Abstract
BACKGROUND
Biosynthesis of extragonadal androgen may contribute to the progression of castration-resistant prostate cancer. We evaluated whether abiraterone acetate, an inhibitor of androgen biosynthesis, prolongs overall survival among patients with metastatic castration-resistant prostate cancer who have received chemotherapy.
METHODS
We randomly assigned, in a 2:1 ratio, 1195 patients who had previously received docetaxel to receive 5 mg of prednisone twice daily with either 1000 mg of abiraterone acetate (797 patients) or placebo (398 patients). The primary end point was overall survival. The secondary end points included time to prostate-specific antigen (PSA) progression (elevation in the PSA level according to prespecified criteria), progression-free survival according to radiologic findings based on prespecified criteria, and the PSA response rate.
RESULTS
After a median follow-up of 12.8 months, overall survival was longer in the abiraterone acetate–prednisone group than in the placebo–prednisone group (14.8 months vs. 10.9 months; hazard ratio, 0.65; 95% confidence interval, 0.54 to 0.77; P<0.001). Data were unblinded at the interim analysis, since these results exceeded the preplanned criteria for study termination. All secondary end points, including time to PSA progression (10.2 vs. 6.6 months; P<0.001), progression-free survival (5.6 months vs. 3.6 months; P<0.001), and PSA response rate (29% vs. 6%, P<0.001), favored the treatment group. Mineralocorticoid-related adverse events, including fluid retention, hypertension, and hypokalemia, were more frequently reported in the abiraterone acetate–prednisone group than in the placebo–prednisone group.
CONCLUSIONS
The inhibition of androgen biosynthesis by abiraterone acetate prolonged overall survival among patients with metastatic castration-resistant prostate cancer who previously received chemotherapy. (Funded by Cougar Biotechnology; COU-AA-301 ClinicalTrials.gov number, NCT00638690.)
For the past 70 years, depleting or blocking the action of androgens has been the standard of care for men with advanced prostate cancer.1 Androgen deprivation results in a decrease in the concentration of prostate-specific antigen (PSA) as well as tumor regression and relief of symptoms in most patients, but the response to treatment is not durable in patients with advanced cancer, and with time, PSA concentrations increase, indicating reactivated androgen-receptor signaling and a transition to a castration-resistant state that is invariably fatal.2 Many endocrine therapies have been evaluated in these patients, but none have prolonged survival.3 Three nonhormonal systemic approaches have been found to prolong survival: docetaxel4 as first-line and cabazitaxel5 as second-line cytotoxic chemotherapy, and active cellular immunotherapy with sipuleucel-T.6
A unique molecular alteration described in castration-resistant prostate cancer is the up-regulation of androgen biosynthesis enzymes, leading to an increase in intratumoral androgen concentrations, which can exceed the levels measured in the blood.7–9 Other alterations include overexpression of androgen receptors, and androgen-receptor mutations leading to androgen-receptor binding by additional ligands that would not stimulate the wild-type receptor.2,10 Abiraterone acetate, a prodrug of abiraterone, is a selective inhibitor of androgen biosynthesis that potently blocks cytochrome P450 c17 (CYP17), a critical enzyme in testosterone synthesis, thereby blocking androgen synthesis by the adrenal glands and testes and within the prostate tumor.11–14 In phase 1–2 trials, treatment with abiraterone acetate, either as a single agent or in combination with low-dose glucocorticoids such as prednisone, resulted in significant antitumor activity among both patients with progressing castration-resistant prostate cancer who had not received chemotherapy and those who had received chemotherapy.15–20 The most common adverse events, which were associated with increased mineralocorticoid levels, included hypokalemia, fluid retention, and hypertension; these events were largely abrogated by coadministering low-dose glucocorticoids. We hypothesized that inhibition of androgen biosynthesis with abiraterone acetate and prednisone would improve overall survival among patients with advanced prostate cancer.
Methods
Patients
Patients were eligible to participate in the study if they had histologically or cytologically confirmed prostate cancer that had previously been treated with docetaxel, disease progression according to the criteria of the Prostate Cancer Working Group21,22 (for trial entry, patients were considered to have disease progression if they had two consecutive increases in the PSA concentration over a reference value) or radiographic evidence of disease progression in soft tissue or bone with or without disease progression on the basis of the PSA value, and ongoing androgen deprivation, with a serum testosterone level of 50 ng per deciliter or less (≤2.0 nmol per liter).
Additional eligibility criteria included an Eastern Cooperative Oncology Group (ECOG)23 performance status score of 2 or less (on a scale from 0 to 5, with 0 indicating that the patient is fully active and able to carry on all predisease activities without restriction; 1 indicating that the patient is restricted in physically strenuous activity but is ambulatory and able to carry out work of a light or sedentary nature, such as light housework or office work; and 2 indicating that the patient is ambulatory and up and about more than 50% of waking hours and is capable of all self-care but unable to carry out any work activities) and hematologic and chemical laboratory values that met predefined criteria, including an albumin level of 3.0 g per deciliter or higher.
Patients were excluded if they had abnormal aminotransferase levels (levels of aspartate aminotransferase or alanine aminotransferase that were ≥2.5 times the upper level of the normal range; patients with known liver metastasis who had levels of aspartate aminotransferase or alanine aminotransferase that were ≤5 times the upper level of the normal range were eligible to participate), serious coexisting nonmalignant disease, active or symptomatic viral hepatitis or chronic liver disease, uncontrolled hypertension, a history of pituitary or adrenal dysfunction, clinically significant heart disease, or previous therapy with ketoconazole.
The review boards at all participating institutions approved the study, which was conducted according to the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonization. All patients provided written informed consent to participate in the study.
