Abstract
Decitabine (DAC) and 5-azacitidine have recently been approved for the treatment of myelodysplastic syndrome. Thepharm acodynamic effects of DAC and 5-azacitidine outsidethe ir known activity as inhibitors of DNA methyltransferases (DNMTs) require further investigation. The purpose of this study was to investigate the effect of DAC on the expression of p21WAF1/CIP1, a gene with a putative CpG island surrounding its promoter region. Promoter methylation analysis of p21WAF1/CIP1 in leukemia cells revealed the absence of CpG methylation. However, DAC upregulated p21WAF1/CIP1 expression in a dose-dependent manner (ED50 = 103.34 nM) and induced G2/M cell cycle arrest in leukemia cells. Sequential application of DAC followed by different histone deacetylase inhibitors induced expression of p21WAF1/CIP1 synergistically. Upregulation of p21WAF1/CIP1 paralleled DAC-induced apoptosis (ED50 = 153 nM). Low doses of DAC induced γ-H2AX expression (ED50 = 16.5 nM) and upregulated p21WAF1/CIP1 in congenic HCT 116 colon cancer cells in a DNMT-independent and p53-dependent fashion. Inhibition of p53 transactivation by pifithrin-α or the kinase activity of ATM by either the specific ATM inhibitor KU-5593 or caffeine abrogated p21WAF1/CIP1 upregulation, indicating that DAC upregulation of p21WAF1/CIP1 was p53- and ATM-dependent in leukemia cells. In conclusion, DAC upregulates p21WAF1/CIP1 in DNMT-independent manner via the DNA damage/ATM/p53 axis.
Keywords: decitabine, apoptosis, DNA methyltransferase inhibitors, histone deacetylase inhibitors, epigenetics, p21
Introduction
Epigenetic modifications play a significant role in tumor suppressor gene silencing and are potentially reversible (Jones and Baylin, 2002; Baylin and Ohm, 2006). Two of the best characterized epigenetic events are aberrant DNA methylation and changes in chromatin structure involving posttranslational modifications of histones (Yoo and Jones, 2006). Methylation of cytosines in the dinucleotide sequence CpG in gene promoters plays a role in silencing genes either by directly inhibiting the interaction of transcription factors with their regulatory sequences or by attracting methylated DNA-binding proteins, which in turn recruit transcriptional corepressors including histone deacetylases (HDACs)an d histone methyltransferase, resulting in transcriptionally inactive chromatin (Galm et al., 2006). DNA methylation is catalysed by DNA methyltransferases (DNMTs), which transfer the methyl moiety from the methyl donor S-adenosyl-methionine to the 5 position on the cytosine ring. Pharmacologic inhibition of DNMT in cancer cells causes reversal of promoter methylation associated with transcriptional activation of methylated tumor suppressor genes including p16, p15 and p73 (Galm et al., 2004). DNMT1 interacts directly with HDAC 1 and 2 as well as the histone methyltransferase SUV39H1 (Vaute et al., 2002). DNMT1 can also activate Sp1 response elements without increasing the protein levels of of Sp1 or Sp3 or even the occupancy of their elements by these proteins (Milutinovic et al., 2004).
Decitabine and its prodrug 5-azacitidine (5AC), approved for the treatment of myelodysplastic syndrome (MDS), have been shown to reactivate the expression of a variety of methylated genes. Gene reexpression can be augmented by the addition of HDAC inhibitors (Cameron et al., 1999). Synergistic reactivation of methylated genes including MLH1, TIMP3, CDKN2B and CDKN2A in human colon cancer and leukemia cells results from the sequential application of low-dose DNMT inhibitors followed by HDAC inhibitors (Cameron et al., 1999).
In a dose-finding study in which patients with acute myeloid leukemia or MDS received 5AC followed by the HDAC inhibitor sodium phenylbutyrate (NaPB), histone acetylation was monitored sequentially in peripheral blood and bone marrow mononuclear cells during the first course of therapy (Gore et al., 2006). Surprisingly, the administration of 5AC alone was associated with induction of acetylated histones H3 and H4 in 11/23 evaluable patients. Coupled with recent studies suggesting that DAC can induce expression of unmethylated genes such as CDKN2D and p21WAF1/CIP1 (Zhu et al., 2001; Schmelz et al., 2005a), these data indicate that molecular mechanisms in addition to the reexpression of methylated genes may contribute to the clinical activity of DNMT inhibitors. This study examines the induction of the expression of the unmethylated cell cycle regulatory gene p21WAF1/CIP1 (Brakensiek et al., 2005; Scott et al., 2006)in response to the DNMT inhibitor DAC.
Results
Impact of DAC administration on p21WAF1/CIP1 expression, cell cycle distribution, DNA damage and apoptosis
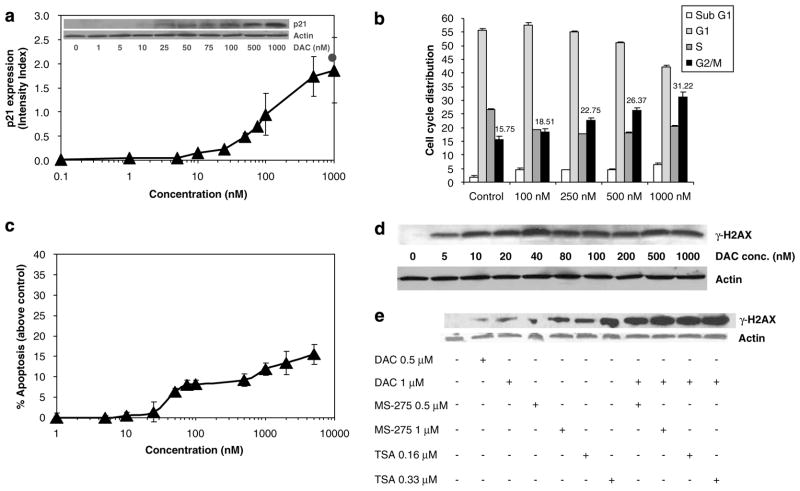
ML-1 myeloid leukemia cells were incubated in the presence or absence of increasing concentrations of DAC for 48 h before collecting cells for determination of p21WAF1/CIP1 expression by western analysis. As seen in Figure 1a, treatment of cells with DAC for 48 h led to a concentration-dependent increase in p21WAF1/CIP1 protein expression. The calculated ED50, 103.34 nM, is well below concentrations typically used in vitro to inhibit DNMT.
