Abstract
Tumor-specific immunosuppression is frequently observed in tumor-bearing hosts. Exosomes are nano-sized, endosomal-derived membrane vesicles secreted by most tumor and hematopoietic cells and have been shown to actively participate in immune regulation. We previously demonstrated that antigen-specific immunosuppressive exosomes could be isolated from the blood plasma of antigen-immunized mice. Here we demonstrate that plasma-derived exosomes isolated from mice bearing OVA-expressing tumors were able to suppress OVA-specific immune response in a mouse delayed-type hypersensitivity model. Enrichment of tumor-derived exosomes in the plasma of mice bearing subcutaneous melanoma was not detected using an exosome-tagging approach. Instead, depletion of MHC Class II+ vesicles from plasma-derived exosomes or using plasma-derived exosomes isolated from MHC Class II deficient mice resulted in significant abrogation of the suppressive effect. These results demonstrate that circulating host-derived, MHC Class II+ exosomes in tumor-bearing hosts are able to suppress the immune response specific to tumor antigens.
Introduction
Exosomes are 30–100 nm membrane vesicles formed in the late endocytic compartment and secreted by a large variety of cells, including tumor cells and most hematopoietic cells [1]. Exosomes are also present in various biological fluids such as blood plasma/serum [2], malignant effusions [3], bronchoalveolar fluid [4] and breast milk [5]. In certain cancer patients, exosomes were found enriched in the peripheral circulation and carry tumor-specific markers [6].
Blood-borne exosomes with immunosuppressive or tolerogenic effects have been observed in different animal models. Plasma- or serum-derived exosomes isolated from antigen-fed animals were found to suppress Th1-dominated delayed-type hypersensitivity (DTH) as well as Th2-type allergic responses [7–9], and plasma-derived exosomes isolated from antigen-immunized mice were shown to induce antigen-specific DTH suppression [10]. In cancer patients, serum-derived exosomes were reported to affect T cell activity and potentially promote tumor progression [11–13]. However, it is still unclear whether circulating exosomes in tumor-bearing hosts play a role in tolerizing against tumor antigens and suppressing tumor-specific immune responses. Also, because of the heterogeneous origin of these exosomes it is undetermined what type(s) of vesicles is actively involved in immune regulation. In this study, we demonstrate in a mouse footpad DTH model that exosomes derived from the blood plasma of tumor-bearing mice are able to confer antigen-specific immunosuppression, and the suppressive effect is conferred predominantly by host-derived, MHC Class II+ exosomes.
Results
Plasma-derived exosomes from mice bearing OVA-expressing tumors suppress OVA-specific DTH responses
We previously demonstrated that exosome-like vesicles isolated from the blood plasma of mice immunized with a soluble antigen can suppress antigen-specific inflammatory response [10]. To investigate whether a similar immunosuppressive effect is conferred by plasma-derived exosomes isolated from tumor-bearing hosts, tumor-bearing mice were generated by subcutaneous inoculation of different tumor cells, including the OVA-expressing MO5 and EG7 cells and their respective OVA-negative parental cells B16 and EL4. Blood was collected from tumor-bearing mice 3 weeks post-inoculation (tumor diameter measures between 10–20 mm) as well as from naïve mice at the same age. Exosomes were isolated from the blood plasma (Figure 1A). Routinely, around 40–60 µg of exosomes were obtained from 1 ml of plasma with no significant difference in the protein amount between tumor-bearing mice and naïve mice.
