Abstract
Activation of the P2Y1 nucleotide receptor in platelets by ADP causes changes in shape and aggregation, mediated by activation of phospholipase C (PLC). Recently, MRS2500 (2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate) was introduced as a highly potent and selective antagonist for this receptor. We have studied the actions of MRS2500 in human platelets and compared these effects with the effects of two acyclic nucleotide analogues, a bisphosphate MRS2298 and a bisphosphonate derivative MRS2496, which act as P2Y1 receptor antagonists, although less potently than MRS2500. Improved synthetic methods for MRS2500 and MRS2496 were devised. The bisphosphonate is predicted to be more stable in general in biological systems than phosphate antagonists due to the non-hydrolyzable C–P bond. MRS2500 inhibited the ADP-induced aggregation of human platelets with an IC50 value of 0.95 nM. MRS2298 and MRS2496 also both inhibited the ADP-induced aggregation of human platelets with IC50 values of 62.8 nM and 1.5 μM, respectively. A similar order of potency was observed for the three antagonists in binding to the recombinant human P2Y1 receptor and in inhibition of ADP-induced shape change and ADP-induced rise in intracellular Ca2+. No substantial antagonism of the pathway linked to the inhibition of cyclic AMP was observed for the nucleotide derivatives, indicating no interaction of these three P2Y1 receptor antagonists with the proaggregatory P2Y12 receptor, which is also activated by ADP. Thus, all three of the bisphosphate derivatives are highly selective antagonists of the platelet P2Y1 receptor, and MRS2500 is the most potent such antagonist yet reported.
Keywords: P2Y1 nucleotide receptor, G protein-coupled receptor, Acyclic nucleotides, Purines, Platelet aggregation, Carbocyclic nucleotides
1. Introduction
The P2 receptors for extracellular nucleotides are divided into two structurally-unrelated superfamilies: the P2Y receptors, which are G protein-coupled receptors (GPCRs), and the P2X receptors, which are ligand-gated ion channels (LGICs) [1,2]. The P2Y1,2,4,6,11 receptors couple preferentially to the activation of phospholipase C (PLC), via heterotrimeric G proteins of the Gq family and therefore induce an increase in intracellular calcium. The P2Y12,13,14 receptors are coupled preferentially to the inhibition of adenylate cyclase via heterotrimeric G proteins of the Gi family. The P2Y receptors are activated by adenine and/or uracil nucleotides [3]. The P2Y2 receptor is the only human subtype that is activated by both ATP and UTP (but not the corresponding 5′-diphosphates). The pyrimidine-selective receptors include the P2Y4, P2Y6 and P2Y14 receptors, which are activated by UTP, UDP and UDP-glucose, respectively [4]. The remaining subtypes (P2Y1, P2Y11, P2Y12 and P2Y13 receptors) are activated by adenine nucleotides.
ADP is released from platelets and endothelial cells to induce aggregation of platelets and promote intravascular coagulation by acting at the P2Y1 and P2Y12 receptors [5-10]. Antagonists of either P2Y receptor subtype display antiaggregatory properties. A mouse line lacking expression of the P2Y1 receptor has been constructed [11,12], and the absence of P2Y1 receptors in platelets of these mice interfered with ADP-promoted aggregation and in vivo thrombus formation. A third nucleotide receptor, the P2X1 receptor, is present on platelets, and its role in aggregation appears to be most evident under conditions of high shear stress [13,31].
Fig. 1 illustrates the chemical structures of a variety of recently-introduced antagonists of the P2Y1 receptor. The structures differ markedly in the nature of the ribose-like moiety to which the adenine nucleobase is attached. MRS2179 (N6-methyl-2′-deoxyadenosine 3′,5′-bisphosphate) 1 and its 2-chloro analogue MRS2216 2 (Fig. 1) have been designed as nucleotide antagonists of the P2Y1 receptor [14-19]. In suspensions of washed human platelets, MRS2179 inhibited ADP-induced platelet shape change, aggregation and Ca2+ rise but had no effect on ADP-induced inhibition of adenylate cyclase [20]. Binding studies using 33P-labeled MRS2179 demonstrated the presence of an average of 134 binding sites per platelet. MRS2179 inhibited both arterial and venous thrombosis, further establishing this receptor as a potential target for antithrombotic drugs [21]. Further structural modification resulted in the highly potent P2Y1 receptor antagonists MRS2279 3 and the 2-iodo analogue MRS2500 4 [19], containing the rigid (N)-methanocarba ring system. MRS2279 has been radiolabeled for use as a high affinity, competitive radiotracer in binding experiments [18], We previously characterized the SAR (structure activity relationship) as P2Y1 receptor antagonists of acyclic analogues of adenine nucleotides, containing two phosphate groups on a symmetrically-branched aliphatic chain, attached at the 9-position of adenine [22,23]. The submicromolar potency of the acyclic P2Y1 receptor antagonists MRS2298 5 and MRS2496 6, both related to a 2-chloro-N6-methyladenine-9-(2-methylpropyl) scaffold, indicated that the ribose ring structure itself is not necessary for receptor recognition but rather serves as a spacer. A significant correlation was observed between the capacity of several of these P2Y1 receptor antagonists to inhibit ADP-induced platelet aggregation in rat platelets [5,23] and the inhibition of P2Y1 receptor-stimulated phospholipase C activity previously determined in turkey erythrocytes [22]. The present study compares the activity of three of these P2Y1 receptor antagonists on aggregatory properties and second messenger (cAMP and PLC) signaling in human platelets.
Fig. 1.
