Abstract
There is increasing evidence that alterations in the focus of attention results in changes in neural responding at the most peripheral levels of the auditory system. To date, however, those studies have not ruled out differences in task demands or overall arousal in explaining differences in responding across intermodal attentional conditions. The present study sought to compare changes in the response of cochlear outer hair cells, employing distortion product otoacoustic emissions (DPOAEs), under different, balanced conditions of intermodal attention. DPOAEs were measured while the participants counted infrequent, brief exemplars of the DPOAE primary tones (auditory attending), and while counting visual targets, which were instances of Gabor gradient phase shifts (visual attending). Corroborating an earlier study from our laboratory, the results show that DPOAEs recorded in the auditory ignoring condition were significantly higher in overall amplitude, compared with DPOAEs recorded while participants attended to the eliciting primaries; a finding in apparent contradiction with more central measures of intermodal attention. Also consistent with our previous findings, DPOAE rapid adaptation, believed to be mediated by the medial olivocochlear efferents (MOC), was unaffected by changes in intermodal attention. The present findings indicate that manipulations in the conditions of attention, through the corticofugal pathway, and its last relay to cochlear OHCs, the MOC, alter cochlear sensitivity to sound. These data also suggest that the MOC influence on OHC sensitivity is composed of two independent processes, one of which is under attentional control.
Keywords: auditory, selective attention, intermodal attention, corticofugal, medial olivocochlear efferent, outer hair cells
Attending to an acoustic stimulus, a simple sinusoid or complex sound, at the expense of competing sensory information, either within or across modalities, has been shown to improve behavioral accuracy and heighten cortical sensitivity to the acoustic target (Schröger and Eimer, 1993). Visual distraction, by contrast, often interferes with auditory processing, impairing the behavioral response to the auditory target and diminishing the cortical signature in event-related potentials (Keil et al., 2007). This pattern of results has been interpreted as reflecting a reduced availability of attentional resources for processing an auditory stimulus in the presence of engaging visual distractors (Wickens et al., 1983; Weissman et al., 2004).
Although a multitude of well-supported models of cross-modal distraction effects exist, many of them describe a restricted pool of attentional resources that limit the ability to process both a distractor and a target with the same accuracy, and that both attentional competition and load are associated with costs in performance (Jolicoeur, 1999; Weissman et al., 2009). Recent findings suggest that this limitation of resources affects sensory registration in the respective cortices (Weissman et al., 2004), whereas other authors have argued for a higher-order cognitive origin of cross-modal interference (Dalton et al., 2009). Consequently, an important question in this literature has been the physiological and temporal locus of auditory attention/distraction effects, including the extent to which manipulations of attention differently affect different brain structures or even sensory processing at the periphery. This issue is also of importance in the context of models of awareness (Dehaene et al., 2006), some of which have postulated that conscious processing of an attended stimulus is characterized by reverberant activity, distributed across sensory and higher-order brain areas. Here we ask whether such conscious, attentive processing - through top-down projection - affects the peripheral sensory organ. To determine the extent to which selectively attending to one modality over another is the critical variable underlying any observed experimental effects, it is imperative that the physical environment be held constant, while changing only the subject’s task (Hillyard, 1993). In addition, it is essential to ensure that the difficulty and nature of the task are comparable across attention conditions.
In the present study, we examined the role of medial olivocochlear efferent (MOC) tracts innervating outer hair cells (OHCs) of the cochlea in intermodal attentional selection. MOC tracts are particularly relevant in this context as they provide the only descending neural connection between the corticofugal tracts originating in the cortex and the cochlea, and have been shown capable of directly altering the cochlear activity in both humans and non-human animals in response to cortical stimulation (Xiao and Suga, 2002; Perrot et al., 2006; Delano et al., 2007; Suga, 2008; Liu et al., 2010); thus, the MOC provides the effective route for top-down modulation of the sensory periphery during distraction and attentional selection.
The role of the corticofugal and MOC systems in mediating a peripheral response in auditory selective attention can be addressed directly by measuring attention-induced changes in distortion product otoacoustic emissions (DPOAEs). DPOAEs are produced by the nonlinear mechanical behavior of cochlear OHCs to sound (Wilson, 1980; Probst et al., 1991; Yates et al., 1992). Because OHCs receive a rich, descending innervation from MOC neurons, DPOAEs offer a valuable, non-invasive measure of MOC control of outer hair cell function (Liberman et al., 1996; Kim et al., 2001; Bassim et al., 2003; Smith et al., 2012) and are one of the most widely employed clinical tools for evaluating the sensitivity of the cochlea.
In a recent study using DPOAEs to characterize changes in OHC function with attention (Smith et al., 2012), we manipulated task modality by instructing participants to either read a book and count the occurrence of the letter “a” (visual task) or count the either short- or long-duration DPOAE-eliciting tones (auditory task). In a second experiment, DPOAEs were recorded while the participants watched muted movies with subtitles (auditory ignoring/visual distraction) and were compared with DPOAEs recorded while the participants again counted the DPOAE-eliciting tones. In both experiments, the level of the DPOAEs recorded during the auditory-ignoring condition were statistically higher than those recorded in the auditory-attending condition. Importantly, because the only corticofugal input to the cochlea is through the MOC, and given the suppressive nature of MOC action on OHCs (c.f., Guinan, 1996, 2006, 2010; Robertson, 2009), any observed effect must result in some reduction in the response to the eliciting tones - in this case, to the attended tones (Smith et al., 2012). These data suggest that the observed alteration in cochlear sensitivity at the DPOAE frequency results from a modulation of inhibitory efferent input, as a function of task properties. To the extent that the DPOAE reflects cochlear activity at a frequency other than the target frequency, one question arising from these findings is whether the attended frequency is suppressed, or alternatively, frequencies competing with the target frequency are suppressed through the MOC. In the present study, however, we first aim to replicate the finding of suppression of DPOAEs in response to the attended modality.