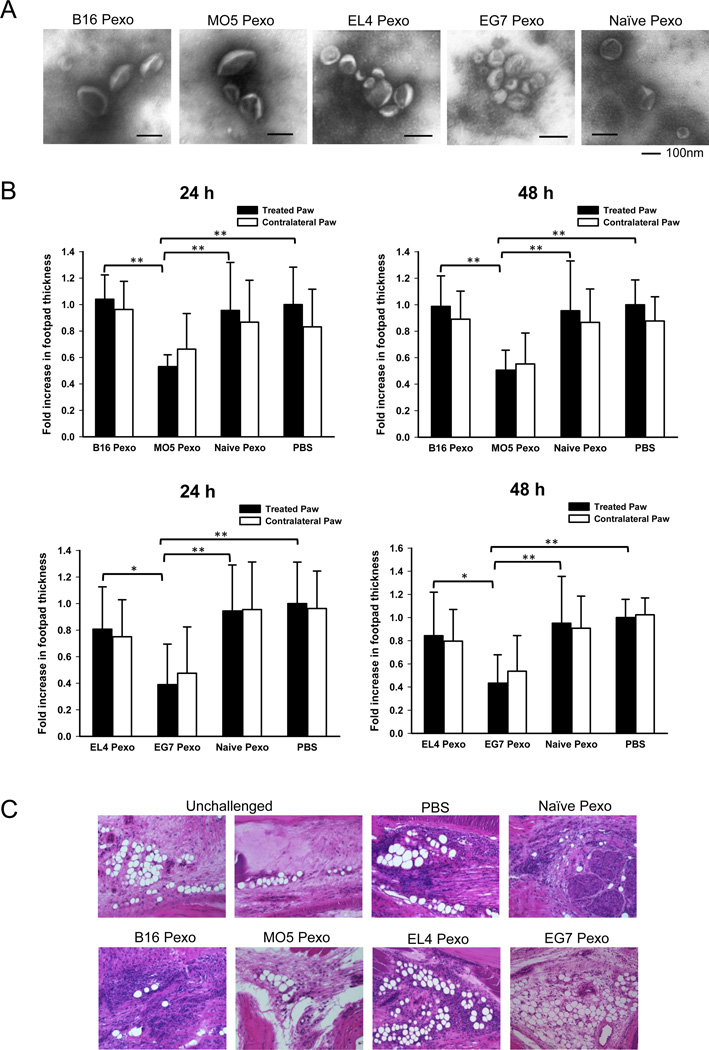
Figure 1. Exosomes isolated from the blood plasma of mice bearing OVA-expressing tumor suppress the OVA-specific DTH response after local administration.
(A) Mice were inoculated with B16, MO5, EL4 or EG7 tumor cells and blood was collected 3 weeks post-inoculation. Exosome-like vesicles isolated from the blood plasma were examined by electron microscopy. (B) Mice pre-sensitized with OVA antigen were injected with 10 µg of plasma-derived exosomes (Pexo) into the right hind paw and challenged with 30 µg of OVA at both hind paws. Paw swelling was measured 24 and 48 h post-challenge. The mean increase of footpad thickness of the treated paws (right paws) in PBS group at each time point was set to 1, and the increases of footpad thickness in the other groups were normalized as fold increase. Data represent the pooled results of two independent experiments and are the means ± SD with 9–11 mice per group. Significance at: **, P<0.01; *, P<0.05. (C) H&E staining of the footpad tissue at 48 h post-challenge. Unchallenged footpad tissue was included as a negative control. Magnification: 20×.
To examine the ability of plasma-derived exosomes to regulate the immune response to the tumor-specific antigen OVA, a mouse footpad DTH model was used. Mice pre-sensitized with OVA were injected with 10 µg of plasma-derived exosomes into the right hind paw and both hind paws were challenged with OVA. The magnitude of DTH response was determined by measuring footpad swelling at 24 and 48 h post-challenge. Treatment with plasma-derived exosomes (Pexo) from MO5 or EG7 tumor-bearing mice significantly reduced paw swelling, whereas treatment with Pexo from B16 or EL4 tumor-bearing mice or naïve mice showed minimal effect (Figure 1B). Comparable reductions of swelling in the untreated, contralateral paws were also observed in mice treated with MO5 or EG7 Pexo (Figure 1B). In addition, a reduction in the mononuclear cell infiltration was observed in the footpad tissue of mice that received EG7 or MO5 Pexo 48 h post-challenge (Figure 1C). These results demonstrate that local administration of plasma-derived exosomes from mice bearing OVA-expressing tumor is able to suppress OVA-specific DTH response. Suppression was not conferred by exosomes isolated from the plasma of naive mice or mice bearing OVA-negative tumors, suggesting that the suppressive effect is antigen-specific.
