Abstract
AIM: To identify the differentially expressed miRNAs and their targets in hepatitis B virus (HBV)-associated hepatocellular carcinoma (HCC).
METHODS: Six hundred and sixty seven human miRNAs were quantitatively analyzed by Taqman low-density miRNA array (TLDA) in HBV-HCC tissues. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were used to analyze the significant function and pathway of the differentially expressed miRNAs in HBV-HCC. TargetScan software was used to predict the targets of deregulated miRNAs. Western blotting and luciferase assay were performed to verify the targets of these miRNAs.
RESULTS: Ten up-regulated miRNAs (miR-217, miR-518b, miR-517c, miR-520g, miR-519a, miR-522, miR-518e, miR-525-3p, miR-512-3p, and miR-518a-3p) and 11 down-regulated miRNAs (miR-138, miR-214, miR-214#, miR-199a-5p, miR-433, miR-511, miR-592, miR-483-3p, miR-483-5p, miR-708 and miR-1275) were identified by Taqman miRNAs array and confirmed quantitatively by reverse transcription polymerase chain reaction in HCC and adjacent non-tumor tissues. GO and KEGG pathway analysis revealed that “regulation of actin cytoskeleton” and “pathway in cancer” are most likely to play critical roles in HCC tumorigenesis. MiR-519a and ribosomal protein S6 kinase polypeptide 3 (RPS6KA3) were predicted as the most significant candidates by miRNA-mRNA network. In addition, cyclin D3 (CCND3) and clathrin heavy chain (CHC), usually up-regulated in HCC tissues, were validated as the direct target of miR-138 and miR-199a-5p, respectively.
CONCLUSION: Our data suggest an importance of miR-138 and miR-199a-5p as well as their targets CCND3 and CHC in HCC tumorigenesis, and may provide more evidence for reliability of integrative bioinformatics analysis.
Keywords: Hepatocellular carcinoma, miR-138, miR-199a-5p, Cyclin D3, Clathrin heavy chain, Bioinformatics, Taqman array
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide[1]. The development and progression of HCC is characterized by a multi-stage process, which is believed to involve the deregulation of genes that are critical to cellular processes such as cell cycle control, cell growth, apoptosis, and cell migration and spreading. In the past decades, studies have focused on investigating the effect of genes and proteins on the development and progression of HCC[2]. Recently, an increasing number of reports have described microRNAs (miRNAs) that are implicated in HCC progression[3].
MiRNAs are endogenous non-coding RNAs (20-22 nucleotides) which regulate gene expression by catalyzing the cleavage of messenger RNA (mRNA) or repressing mRNA translation[4]. Increasing evidence showed that miRNAs play significant roles in cell development, differentiation and communication[5]. Deregulation of miRNAs has also been observed in a wide range of human diseases, including cancer[6]. In human cancer, miRNAs can function as oncogenes or tumor suppressor genes during tumor development and progression[7].
In this study, the expression of 667 miRNAs was profiled in human HCC and adjacent non-tumor tissues. A set of significantly differentially expressed miRNAs was identified in HCC tissues. Furthermore, a global analysis of miRNA-regulated signaling pathways and related genes was performed on the basis of miRNA expression profiles and bioinformatics interpretation. Cyclin D3 (CCND3) and clathrin heavy chain (CHC) which had been previously described in HCC tumorigenesis were further validated to be the direct target of miR-138 and miR-199a-5p, respectively.
MATERIALS AND METHODS
Tissue specimens
Eighteen pairs of human HCC and adjacent non-tumor tissues were obtained from surgical specimens immediately after resection from patients undergoing primary surgical treatment of HCC in the Eastern Hepatobiliary Surgery Hospital, Shanghai, China. No patient had received preoperative irradiation or chemotherapy. The samples were frozen in liquid nitrogen and stored at -80 °C until use. Among these samples, three pairs were used for Taqman low-density miRNA array (TLDA) analysis and all were used for quantitative real-time polymerase chain reaction (qRT-PCR) analysis. Clinical and pathological information was extracted from the patients’ medical charts and pathological reports (Table 1). Written consent for tissue donation (for research purposes) was obtained from the patients before tissue collection and the protocol was approved by the Institutional Review Board of Eastern Hepatobiliary Surgery Hospital and Second Military Medical University.
Table 1.
Characteristics of patients (n = 18)
| No. | Gender | Age (yr) | Tumor size1 (cm) | Cirrhosis | Tumor grade2 | TNM stage |
| 1 | M | 55 | 1.9 | No | G3 | T2N0M0 |
| 2 | M | 40 | 4.1 | Macronodular | G3 | T2N0M0 |
| 3 | M | 42 | 4.6 | No | G3 | T3N0M0 |
| 4 | M | 42 | 5.6 | Micronodular | G2 | T3N0M0 |
| 5 | M | 43 | 5.8 | Micronodular | G1 | T1N0M0 |
| 6 | M | 61 | 1.9 | Micronodular | G3 | T2N0M0 |
| 7 | M | 55 | 12.0 | Micronodular | G2 | T4N0M0 |
| 8 | M | 40 | 4.0 | Macronodular | G2 | T3N0M0 |
| 9 | M | 35 | 2.3 | Macronodular | G1 | T1N0M0 |
| 10 | M | 57 | 5.9 | No | G3 | T1N0M0 |
| 11 | M | 44 | 9.6 | No | G2 | T3N0M0 |
| 12 | M | 45 | 12.2 | Micronodular | G3 | T2N0M0 |
| 13 | M | 46 | 4.4 | Micronodular | G3 | T2N0M0 |
| 14 | F | 46 | 15.8 | Micronodular | G3 | T2N0M0 |
| 15 | M | 63 | 6.1 | No | G3 | T3N0M0 |
| 16 | M | 61 | 4.6 | No | G3 | T2N0M0 |
| 17 | F | 60 | 9.1 | No | G3 | T3N0M0 |
| 18 | F | 50 | 9.3 | No | G3 | T2N0M0 |
Diameter of the biggest nodule.
G1-2: Well-differentiated; G3: Moderately-differentiated; G4: Poorly-differentiated. M: Male; F: Female; TNM: Tumor-node-metastasis.