Figure 1.
Decitabine (DAC)induces p21WAF1/CIP1 expression, G2/M arrest, apoptosis and DNA damage in leukemia cells. (a)ML-1 cells were treated with graded doses of DAC for 48 h, and p21WAF1/CIP1 expression was measured by western blotting and signals quantified by densitometry. Results represent the mean±s.d. for three independent experiments. Closed circle indicates MS275 (1 μM) as a positive control. The inset shows a representative blot. (b)Synchronize d ML-1 cells (serum starvation)were treated with different concentrations of DAC for 48 h for cell cycle analysis by propidium iodide staining. Results represent the mean±s.d. for three independent experiments. G2/M values are shown above each bar. (c)ML-1 cells were treated with different concentrations of DAC for 72 h, and apoptosis was measured as described under Materials and methods. Results represent the mean±s.d. for three independent experiments. (d)ML-1 cells were treated with different concentrations of DAC for 48 h, and the expression of the DNA damage marker γ-H2AX was determined by western blotting. A representative blot of three independent experiments is shown. Actin was used as a loading control. (e)ML-1 cells were sequentially treated with different concentrations of DAC (48 h)followed by MS-275 or trichostatin A (TSA)for 24 h, and the expression of the DNA damage marker γ-H2AX was determined by western blotting. Actin was used as a loading control.
Although others have reported that the p21WAF1/CIP1 gene, which possesses a CpG island in the promoter region, is not methylated in leukemia or MDS (Brakensiek et al., 2005; Scott et al., 2006), it was critical to confirm this in ML-1 and HL60 cells under investigation in this study. The promoter CpG island in both cell lines was unmethylated as indicated by methylation-specific PCR (MSP)(data not shown), confirming the absence of p21WAF1/CIP1 promoter methylation in these human myeloid leukemia cell lines.
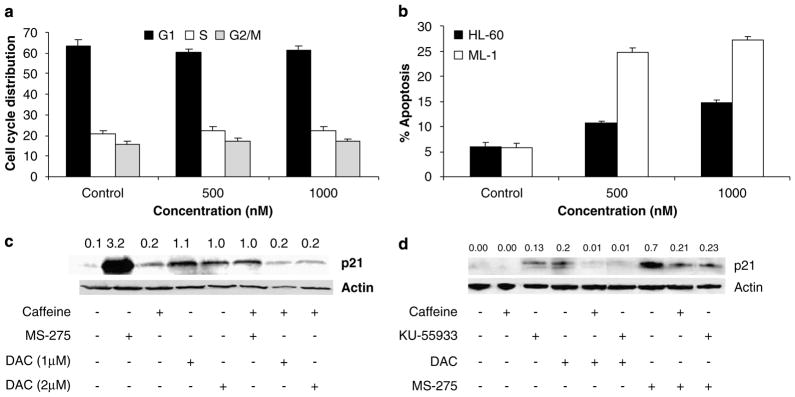
Because p21WAF1/CIP1 can mediate cell cycle arrest in different phases, synchronized ML-1 cells were treated with different concentrations of DAC for 48 h before cell cycle analysis. Treatment with different concentrations of DAC for 48 h significantly increased the percentage of cells in G2/M in a dose-dependent manner, with a concomitant decrease in percentage of cells in S and G1 (at higher concentrations)pha ses (Figure 1b).
The application of DAC and 5AC to induce reexpression of silenced genes may be confounded by their cytotoxic effects. ML-1 cells were incubated with different concentrations of DAC for 72 h and apoptosis was measured. DAC induced apoptosis in treated ML-1 cells with calculated ED50 = 153 nM (Figure 1c).
Genotoxic stress response may upregulate p21WAF1/CIP1 expression to induce cell cycle arrest and allow for DNA repair before cell division. Expression of phosphorylated histone H2AX (γ-H2AX)is a sensitive marker of double-strand breaks in DNA (Rogakou et al., 2000). ML-1 cells were treated with increasing concentrations of DAC for 48 h prior to immunoblotting for γ-H2AX (Figure 1d). Expression of γ-H2AX was observed even at the lowest concentration of DAC studied (5 nM). The calculated ED50 for γ-H2AX induction was 16.5 nM, which is far below the calculated ED50 for apoptosis (153 nM). This implies that low doses of DAC can induce DNA damage in an apoptosis-independent manner. Moreover, the sequential treatment of ML-1 cells with DAC (48 h)followe d by the HDAC inhibitors MS-275 or trichostatin A (TSA; 24 h)induced more DNA damage than DAC or HDAC inhibitors alone (Figure 1e). The sequential treatment induced a similar enhanced DNA-damaging effect in the p53-null HL-60 cells (data not shown).