Study Design and Treatment
This phase 3, multinational, randomized, double-blind, placebo-controlled study was conducted at 147 sites in 13 countries. Patients were enrolled from May 2008 through July 2009 and were stratified according to baseline ECOG performance status score (0 or 1 vs. 2), level of worst pain over the previous 24 hours on the Brief Pain Inventory–Short Form (BPI-SF) (on a scale of 0 to 10, with 0 to 3 indicating that clinically significant pain is absent vs. 4 to 10 indicating that clinically significant pain is present),24,25 number of previous chemotherapy regimens (one vs. two), and type of evidence of disease progression (an increase in the PSA concentration only vs. radiographic evidence of progression with or without an increase in the PSA concentration). Patients were then randomly assigned in a 2:1 ratio to receive either abiraterone acetate and prednisone or placebo and prednisone. Blocked randomization was used.
Patients received 1 g of abiraterone acetate (administered as four 250-mg tablets) or four placebo tablets orally once daily at least 1 hour before or 2 hours after a meal, with prednisone at a dose of 5 mg orally twice daily. Each cycle of treatment was 28 days. Treatment could be continued until disease progression was documented on the basis of the PSA concentration, radiographic imaging, and clinical findings. Safety and dosing compliance were evaluated on day 15 of cycle 1 and on day 1 of each subsequent cycle, at the time of treatment discontinuation if applicable, and at the end-of-study visit.
The primary end point was overall survival, defined as the time from randomization to death from any cause. The prespecified secondary end points included the PSA response rate (defined as the proportion of patients with a decrease of ≥50% in the PSA concentration from the pretreatment baseline PSA value, which was confirmed after ≥4 weeks by an additional PSA evaluation). Other secondary end points included time to PSA progression according to prespecified criteria (in patients in whom the PSA level had not decreased, PSA progression was defined as a 25% increase over the baseline and an increase in the absolute-value PSA level by at least 5 ng per milliliter, which was confirmed by a second value; in patients in whom the PSA had decreased but had not reached response criteria [PSA ≤50%], progressive disease would be considered to have occurred when the PSA level increased 25% over the nadir, provided that the increase was a minimum of 5 ng per milliliter and was confirmed; and if at least a 50% decrease in the PSA level had been achieved, PSA progression would be an increase of 50% above the nadir at a minimum of 5 ng per milliliter), and radiographic evidence of progression-free survival according to prespecified criteria (defined as soft-tissue disease progression according to modified Response Evaluation Criteria in Solid Tumors [RECIST]26 [with a baseline lymph node of ≥2.0 cm considered to be a target lesion] or progression according to bone scans showing two or more new lesions not consistent with tumor flare). A complete response was defined as the disappearance of all target and nontarget lesions, and a partial response as a decrease by at least 30% in the sum of the largest diameter of each target lesion, relative to the corresponding sum at baseline. Stable disease was defined as the absence of shrinkage sufficient for a partial response and the absence of enlargement sufficient for progressive disease, relative to the sum of the largest diameter of each target lesion at baseline, and progressive disease as an increase by at least 20% in the sum of the largest diameter of each target lesion, relative to the smallest corresponding diameter recorded since the start of treatment, or the appearance of one or more new lesions. Definitions of the secondary end points are provided in Table 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Table 1.
Baseline Demographic and Clinical Characteristics of the Patients.*
| Characteristic | Abiraterone Acetate (N = 797) |
Placebo (N = 398) |
|---|---|---|
| Age | ||
| Median (range) — yr | 69 (42–95) | 69 (39–90) |
| ≥75 yr — no. of patients/total no. (%) | 220/797 (28) | 111/397 (28) |
| Disease location — no. of patients/total no. (%) | ||
| Bone | 709/797 (89) | 357/397 (90) |
| Node | 361/797 (45) | 164/397 (41) |
| Liver | 90/797 (11) | 30/397 (8) |
| BPI-SF score for pain† | ||
| No. of patients | 792 | 394 |
| Median score (range) | 3.0 (0–10) | 3.0 (0–10) |
| No. of previous cytotoxic chemotherapy regimens — no. of patients/total no. (%) | ||
| 1 | 558/797 (70) | 275/398 (69) |
| 2 | 239/797 (30) | 123/398 (31) |
| ECOG performance status — no. of patients/total no. (%) | ||
| 0 or 1 | 715/797 (90) | 353/398 (89) |
| 2 | 82/797 (10) | 45/398 (11) |
| Prostate-specific antigen | ||
| No. of patients | 788 | 393 |
| Median (range) — ng/ml | 128.8 (0.4–9253.0) | 137.7 (0.6–10114.0) |
See Table 2 in the Supplementary Appendix for more baseline demographic and clinical characteristics.
The Brief Pain Inventory–Short Form (BPI-SF) rates pain on a scale of 0 to 10, with 0 to 3 indicating that clinically significant pain is absent and 4 to 10 indicating that clinically significant pain is present. The scores shown are for the worst pain over the previous 24 hours.
Study Assessments
Efficacy assessments included the PSA concentration, radiographic imaging, the pain level on the BPI-SF, and analgesic use. Clinical assessments included the patient’s medical history, vital-sign measurements, and body weight; a physical examination; review of concomitant therapy and procedures and of adverse events and serious adverse events, including adverse events detected by means of laboratory tests; blood chemical, hematologic, coagulation, and serum lipid studies; urinalysis; electrocardiography; and measurement of the cardiac ejection fraction. An independent data and safety monitoring committee monitored patient safety at regular intervals.