Plasma-derived exosomes from tumor-bearing mice are not enriched with tumor-derived exosomes
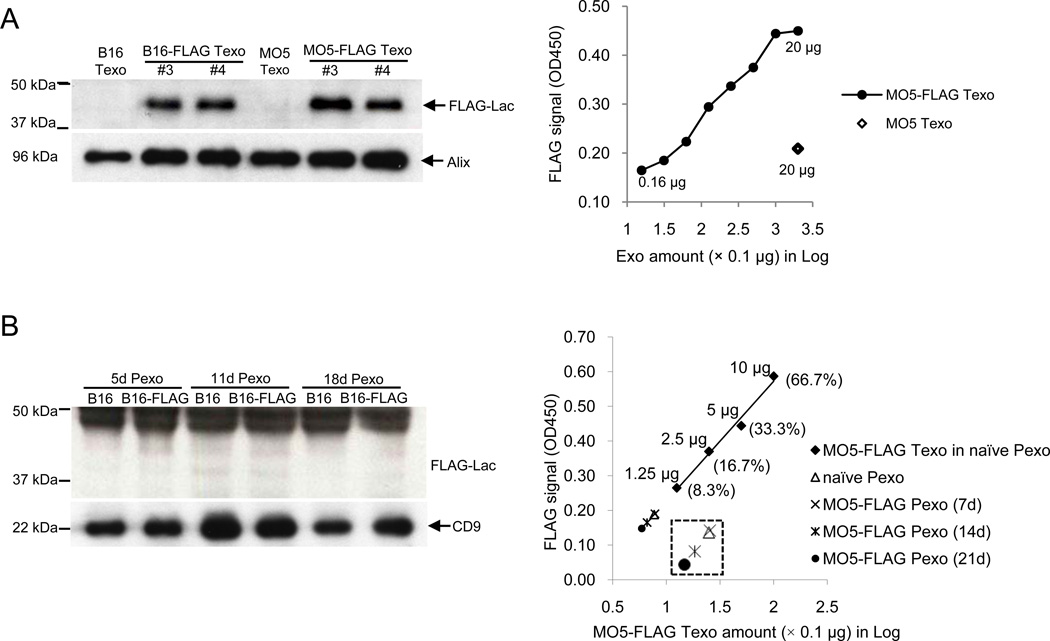
Circulating exosomes in tumor-bearing hosts can be released by different cell types including hematopoietic cells and tumor cells that constantly secrete exosomes into the tumor microenvironment. Tumor-derived exosomes have been shown to exert multiple immunosuppressive effects [1, 14, 15]. To determine if tumor-derived exosomes play a role in mediating the suppressive effect of plasma-derived exosomes, the presence of tumor-derived exosomes in plasma-derived exosomes was examined. To identify exosomes of a tumor origin, a FLAG epitope was targeted to the membrane surface of tumor-derived exosome using the “exosome display” method [16] by infecting cells with retrovirus encoding the mouse lactadherin leader sequence and C1C2 domain with a single FLAG epitope inserted. Stably infected B16 or MO5 cells produce exosomes displaying a membrane-associated FLAG-tagged Lactadherin, which can be easily detected by Western blotting and ELISA (Figure 2A). To determine whether tumor-derived exosomes accumulate in blood plasma during tumor progression, blood was collected from mice bearing subcutaneous B16-FLAG or MO5-FLAG tumors at different time points after tumor inoculation and exosomes were isolated and examined. However, the FLAG signal in B16-FLAG or MO5-FLAG Pexo was below the detection limit of Western blotting even in mice with late stage tumors. In fact, the FLAG signal was similar to that of naïve Pexo at all the time points examined, as shown by ELISA (Figure 2B). These results demonstrate that in our subcutaneous melanoma model, plasma-derived exosomes were not enriched with tumor-derived exosomes, suggesting that tumor-derived exosomes are not directly responsible for the observed suppressive effect conferred by plasma-derived exosomes.
Figure 2. Tumor-derived exosomes are not the major components in plasma-derived exosomes isolated from mice bearing subcutaneous melanoma.