Impact of sequential administration of DNMT and HDAC inhibitors on p21WAF1/CIP1 expression
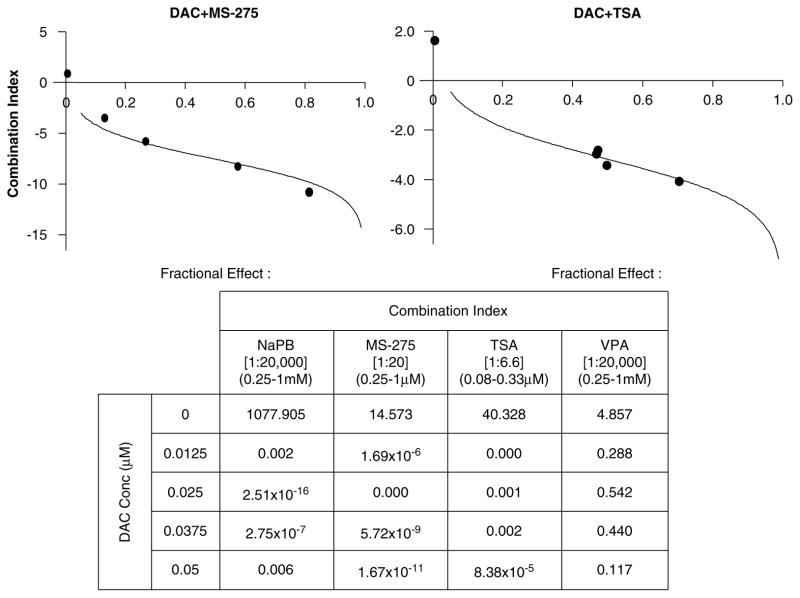
Previous studies examining pharmacodynamic interaction between DNMT and HDAC inhibitors have focused on silenced genes with methylated promoters; in many systems, such genes are synergistically reexpressed when cells are treated with HDAC inhibitors, which alone are ineffective for reexpressing the genes, following brief exposures to DNMT inhibitors (Cameron et al., 1999). To test whether this phenomenon was restricted to genes with methylated promoters, ML-1 cells were pretreated with different DAC concentrations (0.0125–0.05 μM)for 48 h. One of the several HDAC inhibitors was then added for 24 h before harvesting of cells for determination of p21WAF1/CIP1 protein expression. Median effect plot analysis was performed using the following fixed dose ratios of HDAC/DAC inhibitors: MS-275, 20:1; TSA, 6.5:1; NaPB, 20 000:1; and valproic acid, 20 000:1. As with methylated genes, expression of p21WAF1/CIP1 was synergistically induced (Figure 2)by combinations of DAC followed by HDAC inhibitors (combination index ≪1).
Figure 2.
Sequential treatment of decitabine (DAC) and histone deacetylase (HDAC) inhibitors synergistically upregulates p21WAF1/CIP1 expression. ML-1 cells were incubated with different concentrations of DAC (0.0125–0.05 μM)for 48 h prior to addition of sodium phenylbutyrate (NaPB; 0.25–1mM), MS-275 (0.25–1 μM), trichostatin A (TSA; 0.08–0.33 μM)or valproic acid (VPA; 0.25–1mM)for 24 h. p21WAF1/CIP1 expression was analysed by western blotting and densitometry. The mean signal intensity from three independent experiments was used for the median effect analysis. The combination index (CI)as a function of fraction affected was plotted for the combination of DAC with HDAC inhibitors in ML-1 at fixed ratios. Representative graphs for the combination of DAC with MS-275 and TSA are shown along with the calculated CI values for each combination. CI < 1 indicates a synergistic interaction.
Impact of sequential administration of DNMT and HDAC inhibitors on apoptosis
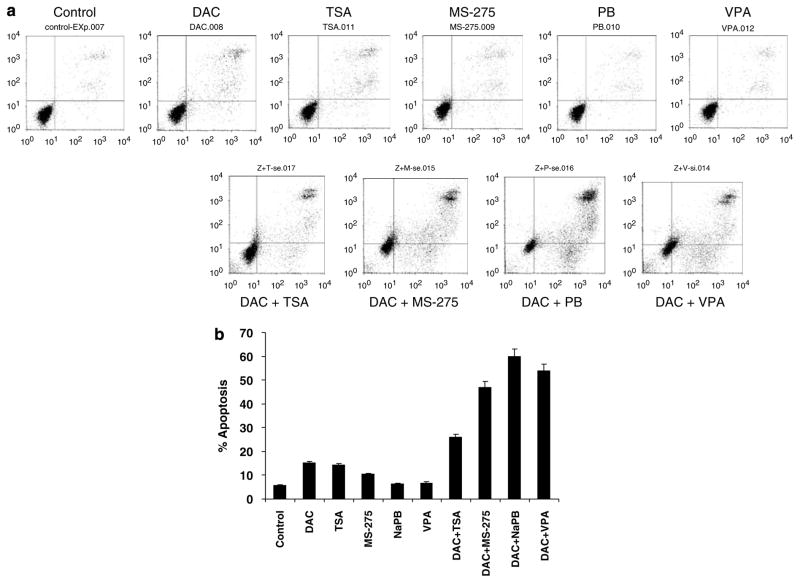
To determine the effect of sequential DNMT/HDAC inhibition on apoptosis in leukemia cells, ML-1 cells were incubated with 1 μM DAC for 48 h followed by HDAC inhibitors for 24 h at the following concentrations: TSA, 0.33 μM; MS-275, 1 μM; NaPB, 1mM; and valproic acid, 1mM. At the concentrations studied, DAC, TSA and MS-275 were moderately apoptogenic (10% above control for DAC and TSA), whereas the short-chain fatty acids induced little or no apoptosis at the concentrations studied (Figures 3a and b). The sequential administration of DAC followed by each of the HDAC inhibitors induced marked apoptosis, ranging from 20% above control for the combination with TSA to 65% above control for the NaPB combination (Figures 3a and b).
Figure 3.
Sequential treatment with decitabine (DAC)and histone deacetylase (HDAC)inhibitors enhances HDAC inhibitor-induced apoptosis. (a)ML-1 cells were treated with DAC (1 μM)for 48 h then treated with trichostatin A (TSA; 0.33 μM), MS-275 (1 μM), sodium phenylbutyrate (NaPB; 1mM)or valproic acid (VPA; 1mM)for 24 h. Apoptosis was measured as described under Materials and methods. A representative experiment is shown. (b)Apoptosis data for three independent experiments are plotted as the mean±s.d. for the different treatments.
