Abstract
Monoclonal antibody directed against the transformation-related protein p53 was microinjected manually into the nuclei of quiescent Swiss 3T3 mouse cells. The cells were subsequently stimulated with 10% fetal calf serum. Microinjection of p53 antibody at or around the time of serum stimulation clearly inhibited the subsequent entry of Swiss 3T3 cells into the S phase of the cell cycle. p53 antibody had no effect on serum-stimulated DNA synthesis when it was microinjected 4 hr or later after serum stimulation. Monoclonal antibody to an unrelated antigen, Lyt-2.2, had no effect on serum-stimulated DNA synthesis regardless of the time it was microinjected. Under similar experimental conditions, p53 antibody had no effect on simian virus 40- or adenovirus 2-induced DNA synthesis. These experiments add strength to the suggestion that p53 is involved in the regulation of cell proliferation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antman K. H., Livingston D. M. Intracellular neutralization of SV40 tumor antigens following microinjection of specific antibody. Cell. 1980 Mar;19(3):627–635. doi: 10.1016/s0092-8674(80)80039-0. [DOI] [PubMed] [Google Scholar]
- DeLeo A. B., Jay G., Appella E., Dubois G. C., Law L. W., Old L. J. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc Natl Acad Sci U S A. 1979 May;76(5):2420–2424. doi: 10.1073/pnas.76.5.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
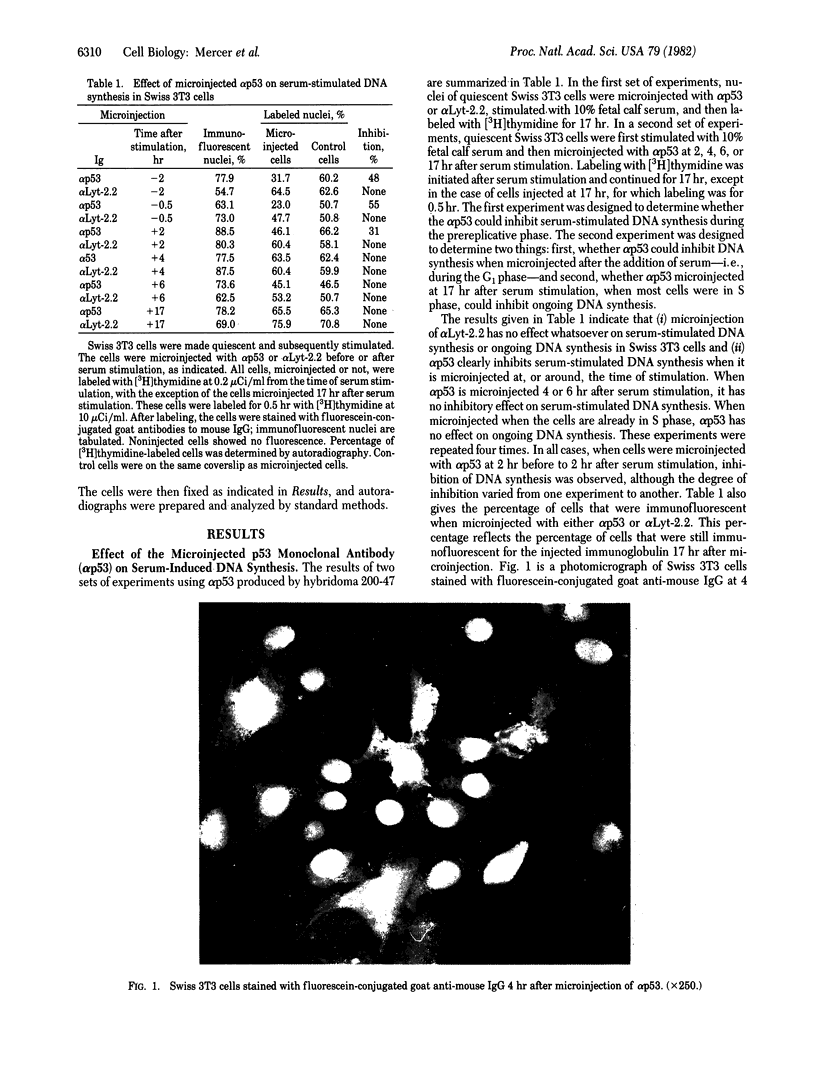
- Dippold W. G., Jay G., DeLeo A. B., Khoury G., Old L. J. p53 transformation-related protein: detection by monoclonal antibody in mouse and human cells. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1695–1699. doi: 10.1073/pnas.78.3.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floros J., Jonak G., Galanti N., Baserga R. Induction of cell DNA replication in G1-specific ts mutants by microinjection of SV40 DNA. Exp Cell Res. 1981 Mar;132(1):215–223. doi: 10.1016/0014-4827(81)90097-5. [DOI] [PubMed] [Google Scholar]
- Graessmann M., Graessman A. "Early" simian-virus-40-specific RNA contains information for tumor antigen formation and chromatin replication. Proc Natl Acad Sci U S A. 1976 Feb;73(2):366–370. doi: 10.1073/pnas.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney E. G., Harrison R. O., Fenno J. Monoclonal antibodies against simian virus 40 T antigens: evidence for distinct sublcasses of large T antigen and for similarities among nonviral T antigens. J Virol. 1980 Jun;34(3):752–763. doi: 10.1128/jvi.34.3.752-763.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay G., DeLeo A. B., Appella E., Dubois G. C., Law L. W., Khoury G., Old L. J. A common transformation-related protein in murine sarcomas and leukemias. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):659–664. doi: 10.1101/sqb.1980.044.01.069. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Levine A. J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979 May;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Luka J., Jörnvall H., Klein G. Purification and biochemical characterization of the Epstein-Barr virus-determined nuclear antigen and an associated protein with a 53,000-dalton subunit. J Virol. 1980 Sep;35(3):592–602. doi: 10.1128/jvi.35.3.592-602.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero J. A., Tur S., Carroll R. B. Host nuclear proteins expressed in simian virus 40-transformed and -infected cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):97–101. doi: 10.1073/pnas.77.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner J., Milner S. SV40-53K antigen: a possible role for 53K in normal cells. Virology. 1981 Jul 30;112(2):785–788. doi: 10.1016/0042-6822(81)90327-5. [DOI] [PubMed] [Google Scholar]
- Novi A. M., Baserga R. Correlation between synthesis of ribosomal RNA and stimulation of DNA synthesis in mouse salivary glands. Lab Invest. 1972 May;26(5):540–547. [PubMed] [Google Scholar]
- Pledger W. J., Hart C. A., Locatell K. L., Scher C. D. Platelet-derived growth factor-modulated proteins: constitutive synthesis by a transformed cell line. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4358–4362. doi: 10.1073/pnas.78.7.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini M., Jonak G. J., Baserga R. Identification of adenovirus 2 early genes required for induction of cellular DNA synthesis in resting hamster cells. J Virol. 1981 Jun;38(3):982–986. doi: 10.1128/jvi.38.3.982-986.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow P. W., Riddle V. G., Pardee A. B. Synthesis of labile, serum-dependent protein in early G1 controls animal cell growth. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4446–4450. doi: 10.1073/pnas.76.9.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter V., Witte O. N., Coffman R., Baltimore D. Abelson murine leukemia virus-induced tumors elicit antibodies against a host cell protein, P50. J Virol. 1980 Nov;36(2):547–555. doi: 10.1128/jvi.36.2.547-555.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Stiles C. D. Cytoplasmic transfer of the mitogenic response to platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4363–4367. doi: 10.1073/pnas.78.7.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaizumi M., Uchida T., Mekada E., Okada Y. Antibodies introduced into living cells by red cell ghosts are functionally stable in the cytoplasm of the cells. Cell. 1979 Dec;18(4):1009–1014. doi: 10.1016/0092-8674(79)90213-7. [DOI] [PubMed] [Google Scholar]
- Zavortink M., Thacher T., Rechsteiner M. Degradation of proteins microinjected into cultured mammalian cells. J Cell Physiol. 1979 Jul;100(1):175–185. doi: 10.1002/jcp.1041000118. [DOI] [PubMed] [Google Scholar]