(A) Infection of B16 and MO5 cells with retroviruses packaged with FLAG-Lactadherin construct generated stable cell lines (B16-FLAG and MO5-FLAG) that constantly release FLAG-tagged tumor exosomes (Texo). Left: Western blotting for FLAG on Texo isolated from tumor culture supernatant (#3 and #4, two individual stable cell lines, #4 were used in the following experiments). Exosomes derived from uninfected B16 and MO5 tumor cells were included as negative controls. The MVB marker Alix was used as a loading control for Texo. 10 µg of protein was loaded per lane. Right: ELISA detection of FLAG on MO5-FLAG Texo (2-fold dilution, 20 µg-0.16 µg) and MO5 Texo (20 µg). (B) Blood were collected from mice bearing B16, B16-FLAG, MO5 or MO5-FLAG tumor at indicated time points after tumor inoculation and plasma-derived exosomes (Pexo) were isolated respectively. Left: Western blotting for FLAG on Pexo isolated from B16 or B16-FLAG tumor mice 5, 11 or 18 days post tumor inoculation. CD9 was used as a loading control for Pexo. Right: ELISA detection of FLAG on Pexo (15 µg) isolated from MO5 or MO5-FLAG tumor mice 7, 14 or 21 days post-inoculation. MO5-FLAG Texo mixed with Pexo of naïve mice (naïve Pexo) at different percentages (10 µg, 5 µg, 2.5 µg and 1.25 µg in a total of 15 µg proteins) were used for a standard curve. Naïve Pexo was used as a negative control.
The immunosuppressive effect of plasma-derived exosomes is predominantly conferred by MHC Class II+ exosomes
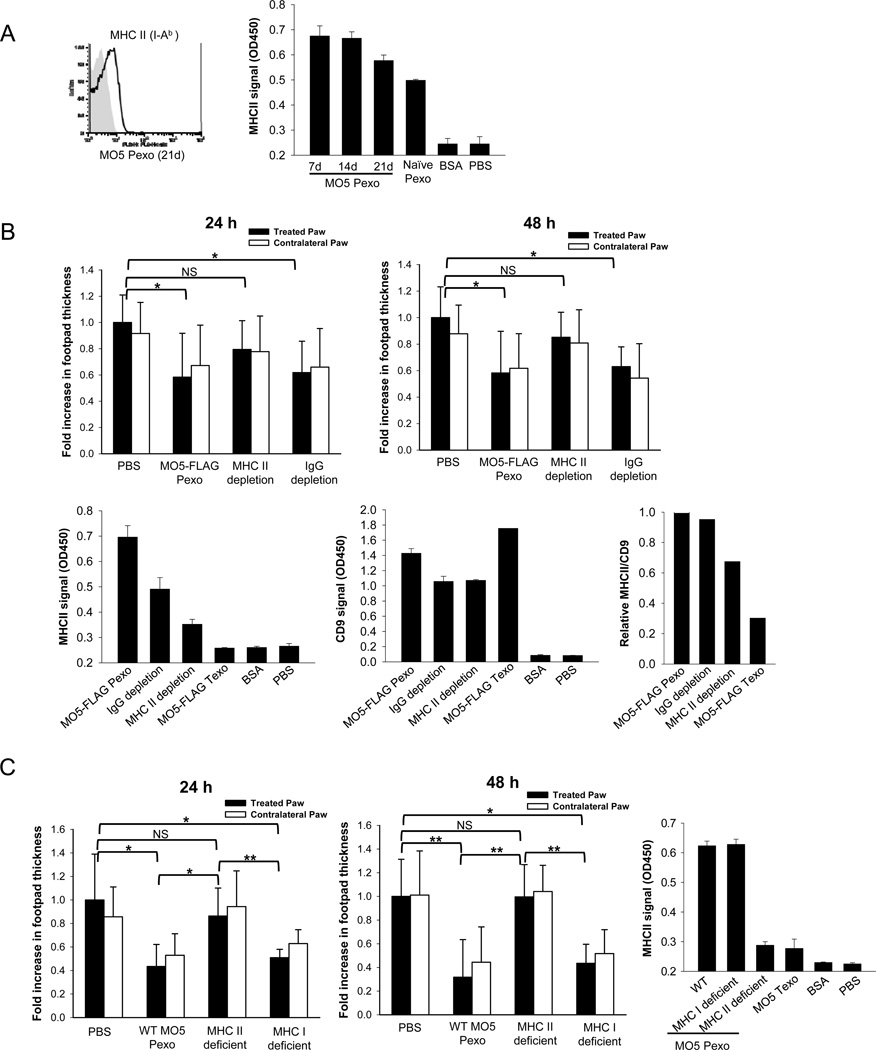
MHC Class II+ vesicles were shown to be an important component of exosomes isolated from the blood plasma and play an important role in mediating antigen-specific immunosuppression/tolerance [2, 8, 10]. Consistent with these observations, we detected MHC Class II expression on plasma-derived exosomes from both MO5 tumor-bearing and naïve mice (Figure 3A), but not on MO5 tumor-derived exosomes (Figure 3B–C). To determine if MHC Class II+ vesicles play a role in suppressing OVA-specific DTH response, MHC Class II+ vesicles were depleted from MO5-FLAG Pexo using MACS beads (Figure 3B). 10 µg of undepleted, MHC II beads-depleted or IgG control beads-depleted MO5-FLAG Pexo were administered into OVA-immunized mice. MHC II-depleted Pexo largely lost the suppressive effect, whereas IgG-depleted Pexo retained the effect (Figure 3B). To further confirm the role of MHC Class II+ exosomes in conferring antigen-specific immunosuppression, MHC Class I or Class II deficient mice were inoculated with MO5 tumor and plasma exosomes were isolated. The Pexo from MHC Class II deficient mice were unable to suppress the DTH response, whereas Pexo from MHC Class I deficient mice were effective in suppression (Figure 3C). Taken together, these results demonstrate that the antigen-specific suppressive effect of plasma exosomes isolated from tumor-bearing mice is dependent on host-derived, MHC Class II+ exosomes.