The observed suppression of DPOAE amplitude to attended auditory stimuli is consistent with the findings from several studies by Michie et al. (1996). In their studies, Michie and colleagues sought to replicate findings from several groups using different attention and stimulus paradigms – all of which reported a relative decrease in the amplitude of ignored responses (Puel et al., 1988; Méric and Collet, 1992, 1994; Giard et al., 1994; Maison et al., 2001); this was the expected peripheral consequence of ignoring a stimulus as this effect is the generally accepted effect observed cortically (Woldorff et al., 1987; Johnson and Zatorre, 2005; Kauramäki et al., 2007). In contrast to data from cortical measures, in four of five experiments, Michie and colleagues demonstrated statistical increases in the level of evoked OAE responses to ignored auditory stimuli, findings we have now corroborated (Smith et al., 2012).
A question arises, however, as to whether the inhibition or suppression of OHCs during auditory attending conditions, as observed by several authors, is related to the fact that the visual attention/auditory ignoring task (often involving silent movies or challenging visual search tasks) is more engaging or arousing than an auditory attention task, which often consists of counting tones or detecting intermittent acoustic targets. In the present study we examined the robustness of the inhibitory effect of attention on OHC responses observed in earlier work by comparing the response of outer hair cells under different, balanced conditions of intermodal attention.
2. Experimental Procedures
2.1 Participants
Eight college-aged students (18–26 years, six females) participated in the experiment. A brief history was taken from each participant to document ear-related complaints, such as current ear congestion or infection, history of ear infections, ear surgery, noise exposure, music player and headphone use and ototoxic and chronic medication use.
All experiments were approved by the Institutional Review Board of the University of Florida.
2.2 Instrumentation and stimulus parameters
The equipment, auditory stimuli and DPOAE recording and analysis procedures have been described in previous reports (c.f., Bassim et. al. 2003 and Smith et. al. 2012). Briefly, two primary tones generated digitally (RX6 and RP2.1 DSP processors, Tucker-Davis Technologies, Gainesville, FL) were fed individually to two transducers in each ear (Etymotic Research, Elk Grove Village, IL). The primaries and the otoacoustic emissions were measured in the ear canal with a low-noise microphone probe (ER 10B+, Etymotic Research), sampled continuously at a rate of 48.83 kHz, digitized (Tucker-Davis Technologies), and stored to the hard drive.
2.2.1 Auditory stimuli
The primary tones, f1 and f2, were presented with a frequency ratio f2/f1 = 1.21 and f1 level = 70 dB SPL, and f2 level = 65 dB SPL. A DP-gram, assessing the sensitivity of the ear across a range of frequencies, was constructed by varying the f2 frequency in a 20-step geometric progression from 1.2 to 10.8 kHz, and the frequency producing the strongest DPOAE level in either ear was selected for further study during the experiment. The primary tones, though chosen to generate the largest DPOAE in one ear, were presented identically in both ears at all times in order to produce the largest DPOAE in the selected ear. Responses from the non-selected ear, though collected, were not further analyzed. The tones were either 1.5 or 3 sec in duration (≈20% 1.5 sec and ≈80% 3 sec), presented in a randomized manner with an inter-trial interval of 3 sec. Using long-duration tones, as opposed to transient or click stimuli, to measure DPOAEs offers the advantage of characterizing the both the presence of MOC activity as well as its onset time course (Liberman et al., 1996; Kim et al., 2001; Bassim et al., 2003; Smith et al., 2012). The rise/fall times of all stimuli were zero, with the primaries beginning at 0° of phase in order to reduce the effects of frequency splatter. This splatter was further minimized by measuring the DPOAE amplitude at the 2f1-f2 frequency peak, discarding the first ≈1 ms, with a bin width of 11.92 Hz.
Once the eartip transducers/microphone assembly was fully seated within the ear canal, the acoustic system output was calibrated at the start of each session, and calibration tests were repeated throughout the session to detect the emergence of small changes in probe placement or orientation. During each session, the primary tone and DPOAE levels were plotted on screen on a stimulus-by-stimulus basis and any sudden, or systematic change in signal levels, usually indicating a displacement of the probe/microphone system in the ear canal, resulted in the session being interrupted. In these situations, the earphones were re-positioned and another calibration was performed.
2.2.2 Visual stimuli
All visual stimuli were presented on a flat-panel monitor, situated outside the sound-attenuating chamber and, through the chamber observation window, directly in front of the participant. During each trial, after presentation of a fixation cross, Gabor patches oriented 45° to the right of vertical were presented in the center of a screen placed 72” (1.83m) away from the subject. Each Gabor patch was composed of 7 alternating black/white sinusoidal bars whose greatest contrast (100% Michelson) was at the center of the stimulus, with a Gaussian decline to the edges. The Gabor patch subtended a visual angle of 2.18° with 3.21 cycles per degree. After a 1.5 or 3 sec delay (presented randomly in a session, ≈20% 1.5 sec and ≈80% 3 sec), the patch was phase shifted by 180°. The visual stimulus was presented for a total of 4 sec in each trial with an inter-stimulus interval of approximately 3 sec. During the inter-stimulus interval, a fixation cross was present occupying 0.8° of visual angle.
