Abstract
Lung cancer (LC) is a leading cause of death worldwide. Recent advances in chemotherapeutic agents have not yielded any significant improvement in the prognosis of patients with LC. The five-year survival rate for all combined disease stages remains about 15%. For this reason, new therapies such as those that inhibit tumor angiogenesis or block activity of growth factor receptors are of special interest in this group of patients. In this review we will summarize the most recent clinical data on biologic therapies that inhibit tumor angiogenesis in LC, focusing on those that are most clinically relevant.
Keywords: lung cancer, angiogenesis, VEGF, antiangiogenesis
Introduction
Lung cancer (LC) is a preventable malignancy; yet it remains a leading cause of death both in women and in men. Worldwide over 1,000,000 new patients are diagnosed with LC each year and more than 900,000 die from the disease.1 A majority of patients present with advanced disease or will recur despite multidisciplinary intervention. Despite recent progress in chemotherapeutic agents, this has not yet yielded any significant improvement in the prognosis of patients with LC. The five-year survival rate for all combined disease stages remains about 15%.2 For this reason, emerging therapeutic modalities such as those that inhibit tumor angiogenesis or block activity of growth factor receptors are of special interest for their potential to improve prognosis in patients with LC. In this review, we will summarize the most recent clinical data on biologic therapies that inhibit tumor angiogenesis in LC patients, focusing on those that are most clinically relevant.
LC – epidemiology and pathology
LC falls into two major categories – non-small-cell (NSCLC) and small-cell lung cancer (SCLC). Prognosis, natural history, pathology, management and response to therapy validate this distinction. NSCLC comprises about 80% of all LC cases and is further subdivided into squamous-cell carcinoma (25–40%), adenocarcinoma (25–40%) and large-cell carcinoma (10–15%). The incidence of adenocarcinoma and bronchioalveolar carcinoma is rising for reasons that are not fully understood.3 The presentation, management and prognosis of different histological subtypes of NSCLC are similar; yet some differences do exist. Squamous-cell tumors are usually located close to the lung hilum and tend to metastasize later, while adenocarcinomas are found more distally in the lung parenchyma, metastasize earlier and respond better to chemotherapy. SCLC is an entirely different clinical and pathological entity characterized by aggressive growth, early distant organ metastases and survival given in weeks when untreated, despite being an initially chemosensitive disease.2
Cigarette smoking is a major etiological factor in more than 85% of all LC cases and contributes greatly to molecular alterations leading to malignancy.4 LC develops by stepwise acquisition of genetic changes resulting in uncontrolled growth, resistance to apoptosis, tumor angiogenesis, tissue invasion and distant metastasis. Once clinically evident, LC cells may already harbor 20 or more genetic mutations facilitating the malignant behavior.5 Genetic changes that are found in LC cells can be divided into chromosomal abnormalities (deletions, amplifications and translocations), somatic genetic mutations and epigenetic changes (e.g. DNA adducts, gene promoter methylation and histone acetylation). Some genetic alterations are characteristic of NSCLC (K-RAS, HER-2/neu and p16INK4a) while others are described more frequently in association with SCLC (p53 and p16/RB).6 Although the knowledge regarding the molecular mechanisms involved in the pathogenesis of LC has recently expanded rapidly, only two molecular pathways are targeted therapeutically in clinical practice today: epithelial growth factor receptor (EGFR) signaling and the vascular endothelial growth factor (VEGF) pathway.