Figure 3. The suppressive effect of plasma-derived exosomes is largely dependent on MHC Class II+ vesicles.
(A) FACS and ELISA analysis of MHC Class II expression on plasma-derived exosomes (Pexo) from MO5 tumor mice. 10 µg of Pexo or BSA (negative control) were used in ELISA. (B) MHC Class II+ vesicles were depleted from MO5-FLAG Pexo (21d) by MACS beads and used in the DTH experiment. Undepleted, MHC Class II-depleted or IgG-depleted MO5-FLAG Pexo (10 µg) were administered into the footpad of OVA-immunized mice. The DTH magnitudes 24 and 48 h post-challenge are shown. n=5 except the IgG depletion group (n=4). *: P<0.05; NS: not significant. MHC Class II and CD9 expression on exosomes (10 µg) before and after depletion were analyzed by ELISA. (C) Wild type, MHC Class II or Class I deficient mice were inoculated with MO5 tumor and Pexo isolated 21 days post-inoculation. OVA-immunized mice were treated with 10 µg of Pexo at the time of antigen challenge. The DTH magnitudes 24 and 48 h post-challenge are shown. n=6. Significance at: **, P<0.01; *, P<0.05; NS: not significant. MHC Class II expression on exosomes (10 µg) was analyzed by ELISA.
Concluding remarks
In this study, we demonstrate that exosomes isolated from the blood plasma of tumor-bearing mice can suppress inflammatory responses induced by a model tumor antigen. This result suggests that circulating exosomes in tumor-bearing hosts may be able to tolerize the immune response against tumor-specific antigens. Although accumulation of tumor-derived exosomes in the peripheral circulation has been correlated with certain advanced-stage malignancies [17–19], exosomes with tumor markers are not always detected in the blood of cancer patients [6, 13]. Here we did not detect the enrichment of circulating tumor-derived exosomes in mice bearing subcutaneous melanoma. It is possible that tumor-derived exosomes are quickly internalized by or have interacted with neighboring cells within the tumor microenvironment before they have access to the blood circulation. It also is likely that the tumor type as well as the tumor site could affect the accumulation of tumor-derived exosomes in peripheral circulation and tumors generating large amounts of malignant effusions may result in more tumor-derived exosomes in the circulating blood [17].
