Abstract
AIM: To characterize the profiles of alveolar hypoventilation during colonoscopies performed under sedoanalgesia with a combination of alfentanil and either midazolam or propofol.
METHODS: Consecutive patients undergoing routine colonoscopy were randomly assigned to sedation with either propofol or midazolam in an open-labeled design using a titration scheme. All patients received 4 μg/kg per body weight alfentanil for analgesia and 3 L of supplemental oxygen. Oxygen saturation (SpO2) was measured by pulse oximetry (POX), and capnography (PcCO2) was continuously measured using a combined dedicated sensor at the ear lobe. Instances of apnea resulting in measures such as stimulation of the patient, a chin lift, a mask maneuver, or withholding of sedation were recorded. PcCO2 values (as a parameter of sedation-induced hypoventilation) were compared between groups at the following distinct time points: baseline, maximal rise, termination of the procedure and 5 min after termination of the procedure. The number of patients in both study groups who regained baseline PcCO2 values (± 1.5 mmHg) five minutes after the procedure was determined.
RESULTS: A total of 97 patients entered this study. The data from 14 patients were subsequently excluded for clinical procedure-related reasons or for technical problems. Therefore, 83 patients (mean age 62 ± 13 years) were successfully randomized to receive propofol (n = 42) or midazolam (n = 41) for sedation. Most of the patients were classified as American Society of Anesthesiologists (ASA) II [16 (38%) in the midazolam group and 15 (32%) in the propofol group] and ASA III [14 (33%) and 13 (32%) in the midazolam and propofol groups, respectively]. A mean dose of 5 (4-7) mg of IV midazolam and 131 (70-260) mg of IV propofol was used during the procedure in the corresponding study arms. The mean SpO2 at baseline (%) was 99 ± 1 for the midazolam group and 99 ± 1 for the propofol group. No cases of hypoxemia (SpO2 < 85%) or apnea were recorded. However, an increase in PcCO2 that indicated alveolar hypoventilation occurred in both groups after administration of the first drug and was not detected with pulse oximetry alone. The mean interval between the initiation of sedation and the time when the PcCO2 value increased to more than 2 mmHg was 2.8 ± 1.3 min for midazolam and 2.8 ± 1.1 min for propofol. The mean maximal rise was similar for both drugs: 8.6 ± 3.7 mmHg for midazolam and 7.4 ± 3.2 mmHg for propofol. Five minutes after the end of the procedure, the mean difference from the baseline values was significantly lower for the propofol treatment compared with midazolam (0.9 ± 3.0 mmHg vs 4.3 ± 3.7 mmHg, P = 0.0000169), and significantly more patients in the propofol group had regained their baseline value ± 1.5 mmHg (32 of 41 vs 12 of 42, P = 0.0004).
CONCLUSION: A significantly higher number of patients sedated with propofol had normalized PcCO2 values five minutes after sedation when compared with patients sedated with midazolam.
Keywords: Colonoscopy, Deep sedation, Propofol, Hypoventilation, Blood gas monitoring, Transcutaneous
INTRODUCTION
Colonoscopies are usually performed under sedation with an intravenous sedative that is often combined with an analgesic drug[1,2]. A combination of the benzodiazepine midazolam with an opioid is the most commonly used regimen in Western countries[3]. During the last few years, propofol (2,6-diisopropylphenol) sedation has gained increasing attention among endoscopists as an alternative sedative in GI endoscopy[4-6]. With a fast onset of action of 30-60 s, a distribution half-life of 2-4 min, and a rapid recovery time, propofol combines the major characteristics of an ideal sedative[7].
In recent years, several randomized trials have compared midazolam vs propofol with regard to patient safety and satisfaction[4,8-10]. In most studies, recovery time, measured as completely regained alertness after the endoscopic procedure, was used as the main endpoint. Although the advantages of propofol regarding these pharmacokinetic properties are unquestionable, there is still an ongoing debate on the potential respiratory impairment hazards of propofol when used by non-anesthesiologists. In contrast to benzodiazepines, a reversal agent for propofol does not exist; consequently, the use of propofol requires special attention. Furthermore, a deeper level of sedation may be reached with propofol that carries the risk of unintentional deep sedation or even general anesthesia[11,12].
Standard monitoring with pulse oximetry (POX) may miss hypoventilation, which is much better reflected by an increase in arterial carbon dioxide tension[13,14]. Arterial blood gas analysis is the ‘gold standard’ method to measure the arterial partial pressure of carbon dioxide (PaCO2). However, arterial sampling, including arterial catheterization, is invasive and expensive. Transcutaneous carbon dioxide tension (PcCO2) measurement is used as a noninvasive surrogate measure of PaCO2 and to estimate PaCO2 or determine trend changes in the measurement. Recently, considerable progress has been made in the technical aspects of PcCO2 monitoring. A single earlobe sensor can now measure PcCO2 and pulse oximetry simultaneously. Transcutaneous carbon dioxide tension measurement has been shown to be a reliable monitoring technique that corresponds well with PaCO2 values measured in arterial blood gas samples[15-17].
