Abstract
Using adenoviruses encoding reporter genes as retrograde tracers, we assessed the capacity of motoneurons to take up and retrogradely transport adenoviral particles injected into the muscles of transgenic mice expressing the G93A human superoxide dismutase mutation, a model of amyotrophic lateral sclerosis. Surprisingly, transgene expression in the motoneurons was significantly higher in symptomatic mice than in control or presymptomatic mice. Using botulinum toxin to induce nerve sprouting at neuromuscular junctions, we showed that the unexpectedly high level of motoneurons retrograde transduction results, at least in part, from newly acquired uptake properties of the sprouts. These findings demonstrate the remarkable uptake properties of amyotrophic lateral sclerosis motoneurons in response to denervation and the rationale of using intramuscular injections of adenoviruses to overexpress therapeutic proteins in motor neuron diseases.
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disorder caused by the progressive death of motoneurons in the cortex, brainstem, and spinal cord, leading to paralysis and death usually within 2–5 years. There is no treatment for this disease, and its etiology remains unknown, although the discovery of missense mutations in the gene for copper-zinc superoxide dismutase (SOD1) in some pedigrees with familial ALS has marked an important advance in the understanding of ALS physiopathology (1, 2). Most ALS cases are sporadic, and, of the 10% autosomal dominant inherited cases, about 20% of kindreds are associated with mutations in the SOD1 gene.
The SOD1 enzyme plays a critical role in preventing cell damage by free radicals by scavenging the superoxide anion radical and converting it into oxygen and hydrogen peroxide (3). Although the mechanism by which mutations in the gene encoding ubiquitous SOD1 protein lead to selective motoneuron degeneration is unknown, some such mutations cause motoneuron disease when expressed in transgenic mice (4–7). For example, transgenic SOD1 mutant mice (G93A, glycine-to-alanine substitution at position 93) develop progressive loss of motoneurons and vacuolar degeneration of mitochondria within motoneuron cell bodies of the spinal cord and the brainstem, leading to a progressive decline in motor function and death at 5–6 months of age (4, 8). Novel cytotoxic properties of the mutated SOD1, rather than a decrease in enzyme activity, are thought to be involved in this neurotoxicity. In particular, the SOD1 mutation may induce misaccumulation of the neurofilaments (NF) (9), as has been described in both human (10–12) and experimental ALS (4, 13).
A gene-delivery method using retrograde axonal transport of adenoviral vectors has been proposed to overexpress therapeutic proteins in motoneurons after i.m. injections of these vectors (14, 15). However, the value of this gene-transfer method for the treatment of motor neuron diseases remains unclear in view of studies reporting denervation and impairment of axonal transport in the mouse models of ALS (13, 16–18). The NF misaccumulations have been implicated in the impairment of both slow and fast axonal transport in G93A mice (16). Although the impairment of fast axonal transport has been described as a very early event in these mice (17), the low level of slow axonal transport has been reported to be the earliest abnormality detected in G37R and G85R mice (18).
The aim of this study was to assess axonal retrograde transport in G93A mice over time by using adenoviruses encoding reporter genes as retrograde tracers. Replication-defective adenoviruses expressing the luciferase and lacZ genes, as well as the retrograde tracer horseradish peroxidase (HRP), were inoculated into the tongue muscles of wild-type (wt), presymptomatic, and symptomatic G93A mice, and the presence of proteins was analyzed in the motoneuron soma in the brainstem. Surprisingly, i.m. injection of the adenoviruses in symptomatic ALS mice resulted in a substantially higher level of transgene expression, consecutive to the transduction of a larger number of motoneurons, than in presymptomatic G93A and wt mice. These findings demonstrate the maintenance of axonal retrograde transport in G93A mice and even its improvement after the onset of the disease. We demonstrate further the remarkable uptake properties of motoneurons in response to ALS denervation and botulinum neurotoxin (BoNT) paralysis. We also discuss the possibility that an alternative endocytosis pathway is induced in these two models.
Materials and Methods
Recombinant Adenoviruses.
Ad-phosphoglycerate kinase (PGK)-luc encodes the luciferase enzyme driven by the PGK promoter (19). Ad-Rous sarcoma virus (RSV)-β-galactosidase (βgal) encodes β-gal targeted to the nucleus by the simian virus 40 nuclear localization signal under the control of the RSV promoter (20). These first-generation adenoviruses were obtained by homologous recombination as described (20). Viral stocks were prepared by standard procedures (19).
Animals and Injection Procedures.