Tumor angiogenesis
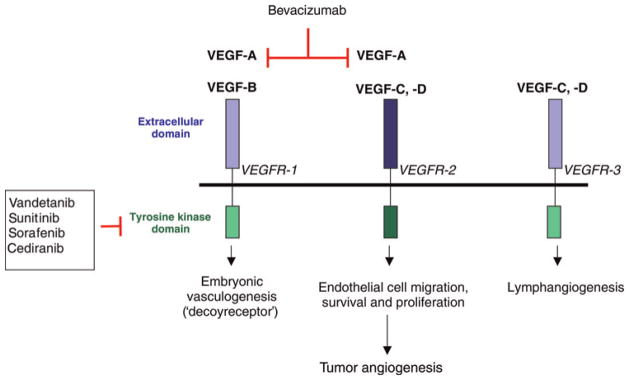
The concept that a tumor stimulates its own blood supply (tumor angiogenesis) extends back to the 1930s.7 In 1971, Folkman proposed the hypothesis that tumors could not grow beyond a certain size without an additional blood supply. He also postulated for the first time that this phenomenon may be used as a therapeutic target, and that by inhibiting tumor angiogenesis it would be possible to prevent local tumor growth and the formation of distant metastases.8 Since then, extensive research has been focused on identification of pro-angiogenic factors produced by tumor cells and methods of blocking their action. One of the best-studied factors that stimulates tumor angiogenesis is VEGF (known also as VEGF-A).9 VEGF is a growth factor that stimulates proliferation and migration, promotes survival, inhibits apoptosis and regulates permeability of vascular endothelial cells (ECs). VEGF belongs to a family of growth factors that includes VEGF-A, -B, -C, -D, -E and placenta growth factor (PlGF).10 Alternative splicing of the VEGF-A gene results in the formation of four major isoforms with variable number of amino acids building the VEGF molecule: 121, 165, 189 and 206 (VEGF-A121, VEGF-A165, VEGF-A189 and VEGF-A206). The most prominent isoform is VEGF-A165, which can be found circulating in the plasma and bound to the extracellular matrix. Biological functions of VEGF are mediated upon binding to receptors with tyrosine kinase activity – VEGF receptor-1, -2 and -3 (VEGFR-1, -2, -3). VEGFR-1 binds VEGF-A, VEGF-B and uniquely PlGF. Its activation mediates embryonic vessel development, hematopoiesis, macrophage chemotaxis and recruitment of EC progenitors to tumor blood vessels from the bone marrow.11 VEGFR-2 is the key mediator of VEGF-driven tumor angio-genesis. Its expression and function is also crucial during embryonic vascular development since heterozygous and homozygous knockout mice die in utero due to significantly disrupted vasculogenesis and hematopoiesis. Upon binding of VEGF to VEGFR-2, the receptor is phosphorylated and downstream effectors including phospholipase C gamma, protein kinase C, Raf and MAP kinase signaling cascade and PI3K and FAK pathways are activated, which leads to EC proliferation, migration and survival (Figure 1).12 VEGFR-3 binds VEGF-C and -D isoforms and is directly involved in the formation of lymphatics during adulthood in healthy tissue and in tumors.13
Figure 1.
VEGF pathway and the site of action of the inhibitors. VEGF, vascular endothelial growth factor. (A color version of this figure is available in the online journal)
VEGF expression is regulated by three major mechanisms: (1) cellular oxygen level; (2) growth factors and cytokines; and (3) oncogene activation/tumor suppressor gene inactivation.12 Hypoxia is known to be one of the most important factors facilitating VEGF expression in tumors. Hypoxia inducible factor-1 is a transcription factor, which regulates expression of hypoxia responsive genes, including a VEGF gene, upon decrease of intracellular oxygen levels.14 VEGF expression is also regulated via paracrine and autocrine release of growth factors and cytokines like platelet-derived growth factor (PDGF), epidermal growth factor (EGF), keratinocyte growth factor, insulin-like growth factor (IGF), transforming growth factors alpha and beta (TGF-α and -β), interleukin-1α, interleukin-6 and prosta-glandins. During tumor progression, certain genetic mutations in the ras oncogene or Wnt-signaling pathways may also lead to overexpression of VEGF through uncontrolled gene transcription.15 Tumor-derived VEGF regulates the function of tumor cells via autocrine signaling and VEGF receptors are expressed in multiple cancer cell lines including LC.16,17
VEGF-mediated angiogenesis in LC
Lung microvascular ECs produce significant amounts of VEGF, which contributes to endothelial maintenance and homeostasis within the lungs in an autocrine manner.18 Finely tuned interactions between ECs, VEGF and lung embryonic mesenchyma are critical to the formation and maturation of lungs.19,20 Disregulated VEGF signaling is found in the pathophysiology of diseases such as asthma, emphysema, pulmonary hypertension and acute respiratory distress syndrome.18
Both NSCLC and SCLC tumors produce VEGF, and high levels of VEGF are correlated with patients’ poor prognosis.21,22 It is unclear however whether VEGF levels in cancer patients can be used as a reliable biomarker of anti-VEGF therapy. Some discrepancies also exist when levels of different isoforms of VEGF are studied. One study involving NSCLC patients showed that the levels of VEGF-A189 provide a better correlation with intra-tumoral mean vascular density (MVD), disease recurrence and overall survival (OS) than the most abundant isoform – VEGF-A165, which did not correlate with clinical out- comes.23 When analyzing the MVD, it has to be noted that vessels are not equally distributed within the tumors. Usually the MVD at the tumor’s periphery is higher than the MVD at the center due to central necrosis. It was shown that peripheral MVD but not central MVD, when combined with blood VEGF levels, correlated with poor prognosis in NSCLC patients.24
SCLC tumors are more vascularized than NSCLC, but histologically assessed MVD does not correlate with the levels of VEGF expression.21 Some experimental data suggest that tumor angiogenesis plays a minor role in the progression of some forms of NSCLC. Tumors can efficiently use already existing host vessels in the alveoli septa and take advantage of the close proximity of lung alveoli for oxygen delivery.25 However, it is not a widespread phenomenon and in the vast majority of cases, tumors are dependent on their own angiogenesis to grow and metastasize. Therefore, assessing circulating VEGF and VEGF expression within NSCLC tumors provides a valid marker of tumor aggressiveness and provides a target for therapy in LC.
