Abstract
Rationale
Little is known about vitamin D status and its effect on asthma pathophysiology in children with severe, therapy-resistant asthma (STRA).
Objectives
Relationships between serum vitamin D, lung function, and pathology were investigated in pediatric STRA.
Methods
Serum 25-hydroxyvitamin D [25(OH)D3] was measured in 86 children (mean age, 11.7 yr): 36 with STRA, 26 with moderate asthma (MA), and 24 without asthma (control subjects). Relationships between 25(OH)D3, the asthma control test (ACT), spirometry, corticosteroid use, and exacerbations were assessed. Twenty-two of 36 children with STRA underwent fiberoptic bronchoscopy, bronchoalveolar lavage, and endobronchial biopsy with assessment of airway inflammation and remodeling.
Measurements and Main Results
25(OH)D3 levels (median [IQR]) were significantly lower in STRA (28 [22–38] nmol/L) than in MA (42.5 [29–63] nmol/L) and control subjects (56.5 [45–67] nmol/L) (P < 0.001). There was a positive relationship between 25(OH)D3 levels and percent predicted FEV1 (r = 0.4, P < 0.001) and FVC (r = 0.3, P = 0.002) in all subjects. 25(OH)D3 levels were positively associated with ACT (r = 0.6, P < 0.001), and inversely associated with exacerbations (r=−0.6, P < 0.001) and inhaled steroid dose (r=−0.39, P = 0.001) in MA and and STRA. Airway smooth muscle (ASM) mass, but not epithelial shedding or reticular basement membrane thickness, was inversely related to 25(OH)D3 levels (r=−0.6, P = 0.008). There was a positive correlation between ASM mass and bronchodilator reversibility (r = 0.6, P = 0.009) and an inverse correlation between ASM mass and ACT (r = −0.7, P < 0.001).
Conclusions
Lower vitamin D levels in children with STRA were associated with increased ASM mass and worse asthma control and lung function. The link between vitamin D, airway structure, and function suggests vitamin D supplementation may be useful in pediatric STRA.
Keywords: vitamin D, asthma, remodeling, airway smooth muscle, pediatrics
For most children with ready access to healthcare, asthma can be controlled with low doses of inhaled steroids. There remains, however, a significant number of individuals with asthma who, despite apparently appropriate treatment, remain symptomatic and at risk of exacerbations. Most assessments have suggested that this difficult-to-treat population represents 5–10% of individuals with asthma (1–3). Even though this high-risk group represents only a small portion of all individuals with asthma, they suffer the greatest morbidity and need for healthcare use.
Exposure to solar ultraviolet radiation within a wavelength band of 290–315 nm, leading to production of vitamin D in the skin, is the primary source of vitamin D for humans (4). Over the last few decades, vitamin D deficiency and insufficiency are increasingly being recognized in the general population, and have been largely attributed to dietary, lifestyle, and behavioral changes (4). The musculoskeletal consequences of vitamin D deficiency are well established; however, a number of pulmonary disorders, including asthma, have now been linked to vitamin D deficiency and insufficiency. Cross-sectional data suggest that low serum vitamin D in children with mild to moderate asthma is associated with poor asthma control, more exacerbations, reduced lung function, and increased medication use (5–7). However, little is known about vitamin D levels and their impact on disease control and airway pathology in children with severe, therapy-resistant asthma (STRA).
An increase in airway smooth muscle (ASM) mass is a key feature of airway remodeling in asthma (8–10). Importantly, increased ASM hypertrophy and hyperplasia have been demonstrated in endobronchial biopsies from children with severe asthma and are significantly related to bronchodilator responsiveness (10). However, to date, there is little evidence that any asthma therapies affect airway remodeling. In vitro studies have shown that vitamin D may influence ASM remodeling by exerting an inhibitory effect on passively sensitized ASM growth and contractility (11–13). However, the relationship between airway pathology in bronchial tissue from individuals with asthma and serum vitamin D levels has not been reported.
We hypothesized that our cohort of children with STRA would have lower serum vitamin D levels than children with moderate asthma (MA) and nonasthmatic control subjects, and that lower serum vitamin D levels would be associated with worse lung function, airway inflammation, and remodeling.
Some of the results of these studies have been previously reported in the form of abstract (14).
METHODS
Detailed methods are described in the online supplement.
Subjects
Children aged 6–16 years with STRA (n = 36) or MA (n = 26) and nonasthmatic control subjects (n = 24) were recruited prospectively (Table 1). The Royal Brompton and Harefield Hospital (London, UK) Research Ethics Committee approved the study. Informed consent was obtained from parents and age-appropriate assent from children.
TABLE 1.