G93A transgenic mice derived from B6SJL-TgN (SOD1-G93A)1Gur (4) and wt littermates were provided by Transgenic Alliance (Iffa Credo, France). Production of normal SOD1 transgenic mice (NS) and control littermates (WTNS) is described in ref. 21. Transgenic progeny were identified by PCR amplification of tail DNA. All animals were maintained and treated according to the guidelines of the European Community.
After deep anesthesia with Rompun (Bayer Pharma)/Ketamine (Virbac, Carros, France), G93A and wt mice were slowly given (2.5 μl⋅min−1) 10 μl of PBS containing 109 pfu of Ad-PGK-luc, Ad-RSV-βgal, or 10% HRP type VI by injection into the tongue (four sites). For BoNT injection, 25 pg of type A BoNT (Sigma) was diluted in 10 μl of PBS and injected into the tongue of wt mice by a procedure similar to that used for adenoviruses. BoNT- or PBS-injected animals were inoculated with Ad-PGK-luc 8 days later. After BoNT injections, mice received standard food pellet bruised in water ad libitum.
Measurement of Luciferase Activity.
Ad-PGK-luc-injected mice were killed by overdose of pentobarbital (Sanofi, Paris), and their brainstems and tongues were removed for measurement of luciferase activity, which was performed as described (19) and normalized according to the amount of protein in cell extracts determined by the Bio-Rad assay (Bio-Rad).
Histochemical Analyses.
Ad-RSV-βgal- and HRP-injected mice were killed by overdose of pentobarbital, and transcardial perfusion with fixative solution consisted of PBS containing 4% paraformaldehyde or 1% paraformaldehyde with 1.25% glutaraldehyde for Ad-RSV-βgal and HRP groups, respectively. Brains were removed, cryoprotected in 30% sucrose overnight, and frozen by immersion in isopentane at −40°C. Brains were cut into 16-μm-thick transversal sections.
Detection of βgal activity was processed by incubating the sections for 2 h with PBS containing the 5-bromo-4-chloro-3-indoyl-β-d-galactosidase substrate (X-Gal, 0.4 mg/ml; Appligene, Strasbourg, France) with 4 mM potassium ferricyanide (Sigma), 4 mM potassium ferrocyanide (Merck), and 2 mM MgCl2 (Merck). The sections then were counterstained with neutral red and mounted.
Peroxidase activity was detected by using tetramethyl benzidine (TMB) as a chromogen as recommended (22). Sections were incubated with a 3,3′, 5,5′-TMB liquid substrate system (Sigma) for 10 min and mounted.
The number of X-Gal-positive, TMB-positive, or Nissl-stained motoneurons in the hypoglossal nucleus was assessed by manual counting on every fourth section of the brainstem (blind-coded slides). The raw values were corrected according to Abercrombie's formula (23).
Neuromuscular Junctions (NMJ) Analyses.
Longitudinal sections of the tongues (50 μm) were labeled with 0.5 μg/ml tetramethylrhodamine-α-bungarotoxin (Molecular Probes) and NF antibody (1:50; Chemicon), detected by using anti-mouse secondary antibody conjugated to fluorescein (1:400; Southern Biotechnology Associates). α-Bungarotoxin and antibodies were diluted in a PBS solution containing 3% BSA, 10% goat serum, and 0.5% Triton X-100 (Sigma). Sections were observed by laser confocal microscopy. For each animal (three wt and four G93A mice), 20–40 endplates were analyzed, and the percentages of sprouted endplates were compared by Student's t test.
Western Blot Analysis.
Brainstem extracts from G93A mice, NS mice, and control littermates were homogenized in a solution of Tris-phosphate (25 mM) containing 0.5% Triton X-100 (Sigma) and a protease inhibitor mixture (Complete Mini; Roche, Gipf-Oberfrick, Switzerland).
One hundred micrograms of protein was electrophoresed in each lane on a 15% polyacrylamide gel and transferred onto a nitrocellulose membrane. Mouse and human SOD1 were detected by using a polyclonal rabbit antiserum raised to mouse and human SOD1 (Annie Nicole, Institut National de la Santé et de la Recherche Médicale, Paris). After preincubation in PBS containing 5% low-fat milk and 0.1% Tween, membranes were incubated with the primary antibody diluted in the preincubation solution (1:2,000) overnight at room temperature. Membranes then were incubated with a secondary anti-rabbit antibody diluted in the preincubation solution (1:10,000) for 30 min at room temperature. SOD1 bands were detected by using chemiluminescence (ECL Plus Western blotting detection system; Amersham Pharmacia).