Anti-VEGF therapy in LC
The VEGF pathway can be targeted therapeutically at various molecular levels. Currently two major concepts are studied in the clinical setting – blocking VEGF from binding to its extracellular receptors with VEGF-specific antibodies and fusion proteins (VEGF-traps), or inhibiting intracellular VEGF signaling using tyrosine kinase inhibitors (TKIs) (Figure 1).26 Bevacizumab (Avastin®) is a recombinant, humanized, VEGF-neutralizing antibody that binds all isoforms of VEGF-A. Adding bevacizumab to standard chemotherapy in patients with metastatic colorectal cancer improved both progression free (PFS) and OS.27,28
In 2004, a phase II clinical trial assessing the use of bevacizumab in newly diagnosed stage IIIB/IV or recurrent NSCLC patients was conducted.29 Patients were randomized to receive bevacizumab at 7.5 or 15 mg/kg with carboplatin (area under the curve; AUC = 6) and paclitaxel 200 mg/m2 every three weeks or chemotherapy alone. The primary endpoints were the median time to progression (TTP) and tumor response rate (RR). High-dose bevacizumab when added to the chemotherapy showed improved TTP (7.4 months) when compared with either chemotherapy alone (4.2 months) or the lower dose of bevacizumab (4.3 months). The patients who received the higher dose of bevacizumab combined with chemotherapy had higher RR (31.5%) when compared with the chemotherapy alone arm (18.8%). There was a trend towards a survival benefit in patients who received the higher dose of bevacizumab with chemotherapy; however it was not statistically significant. Bevacizumab was overall well tolerated. Hypertension, proteinurea, thrombotic events and grade 3/4 leukopenia were increased in patients who received bevacizumab. The most notable side-effect of bevacizumab in this phase II study was the increased frequency of bleeding events. The bleeding in all patients had two distinct patterns: mucocutaneous that was minor and did not require changes in treatment protocol, or major hemoptysis/pulmonary hemorrhage that was fatal in four out of six symptomatic patients. Further analysis showed that the majority of patients (67%) who had serious pulmonary bleeding had tumors with squamous-cell histology and the tumors were centrally located in close vicinity to major blood vessels. In future clinical trials with bevacizumab, patients with squamous histology were excluded from participation based on this data.29
The Eastern Cooperative Oncology Group conducted a phase III clinical trial (E4599) in 878 patients with advanced or recurrent stage IIIB/IV NSCLC.30 Patients were randomized to receive chemotherapy alone (control arm), carboplatin (AUC = 6) and paclitaxel (200 mg/m2) or chemotherapy with bevacizumab at 15 mg/kg once every three weeks. After completion of six cycles of treatment, patients receiving bevacizumab with chemotherapy continued on bevacizumab as a single agent until disease progression or intolerable toxicities occurred. The primary endpoint in this study was OS. Adding bevacizumab to paclitaxel and carboplatin chemotherapy significantly increased not only PFS and RR but also OS. There were 15 deaths in the bevacizumab arm that were related to the toxicity of treatment: five patients had fatal pulmonary hemorrhage, five deaths were related to febrile neutropenia, two patients had fatal gastrointestinal bleeding, two had a stroke and one died of probable pulmonary embolus. In the control arm there were two deaths related to toxic effects of therapy. A retrospective analysis of this trial demonstrated that patients who were over 70 years of age and received bevacizumab experienced significantly higher rates of side-effects when compared with younger patients. The addition of bevacizumab to platinum-doublet chemotherapy did not improve either PFS or OS in this group of patients.31,32 ECOG 4599 trial was the first study in LC patients that showed significant survival benefit by adding an antiangiogenic agent to already established chemotherapy protocol. Recently published data from the AVAiL trial confirmed clinical efficacy of bevacizumab in combination with a different platinum-doublet chemotherapy in patients with stage IIIB/IV NSCLC; however, OS benefit favoring bevacizumab did not reach statistical significance.33