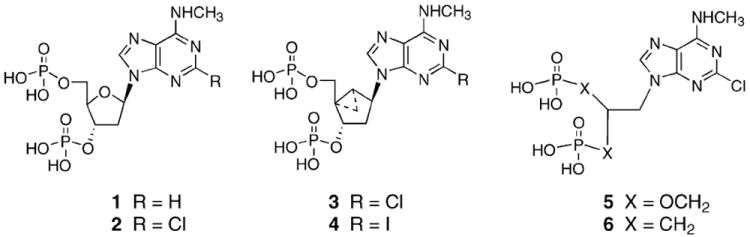
Structures of nucleotide (bisphosphate) derivatives that have been shown to act as inhibitors of P2Y1 receptors. The nucleotide analogues differ mainly in the nature of the ribose-like moiety and include 9-ribosides (1, 2), the (N)-methanocarba ring system, which is sterically-constrained in the North 2′-exo conformation favored by the P2Y1 receptor (3, 4), and acyclic analogues (5, 6).
2. Materials and methods
2.1. Reagents
myo-[3H]Inositol (20 Ci/mmol) was obtained from American Radiolabeled Chemicals. Dowex AG 1-X8 resin was purchased from Bio-Rad. DMEM and FBS were from Life Technologies. All other reagents were purchased from Sigma–Aldrich Chemical Co. HPLC mobile phases consisted of System A: linear gradient solvent system: CH3CN/triethyl ammonium acetate from 5/95 to 60/40 in 20 min, flow rate 1.0 mL/min; System B: linear gradient solvent system: CH3CN/tetrabutyl ammonium phosphate from 20/80 to 60/40 in 20 min, flow rate 1.0 mL/min. Apyrase was a kind gift of R.L. Kinlough-Rathbone (McMaster University, Hamilton, Ontario): the activity of the compound was such that the enzyme (1 μL/mL) converted 0.25 μmol/L ATP to adenosine monophosphate within 120 s at 37 °C.
2.2. Chemical synthesis
The following synthetic methods are depicted schematically in Fig. 2.
Fig. 2.
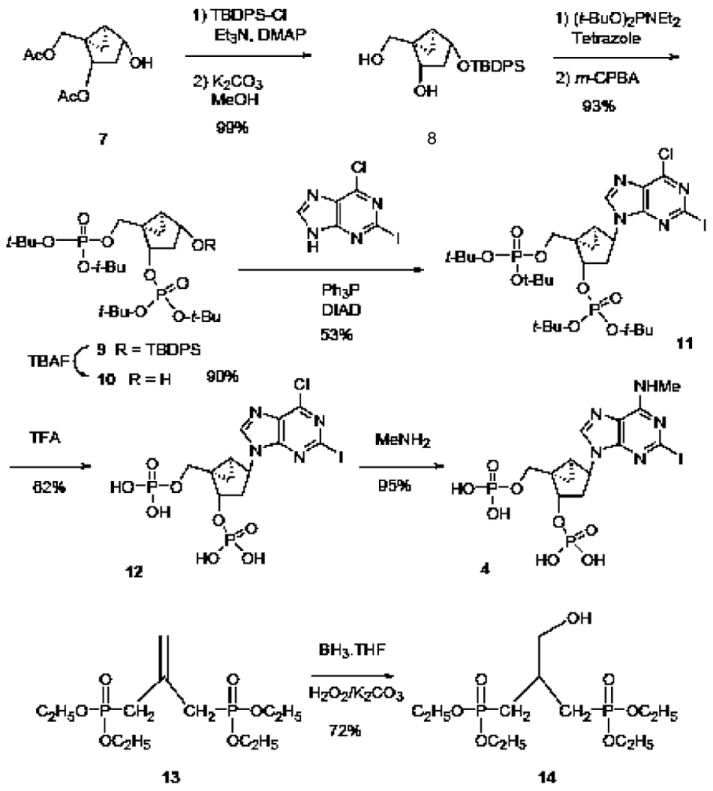
Improved synthetic routes for the preparation of the (N)-methanocarba antagonist MRS2500 4 and the acyclic antagonist MRS2496 6. The route applied to MRS2500 involves phosphorylation of the [3.1.0]bicyclohexane system in 8, condensation with the nucleobase using a Mitsunobu coupling to yield 11, followed by deprotection of the phosphate groups and functional group replacement on the adenine moiety. The single step applied to the preparation of MRS2500 involves hydroboration to form the intermediate 14.
2.2.1. (1′R,2′S,4′R,5′S)-4-(tert-Butyl-diphenyl-silanyloxy)-1-hydroxymethyl-bicyclo[3.1.0]hexan-2-ol (8)
To a solution of acetic acid 1-acetoxymethyl-4-hydroxy-bicyclo [3.1.0]hex-2-yl ester 7 ([24], 750 mg, 3.29 mmol) in CH2C12 (10 mL) was added 4-N,N-dimethylaminopyridine (50 mg, 0.41 mmol), Triethylamine (1.00 mL, 7.17 mmol) and t-butyl-diphenylsilyl chloride (TBDPS, 1.85 mL, 7.11 mol) and the reaction mixture was stirred at rt for 23 h. The reaction mixture was diluted with ethyl acetate (EtOAc) and was washed sequentially with 5% citric acid, brine, sat NaHCO3, and brine. The organic layer was dried over Na2SO4 and the solvent evaporated. The residue was dissolved in MeOH (50 mL) and K2CO3 (166 mg, 1.20 mmol) was added. The reaction mixture was stirred at rt for 3 days and evaporated. The residue was dissolved with AcOEt and washed with 5% citric acid, sat NaHCO3, and brine, and dried over Na2SO4 and the solvent evaporated. The residue obtained was purified by column chromatography (silica gel, eluent: AcOEt/petroleum ether = 1/2 then 1/0), to give 8 (1.24 g, 99%). 1H NMR (CDCl3) δ 7.72–7.62 (m, 4H), 7.43–7.34 (m, 6H), 4.36–4.23 (m, 2H), 3.72 (dd, 1H, J = 5.1, 11.1 Hz), 3.50 (dd, 1H, J = 5.7, 11.1 Hz), 2.17–2.06 (m, 1H), 1.83 (d, 1H, J = 5.1 Hz), 1.57 (m, 1H), 1.32 (m, 2H), 1.04 (s, 9H), 0.55 (t, 1H, J = 7.7 Hz); MS (m/e) (positive-FAB) 381 (M − H)+, 405 (M + Na)+.
