 6 h, in response to continual irradiation with 1.87 μm light, with a 35 μs pulse duration at 13 Hz, the maximum repetition rate for this laser.
III. Results
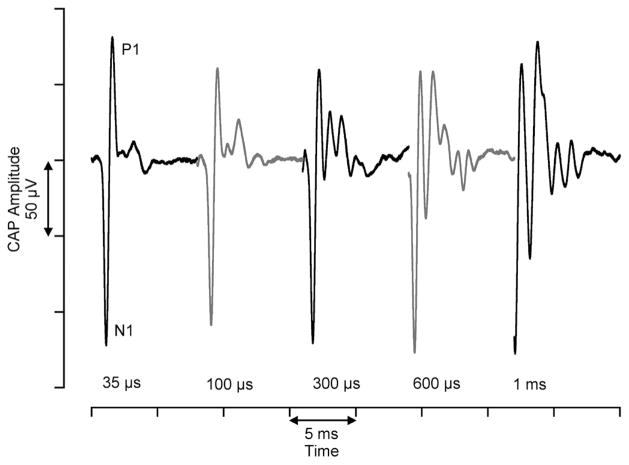
Using an infrared diode laser, operating between 1.844–1.873 μm, we were able to elicit CAPs from neurons in the gerbil cochlea (Fig. 2). A total of 18 animals were used for all experiments presented in this paper. We examined the shape of the CAP as a function of pulse duration of the stimulating laser pulse. At the laser’s shortest pulse duration, 35 μs, the CAP is composed primarily of an initial large negative peak, N1, followed by a large positive peak, P1. At longer pulse durations, subsequent (smaller) peaks appeared, one of which increases in amplitude with increasing pulse duration.
Fig. 2.
Compound action potentials elicited by varying pulse durations. The CAPs evoked by the laser change with different pulse durations. At the shortest pulse durations, the CAP is primarily composed of one negative peak (N1) and one positive peak (P1). As the pulse durations increase, there is a secondary peak that increases in amplitude. These CAPs were measured from the same animal. The amount of time between the onset of data acquisition and onset of CAP decreases as the pulse duration increases due to the data acquisition trigger from the laser.
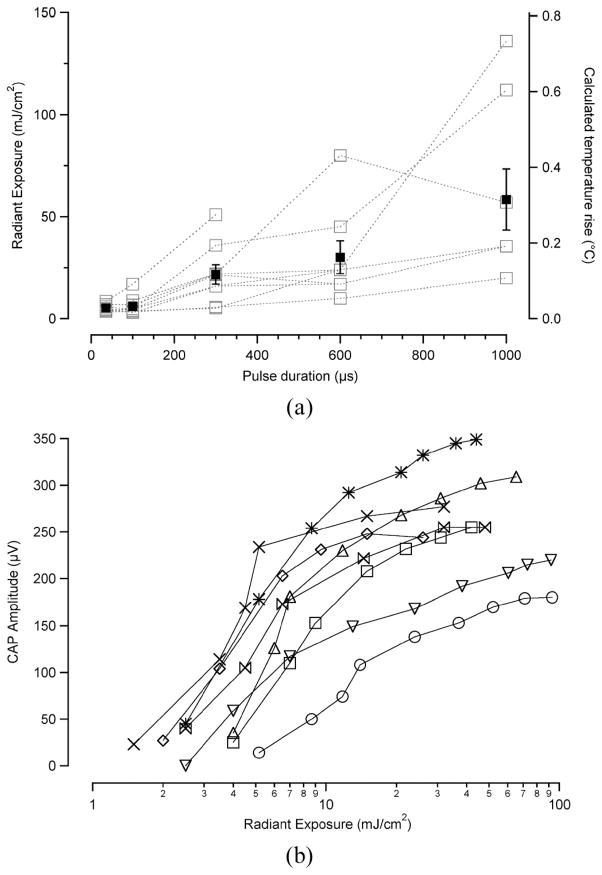
Interestingly, the stimulation level required to evoke a 50-μV CAP is the smallest at the shortest pulse duration, 5.29 ± 0.6 mJ/cm2 (x̄ ± s.e.; n = 8). For the other pulse durations, the stimulation thresholds were: 100 μs, 6.18 ± 1.5 mJ/cm2; 300 μs,21.71 ± 4.8mJ/cm2; 600 μs, 30.15 ± 8.0 mJ/cm2; 1 ms, 58.38 ± 14.9 mJ/cm2 [see Fig. 3(a)]. In other words, the radiant exposure required to stimulate the spiral ganglion cells increases as the pulse duration gets longer. The input-output (I/O) curves indicate a relatively constant increase of evoked CAP with increasing radiant exposure [Fig. 3(b)]. These curves show a dynamic range of approximately 20–30 dB from stimulation onset to stimulation plateau. I/O curves for all pulse durations are similar in shape (data not shown).