Bevacizumab efficacy was also assessed in NSCLC patients in phase II clinical trials in combination with various platinum-based chemotherapy doublets. The number of patients enrolled in these trials varied from 20 to 53 and the overall RR from 74% in bevacizumab plus carboplatin-docetaxel to 30% in bevacizumab plus carboplatin-nanoparticle albumin bound paclitaxel.34
Multi-targeted therapy in NSCLC
There is strong preclinical evidence regarding the close relationship of the EGFR and VEGFR pathways in cancer.35 It is thus reasonable to hypothesize that targeting both pathways with separate drugs would have an additive or synergistic inhibitory effect on tumor growth. The EGFR pathway can be inhibited by monoclonal antibodies blocking EGFR (cetuximab/Erbitux®; panitumumab/Vectibix®) or by small molecule TKIs that interfere with EGFR activation (erlotinib/Tarceva®; gefitinib/Irresa®). The concept of dual blockade in NSCLC patients was evaluated in a phase I/II trial combining bevacizumab and erlotinib in stage IIIB/IV non-squamous NSCLC.36 Out of 40 patients enrolled, eight achieved a partial response and 26 patients had stable disease. There were no pharmacokinetic interactions between bevacizumab and erlotinib and there were no severe adverse reactions noted.36
The combination of bevacizumab and EGFR TKIs was also investigated in patients who progressed during or after platinum-based treatment.37 One hundred and twenty patients were randomized in the phase II trial to one of three arms: (1) bevacizumab combined with either chemotherapy (docetaxel or pemetrexed); or (2) bevacizumab combined with erlotinib; and (3) chemotherapy alone. The median PFS was three months for chemotherapy alone arm and 4.8 and 4.4 months for bevacizumab plus chemotherapy and bevacizumab plus erlotinib arm, respectively. Median OS times were 8.6, 12.6 and 13.7 months for the chemotherapy alone, bevacizumab–chemotherapy and bevacizumab–erlotinib arms, with one-year survival rates of 33.1%, 53.8% and 57.4%, respectively. However, the difference in PFS or OS between the bevacizumab plus chemotherapy and bevacizumab plus erlotinib arms was not significant.37
These findings led to the design of phase III clinical trials utilizing multi-targeted therapy in NSCLC. There are at least two ongoing phase III clinical trials combining bevacizumab and erlotinib in NSCLC patients. The ATLAS trial (AVF3671 g study) is evaluating the efficacy of bevacizumab combined with erlotinib versus bevacizumab alone as a maintenance treatment after four cycles of chemotherapy with bevacizumab in stage IIIB/IV NSCLC. The BeTA Lung trial is evaluating the efficacy of erlotinib combined with bevacizumab versus erlotinib and placebo in the second-line treatment setting.34
Dual blockade of VEGFRs and EGFRs with TKIs
Vandetanib (Zactima, ZD6474) is a small molecule TKI that blocks VEGFR-1, -2, -3 and EGFR. In a phase II trial patients with advanced, previously treated NSCLC were treated with either vandetanib or erlotinib as a single agent. Vandetanib significantly increased PFS to 11.9 weeks when compared with erlotinib to 8.1 weeks.38
Vandetanib has also been evaluated with docetaxel for second-line therapy in patients with advanced NSCLC. Patients were randomized to receive either docetaxel alone or combined with two different doses of vandetanib (100 or 300 mg). Median PFS was 18.7 weeks in patients receiving docetaxel with 100 mg of vandetanib and 12 weeks in patients receiving docetaxel alone. Increasing dose of vandetanib (300 mg) did not improve the PFS and no survival benefit was observed.39