The aim of this study was to evaluate the profile of PcCO2 as a marker of hypoventilation during sedation with propofol or midazolam in colonoscopies.
MATERIALS AND METHODS
This was an open-labeled, blinded, randomized prospective study. Consecutive outpatients undergoing elective colonoscopy and opting for sedation were randomly assigned to receive propofol or midazolam. The patients were assigned using randomly numbered opaque envelopes. As a standard procedure, all patients received 3 L/min supplemental oxygen and analgesia with 4 μg/kg per body weight (BW) alfentanil (Rapifen®, Janssen-Cilag, Baar, Switzerland) prior to sedation[18]. The first bolus of the sedative drug was administered exactly one minute after the alfentanil. Midazolam (Dormicum®, Hoffmann La Roche AG, Basel, Switzerland) was administered in a first dose of 2 mg; further boluses of 1 mg were administered with an interval of at least 1 min or more depending on the clinical outcome. Propofol (Disoprivan®, AstraZeneca, Zug, Switzerland) was administered in two boluses of 20 mg followed by further boluses of 10 mg after an interval of at least 20 s. The sedative drugs were administered by registered nurses under the supervision of the endoscopist based on the clinical response of the patient; the nurses followed our institutional protocol as published elsewhere (nurse-administered propofol sedation or NAPS)[18]. The nurse administering the sedation had no tasks except to monitor the patient and administer sedation. A different nurse assisted the endoscopist with the technical performance of the procedure. Monitoring consisted of the measurement of continuous oxygen saturation, electrocardiography and heart rate, as well as regular measurements of blood pressure. The primary method of monitoring was the nurse’s clinical assessment of the patient, including measurement of respiratory effort by visual assessment and by palpation of the chest wall and abdominal excursion and/or palpation of exhaled breath.
A short personal history was obtained from all the patients, and their general physical condition was assessed using the American Society of Anesthesiologists (ASA) classification. Exclusion criteria were as follows: (1) a known history of intolerance to propofol (including sensitivity to eggs and soybeans); (2) an age less than 18 or more than 85 years; (3) an ASA score of IV or V; (4) a known high grade of aortic (gradient > 80 mmHg) or carotid stenosis (> 75%); and (5) intravenous drug abuse.
All endoscopic examinations were performed according to the department’s standard operating procedures with different types of Pentax video colonoscopies (Pentax, Hamburg, Germany) using regular room air to insufflate the colon. The colonoscopies were conducted by seven different expert endoscopists, including four experienced gastroenterologists and three residents in their last year of gastroenterology training, who all had performed more than 400 colonoscopies each. The procedures were performed in an x-ray suite equipped for fluoroscopy (straightening maneuvers and confirming instrument position when necessary). The decision to perform an ileal intubation or an endoscopic intervention (e.g., polypectomy) depended on the clinical situation and was decided by the endoscopist.
The local ethics committee approved the study protocol, and written informed consent was obtained from the patients before study enrollment.
A short personal history was obtained from all the patients, and their general physical condition was assessed using the American Society of Anesthesiologists (ASA) classification. Exclusion criteria were as follows: (1) a known history of intolerance to propofol (including sensitivity to eggs and soybeans); (2) an age less than 18 or more than 85 years; (3) an ASA score of IV or V; (4) a known high grade of aortic (gradient > 80 mmHg) or carotid stenosis (> 75%); and (5) intravenous drug abuse.
All endoscopic examinations were performed according to the department’s standard operating procedures with different types of Pentax video colonoscopies (Pentax, Hamburg, Germany) using regular room air to insufflate the colon. The colonoscopies were conducted by seven different expert endoscopists, including four experienced gastroenterologists and three residents in their last year of gastroenterology training, who all had performed more than 400 colonoscopies each. The procedures were performed in an x-ray suite equipped for fluoroscopy (straightening maneuvers and confirming instrument position when necessary). The decision to perform an ileal intubation or an endoscopic intervention (e.g., polypectomy) depended on the clinical situation and was decided by the endoscopist.
The local ethics committee approved the study protocol, and written informed consent was obtained from the patients before study enrollment.