Results
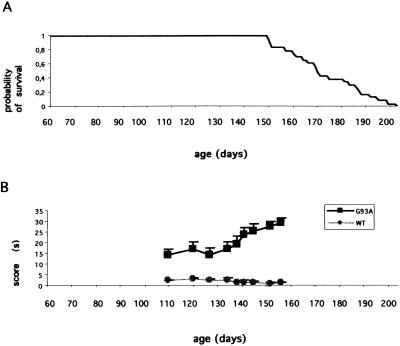
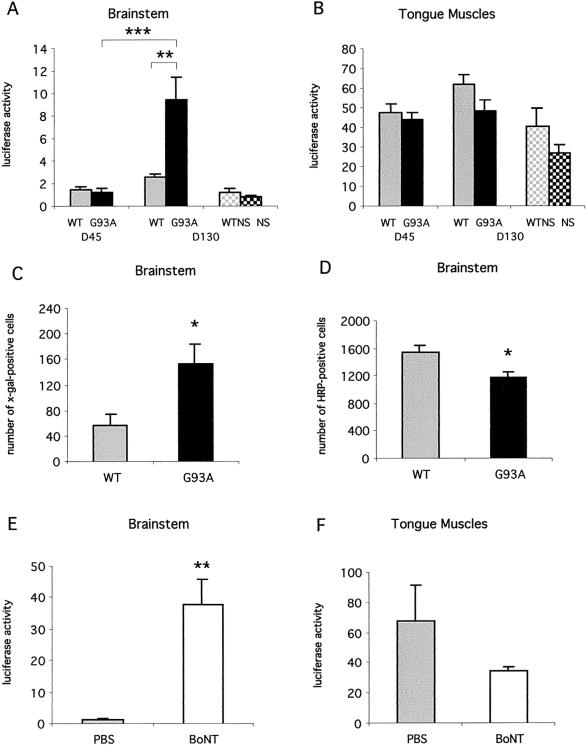
G93A mice began to show signs of weakness at 90 days of age, with progressive motor impairment and death at ≈170 days of age (Fig. 1A). Muscle strength was evaluated over time (beginning at 110 days of age) with the traction test, which is one of the first motor tests to show impairment in these mice (24). Motor performance was significantly lower in G93A mice than in wt mice at each time point tested (Fig. 1B). Forty-five- and 130-day-old ALS mice were arbitrarily defined as presymptomatic and symptomatic animals, respectively. Retrograde axonal transport was investigated in these two age groups by evaluating luciferase expression in the brainstem of the animals 10 days after i.m. injection into the tongues of Ad-PGK-luc. Luciferase activity measured in the brainstem was similar for wt and G93A mice at 45 days of age (Fig. 2A). In contrast, luciferase activity was significantly higher in the brainstems of 130-day-old G93A mice treated with Ad-PGK-luc than in the brainstems of wt littermates (360% of increase) or 45-day-old mutant mice (770% of increase, Fig. 2A). There was no substantial difference of luciferase activity in the inoculated tongues of wt and G93A mice at either 45 days or 130 days of age (Fig. 2B). This result was confirmed with the Ad-RSV-βgal vector. Ten days after adenovirus injection, we found a significantly larger number of motoneurons (270%) expressing the transgene in the hypoglossal nucleus of 130-day-old G93A mice than in those of the other group (Figs. 2C and 3 A–D). Similar results were obtained in five independent experiments. Gene transfer by adenoviral retrograde transport thus appears to be more efficient in symptomatic ALS mice than in wt littermates.
Figure 1.
Cumulative probability of survival for G93A mice (A) and motor performance through time (B) in G93A (solid squares) and wt (shaded circles) mice in a behavioral test assessing muscle strength. Each mouse was suspended by its front legs on a tight rope, and the time used to put one hind leg onto the rope was measured (this time was arbitrarily limited to 30 sec). Data are means ± SEM of the scores for 12 G93A and 12 wt mice. Score means of the two groups were compared by using Student's t test.
Figure 2.
Luciferase activity in the brainstem (A) and in the tongue (B) 10 days after i.m. injection of Ad-PGK-luc in 45-day-old (D45) and 130-day-old (D130) mice. Solid and shaded bars, mutant SOD1 G93A mice (G93A) and control littermates (WT), respectively. Data are means ± SEM from three independent experiments; n = 14 at D45 and n = 11 at D130 in each group. Dark and light checked bars, normal SOD1 mice (NS, n = 3) and control littermates (WTNS, n = 4), respectively. (C and D) Numbers of X-Gal- (C) or TMB-positive (D) motoneurons in the hypoglossal nucleus after injection of Ad-RSV-βgal (C) or HRP (D) into the tongue of 130-day-old G93A (solid bars) and wt (shaded bars) mice. Data are means ± SEM for six (C) or seven (D) animals from two independent experiments. (E and F) Luciferase activity in the brainstem (E) and the tongue (F) 10 days after injection of Ad-PGK-luc into the tongue pretreated with BoNT (striped bars) or PBS (shaded bars). Data are means ± SEM for four animals. Luciferase activity is expressed in RLU/μg of protein (A and B; E and F). Values were compared by Student's t test (*, P < 0.05); **, P < 0.01; ***, P < 0.001.