DEMOGRAPHIC CHARACTERISTICS OF SUBJECTS
| STRA (n = 36) | MA (n = 26) | Control Subjects (n = 24) | P Value | |
|---|---|---|---|---|
| Age, yr | 11.5 (9.5, 14) | 12.5 (11, 13) | 10.5 (9–13) | 0.24* |
| Male | 21 (58%) | 11 (42%) | 15 (62%) | 0.17† |
| BMI, kg/m2 | 19.5 (16, 24) | 19 (16, 25) | 18 (15.7, 23) | 0.16* |
| Ethnicity | 0.056† | |||
| White | 23 (64%) | 22 (84%) | 21 (88%) | |
| Nonwhite | 13 (36%) | 4 (16%) | 3 (12%) | |
| FEV1, % predicted | 76 (63, 85) | 88 (84, 95) | 94 (90, 97) | <0.001* |
| FVC, % predicted | 88 (78, 97) | 103 (96,110) | 96 (94, 108) | <0.002* |
| FEV1/FVC ratio | 73 (68, 84) | 84 (78, 92) | 92 (89, 96) | <0.001* |
| Atopic,‡ % | 32 (88) | 20 (77) | 3 (12) | <0.001† |
| IgE,§ IU/ml | 530 (196, 645) | 717 (254, 1,377) | 12.5 (8, 51) | <0.001* |
| BDR,|| % | 15 (7, 25) | 4 (3.5, 6) (n = 4) | – | 0.065¶ |
| ACT** | 11 (8.5, 14) | 18 (17, 21) | – | <0.001¶ |
| Daily inhaled corticosteroid dose,†† μg/d | 1,600 (1,000–2,000) | 600 (500–800) | – | <0.001¶ |
| Median exacerbations in last 6 mo requiring oral steroids | 3 (2, 4) | 1 (0, 2) | – | <0.001¶ |
Definition of abbreviations: ACT = asthma control test; BDR = bronchodilator response; BMI = body mass index; MA = moderate asthma; STRA = severe therapy-resistant asthma.
Note: Decimal values were approximated to closest integer for ease of exposition.
Note: Values are given as medians (interquartile range) for continuous variables or as number (%) for binary variables.
P value calculated by Kruskal-Wallis test.
P value calculated by chi-square test.
One or more positive allergen-specific IgE responses.
Sum of specific IgE to nine allergens: cat, dog, egg, milk, peanut, grass, tree pollen, Dermatophagoides pteronyssinus, and Aspergillus fumigatus.
Rise in FEV1 after bronchodilator administration (%).
P value calculated by Mann-Whitney test.
Score out of 25.
Beclomethasone equivalent.
A detailed definition of STRA is given in the online supplement. Briefly, these were all children receiving at least 800 μg of inhaled steroids (beclomethasone equivalent) per day and additional controller medications, and had undergone detailed assessments to exclude a wrong diagnosis, asthma with important comorbidities, and difficult asthma (underlying modifiable factors identified) (15, 16). Children with MA were well controlled on a lower dose of inhaled corticosteroids (<800 μg of beclomethasone equivalent per day). Nonasthmatic control subjects comprised either children with no respiratory disease whose parents had consented for blood tests during an elective surgical procedure (n = 18) or children undergoing a clinically indicated bronchoscopy for upper airway symptoms (n = 6).
Asthma Control Test
Symptom control was assessed by childhood asthma control test (ACT) (17) (see the online supplement).
Exacerbations and Medication Use
Regular medications were recorded in MA and STRA. Acute exacerbations were defined as episodes necessitating high-dose oral steroids for at least 3 days, in the previous 6 months.
Lung Function
Spirometry was performed in accordance with American Thoracic Society guidelines (18) (see the online supplement).
Serum 25-Hydroxyvitamin D
Serum levels of 25-hydroxyvitamin D [25(OH)D3] were measured in all subjects, using a two-dimensional high-performance liquid chromatography system–tandem mass spectrometry (2D LC-MS-MS) system (19). Vitamin D deficiency was defined as serum 25(OH)D3 less than 50 nmol/L (20 ng/ml) (4, 20) and serum levels less than 75 nmol/L were defined as insufficient (4, 20) (see the online supplement).
Sputum Eosinophils and Neutrophils
Sputum induction, processing, and cell counts were performed in a subgroup of STRA (n = 22) as previously described (21) (see the online supplement).
Bronchoscopy, Bronchoalveolar Lavage, and Endobronchial Biopsy
Bronchoscopy was performed under general anesthetic (22) in children with STRA (n = 22) as part of clinical investigations (15) (see the online supplement). Details of bronchoalveolar lavage (BAL) and endobronchial biopsy processing are in the online supplement. Eosinophils and neutrophils were quantified in BAL and biopsy, and mast cells were quantified in biopsy alone. Smooth muscle proliferation was quantified by immunohistochemistry for proliferating cell nuclear antigen (PCNA). Reticular basement membrane (RBM) thickness (23), epithelial shedding (24), and airway smooth muscle (ASM) mass (10) were quantified in sections stained with hematoxylin and eosin (see the online supplement).
Statistical Analysis
Differences between three groups were assessed using one way analysis of variance (ANOVA) (normal data) or Kruskal-Wallis test (nonnormal distribution). To assess the association between serum vitamin D levels and severity of disease, linear regression was used for continuous variables and logistic regression was used for categorical variables. When a nonnormal variable remained skewed even after log transformation, this variable was dichotomized and used in the logistic regression. For both types of regression analyses, two models (an unadjusted model and a multivariable model) were constructed. Follow-up tests performed after ANOVA were determined a priori unless specified otherwise. Correlations were assessed by Pearson correlation (normal data) or Spearman’s rank correlation (skewed data). Statistical significance was reported at P < 0.05. Stata version 10.1 (StataCorp, College Station, TX) and GraphPad Prism version 5.02 (GraphPad Software, San Diego, CA) were used (see the online supplement).
RESULTS
Subjects
Demographic data of the children studied are presented in Table 1. There were no significant differences between children with MA, children with STRA, and nonasthmatic control subjects in age, ethnicity, sex distribution, or body mass index.