A miniature video camera, connected to a monitor outside of the sound chamber, permitted monitoring of the subject’s behavior in the test session. Visual stimuli and auditory stimuli were presented simultaneously during each trial, with the visual stimulus onset delayed with respect to the auditory stimulus by a random interval ranging between 500 and 1000 ms from the onset of the auditory stimulus. The random delay interval was introduced to prevent participants’ usage of the other modality, respectively, to identify short-duration targets. Post-experimental assessment of the strategy adopted by the participants suggested that they focused solely on the to-be-attended modality to complete the task.
2.3 DPOAE analysis
The microphone signals were divided and a real-time spectral analysis (Fast Fourier Transform; FFT) was performed by a second computer during each trial to monitor the levels of the primary signals, as well as to monitor for the presence of the 2f1-f2 DPOAE. During data analysis, a two-component exponential was fitted to the DPOAE contours (Sigma Plot; Systat Software, San Jose, CA) to facilitate calculation of the magnitude of adaptation, and well as the adaptation onset time constant (c.f., Kim et al., 2001). A participant’s data were included in the overall analysis only if the observed rapid adaptation was larger than the measured variance in the adaptation contour; using this criterion gave confidence that MOC activity, necessary for any attentional effects, could be observed in that participant’s DPOAE response. Absolute DPOAE levels across attention conditions were estimated by averaging the data points in each trace from 1000 ms to 1500 ms. The results obtained for each participant under the testing conditions were compared statistically using a non-parametric Wilcoxon signed-rank test with alpha set at 5%.
2.4 Experimental procedure
All experiments were conducted inside of an IAC (Industrial Acoustics Corporation, Bronx, NY) sound-attenuating chamber. After initial screening, participants were seated in a comfortable reclining chair within the sound booth, detailed instructions were provided, eartips were inserted in both ears and the calibrations performed. As described above, a DP-gram was then measured for each ear and, to maximize the response signal-to-noise ratio, the f2 frequency producing the largest emission in either ear was selected for further study in a given session; only these chosen responses, and not those from the opposite ear, were further analyzed. Stimulus conditions were optimized to produce the largest DPOAE in one ear for that individual, though the same stimuli were presented binaurally during each trial. DPOAE responses were recorded and saved to disk, and the responses from the ear with the selected DPOAE, were averaged across subjects. Trials with variations in the primary levels and/or DPOAE responses (greater than the magnitude of rapid adaptation) were discarded before calculating averages. At least two sessions under each attention condition were conducted, with 128 trials in each session.
Auditory stimuli of 1.5 (short) or 3 sec (long) duration were pseudo-randomly presented binaurally in each session with ≈20% of the trials of the short duration. Visual stimuli were presented simultaneously on the screen in front of the participant, starting with a short pseudo-randomized delay of 500 – 1000 ms after auditory stimuli onset. The visual stimuli changed phase either 1.5 sec (short) or 3 sec (long) after onset and stayed on the screen for a total of 4 sec before being replaced by a fixation cross. Short visual stimuli were presented in ≈20% of the trials in a pseudo-randomized fashion in a session (the pseudo random order not coupled with that of the auditory stimuli). The inter-trial interval was 3 sec. Participants were instructed to press a response key when a target event (short duration tone in the auditory attend condition; short latency phase change of the Gabor patch in the visual attend condition) was present. During the auditory attending condition, participants were asked to respond to occurrences of short duration auditory stimuli and to ignore the visual stimuli (they were instructed to look at the screen). During the visual attending condition, participants were asked to ignore the auditory stimuli and respond to visual targets.
2.5 Analysis of behavioral data
We extracted the percentage of hits and misses, along with the false alarm and correct rejection rate, for the auditory and visual conditions separately. Response times were disregarded, as subjects were not instructed to respond as fast as possible.
3 Results
3.1 Behavioral results
Mean hit rates were acceptable across modalities, with 94.6% (SD 9.2%) hits for the auditory targets (5.3% false alarms), and 85.0% (SD 21.1%) hits for the visual targets (6.5% false alarms). The resulting d’ (sensitivities) were 3.23 for the auditory and 2.55 for the visual domain. When comparing auditory and visual tasks with a paired t-test, hit rates did not differ, t(7) = 1.6, p=.144; neither did false alarm rates, t(7) = −.63, p = .55.