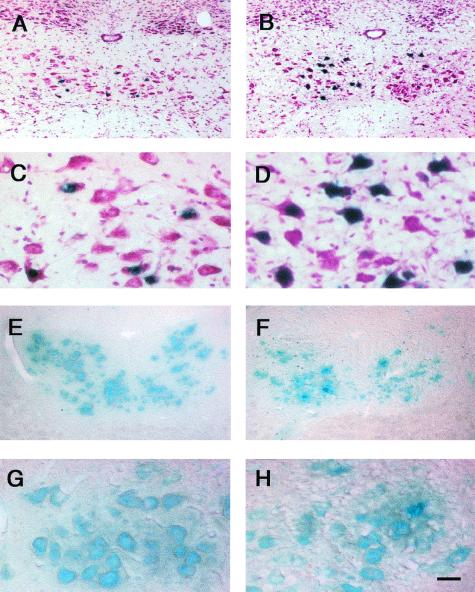
Figure 3.
Detection of X-Gal- (A–D) or TMB-positive (E–H) motoneurons in the hypoglossal nucleus of 130-day-old wt (A, C, E, and G) or G93A (B, D, F, and H) mice. A higher number of transduced motoneurons (expressing intranuclear βgal) was detected in the hypoglossal nucleus of G93A (B and D) than in that of wt (A and C) mice 10 days after i.m. injection of Ad-RSV-βgal. Intracytoplasmic peroxidase activity was detected by using TMB as substrate in the hypoglossal nucleus 48 h after injection of HRP into the tongue. Fewer stained motoneurons were detected in G93A (F and H) than in wt (E and G) mice. The presence of vacuoles, histopathological features of motoneuron degeneration (8), is particularly clear in D and H. [Scale bar = 100 μm (A, B, E, and F) and 25 μm (C, D, G, and H).]
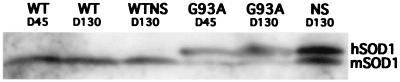
To determine whether enhancement of retrograde transport in symptomatic G93A mice is a consequence of the SOD1 mutation or simply of the high level of SOD1 synthesis reported in these mice (4), we assessed axonal retrograde transport in NS mice (21). NS mice expressed a high level of human SOD1 synthesis, comparable to that of 45- and 130-day-old G93A mice (Fig. 4).
Figure 4.
Mutant and normal SOD1 from brainstem of 45- and 130-day-old G93A mice (G93A D45, “presymptomatic,” and G93A D130, “symptomatic,” respectively), 130-day-old NS mice (NS D130, normal SOD1), and control, nontransgenic littermates. Both mouse (mSOD1) and human (hSOD1) were detected by using a polyclonal antibody cross-reacting with the human and mouse protein. All SOD1 transgenic mice (G93A D45, G93A D130, and NS D130) produced high levels of human SOD1.
Intralingual injection of Ad-PGK-luc resulted in a similar level of luciferase activity in the brainstems of NS and WTNS mice (Fig. 2A). There was no difference between the luciferase activities in the inoculated tongues of NS and WTNS mice (Fig. 2B), despite the high level of SOD1 synthesis detected in the NS mice (Fig. 4). Therefore, the unexpected enhancement of retrograde transport in symptomatic G93A mice is not a consequence of the high level of SOD1 synthesis, but presumably is due to the effect of the SOD1 mutation.
In view of these unexpected findings, we examined in these mice the axonal retrograde transport of HRP, a commonly used retrograde tracer (22). We counted HRP-positive neurons in the hypoglossal nucleus 2 days after injection of the tracer into the tongue of wt and G93A mice. At 130 days of age, there were fewer HRP-positive motoneurons (23% loss) in the G93A mice than in control littermates (Figs. 2D and 3 E–H). Similarly, there were fewer Nissl-stained motoneurons (20%) in the hypoglossal nucleus of G93A mice than that of wt littermates at 130 days of age (data not shown). These experiments suggest a general maintenance of axonal retrograde transport in symptomatic ALS mice despite the early damage to the machinery of transport reported in these mice (16, 17).