2.2.2. (1′R,2′S,4′R,5′S)-Phosphoric acid di-tert-butyl ester 4-(tert-butyl-diphenyl-silanyloxy)-1-(di-tert-butoxy-phosphoryloxymethyl)-bicyclo[3.1.0]hex-2-yl ester (9)
To a stirred solution of 8 (82 mg, 0.21 mmol) and 1H-tetrazole (79 mg, 1.13 mmol) in anhydrous tetrahydrofuran (THF, 2.0 mL) was added di-t-butyl diethylphosphoramidite (0.400 mL, 1.44 mmol), and the reaction mixture was stirred for 3 h at room temperature. The reaction mixture was cooled to −78 °C and treated with a solution of m-chloroperbenzoic acid (70% max, 530 mg) in CH2Cl2 (5.0 mL). The resulting mixture was warmed to room temperature and stirred for 30 min. 5% NaHSO3 (2.0 mL) was added to the reaction mixture, and was stirred for another 10 min at room temperature. The reaction mixture was extracted with AcOEt and the organic phase was subsequently washed with saturated aqueous NaHCO3 and brine and dried over Na2SO4 and filtered. The solvent was removed under reduced pressure. The obtained residue was purified by silica gel column chromatography (AcOEt/petroleum ether = 1/2 to 1/0), to give 9 (152 mg, 93%). 1H NMR (CDC13) δ 7.70–7.60 (m, 4H), 7.43–7.31 (m, 6H), 4.72 (m, 1H), 4.32 (m, 2H), 3.61 (dd, 1H, J = 6.9, 11.4 Hz), 2.27 (dt, 1H, J = 7.5, 13.5 Hz), 1.72 (m, 1H), 1.52 (s, 9H), 1.46 (s, 18H), 1.39 (s, 9H), 1.33 (s, 9H), 1.26 (m, 1H), 1.07(m, 1H), 0.67(m, 1H); MS (m/e) (positive-FAB) 767 (M + H)+.
2.2.3. (1′R,2′S,4′R,5′S)-Phosphoric acid di-tert-butyl ester 1-(di-tert-butoxy-phosphoryloxymethyl)-4-hydroxy-bicyclo[3.1.0]hex-2-yl ester (10)
To a stirred solution of 9 (2.16 g, 2.81 mmol) in 6.0 mL of anhydrous THF was added 1.0 M tetrabutyl ammonium fluoride THF solution (4.3 mL, 4.3 mmol) and the reaction mixture was stirred at room temperature for 24 h. The solvent was removed under reduced pressure. The residue obtained was purified by silica gel column chromatography (MeOH/CHCl3 = 1/10), which furnished 10 (1.34 g, 90%). 1H NMR (CDCl3) 4.89 (q, 1H, J = 7.4 Hz), 4.41 (m, 2H), 3.69 (dd, 1H, J = 6.9, 10.8 Hz), 2.46 (dt, 1H, J = 8.0, 13.5 Hz), 1.80 (m, 1H), 1.49 (s, 18H), 1.48(s, 9H), 1.47 (s, 9H), 1.23 (m, 1H), 1.09 (m, 1H), 0.69 (m, 1H); MS (m/e) (positive-FAB) 529 (M + H)+.
2.2.4. (1′R,2′S,4′S,5′S)-Phosphoric acid di-tert-butyl ester 1-(di-tert-butoxy-phosphoryloxymethyl)-4-(6-chloro-2-iodo-purin-9-yl)-bicyclo[3.1.0]hex-2-yl ester (11)
To a solution of triphenylphosphine (101 mg, 0.385 mmol) in anhydrous THF (1.00 mL) was added diisopropyl azodicarboxylate (0.075 mL, 0.38 mmol) at rt with stirring for 1.5 h. Compound 10 (102 mg, 0.194 mmol) and 6-chloro-2-iodopurine ([19], 70 mg, 0.25 mmol) in THF (2.20 mL) were added to the reaction mixture, and it was stirred at room temperature for 23 h. The solvent was removed under vacuum and the residue obtained was purified by preparative thin-layer chromatography (AcOEt), which furnished 11 (81.3 mg, 53%). 1H-NMR (CDC13) δ 8.44 (s, 1H), 5.34 (dd, 1H, J = 8.1, 15.0 Hz), 5.16 (d, 1H, J = 6.9 Hz), 4.69 (dd, 1H, J = 5.1, 11.4 Hz), 3.94 (dd, 1H, J = 6.6, 11.4 Hz), 2.40–2.30 (m, 1H), 2.22–2.10 (m, 1H), 1.85–1.80 (m, 1H), 1.50 (s, 9H), 1.49 (s, 18H), 1.48 (s, 9H), 1.18–1.14 (m, 1H), 1.09–1.03(m, 1H); MS (m/e) (positive-FAB) 791, 793 (peak height ratio 3:1) (M + H)+.