We used a recently developed combined POX/PcCO2 sensor (V-Sign™, Sentec AG, Therwil, Switzerland) weighing 3 g that was placed at the right earlobe with a dedicated ear clip. We used a recently developed combined POX/PcCO2 sensor (V-Sign™, Sentec AG, Therwil, Switzerland) weighing 3 g that was placed at the right earlobe with a dedicated ear clip[17]. This fully digital sensor combines the elements of an electrochemical Severinghaus-type carbon dioxide tension sensor with those of conventional optical POX sensors, thus providing noninvasive and continuous estimation of PaCO2 and SaO2[19]. The sensor was warmed to a constant surface temperature of 42 °C to improve local arterialization and to accelerate carbon dioxide diffusion. After the sensor was positioned, the SpO2 values were available immediately, whereas the PcCO2 values required an equilibration time of approximately 4-5 min. The system is designed to be “ready-for-use” by automated recalibration every time the sensor is placed on the docking station between measurements. The system was previously shown to deliver accurate and reproducible results for PcCO2 and POX[17]. The system was also shown to have an excellent correlation between oxygen saturation and carbon dioxide measurements when the combined sensor was compared with arterial blood gas analysis[17,20].
Readings from the POX/PcCO2 sensor (placed at the right ear lobe) were continuously recorded and stored on a personal computer. An independent observer who was blinded to the type of sedation recorded the readings. Similarly, the procedural personnel were blinded to the PcCO2 data. All collected data were visualized using statistic graphics software (Igor Pro 4.01, WaveMetrics Inc., Lake Oswego, OR). Values at defined time points were thereafter identified manually. PcCO2 values (as a parameter of sedation-induced hypoventilation) were compared between the study groups at the following distinct time points: at baseline, at the time point of maximal increase, at the time point when the cecum was reached, at the end of the procedure and 5 min after the end of the procedure.
The primary endpoint was defined as the number of patients in both study groups who regained their baseline PcCO2 value (± 1.5 mmHg) five minutes after the end of the procedure. Secondary end points included the mean time lag between the application of the sedative drug and an increase of the PcCO2 curve of more than 2 mm Hg, safety parameters defined as apnea with the need for intervention (in case of a decrease in SaO2 values below 85% for more than 20 s), the frequency of SaO2 decreases below 90% and a decrease of the heart rate below 50 bpm. Apnea was defined as a lack of spontaneous respiratory effort for more than 20 s and was assessed clinically by the nurse administrating the sedation. An increase of PcCO2 above the baseline was defined as hypoventilation. The target sedation level was a quiet patient in both of the groups as implemented using our NAPS protocol, which has been in practice at this center for several years. The recovery time for all of the patients was defined as the time required for completion of the procedure, i.e., a complete withdrawal of the instrument and simultaneous conclusion of all sedation. As a standard of practice, the patients were transferred to a quiet recovery room following the final 5-min recording of thePcCO2 readings in the endoscopy suite.
All of the parameters were analyzed using descriptive statistics (mean, standard deviation). Categorical outcomes were analyzed using the χ2 or F test as appropriate. Continuous parameters were analyzed using an analysis of variance, and for non-continuous parameters, the Mann-Whitney Test was used. P < 0.05 was defined as statistically significant. All tests were two-sided. For all statistical calculations, SPSS software (SPSS for Windows, Version 11.0, SPSS Inc., Chiago, Illinois) was used. The sample size calculation was based on the primary outcome of this study of detecting a PcCO2 difference of < 1.5 mmHg from the baseline at 5 min after the end of the procedure in the propofol group. In a pilot study in a similar population, a difference in values between midazolam and propofol was observed in 4 of 26 patients (15.4%). As we expected a clinical/physiological relevant effect, the sample size was determined to detect an additional increase of 75% in the midazolam group with a power of 80%. Thirty-eight patients in each group would be required to detect such a difference (P = 0.05) (nQuery Advisor, Version 5.0, Statistical Solutions, Saugus, MA, United States).
RESULTS
Among the 133 colonoscopies performed during the study period, 97 patients were successfully randomized to the study. Fourteen randomized patients had to be subsequently excluded for the following reasons: in 5 patients, the procedure was not completed because of incomplete bowel preparation; in 5 patients, a short disconnection of the sensor provided no continuous data; in two patients, a calibration fault occurred because of the prototype calibration unit used, and the endoscopist refrained from recalibration; and in two patients, the sedative drug was not correctly administered according to the protocol. Therefore, the final study population contained 83 patients. Demographic characteristics of the study groups are shown in Table 1. The mean duration of the procedures was 26 ± 13 min for the midazolam group and 27 ± 18 min for the propofol group (not significant).
Table 1.
Demographic data of the randomized groups
| Sedation with Midazolam (n = 42) | Sedation with Propofol (n = 41) | P value | |
| Age (yr, mean ± SD) | 62 ± 13 | 62 ± 13 | NS |
| M : F (n) | 19:23 | 20:21 | NS |
| ASA I | 12 (29%) | 13 (32%) | NS |
| ASA II | 16 (38%) | 15 (37%) | NS |
| ASA III | 14 (33%) | 13 (32%) | NS |
| Smoker | 6 (15%) | 6 (15%) | NS |
| Mean dosage of sedative in mg (range) | 5 (4-7) | 131 (70-260) | - |
ASA: American Society of Anesthesiologists classification; NS: Not significant.