Taqman low-density miRNA array
Total RNA was isolated using mirVana miRNA isolation kit (Ambion, Austin, TX, United States). For miRNA cDNA synthesis, RNA was reversely transcribed using the miRNA reverse transcription kit (Applied Biosystems, Foster City, CA, United States) in combination with the stem-loop Megaplex primer pool (Applied Biosystems). TLDA v2.0 (Applied Biosystems) was performed on the 7900HT real-time PCR system (Applied Biosystems) according to the manufacturer’s protocol (667 small RNAs were profiled for each cDNA sample). PCR cycling conditions were as follows: 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Human U6 small RNA was used as an internal control to normalize RNA input. The data were analyzed using SDS v2.3 software. The Ct value was defined as the fractional cycle number at which the fluorescence passed the fixed threshold. The fold change was calculated using the 2-ΔΔCt method and presented as the fold-expression change in tumors and their adjacent normal tissues after normalization to the endogenous control.
Quantitative real-time PCR
For miRNA expression analysis, synthesis of cDNA and qRT-PCR was carried out with TaqMan microRNA assay kits (Applied Biosystems) according to the manufacturer’s protocol. Briefly, total RNA was extracted using TRIzol Reagent (Invitrogen, Carlsbad, CA, United States) from HCC and adjacent non-tumor tissues and used to synthesize cDNAs with gene-specific primers. Reverse transcriptase reactions contained 100 ng RNA, 50 nmol/L stem-loop RT primers, 1 × RT buffer, 0.25 mmol/L each of the dNTPs, 3.33 U/μL MultiScribe reverse transcriptase and 0.25 U/μL RNase inhibitor. The 15 μL reactions were incubated for 30 min at 16 °C, 30 min at 42 °C, 5 min at 85 °C, and then kept at 4 °C. The cDNA product was used for the following qRT-PCR analysis. The 20 μL PCR reaction included 1.33 μL RT product, 1 × TaqMan universal PCR master mix and 1 μL primers and probe mix of the TaqMan microRNA assay kit. Reactions were incubated in a 96-well optical plate at 95 °C for 5 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. PCR reactions were run on a StepOne Plus real-time PCR machine (Applied Biosystems) and the data were analyzed using SDS v2.3 software, the same as in TLDA.
Prediction of miRNA targets
The target genes of the deregulated miRNAs were predicted by TargetScan (http://www.targetscan.org/).
Gene oncology and Kyoto encyclopedia of genes and genomes pathway analysis based on miRNA expression profile
The miRNA targets were subjected to gene oncology (GO) analysis in order to organize genes into hierarchical categories and uncover the miR-gene regulatory network on the basis of biological process and molecular function[8]. Fisher’s exact test and χ2 test were used to classify the GO category, and the false discovery rate (FDR)[9] was calculated to correct the P value,the lower the FDR, the slight the error in judging the P value. The FDR was defined as
, where Nk refers to the number of Fisher’s test P values less than χ2 test P values. P values were computed for the GOs of all the differential genes. Enrichment provides a measure of the significance of the function: as the enrichment increases, the corresponding function becomes more specific, which can help find those GOs with more concrete function description in the experiment. Within the significant category, the enrichment Re was given by: Re = (nf/n) / (Nf/N) where nf is the number of differential genes within the particular category, n is the total number of genes within the same category, Nf is the number of differential genes in the entire array, and N is the total number of genes in the array[10]. Similarly, pathway analysis was used to find out the significant pathway of the differential genes according to Kyoto encyclopedia of genes and genomes (KEGG), Biocarta and Reatome. The Fisher’s exact test and χ2 test were also used to select the significant pathway, and the threshold of significance was defined by P value and FDR. The enrichment Re was calculated using the same equation mentioned above[11-13]. The network of miRNA-mRNA interaction, representing the critical miRNAs and their targets, was established according to the miRNA degree.
Construction of luciferase reporter plasmids
The fragment of 3’-untranslated region (UTR) of CCND3 (1054-2061nt, Genbank accession no. NM_001136017.2) containing the two putative miR-138 binding sequences (1279-1285nt and 1346-1352nt) was amplified with the primers 5’-CCCTGGAGAGGCCCTCTGGA-3’ and 5’-TTCCAAGAAGCCAAAGCCAG-3’. The partial fragment of 3’-UTR of CHC (5472-6480nt, Genbank accession no. NM_004859) containing the two putative miR-199a-5p binding sequences (5979-5986nt and 5915-5922nt) was amplified with the primers 5’-GATGAAGCGCTGATCCTGTAG-3’ and 5’-TGCCTCCCTAATGCCTCAG-3’. The PCR products were cloned into firefly luciferase reporter vector pGL3 (Promega Corporation, Madison, WI, United States) respectively, termed as pGL3-CCND3-3’UTR or pGL3-CHC-3’UTR. The plasmids carrying the mutated sequence in the complementary sites for the seed region of miR-138 or miR-199a-5p, were generated based on pGL3-CCND3-3’UTR and pGL3-CHC-3’UTR plasmids by site-specific mutagenesis, termed as pGL3-CCND3-3’UTR-mut or pGL3-CHC-3’UTR-mut.
Transfection
The transfection was carried out using FuGene HD transfection reagent (Roche, Indianapolis, IN, United States) following the manufacturer’s protocol. In brief, 2 × 104 HepG2 cells or 5 × 104 HEK293T cells in 24-well plate were transfected with indicated miRNA mimic (50 nmol/L, GenePharma, Shanghai, China) or plasmid DNA (100 ng) and collected 24-48 h after transfection for assay.
Dual-luciferase reporter assay
HEK293T cells were cotransfected with pGL3-CCND3-3’UTR or pGL3-CCND3-3’UTR-mut and miR-138 mimic or nonrelative control RNA duplex [non-relative control (NC) duplex, GenePharma] using FuGene HD transfection reagent. In another well, HEK293T cells were cotransfected with pGL3-CHC-3’UTR or pGL3-CHC-3’UTR-mut and miR-199a-5p mimic or nonrelative control RNA duplex using FuGene HD transfection reagent. The pRL-TK (Promega Corporation, Madison, WI, United States) was also transfected as a normalization control. Cells were collected 48 h after transfection, and luciferase activity was measured using a dual-luciferase reporter assay kit (Promega Corporation) and recorded by multi-plate reader (Synergy 2, BioTek).
Western blotting
Protein extracts from HCC tissues and their adjacent non-tumorous tissues or HepG2 cells were prepared by a modified radioimmunoprecipitation buffer with 0.5% sodium dodecyl sulfate in the presence of proteinase inhibitor cocktail (Complete Mini, Roche). Twenty-five micrograms protein were electrophoresed in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis mini-gels and transferred onto polyvinylidene fluoride membranes (Immobilon P-SQ, Millipore, Billerica, MA, United States). After blocking with 5% nonfat milk, the membranes were incubated with rabbit anti-CCND3 antibody (1:1000 dilution, Epitomics, Inc., Burlingame, CA, United States), rabbit anti-clathrin heavy chain (CHC) antibody (1:1000 dilution, Abcam, Cambridge, United Kingdom) or mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (1:5000 dilution, Epitomics, Inc.) at 4 °C overnight, followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse antibody (1:10 000 dilution, KPL, Gaithersburg, MA, United States) for 1h at room temperature. Finally, signals were developed with Super Signal West Pico chemoluminescent substrate (Pierce, Rockford, Ill, United States) and visualized by the Gene Gnome HR Image Capture System (Syngene, Frederick, MD, United States).