Serum 25(OH)D3 Levels in Subjects with Asthma and Control Subjects
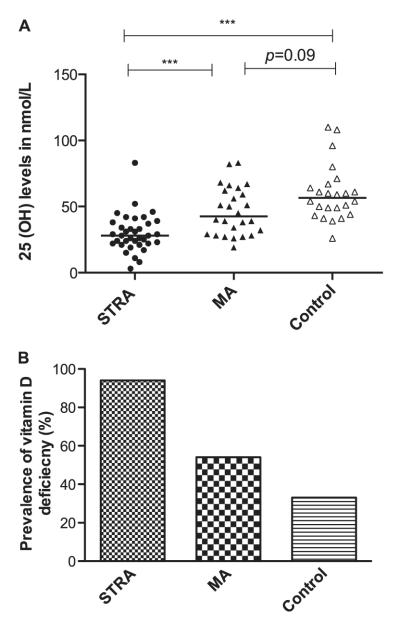
Serum 25(OH)D3 levels (median [IQR] nmol/L) were significantly lower in children with STRA (28 [22–38] nmol/L) than in those with MA (42.5 [29–63] nmol/L) and nonasthmatic control subjects (56.5 [45–67] nmol/L) (P < 0.001 for both) (Figure 1A). The prevalence of vitamin D deficiency [25(OH)D3 level < 50 nmol/L] was 94, 54, and 33% in STRA, MA, and control subjects, respectively (P < 0.001) (Figure 1B) and 97% of children with STRA, 92% of children with MA, and 83% of control subjects had insufficient serum 25(OH)D3 levels [25(OH)D3 level < 75 nmol/L].
Figure 1.
(A) Serum vitamin D levels in subjects with severe therapy-resistant asthma (STRA), subjects with moderate asthma (MA), and control subjects. Serum 25-hydroxyvitamin D levels were lower in subjects with STRA and subjects with MA than in control subjects (Kruskal-Wallis test, P < 0.0001). Horizontal bars represent median values. The Mann-Whitney U test, followed by a Bonferroni correction, was used to compare differences between groups. (B) A higher prevalence of vitamin D deficiency (serum 25-hydroxyvitamin D levels less than 50 nmol/L) is shown in subjects with STRA compared with subjects with MA and control subjects (P < 0.001, calculated by chi-square test). ***P < 0.001.
There was no significant impact of season of sample collection on serum vitamin D level in the three groups (see Figure E1 in the online supplement).
Vitamin D and Atopic Status
Serum 25(OH)D3 was inversely related to serum total IgE (r = −0.3, P = 0.01), specific IgE to cat (r = −0.27, P = 0.01), dog (r = −0.29, P = 0.01), tree pollen (r = −0.28, P = 0.02), Dermatophagoides pteronyssinus (r = −0.3, P = 0.01), and Aspergillus fumigatus (r = −0.36, P = 0.009) (Table E1). In a post hoc analysis, there was a significant inverse relationship between serum 25(OH)D3 levels and sum of specific IgE to aeroallergen (r = −0.28, P = 0.009) but not to sum of specific IgE to food allergens.
Lung Function, Bronchodilator Reversibility, and Vitamin D
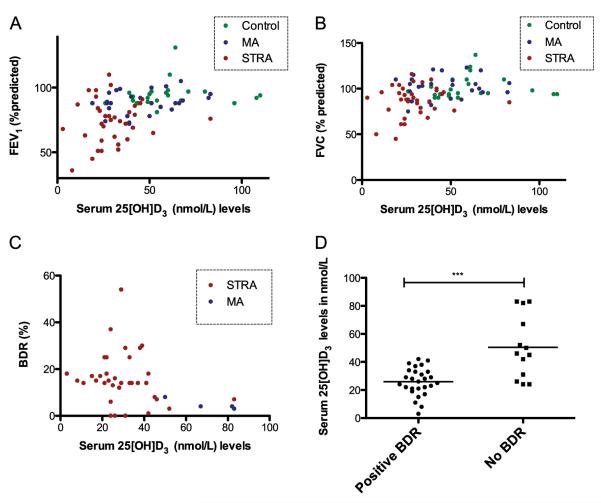
There was a positive correlation between serum 25(OH)D3 levels and percent predicted FEV1 (r = 0.43, P < 0.001) (Figure 2A) and FVC (r = 0.32, P = 0.002) (Figure 2B). Serum 25 (OH)D3 significantly and inversely associated with percent BDR (r = −0.4, P = 0.003) and, in a post hoc analysis, BDR (FEV1 improvement of at least 12%) (Figures 2C and 2D).
Figure 2.
Positive association between serum vitamin D levels and (A) percent predicted FEV1 (R = 0.43, P < 0.001) and (B) FVC (R = 0.32, P = 0.002). Lower serum vitamin D levels were associated with (C) higher bronchodilator response (BDR) (r = −0.40, P = 0.003) and (D) positive BDR (FEV1 improvement of at least 12%) (P < 0.001). BDR (%) = percentage increase in FEV1 after inhalation of 1,000 μg of salbutamol. Correlation was determined by Spearman rank correlation coefficient. The Mann-Whitney U test was used to compare differences between groups. ***P < 0.001. MA = moderate asthma; STRA = severe therapy-resistant asthma.
Asthma Control, Exacerbations, and Vitamin D Levels
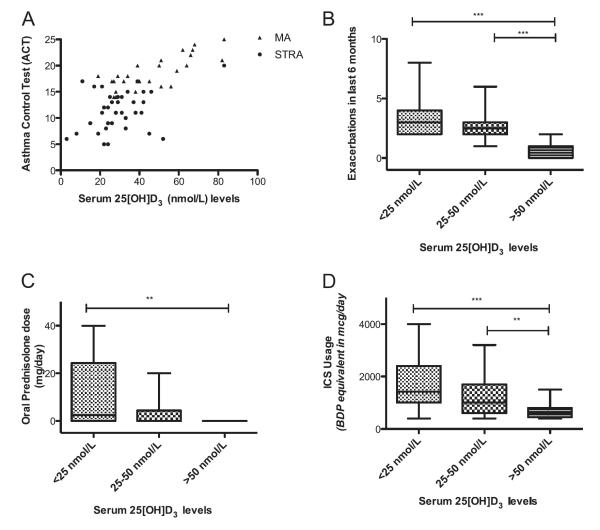
A positive relationship was found between 25(OH)D3 level and ACT (r = 0.6, P < 0.001) (Figure 3A), whereby higher serum 25(OH)D3 was associated with better asthma control. Lower serum 25(OH)D3 levels were associated with increased acute asthma exacerbations in the previous 6 months (r = −0.6, P < 0.001) (Figure 3B).