2.2.5. (1′R,2′S,4′S,5′S)-4-(6-chloro-2-iodo-9H-purin-9-yl)-1-[(phosphato)-methyl]-2-(phosphato)-bicyclo[3.1.0]hexane tetrakis ammonium salt (12)
A mixture of 11 (33.0 mg, 0.042 mmol) in CH2C12 (3 mL) was treated with trifluoroacetic acid (TFA, 0.100 mL) and the reaction mixture was stirred at room temperature for 3 h. After removal of the solvent, the crude 12 was purified with ion-exchange column chromatography with the use of Sephadex-DEAE-A-25 resin with a linear gradient (0.01–0.7 M) of 0.5 M NH4HCO3 as the mobile phase. After lyophilization, 12 (16.3 mg, 62%) was obtained as a white solid. 1H NMR(D2O) δ 8.83 (s, 1H), 5.30–5.20 (m, 1H), 5.16 (d, 1H, J = 6.3 Hz), 4.60–4.50 (m, 1H), 3.75–3.65 (m, 1H), 2.40–2.20 (m, 1H), 2.10–1.95 (m, 1H), 1.95–1.90 (m, 1H), 1.25–1.20 (m, 1H), 1.05–1.00 (m, 1H); 31P NMR (D2O) δ 2.02, 1.40 (2s, 3′-P, 5′-P); MS (m/e) (negative-FAB) 565, 567 (peak height ratio = 3:1) (M − H)+; HPLC 9.8 min (98%) in solvent system A, 16.0 min (98%) in solvent system B.
2.2.6. (1′R,2′S,4′S,5′S)-4-(2-iodo-6-methylamino-purin-9-yl)-l-[(phosphato)-methyl]-2-(phosphato)-bicyclo[3.1.0]hexane (4)
To a solution of 12 (10.9 mg, 0.017 mmol) in water (5.00 mL) was added 40% MeNH2 in water (1.0 mL) and the reaction mixture was stirred for 2 h at room temperature. The reaction was monitored by HPLC. The reaction mixture was subsequently frozen and lyophilized. Purification of the residue obtained was performed on an ion-exchange column packed with Sephadex-DEAE A-25 resin. A linear gradient (0.01–0.7 M) of 0.5 M ammonium bicarbonate was applied as the mobile phase, and UV and HPLC were used to monitor the elution, which furnished 4 (10.2 mg, 95%). 1H NMR (D2O) δ 8.54 (bs, 1H), 5.19 (m, 1H), 5.01 (d, 1H, J = 6.9 Hz), 4.58 (dd, 1H, J = 4.7, 11.3 Hz), 3.73 (dd, 1H, J = 4.4, 11.0 Hz), 3.07 (bs, 3H), 2.28 (dd, 1H, J = 7.7, 14.6 Hz), 1.92–2.09 (m, 2H), 1.26 (dd, 1H, J = 4.1, 6.1 Hz), 1.06 (dd, 1H, J = 9.7, 16.3 Hz). 31P NMR (D2O) 0.651 (s). High-resolution MS (negative-ion FAB) calcd for C13H17N5O8P2I 559.9597, found 559.9604, HPLC 9.8 min (99%) in solvent system A, 15.4 min (99%) in system B.
2.2.7. Tetraethyl 2-hydroxymethyl-1,3-propanebisphosphonate (14)
Compound 13 (3.28 g, 10 mmol) was dissolved in THF (25 mL) and BH3-THF complex (34 mL of 1 M in THF, 34 mmol) was added at 0–5 °C, and the reaction mixture was allowed to warm to room temperature. After stirring for an additional 8 h, the mixture was cooled to 0 °C and treated with solid K2CO3 (10 g) and 30% hydrogen peroxide (20 mL), added slowly. The resulting reaction mixture was stirred at room temperature for 2 h. It was then extracted twice with EtOAc (100 mL × 2), and the combined organic layer was dried over sodium sulfate and concentrated in vacuo. The final purification was effected by silica-gel column chromatography (EtOAc:MeOH, 75:25) to afford 14 (2.49 g, 72%) as an oil. The spectral data of 14 were found to be comparable with a genuine sample prepared previously [21].
2.3. Binding assay
The affinities of bisphosphate analogues for the human P2Y1 receptor were directly determined by using [3H]MRS2279 in a radioligand binding assay, as we recently described in detail [18]. Briefly, the human P2Y1 receptor was expressed in 1321N1 astrocytoma cells. Membranes prepared from these cells were incubated for 30 min at 4 °C in the presence of ~20 nM [3H]MRS2279 and a wide range of concentrations of the nucleotide analogues. Binding reactions were terminated by the addition of ice-cold tris(hydroxymethyl)aminomethane (Tris) wash buffer (10 mM Tris, pH 7.5, and 145 mM NaCl) to the samples followed by rapid filtration over GF/A glass-fiber filters. Each filter was washed with an additional 4 mL of wash buffer, and radioactivity retained by the filters was quantified by liquid scintillation spectrometry. All assays were carried out in triplicate, and competition curves for all molecules were generated in three separate experiments. IC50 values were determined from each competition curve, and a Ki value was calculated for each analogue according to the relationship Ki = IC50/1 + [[3H]MRS2279]/Kd of [3H]MRS2279, where the Kd of [3H]MRS2279 determined in separate experiments was 8 nM.