Together with our previous study showing that plasma-derived exosomes isolated from soluble antigen-immunized mice suppress antigen-specific immune response [10], our current study suggest that immunosuppressive exosomes in plasma can be the result of suppressive APCs acquiring tumor antigen through one or more of several different mechanisms. Consistent with this model, we demonstrated that host-derived, MHC Class II+ exosomes in plasma-derived exosomes are essential for effective immunosuppression, similar to previous studies [8, 10]. We propose that in our subcutaneous tumor model, tumor infiltrating APCs capture tumor-derived exosomes, tumor-secreted soluble OVA or antigens from necrotic or apoptotic tumor cells, and in turn release tolerogenic, MHC Class II+ exosomes that are able to suppress OVA-specific immune responses. How the suppressive effect is conferred by plasma exosomes is still unclear. It is possible that these exosomes carry OVA or present the OVA epitope in MHC Class II to preferentially induce or activate antigen-specific regulatory T cells. This is consistent with a previous report suggesting that tolerogenic serum factor from OVA-fed animals can suppress an established immune response in recipient animals through induction of regulatory CD25+ cells [20]. The suppression of OVA-specific DTH response in the contralateral paws by plasma exosomes suggests that local injection of immunosuppressive MHC Class II+ exosomes indeed induces a systemic suppressive effect.
In conclusion, we have demonstrated that host-derived, MHC Class II+ exosomes able to suppress tumor antigen-specific immune responses are present in the plasma of tumor-bearing mice. Approaches to reduce or modify the activity of circulating MHC Class II+ exosomes would have the potential to improve the outcome of the current immunotherapies for cancer.
Materials and Methods
Cell cultures and mice
The C57BL/6 melanoma line B16 and MO5 and thymoma line EL4 and EG7 were cultured in RPMI-1640. The EG7 and MO5 cells were under G418 selection. Cells were tested to be free of mycoplasma. Female C57BL/6 mice, MHC Class I deficient (B6.129P2-B2mtm1unc/J) mice and MHC Class II deficient (B6.129S2-H2dlAb1-Ea/J) mice were purchased from The Jackson Laboratory. All animal experiments were conducted according to the protocols approved by the Institutional Animal Care and Use Committee.
Exosome purification and administration into the DTH model
Tumor cells (5×105) were injected subcutaneously into mice abdomen and the tumor size was monitored. Blood was collected by submandibular bleeding and centrifuged at 11,000×g for 20 min for 3 times to separate the plasma. Plasma-derived and tumor-derived exosomes were isolated by ultracentrifugation as previously described [10, 15]. 8–9 wk old mice were immunized with 150 µg of OVA (grade V, Sigma) 1:1 emulsified in Freund’s complete adjuvant (Pierce) at the tailbase and boosted with 50 µg of OVA in Freund’s incomplete adjuvant (Pierce) 14 days later. After 7 days, the right hind paw was injected with 10 µg of exosomes and both hind paws were challenged with 30 µg of OVA (grade II, Sigma) in a total of 50 µl PBS. Footpad thickness was measured by a spring-loaded caliper.
Lactadherin-FLAG construct and retroviral infection
The p6mLSC1C2 vector was generously provided by Dr. Alain Delcayre [16]. A single FLAG epitope was inserted between the mouse lactadherin LS and C1C2 using the oligos 5’-GTCTATGGATTACAAGGATGACGACGATAAG-3’ and 5’-CGGTCTTATCGTCGTCATCCTTGTAATCCAT-3’. HEK293T cells were co-transfected with pAmpho and pBABE-puro with LS-FLAG-C1C2 insertion using Lipofectamine2000 (Invitrogen). The retroviral supernatants were collected 48 h post-transfection and used for infection. Stable cell lines were selected by puromycin.
Antibodies and MACS depletion
HRP-anti-FLAG M2 (Sigma), anti-mouse Alix (Biolegend) and anti-mouse CD9 (BD) were used for Western Blotting. PE-anti-I-Ab was used for FACS. An anti-FLAG M2 coated plate and anti-FLAG-BioM2 (Sigma) were used for FLAG ELISA. For MHC Class II ELISA, anti-mouse IA/I-E and anti-mouse I-Ab (Biolegend) were used as capture antibody and Biotin anti-mouse I-Ab (Biolegend) were used as detection antibody. For CD9 ELISA, anti-mouse CD9 (BD) were used as capture antibody and Biotin anti-mouse CD9 (BD) were used as detection antibody. For depletion of MHC II+ vesicles, plasma-derived exosomes were incubated with anti-MHC Class II MACS beads (Miltenyi Biotech, 50 µl in 500 µl sample) and applied onto MACS column. Exosomes were retrieved by ultracentrifugation of the flow-through fractions. Anti-mouse IgG MACS beads were used for depletion control.