Fig. 3.
Stimulation thresholds for various pulse durations. (a) The radiant exposure required to elicit a CAP is smallest at 35-μs pulse duration, 5.29 ± 0.6 mJ/cm2 (x̄ ± s.e., n =8) The stimulation threshold increases with increasing pulse duration. On the right-hand axis, the calculated temperature rise for the corresponding radiant exposures is provided for reference. The average data with standard error bars are shown in black squares. The individual data sets, each measured from a different animal, are shown by the gray squares. (b) I/O functions relating radiant exposure to CAP amplitude for 35-μs pulse duration. There is a steady increase in CAP amplitude with increasing radiant exposure, followed by a plateau of CAP amplitude. Each type of data marker represents a single data point measured on a different animal (n = 8).
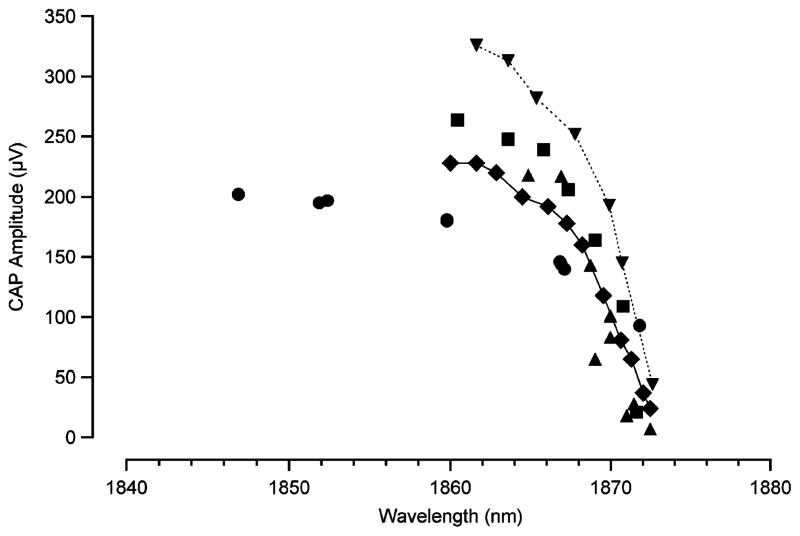
We have also examined the CAP amplitude as a function of wavelength. Beginning with the longest wavelength of 1.873 μm (shortest penetration depth), we selected a radiant exposure that elicited a CAP just above threshold amplitude. Keeping radiant exposure and position of the fiber constant, the wavelength was then decreased [increasing optical penetration depth (OPD)]. OPD is defined as the distance over which the incident light is reduced in magnitude by 1/e and is a function of the absorption coefficient of the tissue. See Table I for OPDs corresponding to each wavelength in Fig. 4. With increasing penetration depth, the CAP amplitude increased substantially over a range of 300 μm. For further increases in penetration depth, there was only a slight increase in CAP amplitude (Fig. 4). Recall from Fig. 1, the length of the optical path through the spiral ganglion cell population in the upper basal portion of the cochlea is approximately 250 μm.
TABLE I.
Wavelength Dependent OPD. Data From [25]
| Wavelength (μm) | Optical penetration depth (μm) |
|---|---|
| 1.84 | 1129 |
| 1.85 | 1016 |
| 1.86 | 819 |
| 1.87 | 520 |
| 1.88 | 308 |
Fig. 4.
Wavelength variability of laser evoked neural response. At longer wavelengths (shorter penetration depths), the CAP amplitude is at a minimum. When the wavelength is decreased (penetration depth increases), the CAP amplitude grows, until it reaches a plateau. Wavelengths between 1.844–1.873 μm were tested. Each type of data marker represents a single data point measured on a different animal. (n = 5).
