Abstract
Objectives
To evaluate whether impaired vasodilator function, an early manifestation of coronary artery disease which precedes angiographic stenosis, accounts for increased risk among patients with moderate to severe renal dysfunction.
Background
Patients with renal dysfunction are at increased risk of adverse cardiac outcomes, even in the absence of overt myocardial ischemia or infarction.
Methods
We included 866 consecutive patients with moderate to severe renal dysfunction referred for rest and stress myocardial perfusion PET and followed them for a median of 1.28 years (inter-quartile range: 0.64–2.34). Regional myocardial perfusion abnormalities were assessed by semiquantitative visual analysis of PET images. Rest and stress myocardial blood flow (MBF) were calculated using factor analysis and a 2-compartment kinetic model, and were used to compute coronary flow reserve (stress/rest MBF). The primary endpoint was cardiac death.
Results
Overall 3-year cardiac mortality was 16.2%. After adjusting for clinical risk, left ventricular ejection fraction, as well as the magnitude of scar and/or ischemia, coronary flow reserve below the median (<1.5) was associated with a 2.1-fold increase in the risk of cardiac death (95%CI 1.3–3.5, P= 0.004). Incorporation of coronary flow reserve into cardiac death risk assessment models resulted in an increase in the c-index from 0.75 to 0.77 (P=0.05) and in a net reclassification improvement (NRI) of 0.142 (95%CI 0.076–0.219). Among patients at intermediate risk based on all data other than coronary flow reserve, the NRI was 0.489 (95%CI 0.192–0.836). Corresponding improvements in risk assessment for mortality from any cause were also demonstrated.
Conclusions
The presence of coronary vascular dysfunction in patients with moderate to severe renal dysfunction, as assessed by PET, is a powerful, independent predictor of cardiac mortality and provides meaningful incremental risk stratification over conventional markers of clinical risk.
Keywords: coronary artery disease, chronic kidney disease, blood flow, imaging, atherosclerosis, ischemia
Introduction
Cardiovascular disease is the leading cause of mortality among patients with moderate to severe renal dysfunction(1). In selected high risk patients, early referral to cardiac catheterization and coronary revascularization may improve outcomes(2). However, acute deterioration of renal function following diagnostic coronary angiography occurs in approximately 10% of patients(3) and up to 30% of patients after percutaneous coronary intervention(4). Contrast medium induced renal dysfunction after coronary procedures carries poor prognosis(4, 5), especially if dialysis becomes necessary(5). In addition, coronary revascularization procedures in patients with renal impairment are associated with markedly higher risks of both fatal and non-fatal adverse outcomes (6, 7). Consequently, careful selection of high risk patients for referral to coronary angiography and revascularization is of paramount importance.
Unfortunately, traditional approaches for cardiac risk assessment, including stress imaging, have been unable to accurately identify low risk individuals in this patient subgroup(8). This may be related, in part, to the fact that noninvasive imaging methods are insensitive for detecting the presence of diffuse atherosclerosis and its impact on coronary epicardial and microcirculatory function and myocardial ischemia. The latter may help explain the increase in biomarkers of myocardial injury including serum N-terminal pro B-type natriuretic peptide and troponin T seen among patients with renal dysfunction, and their effects on prognosis(9). This issue is of relevance because both microvascular dysfunction and myocardial ischemia may be amenable to treatment.
This study was designed to test the hypothesis that in patients with moderate to severe renal dysfunction, coronary vasodilator dysfunction, as measured by positron emission tomography, is prevalent and helps explain the observed excess risk of cardiac mortality in this population.
Methods
Study Population
All patients with moderate to severe renal dysfunction (estimate glomerular filtration rate ≤60 ml/min/1.73 m2) referred for rest/stress cardiac PET at the Brigham & Women's Hospital (Boston, MA) between January 1, 2006 and June 30, 2010 were included in this study, excluding those whose images were missing or uninterpretable due to poor image quality. In cases of repeat PET scans during the study period, only the earliest evaluable study was included. Demographic factors and key elements of the patients' history including risk factors and medication use were ascertained at the time of the study by patient interview and review of medical records. Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease formula(10) based on a mean creatinine in the 90 days preceding the imaging study. Patients being treated with chronic renal replacement therapy, determined by patient interview and billing codes(11), were assumed to have eGFR of 1 ml/min/1.73 m2. The study was approved by the Partners Healthcare Institutional Review Board and conducted in accordance with institutional guidelines.
Positron Emission Tomographic Imaging
Patients were studied using a whole body PET-CT scanner (Discovery RX or STE LightSpeed 64, GE Healthcare, Milwaukee, WI) after an overnight fast. Patients refrained from caffeine and methylxanthine containing substances and drugs for 24 hours prior to their scans. Myocardial blood flow (MBF) was measured during rest and peak stress using 82Rubidium as a perfusion tracer, as described previously(12). Briefly, after transmission imaging and beginning with the intravenous bolus administration of 82Rubidium (1,480–2,200 MBq), list mode images were acquired for seven minutes. Then, a standard intravenous infusion of dipyridamole, adenosine, regadenoson or dobutamine was given. At peak stress, a second dose of 82Rubidium was injected and images were recorded in the same manner. The average radiation exposure per study was 4.6 mSv(13). Heart rate, blood pressure, and 12-lead electrocardiogram were recorded at baseline and every minute during and after pharmacological stress.