Subset analysis of studies with vandetanib showed that female patients experience more clinical benefit from this treatment when compared with men; however, further randomized studies should address this finding. Vandetanib also had a favorable toxicity profile in patients with advanced disease and its once-daily dosing makes it convenient for patients to take orally and also makes it an interesting option for elderly patients with NSCLC. Currently vandetanib is evaluated in combination with chemotherapy (carbolpatin/paclitaxel) in neoadjuvant settings in patients with resectable NSCLC as well as maintenance therapy in advanced NSCLC patients after carboplatin/docetaxel chemotherapy. Phase III clinical trial evaluating efficacy of combination of vandetanib and pemetrexed as a second line treatment in patients with advanced NSCLC is also inititiated.40
Many TKIs target the activity of multiple receptors.41 Sunitinib (Sutent, SU11248), sorafenib (Nexavar, BAY 43–9006) and cediranib (Recentin, AZD2171) are multi-targeting TKIs that block activity of VEGFR-1, -2, -3, as well as PDGF receptors and other kinases like c-Kit and RET. Ongoing clinical trials are evaluating the antitumor activity of these TKIs in LC when combined with classical platinum-based doublet chemotherapy.42
A number of small molecule TKIs with predominantly VEGFR blocking activity like pazopanib, axitinib and mote-sanib (AMG 706) are being evaluated at present in early phase clinical trials in patients with NSCLC.43 The early clinical data from these trials will hopefully shed some more light on the complex issues of molecular targeted therapy of LC.44
Mechanism of action and toxicity of anti-VEGF therapies
The mechanisms of action of antiangiogenic compounds are being actively investigated. One of the hypotheses proposes that anti-VEGF therapy ‘normalizes’ tumor vasculature and transiently improves blood flow within the tumor, thus enhancing the efficacy of chemotherapy.45 The additive affects of bevacizumab and chemotherapy in prolonging survival in patients with metastatic colorectal and LC supports the ‘normalization’ hypothesis. Other mechanisms propose inhibiting the incorporation of circulating vascular progenitor cells into a tumor vasculature, thus blocking tumor angiogenesis.46
VEGF is involved in many physiological processes like hemostasis, vascular homeostasis and integrity, maintenance of endothelial function in kidney glomeruli, ovulation, wound healing, hematopoiesis, immunomodulation and thyroid function.47 As bevacizumab became widely used in the clinics, the toxicity profile became apparent. Its most common side-effects are hypertension, proteinurea, hemorrhagic and thrombotic events and bowel perforations.48 Although the exact pathophysiological mechanism is not yet fully understood, there is evidence coming from both animal and clinical models that bevacizumab increases the risk of renal thrombotic microangiopathy. It has been shown in animal models that after binding to VEGF, bevacizumab–VEGF immune complexes can be deposited in the glomerular basement membrane and contribute to the development of proteinuria and hypertension. VEGF stimulates nitric oxide (NO) synthase, which is a major source of NO. NO is a potent vasodilator and a key molcule involved in functional maintenance of vascular endothelium. Inhibition of VEGF-mediated NO release and production most likely contributes to high rate of hypertension among patients treated with anti-VEGF drugs. Since VEGF is involved in wound healing and tissue repair mechanisms, VEGF blockade impairs the physiological processes of tissue regeneration and most likely explain the high incidence of bowel perforations in patients treated with bevacizumab.49,50 The increased hemorrhagic events and mortality in patients with squamous NSCLC lead to the subsequent exclusion of these patients for bevacizumab therapy. Other potentially serious toxicities reported include nasal septum perforation, reversible posterior leukoencephalopathy syndrome (severe hypertension, cortical blindness and seizures) or osteonecrosis of the jaw; however, these are very rare.51–53 The toxicity profile associated with bevacizumab treatment emphasizes the need to continue closely monitoring undesirable side-effects from antiangiogenic therapy.
