Abstract
Bezoars are concretions of indigestible materials in the gastrointestinal tract. It generally develops in patients with previous gastric surgery or patients with delayed gastric emptying. Cases of periampullary duodenal divericular bezoar are rare. Clinical manifestations by a bezoar vary from no symptom to acute abdominal syndrome depending on the location of the bezoar. Biliary obstruction or acute pancreatitis caused by a bezoar has been rarely reported. Small bowel obstruction by a bezoar is also rare, but it is a complication that requires surgery. This is a case of acute pancreatitis and subsequent duodenal obstruction caused by a large duodenal bezoar migrating from a periampullary diverticulum to the duodenal lumen, which mimicked pancreatic abscess or microperforation on abdominal computerized tomography. The patient underwent surgical removal of the bezoar and recovered completely.
Keywords: Bezoar, Diverticulum, Pancreatitis, Duodenal obstruction
INTRODUCTION
Bezoars are concretions of foreign bodies found in the gastrointestinal tract. They are usually detected after gastric operations because of reduced gastric motor activity and delayed gastric emptying[1]. Bezoars in a periampullary duodenal diverticular without previous surgery are rare. The presence of bezoars may or may not be accompanied by gastrointestinal manifestations, such as epigastric pain and postprandial fullness. Major complications of bezoars include gastritis, gastric ulcer, gastric perforation, and intestinal obstruction[2]. Small bowel obstruction is a rare complication usually due to the migration of gastric bezoars. It could also be caused by formation of primary bezoars in the small bowel in association with underlying diseases such as diverticulum, stricture or tumor[3]. Acute pancreatitis secondary to a bezoar is rare, but can present as a possible complication[4]. However, duodenal obstruction by a diverticular bezoar following acute pancreatitis has not been reported.
We report a case of acute pancreatitis and subsequent duodenal obstruction caused by a large duodenal bezoar migrating from periampullary diverticulum to duodenal lumen, which was difficult to diagnose by radiological study.
CASE REPORT
A 75-year-old female visited our hospital with epigastric pain, nausea and vomiting for 4 d. She was diagnosed with diabetes 15 years ago and was taking oral hypoglycemic agents for the control of blood sugar level. There were no specific findings from her social history. Her blood pressure on admission was 130/80 mmHg, pulse rate 70/min, respiratory rate 20/min, and body temperature 36.4 °C. On physical examination, she appeared acutely ill, with a soft abdomen but with tenderness in the epigastric area and right upper quadrant. Complete blood count test showed a leukocyte count of 10 340/mm3, a hemoglobin level of 13.1 g/dL, and a platelet count of 174 000/mm3. Blood chemistry showed total protein 7.1 g/dL, albumin 4.3 g/dL, total bilirubin 1.1 mg/dL, aspartate aminotransferase 491 IU/L, alanine aminotransferase 375 IU/L, alkaline phosphatase 154 IU/L, γ-glutamyl transpeptidase 213 IU/L, amylase 1730 IU/L, and lipase over 300 IU/L. Abdominal computerized tomography (CT) taken on the day of admission revealed a 5 cm sized subtle rim enhancing mass with air bubbles in the pancreatic head which raised the suspicion of an air forming abscess of the pancreatic head or a duodenal diverticulum of the second duodenal portion (Figure 1A and B). Following this, magnetic resonance cholangiopancreatography (MRCP) showed no evidence of abnormality in the common bile duct or pancreatic duct. The medical treatments including intravenous fluids, parenteral alimentation, and antibiotics were started, and abdominal pain improved thereafter. Additionally, the elevated serum transaminase and pancreatic enzymes progressively improved. However, nine days after admission, the patient’s body temperature rose to 40.4 °C, her blood pressure was 80/50 mmHg, leukocyte count 47 620/mm3, erythrocyte sedimentation rate 66 mm/h, and C-reactive protein 166 mg/dL. The follow-up abdominal CT revealed aggravation of duodenal wall thickening and stranding of the surrounding soft tissues with some tiny extraluminal air bubbles indicating possible micro-perforation (Figure 1C). She received fluid resuscitation through rapid intravenous administration of crystalloid fluid and vasopressor support, and antibiotic therapy with meropenem was started for the management of septic shock. After vigorous treatment, the patient’s state became stabilized, but more than 1500 cc of dark-colored fluid was drained through the Levin tube per day. Esophagogastroduo- denoscopy (EGD) was performed for suspected duodenal obstruction. On entering the duodenum, a huge dark yellowish mass was found obstructing the lumen of the second portion of the duodenum. This mass was thought to be originated from the large periampullary diverticulum and migrated toward the distal portion (Figure 2A). A large broad based inflamed diverticulum was observed, with small secondary diverticula corresponding to the extraluminal air bubbles seen on the follow-up abdominal CT. Endoscope was not able to pass through because of luminal obstruction. We tried to extract the bezoar with the help of a basket and a net, but it was unsuccessful as the bezoar was too hard and big (Figure 2B). On eighteenth hospital day, when the clinical symptoms and the laboratory findings of acute pancreatitis and septic shock had been improved, the patient was transferred to the department of general surgery for operation. A gastrotomy was performed and a huge bezoar (5 cm × 2.5 cm) was removed through the antrum by manually pushing the duodenal bezoar up into the stomach (Figure 3). The patient was discharged on the 6th postoperative day. During the 2-mo follow-up, the patient did not complain of any discomfort and two periampullary diverticula were seen in the upper gastrointestinal series (Figure 4).