The increased transport of recombinant adenoviruses in symptomatic G93A compared with wt mice could be a result of the unaffected motoneurons acquiring the capacity to take up more viral particles than wt motoneurons at the NMJ. In a recent study, the uptake and transport of a retrograde tracer by dorsal root ganglion (DRG) neurons was increased after peripheral axotomy (25), and this was suggested to be linked to the nerve-sprouting process. This led us to investigate whether sprouting and synaptic remodeling at NMJ in ALS mice (26) was associated with increased efficacy of adenoviral gene transfer. We thus inoculated Ad-PGK-luc into the tongue of wt mice treated with BoNT, an agent known to induce synaptic sprouting (27). Pretreatment with BoNT resulted in a large increase of gene transfer efficacy in the brainstem (20-fold, Fig. 2E). Although luciferase activity was slightly lower in BoNT-treated than control tongues, the difference was not significant (Fig. 2F). These results demonstrate that nerve sprouting increases motoneuronal gene transfer after i.m. injection of adenoviral vectors and strongly support the occurrence of this mechanism in symptomatic ALS mice. This also demonstrates in vivo that BoNT does not arrest vesicle endocytosis although it is known to block exocytosis.
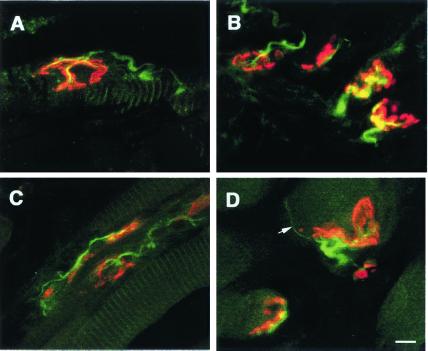
To describe synaptic reinnervation in ALS mice at 130 days of age, we examined the morphology of NMJ in the tongue of both wt and G93A mice (Fig. 5). The percentage of reinnervation and terminal sprouting was significantly higher in the G93A mice (21.7% ± 1.8, P = 0.0005) than in wt littermates (2.3% ± 1.3). Interestingly, the percentage of reinnervation corresponded to the level of motoneurons loss observed in the hypoglossal nucleus (Fig. 2D) and presumably resulted from compensatory processes related to the disease. In ALS mice, the improved transduction of motoneurons after axonal retrograde transport of adenoviral vectors therefore is due, at least in part, to the denervation-induced synaptic reorganization at NMJ.
Figure 5.
Laser confocal-scanned images of neuromuscular synapses in tongue muscles of 130-day-old wt (A) and G93A (B–D) mice. Longitudinal sections were incubated with fluorescein-conjugated anti-NF antibody for detection of nerve (green) and rhodamine-α-bungarotoxin (red) to label acetylcholine receptors. (A and B) Typical morphology of normal endplates in a wt mouse (A) and in an age-matched G93A mouse (B). (C and D) Abnormal synaptic configurations in G93A mice. (C) Reinnervation at numerous endplates on a single muscle fiber. (D) An endplate with a thin, unmyelinated terminal sprout is indicated by a white arrow. (Scale bar = 10 μm.)
Discussion
In ALS mice, progressive loss of neuromuscular synapses combined with nerve sprouting begins very early and correlates with disease progression (26). This is similar to the reinnervation of muscle fibers described previously in ALS patients (28). Here, we demonstrate that denervation-induced sprouting at NMJ modifies the uptake properties of motoneurons in symptomatic ALS mice such that uptake of adenoviruses and resulting motoneuron retrograde transduction is increased after i.m. inoculation of the vectors. Indeed, using BoNT paralysis to induce synaptic sprouting in wt mice before adenoviral injection, we showed that motoneuron transduction was increased considerably. Although BoNT is known to block synaptic vesicle exocytosis, it does not arrest endocytosis, as demonstrated previously in dissociated mouse spinal cord cells (29). Rather, the increase in motoneuron transduction that we observed in BoNT-treated animals demonstrates that vesicle endocytosis is increased by the toxin in vivo.
Similar changes in neuronal uptake recently have been described in the DRG after peripheral axotomy (25). In this model, an increase in neuronal uptake and transport of the cholera toxin B-subunit was induced in the DRG in response to injury. This increase apparently resulted from changes in the regulation of a cell membrane glycoconjugate, the GM1 monoganglioside, known to exert a protective and neuritogenic effect on DRG neurons (25). Similarly, the increased uptake of adenoviral particles that we observed in symptomatic ALS mice probably results from changes in the regulation of cellular molecules.