DAC administration upregulates p21WAF1/CIP1 expression independent of DNMT expression
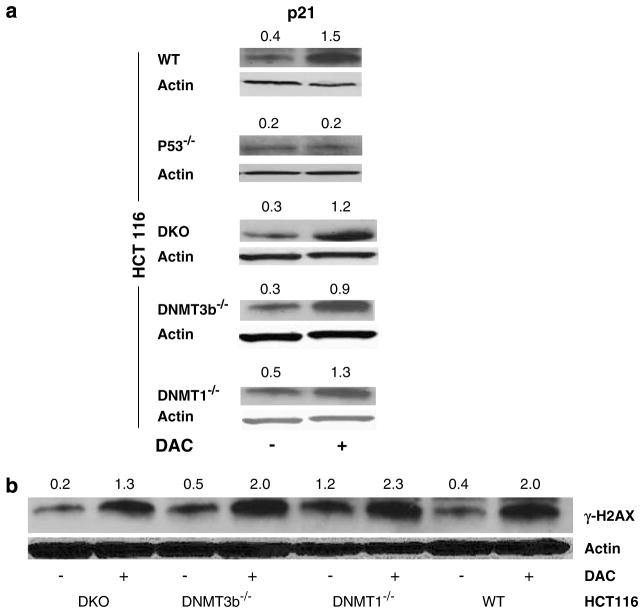
To investigate the mechanism by which DAC induces expression of the unmethylated p21WAF1/CIP1 gene, isogenic HCT116 colorectal cells, genetically manipulated to lack expression of DNMT1 (DNMT1−/−)or DNMT3b (DNMT3b−/−)or both genes (double knockout or DKO)(Rhee et al., 2002)were treated with DAC in parallel with wild-type (WT)HCT 116 cells. It is worth mentioning here that DNMT3a is still expressed in these congenic HCT116 cells; however, its contribution to the process of DNA methylation is extremely minor in comparison to DNMT1 and DNMT3b, which are responsible for 95% or greater of genomic DNA methylation (Rhee et al., 2002). Cells were treated with 1 μM DAC for 72 h prior to harvesting and determination of p21WAF1/CIP1 expression. DAC upregulated p21WAF1/CIP1 expression in all four cell lines (Figure 4a), suggesting that induction of p21WAF1/CIP1 expression by DAC is not mediated through DNMT inhibition. Moreover, DAC treatment of congenic HCT116 cells lacking TP53 gene expression did not induce p21WAF1/CIP1 expression (Figure 4a), indicating that p53 is required for p21WAF1/CIP1 upregulation by DAC.
Figure 4.
Decitabine (DAC)-induced p21WAF1/CIP1 upregulation and DNA damage are DNA methyltransferase (DNMT) independent and p53 dependent. Congenic HCT116 cells were used to investigate the effect of DAC (1 μM)treatment for 72 h on p21WAF1/CIP1 (a)and γ-H2AX (b)by western blotting. DKO indicates double knockout cells (DNMT1 and DNMT3b). Actin was used as a loading control. The figure is representative of four independent experiments. Numerical values above each blot represent the signal intensity measured by densitometry.
In parallel with above experiments, the induction of γ-H2AX in response to DAC (1 μM, 72 h)w as investigated in the congenic HCT116 cells (Figure 4b). Regardless of DNMT expression status, DAC upregulated the expression of γ-H2AX, suggesting that DAC-induced DNA damage is independent on the major acting DNMTs, DNMT1 and DNMT3b. Despite DNA damage induction by DAC, apoptosis induction by the same concentration of DAC was minimal (5–7%)with no significant difference among the congenic HCT116 cells (data not shown).
p21WAF1/CIP1 upregulation by DAC is dependent on the ATM/p53 pathway
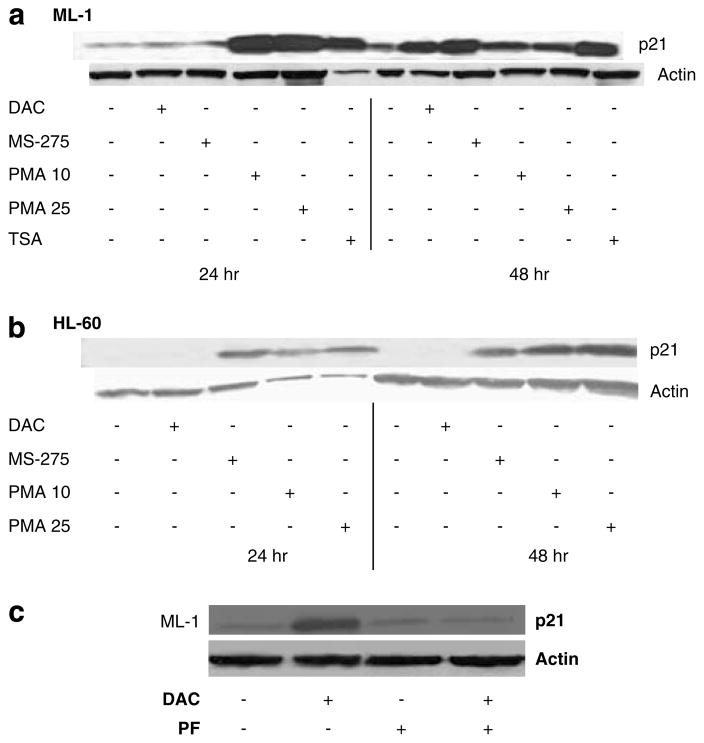
p21WAF1/CIP1 expression is controlled by p53-dependent and p53-independent mechanisms. To investigate the effect of p53 on p21WAF1/CIP1 upregulation by DAC, we investigated the effect of DAC (1 μM), MS-275 (1 μM) and phorbol 12-myristate 13-acetate (PMA)(10 and 25 nM)on p21WAF1/CIP1 expression in ML-1 cells (p53-WT)and HL-60 cells (p53-null)(Wolf and Rotter, 1985). PMA is a protein kinase C activator known to induce p21WAF1/CIP1 in a p53-independent fashion (Biggs et al., 1996). As seen in Figure 5a, all three agents as well as TSA (300 nM)upregula ted p21WAF1/CIP1 within 48 h in the p53-WT ML-1 cells. While p21 was upregulated by MS275 and PMA in the p53-null HL-60 cells, no p21 upregulation occurred in the latter cell line in response to DAC (Figure 5b), supporting a p53-dependent upregulation of p21WAF1/CIP1. Moreover, pretreatment of ML-1 leukemia cells, with 20 μM pifithrin-α for 24 h (p53 transactivation inhibitor)followe d by DAC (1 μM) for 72 h abrogated the upregulation of p21WAF1/CIP1 by DAC (Figure 5c). The same effect on p21WAF1/CIP1 expression was also observed in BV-173 leukemia cells, which expresses WT p53 (data not shown).