3.2 DPOAE results
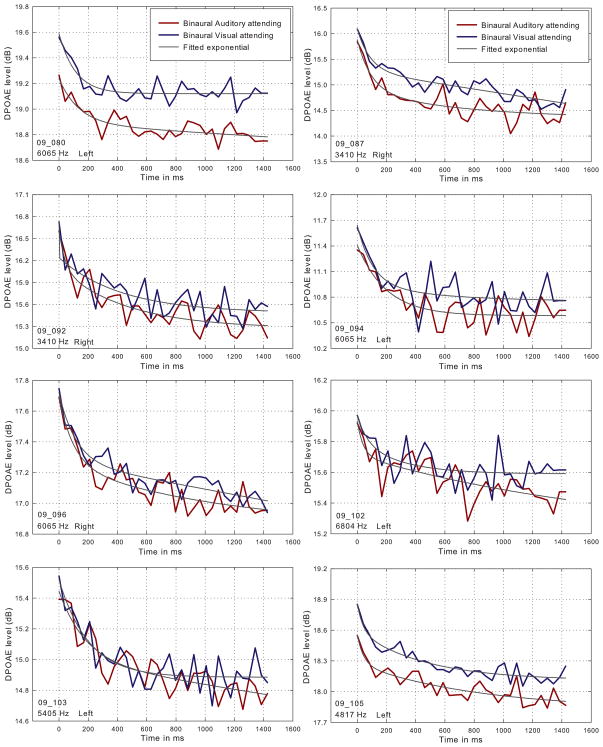
Figure 1 shows individual DPOAE adaptation contours measured for eight listeners under conditions of attending to the DPOAE-eliciting tones while ignoring the Gabor patches (red line), and while attending to the Gabor patches and ignoring the eliciting tones (blue line). For the purposes of estimating the magnitude and onset time constant of DPOAE adaptation, each contour is fitted with a two-component exponential (Kim et al., 2001; Bassim et al., 2003). Though the shape of the contours varied somewhat across subjects, all contours showed the rapid decrease in DPOAE amplitude characteristic of MOC-mediated rapid adaptation (Liberman et al., 1996; Kim et al., 2001; Bassim et al., 2003; Smith et al., 2012). For all subjects, the absolute onset level of the DPOAE contours while attending to the Gabor patches, and ignoring the primary tones, was relatively higher than when attending to the eliciting tones.
Figure 1.
Individual DPOAE adaptation contours measured for eight listeners under conditions of attending to the DPOAE-eliciting tones, while ignoring the Gabor patches (red line), and while attending to the Gabor patches and ignoring the eliciting tones (blue line). The f2 frequency and chosen ear are displayed on the bottom left of each graph.
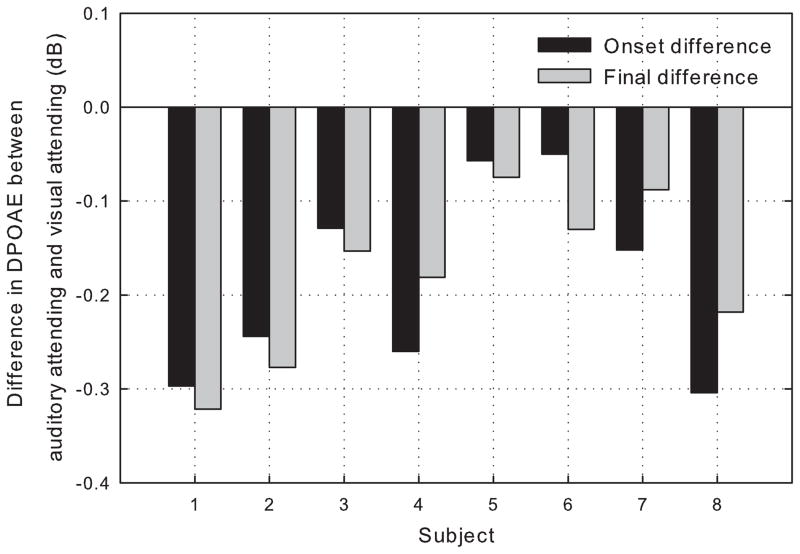
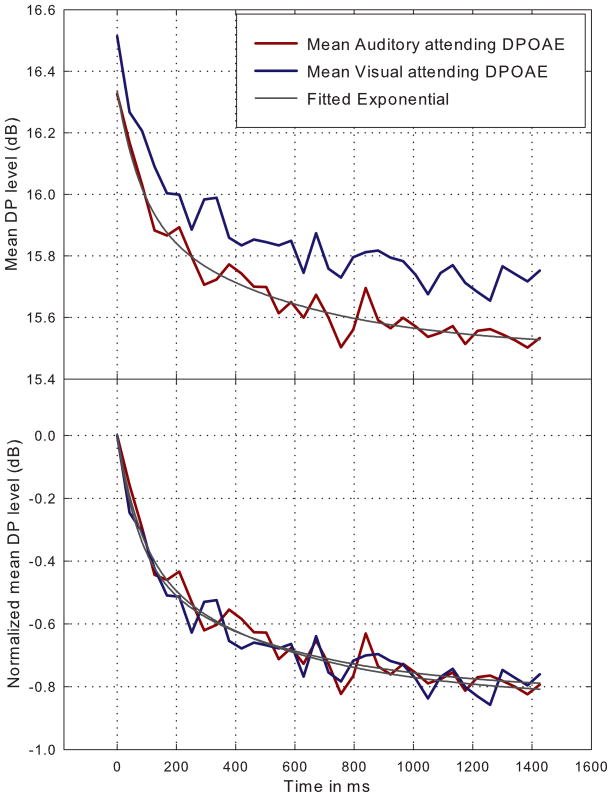
When the decrease in DPOAE level, from the visual-attending DPAOE to the auditory-attending DPOAE, is plotted for both the onset and offset of the primary tones for each subject (Figure 2), it reflects an approximate, parallel, downward shift of the contours by approximately 0.19 dB on average. This parallel shift can also be seen when the contours for each attending condition are averaged across subjects (upper panel, Figure 3). When the mean contours for both attending conditions are normalized to onset level (lower panel, Figure 3), it is apparent that the two contours mimic each other in onset slope and overall shape.
Figure 2.
Difference in DPOAE level, from the visual-attending DPOAE and the auditory-attending DPOAE, plotted for both the onset and offset of the primary tones for each subject.
Figure 3.