Decreased ventilation activity was detected in all patients to whom a sedative was administered; on average, the PcCO2 values increased by 8.0 ± 3.7 mmHg. The mean SpO2 values at baseline and the mean maximal decrease during sedation are shown in Table 2.
Table 2.
Oxygen saturation measured by pulse oximetry at baseline and changes during endoscopy according to sedatives
| Midazolam (n = 42) | Propofol (n = 41) | P value | |
| SpO2 at baseline (%) | 99 ± 1 | 99 ± 1 | NS |
| Mean max decrease of SpO2 (%) | 6 ± 3 | 4 ± 2 | NS |
SpO2: Oxygen saturation measured by pulse oximetry; NS: Not significant.
When comparing midazolam and propofol, different profiles were observed for the PcCO2 readings, as shown in Figure 1. The increase in PcCO2 was mostly related to a short delay in the administration of incremental dosages of the sedatives. The mean time interval after the first application of the drug until the PcCO2 value had increased by more than 2 mmHg was 2.8 ± 1.3 min for midazolam and 2.8 ± 1.1 min for propofol. Although there was no significant difference in the mean increase in PcCO2 following administration of midazolam or propofol, the patients who received midazolam tended to remain in a prolonged state of decreased ventilation when compared with the patients receiving propofol. The difference in PcCO2 values (baseline compared with the end of the procedure) was significantly higher in the patients receiving midazolam. Therefore, the patients who received propofol had a PcCO2 level that was significantly closer to the baseline five minutes after the end of the procedure when compared with the patients who received midazolam (Tables 2, 3, Figure 2).
Figure 1.
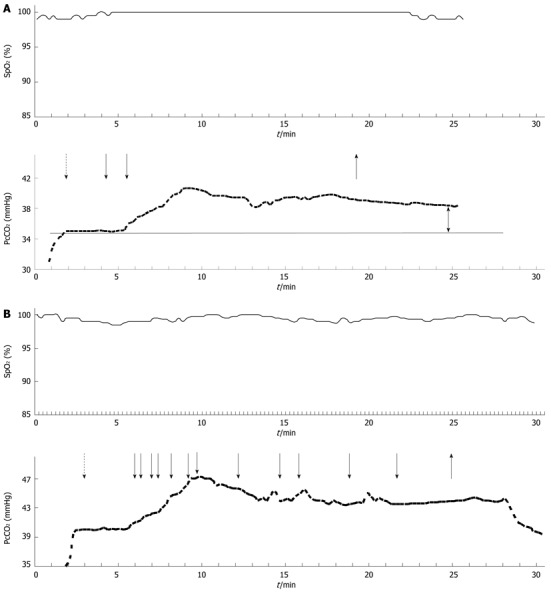
A typical course of oxygen saturation measured by pulse oximetry and transcutaneous carbon dioxide tension following administration of alfentanil (dashed arrow) and midazolam (A) or propofol (B) (solid arrows as indicated). The upright arrow indicates the end of the procedure. The double arrow highlights the difference after termination of the procedure.
Table 3.
Procedure outcomes according to the defined endpoints (n)
| Midazolam (n = 42) | Propofol (n = 41) | P value | |
| ΔPcCO2 < ± 1.5 mmHg (from baseline and five min after end) | 12 | 32 | 0.0004 |
| SpO2 < 85% | 0 | 0 | NS |
| SpO2 < 90% | 6 | 0 | 0.05 |
| HR < 50 bpm | 5 | 1 | NS |
| Decrease MAP > 25% | 17 | 17 | NS |
HR: Heart rate; MAP: Mean arterial pressure; SpO2: Oxygen saturation measured by pulse oximetry; ΔPcCO2: Difference of transcutaneous carbon dioxide tension; NS: Not significant.
Figure 2.
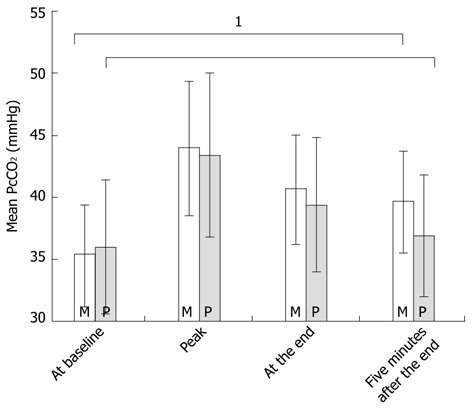
Mean transcutaneous carbon dioxide tension values in mmHg (± SD) at four distinct time points during colonoscopies according to sedative use (for the whole group). 1A significant difference in the transcutaneous carbon dioxide tension values measured five minutes after the end of the procedure when compared with the baseline values. M: Midazolam; P: Propofol.
None of the study patients manifested apnea or hypoxemia below 85%. Drops in oxygen saturation below 90% tended to occur more often during sedation with midazolam than with propofol (Table 3).