2.4. Preparation of washed platelet suspensions
Six volumes of blood were drawn into one volume of acid-citrate-dextrose anticoagulant. Twice washed platelet suspensions were prepared according to the method described by Mustard et al. [25], with the exception that 500 nmol/L PGI2 was added during the first and second wash. The number of platelets was adjusted to 4 × 1011/L in the final suspension, which contained Tyrode’s buffer, 0.35% bovine serum albumin, 0.1% dextrose and 1 μL/mL apyrase.
2.5. Studies of platelet aggregation and shape change
The inhibition of ADP-induced activation of human platelets was measured using aggregometry using washed platelet suspensions. Samples of washed platelet suspensions (0.45 mL) were incubated with vehicle or test compounds at 37 °C for 30 s and stirred at 1000 rpm in an aggregometer (Lumi-Aggregometer, Chrono-log Corp.). After incubation, 10 μL of ADP (final concentration, 5 μmol/L) was added and the aggregation tracings were recorded for 3 min. The initial rapid decrease in oscillations of the basal tracings and increase in optical density were interpreted as being caused by a platelet shape change. Concentration response curves for antagonists were constructed, and the IC50 values determined (n = 3).
2.6. Measurement of intracellular ionized calcium ([Ca2+]i)in platelets
Platelets were loaded with 2 μmol/L fura-2/AM at 37 °C for 45 min as previously described [26]. Aliquots of fura-2/AM-loaded platelets were transferred to quartz cuvettes maintained at 37 °C. Fluorescence was monitored continuously before and after stimulation with ADP, using a spectrofluorimeter (LS50B, Perkin-Elmer Co.). The excitation wavelength was alternatively fixed at 340 and 380 nm, and fluorescence emission was determined at 510 nm.
2.7. Measurement of cyclic AMP (cAMP) in platelets
Platelet cyclic AMP levels were measured by a radioisotopic assay, using a commercially available kit (Amersham). Duplicate samples of 1 mL washed platelets containing theophylline (1 mM) were incubated with: (a) Tyrode’s buffer and PGE1 (1 μmol/L), (b) Tyrode’s buffer, PGE1 and ADP (10 μM) or (c) Tyrode’s buffer alone. After incubation at 37 °C (2 min), 1 mL of 5% trichloroacetic acid was added, and the samples were snap-frozen in dry ice and methanol, thawed at room temperature (22–25 °C), and then shaken at 4 °C for 45 min. After centrifugation at 4 °C for 30 min, the supernatant was extracted three times with 5 mL of water-saturated ether, dried under a stream of nitrogen at 60 °C, and stored at −20 °C. Before assay, the samples were reconstituted with 50 mM Tris buffer containing 4 mM EDTA.
2.8. Statistical analysis
Pharmacological parameters were analyzed by Graph-PAD Prism software. Data were expressed as mean ± standard error.
3. Results
3.1. Chemistry
An improved synthetic method for the preparation of MRS2500 4 is shown in Fig. 2. The previous route of Kim et al. [19] featured a condensation of the N6-methyl-2-iodoadenine with the prephosphorylated and protected (N)-methanocarba ring system 10. In contrast, the present method began with the Mitsunobu coupling of 6-chloro-2-iodoadenine with the same methanocarba ring moiety 10 to provide 11. Following deprotection of the phosphate esters of 11 to yield 12, the 6-chloro was substituted with methylamine resulting in MRS2500 4. This synthetic route also would be amenable to tritium labeling upon treatment of 12 with labeled methylamine, similar to the route used for labeling MRS2279 [18].
We devised an alternate approach to the synthesis of intermediate 10. The previous study used a 1′-acetoxy protection scheme in the phosphorylation step. The present method used the orthogonal protection scheme of transient acetylation of the 3′-and 5′-hydroxyl groups 7, followed by silylation of the 1′-hydroxyl and removal of the esters using potassium carbonate, to provide intermediate 8. Phosphorylation was carried out using the phosphoramidite method as described [19], and subsequent desilylation provided 10 in high yield.
The acyclic phosphonate MRS2496 was prepared by the method reported by Xu et al. [23], except that an improvement was made in the preparation of the intermediate tetraethyl 2-hydroxymethyl-1,3- propane bisphosphonate 14 from diethyl 2-(diethylphosphonomethyl)-allylphosphonate 13. Use of borane-tetrahydrofuran complex in this hydroboration step proved superior to 9-borabicyclo[3.3.1]nonane (9-BBN) in giving higher yields as well as a simpler product isolation procedure.
3.2. Inhibition of radioligand binding at the human P2Y1 receptor
The affinity of the three nucleotide bisphosphate analogues at the human P2Y1 receptor expressed in 1321N1 astrocytoma cells was determined in a binding assay using the recently introduced antagonist radioligand [3H]MRS2279 [18] and Ki values were calclulated (Table 1). The order of affinity based on Ki values was 4 ≫ 5 > 6. Thus, the conformationally constrained analogue 4 was demonstrated to have extremely high receptor affinity, while the two acyclic compounds were of intermediate affinity.
Table 1.