Average DPOAE contours for both auditory-attending and visual-attending conditions (upper panel) and the same conditions when normalized to stimulus onset (lower panel).
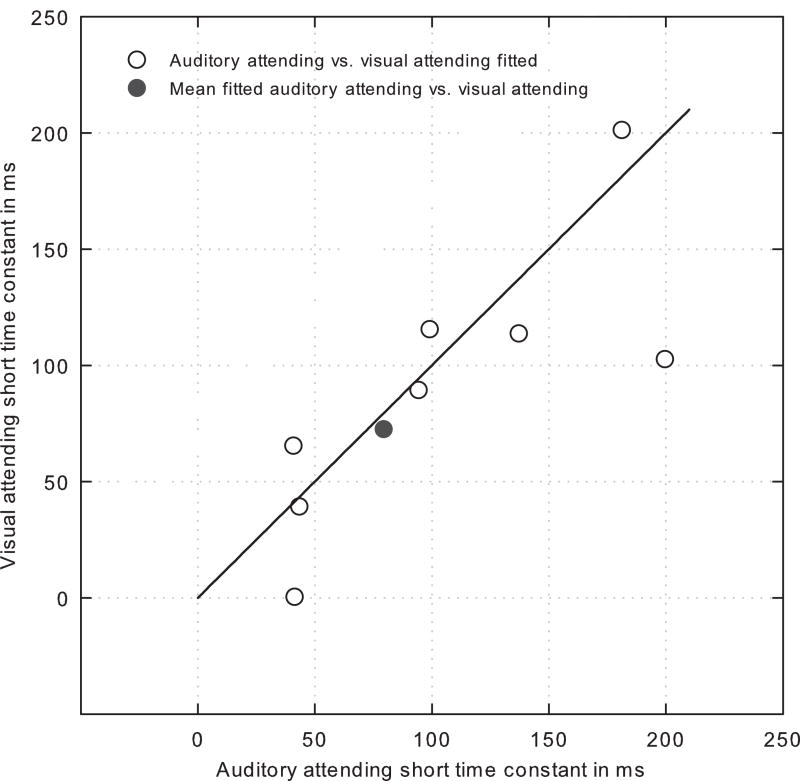
Across individual subjects and attending conditions, the onset time constant, a measure of the slope of the rapid adaptation time course, with a shorter time constant reflecting a steeper onset slope, varied from approximately 40 ms to 200 ms (Figure 4, open symbols). When compared across attention conditions for each subject, the calculated onset time constants, with one exception, were comparable for both attending conditions. As predicted from the similarity of the mean DPOAEs recorded under both attention conditions (Figure 4), a comparison of the mean time constants from each attention condition (solid black circle) shows the time constants were statistically indistinguishable.
Figure 4.
Onset short (i.e., rapid adaptation) time constants compared across auditory- and visual-attending conditions for all subjects.
4 Discussion
The present results demonstrate that attending to the primary, eliciting tones (auditory attending condition), results in DPOAEs that are relatively lower in overall amplitude, compared with DPOAEs recorded from the same primary tones, when the eliciting tones are ignored and the subject is required to report Gabor patch phase shifts (auditory ignoring condition). On average, the effect of attending to the eliciting tones was observed as a parallel, downward shift of approximately 0.19 dB, with an across subject range of approximately 0.08 to 0.35 dB (Figure 1). When the DPOAE contours recorded under both attending and ignoring conditions are superimposed, it is apparent that the magnitude of MOC-mediated rapid adaptation is unaffected by attention.
These findings are consistent with a previous study where DPOAEs were compared under different task conditions, with varying demands and different visual and auditory distractors (Smith et al., 2012). In that work, DPOAEs measured while subjects either read a book and counted the occurrence of the letter “A”, or watched a muted DVD movie and read subtitles, were significantly lower in absolute level compared with when participants were instructed to attend to the same auditory stimuli and to count occurrences of short-duration tones. In that study, as in the present, the observed decrease in overall DPOAE level when attending to the eliciting tones was concomitant with a rapid adaptation component. Rapid adaptation has previously been shown in the cat to be mediated by the MOC (Liberman et al., 1996) and, in the rat, the middle ear reflex (Relkin et al., 2005). In humans, we have argued elsewhere (Bassim et al., 2003) that rapid adaptation, as with the cat, is due to normal MOC function. This argument was based on several factors, including the relatively low-level tones we used to elicit the DPOAEs and the observation that those tones were approximately 20 dB below middle ear reflex threshold in the same subjects (Bassim et al., 2003). These findings suggest that the MOC efferents have two simultaneous functions in influencing inner ear function; first, through the process of rapid adaptation, to reduce the response of OHCs to sustained background noise, while unmasking simultaneous target signals (Winslow and Sachs, 1987) and, second, to alter the sensitivity of the cochlea during focused attention.
At higher levels within the CNS, it is generally believed that attending to a stimulus results in a relatively larger signal in the corresponding cortex, compared with when the same stimulus is ignored (c.f., Woldorff et al., 1987; Johnson and Zatorre, 2005; Kauramäki et al., 2007; Saupe et al., 2009). On initial comparison, our observation of decreases in overall DPOAE level when attention is focused on the presented auditory signals appears inconsistent with this widely accepted viewpoint. The reasons for this discrepancy remain uncertain, but may be either, or both physiological or methodological in nature. The cortex interacts with the cochlea via the corticofugal pathway, originating in the superior temporal gyrus, and its final MOC efferent link between the superior olivary complex and the OHCs in the organ of Corti (c.f., Robles and Delano, 2008). MOC fibers terminate on the subnuclear regions of OHCs, and, when activated, alter membrane resistances to produce a decrease in the OHC resting potential and an overall reduction in the OHC’s mechanical amplification of basilar membrane motion (Mountain, 1980; Siegel and Kim, 1982; Housley and Ashmore, 1991; Murugasu and Russell, 1996; Cooper and Guinan, 2003). Given its mode of action, MOC activity is suppressive in nature, and activation of the descending pathways to the cochlea can only result in a suppression of targeted receptors, as we have observed here and in previous work (Michie et al., 1996; Smith et al., 2012).