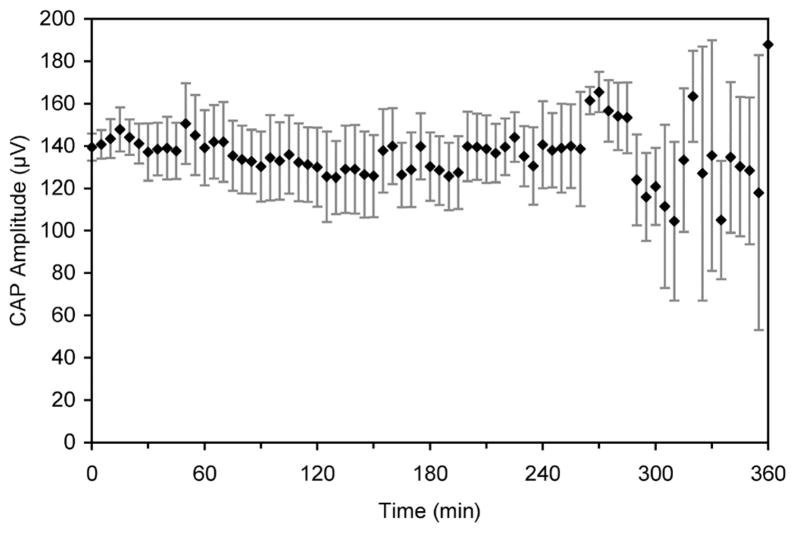
To determine any effect of continual laser stimulation, the gerbil cochlea was stimulated at the maximum repetition rate of the laser, 13 Hz, for extended periods of time. The average CAP amplitude remained steady over the 6 h of continual laser stimulation (Fig. 5). The variability that is seen towards the end of the experiment is similar to variability seen in continual acoustic stimulation experiments [3].
Fig. 5.
Extended stimulation of the gerbil cochlea reveals constant evoked response. The CAP amplitude remains relatively constant over a period of 6 h of continual stimulation (x̄± s.e., n = 6). The laser operated at 1.873 μm, 35 μs, 13 Hz, at a radiant exposure of ~ 10 mJ/cm2.
IV. Discussion
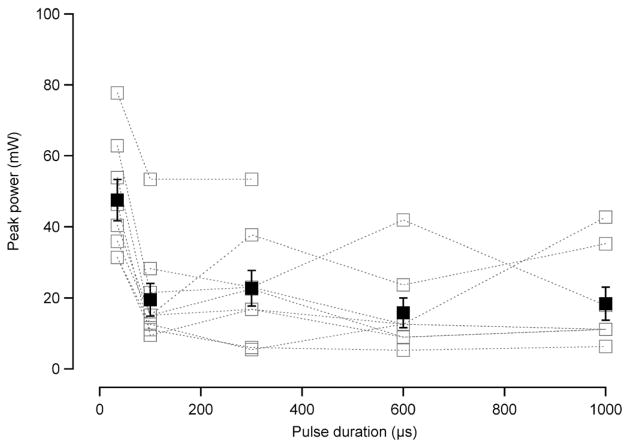
Looking at the stimulation level data as a function of pulse duration, there is a nonconstant amount of energy required to evoke a CAP of the same magnitude, as we vary the pulse duration. It has been suggested that optical stimulation of neural activity occurs via a thermal mechanism [5]. With all other parameters being equal (e.g., wavelength, tissue hydration), the amount of energy absorbed into the tissue governs the temperature increase subsequent to the light absorption. Our results then indicate that it is not the total energy and, therefore, total thermal rise, that governs optical stimulation in the gerbil cochlea. It could be the time over which this energy is deposited that governs optical stimulation. When the stimulation level data [Fig. 3(a)] are calculated in terms of peak optical power, we see another interesting trend of data (Fig. 6). In general, the peak power is constant across all pulse durations, except for 35 μs, in which it increases. This trend could indicate that the time over which the energy is deposited is more important for laser stimulation. However, it is also possible that the rise time of the optical pulse is significant in optical stimulation of the cochlea. At present, we are limited by the fixed pulse rise time of our current laser, but we will investigate this parameter in future experiments.
Fig. 6.