Activities of the adenine nucleotide derivatives as antagonists of functions induced by 10 μM ADP in human platelets and in a competitive binding assay at heterologously expressed human P2Y1 receptors in 1321 astrocytoma cells (n = 3)
| IC50 or Ki (nM) (mean ± S.E.M.)
| ||||
|---|---|---|---|---|
| Compound | Inhibition of P2Y1 receptor bindinga (Ki) | Inhibition of platelet aggregationb (IC50) | Inhibition of Ca2+ rise in plateletsb IC50 | Inhibition of cAMP effects in plateletsb (IC50) |
| 4 MRS2500 | 0.78 ± 0.08 | 0.95 ± 0.16 | 49.1 ± 6.7 | >100,000 |
| 5 MRS2298 | 29.6 ± 3.3 | 62.8 ± 15.6 | 810 ± 207 | >100,000 |
| 6 MRS2496 | 76 ± 10 | 1500 ± 250 | 16,100 ± 4600 | >100,000 |
Using [3H]MRS2279 as radioligand [18].
In washed platelet suspensions.
3.3. Inhibition of ADP-induced platelet responses
Three nucleotide bisphosphate analogues were examined as inhibitors of ADP-induced aggregation of human platelets, using platelet aggregometry [23,26]. Compound 4, the (N)-methanocarba derivative, was highly potent in inhibiting ADP (10 μM)-induced platelet aggregation with an IC50 value of 0.95 nM. Compound 5, the acyclic bisphosphate antagonist MRS2298, and compound 6, the bisphosphonate acyclic nucleotide, also inhibited ADP (10 μM)-induced platelet aggregation in a concentration-dependent manner (Table 1) and with IC50 values of 62.8 nM and 1.5 μM, respectively. The effect of these three P2Y1 receptor antagonists in ADP-promoted Ca2+ mobilization also was examined in human platelets. All three antagonists inhibited ADP (10 μM)-induced increases in intra-cellular calcium, and the order of potency for inhibition (4 > 5 > 6) was the same as observed for inhibition of ADP-promoted aggregation (Table 1).
All three antagonists also inhibited ADP-promoted shape change with an order of potency identical to that observed for aggregation and Ca2+ responses. In contrast to platelet aggregation, the inhibition of ADP (10 μM)-induced platelet shape change was complete at concentrations of the compounds that completely inhibited the ADP-induced intracellular Ca2+ rise (Fig. 3).
Fig. 3.
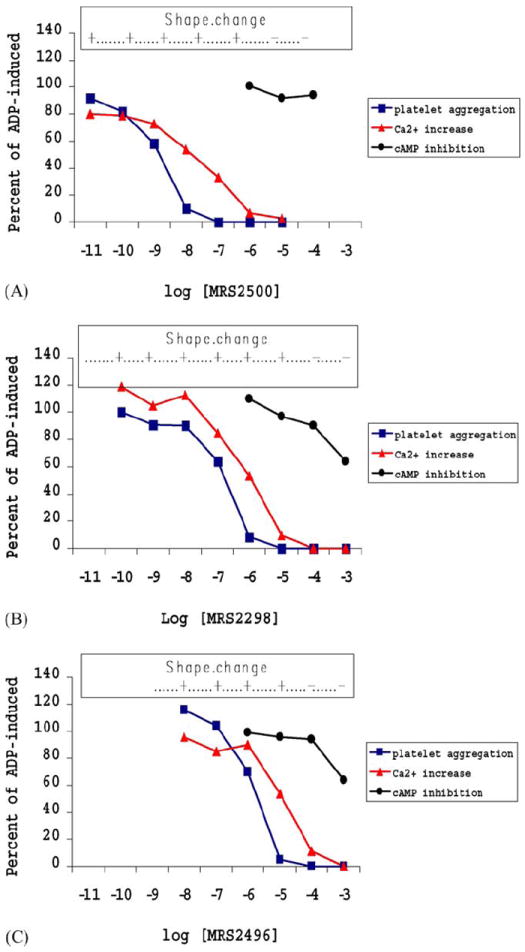
Concentration-response curve for inhibition of ADP-induced processes in human platelets by MRS2500 4 (upper panel), the acyclic bisphosphate derivative 5 (middle panel), and the acyclic bisphosphonate derivative 6 (lower panel). Aggregation was induced in the presence of 10 μM ADP. The antagonism of the following parameters is expressed as percent (mean ± S.D.) of ADP-induced control in the presence of 1% DMSO: aggregation, increase in Ca2+, and the inhibition of adenylate cyclase. An upper bar indicates whether the initial shape change in the platelets was observed at each concentration of the antagonist.
The cAMP inhibitory pathway in platelets [26] is associated with activation of P2Y12 receptors. We examined whether any of these three antagonists for interaction with the P2Y12 receptor-promoted pathway (Table 1 and Fig. 3). No significant effect of compound 4 was observed on the capacity of 10 μM ADP to inhibit PGE1-stimulated cAMP accumulation. Compounds 4 and 6 at the highest concentration tested (1 mM) caused approximately 40% inhibition of the ADP (10 μM)-induced effects on cAMP. Compounds 4, 5, and 6 alone had no effect on basal cAMP levels (not shown).
4. Discussion
We have compared the effects of three antagonists of the P2Y1 receptor on ADP-induced responses in human platelets and at recombinant human P2Y1 receptors. All compounds exhibited selective and potent antagonism of the P2Y1-dependent responses of platelets to ADP. The potency order was consistently 4 > 5 > 6 by a variety of criteria for antagonism of P2Y1 receptors (Table 1). The same order of potency was observed for all three antagonists for the inhibition of ADP-promoted Ca2+ mobilization, shape change, and aggregation. Lower concentrations of antagonists were necessary to inhibit completely platelet aggregation than shape change and Ca2+ mobilization, apparently because platelet aggregation requires a higher degree of receptor occupancy by ADP than shape change and Ca2+ mobilization [27]. In a previous study [23] it was demonstrated the 5 and 6 inhibited the aggregation of rat platetets induced by 3.3 μM ADP in platelet-rich plasma with IC50 values of 300 and 680 nM, respectively. Compound 4 was reported to inhibit the activation of PLC induced by 30 nM 2-methylthio-ADP with an IC50 value of 8.4 nM [19]. Thus, the order of potency is consistent with previous findings.