The greater endocytosis observed in G93A mice may involve Ca2+ activation of calcineurin, which combines with dynamin and amphiphysin in clathrin-mediated endocytosis (30). However, the increase in intracellular Ca2+ levels in the G93A mice (31–33) argues against this hypothesis because the interaction between calcineurin and dynamin is optimal for a small range of intracellular Ca2+ concentration (30). Moreover, calcineurin activity was reported to be either decreased or unchanged in cells overexpressing SOD1 mutants (34, 35). Alternatively, the higher level of endocytosis in G93A mice could be due to the activation of GAP-43, a growth-associated protein present in growth cones and synaptic terminals, which has been implicated in nerve sprouting and endocytosis (36, 37). GAP-43 is bound by calmodulin when Ca2+ levels are low and released when Ca2+ levels rise. During activity-dependent increases in Ca2+ levels, GAP-43 interacts with rabaptin-5, a protein involved in endocytosis (37). Because GAP-43 is negatively regulated by the phosphatase activity of calcineurin (38), the phosphorylation state of GAP-43 may increase secondarily to possible calcineurin inhibition in G93A mice reported in cells overexpressing SOD1 mutants (34). This hypothesis is supported further by the protective effect of wt SOD on Ca2+/calmodulin-dependent calcineurin inactivation (39). Finally, the immunoreactivity of GAP-43 is high in both the spinal cord and nerve terminals of patients with ALS (40, 41) and in motor end plates and axons in BoNT-treated rats (42).
Our study validates the interest of axonal retrograde transport for the administration of therapeutic proteins or of virally transferred therapeutic genes in the central nervous system. Although in the present study, assessment of motoneuron transgene expression was performed 10 days after adenoviral injections, we showed previously that luciferase gene expression was detected in the brainstem for at least 35 days after intralingual injection of the same recombinant adenovirus (Ad-PGK-luc) and of other recombinant adenoviruses expressing luciferase (Ad-NRSE-luc) (19). We also reported in previous studies a long-term expression of other “first-generation” adenoviral vectors injected in different brain structures (persistence of expression at least 6 months after injection) (43, 44). Furthermore, new generations of adenoviruses, from which additional or all viral genes have been deleted (deltaE1/deltaE4 and “gutless”), are expected to increase persistence of transgene expression and to allow secondary vector administration (45, 46). Intramuscular injections of such nonimmunogenic recombinant vectors should be used as a promising therapeutic approach in ALS and other motoneuronal diseases.
Overall, our results and recent data published in the literature suggest the involvement of an alternative endocytosis pathway, possibly regulated by GAP-43, in the increase in uptake observed both in ALS and in BoNT-treated mice. We also demonstrate that axonal retrograde transport of adenoviral vectors can be used successfully to transduce motoneurons in ALS mice. The improved adenovirus gene transfer in the motoneurons of symptomatic ALS mice, despite the misaccumulation of NF and abnormalities in axonal transport reported in these mice, is a striking finding demonstrating the plasticity of ALS motoneurons, which compensate for the loss of nerve fibers by acquiring new capacities for viral particle uptake. This method of gene transfer therefore would be applicable in patients even after the onset of the first clinical signs of disease, allowing diagnosis. As shown here, retrograde gene-transfer efficacy is improved by synaptic sprouting. Thus, the injection of retrogradely transported gene vectors also could be a valuable therapeutic approach in all sprouting-associated neurological diseases such as epilepsy, Parkinson's disease, or Alzheimer's disease.