82Rubidium undergoes very rapid radioactive decay, with a physical half-life of 75 seconds, producing trace quantities of stable, non-radioactive 82Krypton gas, which is passively exhaled by the lungs. Neither renal nor hepatic excretion contributes meaningfully to 82Rubidium elimination. As a result, administered tracer doses and analytic methods do not require adjustment for renal function.
Image Analysis
Semi-quantitative Analysis of Myocardial Perfusion Semi-quantitative 17-segment visual interpretation of the gated myocardial perfusion images was performed by experienced observers using a standard 5-point scoring system(14). Summed rest and stress scores were calculated as the sum of individual segmental scores on the respective images, and their difference was recorded as summed difference score. These were converted to percentages of left ventricular myocardium by dividing by the maximum score, i.e. 68.
Left Ventricular Systolic Function
Rest and stress LV ejection fraction (LVEF) were calculated from gated myocardial perfusion images using commercially available software. Left ventricular ejection fraction reserve was considered present when LVEF increased from rest to stress.
Quantitative Myocardial Blood Flow and Flow Reserve
Absolute MBF (in ml/g/min) was computed from the dynamic rest and stress images using commercially available software (Corridor4DM; Ann Arbor, Michigan) and previously validated methods(15). Automated factor analysis was used to generate blood pool (arterial input function) and tissue time-activity curves. Regional and global rest and peak stress MBF were calculated by fitting the 82Rubidium time-activity curves to a two-compartment tracer kinetic model as described previously(15). Per-patient global coronary flow reserve (CFR) was calculated as the ratio of absolute MBF at stress over rest for the entire left ventricle. Quantitation of MBF was performed by four operators. The intra-class correlation coefficient(16) for CFR among these four readers was 0.94 (95%CI 0.88–0.98), indicating excellent reproducibility.
Assessment of Outcomes
The primary outcome was death from any cardiac cause. Patients who died from non-cardiac causes were censored. Mortality from any cause was used as a secondary endpoint. Vital status of all patients was ascertained by integrating data from the Social Security Death Index, the National Death Index and the Partners Healthcare Research Patient Data Registry. Cause of death was determined by blinded adjudication of hospital records and death certificates. Early revascularization (within 90 days) was ascertained from the Partners Healthcare Research Patient Data Registry and hospital records.
Statistical Analysis
Statistical significance was assessed using Wilcoxon tests, Fisher exact and chi-square tests for continuous, dichotomous and categorical variables, respectively. Two sided p-values <0.05 were considered significant. All statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC).
Multivariable Modeling
The Cox proportional hazards model was used to assess the impact of CFR on cardiac mortality after controlling for the effects of critical covariates. A series of models were developed starting with the Duke Clinical Score, an index of CAD likelihood and prognosis based on clinical covariates(17). Rest LVEF, combined extent and severity of scar and ischemia, stress-induced LVEF augmentation (LVEF reserve) and CFR (as a continuous variable or dichotomized at the median) were then sequentially incorporated into the model. In order to investigate the effects of absolute peak stress MBF we generated an additional model containing absolute stress MBF instead of CFR. The models were examined for the validity of the proportional hazards assumption and additive value, taking care to avoid over-fitting. Survival was plotted using direct adjusted survival probabilities(18) from the Cox survival model.
To assess for biases introduced by early revascularization, analyses were repeated censoring all patients who underwent early revascularization(19). In an exploratory analysis, we considered the effect of any revascularization, including those >90 days after the PET scan, as a time-dependent covariate.
Assessment of Incremental Value
Incremental prognostic value of CFR was assessed with the likelihood ratio test to determine the improvement in prediction power of each sequential Cox model. The c-index was calculated for each model(20) with comparisons using the method of Antolini and colleagues(21). The potential impact of CFR on risk stratification was assessed by net reclassification improvement (NRI)(22) at 2-years using threshold annual rates of cardiac mortality of 2% and 4%. These thresholds were selected to be slightly higher than ACC/AHA guidelines for management of chronic stable angina(23) because of the higher periprocedural morbidity and mortality of coronary angiography and revascularization in patients with moderate to severe renal impairment(3–7). Reclassification metrics using guideline derived(23) 1 and 3% thresholds were also computed as a secondary analysis.
Results
Patient Characteristics
A total of 866 consecutive patients met inclusion and exclusion criteria during the study period and were followed for a median of 1.28 years (inter-quartile range: 0.64–2.34 years). Baseline characteristics are given in Table 1. The most common indications for testing were evaluation for chest pain, dyspnea, or their combination. Approximately half of all studies were normal by semi-quantitative visual analysis.
Table 1.