Each of the three compounds has a unique chemical character. The most potent antagonist, MRS2500, contains a novel rigid ring system, which has been shown to enhance P2Y1 receptor selectivity and impede the hydrolysis of pendant 5-phosphate esters [2]. MRS2298, the flexible, yet structurally optimized acyclic phosphate ester [22], was intermediate in potency. Finally, a bisphosphonate MRS2496 [23], structurally similar to MRS2298, except more chemically and biologically stable due to the present of C-P bonds rather than C-O-P bonds, also inhibited P2Y1-receptor mediated platelet function at micromolar concentrations.
No substantial antagonism of the pathway linked to the inhibition of cyclic AMP was observed with these three P2Y1 receptor antagonists, indicating no interaction with the proaggregatory P2Y12 receptor, which is also activated by ADP. Thus, we have demonstrated the functional selectivity of the derivatives for the P2Y1 receptor in human platelets. It will be important to examine the activity of these compounds in isolated organs and in vivo. This would further clarify the feasibility of using P2Y1 receptor antagonists as antithrombotic agents, which has already been established experimentally. A previously reported P2Y1 receptor antagonist, the riboside derivative MRS2179, was studied in the isolated guinea pig heart [28], to demonstrate that vasodilation induced by endogenous ADP occurs principally through the P2Y1 receptor rather than the adenosine A2A receptor (as a result of the breakdown of ADP to adenosine). These compounds will also be useful in determining the relative importance of the two metabotropic ADP receptors in platelet activation in vivo [29]. Since there are other known sites of the P2Y1 receptor in adult and developing mammals [30], both in the brain and in the periphery (i.e. endothelial cells, bone cells, skeletal muscle), it would be important to assess possible side effects of such proposed therapeutic use using potent and relatively stable analogues. In summary, these selective antagonists potentially are valuable as pharmacological tools for defining the role of P2Y1 receptors in platelet function and in other physiological processes.
Acknowledgments
B.V. Joshi thanks Gilead Sciences (Foster City, CA) for financial support. We also thank Toray Industries, Inc., Pharmaceutical Research Laboratories (Kamakura, Japan) for financial support. We thank Dr. G. Cook and G. Kirschenheuter (formerly of Gilead Sciences) and Heng T. Duong (NIDDK) for helpful discussion and Savitri Maddileti (University of North Carolina, Chapel Hill, NC) for technical assistance.
Abbreviations
- MRS2279
2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate
- MRS2298
2-[2-(2-chloro-6-methylamino-purin-9-yl)methyl]propane-1,3-bisoxy(diammoniumphosphate)
- MRS2496
[3-(2-chloro-6-methylamino-purin-9-yl)-2-phosphonomethyl-propyl]-phosphonic acid ammonium salt
- MRS2500
2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate
- PLC
phospholipase C
- Tris
Tris(hydroxymethyl)aminomethane
References
- 1.Fredholm BB, Abbracchio MP, Burnstock G, Dubyak GR, Harden TK, Jacobson KA, et al. Towards a revised nomenclature for PI and P2 receptors. Trends Pharmacol Sci. 1997;18:79–82. doi: 10.1016/s0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson KA, Costanzi S, Ohno M, Joshi BV, Besada P, Xu B, et al. Molecular recognition at purine and pyrimidine nucleotide (P2) receptors. Curr Trends Med Chem. 2004;4:805–19. doi: 10.2174/1568026043450961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Kügelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:310–23. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- 4.Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, et al. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–5. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leon C, Ravanat C, Freund M, Cazenave JP, Gachet C. Differential involvement of the P2Y1 and P2Y12 receptors in platelet procoagulant activity. Arterioscler Thromb Vasc Biol. 2003;23:1941–7. doi: 10.1161/01.ATV.0000092127.16125.E6. [DOI] [PubMed] [Google Scholar]
- 6.Kunapuli SP, Ding Z, Dorsam RT, Kim S, Murugappan S, Quinton TM. ADP receptors—argets for developing anti thrombotic agents. Curr Pharm Des. 2003;9:2303–16. doi: 10.2174/1381612033453947. [DOI] [PubMed] [Google Scholar]
- 7.Leon C, Alex M, Klocke A, Morgenstern E, Moosbauer C, Eckly A, et al. Platelet ADP receptors contribute to the initiation of intravascular coagulation. Blood. 2004;103:594–600. doi: 10.1182/blood-2003-05-1385. [DOI] [PubMed] [Google Scholar]
- 8.Webb TE, Simon J, Krishek BJ, Bateson AN, Smart TG, King BF, et al. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993;324:219–25. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- 9.Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–7. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 10.Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–8. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fabre JE, Nguyen M, Latour A, Keifer JA, Audoly LP, Coffman TM, et al. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat Med. 1999;5:1199–202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- 12.Leon C, Hechler B, Freund M, Eckly A, Vial C, Ohlmann P, et al. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y1 receptor-null mice. J Clin Invest. 1999;104:1731–7. doi: 10.1172/JCI8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cattaneo M, Marchese P, Jacobson KA, Ruggeri ZM. The P2X1 receptor for ATP plays an essential role in platelet aggregation at high shear. Haematol J. 2003;4(Suppl 2):150. abstract. [Google Scholar]
