Abstract
Primary hepatic leiomyosarcoma is a particularly rare tumor with a poor prognosis. Curative resection is currently the only effective treatment, and the efficacy of chemotherapy is unclear. This represents the first case report of a patient with primary hepatic leiomyosarcoma co-existing with metastatic liver carcinoma. We present a 59-year-old man who was diagnosed preoperatively with rectal cancer with multiple liver metastases. He underwent a curative hepatectomy after a series of chemotherapy regimens with modified FOLFOX6 consisting of 5-fluorouracil, leucovorin and oxaliplatin plus bevacizumab, FOLFIRI consisting of 5-fluorouracil, leucovorin and irinotecan plus bevacizumab, and irinotecan plus cetuximab. One of the liver tumors showed a different response to chemotherapy and was diagnosed as a leiomyosarcoma following histopathological examination. This case suggests that irinotecan has the potential to inhibit the growth of hepatic leiomyosarcomas. The possibility of comorbid different histological types of tumors should be suspected when considering the treatment of multiple liver tumors.
Keywords: Leiomyosarcoma, Rectal cancer, Metastasis, Chemotherapy, Surgery
INTRODUCTION
Hepatic sarcomas constitute only 1%-2% of primary malignant tumors of the liver, and primary hepatic leiomyosarcoma is particularly rare[1]. The prognosis of primary hepatic leiomyosarcoma is poor, but long-term survival is possible if complete resection can be achieved[2]. We herein report a case of primary hepatic leiomyosarcoma co-existing with synchronous liver metastases of rectal cancer. The patient was treated by a curative hepatectomy following chemotherapy.
CASE REPORT
A 59-year-old man with a history of hypertension and hyperuricemia demonstrated fecal occult blood and multiple liver tumors in a comprehensive medical examination in October 2009 and was observed at our hospital. There were no remarkable physical findings. Laboratory examinations revealed a slight increase in uric acid (7.4 mg/dL). Hepatitis B and C viral markers were negative. Of the tested tumor markers, only carcinoembryonic antigen was elevated (17.9 ng/mL).
Dynamic computed tomography (CT) of the liver revealed a well-defined, hypo- or isodense tumor, 42 mm in diameter, with early enhancement and delayed washout or gradual enhancement in segment 4. Segment 8 contained an ill-defined, hypodense tumor, 52 mm in diameter, with continuous peripheral enhancement, and segment 3 contained a hypodense tumorthat was 11 mm in diameter (Figure 1).
Figure 1.
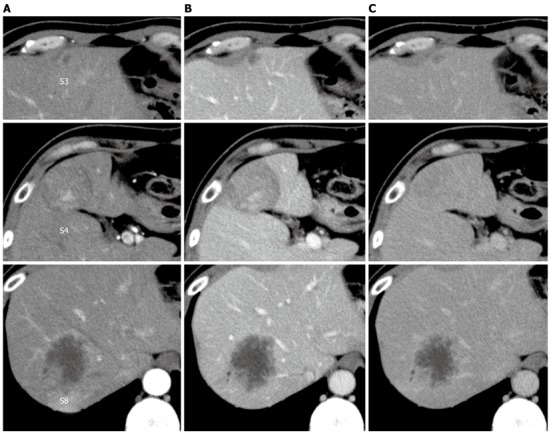
Dynamic computed tomography of the liver. A: Arterial phase; B: Portal phase; C: Venous phase. A hypo- or iso-dense tumor with heterogeneous enhancement in segment 4, a hypodense tumor with peripheral enhancement in segment 8, and a small hypodense tumor in segment 3.
The tumor in segment 4 appeared to be isointense throughout the dynamic phase on gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (MRI), while the tumors in segments 3 and 8 appeared hypointense. All of the tumors appeared hypointense in pre-enhancement scans and the hepatocellular phase on T1-weighted images (Figure 2). Additionally, all of the tumors appeared hyperintense on T2-weighted images and diffusion-weighted images. Ultrasonography demonstrated that the tumors were hypo- or isoechoic heterogeneous masses without halos (Figure 3).
Figure 2.
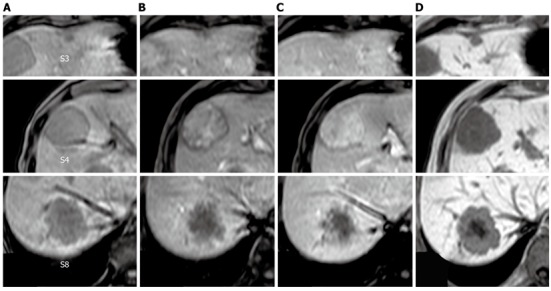
T1-weighted gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging. A: Pre-enhancement; B: 30 s; C: 120 s; D: Hepatocellular phase. The tumor in segment 4 showed heterogeneous enhancement in the dynamic phase. The tumors in segments 3 and 8 showed gradual peripheral enhancement.