Other assessments for analyses of exploratory end points included the score on the Functional Assessment of Cancer Therapy–Prostate questionnaire27; the score for fatigue, as evaluated by means of the Brief Fatigue Inventory instrument28; information on medical resource utilization29; and counts of circulating tumor cells.30
Study Oversight
This study was designed by both the academic authors and employees of the sponsor, the Ortho Biotech Oncology Research and Development Unit of Cougar Biotechnology. The first draft of the manuscript was written by some of the academic authors and employees of the sponsor; the draft was then completed and approved by the other coauthors. All authors were responsible for writing the manuscript and for the decision to submit the manuscript for publication, and all authors assume responsibility for the completeness and integrity of the data. The blinded database was held at a third-party contract clinical research organization, and queries were issued by both the sponsor and the staff of the clinical research organization. The statistician employed by the independent clinical research organization provided the analysis to the independent data and safety monitoring committee, whose members were invited by the sponsor. After the independent data and safety monitoring committee recommended unblinding of the data, analyses of the data were performed by a statistician employed by the sponsor, and the results were reviewed by the authors.
Statistical Analysis
The planned sample of approximately 1158 patients provided 85% power to detect a hazard ratio of 0.80 for death in the group receiving abiraterone acetate plus prednisone as compared with the group receiving placebo plus prednisone. This sample size was calculated by assuming a median survival of 15 months for the abiraterone acetate group and 12 months for the placebo group, with a two-sided significance level (alpha) of 0.05, an enrollment period of approximately 13 months, and a total study duration of approximately 30 months to observe the required 797 total events.
One interim analysis was planned after 534 deaths were observed (67% of 797 total events) in a group-sequential design with the use of the O’Brien–Fleming stopping boundary. Distributions of time-to-event variables and associated 95% confidence intervals were estimated with the use of the Kaplan–Meier product-limit method. The stratified log-rank test was used as the primary analysis for comparison of treatment groups. Statistical inference was evaluated with the use of the chi-square statistic. Analyses of overall survival with the use of the nonstratified log-rank test and Cox proportional-hazards model were also performed as supportive analyses. Subgroup analyses were carried out to assess whether treatment effects were consistent across subgroups.
Results
Patients and Treatment
We randomly assigned 1195 patients to receive abiraterone acetate plus prednisone (797 patients) or placebo plus prednisone (398 patients) (Fig. 1 in the Supplementary Appendix). Baseline demographic and other characteristics were well-balanced between the two treatment groups (Table 1). Most patients (67%) had radiographic evidence of disease progression before study entry. The median duration of treatment was 8 months in the group that received abiraterone acetate plus prednisone (hereinafter referred to as the abiraterone acetate group) and 4 months in the group that received placebo plus prednisone (hereinafter referred to as the placebo group). The median follow-up in the overall study population was 12.8 months.
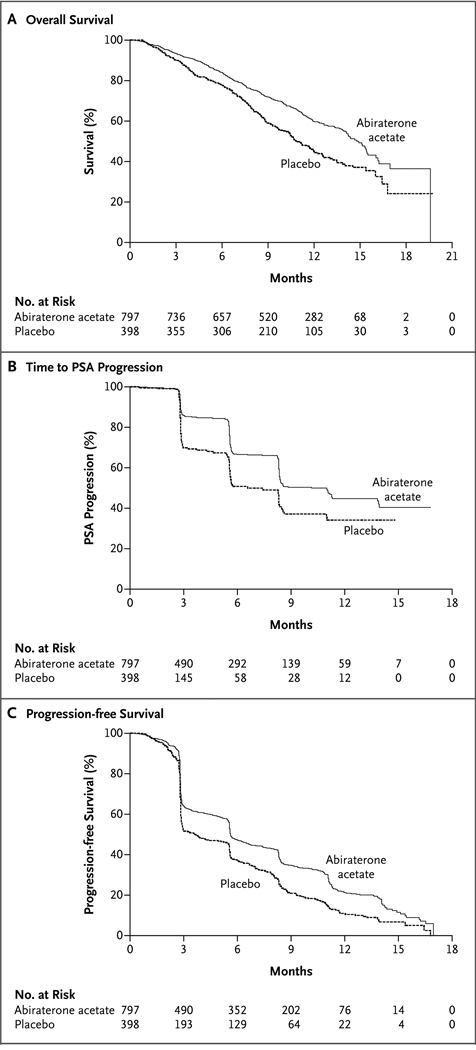
Figure 1. Kaplan–Meier Estimates of Overall Survival, Time to PSA Progression, and Progression-free Survival According to Radiographic Evidence in the Intention-to-Treat Population.