Peak power at stimulation level as a function pulse duration. The peak power measured for a 50-μ CAP is constant for pulse durations 100 μs−1 ms, but increases at a pulse duration of 35 μs. These are the same experiments as shown in Fig. 3(a) calculated as a function of the pulse duration. The data are averages with standard error bars.
When examining the CAPs, it is clear that the CAPs evoked from laser pulses at 35, 100, and 300 μs are all primarily composed of N1/P1 peaks. However, at 600μs and 1-ms pulse durations, there is a large secondary peak that is evident in the CAPs. The large secondary peak could account for the variability in the error estimates seen in the stimulation measurements at these pulse durations. The second maximum could be explained by a second action potential from some of the nerve fibers that contribute to the CAP. There’s also the possibility that a different subpopulation of nerve fibers that do not contribute to the CAP are responsible for the second maximum. In depth studies of single auditory nerve fibers are underway and will help determine the sources contributing to the CAP.
Other data that were acquired during these experiments examined the influence of wavelength on the evoked response, at a pulse width of 35 μs. Starting at the shortest possible penetration depth and increasing, the CAP amplitude increased significantly for approximately 300 μm, then almost plateaued for the remaining increase in OPD (Fig. 4). (OPD is defined as the distance over which the incident light is reduced in magnitude by 1/e and is a function of the absorption coefficient of the tissue.) The steady increase in CAP amplitude is most likely due to an increasing number of neurons in the optical path that receive suprathreshold irradiation as the penetration depth increases. Notice that the OPD distance over which the CAP increases, 300 μm, corresponds well with the approximate length of the optical path through the spiral ganglion cell population in the upper basal turn of the gerbil cochlea, ~ 250 μm. Further into the optical path from the spiral ganglion cells in the upper base, there is supporting tissue that is nonneural. Therefore, as the penetration depth is increased beyond the initial spiral ganglion cell population, there are no further neurons to receive suprathreshold irradiation, which likely contributes to the plateauing of the data. For use in human cochlear implants, it is possible that a different wavelength, corresponding to a longer OPD, will be better suited to stimulating the auditory neurons as the human cochlea is larger than that of a gerbil [26]. However, this parameter will ultimately be determined by the location for the optical implant array in the scala tympani.
The continual stimulation experiments conducted for 6 h at 13 Hz, the upper limit of this laser, indicate that we can evoke a very stable CAP. The CAP is a very sensitive marker for the physiological state of the cochlea, such as cochlear damage and changes in temperature [27], [28]. Acoustic tones activate a segment of neurons along the length of the cochlea and the CAP thresholds reflect the synchronous activation of neurons in this small cochlear segment. In contrast to stimulating with acoustic stimuli, for optical radiation, neural stimulation occurs over the depth of the optical path and the corresponding evoked CAP amplitudes are governed by the number of cells that receive suprathreshold stimulus levels. In case optical irradiation damages the cells, the number of neurons that respond to optical radiation should decrease and consequently the peak-to-peak amplitude of the CAP. Variability in CAP amplitude towards the end of the experiments may be attributed to changes in cochlear function caused by the surgery, by inadvertent cooling of the cochlea, or by effects on the animal from anesthesia. The same type of effect was seen in the extended acoustic stimulation previously [3].
In order to clinically implement an optical cochlear implant, certain design criteria must be met first. The most important objectives are 1) improved spatial selectivity of stimulation, 2) ability to optically stimulate at rates that mimic the native firing rates of the auditory system, and 3) safe optical stimulation for the aforementioned parameters. In terms of the spatial objective, mid-infrared light does not spread laterally upon incidence into tissue as does electric current. Only tissue that is directly in the optical path absorbs the light [29], [30]. We have demonstrated selective optical stimulation of the auditory system in comparison to electric stimulation [4].