Acknowledgments
We gratefully acknowledge Jeanine Koenig and Daniel Hantaï from the Institut National pour la Santé et la Recherche Médicale (U523) for expertise in the study of NMJ, Vincent Navarro for helpful advice and critical reading of the manuscript, and Nicole Faucon for expertise in Western blot analysis. We also thank Sylvie Carpaye and Philippe Colin for their care of the animals and Annie Nicole for providing us with the SOD1 antibody. S.M. was supported by the Association Française contre les Myopathies, and M.B. was supported by the Association France Alzheimer. This work was supported by grants from Institut de Recherche sur la Moelle Epinière, Centre National de la Recherche Scientifique, Aventis, Retinis Pigmentosa, and the Conseil Régional d'Ile-de-France.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- βgal
β-galactosidase
- BoNT
botulinum toxin
- DRG
dorsal root ganglion
- HRP
horseradish peroxidase
- NF
neurofilaments
- NMJ
neuromuscular junctions
- PGK
phosphoglycerate kinase
- RSV
Rous sarcoma virus
- SOD1
superoxide dismutase
- TMB
tetramethyl benzidine
- wt
wild type
- NS
normal SOD1 transgenic mice
- WTNS
control SOD1 mice
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Deng H X, Hentati A, Tainer J A, Iqbal Z, Cayabyab A, Hung W Y, Getzoff E D, Hu P, Herzfeldt B, Roos R P, et al. Science. 1993;261:1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- 2.Rosen D R, Siddique T, Patterson D, Figlewicz D A, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan J P, Deng H X, et al. Nature (London) 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 3.Gutteridge J M, Halliwell B. Ann NY Acad Sci. 2000;899:136–147. doi: 10.1111/j.1749-6632.2000.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 4.Gurney M E, Pu H, Chiu A Y, Dal Canto M C, Polchow C Y, Alexander D D, Caliendo J, Hentati A, Kwon Y W, Deng H X, et al. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 5.Wong P C, Pardo C A, Borchelt D R, Lee M K, Copeland N G, Jenkins N A, Sisodia S S, Cleveland D W, Price D L. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 6.Ripps M E, Huntley G W, Hof P R, Morrison J H, Gordon J W. Proc Natl Acad Sci USA. 1995;92:689–693. doi: 10.1073/pnas.92.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruijn L I, Becher M W, Lee M K, Anderson K L, Jenkins N A, Copeland N G, Sisodia S S, Rothstein J D, Borchelt D R, Price D L, et al. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- 8.Dal Canto M C, Gurney M E. Brain Res. 1995;676:25–40. doi: 10.1016/0006-8993(95)00063-v. [DOI] [PubMed] [Google Scholar]
- 9.Chou S M, Wang H S, Komai K. J Chem Neuroanat. 1996;10:249–258. doi: 10.1016/0891-0618(96)00137-8. [DOI] [PubMed] [Google Scholar]
- 10.Hirano A, Donnenfeld H, Sasaki S, Nakano I. J Neuropathol Exp Neurol. 1984;43:461–470. doi: 10.1097/00005072-198409000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Hirano A, Nakano I, Kurland L T, Mulder D W, Holley P W, Saccomanno G. J Neuropathol Exp Neurol. 1984;43:471–480. doi: 10.1097/00005072-198409000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Rouleau G A, Clark A W, Rooke K, Pramatarova A, Krizus A, Suchowersky O, Julien J P, Figlewicz D. Ann Neurol. 1996;39:128–131. doi: 10.1002/ana.410390119. [DOI] [PubMed] [Google Scholar]
- 13.Tu P H, Raju P, Robinson K A, Gurney M E, Trojanowski J Q, Lee V M. Proc Natl Acad Sci USA. 1996;93:3155–3160. doi: 10.1073/pnas.93.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finiels F, Gimenez y Ribotta M, Barkats M, Samolyk M L, Robert J J, Privat A, Revah F, Mallet J. NeuroReport. 1995;7:373–378. [PubMed] [Google Scholar]
- 15.Ghadge G D, Roos R P, Kang U J, Wollmann R, Fishman P S, Kalynych A M, Barr E, Leiden J M. Gene Ther. 1995;2:132–137. [PubMed] [Google Scholar]
- 16.Zhang B, Tu P, Abtahian F, Trojanowski J Q, Lee V M. J Cell Biol. 1997;139:1307–1315. doi: 10.1083/jcb.139.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warita H, Itoyama Y, Abe K. Brain Res. 1999;819:120–131. doi: 10.1016/s0006-8993(98)01351-1. [DOI] [PubMed] [Google Scholar]
- 18.Williamson T L, Cleveland D W. Nat Neurosci. 1999;2:50–56. doi: 10.1038/4553. [DOI] [PubMed] [Google Scholar]