Statistical analysis
Data were presented as mean ± SD. Comparisons were made using a two-tailed t test or one-way analysis of variance for experiments with more than two subgroups. P < 0.01 was considered statistically significant.
RESULTS
Patient characteristics
HCC and adjacent non-tumor tissues were obtained from 18 patients, whose average age was 48.5 years (ranged from 35 years to 71 years) (Table 1). All of the patients were HBsAg positive and with the diagnosis of HCC. In tumor grades, 7 (38.9%) cases were well differentiated (G1 + G2) and 11 (61.1%) cases were moderately differentiated (G3) HCC. In clinical stage, 4 (22.2%) were at invasion T1, 7 (38.9%) at invasion T2, and 6 (33.3%) at invasion T3 and 1 (5.6%) at invasion T4.
Differential expression of miRNAs in HCC
There were 86 deregulated miRNAs in total between HCC tumor and non-tumor tissues by TLDA analysis. In order to select the most significant candidates, miRNAs altered by at least 3-fold in all three pairs of the samples were selected. Under these strict criteria, 11 up-regulated miRNAs and 13 down-regulated miRNAs were identified (Figure 1A and Table 2). To validate the miRNA array data, qRT-PCR was performed in 18 pairs of HCC tissues. Four up-regulated (miR-217, miR-520g, miR-522 and miR-525-3p) (Figure 1B) and 4 down-regulated miRNAs (miR-199a-5p, miR-138, miR-483-5p and miR-511) showed consistent changes in more than 70% tumorous tissues (Figure 1C). Six up-regulated (miR-517c, miR-512-3p, miR-518a-3p, miR-519a, miR-518e and miR-518b) and 7 down-regulated miRNAs (miR-214, miR-214#, miR-592, miR-483-3p, miR-433, miR-708 and miR-1275) showed consistent changes in more than 50% tumorous tissues (data not shown). No significant difference was found between one up-regulated (miR-888) and two down-regulated miRNAs (miR-21# and miR-27a#) in paired tumorous tissues (data not shown).
Figure 1.
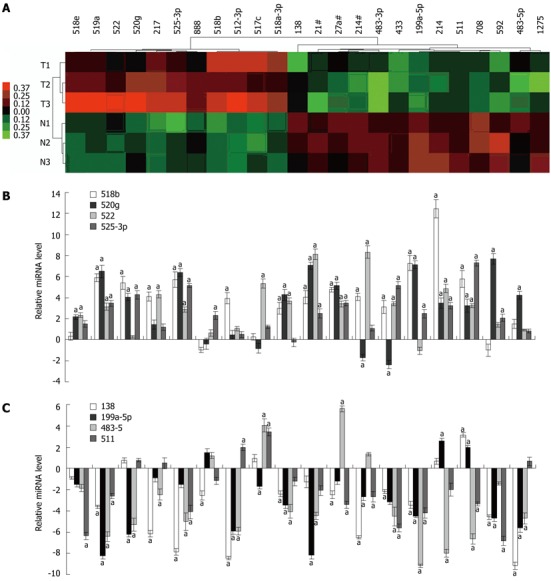
MiRNA profiles differentiate hepatitis B virus-associated hepatocellular carcinoma from adjacent non-tumor tissues. A: The cluster analysis of down-regulated (green) and up-regulated (red) miRNAs identified in hepatocellular carcinoma (hepatitis B virus-hepatocellular carcinoma). Samples consist of paired samples from three patients; B: Validation of Taqman array data using quantitative reverse transcription polymerase chain reaction (RT-PCR) for up-regulated miRNA; C: Validation of Taqman array data using quantitative RT-PCR for down-regulated miRNA. Triplicate assays were done for each RNA sample and the relative amount of each miRNA was normalized to U6 snRNA. aP < 0.01 vs control group.
Table 2.
Deregulated miRNA in hepatitis B virus-associated hepatocellular carcinoma n (%)
| miRNA | Fold change | P value | Validation1 |
| Up-regulated miRNAs | |||
| hsa-miR-520g | 9475.09 | 0.00 | 13 (72.2) |
| hsa-miR-519a | 8204.48 | 0.00 | 10 (55.6) |
| hsa-miR-522 | 6178.34 | 0.00 | 13 (72.2) |
| hsa-miR-518e | 4096.74 | 0.00 | 11 (61.1) |
| hsa-miR-525-3p | 1489.49 | 0.00 | 14 (77.8) |
| hsa-miR-217 | 1080.33 | 0.00 | 11 (59.4) |
| hsa-miR-518b | 982.85 | 0.00 | 14 (77.8) |
| hsa-miR-512-3p | 197.21 | 0.00 | 10 (55.6) |
| hsa-miR-517c | 130.92 | 0.00 | 11 (61.1) |
| hsa-miR-518a-3p | 46.84 | 0.00 | 10 (55.6) |
| Down-regulated miRNAs | |||
| hsa-miR-199a-5p | 0.00237 | 0.00050 | 13 (72.2) |
| hsa-miR-433 | 0.00310 | 0.00270 | 10 (55.6) |
| hsa-miR-592 | 0.01137 | 0.00350 | 11 (59.4) |
| hsa-miR-214# | 0.01327 | 0.00610 | 11 (59.4) |
| hsa-miR-483-5p | 0.03310 | 0.00340 | 14 (77.8) |
| hsa-miR-483-3p | 0.03323 | 0.00510 | 12 (72.2) |
| hsa-miR-138 | 0.03727 | 0.00190 | 14 (77.8) |
| hsa-miR-214 | 0.04213 | 0.00530 | 11 (59.4) |
| hsa-miR-511 | 0.07957 | 0.00490 | 13 (72.2) |
| hsa-miR-708 | 0.02135 | 0.00480 | 10 (55.6) |
| hsa-miR-1275 | 0.06296 | 0.00530 | 10 (55.6) |
Quantitative reverse-transcription polymerase chain reaction was performed in 18 pairs of tumor and non-tumor tissues in hepatitis B virus-associated hepatocellular carcinoma.