Figure 5.
Decitabine (DAC)-induced p21WAF1/CIP1 upregulation is p53 dependent. DAC (1 μM)was used to treat p53-wild-type (WT) ML-1 cells (a)and p53-null HL-60 cells (b)for 24 and 48 h. The expression of p21WAF1/CIP1 was measured by western blotting. MS-275 (1 μM), phorbol 12-myristate 13-acetate (PMA; 10–25 nM)and trichostatin A (TSA; 330 nM)were used as positive controls. Actin was used as a loading control. The figure is a representative of three independent experiments. (c)ML-1 cells pretreated with 20 μM pifithrin-α (PF)for 24 h followed by DAC (1 μM)for 72 h. p21WAF1/CIP1 expression was measured by western blotting. Actin was used a loading control.
Since DAC did not induce p21WAF1/CIP1 in HL-60 cells, we next tested whether DAC could induce cell cycle arrest in the p53-null HL-60 cells. As seen in Figure 6a, treatment of HL60 cells with different doses of DAC for 48 h led to no alteration in cell cycle populations, in marked contrast to ML-1 cells (Figure 1b). Similarly, DAC treatment for 72 h induced minimal apoptosis in the p53-null HL60 cells in contrast to ML-1 cells (Figure 6b).
Figure 6.
Decitabine (DAC)-induced G2/M arrest is p53 dependent and p21WAF1/CIP1 upregulation is ATM dependent. (a)Synchronize d HL-60 cells were treated with DAC (0.5, 1 μM)for 48 h and cell cycle analysis was performed as described under Materials and methods. Results represent the mean±s.d. for three independent experiments. (b)HL-60 and ML-1 cells were treated with DAC (500, 1000 nM)for 72 h. Apoptosis was measured as described under Materials and methods. Results represent the mean±s.d. for three independent experiments. (c)ML-1 cells were treated with DAC (1, 2 μM), caffeine (1mM), MS-275 (1 μM)or cotreated with DAC + caffeine or MS-275 + caffeine for 48 h. The expression of p21WAF1/CIP1 was measured by western blotting. Actin was used as a loading control. Numerical values above each blot represent the signal intensity measured by densitometry. (d)ML-1 cells were treated with DAC (1 μM), caffeine (1mM), MS-275 (1 μM), KU-5593 (20 μM)alone or in combination for 48 h. Caffeine was used as a cotreatment, while KU-5593 was used as a pretreatment for 1 h.
Further evidence for the involvement of DNA damage in DAC-induction of p21WAF1/CIP1 was sought by inhibition of ATM, which phosphorylates and activates downstream target proteins involved in DNA repair and cell cycle regulation. The kinase activity of ATM can be inhibited by the specific ATM inhibitor 2-morpholin-4-yl-6-thianthren-1-yl-pyran-4-one (KU-55933)or caffeine (Sarkaria et al., 1999).ML-1 leukemia cells were thus either cotreated with caffeine (1mM)an d DAC (1–2 μM)for 48 h or pretreated with 20 μM of KU-55933 for 1 h followed by DAC (1 μM)for 48 h. Caffeine completely abrogated DAC-induced p21WAF1/CIP1 upregulation and also inhibited induction of p21WAF1/CIP1 by MS-275 (Figures 6c and d), which is known to be ATM-dependent (Ju and Muller, 2003). Although KU-55933 upregulated p21WAF1/CIP1 by itself (Figure 6d), it inhibited p21WAF1/CIP1 upregulation upon treatment with DAC or MS275 (Figure 6d), indicating the essential role of ATM in DAC-induced p21WAF1/CIP1 upregulation in leukemia cells.
Discussion
In this study, we investigated the effect of DAC on the expression of the unmethylated p21WAF1/CIP1 gene. DAC upregulated the expression of p21WAF1/CIP1 in leukemia cells in a dose-dependent and p53-dependent manner. Induction of p21WAF1/CIP1 by DAC was independent on DNMT1 and DNMT3b (the major contributors of genomic DNA methylation); rather DAC mediated p21WAF1/CIP1 induction through DNA damage along the ATM/p53 axis. These data highlight the importance of DAC-induced DNA damage and how this effect can modulate gene expression in a DNMT-independent manner.
DAC induced p21WAF1/CIP1 expression in other systems. DAC induction of p21WAF1/CIP1 expression in lung and colon cancer cells was p53 dependent and not associated with chromatin remodeling events (Karpf et al., 2001; Zhu et al., 2004). On the other hand, chromatin immunoprecipitation experiments suggested that treatment of KG-1 and KG-1a leukemia cells with DAC was associated with an increase in H3 acetylation at the unmethylated p21WAF1/CIP1 promoter (Scott et al., 2006). It is also notable that clinical administration of 5AC was associated with increases in global histone acetylation (Gore et al., 2006), suggesting that histone acetylation at the p21WAF1/CIP1 promoter in response to DAC may not be specific. However, in this study, treatment of ML-1 cells with DAC in vitro does not lead to detectable changes in histone acetylation neither globally nor at the promoter region of p21WAF1/CIP1 as demonstrated by chromatin immunoprecipitation using acetylated H3 antibody (data not shown). p21WAF1/CIP1 induction by DAC was also observed in p53-mutated leukemia cell lines and was dependent on reversal of methylation of the tumor suppressor p73 (Schmelz et al., 2005b; Tamm et al., 2005). However, in ML-1 cells, we could not detect p73 promoter methylation by MSP (data not shown).