Single auditory neurons have maximum firing rates of ~300–400 Hz [31], [32]. To adequately restore a sense of hearing, an optical cochlear implant will need to operate up to this stimulation rate without causing damage. To make a conservative estimate of the likelihood of heat accumulation following optical stimulation, we can use an equation for the tissue thermal relaxation time, τtherm, which describes the time-dependent heat transport out of the tissue by diffusion following laser irradiation (this term is independent of the incident laser energy)
| (1) |
where κ is the temperature conductivity, which has a value of ~ 1.4*10−7 m2/s in most tissues [33], and α is the wavelength-dependent absorption coefficient of the material. Based on the wavelengths used here, we would arrive at a thermal relaxation time of ~0.5 s, which corresponds to 2 Hz. By these conservative calculations, 2 Hz would be the upper limit to the safe region in which we could stimulate without causing a build-up of heat and, therefore, tissue damage. However, we have shown that it is possible to optically stimulate the cochlea at much higher rate of stimulation with no apparent thermal effects. The ability to stimulate at high rates with no damage is likely due to the very low energies that we are using. The types of laser-tissue interactions for which the thermal relaxation equation is typically used are higher powered irradiation, for applications such as coagulation and ablation. It is also possible that the perfusion of the cochlea, which is not incorporated into the equation, increases the ability to dissipate thermal energy, thereby decreasing the time parameter above. We have recently acquired a pulsed diode laser that can operate up to 1-kHz repetition rate and experiments are underway to examine high rate optical stimulation of the auditory system. While we have measured a steady CAP in response to optical stimulation at 400 Hz over several hours, we are conducting further studies to validate these data using single fiber population studies, which will be the topic of a future manuscript. We will also explore the safety of an optical cochlear implant with chronic animal studies, in which the cochlea is optically stimulated over a period of months. Electrophysiology and histology data will reveal the safe parameters for optical stimulation in a cochlear implant.
To apply optical stimulation for use in other neuroprostheses, two general goals must be met: 1) improvement of optical stimulation versus electrical stimulation for the application; 2) safely optically stimulate to achieve desired function. Other likely candidates for optical stimulation in a sensory system include the dorsal root ganglia, which carries sensory information such as tactile, pain, and temperature sensations from the periphery to the central nervous system; and the retinal ganglion cells, which transmit the signals from the photoreceptors to the brain. Each of these applications would require unique design criteria, for instance the physical size of an implant, the method of delivering the light, and the as-yet unknown optical parameters required to evoke a response from the neurons. Introducing optical stimulation into a neuroprosthesis for the tactile sensory system could allow information feedback for individuals with motor prostheses. A retinal prosthesis incorporating optical stimulation could improve the resolution of vision that is restored to the blind individual. These are just a few of the many applications of optical stimulation of neural tissue.
V. Conclusion
We have investigated the effect of pulse duration, wavelength, and repetition rate on neural activity evoked from the gerbil auditory system. Results showed that pulse durations as short as 35 μs can evoke neural activity and that this response is stable for continual stimulation at a repetition rate of 13 Hz. The parameters, 35 μs pulse duration and 13-Hz repetition rate, are at the limits of the laser. It would be beneficial to determine the shortest pulse duration that will successfully evoke neural response, thereby leading to a smaller laser energy. Additionally, a laser that allows higher repetition rates of stimulation will enable a more thorough investigation of parameters that mimic the native firing patterns of the auditory system and electrical stimulation rates in cochlear implants.
Acknowledgments
This work was supported in part by the ER Capita Foundation and in part by the National Institutes of Health (NIH) under Grant F31 DC008246–01, Grant R41 DC008515–01, and Grant HHN-260–2006–00006-C.
Biographies

Agnella D. Izzo was born in New Haven, CT and raised in Fairfield, CT. She received the B.E. degree in biomedical engineering from Vanderbilt University, Nashville, TN, in 2001, and the M.S. and Ph.D. degrees in biomedical engineering from Northwestern University, Evanston, IL, in 2003 and 2006, respectively.
She is currently a Postdoctoral Fellow in the Department of Otolaryngology at Feinberg Medical School, Northwestern University. Her research interests fall under the broad category of medical use of lasers. Her previous projects include optical imaging of wound healing after laser surgery and bactericidal effects of low-level irradiation. Her current research focus is on laser stimulation of neural tissue, specifically the auditory system.
Dr. Izzo is a member of the Association for Research in Otolaryngology (ARO) and the Society of Photo-Optical Instrumentation Engineers (SPIE).

Joseph T. Walsh, Jr. was born in Baltimore, MD in 1959. He received the B.S.E.E. and M.S.E.E. degrees from the Massachusetts Institute of Technology (MIT). He received the Ph.D. degree from the Harvard-MIT Division of Health Science and Technology in 1988.