The question remains, however, if some methodological considerations can explain the discordant findings. For example, so far as we are aware, all previous studies of the cortical effects of attention rely on dichotic, or monaural stimulation paradigms (Näätänen, 1990; Woldorff and Hillyard, 1991; Woldorff et al., 1993), where as our studies of the peripheral consequences of alterations in focused attention have employed binaural stimulation; binaural stimulation is widely known to increase the magnitude of MOC-mediated suppression because BOTH crossed and uncrossed MOC tracts are activated (c.f., Liberman et al., 1996; Bassim et al., 2003) and result in a greater suppression of DPOAEs compared with monaural stimulation. Thus, it is intuitive that attentional studies employing binaural acoustic signals, as we have employed, activating both crossed- and uncrossed MOC systems, would result in relatively lower DPOAE levels compared with monaural stimulation protocols, as has been employed for more central or cortical studies of attention (Woldorff and Hillyard, 1991).
Another consideration, given the role of the MOC in suppressing the response of the OHCs to background noise (Winslow and Sachs, 1987; Kawase and Liberman, 1993; Lima da Costa et al., 1997; Voytenko and Galazyuk, 2010), is that attentionally-driven MOC action might function to suppress OHC activity to endogenous noise (e.g., heart sounds, respiration, vascular noise, myogenic noise, etc.) and, as a consequence increase the signal-to-noise ratio, and the amplitude, of the attended signal cortical potential, which might not be apparent in a DPOAE measure. Similarly, many reports have shown that MOC activity is tonotopically tuned (Cody and Johnstone, 1982; Liberman and Brown, 1986; Brown, 1989). In the present experiment, participants are instructed to attend to the DPOAE primary tones, but the 2f1-f2 DPOAE is at a frequency some cochlear distance below the primaries and, depending on the sharpness of the tuning, attending to the primaries might result in a suppression of the DPOAE response (Greenberg and Larkin, 1968; Dai et al., 1991; Strickland and Viemeister, 1995). Further research presently underway will examine the role of the target frequency and may co-register DPOAEs and electrocortical measures to address this question.
Participants attended to either auditory pure tone or visual Gabor patch stimuli.
Cochlear OHC activity was compared under the two attention conditions using DPOAEs.
DPOAE amplitude was higher during auditory ignoring than during auditory attending.
Efferent mediated DPOAE rapid adaptation was not affected by attention condition.
Acknowledgments
The authors wish to thank Fletcher Osborne, Jennifer Wong and Colin Cerwonka for assistance in the conduct of the experimental sessions. This research was supported by a grant from National Institute of Mental Health (R01 MH084932 - 02) to A. Keil.
Footnotes
Disclosure
Author Contributions:
A.K., and D.W.S. conception and design of research; S.S., K.S., and K.L.W.C. performed experiments; S.S., A.K, and D.W.S. analyzed data; S.S., A.K., and D.W.S. interpreted results of experiments; S.S. prepared figures; S.S., A.K., and D.W.S. drafted manuscript; A.K., and D.W.S. edited and revised manuscript; S.S., A.K., K.S., K.L.W.C., and D.W.S. approved final version of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bassim MK, Miller RL, Buss E, Smith DW. Rapid adaptation of the cubic distortion tone emission in humans: Monaural, binaural and contralateral stimulation effects. Hear Res. 2003;182:140–152. doi: 10.1016/s0378-5955(03)00190-4. [DOI] [PubMed] [Google Scholar]
- Brown M. Morphology and response properties of single olivocochlear fibers in the guinea pig. Hear Res. 1989;40(1–2):93–109. doi: 10.1016/0378-5955(89)90103-2. [DOI] [PubMed] [Google Scholar]
- Cody A, Johnstone B. Acoustically evoked activity of single efferent neurons in the guinea pig cochlea. J Acoust Soc Am. 1982;72:280–282. doi: 10.1121/1.387993. [DOI] [PubMed] [Google Scholar]
- Cooper NP, Guinan JJ., Jr Separate mechanical processes underlie fast and slow effects of medial olivocochlear efferent activity. J Physiol. 2003;548:307–312. doi: 10.1113/jphysiol.2003.039081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Scharf B, Buus S. Effective attenuation of signals in noise under focused attention. J Acoust Soc Am. 1991;89:2837–2842. doi: 10.1121/1.400721. [DOI] [PubMed] [Google Scholar]
- Dalton P, Santangelo V, Spence C. The role of working memory in auditory selective attention. Q J Exp Psychol(Colchester) 2009;62(11):2126–2132. doi: 10.1080/17470210903023646. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: a testable taxonomy. Trends Cogn Sci. 2006;10(5):204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Delano PH, Elgueda D, Hamame CM, Robles L. Selective attention to visual stimuli reduces cochlear sensitivity in chinchillas. J Neurosci. 2007;27:4146–4153. doi: 10.1523/JNEUROSCI.3702-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard MH, Collet L, Bouchet P, Pernier J. Auditory selective attention in the human cochlea. Brain Res. 1994;633:353–356. doi: 10.1016/0006-8993(94)91561-x. [DOI] [PubMed] [Google Scholar]