Patient Characteristics
| Variable | No Cardiac Death (n=778) | Cardiac Death (n=88) | All Patients (n=866) | P-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age (y) | 60.7 [51.8–70.5] | 76 [65.8–82.1] | 71.1 [61.3–79.8] | 0.004 |
| Male gender | 380 (48.8) | 55 (62.5) | 435 (50.2) | 0.02 |
| Hispanic | 76 (9.8) | 3 (3.4) | 79 (9.1) | 0.05 |
| Race | 0.19 | |||
| White | 478 (61.4) | 63 (71.6) | 541 (62.5) | |
| Black | 138 (17.7) | 11 (12.5) | 149 (17.2) | |
| Other/Unknown | 162 (20.8) | 14 (15.9) | 176 (20.3) | |
| Risk Factors | ||||
| BMI (kg/m2) | 28.4 [24.5–33.3] | 26.4 [23.1–30.3] | 28.2 [24.4–33.0] | 0.003 |
| BMI ≥30 kg/m2 | 324 (41.6) | 23 (26.1) | 347 (40.1) | 0.01 |
| Hypertension | 714 (91.8) | 76 (86.4) | 790 (91.2) | 0.11 |
| Dyslipidemia | 540 (69.4) | 66 (75.0) | 606 (70.0) | 0.33 |
| Diabetes | 338 (43.4) | 50 (56.8) | 388 (44.8) | 0.02 |
| Family history of CAD | 166 (21.3) | 23 (26.1) | 189 (21.8) | 0.34 |
| Tobacco Use | 66 (8.5) | 9 (10.2) | 75 (8.7) | 0.55 |
| Duke Clinical Risk (%) | 56.6 [26.8 86.8] | 80 [55.1–95.6] | 60.5 [28–87.8] | <0.0001 |
| Renal Function | 0.11 | |||
| CKD Stage 3 | 508 (65.3) | 51 (58.0) | 559 (64.6) | |
| CKD Stage 4 | 89 (11.4) | 17 (19.3) | 106 (12.2) | |
| CKD Stage 5 | 181 (23.3) | 20 (22.7) | 201 (23.2) | |
| Dialysis | 138 (17.7) | 15 (17) | 153 (17.7) | 1.00 |
| eGFR MDRD (ml/min/1.73 m2) | 41.9 [16.6–52.1] | 33.5 [19.7–44.7] | 40.7 [17.3–51.8] | 0.03 |
| Medications | ||||
| Aspirin | 491 (63.1) | 62 (70.5) | 553 (63.9) | 0.20 |
| β-adrenergic blockers | 573 (73.7) | 70 (79.5) | 643 (74.2) | 0.25 |
| Cholesterol agents | 546 (70.2) | 63 (71.6) | 609 (70.3) | 0.90 |
| Insulin | 167 (21.5) | 27 (30.7) | 194 (22.4) | 0.06 |
| Oral hypoglycemic agents | 67 (8.6) | 6 (6.8) | 73 (8.4) | 0.69 |
| Ca-channel blockers | 253 (32.5) | 16 (18.2) | 269 (31.1) | 0.01 |
| ACE inhibitors | 320 (41.1) | 41 (46.6) | 361 (41.7) | 0.36 |
| Nitrates | 121 (15.6) | 23 (26.1) | 144 (16.6) | 0.02 |
| Diuretics | 366 (47.0) | 53 (60.2) | 419 (48.4) | 0.02 |
| Indications | ||||
| Chest Pain | 274 (35.2) | 20 (22.7) | 294 (33.9) | 0.02 |
| Dyspnea | 242 (31.1) | 41 (46.6) | 283 (32.7) | 0.01 |
| Post-MI | 112 (14.4) | 19 (21.6) | 131 (15.1) | 0.08 |
| Pre-operative | 113 (14.5) | 16 (18.2) | 129 (14.9) | 0.35 |
| Cardiovascular History | ||||
| Any prior CAD | 405 (52.1) | 70 (79.5) | 475 (54.8) | <0.0001 |
| Recent MI (≤30 days) | 137 (17.6) | 27 (30.7) | 164 (18.9) | 0.01 |
| Remote MI (>30 days) | 163 (21.0) | 33 (37.5) | 196 (22.6) | 0.001 |
| Prior PCI | 183 (23.5) | 32 (36.4) | 215 (24.8) | 0.01 |
| Prior CABG | 125 (16.1) | 35 (39.8) | 160 (18.5) | <0.0001 |
| Cerebrovascular Disease | 64 (8.2) | 5 (5.7) | 69 (8.0) | 0.53 |
| Peripheral Vascular Disease | 60 (7.7) | 19 (21.6) | 79 (9.1) | 0.00 |
| Early Revascularization (≤90 days post-PET) | 76 (9.8) | 12 (13.6) | 88 (10.2) | 0.26 |
| Imaging Parameters | ||||
| Rest LVEF | 55 [44–63] | 35 [26–54] | 54 [41–63] | <0.0001 |
| LVEF Reserve | 569 (73.1) | 57 (64.8) | 626 (72.3) | 0.10 |
| Ischemia+Scar (%) | 2.9 [0–14.7] | 16.9 [5.1–33.8] | 4.4 [0–16.2] | <0.0001 |
| Ischemia (%) | 0 [0–5.9] | 4.4 [0–8.8] | 0 [0–7.4] | 0.0004 |
| Global CFR | 1.53 [1.19–1.96] | 1.30 [1.08–1.5] | 1.49 [1.18–1.92] | <0.0001 |
| Stress Global MBF (ml/g/min) | 1.60 [1.11–2.23] | 1.21 [0.93–1.77] | 1.55 [1.08–2.18] | <0.0001 |
| Rest Global MBF (ml/g/min) | 0.99 [0.79–1.32] | 1.03 [0.77–1.29] | 1.00 [0.79–1.32] | 0.51 |
| CFR <1.5 | 405 (52.1) | 22 (25.0) | 427 (49.3) | <0.0001 |
Continuous variables are presented as median (inter-quartile range). Dichotomous variables are presented as number (%). Patients whose LVEF at stress was greater than that at rest were considered to have positive stress-induced increase in LVEF. BMI = body mass index. ACE = angiotensin converting enzyme. MI = myocardial infarction. CAD = coronary artery disease. CKD = Chronic Kidney Disease. eGFR MDRD = estimated glomerular filtration rate by modification of diet in renal disease formula. PCI = percutaneous coronary intervention. CABG = coronary artery bypass graft. LVEF = left ventricular ejection fraction. CFR = coronary flow reserve. MBF = myocardial blood flow.