He is now a Professor of Biomedical Engineering and the Senior Associate Dean for Northwestern University’s McCormick School of Engineering School and Applied Science in Evanston, IL. His research area involves light-tissue interactions. More specifically, he is interested in analytic and experimental analysis of the interaction of laser radiation with tissue and developing diagnostic and therapeutic applications of lasers. He has been a program chairman for 6 major conferences in his field in the past 14 years; most recently as the program chairman for the 2006 Annual Meeting of the Biomedical Engineering Society.
Dr. Walsh is a recent Past President of the American Society for Laser Medicine and Surgery (ASLMS).

E. Duco Jansen received the M.S. (Drs) degree in medical sciences from the University of Utrecht, Utrecht, The Netherlands, in 1990, and the M.S. and Ph.D. degrees in biomedical engineering from the University of Texas at Austin in 1992 and 1994, respectively.
He joined Vanderbilt University in 1997 and is currently Associate Professor of Biomedical Engineering and Neurosurgery at and a faculty member in the Vanderbilt University Institute of Imaging Sciences. His research interests include novel approaches to optically stimulate neurons, mechanisms of pulsed laser ablation of biological tissue, cellular and biochemical responses of biological tissue to laser radiation, medical applications of lasers. Additionally, his research involves development and application of in vivo optical imaging of tissue ultrastructure and cellular and molecular processes, including gene expression. He is also actively involved in the NSF-funded Engineering Research Center (ERC) for Biomedical Engineering Education Technology.
Dr. Jansen is a member of ASLMS, Optical Society of America (OSA), and SPIE.

Mark Bendett (S’80–M’84) received the B.S. degree in physics from Haverford College, Haverford, PA, and the M.S.E.E. and Ph.D. degrees from the University of Delaware, Newark, in 1981 and 1984, respectively.
Over the last 20 years, his primary areas of technical work have been in integrated-optics, fiber-optic sensors and systems, optical interconnects, and laser-based medical systems. He is currently the Director of Product Development and heads the medical program at Aculight Corporation, Bothell, WA. He holds more than 15 patents and has 30 publications in the integrated-optics and optical systems areas.
Dr. Bendett is a member of OSA, SPIE, and the American Society for Cataract and Refractive Surgery.

Jim Webb received the B.S. degree in mechanical engineering from the University of Wisconsin-Madison in 1995.
Currently, he is the Project Manager at Aculight Corporation, Bothell, WA, where he is developing medical laser products. He has also developed thermal and mechanical solutions for various solid state laser applications. Previously, he has worked at Terabeam Corporation developing free space telecommunication devices and Intel Corporation developing processor packaging. He holds more than 12 patents.

Heather Ralph received the B.Sc. degree in mechanical engineering from Tufts University, Boston, MA, in 1996 and the M.Sc. degree in mechanical engineering from the University of Washington, Seattle, in 1999.
She is currently a Development Engineer at Aculight Corporation, Bothell, WA, where she researches lasers for medical, lidar, and infrared counter measure systems.

Claus-Peter Richter received the M.D. degree from the Johann Wolfgang Goethe-University, Frankfurt, Germany, in 1990. He received the M.S. degree in physics and the Habilitation in physiology from the Johann Wolfgang Goethe-University in 1990 and 2000, respectively.
Following his postdoctoral research in the Department of Physiology at the Johann Wolfgang Goethe-University, he joined Northwestern University in 1996, and became full time faculty at Northwestern University Feinberg School of Medicine in 2002. He is the Director of Resident Research in the Department of Otolaryngology Head—and Neck Surgery. His primary research interests are the development and improvement of cochlear implant electrodes, and the biophysics of the inner ear. His previous research projects include the micromechanics of the cochlea, and current spread in the cochlea and its possible effects on cochlear implant performance. His current research focuses on improving cochlear implants using optical radiation.
Dr. Richter is Member of the Deutsche Physiologische Gesellschaft, the American Physiological Society, and ARO.
Contributor Information
Agnella D. Izzo, Email: a-izzo@northwestern.edu, Department of Biomedical Engineering, Northwestern University, Evanston, IL 60208 USA. She is also with the Department of Otolaryngology, Feinberg Medical School, Northwestern University, Chicago, IL 60611 USA.