- Greenberg GZ, Larkin WD. Frequency-response characteristic of auditory observers detecting signals of a single frequency in noise: The probe-signal method. J Acoust Soc Am. 1968;44:1513–1523. doi: 10.1121/1.1911290. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr . Physiology of olivocochlear efferents. In: Dallos P, Popper AN, Fay RR, editors. The Cochlea. New York: Springer-Verlag; 1996. pp. 435–502. [Google Scholar]
- Guinan JJ., Jr Olivocochlear efferents: Anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear. 2006;27:589–607. doi: 10.1097/01.aud.0000240507.83072.e7. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr Cochlear efferent innervation and function. Curr Opin Otolaryngol Head Neck Surg. 2010;18:447–453. doi: 10.1097/MOO.0b013e32833e05d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA. Electrical and magnetic brain recordings: Contributions to cognitive neuroscience. Curr Opin Neurobiol. 1993;3:217–224. doi: 10.1016/0959-4388(93)90213-i. [DOI] [PubMed] [Google Scholar]
- Housley GD, Ashmore JF. Direct measurement of the action of acetylcholine on isolated outer hair cells of the guinea-pig cochlea. Proc R Sot Lond [Biol] 1991;244:161–167. doi: 10.1098/rspb.1991.0065. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Zatorre RJ. Attention to simultaneous unrelated auditory and visual events: Behavioral and neural correlates. Cereb Cortex. 2005;15:1609–1620. doi: 10.1093/cercor/bhi039. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P. Restricted attentional capacity between sensory modalities. Psychon Bull Rev. 1999;6(1):87–92. doi: 10.3758/bf03210813. [DOI] [PubMed] [Google Scholar]
- Kawase T, Liberman MC. Antimasking effects of the olivocochlear reflex. I. Enhancement of compound action potentials to masked tones. J Neurophysiol. 1993;70:2519–2532. doi: 10.1152/jn.1993.70.6.2519. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Junghoefer M, Russmann T, Lowenthal W, Lang PJ. Cross-modal Attention Capture by Affective Stimuli: Evidence from Event-Related Potentials. Cogn Affect Behav Neurosci. 2007;7(1):18–24. doi: 10.3758/cabn.7.1.18. [DOI] [PubMed] [Google Scholar]
- Kim DO, Dorn PA, Neely ST, Gorga MP. Adaptation of distortion product otoacoustic emissions in humans. J Assoc Res Otolaryngol. 2001;2:31–40. doi: 10.1007/s101620010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauramäki J, Jääskeläinen IP, Sams M. Selective attention increases both gain and feature selectivity of the human auditory cortex. PLoS One. 2007;2:e909. doi: 10.1371/journal.pone.0000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Brown M. Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res. 1986;24(1):17–36. doi: 10.1016/0378-5955(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Puria S, Guinan JJ., Jr The ipsilaterally evoked olivocochlear reflex causes rapid adaptation of the 2f1-f2 distortion product otoacoustic emission. J Acoust Soc Am. 1996;99:2572–2584. doi: 10.1121/1.414956. [DOI] [PubMed] [Google Scholar]
- Lima da Costa D, Erre JP, Charlet de Sauvage R, Popelar J, Aran JM. Bioelectrical cochlear noise and its contralateral suppression: relation to background activity of the eighth nerve and effects of sedation and anesthesia. Exp Brain Res. 1997;116(2):259–269. doi: 10.1007/pl00005754. [DOI] [PubMed] [Google Scholar]
- Liu X, Yan Y, Wang Y, Yan J. Corticofugal modulation of initial neural processing of sound information from the ipsilateral ear in the mouse. PLoS One. 2010;5:e14038. doi: 10.1371/journal.pone.0014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison S, Micheyl C, Collet L. Influence of focused auditory attention on cochlear activity in humans. Psychophysiology. 2001;38:35–40. [PubMed] [Google Scholar]
- Méric C, Collet L. Visual attention and evoked otoacoustic emissions: A slight but real effect. Int J Psychophysiol. 1992;12:233–235. doi: 10.1016/0167-8760(92)90061-f. [DOI] [PubMed] [Google Scholar]
- Méric C, Col let L. Differential effects of visual attention on spontaneous and evoked otoacoustic emissions. Int J Psychophysiol. 1994;17:281–289. doi: 10.1016/0167-8760(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Michie PT, LePage EL, Solowij N, Haller M, Terry L. Evoked otoacoustic emissions and auditory selective attention. Hear Res. 1996;98:54–67. doi: 10.1016/0378-5955(96)00059-7. [DOI] [PubMed] [Google Scholar]
- Mountain DC. Changes in endolymphatic potential and crossed olivocochlear bundle stimulations alter cochlear mechanics. Science. 1980;210:71–72. doi: 10.1126/science.7414321. [DOI] [PubMed] [Google Scholar]
- Murugasu E, Russell IJ. The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J Neurosci. 1996;16:325–332. doi: 10.1523/JNEUROSCI.16-01-00325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behav Brain Sci. 1990;13:201–233. [Google Scholar]