Gene oncology and Kyoto encyclopedia of genes and genomes pathway analysis of the deregulated miRNAs
The targets of the 21 deregulated miRNAs (Table 2) were predicted by TargetScan. To identify the most significant candidates and investigate the cellular function, the signaling pathway and GOs of the target genes were analyzed. The results showed that a wide variety of cellular processes were featured significantly in signaling pathways (Figure 2A and B). Many of these signaling pathways, such as insulin, MAPK, TGF-β and Wnt signaling pathway, participated in the tumorigenesis[14-17]. However, some other signaling pathways have never been reported to play a role in tumorigenesis, e.g., axon guidance. Among all these differentially regulated signaling pathways, “regulation of actin cytoskeleton” and “pathway in cancer” appeared to be the most enriched one among both up-regulated and down-regulated miRNA groups. A similar phenomenon was observed in GOs analysis. Many cellular functions were featured significantly, of which the “signal transduction” appeared to be the most enriched one (Figure 2C and D). The miRNA-mRNA interaction network analysis integrated these miRNAs and GOs by outlining the interactions of miRNA and GO-related genes (Figure 3A). MiR-519a and miR-199a-5p showed the target genes of 53 (degree 53) in up-regulated miRNAs and target genes of 32 (degree 32) in down-regulated miRNAs. MiR-138 had a degree of 30, which was the second place in down-regulated miRNAs. These results indicated that miR-138 and miR-199a-5p as well as their targets, might be of great importance to the HCC tumorigenesis. Twenty-six target genes, including RPS6KA3, SMAD4, ACVR2A, CHC, and MAPK1, etc., had more than 3 miRNAs (degree > 3) (Figure 3B).
Figure 2.
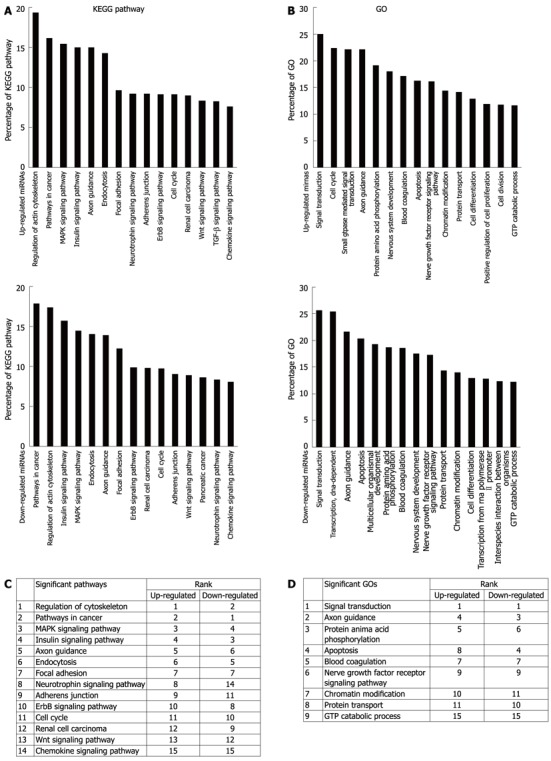
Gene oncology and kyoto encyclopedia of genes and genomes pathway analysis based on miRNA targeted genes. A: The upper panel showing significant pathways targeted by up-regulated miRNA and the lower panel showing significant pathways targeted by down-regulated miRNA; B: The upper panel showing significant GOs targeted by up-regulated miRNA and the lower panel showing significant GOs targeted by down-regulated miRNA. The vertical axis is the pathway or GO category, and the horizontal axis is the enrichment of pathways or GOs; C, D: Summary data of A and B respectively. KEGG: Kyoto Encyclopedia of Genes and Genomes; GO: Gene ontology.
Figure 3.

MiRNAs-mRNA network. A: Orange box nodes represent up-regulated miRNAs, blue box nodes represent down-regulated miRNAs, and cyan cycle nodes represent mRNA. Green lines show the inhibitory effect of miRNAs on target mRNAs; B: Summary data of A.
CCND3 is a direct target of miR-138
Among these deregulated miRNAs, miR-138 was most abundant in non-tumor tissues and miR-199a-5p was most significant in paired HCC tissues (Figure 4A). Thus, miR-138 and miR-199a-5p were selected for the further study. CCND3 was predicted as a potential target of miR-138 by TargetScan. The 3’-UTR of CCND3 mRNA contained a complementary site for the seed region of miR-138 (Figure 4B). CCND3 was found to be up-regulated in 4 specimens of HCC tissues compared with adjacent non-tumor liver tissues (Figure 4C), showing a negative correlation with down-regulated miR-138. These results indicated that miR-138 may be associated with CNND3 and both of them may be involved in HCC tumorigenesis.
Figure 4.
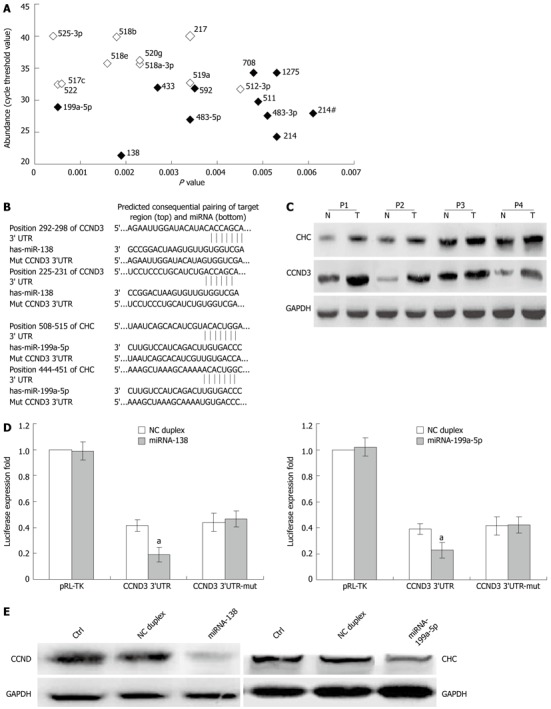
Cyclin D3 and clathrin heavy chain are the direct target of miR-138 and miR-199a-5p. A: Abundance of deregulated miRNAs in hepatitis B virus (HBV)-hepatocellular carcinoma (HCC) non-tumor tissues; B: The putative miR-138 or miR-199a-5p binding sequence in the 3’-UTR of cyclin D3 (CCND3) or clathrin heavy chain (CHC) mRNA; C: The expression of CCND3 and CHC in 4 paired HCCs (T) and adjacent non-tumor tissues (N); D: Suppressed luciferase activity of wild type 3’UTR of CCND3 or CHC by miR-138 or miR-199a-5p mimic. Firefly luciferase activity of each sample was mea sured 48 h after transfection and normalized to Renilla luciferase activity; E: Suppressed expression of endogenous CCND3 or CHC in HepG2 cells by miR-138 or miR-199a-5p mimic, respectively. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. Column, mean of three independent experiments; bars, SD; aP < 0.01 vs control group. NC: Non-relative control.