Patient Outcomes
Mortality from any cause occurred in 155 (17.9%) patients, of which 88 (56.8%) were due to cardiac causes (Supplement, Table 2). Three-year cardiac mortality was 16.2%. Compared to patients without cardiac death, those who experienced cardiac death were older, more likely to be male, have diabetes, had a lower body mass index, more likely to be referred for dyspnea evaluation, have prior CAD, lower rest LVEF, and larger abnormalities on PET scans (Table 1). The annualized rate of cardiac death increased with increasing extent and severity of perfusion abnormalities (Figure 1a) and, importantly, was 2.7% per year among patients with a visually normal PET scan. Furthermore, in each category of abnormality on PET scanning (combined ischemia and scar extent), an impaired CFR identified higher risk subgroups, including among those with visually normal scans (Figure 1b). Likewise, in each category of LVEF, a higher CFR was associated with a decrease in the risk of cardiac mortality (Figure 1c).
Figure 1. Unadjusted Cardiac Mortality.
Unadjusted annualized cardiac mortality in categories of total extent of myocardial ischemia and scar (panel A); and by CFR above and below median (1.5) and categories of total extent of myocardial ischemia and scar (panel B); and by CFR above and below median and categories of left ventricular ejection fraction (panel C). The annual rate of cardiac death increased with increasing extent of ischemia and scar, decreasing LVEF and CFR. Importantly, lower CFR consistently identified higher risk patients at every level of ischemia and scar extent and LVEF, including among those with visually normal PET scans and normal LV function.
Univariate Predictors of Cardiac Mortality
CFR values below the median were associated with a 3.3-fold increased risk of cardiac death. Other significant predictors of increased risk included age, male gender, diabetes and prior CAD (Supplement, Figure 2). Somewhat surprisingly, chest pain as a reason for testing was associated with a decreased risk, possibly reflecting confounding. In addition, dyspnea, a decrease in rest LVEF, as well as increasing burden of scar, ischemia or their combination on semi-quantitative visual analysis were all significantly associated with increased risk.
Multivariable Survival Analysis and Incremental prognostic Value
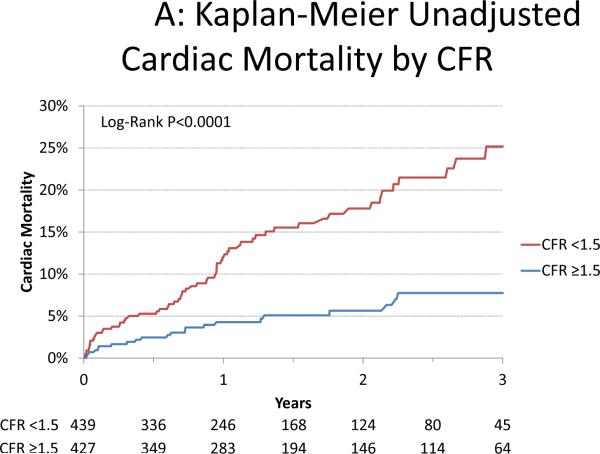
A series of multivariable models were then constructed to assess the incremental value of CFR after adjustment for critical covariates known to be associated with increased risk of cardiac mortality (Table 2). Addition of CFR to a model including the Duke clinical risk score, early revascularization, eGFR, rest LVEF, LVEF reserve and the total burden of ischemia and scar was associated with a significant increase in global χ2 and decrease in Akaike information criterion, indicating improved model fit, improved model calibration and a borderline significant increase in the c-index from 0.75 to 0.77 (p=0.05). Dichotomization of CFR at the median value led to better model fit than as a continuous variable. Compared to those with CFR ≥1.5, the fully-adjusted hazard ratio for cardiac death was 2.1 (95%CI 1.3–3.5, p=0.0004) for those with CFR <1.5 (Figure 2).
Table 2.