Figure 3.
Positive association between serum vitamin D level and asthma control test (ACT) (r = 0.6, P < 0.001). (A) Children with higher serum vitamin D levels had fewer asthma-related symptoms. Correlation was determined by Spearman rank correlation coefficient. (B) Children with lower serum vitamin D levels had more acute exacerbations in the last 6 months. Lower serum vitamin D levels were associated with increased (B) oral and (C) inhaled corticosteroid (ICS) use. The Kruskal-Wallis test and Mann-Whitney U test, followed by a Bonferroni correction, were used to compare differences between groups. **P < 0.01; ***P < 0.001. BDP = beclomethasone dipropionate; MA = moderate asthma; STRA = severe therapy-resistant asthma.
Medication Dose and Vitamin D Levels
Of the various therapies received by children with MA and STRA, the use of daily maintenance oral steroids (P < 0.001), oral theophyllines (P = 0.02), and leukotriene receptor antagonists (P = 0.005) was significantly associated with lower 25(OH)D3 levels, but there was no association between antireflux therapy and serum 25(OH)D3 level (Table 2). Moreover, the daily doses of inhaled corticosteroid (r = −0.39, P = 0.001) and oral maintenance (r = −0.43, P < 0.001) were inversely related to serum 25(OH)D3 levels (Figures 3C and 3D). There was no association between use of anti-reflux medications and serum 25(OH)D3 levels.
TABLE 2.
SERUM VITAMIN D LEVELS AND MEDICATION USE IN SUBJECTS WITH MODERATE ASTHMA AND SUBJECTS WITH SEVERE THERAPY-RESISTANT ASTHMA
| Medication(s) Used | Median 25-Hydroxyvitamin D Levels (IQR) | P Value* |
|---|---|---|
| Oral CS | Oral CS: 28 (21–33) | <0.001 |
| No oral CS: 45 (21–61) | ||
| LTRA | LTRA: 33.7 (27–39) | 0.005 |
| No LTRA: 47 (40–53) | ||
| Theophylline | Theophylline: 31 (21.5–39.5) | 0.02 |
| No Theophylline: 38.5 (27–59) | ||
| Antireflux medications | Yes: 39 (33–44) | 0.60 |
| No: 44.2 (32–57) |
Definition of abbreviations: CS = corticosteroid; IQR = interquartile range; LTRA = leukotriene receptor antagonist.
The Wilcoxon test was used for median value differences for categorical variables.
Serum Vitamin D Levels and Asthma Pathology
Airway inflammation
Table E3 (see the online supplement) outlines the relationship between serum 25(OH)D3 levels and measures of airway inflammation in children with STRA. There was no significant correlation between serum 25(OH)D3 levels and eosinophils or neutrophils in induced sputum (n = 18), BAL (n = 22), or endobronchial biopsy (n = 19). There was also no association between tissue mast cells and serum 25(OH)D3.
Airway remodeling
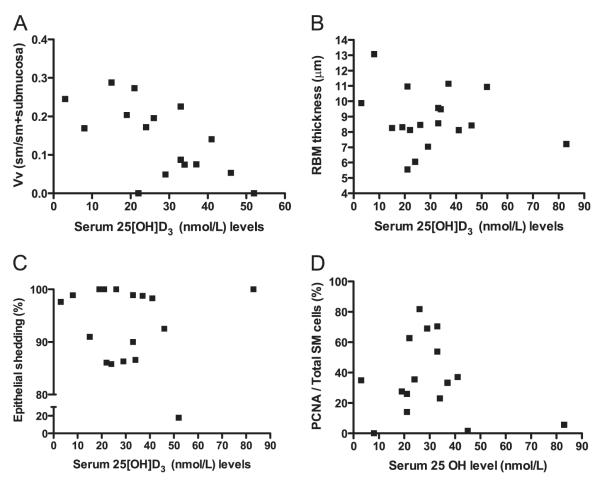
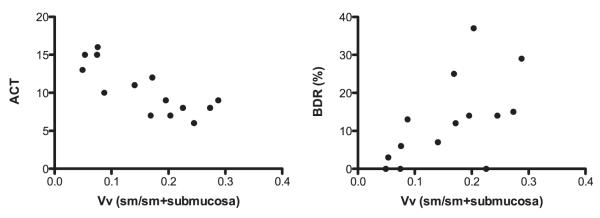
Endobronchial biopsies were of sufficient quality to quantify airway remodeling in 19 of 21 children with STRA. Median volume fraction of smooth muscle was 0.16 (range, 0.07–0.20). There was a significant negative correlation between serum 25(OH)D3 and volume fraction of ASM (r = −0.63, P = 0.007) (Figure 4A). Median RBM thickness in children with STRA was 8.4 (range, 7.9–9.7) μm. There was no relationship between serum 25(OH)D3 and RBM thickness (r = −0.12, P = 0.62) (Figure 4B). Median percent epithelial shedding in the biopsies was 98% (range, 87.4–99.7%). There was no significant relationship between epithelial shedding and serum 25(OH)D3 (r = −0.09, P 0.69) (Figure 4C). Smooth muscle proliferation was assessed by quantifying the proportion of PCNA-positive smooth muscle cells (Figure E2). Median myocyte proliferation was 35.3% (range, 25.3–64.3%). There was no relationship between serum 25(OH)D3 and percentage of smooth muscle cells positive for PCNA (r = 0.007, P = 0.07) (Figure 4D). There was a significant positive correlation between volume fraction of ASM and BDR (r = 0.6, P = 0.009) (Figure 5A) and a negative correlation between volume fraction of ASM and ACT (r = −0.7, P < 0.001) (Figure 5B).