Figure 1.
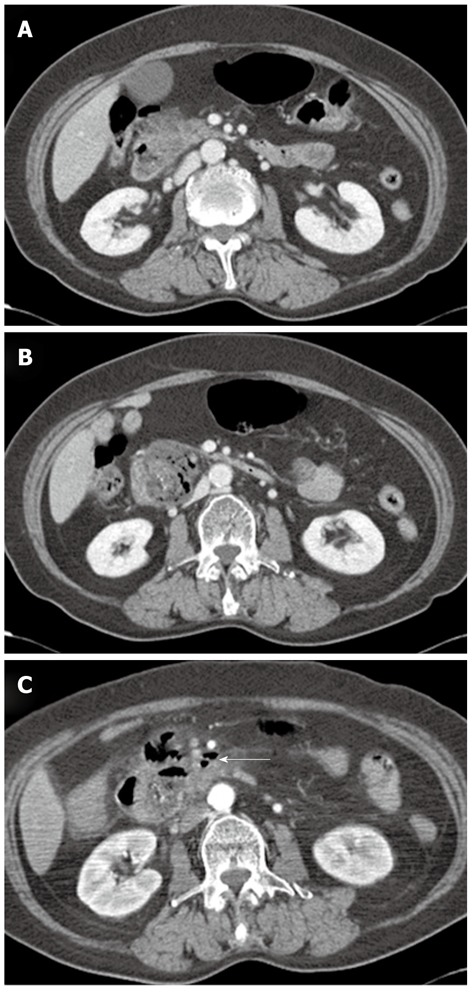
Abdominal computed tomography findings. A: The pancreas head showed swelling with irregular contour of the pancreatic margin and mild peripancreatic infiltration; B: There is a 5 cm size subtle rim enhancing mass with air bubbles, indicating acute diverticulitis in the second duodenal portion or air forming abscess of the pancreatic head; C: After nine days, a few extraluminal air bubbles (arrow) suspicious for microperforation were found.
Figure 2.
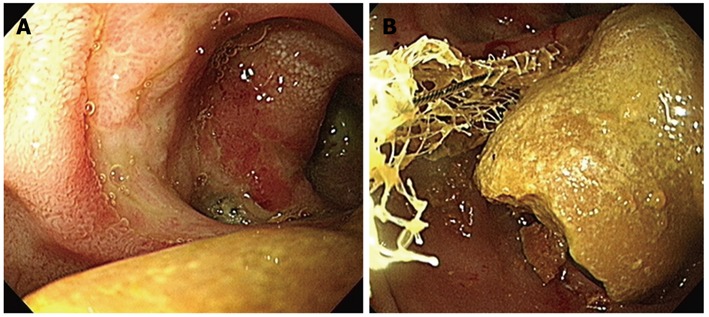
Endoscopic findings. A: There is a huge luminal obstructing bezoar near the duodenal diverticulum; B: The bezoar was too large to be captured and removed by an endoscopic basket or net.
Figure 3.
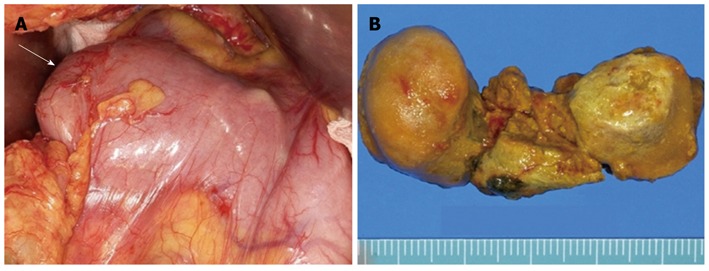
Surgical findings. A: Obstructed duodenum with impacted huge bezoar (arrow); B: Surgical specimen of the divided bezoar, 5 cm long, 2.5 cm in diameter.
Figure 4.