Efficacy
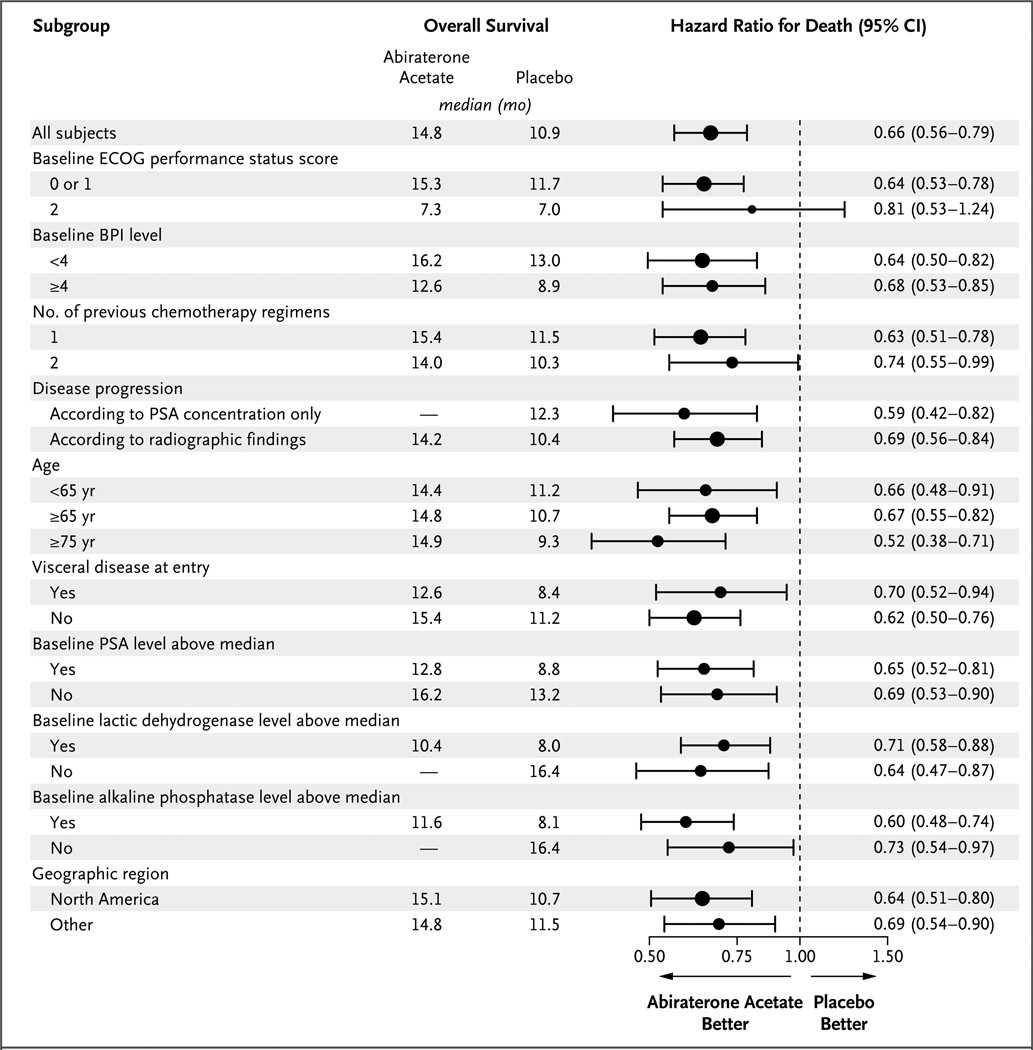
At the time of the preplanned interim analysis, treatment with abiraterone acetate plus prednisone resulted in a 35.4% reduction in the risk of death as compared with placebo plus prednisone (hazard ratio, 0.65; 95% confidence interval [CI], 0.54 to 0.77; P<0.001). A total of 552 patients in the intention-to-treat population died: 333 patients in the abiraterone acetate group (42%) and 219 patients in the placebo group (55%). The median overall survival was 14.8 months in the abiraterone acetate group and 10.9 months in the placebo group (Fig. 1A). The effect of abiraterone acetate and prednisone on overall survival was consistent across all subgroups (Fig. 2), and the significance of the treatment effect on overall survival was robust after adjustment for stratification factors in a multivariate analysis (hazard ratio for death, 0.66; 95% CI, 0.55 to 0.78; P<0.001) (Table 2). These data led the independent data and safety monitoring committee to recommend unblinding of the study data, with patients in the placebo group receiving abiraterone acetate if they met the criteria for crossover treatment specified in protocol amendment 3.0 (see the protocol, available at NEJM.org).
Figure 2. Hazard Ratios for the Risk of Death, According to Subgroup.
Hazard ratios are based on a nonstratified proportional-hazards model. The Eastern Cooperative Oncology Group (ECOG) grades the performance status of patients with respect to activities of daily living, with 0 indicating that the patient is fully active and able to carry on all predisease activities without restriction; 1 indicating that the patient is restricted in physically strenuous activity but is ambulatory and able to carry out work of a light or sedentary nature, such as light housework or office work; and 2 indicating that the patient is ambulatory and up and about more than 50% of waking hours and is capable of all self-care but unable to carry out any work activities. Dashes indicate that the median time to death had not been reached for the indicated patient subgroup. The size of the circles is proportional to the size of the subgroup. BPI denotes Brief Pain Inventory–Short Form, CI confidence interval, and PSA prostate-specific antigen.
Table 2.
Results of Multivariate Analysis of Overall Survival in the Intention-to-Treat Population.*
| Variable | Model Fit | Hazard Ratio for Death (95% CI) |
|
|---|---|---|---|
| Coefficient | P Value | ||
| Treatment: abiraterone acetate vs. placebo | −0.4±0.09 | <0.001 | 0.66 (0.55–0.78) |
| ECOG score: 0 or 1 vs. 2 | −0.9±0.12 | <0.001 | 0.40 (0.32–0.50) |
| Pain: absent vs. present | −0.4±0.09 | <0.001 | 0.67 (0.56–0.79) |
| Previous chemotherapy regimens: 1 vs. 2 | −0.2±0.09 | 0.006 | 0.78 (0.66–0.93) |
| Evidence of progression: PSA concentration only vs. radiographic findings | −0.3±0.10 | 0.01 | 0.78 (0.64–0.94) |
Data on patients who had not died by the time of analysis were censored on the last date the patient was known to be alive or was available for follow-up. Each test was carried out at a significance level of 0.05. CI denotes confidence interval, ECOG Eastern Cooperative Oncology Group, and PSA prostate-specific antigen.