Figure 4.
Relationships between serum 25-hydroxyvitamin D [25(OH)D3] levels and airway remodeling. (A) There was a significant negative correlation between serum 25(OH)D3 and volume fraction of airway smooth muscle (ASM) (r = −0.6, P = 0.008). There was no significant correlation between serum 25(OH)D3 and (B) reticular basement membrane (RBM) thickness or (C) epithelial shedding. (D) There was no relationship between serum 25(OH)D3 and proliferating cell nuclear antigen (PCNA)–positive smooth muscle cells. Correlation was determined by the Spearman rank correlation coefficient. Vv (sm/sm + submucosa) = volume fraction of smooth muscle indexed to volume of submucosal tissue PCNA SM cells per total SM cells; (%) = positively stained smooth muscle nuclei were counted in every biopsy at × 400 magnification and divided by the total number of smooth muscle nuclei and expressed as a percentage.
Figure 5.
Relationship between volume fraction of airway smooth muscle (ASM) in endobronchial biopsies from children with severe therapy-resistant asthma (STRA), and asthma control test (ACT) and bronchodilator response (BDR). (A) A significant negative relationship was found between volume fraction of ASM and ACT (r = −0.7, P < 0.001). (B) A significant positive relationship was present between BDR and volume fraction of ASM (r = 0.6, P = 0.009). BDR (%) = percentage increase in FEV1 after inhalation of 1,000 μg of salbutamol; Vv (sm/sm + submucosa) = volume fraction of smooth muscle indexed to volume of submucosa tissue.
DISCUSSION
We have shown for the first time that children with STRA have significantly lower serum 25(OH)D3 levels than do children with MA. Lower serum 25(OH)D3 levels were associated with worse lung function, poor asthma control, and more steroid use in MA and STRA. Within STRA, low 25(OH)D3 levels were associated with increased ASM mass, but not with other parameters of airway remodeling, nor with airway inflammation despite an association with aeroallergen sensitization.
Importantly, the children in this study with STRA had been carefully assessed such that their basic management had been optimized, and any “difficult asthmatics” (whose asthma was uncontrolled because of modifiable factors such as poor adherence to treatment) had been excluded. The detailed multidisciplinary assessment to ensure as far as possible that basic management is correct is one of the novel features of this study.
In this group with STRA, a significant negative association was present between volume fraction of ASM and 25(OH)D3 levels. Of note, however, there was no association between RBM thickness or epithelial shedding and vitamin D. Although a negative relationship between ASM mass and lung function has been reported in pediatric cases with difficult asthma (10), this is the first demonstration of an association between low serum 25(OH)D3, poor lung function and asthma control, increased BDR and ASM mass. It is therefore plausible that the link between ASM mass and lung function in severe asthma may partly be explained by low 25(OH)D3 levels. The association between increased ASM mass and low 25(OH)D3 is supported by in vitro studies, which have shown that vitamin D inhibits smooth muscle proliferation (11–13). Vitamin D blocked smooth muscle proliferation in a concentration-dependent manner in human smooth muscle cells sensitized with asthmatic serum (12), and it inhibited ASM cell proliferation by preventing progression of the cell cycle, not by inducing apoptosis (13). Furthermore, vitamin D inhibits cell growth in muscle cell cultures (13). Moreover, vitamin D increases glucocorticoid bioavailability in bronchial smooth muscle cells (25). In contrast to the published in vitro studies, there was no relationship between serum 25 (OH)D3 levels and ASM proliferation assessed by myocyte PCNA staining in our subjects. However, all in vitro work has been done in adult ASM, and mechanisms may be different in children. For example, ASM apoptosis may be reduced. Importantly, ASM mass is still increasing as part of normal growth and development in children (26), and therefore the influence of superimposed pathological abnormalities is likely to be different from those in adults. Further work is needed to determine the mechanistic effects of 25(OH)D3 on pediatric ASM.
The cross-sectional nature of the biopsy data prevent us from being certain whether the relationship between increased ASM mass and vitamin D is a result of severe asthma, or whether the increased ASM mass may have been present before the development of disease and caused the asthma. It is possible that a developmental structural defect of the airway wall, such as ASM hypertrophy in children with STRA, results from vitamin D deficiency in utero, and that may have led to asthma in the first place. It may be that exaggerated ASM hypertrophy is a cause of their asthma, rather than a consequence, as a result of in utero (27, 28) and postnatal vitamin D deficiency. The effects of vitamin D deficiency in utero (27, 28) could be in addition to, or independent of, airway remodeling. This is especially important as a randomized controlled trial of vitamin D therapy in children with STRA could potentially reverse the ASM hypertrophy and change the course or natural history of these patients’ asthma. However, if vitamin D induces a smooth muscle developmental defect in utero, then it may prove more challenging to reverse. Importantly, it should be noted that in a previous study of infants with severe wheeze at a median age of 1 year (29), there was no increase in ASM mass on biopsy (30). Vitamin D levels were not measured in that study, but the findings mitigate against, although do not exclude, the developmental hypothesis.