Multivariable Survival Analysis for Cardiac Mortality
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fit Statistic | p-value | Fit Statistic | p-value | Fit Statistic | p-value | Fit Statistic | p-value | Fit Statistic | p-value | Fit Statistic | p-value | |
| Global χ2 | 26.78 | - | 31.47 | 0.03 | 72.4 | <0.0001 | 84.85 | 0.0004 | 85.07 | 0.64 | 94.09 | 0.003 |
| AIC | 1081.33 | - | 1078.64 | 0.10 | 1039.71 | <0.0001 | 1029.25 | 0.001 | 1031.04 | 0.18 | 1024.02 | 0.008 |
| Calibration χ2 | 6.83 | 0.65 | 3.22 | 0.95 | 11.31 | 0.26 | 5.83 | 0.76 | 6.92 | 0.65 | 8.00 | 0.53 |
| C-index | 0.65 (0.59–0.71) | ref | 0.66 (0.6–0.72) | 0.31 | 0.74 (0.68–0.8) | 0.01 | 0.75 (0.7–0.81) | 0.17 | 0.75 (0.7–0.81) | 0.79 | 0.77 (0.72–0.82) | 0.05 |
| Covariate | HR | p-value | HR | p-value | HR | p-value | HR | p-value | HR | p-value | HR | p-value |
|
| ||||||||||||
| Duke Clinical Score | 6.52 (2.99–14.22) | <0.0001 | 7.54 (3.40–16.69) | <0.0001 | 3.72 (1.66–8.36) | 0.002 | 2.84 (1.25–6.42) | 0.01 | 2.88 (1.27–6.52) | 0.01 | 3.00 (1.32–6.84) | 0.009 |
| Early Revascularization | 1.13 (0.61–2.10) | 0.69 | 1.17 (0.63–2.18) | 0.61 | 0.97 (0.52–1.80) | 0.92 | 0.63 (0.33–1.24) | 0.18 | 0.64 (0.33–1.25) | 0.19 | 0.57 (0.29–1.11) | 0.1 |
| eGFR | 0.99 (0.98–1.00) | 0.03 | 0.99 (0.98–1.00) | 0.03 | 0.99 (0.98–1.00) | 0.01 | 0.99 (0.98–1.00) | 0.01 | 0.99 (0.98–1.00) | 0.05 | ||
| Rest LVEF | 0.96 (0.94–0.97) | <0.0001 | 0.97 (0.95–0.98) | <0.0001 | 0.97 (0.95–0.98) | <0.0001 | 0.97 (0.96–0.99) | 0.0002 | ||||
| Ischemia+Scar (%) | 1.03 (1.01–1.04) | 0.0003 | 1.03 (1.01–1.04) | 0.0003 | 1.03 (1.01–1.04) | 0.0007 | ||||||
| LVEF Reserve | 1.11 (0.71–1.76) | 0.64 | 1.23 (0.78–1.96) | 0.38 | ||||||||
| CFR <1.5 | 2.11 (1.27–3.51) | 0.004 | ||||||||||
Summary of characteristics of five nested models for cardiac mortality. Hazard ratios are presented per unit change for each continuous variables.
P-values for fit statistics are for comparison of each model to the next simpler model (e.g. model 6 vs. model 5). C-indices and Hosmer-Lemeshow statistics are calculated for 2-year event data. Global χ2 = likelihood ratio chi-squared statistic for the entire model. AIC = Akaike Information Criterion. LVEF = left ventricular ejection fraction. CFR = coronary flow reserve.
Figure 2. Cardiac Mortality.
Incidence of cardiac mortality for patients coronary flow reserve (CFR) above and below the median (1.5) presented in Kaplan-Meier form (panel A) showing significantly increased risk of cardiac mortality with CFR <1.5 (p<0.0001) which continued after adjustment(18) for clinical risk (Duke clinical score(17)), early revascularization, rest left ventricular ejection fraction (LVEF), extent of myocardial ischemia and scar and LVEF reserve (panel B; p=0.0004). Graphs are censored at 3-years for simplicity. HR = hazard ratio.
Risk Reclassification
Addition of CFR estimates to the model resulted in the reclassification of 14%, 36%, and 12% of patients at low (<2% annualized cardiac mortality), intermediate (2–4% annualized cardiac mortality), and high cardiac risk (≥4% annualized cardiac mortality), respectively, based on the pre-CFR model (Table 2, Model 5) (Figure 3). The benefit of CFR on risk reclassification was greatest among patients with an intermediate pre-CFR risk, in whom addition of CFR to risk estimation downgraded risk in 21% (0% annualized cardiac mortality) and upgraded it in 15% (9.8% annualized cardiac mortality).
Figure 3. Risk Reclassification.
Illustration of risk reclassification by addition of coronary flow reserve (CFR) to a model containing clinical risk factors, left ventricular ejection fraction (LVEF), LVEF reserve and combined extent of myocardial ischemia and scar. The height of each bar is proportional to the number of patients in each pre-CFR risk category (<2, 2–4 and >4% per year risk of cardiac death) as estimated by a model containing clinical risk factors, rest LVEF, LVEF reserve and extent of myocardial ischemia and scar (Model 4, Table 2). Each of these bars is subdivided proportionate to the number of patients reclassified as <2 (green), 2–4 (blue) and >4% (pink) per year risk of cardiac death categories after the addition of CFR to the risk model (Model 5, Table 2). The horizontal bar charts at right represent the observed annualized rates of cardiac mortality in each of the post-CFR risk categories.
In the entire cohort, 19.5% of patients were reclassified (7.7% upward and 11.8% downward) into more accurate risk categories. The net reclassification improvement (NRI) was 0.142 (95%CI 0.076–0.219) across clinical risk categories of <2, 2–4, and ≥4% annual rate of cardiac death (Supplement, Table 2). The effect of reclassification was most pronounced among those patients classified as low (NRI=0.914; 95%CI 0.817–1.11) or intermediate risk without CFR (NRI=0.489; 95%CI 0.192–0.836). However, significant reclassification was also seen among patients classified as high risk without CFR (NRI=0.145; 95%CI 0.106–0.185). Using thresholds of <1, 1–3, and ≥3% annual rate of cardiac death, the NRI for all patients was 0.098 (95%CI 0.055–0.148). The continuous NRI, which measures discriminatory potential, was 0.390 (95%CI 0.131–0.639).