DISCUSSION
Achieving higher safety standards for sedation in routine endoscopy has been a priority over the last few years. In keeping with this objective, the present study showed that patients undergoing colonoscopy under sedation developed relative hypoventilation (as reflected by retention of CO2) that persisted for a significantly longer period in patients sedated with midazolam than in patients sedated with propofol. The results support the findings of a meta-analysis that suggested that propofol sedation during colonoscopy is associated with a lower risk of complications when compared with traditional sedative agents[21].
Although the use of oxygen saturation monitoring during sedation is routinely used by most gastroenterologists and the administration of supplemental oxygen has become a widely accepted practice, little attention has been paid to the development of alveolar hypoventilation[17]. Because of the buildup of CO2 in the patient’s alveoli and blood, hypoventilation can be effectively detected by capnography, which has recently become a focus of interest as an additional monitoring parameter during gastrointestinal procedures. This procedure has become relevant as the use of newer anesthetic drugs such as propofol has increased[15].
Several studies to date have evaluated the importance of CO2 buildup during endoscopic procedures[14-16,22]. Freeman et al[15] were the first to show that profound hypoventilation may frequently occur undetected during a gastrointestinal endoscopy, especially if additional oxygen is given and the decline of oxygen saturation is thus prevented; we observed a similar effect in our study. Freeman et al[15] also found that the degree of hypoventilation was more closely related to the sedative drug dose than to the underlying medical illness. In 30 colonoscopies, with 90% receiving fentanyl and 37% additionally receiving midazolam, Freeman et al[15] recorded a mean PCO2 increase of 6.4 ± 3.8 mmHg, whereas during ERCP with a higher rate of deeper sedation, the mean values were 14.2 ± 10.2 mmHg. Our results showed a mean increase in PcCO2 of 8.6 ± 3.7 mmHg despite a continuous SpO2 above 90%; these results are similar to the experience of Freeman et al[15].
The pattern of the registered PcCO2 readings differed noticeably in our study according to the pharmacological properties of the administered drugs. The pattern of the initial rise of PcCO2 was similar, but the PcCO2 level decreased much earlier after the administration of propofol than after the administration of midazolam. Although propofol had to be administered more often in a repeated fashion to maintain the desired sedation level, its effect ceased much faster than the effect of midazolam. This pharmacologic pattern seems to be reflected by the shape of the PcCO2 curve.
We did not observe severe hypoxemia or apnea in either of the study groups; the increase in CO2 could, however, indicate silent risk during poor sedative practice. In the study by Freeman et al[15]. We did not observe severe hypoxemia or apnea in either of the study groups; the increase in CO2 could, however, indicate silent risk during poor sedative practice. In the study by Freeman et al[15], one case demonstrated an increase of the PcCO2 curve above 80 mmHg prior to respiratory arrest. Nelson et al[16] showed that the monitoring of PcCO2 may be useful for the endoscopist to guide sedation using midazolam and fentanyl and that this monitoring can help to prevent severe carbon dioxide retention. Thus, we believe that determining the PcCO2 level can be helpful for the endoscopist when deciding whether to administer a further incremental dose of the sedative.
The combination used in this study of alfentanil, which is a potent opioid with a rapid onset of action, and midazolam is uncommon. Typically, this substance alfentanil is administered in combination with propofol for patient-controlled sedation or for short sedoanalgesia in emergency medicine[23]. The rationale to use this substance alfentanil as a single bolus at the beginning of the procedure was (1) to provide the patients with optimal analgesia during the most painful passage of the sigmoid colon; and (2) to determine whether differences in alveolar hypoventilation at the end of the procedure can be completely attributed to the pharmacologic effect of the sedatives. Furthermore, if propofol is used in outpatient procedures, alfentanil may be an ideal drug to use in combination because of its pharmacokinetic properties and analogous profile of action. Because alfentanil (similarly to all opioids) can induce or enhance alveolar hypoventilation, our protocol prescribed a single low dose of 4 μg/kg per BW of alfentanil and a strict time interval of administration one minute before the first titration dose of the sedative was given.
Alveolar hypoventilation exists when the arterial PaCO2 increases above 45 mmHg, which can occur as the result of various underlying factors. Sedation directly or indirectly influences alveolar hypoventilation by a predominant central effect, thereby causing an increase in the PaCO2. Therefore, recognition and adequate monitoring of this physiological change through indirect means such as transcutaneous monitoring of PcCO2 could play an important role during sedation. The peak PcCO2 value may not be clinically relevant; however, the time period during which reduced ventilation occurs may represent a period of increased risk for some patients. Because of the S-shape of the oxygen dissociation-curve, hypoventilation accompanied by a decrease in PaO2 may remain unnoticed over time. Although a patient would have adequate arterial saturation with the administration of supplemental oxygen, an adverse physiologic trend that may be reflected by changes in the PaCO2 may go unnoticed.