Interestingly, we did not find an association between any of the inflammatory cells quantified (eosinophils, neutrophils, or mast cells) and serum 25(OH)D3 levels. This remained true for both luminal inflammation (BAL and sputum) and tissue inflammation (endobronchial biopsy). It is possible that the substantial antiinflammatory treatment prescribed for these children may have masked a relationship between vitamin D and airway inflammation in STRA. Having established a link between serum 25(OH)D3 levels and lung function and asthma control, and importantly, having now seen a novel link between serum vitamin D levels and airway smooth muscle alone, we suggest that vitamin D supplementation in children with STRA and low 25(OH)D3 levels may be a novel therapeutic target directed against some aspects of remodeling. Of note, there are currently no treatments that inhibit or prevent airway remodeling.
Even after adjusting for confounding factors including age, sex, body mass index, FEV1, and ethnicity, a significant relationship between serum vitamin D levels and asthma control, exacerbations, inhaled and oral steroid use, and positive BDR remained (Table 3). Some of our findings concur with reports in children and adults with much less severe asthma. These include the associations found between low 25(OH)D3 levels and asthma control and exacerbations (5–7), lower lung function (6, 31–33), increased reversibility to bronchodilator (5, 33), and greater antiinflammatory medication (inhaled corticosteroid, oral steroid, and leukotriene receptor antagonist) use (5, 31).
TABLE 3.
SERUM VITAMIN D LEVELS AND DISEASE SEVERITY IN SUBJECTS WITH MODERATE ASTHMA AND SUBJECTS WITH SEVERE THERAPY-RESISTANT ASTHMA
| A. Linear Regression Used for Continuous Measures of Disease Severity | ||
|---|---|---|
| Beta Coefficient* [95% Confidence Interval] (P Value) |
||
| Outcome | Unadjusted | Multivariate Model† |
| ACT | 0.18 [0.13 to 0.24] (<0.001) | 0.15 [0.09 to 0.20] (<0.001) |
| Daily inhaled corticosteroid dose, μg/d | −0.006 [−0.01 to −0.003] (0.001) | −0.004 [−0.007 to 0.0001] (0.05) |
|
| ||
|
B. Logistic Regression Used for Categorical Variables | ||
| Odds Ratio‡ [95% Confidence Interval] (P Value) |
||
| Outcome | Unadjusted | Multivariate Model§ |
|
| ||
| Positive BDR|| | 0,91 [0.84 to 0.98] (0.01) | 0.86 [0.76 to 0.97] (0.01) |
| Oral CS¶ | 0.92 [0.88 to 0.98] (0.004) | 0.92 [0.86 to 0.98] (0.006) |
| Exacerbation** | 0.87 [0.80 to 0.95] (0.001) | 0.79 [0.64 to 0.97] (0.02) |
Definition of abbreviations: ACT = asthma control test; BDR = bronchodilator response; CS = corticosteroid.
Note: Decimal values were approximated to closed integer for ease of exposition.
Beta coefficient is for each nmol/L increase in serum vitamin D levels.
Multivariate model adjusted for age, sex, BMI, FEV1, and ethnicity.
Odds ratio are for each 1-nmol/L increase in serum vitamin D levels.
Multivariate model adjusted for age, sex, BMI, FEV1, and ethnicity.
Positive bronchodilator response (BDR) = FEV1 improvement of at least 12% after inhalation of 1,000 μg of salbutamol.
CS = corticosteroid; use of maintenance daily oral corticosteroids.
Exacerbation = any acute exacerbations in the last 6 months requiring oral steroids.
Although total serum IgE levels and specific IgE to cat, dog, pollen, Dermatophagoides pteronyssinus, and Aspergillus fumigatus were inversely related to 25(OH)D3 levels, there was no relationship between 25(OH)D3 and serum, BAL, or biopsy eosinophils. Some (5, 31), but not all (6), investigators have found correlations between lower 25(OH)D3 levels and markers of allergy in childhood asthma. A U-shaped association between low and high 25(OH)D3 levels and serum total IgE levels has been demonstrated (34). An association between 25(OH)D3 deficiency and increased sensitization to 11 of 17 environmental and food allergens in children (n = 3,136), but not in adults (n = 3,454), in the National Health and Nutrition Examination Survey has also been shown (35). Our results are in agreement with this report (35) that children with low serum 25(OH)D3 levels are more likely to have allergic sensitization. Interestingly, we have only found an association between sensitization to aeroallergens and 25(OH)D3 levels, but not food allergens.
In terms of ethnicity, because numbers were small we allocated the children into “white” and “nonwhite” groups, but did not attempt to classify ethnicity in more detail. There were more nonwhite children in the STRA group (36%) compared with the MA group (16%) and control subjects (12%) (P = 0.056). Children with nonwhite skin had lower serum vitamin D levels (Figure E3A), as reported by others (4). Moreover, nonwhite children had more severe asthma compared with white children (Figure E4). However, the ANOVA results have shown that there is no interaction between ethnicity and disease severity for levels of vitamin D (P = 0.20). This could be due to the small number of subjects in the study. Importantly, race is a proxy for skin color, and although skin color is a proxy for low vitamin D levels, our conclusions relate to the relationship between low serum vitamin D levels, whether driven by diet, sunlight, ethnicity or another factor, and asthma severity and pathology.
The cross-sectional design of this study made it impossible to determine whether low 25(OH)D3 levels result in severe asthma in children, or whether children with severe asthma have low 25(OH)D3 levels because, for example, they are unable to go outside and exercise normally. Also, because of the ethics of performing bronchoscopy for research in children, we could not perform invasive endobronchial biopsies in control subjects and subjects with MA, and this is a potential source of information bias. One of the other limitations of this study was that BDR and ACT were not performed in normal control subjects. Although the potential to correlate ASM mass with the results of airway challenge would be of interest, given the subjects’ disease severity, this was thought to be both unsafe and unethical.