Future directions
New therapies are needed for improved treatment of LC.54 Targeting tumor angiogenesis by combining bevacizumab with chemotherapy is an attractive new therapeutic approach for LC. There are many ongoing clinical trials worldwide that combine bevacizumab with different chemotherapy protocols in LC patients. Bevacizumab is being currently studied in the neoadjuvant setting in resectable stage IB-IIIA NSCLC tumors and in the adjuvant setting in patients with completely resected stage IB-IIIA NSCLC tumors (ECOG 1505 trial).55 Since a significant proportion of NSCLC patients are not currently eligible for bevacizumab treatment either because of brain metastases or squamous histology of the primary lung tumor, bevacizumab is being investigated in phase II clinical trials with modified protocols in these patients. The BRIDGE trial is recruiting patients with squamous histology for treatment that will combine bevacizumab with carboplatin and paclitaxel chemotherapy after initial two cycles of chemotherapy alone are delivered. The AVASQ (Avastin in Squamous NSCLC) trial is investigating safety and efficacy of chemor-adiation with bevacizumab in first- and second-line treatment of advanced squamous NSCLC. The PASSPORT trial is evaluating use of bevacizumab with first- and second-line treatment in patients with treated brain metastases from non-squamous NSCLC.34
A new approach to anti-VEGF therapy is also being evaluated using genetically engineered proteins called fusion proteins.56 These function as molecular ‘traps’ for VEGF. Aflibercept is a recombinant fusion protein that binds both VEGF and PlGF with high affinity. It is composed of VEGFR1 and VEGFR2 domains that are fused to a human IgG Fc region. A phase II study with aflibercept as a single agent in NSCLC (VITAL study) has completed accrual of almost 100 patients with platinum- and erlotinib-resistant, locally advanced or metastatic NSCLC. Results from the first 37 patients demonstrated aflibercept to be well tolerated and two partial responses were noted.57 A phase III trial is currently randomizing NSCLC patients who progressed through platinum-based chemotherapy to docetaxel versus docetaxel plus aflibercept.
Results of these trials are highly anticipated and will hopefully demonstrate the ability of antiangiogenic therapy to bring further improvements in the management of LC.
Acknowledgments
This work was supported in part by the Randall Bridwell Lung Cancer Research Grant and the Effie Marie Cain Scholarship in Angiogenesis Research to RB.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;350:379–92. doi: 10.1056/NEJMra035536. [DOI] [PubMed] [Google Scholar]
- 3.Read WL, Page NC, Tierney RM, Piccirillo JF, Govindan R. The epidemiology of bronchioloalveolar carcinoma over the past two decades: analysis of the SEER database. Lung Cancer. 2004;45:137–42. doi: 10.1016/j.lungcan.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Cespedes M, Ahrendt SA, Piantadosi S, Rosell R, Monzo M, Wu L, Westra WH, Yang SC, Jen J, Sidransky D. Chromosomal alterations in lung adenocarcinoma from smokers and nonsmokers. Cancer Res. 2001;61:1309–13. [PubMed] [Google Scholar]
- 5.Fong KM, Sekido Y, Gazdar AF, Minna JD. Lung cancer. 9: molecular biology of lung cancer: clinical implications. Thorax. 2003;58:892–900. doi: 10.1136/thorax.58.10.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salgia R, Skarin AT. Molecular abnormalities in lung cancer. J Clin Oncol. 1998;16:1207–17. doi: 10.1200/JCO.1998.16.3.1207. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 8.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 9.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–5. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 11.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 13.He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T, Alitalo K. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739–46. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- 14.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–7. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 15.Okada F, Rak JW, Croix BS, Lieubeau B, Kaya M, Roncari L, Shirasawa S, Sasazuki T, Kerbel RS. Impact of oncogenes in tumor angiogenesis: mutant K-ras up-regulation of vascular endothelial growth factor/ vascular permeability factor is necessary, but not sufficient for tumorigenicity of human colorectal carcinoma cells. Proc Natl Acad Sci USA. 1998;95:3609–14. doi: 10.1073/pnas.95.7.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wey JS, Stoeltzing O, Ellis LM. Vascular endothelial growth factor receptors: expression and function in solid tumors. Clin Adv Hematol Oncol. 2004;2:37–45. [PubMed] [Google Scholar]