Statistics
Statistical analysis was performed by Student’s t-test (between two groups) or one-way ANOVA (multiple groups) using the SPSS software (SPSS). A value of p < 0.05 was considered statistically significant.
Acknowledgements
This work was supported in part by Department of Defense grants 17-03-1-0488 and 17-03-0412 and U01 NS058451, P30 AG024827, P01 CA100327, R01 AR051456 and R21 AG033907 grants from the National Institutes of Health to P.D.R.
Footnotes
Conflicts of Interest
The authors declare no competing financial interests.
Reference
- 1.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 2.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 3.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 4.Prado N, Marazuela EG, Segura E, Fernandez-Garcia H, Villalba M, Thery C, Rodriguez R, Batanero E. Exosomes from bronchoalveolar fluid of tolerized mice prevent allergic reaction. J Immunol. 2008;181:1519–1525. doi: 10.4049/jimmunol.181.2.1519. [DOI] [PubMed] [Google Scholar]
- 5.Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179:1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- 6.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlsson M, Lundin S, Dahlgren U, Kahu H, Pettersson I, Telemo E. "Tolerosomes" are produced by intestinal epithelial cells. Eur J Immunol. 2001;31:2892–2900. doi: 10.1002/1521-4141(2001010)31:10<2892::aid-immu2892>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Ostman S, Taube M, Telemo E. Tolerosome-induced oral tolerance is MHC dependent. Immunology. 2005;116:464–476. doi: 10.1111/j.1365-2567.2005.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almqvist N, Lonnqvist A, Hultkrantz S, Rask C, Telemo E. Serum-derived exosomes from antigen-fed mice prevent allergic sensitization in a model of allergic asthma. Immunology. 2008;125:21–27. doi: 10.1111/j.1365-2567.2008.02812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. MHC class II+ exosomes in plasma suppress inflammation in an antigen-specific and Fas ligand/Fas-dependent manner. J Immunol. 2007;179:2235–2241. doi: 10.4049/jimmunol.179.4.2235. [DOI] [PubMed] [Google Scholar]
- 11.Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11:1010–1020. [PubMed] [Google Scholar]
- 12.Bergmann C, Strauss L, Wieckowski E, Czystowska M, Albers A, Wang Y, Zeidler R, Lang S, Whiteside TL. Tumor-derived microvesicles in sera of patients with head and neck cancer and their role in tumor progression. Head Neck. 2009;31:371–380. doi: 10.1002/hed.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keller S, Konig AK, Marme F, Runz S, Wolterink S, Koensgen D, Mustea A, Sehouli J, Altevogt P. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 2009;278:73–81. doi: 10.1016/j.canlet.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 14.Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67:2912–2915. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 15.Yang C, Kim SH, Bianco NR, Robbins PD. Tumor-derived exosomes confer antigen-specific immunosuppression in a murine delayed-type hypersensitivity model. PLoS One. 2011;6:e22517. doi: 10.1371/journal.pone.0022517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delcayre A, Estelles A, Sperinde J, Roulon T, Paz P, Aguilar B, Villanueva J, Khine S, Le Pecq JB. Exosome Display technology: applications to the development of new diagnostics and therapeutics. Blood Cells Mol Dis. 2005;35:158–168. doi: 10.1016/j.bcmd.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Sherman-Baust CA, Tsai-Turton M, Bristow RE, Roden RB, Morin PJ. Claudin-containing exosomes in the peripheral circulation of women with ovarian cancer. BMC Cancer. 2009;9:244. doi: 10.1186/1471-2407-9-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toth B, Nieuwland R, Liebhardt S, Ditsch N, Steinig K, Stieber P, Rank A, Gohring P, Thaler CJ, Friese K, Bauerfeind I. Circulating microparticles in breast cancer patients: a comparative analysis with established biomarkers. Anticancer Res. 2008;28:1107–1112. [PubMed] [Google Scholar]
- 20.Karlsson MR, Kahu H, Hanson LA, Telemo E, Dahlgren UI. An established immune response against ovalbumin is suppressed by a transferable serum factor produced after ovalbumin feeding: a role of CD25+ regulatory cells. Scand J Immunol. 2002;55:470–477. doi: 10.1046/j.1365-3083.2002.t01-1-01079.x. [DOI] [PubMed] [Google Scholar]