To validate whether CCND3 is a direct target of miR-138, a human CCND3 3’-UTR fragment containing wild-type or mutant miR-138 binding sequence (Figure 4B) was cloned downstream of the firefly luciferase reporter gene in pGL3. In HEK293 cells cotransfected with the reporter plasmids and miR-138 mimic or NC duplex, the luciferase activity of the reporter that contained wild-type 3’-UTR was significantly suppressed by miR-138 mimic, but the luciferase activity of mutant reporter was unaffected (Figure 4D), indicating that miR-138 may suppress gene expression through miR-138 binding sequence at the 3’-UTR of CCND3. Furthermore, transfection of miR-138 mimic decreased CCND3 expression in HepG2 cells at protein level (Figure 4E). All these results showed that miR-138 could regulate the expression of endogenous human CCND3 by directly targeting the 3’-UTR of CCND3 mRNA and human CCND3 was a new target of miR-138.
Clathrin heavy chain is a direct target of miR-199a-5p
CHC was found to be a direct target of miR-199a-5p. CHC was predicted as a potential target of miR-199a-5p by TargetScan (Figure 4B). CHC was up-regulated in 4 specimens of HCC tissues as compared with adjacent non-tumor liver tissues (Figure 4C). The luciferase activity of the reporter containing wild-type 3’-UTR of CHC was significantly suppressed by miR-199a-5p mimic (Figure 4D). Furthermore, transfection of miR-199a-5p mimic decreased CHC expression in HepG2 cells at protein level (Figure 4E). The results showed that miR-199a-5p could regulate the expression of endogenous human CHC by directly targeting the 3’-UTR of CHC mRNA, and human CHC is a new target of miR-199a-5p.
DISCUSSION
MiRNAs were frequently deregulated in HCC, and some specific miRNAs were associated with the clinicopathological features of HCC, such as metastasis, recurrence, and prognosis[18-20]. Moreover, compelling evidence has demonstrated that miRNAs play an important role in HCC progression and directly contribute to the cell proliferation, avoidance of apoptosis, and metastasis of HCC. Identifying the miRNAs and their targets that are essential for HCC progression may provide promising therapeutic opportunities.
In this study, with Taqman miRNAs array and real-time RT-PCR confirmation, 10 up-regulated miRNAs (miR-217, miR-518b, miR-517c, miR-520g, miR-519a, miR-522, miR-518e, miR-525-3p, miR-512-3p, and miR-518a-3p) and 11 down-regulated miRNAs (miR-138, miR-214, miR-214#, miR-199a-5p, miR-433, miR-511, miR-592, miR-483-5p, miR-483-3p, miRNA-708 and miRNA-1275) were identified in HCC. More importantly, of these 21 deregulated miRNAs, only miR-199a-5p was involved in HCC[21,22], and the other 20 deregulated miRNAs were first reported to be involved in HCC tumorigenesis. No report of MiR-214#, miR-518a-3p and miR-518e has been available in the literature. The rest 17 miRNAs were reported in various cancer but not HCC. For example, the up-regulated miR-512-3p and miR-525-3p were associated with a cisplatin resistant phenotype in human germ cell tumors[23]. The up-regulated miR-519a and down-regulated miR-511 and miR-485-5p were associated with histological subtypes in ovarian cancers[24]. MiR-517c and 520 g promote in vitro and in vivo oncogenicity, modulates cell survival, and robustly enhances growth of untransformed human neural stem cells (hNSCs) in neuroectodermal brain tumors[25]. MiR-433 could regulate tumor-associated proteins GRB2 in gastric carcinoma[26]. MiR-592 was reported to be associated with the stepwise progression for transformation from normal colon to carcinoma[27].
On the contrary, some well known HCC-related miRNAs were not found in this study, possibly due to the very strict criteria of selection defined in TLDA (significant difference in all three pairs of HCC tissues). For example, miR-21[28], miR-122[29,30], miR-16[31] and miR-29[32,33] were excluded for their significantly differential expression only in 2 pairs of HCC tissues, and miR-181[34], miR-221[35,36], miR-125[37] and miR-101[38] were excluded for their differential expression in only 1 pair of HCC tissues or no differential expression at all. Using such strict criteria, we might miss some important candidates, but catch some unique ones. Fortunately, quantitative RT-PCR and follow-up studies proved that our strategy helped find the above new deregulated miRNAs. All of the HCC patients in this study were HBsAg positive. Therefore, the expression patterns of identified miRNAs may mainly represent the alterations in hepatitis B virus (HBV)-positive HCC, which may partially account for the inconsistency between our results and results from other studies.
KEGG pathway and GO enrichment analysis based on the reported and predicted target genes of these deregulated miRNAs, was applied to identify which particular functions and pathways were enriched among the genes controlling distinctive characters between HCC and adjacent non-tumor tissues. As a result, KEGG pathway analysis showed that proliferative (cell cycle, MAPK and Wnt), adhesive (actin cytoskeleton, adherens junction and focal adhesion), survival (TGF-β and ErbB) and oncogenic (renal cell carcinoma and pancreatic cancer) signaling pathways were abundant among the significantly enriched ones. Furthermore, the GOs related to signal transduction (signal transduction, small GTPase-mediated signal transduction, protein amino acid phosphorylation and nerve growth factor receptor signaling pathway) and cell growth (cell differentiation, cell division, positive regulation of cell proliferation, cell cycle and multicellular organism development) represented up to 37% of the significantly enriched GOs. As expected, various cell process and signal pathways were involved in HCC tumorigenesis. To narrow the scope of study and evaluate the most significant candidates, miRNAs and their target genes which were in the intersection of “signal transduction” and “regulation of actin cytoskeleton and pathway in cancer” might be the focus of the future studies.