Figure 3.
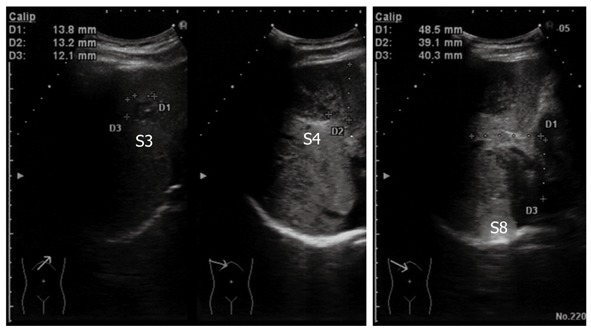
Ultrasonography of the abdomen. All of the tumors showed hypo- or isoechoic heterogeneous masses without halos.
Colonoscopy revealed a Borrmann type 2 tumor at the rectosigmoid junction. Histology of the biopsy tissue revealed a moderately differentiated adenocarcinoma.
The patient was diagnosed with rectal cancer and multiple liver metastases. Because the simultaneous resection of the primary tumor and liver metastases would be a high-risk procedure, we performed an initial high anterior resection with D3 dissection in December 2009. A histopathological examination revealed extraserosal invasion by a moderately differentiated adenocarcinoma and metastases in 8 of 14 resected lymph nodes. A genetic analysis confirmed wild-type KRAS. The patient received chemotherapy with modified FOLFOX6 (mFOLFOX6) plus bevacizumab after the initial surgery. The liver tumor in segment 3 vanished, and the tumor in segment 8 decreased in size from 52 mm to 25 mm in diameter. However, the tumor in segment 4 increased in size from 42 mm to 60 mm in diameter. He then underwent seven courses of FOLFIRI plus bevacizumab, followed by a course of irinotecan plus cetuximab; the tumor in segment 4 decreased in size from 60 mm to 55 mm in diameter, and the tumor in segment 8 decreased in size from 25 mm to 22 mm in diameter (Figure 4). With the exception of the liver tumors, positron emission tomography-CT after the chemotherapy series showed no significant uptake of fluorodeoxyglucose.
Figure 4.
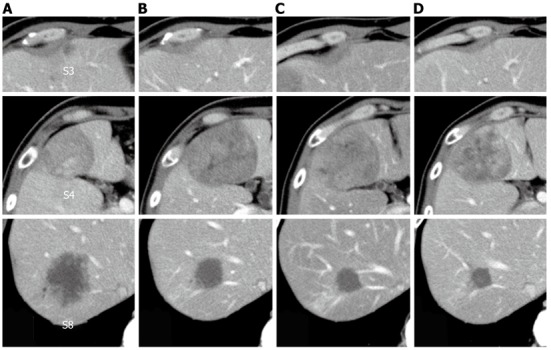
Change in tumor size observed using computed tomography during the clinical course. A: Before treatment; B: After six courses of modified FOLFOX6 plus bevacizumab; C: After seven courses of FOLFIRI plus bevacizumab; D: After one course of irinotecan plus cetuximab.
The patient then underwent a central two-segment resection and a partial resection of segment 3 in September 2010. The resected specimen from the two central segments weighed 380 g, while that from segment 3 weighed 3.8 g. The tumors from segments 4, 8 and 3 measured 56 mm × 44 mm, 24 mm × 22 mm and 7 mm × 4 mm, respectively (Figure 5). A histopathological examination revealed fibrosis and calcification in the tumors from segments 3 and 8, with a few degenerated residual adenocarcinoma cells, which was compatible with rectal cancer metastasis. In contrast, the tumor from segment 4 consisted of irregular fascicles of spindle-shaped cells with eosinophilic cytoplasms and nuclear atypia (Figure 6). Chemotherapy had no apparent effect histologically. An immunohistochemical examination demonstrated that the tumor cells in segment 4 were positive for α-smooth muscle actin and desmin, and negative for CD34, S-100, c-kit and cytokeratin AE1/3 (Figure 7). Based on these findings, the tumor in segment 4 was diagnosed as a leiomyosarcoma.
Figure 5.
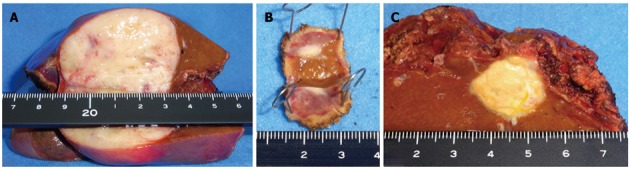
A macroscopic view of the cut-end surfaces of the resected specimens. A: Segment 3; B: Segment 8; C: Segment 4.
Figure 6.