Transcutaneous CO2 monitoring in adults has yielded conflicting results because of technological limitations, such as the time required for calibration, the need to warm the skin to 42 degrees, the effect of sweating and the influence of skin metabolism and thickness. Technical problems precluded an accurate interpretation of the data in 7 of the 97 patients and thus represent a limitation in our study.
A predominant central effect, thereby causing an increase in the PaCO2. Therefore, recognition and adequate monitoring of this physiological change through indirect means such as transcutaneous monitoring of PcCO2 could play an important role during sedation. The peak PcCO2 value may not be clinically relevant; however, the time period during which reduced ventilation occurs may represent a period of increased risk for some patients. Because of the S-shape of the oxygen dissociation-curve, hypoventilation accompanied by a decrease in PaO2 may remain unnoticed over time. Although a patient would have adequate arterial saturation with the administration of supplemental oxygen, an adverse physiologic trend that may be reflected by changes in the PaCO2 may go unnoticed.
Transcutaneous CO2 monitoring in adults has yielded conflicting results because of technological limitations, such as the time required for calibration, the need to warm the skin to 42 degrees, the effect of sweating and the influence of skin metabolism and thickness. Technical problems precluded an accurate interpretation of the data in 7 of the 97 patients and thus represent a limitation in our study.
The main focus of this study was to evaluate the impairment of ventilation induced by midazolam or propofol during colonoscopies. Most trials comparing the use of propofol and midazolam in the endoscopy suite have focused on differences in recovery time (assessed using a discharge scoring system, for example)[24]. The present study suggests that there is also a significant difference in the duration of hypoventilation during the post-procedural period. Although alveolar hypoventilation is generally well tolerated by most patients, it may nevertheless be of clinical relevance in patients with compromised health. Iber et al[25] showed that in 4% of patients sedated with midazolam, a relevant decrease of oxygen saturation below 89% occurred during the 30 min after the endoscopic procedure, which is contrast to our practical experience with propofol, where the effect occurs exclusively during the time when the attention on the patient is greatest. During the endoscopic procedure, the PcCO2 monitoring indicated no increased hypoventilation risk for propofol when compared with midazolam.
Insufflation of the colon with carbon dioxide (CO2) rather than air has been shown to reduce pain and discomfort because CO2 is rapidly absorbed by the intestinal lining. In previous studies, measurement of end tidal CO2 (ETCO2) and the mean pCO2 demonstrated these procedures to be safe. However, no studies have used transcutaneous continuous pCO2 monitoring, which could be valuable given the increasing use of this insufflation technique for pain relief during colonoscopies[26].
A metanalysis by Qadeer et al[21] showed that propofol sedation had a lower rate of cardiopulmonary complications than traditional agents used during colonoscopy procedures. This current study highlights another physiological mechanism that may be detrimental when propofol is used in larger cohorts. Therefore, assessing the PcCO2 during sedation could serve as an added safety measure to detect alveolar hypoventilation.
In conclusion, hypoventilation occurs frequently during sedation for colonoscopy and is often undetected during routine pulse oximetry. A significantly higher number of patients sedated with propofol had normalized PcCO2 values five minutes after sedation when compared with patients sedated with midazolam. Understanding the role of CO2 retention will be important in increasing the further safety standards of sedation during endoscopy. More studies are required to identify and prevent hypercapnia and thus ensure the safe practice of sedation during routine gastrointestinal endoscopies.
ACKNOWLEDGMENTS
The authors thank Sentec Inc., Therwil, Switzerland for providing the PcCO2/SPO2 monitoring system, and Joseph Hayoz, PhD, Prashant N Chhajed, MD, Thomas Hirt, MD, and Jurgen Drewe, MD for technical and statistical support.
COMMENTS
Background
Colonoscopies are usually performed under sedation and monitored by pulse oximetry. With the increasing use of newer sedative agents such as propofol, there is an ongoing discussion about safety and monitoring requirements.
Research frontiers
The surveillance of carbon dioxide tension (e.g., measuring end tidal CO2 by capnography) could provide more accurate information than pulse oximetry regarding ventilation impairment. Until now, little experience has been reported for transdermal CO2 measurement systems used for this purpose.
Innovations and breakthroughs
Monitoring studies with capnography showed that hypoventilation and even short apneas that are not detected by pulse oximetry (POX) may occur during endoscopic sedation. However, an increasing body of scientific data shows that sedation during endoscopy with benzodiazepines and propofol is a safe procedure. The development of a new sensor combining transcutaneous carbon dioxide and pulse oximetry measurements provides the opportunity to explore a new monitoring method during endoscopic sedation.
Applications
The study results suggest that hypoventilation occurs often during endoscopic sedation but lasts for a shorter period if patients are sedated with propofol rather than midazolam.