Although computational analysis indicates that one miRNA may directly modulate hundreds of mRNAs, and a single gene may be regulated by multiple miRNAs, such regulation has not been convincingly demonstrated experimentally. As shown in Figure 3, miR-138 had 30 target genes and miR-199a-5p had 32 target genes. The target genes are involved in different cellular processes, thus individual miRNAs play multi-faceted roles in HCC progression. Down-regulated miR-138 has been observed in different types of cancers but not in HCC[39-43]. MiR-138 plays an important role in tongue squamous cell carcinoma cell migration and invasion by concurrently targeting RhoC and ROCK2[39]. MiR-138 could inhibit the expression of HIF-1a and regulate the apoptosis and migration of clear-cell renal cell carcinoma 786-O cells[40]. MiR-138 enhanced cell migration and invasion by targeting enhancer of zeste homologue 2 (EZH2) in squamous cell carcinoma cell lines[41]. MiR-138 may play an important role in cancer initiation and progression by regulating Fos-like antigen 1 in squamous cell carcinoma[42] or G protein alpha inhibiting activity polypeptide 2(GNAI2) in tongue squamous cell carcinoma[43]. Different from miR-138, the down-regulated miR-199a-5p has been reported in HCC[21,22]. MiR-199a-5p contributes to the increase of cell invasion by functional deregulation of discoidin domain receptor-1 (DDR1) activity in HCC[21]. MiR-199a-5p regulates Brm subunit of SWI/SNF in human cancers[22]. We identified CCND3 as a target of miR-138 and CHC as a target of miR-199a-5p in HCC, which may provide new insights into the mechanisms underlying tumorigenesis. CCND3 is expressed in nearly all proliferating cells and can promote the cell cycle progression[44,45]. Liu Q reported that miR-16 family (including miR-16, miR-195 and miR-424) could induce cell cycle arrest by targeting CCND3, CCNE1 and Cdk6[46]. CHC is a part of clathrin expressed ubiquitously and exerts important functions in endocytosis and mitosis[47]. CHC has been reported to play an important role in inflammation disorder and tumorigenesis[48-51]. CHC functions as a coactivator for p53[48] and contributes to the regulation of basal NF-κB activity in epithelial cells[49]. CHC was identified as immunohistochemical tumor markers for primary HCC[50] and served as a biomarker for early diagnosis of small HCC[51]. Whether CCND3 and CHC regulated by miR-138 and miR-199a-5p respectively are involved in HCC tumorigenesis or other cell functions needs further studies.
In summary, we reported 21 deregulated miRNAs in HCC and CCND3 as the target of miR-138 and CHC as the target of miR-199a-5p. Our findings indicated that the novel miRNAs might be involved in HCC tumorigenesis and provide more evidence for the reliability of integrative bioinformatics analysis.
COMMENTS
Background
MiRNAs are endogenous non-coding RNAs (20-22 nucleotides) that have been identified as post-transcriptional regulators of gene expression. The miRNAs mainly bind to the 3’ untranslated regions (UTRs) of target mRNAs, resulting in mRNA degradation or the blockade of mRNA translation. Increasing evidence has demonstrated that miRNAs play an important role in hepatocellular carcinoma (HCC) progression and directly contribute to the cell proliferation, avoidance of apoptosis, and metastasis of HCC. Identifying the miRNAs and their targets that are essential for HCC progression may provide promising therapeutic opportunities.
Research frontiers
Most recently identified miRNAs were found to be frequently deregulated in HCC, and some specific miRNAs were found to be associated with the clinicopathological features of HCC, such as metastasis, recurrence, and prognosis. Moreover, compelling evidence has demonstrated that miRNAs play an important role in HCC progression and directly contribute to the cell proliferation, avoidance of apoptosis, and metastasis of HCC.
Innovations and breakthroughs
In this study, 10 up-regulated miRNAs (miR-217, miR-518b, miR-517c, miR-520g, miR-519a, miR-522, miR-518e, miR-525-3p, miR-512-3p, and miR-518a-3p) and 10 down-regulated miRNAs (miR-138, miR-214, miR-214#, miR-27a#, miR-199a-5p, miR-433, miR-511, miR-592, miR-483-5p and miR-483-3p) were identified in HCC. Of the 20 deregulated miRNAs, only miR-199a-5p was reported to contribute to the increase of cell invasion by functional deregulation of discoidin domain receptor-1 activity in HCC and regulate Brm subunit of SWI/SNF in human cancers, and the other 19 deregulated miRNAs were first reported to be involved in HCC tumorigenesis. MiR-27a#, miR-214#, miR-518a-3p and miR-518e have never been reported in literatures. The rest 15 miRNAs were reported in various cancers, but not in HCC. Compared with the previous version, the newly found miRNAs (337 updated miRNAs) were profiled in Taqman low-density miRNA array v2.0, which helped the authors identify many new differentially expressed miRNAs in this study.
Applications
This study provides new insights into the understanding of the molecular mechanisms of hepatic carcinogenesis regulated by miRNA, and helps develop personalized miRNA-based therapeutics against HCC.
Terminology
MiRNAs are endogenous non-coding 20 to 22 nucleotide RNAs that have been identified as post-transcriptional regulators of gene expression. MiRNAs are processed from precursor molecules (pri-miRNAs), which are either transcribed from independent miRNA genes or are portions of introns of protein-coding RNA polymerase II transcripts. A single pri-miRNA often contains sequences of several different miRNAs. Pri-miRNAs fold into hairpin structures containing imperfectly base-paired stems and are processed in two steps, catalyzed by the RNase III type endonucleases Drosha (also known as RN3) and Dicer. The Drosha-DGCR8 complex processes pri-miRNAs to ~70-nucleotide hairpins known as pre-miRNAs. In animals, premiRNAs are transported to the cytoplasm by exportin5, where they are cleaved by Dicer to yield ~20-bp miRNA duplexes. One strand is then selected to function as a mature miRNA, while the other strand is degraded. Occasionally, both arms of the pre-miRNA hairpin give rise to mature miRNAs.
Peer review
This article aimed to reveal microRNAs and target genes associated with hepatocellular carcinogenesis using miRNA array and network/pathway analyses combined with integrative bioinformatical analysis. The topic is of significant clinical importance as HCC is a very common and usually lethal liver tumor, and all researches that may reveal some potential target genes/miRNAs that offer new therapeutic possibilities are useful for the better understanding of the disease and related molecular biological mechanisms.