It is challenging to propound a unifying hypothesis to account for ASM changes being the sole manifestation of vitamin D deficiency. In terms of remodeling, we speculate that this may be due to a heightened sensitivity of ASM to vitamin D deficiency as compared with other airway wall components. The lack of any effect on inflammation may in part be due to high-dose inhaled and oral steroid therapy. Although vitamin D deficiency causes a degree of steroid insensitivity, the high doses used in the children with STRA may have overcome this. However, we acknowledge that these ideas remain speculative at the present time and the determination of the exact mechanism between low 25(OH)D3 and airway remodeling in STRA will require intervention studies.
In summary, in this cross-sectional study, children with genuine severe asthma had significantly lower serum vitamin D levels than children with MA and control subjects. Lower serum vitamin D levels were associated with worse parameters of asthma severity, and we propose that a contributory mechanism may be via an effect on ASM. As numbers are small, and represent a selected population, the conclusions drawn must be tentative. However, these findings suggest that detecting and treating low serum vitamin D levels in children with STRA may aid in the treatment of specific structural airway changes.
Supplementary Material
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Children with mild to moderate asthma are more likely to have vitamin D insufficiency. Epidemiologic data suggest that low serum vitamin D in children with asthma is associated with poor asthma control, reduced lung function, and increased medication use. However, little is known about the relationship between serum vitamin D levels and pathophysiology in children with severe therapy-resistant asthma (STRA).
What This Study Adds to the Field
Children with STRA have lower serum vitamin D levels compared with children with moderate asthma and control subjects. Reduced vitamin D levels in STRA are associated with lower lung function, poor asthma control, increased medication use, and asthma exacerbations. The serum vitamin D level is inversely associated with airway smooth muscle mass.
Acknowledgment
The authors gratefully acknowledge the following people for their invaluable help: Dr. M. Rosenthal, Dr. I. Balfour-Lynn, Dr. C. Hogg, and Dr. J. Davies for performing the bronchoscopies; J. Donovan and Sophy Smith for helping with the vitamin D assay; and the pediatric cardiology team at RBHT, especially Dr. J. Till, for helping to recruit control subjects. The authors are grateful to Timothy Oates for performing the special stains on endobronchial biopsies. The authors acknowledge support from the National Institute for Health Research (NIHR) Respiratory Biomedical Research unit at Royal Brompton Hospital, London.
Supported by a British Medical Association James Trust Fellowship (A.G.) and a Wellcome Trust Clinical Intermediate Fellowship (S.S.). C.H. acknowledges financial and technical support from the Department of Health via an NIHR comprehensive Biomedical Research Centre award to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London.
Footnotes
Author Disclosures are available with the text of this article at www.atsjournals.org.
This article has an online supplement, which is available from this issue’s table of contents at www.atsjournals.org
References
- 1.Bush A, Zar HJ. WHO universal definition of severe asthma. Curr Opin Allergy Clin Immunol. 2011;11:115–121. doi: 10.1097/ACI.0b013e32834487ae. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 3.Chung KF, Godard P, Adelroth E, Ayres J, Barnes N, Barnes P, Bel E, Burney P, Chanez P, Connett G, et al. ERS Task Force on Difficult/Therapy-resistant Asthma. European Respiratory Society Difficult/therapy-resistant asthma: the need for an integrated approach to define clinical phenotypes, evaluate risk factors, understand pathophysiology and find novel therapies. Eur Respir J. 1999;13:1198–1208. doi: 10.1034/j.1399-3003.1999.13e43.x. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 5.Brehm JM, Celedon JC, Soto-Quiros ME, Avila L, Hunninghake GM, Forno E, Laskey D, Sylvia JS, Hollis BW, Weiss ST, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179:765–771. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, Weiss ST, Litonjua AA. Serum vitamin D levels and severe asthma exacerbations in the childhood asthma management program study. J Allergy Clin Immunol. 2010;126:52–58. doi: 10.1016/j.jaci.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinellato I, Piazza M, Sandri M, Peroni D, Piacentini G, Boner AL. Vitamin D serum levels and markers of asthma control in Italian children. J Pediatr. 2011;158:437–441. doi: 10.1016/j.jpeds.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 8.Woodruff PG, Dolganov GM, Ferrando RE, Donnelly S, Hays SR, Solberg OD, Carter R, Wong HH, Cadbury PS, Fahy JV. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med. 2004;169:1001–1006. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- 9.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–1368. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 10.Regamey N, Ochs M, Hilliard TN, Muhlfeld C, Cornish N, Fleming L, Saglani S, Alton EW, Bush A, Jeffery PK, et al. Increased airway smooth muscle mass in children with asthma, cystic fibrosis, and non–cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2008;177:837–843. doi: 10.1164/rccm.200707-977OC. [DOI] [PubMed] [Google Scholar]