All the secondary end points analyzed provided support for the superiority of abiraterone acetate over placebo (Table 3), including the confirmed PSA response rate (29% vs. 6%, P<0.001), the objective response rate on the basis of RECIST among patients with measurable disease at baseline (14% vs. 3%, P<0.001), time to PSA progression (10.2 months vs. 6.6 months), and median progression-free survival on the basis of radiographic evidence (5.6 vs. 3.6 months). On the basis of the PSA concentration, abiraterone acetate was associated with a 42% reduction in the risk of disease progression (hazard ratio, 0.58; 95% CI, 0.46 to 0.73; P<0.001), and on the basis of radiographic imaging, it was associated with a 33% reduction in the risk of progression (hazard ratio, 0.67; 95% CI, 0.58 to 0.78; P<0.001) (Table 3 and Fig. 1B and 1C).
Table 3.
Secondary End Points.*
| Variable | Abiraterone Acetate (N = 797) |
Placebo (N = 398) |
Hazard Ratio (95% CI) |
P Value |
|---|---|---|---|---|
| Time to PSA progression (mo) | 10.2 | 6.6 | 0.58 (0.46–0.73) | <0.001 |
| Progression-free survival according to radiographic evidence (mo) | 5.6 | 3.6 | 0.67 (0.59–0.78) | <0.001 |
| PSA response rate (%) | ||||
| Total | 38.0 | 10.1 | <0.001 | |
| Confirmed response on the basis of the PSA concentration | 29.1 | 5.5 | <0.001 | |
| Objective response on the basis of imaging studies | 14.0 | 2.8 | <0.001 |
PSA denotes prostate-specific antigen.
Evaluations of exploratory end points at the interim analysis also favored abiraterone acetate relative to placebo, including the time to 25% of the patients having a skeletal event (9.9 vs. 4.9 months) and the rate of pain palliation among patients with a baseline pain score of 4 or more and at least one post-baseline pain score (44% vs. 27%, P = 0.002). Patients in the abiraterone acetate group had consistently improved pain palliation as compared with those in the placebo group.
Safety
The most common adverse event was fatigue, which occurred at a similar frequency in the two treatment groups (Table 4). Other common adverse events in both groups were back pain (30% in the abiraterone acetate group and 33% in the placebo group), nausea (30% and 32%, respectively), constipation (26% and 31%), bone pain (25% and 28%), and arthralgia (27% and 23%). Most of these events were grade 1 or 2. Urinary tract infection was more frequent in the abiraterone acetate group (12%, vs. 7% in the placebo group; P = 0.02); these infections were also primarily grade 1 or 2 events. Adverse events resulting in treatment discontinuation occurred with similar frequency in the abiraterone acetate and placebo groups (19% and 23%, respectively; P = 0.09). The incidence of adverse events leading to dose modification or interruption was also similar in the two groups (Table 3 in the Supplementary Appendix).
Table 4.
Adverse Events.
| Event | Abiraterone Acetate (N = 791) | Placebo (N =394) | ||||
|---|---|---|---|---|---|---|
| All Grades | Grade 3 | Grade 4 | All Grades | Grade 3 | Grade 4 | |
| number (percent) | ||||||
| Anemia | 178 (23) | 51 (6) | 8 (1) | 104 (26) | 23 (6) | 6 (2) |
| Thrombocytopenia | 28 (4) | 8 (1) | 3 (<1) | 13 (3) | 1 (<1) | 1 (<1) |
| Neutropenia | 7 (1) | 1 (<1) | 0 | 1 (<1) | 1 (<1) | 0 |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 139 (18) | 5 (1) | 0 | 53 (14) | 5 (1) | 0 |
| Fatigue | 346 (44) | 64 (8) | 2 (<1) | 169 (43) | 36 (9) | 3 (1) |
| Asthenia | 104 (13) | 18 (2) | 0 | 52 (13) | 7 (2) | 1 (<1) |
| Back pain | 233 (30) | 44 (6) | 3 (<1) | 129 (33) | 37 (9) | 1 (<1) |
| Nausea | 233 (30) | 12 (2) | 1 (<1) | 124 (32) | 10 (3) | 0 |
| Vomiting | 168 (21) | 13 (2) | 1 (<1) | 97 (25) | 11 (3) | 0 |
| Hematuria | 65 (8) | 11 (1) | 0 | 31 (8) | 9 (2) | 0 |
| Abdominal pain | 95 (12) | 16 (2) | 0 | 44 (11) | 6 (2) | 0 |
| Pain in arm or leg | 134 (17) | 18 (2) | 1 (<1) | 79 (20) | 20 (5) | 0 |
| Dyspnea | 102 (13) | 8 (1) | 2 (<1) | 46 (12) | 7 (2) | 2 (<1) |
| Constipation | 206 (26) | 8 (1) | 0 | 120 (31) | 4 (1) | 0 |
| Pyrexia | 71 (9) | 3 (<1) | 0 | 35 (9) | 5 (1) | 0 |
| Arthralgia | 215 (27) | 33 (4) | 0 | 89 (23) | 16 (4) | 0 |
| Urinary tract infection | 91 (12) | 17 (2) | 0 | 28 (7) | 2 (<1) | 0 |
| Pain | 13 (2) | 5 (1) | 0 | 19 (5) | 6 (2) | 1 (<1) |
| Bone pain | 194 (25) | 42 (5) | 2 (<1) | 110 (28) | 25 (6) | 4 (1) |
| Fluid retention and edema | 241 (31) | 16 (2) | 2 (<1) | 88 (22) | 4 (1) | 0 |
| Hypokalemia | 135 (17) | 27 (3) | 3 (<1) | 33 (8) | 3 (1) | 0 |
| Cardiac disorder* | 106 (13) | 26 (3) | 7 (1) | 42 (11) | 7 (2) | 2 (<1) |
| Liver-function test abnormalities | 82 (10) | 25 (3) | 2 (<1) | 32 (8) | 10 (3) | 2 (<1) |
| Hypertension | 77 (10) | 10 (1) | 0 | 31 (8) | 1 (<1) | 0 |
Cardiac disorders associated with abiraterone acetate treatment, as defined with the use of the standardized Medical Dictionary for Regulatory Activities (version 11.0) queries, included ischemic heart disease, myocardial infarction, supraventricular tachyarrhythmias, ventricular tachyarrhythmias, cardiac failure, and possible arrhythmia-related tests, signs, and symptoms.