Footnotes
Supported by The Key Programs of the Ministry of Science and Technology, No. 2012ZX10002009-004; Shanghai Leading Academic Discipline Project (B901) and Science Fund for Creative Research Groups, NSFC, China, No. 30921006
Peer reviewer: Ferenc Sipos, MD, PhD, Cell Analysis Laboratory, 2nd Department of Internal Medicine, Semmelweis University, Szentkirályi u. 46, 1088 Budapest, Hungary
S- Editor Lv S L- Editor Ma JY E- Editor Zhang DN
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48:2047–2063. doi: 10.1002/hep.22580. [DOI] [PubMed] [Google Scholar]
- 3.Nelson KM, Weiss GJ. MicroRNAs and cancer: past, present, and potential future. Mol Cancer Ther. 2008;7:3655–3660. doi: 10.1158/1535-7163.MCT-08-0586. [DOI] [PubMed] [Google Scholar]
- 4.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504–511. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 7.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 8.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupuy D, Bertin N, Hidalgo CA, Venkatesan K, Tu D, Lee D, Rosenberg J, Svrzikapa N, Blanc A, Carnec A, et al. Genome-scale analysis of in vivo spatiotemporal promoter activity in Caenorhabditis elegans. Nat Biotechnol. 2007;25:663–668. doi: 10.1038/nbt1305. [DOI] [PubMed] [Google Scholar]
- 10.Schlitt T, Palin K, Rung J, Dietmann S, Lappe M, Ukkonen E, Brazma A. From gene networks to gene function. Genome Res. 2003;13:2568–2576. doi: 10.1101/gr.1111403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32:D277–D280. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yi M, Horton JD, Cohen JC, Hobbs HH, Stephens RM. WholePathwayScope: a comprehensive pathway-based analysis tool for high-throughput data. BMC Bioinformatics. 2006;7:30. doi: 10.1186/1471-2105-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draghici S, Khatri P, Tarca AL, Amin K, Done A, Voichita C, Georgescu C, Romero R. A systems biology approach for pathway level analysis. Genome Res. 2007;17:1537–1545. doi: 10.1101/gr.6202607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005. doi: 10.1038/onc.2010.236. [DOI] [PubMed] [Google Scholar]
- 15.Min L, He B, Hui L. Mitogen-activated protein kinases in hepatocellular carcinoma development. Semin Cancer Biol. 2011;21:10–20. doi: 10.1016/j.semcancer.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Mishra L, Banker T, Murray J, Byers S, Thenappan A, He AR, Shetty K, Johnson L, Reddy EP. Liver stem cells and hepatocellular carcinoma. Hepatology. 2009;49:318–329. doi: 10.1002/hep.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takigawa Y, Brown AM. Wnt signaling in liver cancer. Curr Drug Targets. 2008;9:1013–1024. doi: 10.2174/138945008786786127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braconi C, Patel T. MicroRNA expression profiling: a molecular tool for defining the phenotype of hepatocellular tumors. Hepatology. 2008;47:1807–1809. doi: 10.1002/hep.22326. [DOI] [PubMed] [Google Scholar]
- 19.Ladeiro Y, Couchy G, Balabaud C, Bioulac-Sage P, Pelletier L, Rebouissou S, Zucman-Rossi J. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955–1963. doi: 10.1002/hep.22256. [DOI] [PubMed] [Google Scholar]
- 20.Mott JL. MicroRNAs involved in tumor suppressor and oncogene pathways: implications for hepatobiliary neoplasia. Hepatology. 2009;50:630–637. doi: 10.1002/hep.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen Q, Cicinnati VR, Zhang X, Iacob S, Weber F, Sotiropoulos GC, Radtke A, Lu M, Paul A, Gerken G, et al. Role of microRNA-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasion. Mol Cancer. 2010;9:227. doi: 10.1186/1476-4598-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakurai K, Furukawa C, Haraguchi T, Inada K, Shiogama K, Tagawa T, Fujita S, Ueno Y, Ogata A, Ito M, et al. MicroRNAs miR-199a-5p and -3p target the Brm subunit of SWI/SNF to generate a double-negative feedback loop in a variety of human cancers. Cancer Res. 2011;71:1680–1689. doi: 10.1158/0008-5472.CAN-10-2345. [DOI] [PubMed] [Google Scholar]
- 23.Port M, Glaesener S, Ruf C, Riecke A, Bokemeyer C, Meineke V, Honecker F, Abend M. Micro-RNA expression in cisplatin resistant germ cell tumor cell lines. Mol Cancer. 2011;10:52. doi: 10.1186/1476-4598-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim TH, Kim YK, Kwon Y, Heo JH, Kang H, Kim G, An HJ. Deregulation of miR-519a, 153, and 485-5p and its clinicopathological relevance in ovarian epithelial tumours. Histopathology. 2010;57:734–743. doi: 10.1111/j.1365-2559.2010.03686.x. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Lee KF, Lu Y, Clarke I, Shih D, Eberhart C, Collins VP, Van Meter T, Picard D, Zhou L, et al. Frequent amplification of a chr19q13.41 microRNA polycistron in aggressive primitive neuroectodermal brain tumors. Cancer Cell. 2009;16:533–546. doi: 10.1016/j.ccr.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo H, Zhang H, Zhang Z, Zhang X, Ning B, Guo J, Nie N, Liu B, Wu X. Down-regulated miR-9 and miR-433 in human gastric carcinoma. J Exp Clin Cancer Res. 2009;28:82. doi: 10.1186/1756-9966-28-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberg AL, French AJ, Sarver AL, Subramanian S, Morlan BW, Riska SM, Borralho PM, Cunningham JM, Boardman LA, Wang L, et al. miRNA expression in colon polyps provides evidence for a multihit model of colon cancer. PLoS One. 2011;6:e20465. doi: 10.1371/journal.pone.0020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18:350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 29.Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW, Chen CM, Lin CD, Liao YL, Wang JL, Chau YP, et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009;49:1571–1582. doi: 10.1002/hep.22806. [DOI] [PubMed] [Google Scholar]
- 30.Bai S, Nasser MW, Wang B, Hsu SH, Datta J, Kutay H, Yadav A, Nuovo G, Kumar P, Ghoshal K. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J Biol Chem. 2009;284:32015–32027. doi: 10.1074/jbc.M109.016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsang TY, Tang WY, Chan JY, Co NN, Au Yeung CL, Yau PL, Kong SK, Fung KP, Kwok TT. P-glycoprotein enhances radiation-induced apoptotic cell death through the regulation of miR-16 and Bcl-2 expressions in hepatocellular carcinoma cells. Apoptosis. 2011;16:524–535. doi: 10.1007/s10495-011-0581-5. [DOI] [PubMed] [Google Scholar]