All-Cause Mortality
Analyses were repeated using mortality from any cause as a secondary outcome and the results were similar. After correction for clinical risk, left ventricular systolic function, extent of ischemia and scar, and stress induced LVEF augmentation, CFR remained a significant predictor of mortality with CFR <1.5 associated with a hazard ratio of 1.9 (95%CI 1.3–2.8, p=0.0004). Addition of CFR was associated with favorable risk reclassification for all-cause mortality (continuous NRI=0.461; 95%CI 0.257–0.658). Using risk thresholds of 4 and 8% per year (double those for cardiac mortality), the NRI was 0.129 (95%CI 0.044–0.222).
Discussion
The principal finding of this study is that the severity of coronary vascular dysfunction, as assessed by PET, is an independent predictor of cardiac death in patients with moderate or severe renal impairment. We observed that the failure of myocardial blood flow to increase adequately on demand identified patients with renal impairment who experienced a significantly higher rate of cardiac mortality (10.7 vs. 3.2%/year in those with relatively preserved coronary vasodilator reserve, p<0.0001). Importantly, identification of coronary vasodilator dysfunction improved risk stratification beyond comprehensive clinical assessment, LV systolic function and semi-quantitative measures of myocardial ischemia and scar. Indeed, a quantitative estimate of coronary vasodilator reserve in this cohort was able to improve risk stratification in more than one third of patients with intermediate risk, appropriately downgrading risk in 15% and upgrading it in 21% of patients.
Prior studies have shown that coronary vasodilator function assessment improves prognostic assessment(24). This study demonstrates the benefits of improved risk stratification by quantitative measures of coronary vascular dysfunction also apply to patients with moderate-severe renal dysfunction, who are among the highest risk cohorts for CAD complications. Although future studies will be required to determine how coronary flow reserve metrics should best be incorporated into treatment strategies, more aggressive medical treatment of patients with visually normal perfusion but impaired flow reserve could potentially improve outcomes. Similarly, it is possible that avoidance of angiography and revascularization in persons with myocardial scar and/or ischemia but with preserved coronary flow reserve may decrease nephrotoxicity without compromising safety.
Noninvasive measures of coronary vasodilator reserve integrate the hemodynamic effects of focal epicardial coronary stenoses, the fluid dynamic effects of diffuse atherosclerosis, and the presence of coronary microvascular dysfunction. Thus, the close relationship between the blunting of the increase in myocardial blood flow with stress and prognosis could be due to any or all of these. Patients with renal impairment may be more likely to have advanced multi-vessel epicardial coronary disease(25) and more rapid progression of disease(26), both of which may contribute to adverse prognosis(27). Prior investigations have also suggested that renal disease is associated with abnormal coronary vasodilator function(28), which may result from multiple mechanisms(29) including decreased capillary density(30) leading to microvascular dysfunction as well as vascular remodeling in epicardial arteries(31). Our demonstration of increased cardiac mortality in patients who failed to augment myocardial blood flow in response to stress in the absence of overt evidence of myocardial ischemia (5.9% vs. 1.1% per year for those with relatively preserved flow reserve, p=0.002) provides new evidence that microvascular abnormalities play a role in the increased cardiovascular risk of patients with renal impairment. This is supported by prior studies showing that angiographic measures of coronary disease severity could not fully explain the increased risk observed in patients with renal dysfunction(32). Consequently, it is likely that either diffuse atherosclerosis, microvascular dysfunction or both together account for at least part of the increased risk observed in patients with poor coronary vasodilator function.
The current study is a single-center, non-randomized, observational study and carries all of the inherent limitations of that study design. As such, it is likely that some amount of residual confounding remains, despite careful adjustment for clinically relevant covariates. On the other hand, compared to data derived from patients selectively enrolled in a randomized trial, these data, with very limited exclusion criteria, may be more representative of patients seen in routine clinical practice. Although the MDRD formula for estimated GFR has been extensively validated, it represents an estimate of renal function at a single time point. However, for 99% of patients in this study, ≥2 creatinine values were averaged, reducing the impact of fluctuations. At present, cardiac PET is only available at a relatively small number of institutions compared to other stress testing modalities such as single photon emission tomography (SPECT) and echocardiography. However, with increased PET scanner availability and emerging longer half-life tracers for myocardial perfusion imaging available for unit dose distribution (33), accessibility of cardiac PET is likely to continue to improve(34).
Other methods of stress imaging are not able to routinely quantify myocardial perfusion. Advances in SPECT imaging technology may enable this in the near future. Routine stress echocardiography can detect impaired myocardial perfusion once severe enough to result in overt systolic dysfunction, quantification of subclinical abnormalities in myocardial perfusion requires more advanced methods than are available at most sites. One approach, Doppler interrogation of coronary flow velocities, typically in the left anterior descending artery, has been demonstrated to identify patients at risk of future coronary events(35). This method requires excellent acoustic windows, which are often not available due to body habitus or lung disease. Furthermore, this technique can only evaluate a subset of the coronary tree and may thus underestimate disease burden. Myocardial contrast echocardiography may increase the proportion of evaluable myocardium, but lacks regulatory approval in the United States.