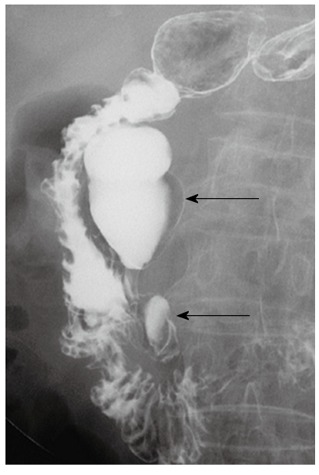
Upper gastrointestinal series show two periampullary diverticula (arrows) in the second duodenal portion.
DISCUSSION
Bezoars are composed of vegetable matter (phytobezoar), hair (trichobezoar), or more unusual materials[1]. They are usually related to previous gastric operations, such as vagotomy or partial gastrectomy, which reduce gastric motor activity and delay gastric emptying. In one report, 70% of patients with bezoar had undergone previous surgery[2]. Bezoars also can be induced by gastroparesis caused by hypothyroidism or diabetes mellitus, and poor mastication or ingestion of indigestible materials[3].
Most bezoars occur in the stomach, but may be encountered elsewhere, including the small bowel and even the esophagus and rectum. Clinical manifestations vary depending on the location of the bezoar from showing no symptom to acute abdominal syndrome, such as, epigastric distention, abdominal pain, and acid regurgitation. Major complications of bezoars include intestinal obstruction, gastric perforation, gastric ulcer, and gastritis[5]. Small bowel obstruction is a severe complication requiring surgery[6]. Small bowel obstructions were usually due to migration of gastric bezoars, but it could also be caused by primary bezoars formed in the small bowel in association with stricture, tumor or diverticulum[3]. Biliary obstruction or acute pancreatitis caused by bezoars has seldom been reported[4,7,8]. In our case, a large duodenal bezoar had originated from an inflamed periampullary diverticulum, causing acute pancreatitis. This was then followed by a spontaneous migration of the bezoar, resulting in a duodenal obstruction and septic shock. The duodenal obstruction due to large gallstone migrated through a cholecysto-enteric fistula can give a similar radiological and endoscopic appearance as our case[9]. However, there was no cholecysto-duodenal fistula or air in the gallbladder in the present case.
Although duodenal bezoars are rare, they can develop in patients with previous gastric surgery[8], deformed duodenal bulb[10], superior mesenteric artery syndrome[11], and duodenal diverticulum[12] as in our case. Most cases of pancreatitis caused by duodenal bezoars are trichobezoars also known as the “Rapunzel syndrome”[4]. This was a case of having unusually long hairballs that extend from the stomach into the jejunum or ileum. It may cause extrinsic compression of the ampulla, common bile duct or pancreatic head. It is thought that bezoars in the periampullary diverticulum may also cause acute pancreatitis through similar mechanisms[13].
Several studies suggested that patients with periampullary diverticulum had higher frequencies of pancreatitis. It is still controversial whether or not pancreatitis is induced by periampullary diverticulum per se. Periampullary diverticulum is associated with choledocholithiasis, and it predispose to gallstone pancreatitis[14]. Our patient showed no evidence of stones in the common bile duct on abdominal CT and MRCP, thus acute pancreatitis did not result from bile duct stones. In fact, the large bezoar had caused compression of major ampulla leading to pancreatitis.
Contrast-enhanced abdominal CT is the most useful tool for diagnosis of duodenal diverticulitis, but it can be misinterpreted as acute pancreatitis or its complications (phlegmon, pseudocyst, abscess), cystic pancreatic head neoplasms, and peripancreatic lymphadenopathy[15]. In our case, the enhanced CT showed findings that were suspicious for pancreatic abscess or microperforation of duodenal diverticulum adjacent to the pancreatic head. After EGD, the condition was recognized as periampullary diverticulitis with secondary diverticula inside. Endoscopy is an important diagnostic modality for diagnosis of diverticular diseases. The side-viewing endoscopy can be beneficial compared to the forward-viewing endoscopy, because acute diverticulitis causes mucosal swelling and narrowing of the diverticular orifice that further hampers the diagnostic yield of a forward-viewing endoscopy[16].
Bezoars are treated by endoscopy or surgery. If the bezoar is large, fragmentation is tried with the use of overtube, baskets, lithotripsic equipment, paraffin, cellulose, acetylcysteine, Coca-Cola lavage, and even lasers. If the bezoar is accompanied by complications such as small bowel obstruction, gastric perforation or gastric hemorrhage, the patient needs urgent treatment, such as surgery[3].
In conclusion, we report a case of a large duodenal bezoar, which had originated from the periampullary diverticulum, causing acute pancreatitis, with subsequent duodenal obstruction by migration. Complications caused by duodenal bezoar, in particular, pancreatitis and subsequent duodenal obstructions are rare, but a high index of suspicion is required for the correct diagnosis and proper management.