Terminology
PcCO2: Transcutaneous carbon dioxide tension is measured electrochemically using a Severinghaus-type sensor placed on the earlobe; POX: Pulse oximetry measures the oxygen saturation of the blood using an optical sensor.
Peer review
In this study, the authors investigated carbon dioxide accumulation after sedation with propofol or midazolam during colonoscopies. Non-invasive, continuous transcutaneous carbon dioxide tension (PcCO2) monitoring was performed using a recently developed POX/PcCO2 sensor that was placed at the earlobe. The results of the study show that a significantly higher number of patients sedated with propofol had normalized PcCO2 values five minutes after sedation when compared with the patient group sedated with midazolam. The study is well designed and performed, and the POX/PcCO2 sensor could be used in future studies rather than capnography.
Footnotes
Peer reviewer: Spiros Ladas, Professor, 1st Department of Internal Medicine-Propaedeutic, Medical School, Athens University, “Laiko” General Hospital, Agiou Thoma 17, Athens 11527, Greece
S- Editor Lv S L- Editor A E- Editor Zhang DN
References
- 1.Fanti L, Testoni PA. Sedation and analgesia in gastrointestinal endoscopy: what’s new? World J Gastroenterol. 2010;16:2451–2457. doi: 10.3748/wjg.v16.i20.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladas SD, Satake Y, Mostafa I, Morse J. Sedation practices for gastrointestinal endoscopy in Europe, North America, Asia, Africa and Australia. Digestion. 2010;82:74–76. doi: 10.1159/000285248. [DOI] [PubMed] [Google Scholar]
- 3.Benson AA, Cohen LB, Waye JD, Akhavan A, Aisenberg J. Endoscopic sedation in developing and developed countries. Gut Liver. 2008;2:105–112. doi: 10.5009/gnl.2008.2.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vargo JJ, Zuccaro G, Dumot JA, Shermock KM, Morrow JB, Conwell DL, Trolli PA, Maurer WG. Gastroenterologist-administered propofol versus meperidine and midazolam for advanced upper endoscopy: a prospective, randomized trial. Gastroenterology. 2002;123:8–16. doi: 10.1053/gast.2002.34232. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK, Heuss LT, Walker JA, Qi R. Trained registered nurses/endoscopy teams can administer propofol safely for endoscopy. Gastroenterology. 2005;129:1384–1391. doi: 10.1053/j.gastro.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Gasparović S, Rustemović N, Opacić M, Premuzić M, Korusić A, Bozikov J, Bates T. Clinical analysis of propofol deep sedation for 1,104 patients undergoing gastrointestinal endoscopic procedures: a three year prospective study. World J Gastroenterol. 2006;12:327–330. doi: 10.3748/wjg.v12.i2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson DB, Barkun AN, Block KP, Burdick JS, Ginsberg GG, Greenwald DA, Kelsey PB, Nakao NL, Slivka A, Smith P, et al. Propofol use during gastrointestinal endoscopy. Gastrointest Endosc. 2001;53:876–879. doi: 10.1016/s0016-5107(01)70311-2. [DOI] [PubMed] [Google Scholar]
- 8.Koshy G, Nair S, Norkus EP, Hertan HI, Pitchumoni CS. Propofol versus midazolam and meperidine for conscious sedation in GI endoscopy. Am J Gastroenterol. 2000;95:1476–1479. doi: 10.1111/j.1572-0241.2000.02080.x. [DOI] [PubMed] [Google Scholar]
- 9.Chin NM, Tai HY, Chin MK. Intravenous sedation for upper gastrointestinal endoscopy: Midazolam versus propofol. Singapore Med J. 1992;33:478–480. [PubMed] [Google Scholar]
- 10.Zuo XL, Li Z, Liu XP, Li CQ, Ji R, Wang P, Zhou CJ, Liu H, Li YQ. Propofol vs midazolam plus fentanyl for upper gastrointestinal endomicroscopy: a randomized trial. World J Gastroenterol. 2012;18:1814–1821. doi: 10.3748/wjg.v18.i15.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dumonceau JM, Riphaus A, Aparicio JR, Beilenhoff U, Knape JT, Ortmann M, Paspatis G, Ponsioen CY, Racz I, Schreiber F, et al. European Society of Gastrointestinal Endoscopy, European Society of Gastroenterology and Endoscopy Nurses and Associates, and the European Society of Anaesthesiology Guideline: Non-anesthesiologist administration of propofol for GI endoscopy. Endoscopy. 2010;42:960–974. doi: 10.1055/s-0030-1255728. [DOI] [PubMed] [Google Scholar]