Adverse events associated with elevated mineralocorticoid levels due to CYP17 blockade (fluid retention and edema, hypokalemia, and hypertension), as well as cardiac disorders and liver-function test abnormalities (Table 4), were deemed of special interest and were more common in the abiraterone acetate group than in the placebo group (55% vs. 43%, P<0.001). The incidence of fluid retention and edema was higher in the abiraterone acetate group (31%, vs. 22% in the placebo group; P = 0.04). Grade 1 or 2 peripheral edema accounted for most of these events. Hypokalemia also occurred in a higher proportion of patients in the abiraterone acetate group (17%, vs. 8% in the placebo group; P<0.001).
Cardiac events (primarily grade 1 or 2) occurred at a higher rate in the abiraterone acetate group than in the placebo group (13% vs. 11%, P = 0.14), but the difference was not significant. The most frequently reported cardiac events were tachycardia (3% in the abiraterone acetate group and 2% in the placebo group, P = 0.22) and atrial fibrillation (2% and 1%, respectively; P = 0.29). All tachycardia events were grade 1 or 2; atrial fibrillation events were grade 3 or lower. Despite the slightly higher incidence of cardiac events in the abiraterone acetate group than in the placebo group, there was no significant increase in fatal cardiac events in the abiraterone acetate group (1.1%, vs. 1.3% in the placebo group). No individual grade 4 adverse events occurred in 2% or more of patients in either treatment group.
Abiraterone acetate treatment has been associated with an elevation in aminotransferase levels. A grade 4 elevation in an aminotransferase level early in the study led to a protocol amendment specifying more frequent monitoring with liver-function tests during the first 12 weeks of treatment. Overall, however, abnormalities in liver-function tests occurred at a similar frequency in the abiraterone acetate and placebo groups, including changes of any grade in liver-function tests (10% and 8%, respectively), grade 3 or 4 changes in liver-function tests (3.5% and 3.0%), grade 3 or 4 elevations in aspartate aminotransferase levels (1.4% and 1.6%), grade 3 or 4 elevations in alanine aminotransferase levels (1.0% and 1.1%), and grade 4 elevations in aminotransferase levels (0.3% and 0.5%).
A total of 11% of patients in the abiraterone acetate group and 13% of patients in the placebo group died within 30 days after the last dose of study medication, primarily as a result of disease progression. A lower proportion of patients in the abiraterone acetate group than in the placebo group had an adverse event that resulted in death (12% vs. 15%).
Discussion
In this phase 3 study of abiraterone acetate, an inhibitor of androgen biosynthesis, survival was prolonged among patients with metastatic castration-resistant prostate cancer who had received chemotherapy. Increased survival was observed in all patient subgroups and the superiority of the treatment group was shown across all prespecified secondary end points. The use of hormonal agents is typically not considered in patients who have received chemotherapy. These results show that continued androgen-receptor signaling contributes to disease progression, and they provide support for the evaluation of other endocrine therapies in this stage of the disease.2,10,31
Multiple studies have shown that despite treatment with medical or surgical castration, prostate cancers continue to have sufficient levels of androgens to drive tumor growth.32–34 Several studies have provided evidence that this disease progression may be due in part to intratumoral synthesis of androgens, with castration-resistant prostate-cancer cells overexpressing the enzymes required for androgen biosynthesis.7,35–38 Surgical orchiectomy or pharmacologic castration with a gonadotropin-releasing hormone analogue (with or without an antiandrogen) leads to serum testosterone levels in the range of 20 to 50 ng per deciliter, as assessed with the use of radioimmunoassays. Abiraterone acetate further reduces androgen levels to the range of 1 to 2 ng per deciliter, as assessed with the use of supersensitive assays involving mass spectroscopy, redefining the concept of “castrate-range” testosterone levels.39 None of the other second-line hormonal agents, including the nonspecific CYP17 inhibitors ketoconazole and aminoglutethimide, improve survival, and each has a less favorable safety profile than abiraterone acetate. This study validates the hypothesis that the biosynthesis of steroid hormones downstream of CYP17 contributes to progression of castration-resistant prostate cancer in a subgroup of men for whom this disease remains driven by steroid ligands and should not be described as “hormone refractory.” Since these men cannot be identified a priori, continuing to call this disease “androgen independent” or “hormone refractory” is imprecise.22 As our study shows, blocking androgen synthesis by inhibiting CYP17 can produce tumor responses in patients who no longer have a response to standard hormonal therapies and who had received docetaxel-based chemotherapy.