Footnotes
Peer reviewers: Juhani Sand, MD, PhD, Department of Gastroenterology and Alimentary Tract Surgery, Tampere University Hospital, Teiskontie 35, 33521 Tampere, Finland; Ross Cyril Smith, Professor, Department of Surgery, University of Sydney, Suite 5 Level 5 North Shore Private Hospital, StLeonards 2065, Australia
S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Andrus CH, Ponsky JL. Bezoars: classification, pathophysiology, and treatment. Am J Gastroenterol. 1988;83:476–478. [PubMed] [Google Scholar]
- 2.Robles R, Parrilla P, Escamilla C, Lujan JA, Torralba JA, Liron R, Moreno A. Gastrointestinal bezoars. Br J Surg. 1994;81:1000–1001. doi: 10.1002/bjs.1800810723. [DOI] [PubMed] [Google Scholar]
- 3.Erzurumlu K, Malazgirt Z, Bektas A, Dervisoglu A, Polat C, Senyurek G, Yetim I, Ozkan K. Gastrointestinal bezoars: a retrospective analysis of 34 cases. World J Gastroenterol. 2005;11:1813–1817. doi: 10.3748/wjg.v11.i12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katapadi M, Kostandy G, Wang A, Gutierrez R, Malik A, Pachter BR. Can a bezoar cause acute pancreatitis? J Clin Gastroenterol. 1997;24:120–121. doi: 10.1097/00004836-199703000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Zhang RL, Yang ZL, Fan BG. Huge gastric disopyrobezoar: a case report and review of literatures. World J Gastroenterol. 2008;14:152–154. doi: 10.3748/wjg.14.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosheh B, Salameh JR. Laparoscopic approach to acute small bowel obstruction: review of 1061 cases. Surg Endosc. 2007;21:1945–1949. doi: 10.1007/s00464-007-9575-3. [DOI] [PubMed] [Google Scholar]
- 7.van der Linde K, van der Linden GH, Beukers R, Cleophas TA. Food impaction in a duodenal diverticulum as an unusual cause of biliary obstruction: case reports and review of the literature. Eur J Gastroenterol Hepatol. 1997;9:635–639. doi: 10.1097/00042737-199706000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Choi HS, Kim CD, Kang HS, Yoon SB, Jeen YT, Chun HJ, Um SH, Ryu HS. A Bezoar That Caused Afferent Loop Syndrome and Pancreatitis. Korean J Gastrointest Endosc. 2009;39:291–295. [Google Scholar]
- 9.Fenchel RF, Krige JE, Bornman PC. Bouveret’s syndrome complicated by acute pancreatitis. Dig Surg. 1999;16:525–527. doi: 10.1159/000018782. [DOI] [PubMed] [Google Scholar]
- 10.Chiu HH, Li JH. Gastric outlet obstruction caused by a dumbbell-shaped phytobezoar impacted in a deformed duodenal bulb. Gastrointest Endosc. 2007;65:322–323; discussion 323. doi: 10.1016/j.gie.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 11.Fuhrman MA, Felig DM, Tanchel ME. Superior mesenteric artery syndrome with obstructing duodenal bezoar. Gastrointest Endosc. 2003;57:387. doi: 10.1067/mge.2003.91. [DOI] [PubMed] [Google Scholar]
- 12.Franzen D, Gürtler T, Metzger U. [Solitary duodenal diverticulum with enterolith as a rare cause of acute abdomen] Swiss Surg. 2002;8:277–279. doi: 10.1024/1023-9332.8.6.277. [DOI] [PubMed] [Google Scholar]
- 13.Srikiran P, Bashar AM, Frida A, Shailaja B, Tariq R. Bezoar in a periampullary extraluminal duodenal diverticulum causing acute pancreatitis. Am J Gastroenterol. 2000;95:2622–2622. [Google Scholar]
- 14.Egawa N, Anjiki H, Takuma K, Kamisawa T. Juxtapapillary duodenal diverticula and pancreatobiliary disease. Dig Surg. 2010;27:105–109. doi: 10.1159/000286520. [DOI] [PubMed] [Google Scholar]
- 15.Pearl MS, Hill MC, Zeman RK. CT findings in duodenal diverticulitis. AJR Am J Roentgenol. 2006;187:W392–W395. doi: 10.2214/AJR.06.0215. [DOI] [PubMed] [Google Scholar]
- 16.Schnueriger B, Vorburger SA, Banz VM, Schoepfer AM, Candinas D. Diagnosis and management of the symptomatic duodenal diverticulum: a case series and a short review of the literature. J Gastrointest Surg. 2008;12:1571–1576. doi: 10.1007/s11605-008-0549-0. [DOI] [PubMed] [Google Scholar]