- 11.Damera G, Fogle HW, Lim P, Goncharova EA, Zhao H, Banerjee A, Tliba O, Krymskaya VP, Panettieri RA., Jr Vitamin D inhibits growth of human airway smooth muscle cells through growth factor–induced phosphorylation of retinoblastoma protein and checkpoint kinase 1. Br J Pharmacol. 2009;158:1429–1441. doi: 10.1111/j.1476-5381.2009.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song Y, Qi H, Wu C. Effect of 1,25-(OH)2D3 (a vitamin D analogue) on passively sensitized human airway smooth muscle cells. Respirology. 2007;12:486–494. doi: 10.1111/j.1440-1843.2007.01099.x. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee A, Damera G, Bhandare R, Gu S, Lopez-Boado Y, Panettieri R, Jr, Tliba O. Vitamin D and glucocorticoids differentially modulate chemokine expression in human airway smooth muscle cells. Br J Pharmacol. 2008;155:84–92. doi: 10.1038/bjp.2008.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta A, Bush A, Richards D, Hawrylowicz C, Saglani S. Serum vitamin D levels and severe therapy resistant asthma in children [abstract] Arch Dis Child. 2011;96:A13. [Google Scholar]
- 15.Bush A, Saglani S. Management of severe asthma in children. Lancet. 2010;376:814–825. doi: 10.1016/S0140-6736(10)61054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bracken M, Fleming L, Hall P, Van Stiphout N, Bossley C, Biggart E, Wilson NM, Bush A. The importance of nurse-led home visits in the assessment of children with problematic asthma. Arch Dis Child. 2009;94:780–784. doi: 10.1136/adc.2008.152140. [DOI] [PubMed] [Google Scholar]
- 17.Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, Rosenzweig JC, Manjunath R. Development and cross-sectional validation of the childhood asthma control test. J Allergy Clin Immunol. 2007;119:817–825. doi: 10.1016/j.jaci.2006.12.662. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 19.Stepman HC, Vanderroost A, Van Uytfanghe K, Thienpont LM. Candidate reference measurement procedures for serum 25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 by using isotope-dilution liquid chromatography–tandem mass spectrometry. Clin Chem. 2011;57:441–448. doi: 10.1373/clinchem.2010.152553. [DOI] [PubMed] [Google Scholar]
- 20.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 21.Lex C, Ferreira F, Zacharasiewicz A, Nicholson AG, Haslam PL, Wilson NM, Hansel TT, Payne DN, Bush A. Airway eosinophilia in children with severe asthma: predictive values of noninvasive tests. Am J Respir Crit Care Med. 2006;174:1286–1291. doi: 10.1164/rccm.200603-352OC. [DOI] [PubMed] [Google Scholar]
- 22.Payne D, McKenzie SA, Stacey S, Misra D, Haxby E, Bush A. Safety and ethics of bronchoscopy and endobronchial biopsy in difficult asthma. Arch Dis Child. 2001;84:423–426. doi: 10.1136/adc.84.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan P, Stephens D, Ansari T, Costello J, Jeffery P. Variation in the measurements of basement membrane thickness and inflammatory cell number in bronchial biopsies. Eur Respir J. 1998;12:811–815. doi: 10.1183/09031936.98.12040811. [DOI] [PubMed] [Google Scholar]
- 24.Barbato A, Turato G, Baraldo S, Bazzan E, Calabrese F, Panizzolo C, Zanin ME, Zuin R, Maestrelli P, Fabbri LM, et al. Epithelial damage and angiogenesis in the airways of children with asthma. Am J Respir Crit Care Med. 2006;174:975–981. doi: 10.1164/rccm.200602-189OC. [DOI] [PubMed] [Google Scholar]
- 25.Clifford RL, Knox AJ. Vitamin D—a new treatment for airway remodelling in asthma? Br J Pharmacol. 2009;158:1426–1428. doi: 10.1111/j.1476-5381.2009.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hislop AA, Haworth SG. Airway size and structure in the normal fetal and infant lung and the effect of premature delivery and artificial ventilation. Am Rev Respir Dis. 1989;140:1717–1726. doi: 10.1164/ajrccm/140.6.1717. [DOI] [PubMed] [Google Scholar]
- 27.Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med. 2011;183:1336–1343. doi: 10.1164/rccm.201010-1596OC. [DOI] [PubMed] [Google Scholar]
- 28.Weiss ST, Litonjua AA. The in utero effects of maternal vitamin D deficiency: how it results in asthma and other chronic diseases. Am J Respir Crit Care Med. 2011;183:1286–1287. doi: 10.1164/rccm.201101-0160ED. [DOI] [PubMed] [Google Scholar]
- 29.Saglani S, Malmstrom K, Pelkonen AS, Malmberg LP, Lindahl H, Kajosaari M, Turpeinen M, Rogers AV, Payne DN, Bush A, et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171:722–727. doi: 10.1164/rccm.200410-1404OC. [DOI] [PubMed] [Google Scholar]
- 30.O’Reilly ROT, Zhu J, Jeffery PK, Bush A, Saglani S. Increased reticular basement membrane thickness but not airway smooth muscle in endobronchial biopsies of severe preschool wheezers. Thorax. 2010;65:A2. [Google Scholar]
- 31.Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125:995–1000. doi: 10.1016/j.jaci.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the third National Health and Nutrition Examination Survey. Chest. 2005;128:3792–3798. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 33.Sutherland ER, Goleva E, Jackson LP, Stevens AD, Leung DY. Vitamin D levels, lung function, and steroid response in adult asthma. Am J Respir Crit Care Med. 2010;181:699–704. doi: 10.1164/rccm.200911-1710OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hypponen E, Berry DJ, Wjst M, Power C. Serum 25-hydroxyvitamin D and IgE—a significant but nonlinear relationship. Allergy. 2009;64:613–620. doi: 10.1111/j.1398-9995.2008.01865.x. [DOI] [PubMed] [Google Scholar]
- 35.Sharief S, Jariwala S, Kumar J, Muntner P, Melamed ML. Vitamin D levels and food and environmental allergies in the United States: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2011;127:1195–1202. doi: 10.1016/j.jaci.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.