The safety profile observed in the group of patients who received abiraterone acetate was similar to that observed in earlier clinical studies.15,17,20 Toxic effects were predominantly grade 1 or 2, with a low rate of drug discontinuation or dose reduction, and were largely mechanism-based and secondary to mineralocorticoid excess resulting from blockade of CYP17. These adverse events included hypokalemia, hypertension, and fluid retention, which were largely abrogated by the use of low-dose prednisone or prednisolone (5 mg twice a day), similar to the mitigation of hypertension and hypokalemia observed with the use of corticosteroids in the well-characterized syndrome of congenital CYP17 deficiency.40,41 Treatment with abiraterone acetate did not appear to increase the risk of metabolic changes or symptoms associated with chronic androgen deprivation.42–45 Nevertheless, longer follow-up is warranted to evaluate late toxic effects. Although grade 4 elevations in aminotransferase levels were observed early in the study, no additional cases were observed after the protocol amendment that mandated more frequent monitoring with liver-function tests during the first 12 weeks, with suspension of treatment in the event of grade 3 elevations in serum aminotransferase levels and reintroduction of abiraterone acetate at a reduced dose when liver enzyme levels returned to baseline values. Overall, compliance with abiraterone acetate treatment was high, and side effects were easily manageable and reversible, despite the advanced age and level of frailty of the study population.46
In conclusion, this study showed that abiraterone acetate plus prednisone, as compared with placebo plus prednisone, prolonged survival among patients with metastatic castration-resistant prostate cancer who had disease progression after docetaxel-based chemotherapy, with a low frequency of additional treatment-related toxic effects.
Supplementary Material
Acknowledgments
Supported by Ortho Biotech Oncology Research and Development (a unit of Cougar Biotechnology) and grants from the Medical Research Council of the United Kingdom, Experimental Cancer Medical Centre, National Institute for Health Research Biomedical Research Centre, and Prostate Cancer Foundation (to Dr. Scher).
Dr. de Bono reports being an employee of the Institute of Cancer Research, where abiraterone was first designed and synthesized and which has a commercial interest in abiraterone, and receiving consulting fees from Ortho Biotech Oncology Research and Development (a unit of Cougar Biotechnology), consulting fees and travel support from Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Dendreon, Enzon, Exelixis, Genentech, GlaxoSmithKline, Medivation, Merck, Novartis, Pfizer, Roche, Sanofi-Aventis, Supergen, and Takeda, and grant support from AstraZeneca; Dr. Logothetis, receiving consulting fees and travel support from Ortho Biotech Oncology Research and Development; Drs. Molina, Haqq, and Kheoh and Ms. Chieffo, being employees of and holding stock options in Johnson & Johnson; Dr. Fizazi, receiving lecture fees from Janssen-Cilag and consulting fees from Ortho Biotech Oncology Research and Development; Dr. North, receiving consulting fees from AstraZeneca, Pfizer, Sanofi-Aventis, Novartis, Abraxis, Ortho Biotech, Amgen, and GlaxoSmithKline and lecture fees from Novartis and Ortho Biotech; Dr. Chu, holding stock in Johnson & Johnson, GlaxoSmithKline, Eli Lilly, Novartis, Rigel Pharmaceuticals, and Sanofi-Aventis; Dr. Jones, receiving grant and travel support from Ortho Biotech Oncology Research and Development; Dr. Goodman, receiving consulting and lecture fees from Veridex (a division of Johnson & Johnson); Dr. Saad, receiving consulting fees from Amgen, Novartis, Sanofi-Aventis, and Astra- Zeneca and receiving payment for the development of educational presentations from Amgen and Novartis; Dr. Staffurth, receiving consulting fees from Pierre Fabre Laboratories and Ortho Biotech Oncology Research and Development, receiving travel support from Sanofi-Aventis, and holding stock in Johnson & Johnson; Dr. Harland, receiving consulting fees from Sanofi-Aventis and travel support from Ortho Biotech Oncology Research and Development; Dr. Flaig, receiving consulting fees from Sanofi-Aventis and lecture fees from Amgen; Dr. Sternberg, receiving consulting fees and travel support from Ortho Biotech Oncology Research and Development; Dr. Fléhon, receiving travel support from Novartis, Bayer, Pfizer, and Ortho Biotech Oncology Research and Development, serving as a board member for Novartis, Sanofi-Aventis, and Ferring, and receiving payment for the development of educational presentations from Novartis, AstraZeneca, Bayer, and Pfizer; Dr. Efstathiou, receiving consulting fees and travel support from Janssen-Cilag; and Dr. Scher, receiving consulting fees from Aragon, Bristol-Myers Squibb, Exelixis, Foundation Medicine, Genentech, and Medivation, consulting fees and travel support from Amgen, Ortho Biotech Oncology Research and Development, Dendreon, Enzon, Millenium, Novartis, Roche, and Sanofi-Aventis, travel support from AstraZeneca, Bristol-Myers Squibb, Exelixis, Genentech, Medivation, and Veridex, and grant support from Bristol-Myers Squibb, Ortho Biotech Services, Medivation, and Veridex, and holding stock in Eli Lilly, Genta, and Johnson & Johnson. No other potential conflict of interest relevant to this article was reported. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the patients who volunteered to participate in this study; the study-site staff members who cared for them; the members of the independent data and safety monitoring committee: Ian Tannock, M.D., Ph.D. (chair), Nick Thatcher, M.D., Sten Nilsson, M.D., and Ralph Harkins, Ph.D.; the members of the Cougar scientific advisory board: Arie Belldegrun, M.D., Eric Small, M.D., Phil Kantoff, M.D., Matthew Smith, M.D., Ph.D., Tia Higano, M.D., Nicholas Vogelzang, M.D., Michael Carducci, M.D., William Kelly, D.O., and Richard Auchus, M.D.; Carlo Messina, M.D., for assistance with the protocol; the sponsor staff involved in data collection and analyses: Esther Welkowsky, B.S., Antonieta Sosa, M.Sc., Amy Goodowitz Levin, R.N., B.S.N., Denise Kimball R.N., B.S.N., and Youn Choi Park, Ph.D.; and Namit Ghildyal, Ph.D., for editorial assistance in the development of the manuscript.
Footnotes
Additional COU-AA-301 investigators are listed in the Supplementary Appendix, available at NEJM.org.