Joseph T. Walsh, Jr., Email: jwalsh@northwestern.edu, Department of Biomedical Engineering, Northwestern University, Evanston, IL 60208 USA.
E. Duco Jansen, Email: duco.jansen@vanderbilt.edu, Department of Biomedical Engineering, Vanderbilt University, Nashville, TN 37235 USA.
Mark Bendett, Email: mark.bendett@aculight.com, Aculight Corporation, Bothell, WA 98011 USA.
Jim Webb, Email: jim.webb@aculight.com, Aculight Corporation, Bothell, WA 98011 USA.
Heather Ralph, Email: heather.ralph@aculight.com, Aculight Corporation, Bothell, WA 98011 USA.
Claus-Peter Richter, Email: cri529@northwestern.edu, Department of Otolaryngology, Feinberg Medical School, Northwestern University, Chicago, IL 60611 USA.
References
- 1.Wells J, Kao C, Mariappan K, Albea J, Jansen ED, Konrad P, Mahadevan-Jansen A. Optical stimulation of neural tissue in vivo. Optics Lett. 2005;31:235–238. doi: 10.1364/ol.30.000504. [DOI] [PubMed] [Google Scholar]
- 2.Wells J, Kao C, Jansen ED, Konrad P, Mahadevan-Jansen A. Application of infrared light for in vivo neural stimulation. J Biomed Opt. 2005;10(6):064003. doi: 10.1117/1.2121772. [DOI] [PubMed] [Google Scholar]
- 3.Izzo AD, Richter CP, Jansen ED, Walsh JT. Laser stimulation of the auditory nerve. Laser Surg Med. 2006;38(8):745–753. doi: 10.1002/lsm.20358. [DOI] [PubMed] [Google Scholar]
- 4.Izzo AD, Suh E, Pathria J, Whitlon DS, Walsh JT, Richter C-P. Selectivity of neural stimulation in the auditory system: A comparison of optic and electric stimuli. J Biomed Opt. 2007;12(2) doi: 10.1117/1.2714296. manuscript #021008. [DOI] [PubMed] [Google Scholar]
- 5.Wells J, Kao C, Konrad P, Mahadevan-Jansen A, Jansen ED. Biophysical mechanisms responsible for pulsed low-level laser excitation of neural tissue. Proc SPIE. 2006;6084 [Google Scholar]
- 6.von Békésy G. Experiments in Hearing. New York: McGraw-Hill; 1960. [Google Scholar]
- 7.Greenwood DD. A cochlear frequency-position function for several species-29 years later. J Acoust Soc Am. 1990 Jun;87(6):2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- 8.Geier LL, Norton SJ. The effects of limiting the number of Nucleus 22 cochlear implant electrodes programmed on speech perception. Ear Hear. 1992;13(5):340–348. doi: 10.1097/00003446-199210000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: Comparison of acoustic hearing and cochlear implants. J Acoust Soc Am. 2001;110(2):1150–1163. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- 10.Lawson D, Wilson BS, Finley C. New processing strategies for multichannel cochlear prosthesis. Prog Brain Res. 1993;97:313–321. doi: 10.1016/s0079-6123(08)62291-8. [DOI] [PubMed] [Google Scholar]
- 11.Lawson D. Speech Processors of Auditory Prostheses. Research Triangle Institute; 1996. NIH Contract 1-DC-5–2103. [Google Scholar]
- 12.van den Honert C, Stypulkowski P. Single fiber mapping of spatial excitation patterns in the electrically stimulated auditory nerve. Hear Res. 1987;29(2–3):195–206. doi: 10.1016/0378-5955(87)90167-5. [DOI] [PubMed] [Google Scholar]
- 13.Busby P, Whitford L, Blamey P, Richardson L, Clark G. Pitch perception for different modes of stimulation using the Cochlear multiple-electrode prosthesis. J Acoust Soc Am. 1994;95:2658–2669. doi: 10.1121/1.409835. [DOI] [PubMed] [Google Scholar]
- 14.Frijns JH, de Snoo SL, ten Kate JH. Spatial selectivity in a rotationally symmetric model of the electrically stimulated cochlea. Hear Res. 1996 May;95(1–2):33–48. doi: 10.1016/0378-5955(96)00004-4. [DOI] [PubMed] [Google Scholar]