Previous studies have demonstrated synergistic induction of reexpression of a variety of genes by DAC followed by HDAC inhibitors (Cameron et al., 1999). Most such studies have focused on the expression of methylated genes, in which HDAC alone cannot induce gene expression and pinpointed the differential effect of HDAC inhibitors on methylated versus unmethylated genes. In this study, we observed a synergistic upregulation of p21WAF1/CIP1 by DAC and HDAC inhibitors despite the unmethylated status of its promoter. Recent studies indicated that decitabine (DAC)c an induce regional chromatin remodeling of other unmethylated genes, including RPGR, CD14, PTPN22 and calgranulin, which could explain the above-mentioned synergy (Schmelz et al., 2005a). However, the synergistic interaction between DNMT and HDAC inhibitors may stem from other potentially non-epigenetic mechanisms in addition to the inhibition of HDAC recruited directly by methyl-binding proteins to methylated promoters (Bird and Wolffe, 1999). It is noteworthy that in the p53-null HL60 cells, no such synergy was found for p21WAF1/CIP1 induction (data not shown), suggesting that DNA damage in response to either the DNMT inhibitor, HDAC inhibitor or both may lead to the synergistic expression of this gene, as well as the augmentation of apoptosis demonstrated in the current study.
DAC-induced DNA damage could play a role in synergizing HDAC inhibitors p21WAF1/CIP1 upregulation through the DNA damage/p53 axis. The inhibition of p21WAF1/CIP1 upregulation by Pifithrin-α and the lack of p21WAF1/CIP1 upregulation in the p53-null HL-60 cells support a major role of the DNA damage/p53 axis in this effect, which is consistent with the effect of DAC on human lung cancer cells (Zhu et al., 2004). Also, p21WAF1/CIP1 upregulation in HCT116 cells in a DNMT1- and DNMT3b-independent manner further supports the hypothesis of DNA damage-induced upregulation of p21WAF1/CIP1.
Chemotherapeutic agents frequently act through the mechanism of DNA damage, and p53 plays an important role in the induction of cell cycle arrest and apoptosis in response to DNA damage (Kastan et al., 1991). In this report, we observed both cell cycle arrest and apoptosis in ML-1 leukemia cells as a consequence of DNA damage in response to DAC. Although DAC was shown to induce DNA damage because of the structural instability at its incorporation site or by obstructing DNA synthesis (Juttermann et al., 1994), the exact mechanism of DNA damage by DAC requires further investigation. It is likely that p21WAF1/CIP1 induction may contribute to the cell cycle arrest (although G1 arrest is more commonly associated with p21WAF1/CIP1); however, the observed G2/M arrest could alternatively be p21WAF1/CIP1 independent and mediated by ATM in a p53-independent manner (Taylor and Stark, 2001). The lack of G2/M cell cycle arrest and p21WAF1/CIP1 upregulation after DAC treatment in the p53-null HL-60 cells further supports a role for p21WAF1/CIP1 in the observed G2/M arrest. The observed γ-H2AX upregulation at very low concentrations of DAC was not accompanied by G2/M cell cycle arrest because G2/M arrest was not statistically significant at doses below 100 nM. However, the different sensitivity of the methods used to measure γ-H2AX expression and cell cycle distribution could explain this. The fact that apoptosis induction by DAC in ML1 cells (~20% after 72 h)was limited, may suggests the involvement of other mechanisms like induction of differentiation in mediating the antileukemic action of DAC. However, longer exposure to DAC (96 h)induced more apoptosis (45%) in ML1 cells and 85% in BV-173 cells (data not shown).
The relationship between p21WAF1/CIP1 induction and apoptosis is highly controversial. In our study, the synergistic effect of DAC and HDAC inhibitors on p21WAF1/CIP1 expression parallels the enhanced effect of DAC pretreatment on HDAC inhibitor-induced apoptosis and may indirectly support a proapoptotic role for p21WAF1/CIP1. However, it is possible that the p21WAF1/CIP1 upregulation event is not linked at all to the enhanced apoptotic effect. Although p21WAF1/CIP1 is known mostly as an inhibitor of apoptosis (Gartel and Tyner, 2002), in some other cases it can promote apoptosis (Gartel, 2005; Gartel and Radhakrishnan, 2005). Further studies that include p21WAF1/CIP1 knockdown by siRNA and isogenic cells modified to lack p21WAF1/CIP1 expression could provide a direct answer for the exact role of p21WAF1/CIP1 in enhancing the apoptotic effect of HDAC inhibitors by DAC pretreatment.
The marked activity of DAC and its congener 5AC in the treatment of MDS and acute myeloid leukemia has been attributed likely to the impact of these drugs on reversal of methylation and induction of silenced gene expression. Recently, we reported that six MDS/acute myeloid leukemia responders to 5AC and NaPB sequential treatment showed reversal of promoter methylation of p15 in bone marrow mononuclear cells, whereas six nonresponders did not (Gore et al., 2006). However, a previous study did not detect any correlation between p15 methylation in peripheral blood mononuclear cells and clinical response to DAC (Issa et al., 2004). Clinical studies of sequential DNMT-HDAC inhibitor combinations are based on an epigenetic paradigm explaining the interaction between these two classes of agents. However, the present data suggest that other mechanisms of activity of both classes of drugs need to be considered. In particular, the potential importance of signaling in response to DNA damage in response to DNMT and/or HDAC inhibitors in the mediation of the clinical activity of these agents requires investigation. Understanding the relevant mechanisms of the clinical activity of these drugs will be critical for further development of compounds targeting epigenetic changes and for the extension of such approaches to other cancers.
Materials and methods
Cell culture
ML-1 and HL-60 leukemia cell lines were grown in suspension in RPMI-1640 (Invitrogen, Carlsbad, CA, USA)supplemented with 10% fetal calf serum, 0.1 mg ml−1 gentamicin and 2mM L-glutamine. WT and genetically altered HCT116 colon cancer cells (generous gifts from Dr Bert Vogelstein, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins)were cultured in McCoy’s 5A (Mediatech Inc., Herndon, VA, USA)supplemented with 10% fetal bovine serum (Sigma, St Louis, MO, USA)and 1% penicillin–streptomycin (Invitrogen). All cultures were incubated in a humidified atmosphere containing 5% CO2 at 37 °C.