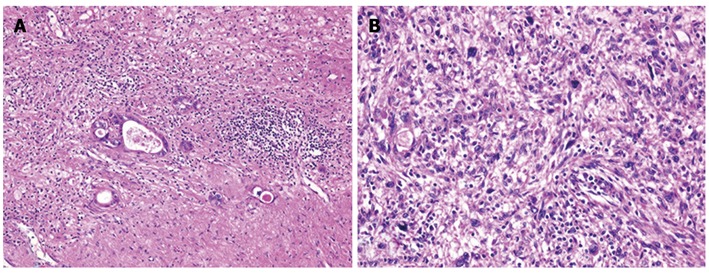
Histological findings of the tumors and hematoxylin and eosin staining. A: The tumor in segment 8 demonstrated fibrosis and calcification, with a few degenerated residual adenocarcinoma cells; B: The tumor in segment 4 had irregular fascicles of spindle-shaped cells with eosinophilic cytoplasm and nuclear atypia.
Figure 7.
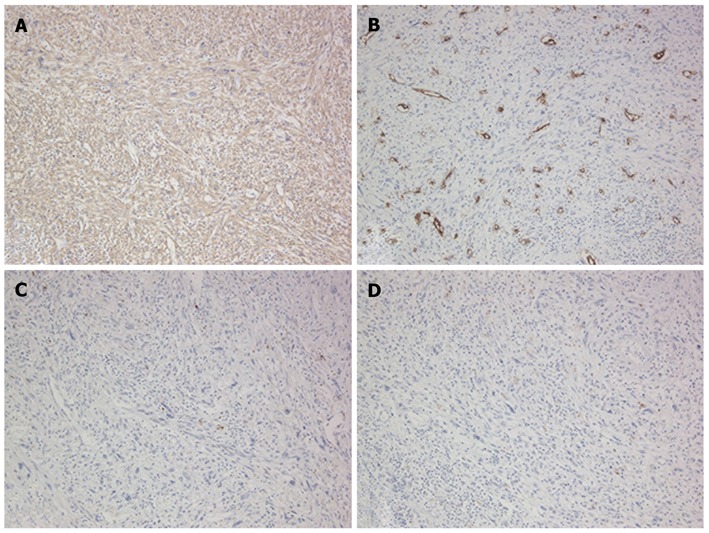
Immunohistochemical features of the tumor in segment 4. A: Positive for α-smooth muscle actin; B: Negative for CD34; C: Negative for S-10; D: Negative for c-kit.
The patient underwent six courses of FOLFIRI plus bevacizumab as adjuvant chemotherapy, and had no recurrence of leiomyosarcoma or rectal cancer at 16 mo after the hepatectomy.
DISCUSSION
Primary hepatic leiomyosarcoma is a particularly rare tumor. Most hepatic leiomyosarcomas are metastatic tumors, and the exclusion of metastatic leiomyosarcoma is therefore essential for an accurate diagnosis[3]. To our knowledge, this represents the first case report in the literature to describe a primary hepatic leiomyosarcoma co-existing with metastatic liver tumors of another origin, but the clinical and histological relevance is unclear. Surgery is considered to be the best treatment for primary hepatic leiomyosarcomas, if an R0 resection can be performed[3,4]. Doxorubicin has been the standard chemotherapy for soft tissue leiomyosarcomas, with gemcitabine and docetaxel or trabectedin as possible alternatives[5,6]. However, the efficacy of chemotherapy for primary hepatic leiomyosarcoma has not been confirmed. In our case, mFOLFOX6 plus bevacizumab did not inhibit the growth of the leiomyosarcoma, while FOLFIRI plus bevacizumab and irinotecan plus cetuximab did. Some studies have demonstrated the efficacy of irinotecan combined with other anticancer agents in some types of sarcomas, such as Ewing’s sarcoma[7] or rhabdomyosarcoma[8], but little is known about the effect of irinotecan on leiomyosarcomas. The present case suggests that irinotecan exerted a growth-inhibiting effect on this leiomyosarcoma.
In a randomized controlled trial, Portier et al[9] demonstrated a disease-free survival benefit of adjuvant chemotherapy with 5-fluorouracil and leucovorin after liver resection for patients with liver metastases from colorectal cancer compared to surgery alone. In another randomized controlled trial, Nordlinger et al[10] demonstrated that perioperative chemotherapy with FOLFOX4 in patients with resectable liver metastases improved progression-free survival compared to surgery alone. However, adjuvant chemotherapy after hepatectomy for patients converted from unresectable liver metastases is still controversial. We selected FOLFIRI plus bevacizumab for adjuvant chemotherapy with the expectation of an effect of irinotecan on the leiomyosarcoma and because the patient developed grade 3 peripheral neuropathy with mFOLFOX6.