- 19.Millecamps S, Kiefer H, Navarro V, Geoffroy M C, Robert J J, Finiels F, Mallet J, Barkats M. Nat Biotechnol. 1999;17:865–869. doi: 10.1038/12849. [DOI] [PubMed] [Google Scholar]
- 20.Stratford-Perricaudet L D, Makeh I, Perricaudet M, Briand P J. Clin Invest. 1992;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceballos-Picot I, Nicole A, Briand P, Grimber G, Delacourte A, Defossez A, Javoy-Agid F, Lafon M, Blouin J L, Sinet P M. Brain Res. 1991;552:198–214. doi: 10.1016/0006-8993(91)90084-9. [DOI] [PubMed] [Google Scholar]
- 22.Mesulam M M. J Histochem Cytochem. 1978;26:106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- 23.Abercrombie M. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 24.Barneoud P, Lolivier J, Sanger D J, Scatton B, Moser P. NeuroReport. 1997;8:2861–2865. doi: 10.1097/00001756-199709080-00012. [DOI] [PubMed] [Google Scholar]
- 25.Tong Y G, Wang H F, Ju G, Grant G, Hokfelt T, Zhang X. J Comp Neurol. 1999;404:143–158. [PubMed] [Google Scholar]
- 26.Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. J Neurosci. 2000;20:2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson W E. J Physiol. 1969;202:611–630. doi: 10.1113/jphysiol.1969.sp008830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjornskov E K, Norris F H, Jr, Mower-Kuby J. Arch Neurol. 1984;41:527–530. doi: 10.1001/archneur.1984.04050170073021. [DOI] [PubMed] [Google Scholar]
- 29.Neale E A, Bowers L M, Jia M, Bateman K E, Williamson L C. J Cell Biol. 1999;147:1249–1260. doi: 10.1083/jcb.147.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai M M, Hong J J, Ruggiero A M, Burnett P E, Slepnev V I, De Camilli P, Snyder S H. J Biol Chem. 1999;274:25963–25966. doi: 10.1074/jbc.274.37.25963. [DOI] [PubMed] [Google Scholar]
- 31.Carri M T, Ferri A, Battistoni A, Famhy L, Gabbianelli R, Poccia F, Rotilio G. FEBS Lett. 1997;414:365–368. doi: 10.1016/s0014-5793(97)01051-x. [DOI] [PubMed] [Google Scholar]
- 32.Roy J, Minotti S, Dong L, Figlewicz D A, Durham H D. J Neurosci. 1998;18:9673–9684. doi: 10.1523/JNEUROSCI.18-23-09673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruman I I, Pedersen W A, Springer J E, Mattson M P. Exp Neurol. 1999;160:28–39. doi: 10.1006/exnr.1999.7190. [DOI] [PubMed] [Google Scholar]
- 34.Ferri A, Gabbianelli R, Casciati A, Paolucci E, Rotilio G, Carri M T. J Neurochem. 2000;75:606–613. doi: 10.1046/j.1471-4159.2000.0750606.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee J P, Palfrey H C, Bindokas V P, Ghadge G D, Ma L, Miller R J, Roos R P. Proc Natl Acad Sci USA. 1999;96:3251–3256. doi: 10.1073/pnas.96.6.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aigner L, Arber S, Kapfhammer J P, Laux T, Schneider C, Botteri F, Brenner H R, Caroni P. Cell. 1995;83:269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 37.Neve R L, Coopersmith R, McPhie D L, Santeufemio C, Pratt K G, Murphy C J, Lynn S D. J Neurosci. 1998;18:7757–7767. doi: 10.1523/JNEUROSCI.18-19-07757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y C, Storm D R. J Biol Chem. 1989;264:12800–12804. [PubMed] [Google Scholar]
- 39.Wang X, Culotta V C, Klee C B. Nature (London) 1996;383:434–437. doi: 10.1038/383434a0. [DOI] [PubMed] [Google Scholar]
- 40.Ikemoto A, Hirano A, Akiguchi I. Acta Neuropathol. 1999;98:367–373. doi: 10.1007/s004010051096. [DOI] [PubMed] [Google Scholar]
- 41.Ueki A, Namba Y, Otsuka M, Okuno M, Nishimura M, Oda M, Ikeda K. Ann Neurol. 1993;33:226–227. doi: 10.1002/ana.410330217. [DOI] [PubMed] [Google Scholar]
- 42.Hassan S M, Jennekens F G, Wieneke G, Veldman H. Neuromuscul Disord. 1994;4:489–496. doi: 10.1016/0960-8966(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 43.Corti O, Sanchez-Capelo A, Colin P, Hanoun N, Hamon M, Mallet J. Proc Natl Acad Sci USA. 1999;96:12120–12125. doi: 10.1073/pnas.96.21.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navarro V, Millecamps S, Geoffroy M C, Robert J J, Valin A, Mallet J, Le Gal La Salle G. Gene Ther. 1999;6:1884–1892. doi: 10.1038/sj.gt.3301008. [DOI] [PubMed] [Google Scholar]
- 45.Wang Q, Finer M H. Nat Med. 1996;2:714–716. doi: 10.1038/nm0696-714. [DOI] [PubMed] [Google Scholar]
- 46.Engelhardt J F, Ye X, Doranz B, Wilson J M. Proc Natl Acad Sci USA. 1994;91:6196–6200. doi: 10.1073/pnas.91.13.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]