- 15.Collins M, Hawthorne M, el Hmd K. Cochlear implantation in a district general hospital: Problems and complications in the first five years. J Laryngol Otol. 1997;111:325–332. doi: 10.1017/s0022215100137235. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee M, Shannon R. Forward masked excitation patterns in multielectrode electrical stimulation. J Acoust Soc Am. 1998;103:2565–2572. doi: 10.1121/1.422777. [DOI] [PubMed] [Google Scholar]
- 17.Nelson DA, Donaldson GS. Psychophysical recovery from pulse-train forward masking in electrical hearing. J Acoust Soc Am. 2002;112(6):2932–2947. doi: 10.1121/1.1514935. [DOI] [PubMed] [Google Scholar]
- 18.Throckmorton CS, Collins LM. The effect of channel interactions on speech recognition in cochlear implant subjects: Predictions from an acoustic model. J Acoust Soc Am. 2002;112(1):285–296. doi: 10.1121/1.1482073. [DOI] [PubMed] [Google Scholar]
- 19.Black RC, Clark GM. Differential electrical excitation of the auditory nerve. J Acoust Soc Am. 1980;67(3):868–874. doi: 10.1121/1.383966. [DOI] [PubMed] [Google Scholar]
- 20.Spelman F, Clopton B, Pfingst B. Tissue impedance and current flow in the implanted ear. implications for the cochlear prosthesis. Ann Otol Rhinol Laryngol Suppl. 1982;98:3–8. [PubMed] [Google Scholar]
- 21.O’Leary S, Black R, Clark GM. Current distributions in the cat cochlea: A modelling and electrophysiologic study. Hear Res. 1985;18(3):273–281. doi: 10.1016/0378-5955(85)90044-9. [DOI] [PubMed] [Google Scholar]
- 22.Emadi G, Richter CP, Dallos P. Stiffness of the gerbil basilar membrane: Radial and longitudinal variations. J Neurophysiol. 2004;91:474–488. doi: 10.1152/jn.00446.2003. [DOI] [PubMed] [Google Scholar]
- 23.Taylor M, Creelman C. PEST: Efficient estimates on probability functions. J Acoust Soc Am. 1967;41:782–787. [Google Scholar]
- 24.Gummer A, Smolders J, Kline R. Basilar membrane motion in the pigeon measured with the Mössbauer technique. Hear Res. 1987;29:63–92. doi: 10.1016/0378-5955(87)90206-1. [DOI] [PubMed] [Google Scholar]
- 25.Walsh JT, Cummings J. Effect of the dynamic optical properties of water on midinfrared laser ablation. Laser Surg Med. 1994;15(3):295–305. doi: 10.1002/lsm.1900150310. [DOI] [PubMed] [Google Scholar]
- 26.Leake PA, Rebscher SJ. Anatomical considerations and long-term effects of electrical stimulation. In: Zeng F-G, Popper AN, Fay RR, editors. Cochlear Implants: Auditory Prostheses and Electrical Hearing. New York: Springer; 2004. pp. 101–148. [Google Scholar]
- 27.Kahana L, Rosenblith W, Galambos R. Effect of temperature change on round-window response in the hamster. Am J Physiol. 1950;163(2):213–223. doi: 10.1152/ajplegacy.1950.163.2.213. [DOI] [PubMed] [Google Scholar]
- 28.Ohlemiller K, Siegel JH. Cochlear basal and apical differences reflected in the effects of cooling on responses of single auditory nerve fibers. Hear Res. 1994;80(2):174–190. doi: 10.1016/0378-5955(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 29.Welch AJ, van Gemert MJ, editors. Optical-Thermal Response of Laser-Irradiated Tissue. New York: Plenum; 1995. [Google Scholar]
- 30.Niemz MH. Laser-Tissue Interactions: Fundamentals and Applications. 2. New York: Springer; 2004. [Google Scholar]
- 31.Sachs M, Abbas PJ. Rate versus level functions for auditory nerve fibers in cats: Tone-burst stimuli. J Acoust Soc Am. 1974;56:1835–1847. doi: 10.1121/1.1903521. [DOI] [PubMed] [Google Scholar]
- 32.Liberman M. Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am. 1978;63:442–455. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- 33.Boulnois JL. Photophysical processes in recent medical laser developments: A review. Lasers Med Sci. 1986;1:47–66. [Google Scholar]