The preoperative diagnosis of primary hepatic leiomyosarcoma is challenging because of the non-specific nature of the symptoms and the lack of serological markers[11]. CT usually shows a well-defined hypodense heterogeneous mass with peripheral enhancement. MRI shows hypointensity on T1-weighted images and hyperintensity on T2-weighted images[12]. Some cases of hepatic leiomyosarcoma diagnosed by fine-needle aspiration cytology have been reported[13]. However, the preoperative histological diagnosis of liver tumors, especially hepatocellular carcinoma, is controversial because of the risk of needle-track seeding. In the current case, the complication of liver metastases from rectal cancer made preoperative diagnosis more difficult. In retrospect, however, the leiomyosarcoma lesion showed slightly different CT and MRI findings compared to the metastatic tumors.
In conclusion, this case suggests the potential of irinotecan to inhibit the growth of hepatic leiomyosarcomas. Irinotecan may be a chemotherapy option for hepatic leiomyosarcomas. The possibility of different histological types of tumors should be considered when planning the treatment of multiple liver tumors, especially when the tumors might have heterogeneous responses to chemotherapy.
Footnotes
Peer reviewers: Dr. Marek Bebenek, Department of Surgical Oncology, Regional Comprehensive Cancer Center, 53-413 Wroclaw, Poland; Dr. Benjamin Perakath, Professor, Department of Colorectal Surgery, Christian Medical College, Vellore 632004, India
S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Fong JA, Ruebner BH. Primary leiomyosarcoma of the liver. Hum Pathol. 1974;5:115–119. doi: 10.1016/s0046-8177(74)80105-x. [DOI] [PubMed] [Google Scholar]
- 2.Matthaei H, Krieg A, Schmelzle M, Boelke E, Poremba C, Rogiers X, Knoefel WT, Peiper M. Long-term survival after surgery for primary hepatic sarcoma in adults. Arch Surg. 2009;144:339–344; discussion 344. doi: 10.1001/archsurg.2009.30. [DOI] [PubMed] [Google Scholar]
- 3.Shivathirthan N, Kita J, Iso Y, Hachiya H, Kyunghwa P, Sawada T, Kubota K. Primary hepatic leiomyosarcoma: Case report and literature review. World J Gastrointest Oncol. 2011;3:148–152. doi: 10.4251/wjgo.v3.i10.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gates LK, Cameron AJ, Nagorney DM, Goellner JR, Farley DR. Primary leiomyosarcoma of the liver mimicking liver abscess. Am J Gastroenterol. 1995;90:649–652. [PubMed] [Google Scholar]
- 5.Eriksson M. Histology-driven chemotherapy of soft-tissue sarcoma. Ann Oncol. 2010;21 Suppl 7:vii270–vii276. doi: 10.1093/annonc/mdq285. [DOI] [PubMed] [Google Scholar]
- 6.Scurr M. Histology-driven chemotherapy in soft tissue sarcomas. Curr Treat Options Oncol. 2011;12:32–45. doi: 10.1007/s11864-011-0140-x. [DOI] [PubMed] [Google Scholar]
- 7.Casey DA, Wexler LH, Merchant MS, Chou AJ, Merola PR, Price AP, Meyers PA. Irinotecan and temozolomide for Ewing sarcoma: the Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2009;53:1029–1034. doi: 10.1002/pbc.22206. [DOI] [PubMed] [Google Scholar]
- 8.Mascarenhas L, Lyden ER, Breitfeld PP, Walterhouse DO, Donaldson SS, Paidas CN, Parham DM, Anderson JR, Meyer WH, Hawkins DS. Randomized phase II window trial of two schedules of irinotecan with vincristine in patients with first relapse or progression of rhabdomyosarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2010;28:4658–4663. doi: 10.1200/JCO.2010.29.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Portier G, Elias D, Bouche O, Rougier P, Bosset JF, Saric J, Belghiti J, Piedbois P, Guimbaud R, Nordlinger B, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976–4982. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 10.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, Bechstein WO, Primrose JN, Walpole ET, Finch-Jones M, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shamseddine A, Faraj W, Mukherji D, El Majzoub N, Khalife M, Soubra A, Shamseddine A. Unusually young age distribution of primary hepatic leiomyosarcoma: case series and review of the adult literature. World J Surg Oncol. 2010;8:56. doi: 10.1186/1477-7819-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrozzi F, Bova D, Zangrandi A, Garlaschi G. Primary liver leiomyosarcoma: CT appearance. Abdom Imaging. 1996;21:157–160. doi: 10.1007/s002619900034. [DOI] [PubMed] [Google Scholar]
- 13.Smith MB, Silverman JF, Raab SS, Towell BD, Geisinger KR. Fine-needle aspiration cytology of hepatic leiomyosarcoma. Diagn Cytopathol. 1994;11:321–327. doi: 10.1002/dc.2840110403. [DOI] [PubMed] [Google Scholar]