- Perrot X, Ryvlin P, Isnard J, Guénot M, Catenoix H, Fischer C, Mauguèie F, Collet L. Evidence for corticofugal modulation of peripheral auditory activity in humans. Cereb Cortex. 2006;16:941–948. doi: 10.1093/cercor/bhj035. [DOI] [PubMed] [Google Scholar]
- Probst R, Lonsbury-Martin BL, Martin GK. A review of otoacoustic emissions. J Acoust Soc Am. 1991;89:2027–2067. doi: 10.1121/1.400897. [DOI] [PubMed] [Google Scholar]
- Puel JL, Bonfils P, Pujol R. Selective attention modifies the active micromechanical properties of the cochlea. Brain Res. 1988;44:380–383. doi: 10.1016/0006-8993(88)91144-4. [DOI] [PubMed] [Google Scholar]
- Relkin EM, Sterns A, Azeredo W, Prieve BA, Woods CI. Physiological Mechanisms of Onset Adaptation and Contralateral Suppression of DPOAEs in the Rat. J Assoc Res Otolaryngol. 2005;6:119–135. doi: 10.1007/s10162-004-5047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. Centrifugal control in mammalian hearing. Clin Exp Pharmacol Physiol. 2009;36:603–611. doi: 10.1111/j.1440-1681.2009.05185.x. [DOI] [PubMed] [Google Scholar]
- Robles L, Delano PH. Efferent system. In: Dallos P, Oertel D, editors. The senses: a comprehensive reference. New York: Academic; 2008. pp. 413–445. [Google Scholar]
- Saupe K, Schröger E, Andersen SK, Müller MM. Neural mechanisms of intermodal sustained selective attention with concurrently presented auditory and visual stimuli. Front Hum Neurosci. 2009;3:58. doi: 10.3389/neuro.09.058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröger E, Eimer M. Effects of transient spatial attention on auditory event -related potentials. Neuroreport. 1993;4(5):588–590. doi: 10.1097/00001756-199305000-00033. [DOI] [PubMed] [Google Scholar]
- Siegel JH, Kim DO. Efferent neural control of cochlear mechanics? Olivocochlear bundle stimulation affects cochlear biomechanical nonlinearity. Hear Res. 1982;6:171–182. doi: 10.1016/0378-5955(82)90052-1. [DOI] [PubMed] [Google Scholar]
- Smith DW, Aouad RK, Keil A. Cognitive task demands modulate the sensitivity of the human cochlea. Front Psychol. 2012;3:30. doi: 10.3389/fpsyg.2012.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland EA, Viemeister NF. An attempt to find psychophysical evidence for efferent action in humans. Abstr Midwinter Res Meet Assoc Res Otolaryngol. 1995:173(A). [Google Scholar]
- Suga N. Role of corticofugal feedback in hearing. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008;194:169–183. doi: 10.1007/s00359-007-0274-2. [DOI] [PubMed] [Google Scholar]
- Voytenko SV, Galazyuk AV. Suppression of spontaneous firing in inferior colliculus neurons during sound processing. J Neurosci. 2010;165(4):1490–1500. doi: 10.1016/j.neuroscience.2009.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Warner LM, Woldorff MG. The neural mechanisms for minimizing cross-modal distraction. J Neurosci. 2004;24(48):10941–10949. doi: 10.1523/JNEUROSCI.3669-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Warner LM, Woldorff MG. Momentary reductions of attention permit greater processing of irrelevant stimuli. Neuroimage. 2009;48(3):609–615. doi: 10.1016/j.neuroimage.2009.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens C, Kramer A, Vanasse L, Donchin E. Performance of concurrent tasks: a psychophysiological analysis of the reciprocity of information-processing resources. Science. 1983;221(4615):1080–1082. doi: 10.1126/science.6879207. [DOI] [PubMed] [Google Scholar]
- Wilson JP. Evidence for a cochlear origin for acoustic re-emissions, threshold fine-structure and tonal tinnitus. Hear Res. 1980;2:233–252. doi: 10.1016/0378-5955(80)90060-x. [DOI] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB. Effect of electrical stimulation of the crossed olivocochlear bundle on auditory nerve response to tones in noise. J Neurophysiol. 1987;57:1002–1021. doi: 10.1152/jn.1987.57.4.1002. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Gallen CC, Hampson SA, Hillyard SA, Pantev C, Sobel D, Bloom FE. Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proc Natl Acad Sci U S A. 1993;90:8722–8726. doi: 10.1073/pnas.90.18.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldorff MG, Hansen JC, Hillyard SA. Evidence for effects of selective attention in the mid-latency range of the human auditory event-related potential. In: Johnson R Jr, Rohrbaugh JW, Parasuraman R, editors. Electroencephalogr Clin Neurophysiol Suppl, EEG. Suppl 40. Amsterdam, Netherlands: Elsevier Science Publishers; 1987. pp. 146–154. [PubMed] [Google Scholar]
- Woldorff MG, Hillyard SA. Modulation of early auditory processing during selective listening to rapidly presented tones. Electroencephalogr Clin Neurophysiol. 1991;79:170–191. doi: 10.1016/0013-4694(91)90136-r. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Suga N. Modulation of cochlear hair cells by the auditory cortex in the mustached bat. Nat Neurosci. 2002;5:57–63. doi: 10.1038/nn786. [DOI] [PubMed] [Google Scholar]
- Yates GK, Johnstone BM, Patuzzi RB, Robertson D. Mechanical processing in the mammalian cochlea. Trends Neurosci. 1992;15:57–61. doi: 10.1016/0166-2236(92)90027-6. [DOI] [PubMed] [Google Scholar]