- 17.Decaussin M, Sartelet H, Robert C, Moro D, Claraz C, Brambilla C, Brambilla E. Expression of vascular endothelial growth factor (VEGF) and its two receptors (VEGF-R1-Flt1 and VEGF-R2-Flk1/KDR) in non-small cell lung carcinomas (NSCLCs): correlation with angiogenesis and survival. J Pathol. 1999;188:369–77. doi: 10.1002/(SICI)1096-9896(199908)188:4<369::AID-PATH381>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 18.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006;290:L209–21. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- 19.Akeson AL, Greenberg JM, Cameron JE, Thompson FY, Brooks SK, Wiginton D, Whitsett JA. Temporal and spatial regulation of VEGF-A controls vascular patterning in the embryonic lung. Dev Biol. 2003;264:443–55. doi: 10.1016/j.ydbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Majka S, Fox K, McGuire B, Crossno J, Jr, McGuire P, Izzo A. Pleiotropic role of VEGF-A in regulating fetal pulmonary mesenchymal cell turnover. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1183–92. doi: 10.1152/ajplung.00175.2005. [DOI] [PubMed] [Google Scholar]
- 21.Stefanou D, Batistatou A, Arkoumani E, Ntzani E, Agnantis NJ. Expression of vascular endothelial growth factor (VEGF) and association with microvessel density in small-cell and non-small-cell lung carcinomas. Histol Histopathol. 2004;19:37–42. doi: 10.14670/HH-19.37. [DOI] [PubMed] [Google Scholar]
- 22.Herbst RS, Sandler AB. Non-small cell lung cancer and antiangiogenic therapy: what can be expected of bevacizumab? Oncologist. 2004;9(Suppl 1):19–26. doi: 10.1634/theoncologist.9-suppl_1-19. [DOI] [PubMed] [Google Scholar]
- 23.Yuan A, Yu CJ, Kuo SH, Chen WJ, Lin FY, Luh KT, Yang PC, Lee YC. Vascular endothelial growth factor 189 mRNA isoform expression specifically correlates with tumor angiogenesis, patient survival, and postoperative relapse in non-small-cell lung cancer. J Clin Oncol. 2001;19:432–41. doi: 10.1200/JCO.2001.19.2.432. [DOI] [PubMed] [Google Scholar]
- 24.Ushijima C, Tsukamoto S, Yamazaki K, Yoshino I, Sugio K, Sugimachi K. High vascularity in the peripheral region of non-small cell lung cancer tissue is associated with tumor progression. Lung Cancer. 2001;34:233–41. doi: 10.1016/s0169-5002(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 25.Passalidou E, Trivella M, Singh N, Ferguson M, Hu J, Cesario A, Granone P, Nicholson AG, Goldstraw P, Ratcliffe C, Tetlow M, Leigh I, Harris AL, Gatter KC, Pezzella F. Vascular phenotype in angiogenic and non-angiogenic lung non-small cell carcinomas. Br J Cancer. 2002;86:244–9. doi: 10.1038/sj.bjc.6600015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 27.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 28.Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB, III Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–44. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 29.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF, III, Gaudreault J, Damico LA, Holmgren E, Kabbinavar F. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–91. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 30.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 31.Ramalingam SS, Dahlberg SE, Langer CJ, Gray R, Belani CP, Brahmer JR, Sandler AB, Schiller JH, Johnson DH Eastern Cooperative Oncology Group. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol. 2008;26:60–5. doi: 10.1200/JCO.2007.13.1144. [DOI] [PubMed] [Google Scholar]
- 32.Merza T, Howard LM, Junagadhwalla M, Gajra A. Exclusions to use of bevacizumab in elderly veterans with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2007;25(Suppl):687s, abstr. 18046. [Google Scholar]
- 33.Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–34. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 34.Gridelli C, Maione P, Rossi A, De Marinis F. The role of bevacizumab in the treatment of non-small cell lung cancer: current indications and future developments. Oncologist. 2007;12:1183–93. doi: 10.1634/theoncologist.12-10-1183. [DOI] [PubMed] [Google Scholar]
- 35.Ellis LM. Epidermal growth factor receptor in tumor angiogenesis. Hematol Oncol Clin North Am. 2004;18:1007–21. viii. doi: 10.1016/j.hoc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Herbst RS, Johnson DH, Mininberg E, Carbone DP, Henderson T, Kim ES, Blumenschein G, Jr, Lee JJ, Liu DD, Truong MT, Hong WK, Tran H, Tsao A, Xie D, Ramies DA, Mass R, Seshagiri S, Eberhard DA, Kelley SK, Sandler A. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:2544–55. doi: 10.1200/JCO.2005.02.477. [DOI] [PubMed] [Google Scholar]