References
- 1.Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin. 2002;52:154–179. doi: 10.3322/canjclin.52.3.154. [DOI] [PubMed] [Google Scholar]
- 2.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 3.Lam JS, Leppert JT, Vemulapalli SN, Shvarts O, Belldegrun AS. Secondary hormonal therapy for advanced prostate cancer. J Urol. 2006;175:27–34. doi: 10.1016/S0022-5347(05)00034-0. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 6.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 7.Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attard G, Cooper CS, de Bono JS. Steroid hormone receptors in prostate cancer: a hard habit to break? Cancer Cell. 2009;16:458–462. doi: 10.1016/j.ccr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Potter GA, Barrie SE, Jarman M, Rowlands MG. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J Med Chem. 1995;38:2463–2471. doi: 10.1021/jm00013a022. [DOI] [PubMed] [Google Scholar]
- 12.Attard G, Belldegrun AS, de Bono JS. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 2005;96:1241–1246. doi: 10.1111/j.1464-410X.2005.05821.x. [DOI] [PubMed] [Google Scholar]
- 13.Barrie SE, Haynes BP, Potter GA, et al. Biochemistry and pharmacokinetics of potent non-steroidal cytochrome P450(17alpha) inhibitors. J Steroid Biochem Mol Biol. 1997;60:347–351. doi: 10.1016/s0960-0760(96)00225-7. [DOI] [PubMed] [Google Scholar]
- 14.Jarman M, Barrie SE, Llera JM. The 16,17-double bond is needed for irreversible inhibition of human cytochrome p45017alpha by abiraterone (17-(3-pyridyl) androsta-5, 16-dien-3beta-ol) and related steroidal inhibitors. J Med Chem. 1998;41:5375–5381. doi: 10.1021/jm981017j. [DOI] [PubMed] [Google Scholar]
- 15.Attard G, Reid AH, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attard G, Reid AH, de Bono JS. Abiraterone acetate is well tolerated without concomitant use of corticosteroids. J Clin Oncol. 2010;28(29):e560–e561. doi: 10.1200/JCO.2010.29.5170. [DOI] [PubMed] [Google Scholar]
- 17.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 18.Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010;28:1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid AH, Attard G, Danila DC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010;28:1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–1488. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [Errata, J Clin Oncol 2000;18:2644, 2007;25:1154.] [DOI] [PubMed] [Google Scholar]
- 22.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 24.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 25.Harris K, Li K, Flynn C, Chow E. Worst, average or current pain in the Brief Pain Inventory: which should be used to calculate the response to palliative radiotherapy in patients with bone metastases? Clin Oncol (R Coll Radiol) 2007;19:523–527. doi: 10.1016/j.clon.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 27.Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920–928. doi: 10.1016/S0090-4295(97)00459-7. [DOI] [PubMed] [Google Scholar]
- 28.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 29.Bennett CL, Armitage JL, Buchner D, Gulati S. Economic analysis in phase III clinical cancer trials. Cancer Invest. 1994;12:336–342. doi: 10.3109/07357909409023033. [DOI] [PubMed] [Google Scholar]
- 30.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [Erratum, Clin Cancer Res 2009;15:1506.] [DOI] [PubMed] [Google Scholar]
- 31.Molina A, Belldegrun AS. Novel therapeutic strategies for castration-resistant prostate cancer: inhibition of persistent androgen production and androgen receptor mediated signaling. J Urol. 2011;185:787–794. doi: 10.1016/j.juro.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 32.Geller J, Albert JD, Nachtsheim DA, Loza D. Comparison of prostatic cancer tissue dihydrotestosterone levels at the time of relapse following orchiectomy or estrogen therapy. J Urol. 1984;132:693–696. doi: 10.1016/s0022-5347(17)49829-6. [DOI] [PubMed] [Google Scholar]
- 33.Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–424. doi: 10.1056/NEJM198908173210702. [Erratum, N Engl J Med 1989;321:1420.] [DOI] [PubMed] [Google Scholar]
- 34.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 35.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 36.Mohler JL, Gregory CW, Ford OH, III, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 37.Mostaghel EA, Page ST, Lin DW, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 38.Page ST, Lin DW, Mostaghel EA, et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91:3850–3856. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- 39.Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 40.Costa-Santos M, Kater CE, Auchus RJ. Two prevalent CYP17 mutations and genotype-phenotype correlations in 24 Brazilian patients with 17-hydroxylase deficiency. J Clin Endocrinol Metab. 2004;89:49–60. doi: 10.1210/jc.2003-031021. [DOI] [PubMed] [Google Scholar]
- 41.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32:81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levine GN, D’Amico AV, Berger P, et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. CA Cancer J Clin. 2010;60:194–201. doi: 10.3322/caac.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michaelson MD, Cotter SE, Gargollo PC, Zietman AL, Dahl DM, Smith MR. Management of complications of prostate cancer treatment. CA Cancer J Clin. 2008;58:196–213. doi: 10.3322/CA.2008.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michaelson MD, Marujo RM, Smith MR. Contribution of androgen deprivation therapy to elevated osteoclast activity in men with metastatic prostate cancer. Clin Cancer Res. 2004;10:2705–2708. doi: 10.1158/1078-0432.ccr-03-0735. [DOI] [PubMed] [Google Scholar]
- 46.de Bono JS, Logothetis CJ, Fizazi K, et al. Abiraterone acetate (AA) plus low dose prednisone (P) improves overall survival (OS) in patients (pts) with metastatic castration-resistant prostate cancer who have progressed after docetaxel-based chemotherapy (chemo): results of COU-AA-301, a randomized double-blind placebo-controlled phase 3 study. Ann Oncol. 2010;21(Suppl):LBA5. abstract. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.