- 32.Wang CM, Wang Y, Fan CG, Xu FF, Sun WS, Liu YG, Jia JH. miR-29c targets TNFAIP3, inhibits cell proliferation and induces apoptosis in hepatitis B virus-related hepatocellular carcinoma. Biochem Biophys Res Commun. 2011;411:586–592. doi: 10.1016/j.bbrc.2011.06.191. [DOI] [PubMed] [Google Scholar]
- 33.Fang JH, Zhou HC, Zeng C, Yang J, Liu Y, Huang X, Zhang JP, Guan XY, Zhuang SM. MicroRNA-29b suppresses tumor angiogenesis, invasion, and metastasis by regulating matrix metalloproteinase 2 expression. Hepatology. 2011;54:1729–1740. doi: 10.1002/hep.24577. [DOI] [PubMed] [Google Scholar]
- 34.Arzumanyan A, Friedman T, Ng IO, Clayton MM, Lian Z, Feitelson MA. Does the hepatitis B antigen HBx promote the appearance of liver cancer stem cells? Cancer Res. 2011;71:3701–3708. doi: 10.1158/0008-5472.CAN-10-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon SO, Chun SM, Han EH, Choi J, Jang SJ, Koh SA, Hwang S, Yu E. Deregulated expression of microRNA-221 with the potential for prognostic biomarkers in surgically resected hepatocellular carcinoma. Hum Pathol. 2011;42:1391–1400. doi: 10.1016/j.humpath.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Gramantieri L, Fornari F, Ferracin M, Veronese A, Sabbioni S, Calin GA, Grazi GL, Croce CM, Bolondi L, Negrini M. MicroRNA-221 targets Bmf in hepatocellular carcinoma and correlates with tumor multifocality. Clin Cancer Res. 2009;15:5073–5081. doi: 10.1158/1078-0432.CCR-09-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W, Xie L, He X, Li J, Tu K, Wei L, Wu J, Guo Y, Ma X, Zhang P, et al. Diagnostic and prognostic implications of microRNAs in human hepatocellular carcinoma. Int J Cancer. 2008;123:1616–1622. doi: 10.1002/ijc.23693. [DOI] [PubMed] [Google Scholar]
- 38.Chiang CW, Huang Y, Leong KW, Chen LC, Chen HC, Chen SJ, Chou CK. PKCalpha mediated induction of miR-101 in human hepatoma HepG2 cells. J Biomed Sci. 2010;17:35. doi: 10.1186/1423-0127-17-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang L, Liu X, Kolokythas A, Yu J, Wang A, Heidbreder CE, Shi F, Zhou X. Downregulation of the Rho GTPase signaling pathway is involved in the microRNA-138-mediated inhibition of cell migration and invasion in tongue squamous cell carcinoma. Int J Cancer. 2010;127:505–512. doi: 10.1002/ijc.25320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song T, Zhang X, Wang C, Wu Y, Cai W, Gao J, Hong B. MiR-138 suppresses expression of hypoxia-inducible factor 1α (HIF-1α) in clear cell renal cell carcinoma 786-O cells. Asian Pac J Cancer Prev. 2011;12:1307–1311. [PubMed] [Google Scholar]
- 41.Liu X, Wang C, Chen Z, Jin Y, Wang Y, Kolokythas A, Dai Y, Zhou X. MicroRNA-138 suppresses epithelial-mesenchymal transition in squamous cell carcinoma cell lines. Biochem J. 2011;440:23–31. doi: 10.1042/BJ20111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin Y, Wang C, Liu X, Mu W, Chen Z, Yu D, Wang A, Dai Y, Zhou X. Molecular characterization of the microRNA-138-Fos-like antigen 1 (FOSL1) regulatory module in squamous cell carcinoma. J Biol Chem. 2011;286:40104–40109. doi: 10.1074/jbc.C111.296707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang L, Dai Y, Liu X, Wang C, Wang A, Chen Z, Heidbreder CE, Kolokythas A, Zhou X. Identification and experimental validation of G protein alpha inhibiting activity polypeptide 2 (GNAI2) as a microRNA-138 target in tongue squamous cell carcinoma. Hum Genet. 2011;129:189–197. doi: 10.1007/s00439-010-0915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin J, Jinno S, Okayama H. Cdk6-cyclin D3 complex evades inhibition by inhibitor proteins and uniquely controls cell’s proliferation competence. Oncogene. 2001;20:2000–2009. doi: 10.1038/sj.onc.1204375. [DOI] [PubMed] [Google Scholar]
- 45.Grillo M, Bott MJ, Khandke N, McGinnis JP, Miranda M, Meyyappan M, Rosfjord EC, Rabindran SK. Validation of cyclin D1/CDK4 as an anticancer drug target in MCF-7 breast cancer cells: Effect of regulated overexpression of cyclin D1 and siRNA-mediated inhibition of endogenous cyclin D1 and CDK4 expression. Breast Cancer Res Treat. 2006;95:185–194. doi: 10.1007/s10549-005-9066-y. [DOI] [PubMed] [Google Scholar]
- 46.Liu Q, Fu H, Sun F, Zhang H, Tie Y, Zhu J, Xing R, Sun Z, Zheng X. miR-16 family induces cell cycle arrest by regulating multiple cell cycle genes. Nucleic Acids Res. 2008;36:5391–5404. doi: 10.1093/nar/gkn522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blixt MK, Royle SJ. Clathrin heavy chain gene fusions expressed in human cancers: analysis of cellular functions. Traffic. 2011;12:754–761. doi: 10.1111/j.1600-0854.2011.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohata H, Ota N, Shirouzu M, Yokoyama S, Yokota J, Taya Y, Enari M. Identification of a function-specific mutation of clathrin heavy chain (CHC) required for p53 transactivation. J Mol Biol. 2009;394:460–471. doi: 10.1016/j.jmb.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 49.Kim ML, Sorg I, Arrieumerlou C. Endocytosis-independent function of clathrin heavy chain in the control of basal NF-κB activation. PLoS One. 2011;6:e17158. doi: 10.1371/journal.pone.0017158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seimiya M, Tomonaga T, Matsushita K, Sunaga M, Oh-Ishi M, Kodera Y, Maeda T, Takano S, Togawa A, Yoshitomi H, et al. Identification of novel immunohistochemical tumor markers for primary hepatocellular carcinoma; clathrin heavy chain and formiminotransferase cyclodeaminase. Hepatology. 2008;48:519–530. doi: 10.1002/hep.22364. [DOI] [PubMed] [Google Scholar]
- 51.Di Tommaso L, Destro A, Fabbris V, Spagnuolo G, Laura Fracanzani A, Fargion S, Maggioni M, Patriarca C, Maria Macchi R, Quagliuolo M, et al. Diagnostic accuracy of clathrin heavy chain staining in a marker panel for the diagnosis of small hepatocellular carcinoma. Hepatology. 2011;53:1549–1557. doi: 10.1002/hep.24218. [DOI] [PubMed] [Google Scholar]