Chemicals and antibodies
Decitabine was purchased from Calbiochem (San Diego, CA, USA), NaPB from Triple Crown America (Perkasie, PA, USA), TSA from Wako Pure Chemicals (Richmond, VA, USA), sodium valproate, caffeine, PMA and propidium iodide from Sigma. MS-275 was supplied by Mitsui Pharmaceuticals (Chiba, Japan). KU55933 and Pifithrin-α, a cell permeable inhibitor of p53 transactivation, were purchased from EMD Biosciences Inc. (San Diego, CA, USA).
Drugs were dissolved in DMSO as stock solutions and diluted before the experiment. DMSO was added to the control cells in the same concentration as the treated cells. Rabbit polyclonal antibodies directed against acetylated-histone H3 (catalog no. 06-599), H4 (catalog no. 06-598) and phosphorylated histone γ-H2AX (Clone JBW301)were from Upstate Biotechnologies (Charlottesville, VA, USA). The mouse β-actin (Clone JLA20)monoclonal antibody from Oncogene Research Products (San Diego, CA, USA), and the mouse p21Waf1/CIP1 (Clone BXM30)monoclonal antibody was from BD pharmingen (San Diego, CA, USA).
Protein extraction and immunoblotting
Nuclear histones were extracted as previously described (Gore et al., 2006). For protein extraction, cells were lysed in RIPA lysis buffer containing an EDTA-free protease inhibitor cocktail, at 4 °C for 30 min. Lysates were collected by centrifugation at 14 000 r.p.m. for 15 min. Protein concentration was determined by a bicinchoninic acid (BCA)assay kit (Pierce, Rockford, IL, USA).
Proteins (10 μg for histone or 30 μg for p21 or γ-H2AX)we re separated by 15% SDS–polyacrylamide gel electrophoresis and immunoblotted using antibodies for acetyl-histone H3 (1:1000), acetyl-histone H4 (1:500), γ-H2AX (1:1000), p21 (1:500)or β-actin (1:3000). The immunoreactive proteins were detected using ECL western blotting analysis system (GE Healthcare, Piscataway, NJ, USA). Signals were quantified by UN-SCAN-IT software from Silk ScientificMedian (Orem, Utah).
Methylation-specific PCR
Methylation-specific PCR was done as previously described with slight modifications (Herman et al., 1996). EZ DNA methylation kit (ZymoResearch, Orange, CA, USA)was used for the bisulfite treatment of DNA as per the manufacturer’s instructions. PCR was performed in 25 μl reaction volume using 3 μl of the bisulfite-treated DNA. The following primers were used to amplify methylated p21WAF1/CIP1 (sense: TTC GGGGAGGGCGGTTTC; antisense: CTCAAAAAAACG AAACCCGCG), unmethylated p21WAF1/CIP1 (sense: GGTTTT GGGGAGGGTGGTTT; antisense: CACCTCAAAAAAA CAAAACCCACA), methylated p73 (sense: GGACGTAGC GAAATCGGGGTTC; antisense: ACCCCGAACATCGAC GTCCG)and unmethylated p73 (sense: AGGGGATGTAGT GAAATTGGGGTTT; antisense: ATCACAACCCCAAAC ATCAACATCCA). The annealing temperature was 58 °C and PCR amplification was done for 35 cycles. The PCR products were resolved on a 6% non-denaturing polyacrylamide gel and poststained with ethidium bromide.
Flow cytometric analyses of cell cycle and apoptosis
For cell cycle analysis, cells were synchronized by overnight serum starvation and DAC was added to the cells growing in regular media for 48 or 72 h. A total of 1 × 106 cells were washed once with 1 × phosphate-buffered saline, fixed with 70% alcohol at 4 °C for at least 30 min and incubated with propidium iodide solution (50 μgml−1)containing RNase (10Uml−1)at 37 °C for 30 min. DNA fluorescence was measured using a Becton Dickinson FACScan flow cytometer and analysed by CellQuest software (BD Biosciences, San Jose, CA, USA). Apoptosis was measured using the Annexin V-FITC detection kit (BD Pharmingen)as per the manufacturer’s instructions.
Chromatin immunoprecipitation
Precipitating anti-acetyl-H3 (K9/K14)and all other required reagents were purchased from Upstate Biotechnology (Long Island, NY, USA). Chromatin immunoprecipitation was performed as described previously (Scott et al., 2006). PCR amplification of the p21WAF1/CIP1 promoter was performed using the following primers: F, 5′-GGGCGGGGCGGTTGTA TATCAG-3′ and R, 5′-GTCTGCCGCCGCTCTCTCACCT-3′ located between positions −60 and +90 of p21WAF1/CIP1 (GenBank accession, U24170)relative to the transcriptional starting point. PCR amplification was performed for 35 cycles and the annealing temperature was 60 °C.
Statistical analysis
Student’s t-test for paired samples was used to test for differences between means within experiments using the same cell line. Linear regression was used to analyse log-linear dose–response relationships. The calculated regression line was then used to calculate the dose responsible for 50% maximal pharmacodynamic effect (ED50). Interactions between drugs were tested using the median effect plot analysis method of Chou and Talaly (1977) using CalcuSyn software (Biosoft, Cambridge, UK).
Acknowledgments
We gratefully acknowledge the gift of congenic HCT116 cells from Dr Bert Vogelstein of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. We thank Drs Robert Arceci, Feyruz Rassool and Michael McDevitt for helpful discussions. This work was supported in part by NCI Grants K24CA111717 and P30 CA06973, translational research award from the Leukemia and Lymphoma Society of America and a grant from the Commonwealth Fund. This work was presented in part at the 2005 meeting of the American Association for Cancer Research (AACR).