In summary, among patients with moderate to severe renal dysfunction non-invasive assessment of coronary vasodilator function provides incremental risk stratification beyond routine measures of clinical risk, including estimates of LV systolic function and the extent and severity of myocardial ischemia and scar, and results in a meaningful risk reclassification of one in five patients with known or suspected CAD. These findings have potentially important implications for optimal identification of high risk individuals and selection of more aggressive management strategies, especially given the markedly higher rates of morbidity and mortality associated with cardiac catheterization and revascularization in patients with renal impairment(6, 7).
Supplementary Material
Acknowledgements
Funding Sources The study was funded in part by grants from the United States National Institutes of Health (RC1 HL101060-01 and T32 HL094301-01A1).
Disclosures Dr. Di Carli receives research grant support from Toshiba. Dr Charytan was supported by a Carl S. Gottschalk award from the American Society of Nephrology. Dr. Murthy owns equity in General Electric.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Conception and Design (VLM and MDC), Acquisition of Data (VLM, MN, CRF, JH and MG), Analysis and Interpretation of Data (VLM, MN, SD, DMC, RB and MDC), Drafting of Manuscript (VLM and MDC), Critical Revision of Manuscript (VLM, MN, CRF, JH, MG, SD, DMC, RB and MDC), Statistical Analysis (VLM), Obtaining Funding (MDC), Administrative, Technical or Material Support (JH, MG, MDC), Supervision (MDC). VLM had full access to all of the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.U.S. Renal Data System . USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2010. Available at: http://www.usrds.org/adr.htm. [Google Scholar]
- 2.Charytan DM, Wallentin L, Lagerqvist B, et al. Early Angiography in Patients with Chronic Kidney Disease: A Collaborative Systematic Review. Clinical Journal of the American Society of Nephrology. 2009;4(6):1032–1043. doi: 10.2215/CJN.05551008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin D-H, Choi D-J, Youn T-J, et al. Comparison of Contrast-Induced Nephrotoxicity of Iodixanol and Iopromide in Patients With Renal Insufficiency Undergoing Coronary Angiography. The American Journal of Cardiology. 2011;108(2):189–194. doi: 10.1016/j.amjcard.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Rihal CS, Textor SC, Grill DE, et al. Incidence and Prognostic Importance of Acute Renal Failure After Percutaneous Coronary Intervention. Circulation. 2002;105(19):2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 5.Gruberg L, Mintz GS, Mehran R, et al. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. Journal of the American College of Cardiology. 2000;36(5):1542–1548. doi: 10.1016/s0735-1097(00)00917-7. [DOI] [PubMed] [Google Scholar]
- 6.Cooper WA, O'Brien SM, Thourani VH, et al. Impact of Renal Dysfunction on Outcomes of Coronary Artery Bypass Surgery. Circulation. 2006;113(8):1063–1070. doi: 10.1161/CIRCULATIONAHA.105.580084. [DOI] [PubMed] [Google Scholar]
- 7.Naidu SS, Selzer F, Jacobs A, et al. Renal insufficiency is an independent predictor of mortality after percutaneous coronary intervention. The American Journal of Cardiology. 2003;92(10):1160–1164. doi: 10.1016/j.amjcard.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Al-Mallah MH, Hachamovitch R, Dorbala S, Di Carli MF. Incremental Prognostic Value of Myocardial Perfusion Imaging in Patients Referred to Stress Single-Photon Emission Computed Tomography With Renal Dysfunction. Circulation: Cardiovascular Imaging. 2009;2(6):429–436. doi: 10.1161/CIRCIMAGING.108.831164. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJV, Uno H, Jarolim P, et al. Predictors of fatal and nonfatal cardiovascular events in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia: An analysis of the Trial to Reduce cardiovascular Events with Aranesp (darbepoetin-alfa) Therapy (TREAT) American Heart Journal. 2011;162(4):748–755. e3. doi: 10.1016/j.ahj.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 10.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. 2002;39(2 Suppl 1):S1–266. Anon. [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, et al. A More Accurate Method To Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Annals of Internal Medicine. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.El Fakhri G, Sitek A, Guerin B, Kijewski MF, Di Carli MF, Moore SC. Quantitative Dynamic Cardiac 82Rb PET Using Generalized Factor and Compartment Analyses. J Nucl Med. 2005;46(8):1264–1271. [PubMed] [Google Scholar]