- 14.Nandanan E, Jang SY, Moro S, Kim H, Siddiqui MA, Russ P, et al. Synthesis, biological activity, and molecular modeling of ribose-modified adenosine bisphosphate analogues as P2Y1 receptor ligands. J Med Chem. 2000;43:829–42. doi: 10.1021/jm990249v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown SG, King BF, Kim Y-CC, Burnstock G, Jacobson KA. Activity of novel adenine nucleotide derivatives as agonists and antagonists at recombinant rat P2X receptors. Drug Dev Res. 2000;49:253–9. doi: 10.1002/1098-2299(200004)49:4<253::AID-DDR4>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyer J, Adams M, Ravi RG, Jacobson KA, Harden TK. 2-Chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate is a selective high affinity P2Y1 receptor antagonist. Br J Pharmacol. 2002;135:2004–10. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyer J, Adams M, Ravi RG, Jacobson KA, Harden TK. 2-Chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate is a selective high affinity P2Y1 receptor antagonist. Br J Pharmacol. 2002;135:2004–10. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji XD, et al. Quantitation of the P2Y1 receptor with a high affinity radiolabeled antagonist. Mol Pharmacol. 2002;62:1249–57. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HS, Ohno M, Xu B, Kim HO, Choi Y, Ji XD, et al. 2-Substitution of adenine nucleotide analogues containing a bicyclo[3.1.0]hexane ring system locked in a Northern conformation: Enhanced potency as P2Y1 receptor antagonists. J Med Chem. 2003;46:4974–87. doi: 10.1021/jm030127+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baurand A, Raboisson P, Freund M, Leon C, Cazenave JP, Bourguignon JJ, et al. Inhibition of platelet function by administration of MRS2179, a P2Y1 receptor antagonist. Eur J Pharmacol. 2001;412:213–21. doi: 10.1016/s0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]
- 21.Lenain N, Freund M, Leon C, Cazenave JP, Gachet C. Inhibition of localized thrombosis in P2Y1-deficient mice and rodents treated with MRS2179, a P2Y1 receptor antagonist. J Thromb Haemost. 2003;1:1144–9. doi: 10.1046/j.1538-7836.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, Barak D, Harden TK, Boyer JL, Jacobson KA. Acyclic and cyclopropyl analogues of adenosine bisphosphate antagonists of the P2Y1 receptor: structure activity relationships and receptor docking. J Med Chem. 2001;44:3092–108. doi: 10.1021/jm010082h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu B, Stephens A, Kirschenheuter G, Greslin AF, Cheng X, Sennelo J, et al. Acyclic analogues of adenosine bisphosphates as P2Y receptor antagonists: Phosphate substitution leads to multiple pathways of inhibition of platelet aggregation. J Med Chem. 2002;45:5694–709. doi: 10.1021/jm020173u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshimura Y, Moon HR, Choi Y, Marquez VE. Enantioselective synthesis of bicyclo[3.1.0]hexane carbocyclic nucleosides via a lipase-catalyzed asymmetric acetylation characterization of an unusual acetal byproduct. J Org Chem. 2002;67:5938–45. doi: 10.1021/jo020249u. [DOI] [PubMed] [Google Scholar]
- 25.Mustard JF, Perry DW, Ardlie NG, Packham MA. Preparation of suspensions of washed platelets from humans. Br J Haematol. 1972;22:193–204. doi: 10.1111/j.1365-2141.1972.tb08800.x. [DOI] [PubMed] [Google Scholar]
- 26.Cattaneo M, Lecchi A, Lombardi R, Gachet C, Zighetti ML. Platelets from a patient heterozygous for the defect of P2CYC receptors for ADP have a secretion defect despite normal thromboxane A2 production and normal granule stores: further evidence that some cases of platelet ‘primary secretion defect’ are heterozygous for a defect of P2CYC receptors. Arterioscler Thromb Vasc Biol. 2000;20:101–6. doi: 10.1161/01.atv.20.11.e101. [DOI] [PubMed] [Google Scholar]
- 27.Cattaneo M, Lecchi A, Randi AM, McGregor JL, Mannucci PM. Identification of a new congenital defect of platelet function characterized by severe impairment of platelet responses to adenosine diphosphate. Blood. 1992;80:2787–96. [PubMed] [Google Scholar]
- 28.Gorman MW, Ogimoto K, Savage MV, Jacobson KA, Feigl E. Nucleotide coronary vasodilation in guinea pig hearts. Am J Physiol. 2003;285:H1040–7. doi: 10.1152/ajpheart.00981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nylander S, Mattsson C, Ramstrom S, Lindahl TL. The relative importance of the ADP receptors, P2Y12 and P2Y1, in thrombin-induced platelet activation. Thromb Res. 2003;111:65–73. doi: 10.1016/j.thromres.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Cheung KK, Ryten M, Burnstock G. Abundant and dynamic expression of G protein-coupled P2Y receptors in mammalian development. Dev Dyn. 2003;228:254–66. doi: 10.1002/dvdy.10378. [DOI] [PubMed] [Google Scholar]
- 31.Hechler B, Lenain N, Marchese P, Vial C, Heim V, Freund M, et al. A role of the fast ATP-gated P2X1 cation channel in thrombosis of small arteries in vivo. J Exp Med. 2003;198:661–7. doi: 10.1084/jem.20030144. [DOI] [PMC free article] [PubMed] [Google Scholar]