- 12.Perel A. Non-anaesthesiologists should not be allowed to administer propofol for procedural sedation: a Consensus Statement of 21 European National Societies of Anaesthesia. Eur J Anaesthesiol. 2011;28:580–584. doi: 10.1097/EJA.0b013e328348a977. [DOI] [PubMed] [Google Scholar]
- 13.Vargo JJ, Zuccaro G, Dumot JA, Conwell DL, Morrow JB, Shay SS. Automated graphic assessment of respiratory activity is superior to pulse oximetry and visual assessment for the detection of early respiratory depression during therapeutic upper endoscopy. Gastrointest Endosc. 2002;55:826–831. doi: 10.1067/mge.2002.124208. [DOI] [PubMed] [Google Scholar]
- 14.Qadeer MA, Vargo JJ, Dumot JA, Lopez R, Trolli PA, Stevens T, Parsi MA, Sanaka MR, Zuccaro G. Capnographic monitoring of respiratory activity improves safety of sedation for endoscopic cholangiopancreatography and ultrasonography. Gastroenterology. 2009;136:1568–1756; quiz 1568-1576. doi: 10.1053/j.gastro.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Freeman ML, Hennessy JT, Cass OW, Pheley AM. Carbon dioxide retention and oxygen desaturation during gastrointestinal endoscopy. Gastroenterology. 1993;105:331–339. doi: 10.1016/0016-5085(93)90705-h. [DOI] [PubMed] [Google Scholar]
- 16.Nelson DB, Freeman ML, Silvis SE, Cass OW, Yakshe PN, Vennes J, Stahnke LL, Herman M, Hodges J. A randomized, controlled trial of transcutaneous carbon dioxide monitoring during ERCP. Gastrointest Endosc. 2000;51:288–295. doi: 10.1016/s0016-5107(00)70357-9. [DOI] [PubMed] [Google Scholar]
- 17.Heuss LT, Chhajed PN, Schnieper P, Hirt T, Beglinger C. Combined pulse oximetry/cutaneous carbon dioxide tension monitoring during colonoscopies: pilot study with a smart ear clip. Digestion. 2004;70:152–158. doi: 10.1159/000081515. [DOI] [PubMed] [Google Scholar]
- 18.Heuss LT, Schnieper P, Drewe J, Pflimlin E, Beglinger C. Risk stratification and safe administration of propofol by registered nurses supervised by the gastroenterologist: a prospective observational study of more than 2000 cases. Gastrointest Endosc. 2003;57:664–671. doi: 10.1067/mge.2003.191. [DOI] [PubMed] [Google Scholar]
- 19.Severinghaus JW, Bradley AF. Electrodes for blood pO2 and pCO2 determination. J Appl Physiol. 1958;13:515–520. doi: 10.1152/jappl.1958.13.3.515. [DOI] [PubMed] [Google Scholar]
- 20.Chhajed PN, Miedinger D, Baty F, Bernasconi M, Heuss LT, Leuppi JD, Tamm M. Comparison of combined oximetry and cutaneous capnography using a digital sensor with arterial blood gas analysis. Scand J Clin Lab Invest. 2010;70:60–64. doi: 10.3109/00365510903450106. [DOI] [PubMed] [Google Scholar]
- 21.Qadeer MA, Vargo JJ, Khandwala F, Lopez R, Zuccaro G. Propofol versus traditional sedative agents for gastrointestinal endoscopy: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3:1049–1056. doi: 10.1016/s1542-3565(05)00742-1. [DOI] [PubMed] [Google Scholar]
- 22.Qadeer MA, Lopez AR, Dumot JA, Vargo JJ. Hypoxemia during moderate sedation for gastrointestinal endoscopy: causes and associations. Digestion. 2011;84:37–45. doi: 10.1159/000321621. [DOI] [PubMed] [Google Scholar]
- 23.Mazanikov M, Udd M, Kylänpää L, Mustonen H, Lindström O, Halttunen J, Färkkilä M, Pöyhiä R. Patient-controlled sedation for ERCP: a randomized double-blind comparison of alfentanil and remifentanil. Endoscopy. 2012;44:487–492. doi: 10.1055/s-0031-1291655. [DOI] [PubMed] [Google Scholar]
- 24.Sipe BW, Rex DK, Latinovich D, Overley C, Kinser K, Bratcher L, Kareken D. Propofol versus midazolam/meperidine for outpatient colonoscopy: administration by nurses supervised by endoscopists. Gastrointest Endosc. 2002;55:815–825. doi: 10.1067/mge.2002.124636. [DOI] [PubMed] [Google Scholar]
- 25.Iber FL, Sutberry M, Gupta R, Kruss D. Evaluation of complications during and after conscious sedation for endoscopy using pulse oximetry. Gastrointest Endosc. 1993;39:620–625. doi: 10.1016/s0016-5107(93)70211-4. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Hu B. The role of carbon dioxide insufflation in colonoscopy: a systematic review and meta-analysis. Endoscopy. 2012;44:128–136. doi: 10.1055/s-0031-1291487. [DOI] [PubMed] [Google Scholar]