- 13.Senthamizhchelvan S, Bravo PE, Lodge MA, Merrill J, Bengel FM, Sgouros G. Radiation Dosimetry of 82Rb in Humans Under Pharmacologic Stress. J Nucl Med. 2011;52(3):485–491. doi: 10.2967/jnumed.110.083477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart: A Statement for Healthcare Professionals From the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 15.El Fakhri G, Kardan A, Sitek A, et al. Reproducibility and Accuracy of Quantitative Myocardial Blood Flow Assessment with 82Rb PET: Comparison with 13N-Ammonia PET. J Nucl Med. 2009;50(7):1062–1071. doi: 10.2967/jnumed.104.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 17.Pryor DB, Shaw L, McCants CB, et al. Value of the History and Physical in Identifying Patients at Increased Risk for Coronary Artery Disease. Annals of Internal Medicine. 1993;118(2):81–90. doi: 10.7326/0003-4819-118-2-199301150-00001. [DOI] [PubMed] [Google Scholar]
- 18.Nieto FJ, Coresh J. Adjusting Survival Curves for Confounders: A Review and a New Method. American Journal of Epidemiology. 1996;143(10):1059–1068. doi: 10.1093/oxfordjournals.aje.a008670. [DOI] [PubMed] [Google Scholar]
- 19.Hachamovitch R, Di Carli MF. Methods and Limitations of Assessing New Noninvasive Tests: Part II: Outcomes-Based Validation and Reliability Assessment of Noninvasive Testing. Circulation. 2008;117(21):2793–2801. doi: 10.1161/CIRCULATIONAHA.107.714006. [DOI] [PubMed] [Google Scholar]
- 20.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Statist. Med. 2004;23(13):2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 21.Antolini L, Nam B-H, D'Agostino RB. Inference on Correlated Discrimination Measures in Survival Analysis: A Nonparametric Approach. Communications in Statistics: Theory & Methods. 2004;33(9):2117–2135. [Google Scholar]
- 22.Pencina MJ, D' Agostino RB, Sr, D' Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Statistics in Medicine. 2008;27(2):157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 23.Gibbons RJ, Chatterjee K, Daley J, et al. ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina) J. Am. Coll. Cardiol. 1999;33(7):2092–2197. doi: 10.1016/s0735-1097(99)00150-3. [DOI] [PubMed] [Google Scholar]
- 24.Murthy VL, Naya M, Foster CR, et al. Improved Cardiac Risk Assessment With Noninvasive Measures of Coronary Flow Reserve. Circulation. 2011;124(20):2215–2224. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joki N, Hase H, Nakamura R, Yamaguchi T. Onset of coronary artery disease prior to initiation of haemodialysis in patients with end-stage renal disease. Nephrology Dialysis Transplantation. 1997;12(4):718–723. doi: 10.1093/ndt/12.4.718. [DOI] [PubMed] [Google Scholar]
- 26.Gradaus F, Ivens K, Peters AJ, et al. Angiographic progression of coronary artery disease in patients with end - stage renal disease. Nephrology Dialysis Transplantation. 2001;16(6):1198–1202. doi: 10.1093/ndt/16.6.1198. [DOI] [PubMed] [Google Scholar]
- 27.Joki N, Hase H, Takahashi Y, et al. Angiographical severity of coronary atherosclerosis predicts death in the first year of hemodialysis. Int Urol Nephrol. 2003;35(2):289–297. doi: 10.1023/b:urol.0000020356.82724.37. [DOI] [PubMed] [Google Scholar]
- 28.Chade AR, Brosh D, Higano ST, Lennon RJ, Lerman LO, Lerman A. Mild renal insufficiency is associated with reduced coronary flow in patients with non-obstructive coronary artery disease. Kidney Int. 2006;69(2):266–271. doi: 10.1038/sj.ki.5000031. [DOI] [PubMed] [Google Scholar]
- 29.Amann K, Ritz E. Microvascular disease—the Cinderella of uraemic heart disease. Nephrology Dialysis Transplantation. 2000;15(10):1493–1503. doi: 10.1093/ndt/15.10.1493. [DOI] [PubMed] [Google Scholar]
- 30.Amann K, Breitbach M, Ritz E, Mall G. Myocyte/capillary mismatch in the heart of uremic patients. Journal of the American Society of Nephrology. 1998;9(6):1018–1022. doi: 10.1681/ASN.V961018. [DOI] [PubMed] [Google Scholar]
- 31.Schwarz U, Buzello M, Ritz E, et al. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrology Dialysis Transplantation. 2000;15(2):218–223. doi: 10.1093/ndt/15.2.218. [DOI] [PubMed] [Google Scholar]
- 32.Yiu KH, de Graaf FR, Schuijf JD, et al. Prognostic Value of Renal Dysfunction for the Prediction of Outcome Versus Results of Computed Tomographic Coronary Angiography. The American Journal of Cardiology. 2011;108(7):968–972. doi: 10.1016/j.amjcard.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 33.Maddahi J, Czernin J, Lazewatsky J, et al. Phase I, first-in-human study of BMS747158, a novel 18F-labeled tracer for myocardial perfusion PET: dosimetry, biodistribution, safety, and imaging characteristics after a single injection at rest. J. Nucl. Med. 2011;52(9):1490–1498. doi: 10.2967/jnumed.111.092528. [DOI] [PubMed] [Google Scholar]
- 34.Di Carli MF, Murthy VL. Cardiac PET/CT for the evaluation of known or suspected coronary artery disease. Radiographics. 2011;31(5):1239–1254. doi: 10.1148/rg.315115056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortigiani L, Rigo F, Galderisi M, et al. Diagnostic and prognostic value of Doppler echocardiographic coronary flow reserve in the left anterior descending artery in hypertensive and normotensive patients [corrected] Heart. 2011;97(21):1758–1765. doi: 10.1136/heartjnl-2011-300178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.