References
- Baylin SB, Ohm JE. Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- Biggs JR, Kudlow JE, Kraft AS. The role of the transcription factor Sp1 in regulating the expression of the WAF1/CIP1 gene in U937 leukemic cells. J Biol Chem. 1996;271:901–906. doi: 10.1074/jbc.271.2.901. [DOI] [PubMed] [Google Scholar]
- Bird AP, Wolffe AP. Methylation-induced repression—belts, braces, and chromatin. Cell. 1999;99:451–454. doi: 10.1016/s0092-8674(00)81532-9. [DOI] [PubMed] [Google Scholar]
- Brakensiek K, Langer F, Kreipe H, Lehmann U. Absence of p21(CIP 1), p27(KIP 1)and p 57(KIP 2)methylation in MDS and AML. Leuk Res. 2005;29:1357–1360. doi: 10.1016/j.leukres.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- Chou TC, Talaly P. A simple generalized equation for the analysis of multiple inhibitions of Michaelis–Menten kinetic systems. J Biol Chem. 1977;252:6438–6442. [PubMed] [Google Scholar]
- Galm O, Herman JG, Baylin SB. The fundamental role of epigenetics in hematopoietic malignancies. Blood Rev. 2006;20:1–13. doi: 10.1016/j.blre.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Galm O, Wilop S, Reichelt J, Jost E, Gehbauer G, Herman JG, et al. DNA methylation changes in multiple myeloma. Leukemia. 2004;18:1687–1692. doi: 10.1038/sj.leu.2403434. [DOI] [PubMed] [Google Scholar]
- Gartel AL. The conflicting roles of the cdk inhibitor p21(CIP1/WAF1)in apoptosis. Leuk Res. 2005;29:1237–1238. doi: 10.1016/j.leukres.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- Gartel AL, Tyner AL. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther. 2002;1:639–649. [PubMed] [Google Scholar]
- Gore SD, Baylin S, Sugar E, Carraway H, Miller CB, Carducci M, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa JP, Garcia-Manero G, Giles FJ, Mannari R, Thomas D, Faderl S, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2′-deoxycytidine (decitabine)in hematopoietic malignancies. Blood. 2004;103:1635–1640. doi: 10.1182/blood-2003-03-0687. [DOI] [PubMed] [Google Scholar]
- Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- Ju R, Muller MT. Histone deacetylase inhibitors activate p21(WAF1)expression via ATM. Cancer Res. 2003;63:2891–2897. [PubMed] [Google Scholar]
- Juttermann R, Li E, Jaenisch R. Toxicity of 5-aza-2′-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc Natl Acad Sci USA. 1994;91:11797–11801. doi: 10.1073/pnas.91.25.11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpf AR, Moore BC, Ririe TO, Jones DA. Activation of the p53 DNA damage response pathway after inhibition of DNA methyltransferase by 5-aza-2′-deoxycytidine. Mol Pharmacol. 2001;59:751–757. [PubMed] [Google Scholar]
- Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- Milutinovic S, Brown SE, Zhuang Q, Szyf M. DNA methyltransferase 1 knock down induces gene expression by a mechanism independent of DNA methylation and histone deacetylation. J Biol Chem. 2004;279:27915–27927. doi: 10.1074/jbc.M312823200. [DOI] [PubMed] [Google Scholar]
- Rhee I, Bachman KE, Park BH, Jair KW, Yen RW, Schuebel KE, et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552–556. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem. 2000;275:9390–9395. doi: 10.1074/jbc.275.13.9390. [DOI] [PubMed] [Google Scholar]
- Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, et al. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- Schmelz K, Sattler N, Wagner M, Lubbert M, Dorken B, Tamm I. Induction of gene expression by 5-aza-2′-deoxycytidine in acute myeloid leukemia (AML)and myelodysplastic syndrome (MDS)but not epithelial cells by DNA-methylation-dependent and - independent mechanisms. Leukemia. 2005a;19:103–111. doi: 10.1038/sj.leu.2403552. [DOI] [PubMed] [Google Scholar]
- Schmelz K, Wagner M, Dorken B, Tamm I. 5-aza-2′-deoxycytidine induces p21WAF expression by demethylation of p73 leading to p53-independent apoptosis in myeloid leukemia. Int J Cancer. 2005b;114:683–695. doi: 10.1002/ijc.20797. [DOI] [PubMed] [Google Scholar]
- Scott SA, Dong WF, Ichinohasama R, Hirsch C, Sheridan D, Sanche SE, et al. 5-aza-2′-deoxycytidine (decitabine)can relieve p21WAF1 repression in human acute myeloid leukemia by a mechanism involving release of histone deacetylase 1 (HDAC1) without requiring p21WAF1 promoter demethylation. Leuk Res. 2006;30:69–76. doi: 10.1016/j.leukres.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Tamm I, Wagner M, Schmelz K. Decitabine activates specific caspases downstream of p73 in myeloid leukemia. Ann Hematol. 2005;84(Suppl 13):47–53. doi: 10.1007/s00277-005-0013-0. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- Vaute O, Nicolas E, Vandel L, Trouche D. Functional and physical interaction between the histone methyl transferase Suv39H1 and histone deacetylases. Nucleic Acids Res. 2002;30:475–481. doi: 10.1093/nar/30.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D, Rotter V. Major deletions in the gene encoding the p53 tumor antigen cause lack of p53 expression in HL-60 cells. Proc Natl Acad Sci USA. 1985;82:790–794. doi: 10.1073/pnas.82.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- Zhu WG, Dai Z, Ding H, Srinivasan K, Hall J, Duan W, et al. Increased expression of unmethylated CDKN2D by 5-aza-2′-deoxycytidine in human lung cancer cells. Oncogene. 2001;20:7787–7796. doi: 10.1038/sj.onc.1204970. [DOI] [PubMed] [Google Scholar]
- Zhu WG, Hileman T, Ke Y, Wang P, Lu S, Duan W, et al. 5-aza-2′-deoxycytidine activates the p53/p21Waf1/Cip1 pathway to inhibit cell proliferation. J Biol Chem. 2004;279:15161–15166. doi: 10.1074/jbc.M311703200. [DOI] [PubMed] [Google Scholar]