- 37.Herbst RS, O’Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, Melnyk O, Ramies D, Lin M, Sandler A. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol. 2007;25:4743–50. doi: 10.1200/JCO.2007.12.3026. [DOI] [PubMed] [Google Scholar]
- 38.Natale RB. Dual targeting of the vascular endothelial growth factor receptor and epidermal growth factor receptor pathways with vandetinib (ZD6474) in patients with advanced or metastatic non-small cell lung cancer. J Thorac Oncol. 2008;3(Suppl 2):S128–30. doi: 10.1097/JTO.0b013e318174e95a. [DOI] [PubMed] [Google Scholar]
- 39.Heymach JV, Johnson BE, Prager D, Csada E, Roubec J, Pesek M, Spa ‘sova’ I, Belani CP, Bodrogi I, Gadgeel S, Kennedy SJ, Hou J, Herbst RS. Randomized, placebo-controlled phase II study of vandetanib plus docetaxel in previously treated non small-cell lung cancer. J Clin Oncol. 2007;25:4270–7. doi: 10.1200/JCO.2006.10.5122. [DOI] [PubMed] [Google Scholar]
- 40.Rossi A, Maione P, Colantuoni G, Ferrara C, Rossi E, Guerriero C, Nicolella D, Falanga M, Palazzolo G, Gridelli C. Recent developments of targeted therapies in the treatment of non-small cell lung cancer. Curr Drug Discov Technol. 2009;6:91–102. doi: 10.2174/157016309788488339. [DOI] [PubMed] [Google Scholar]
- 41.Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–87. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 42.Lynch TJ, Jr, Blumenschein GR, Jr, Engelman JA, Espinoza-Delgado I, Govindan R, Hanke J, Hanna NH, Heymach JV, Hirsch FR, Janne PA, Lilenbaum RC, Natale RB, Riely GJ, Sequist LV, Shapiro GI, Shaw A, Shepherd FA, Socinski M, Sorensen AG, Wakelee HA, Weitzman A. Summary statement novel agents in the treatment of lung cancer: Fifth Cambridge Conference assessing opportunities for combination therapy. J Thorac Oncol. 2008;3(Suppl 2):S107–12. doi: 10.1097/JTO.0b013e318174e9d3. [DOI] [PubMed] [Google Scholar]
- 43.Cabebe E, Wakelee H. Role of anti-angiogenesis agents in treating NSCLC: focus on bevacizumab and VEGFR tyrosine kinase inhibitors. Curr Treat Options Oncol. 2007;8:15–27. doi: 10.1007/s11864-007-0022-4. [DOI] [PubMed] [Google Scholar]
- 44.Riely GJ. Second-generation epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. J Thorac Oncol. 2008;3(Suppl 2):S146–9. doi: 10.1097/JTO.0b013e318174e96e. [DOI] [PubMed] [Google Scholar]
- 45.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 46.Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T, Rawn K, Voskas D, Dumont DJ, Ben-David Y, Lawler J, Henkin J, Huber J, Hicklin DJ, D’Amato RJ, Kerbel RS. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7:101–11. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 47.Verheul HM, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer. 2007;7:475–85. doi: 10.1038/nrc2152. [DOI] [PubMed] [Google Scholar]
- 48.Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis. 2007;49:186–93. doi: 10.1053/j.ajkd.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 49.Cao Y, Zhong W, Sun Y. Improvement of antiangiogenic cancer therapy by understanding the mechanisms of angiogenic factor interplay and drug resistance. Semin Cancer Biol. 2009;19:338–43. doi: 10.1016/j.semcancer.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Higa GM, Abraham J. Biological mechanisms of bevacizumab-associated adverse events. Expert Rev Anticancer Ther. 2009;9:999–1007. doi: 10.1586/era.09.68. [DOI] [PubMed] [Google Scholar]
- 51.Traina TA, Norton L, Drucker K, Singh B. Nasal septum perforation in a bevacizumab-treated patient with metastatic breast cancer. Oncologist. 2006;11:1070–1. doi: 10.1634/theoncologist.11-10-1070. [DOI] [PubMed] [Google Scholar]
- 52.Ozcan C, Wong SJ, Hari P. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:980–2. [PubMed] [Google Scholar]
- 53.Estilo CL, Fornier M, Farooki A, Carlson D, Bohle G, III, Huryn JM. Osteonecrosis of the jaw related to bevacizumab. J Clin Oncol. 2008;26:4037–8. doi: 10.1200/JCO.2007.15.5424. [DOI] [PubMed] [Google Scholar]
- 54.Carney DN. Lung cancer – time to move on from chemotherapy. N Engl J Med. 2002;346:126–8. doi: 10.1056/NEJM200201103460211. [DOI] [PubMed] [Google Scholar]
- 55.Rizvi NA, Rusch V, Zhao B, Senturk E, Schwartz L, Fury M, Downey R, Rizk N, Krug L, Kris MG. Single agent bevacizumab and bevacizumab in combination with docetaxel and cisplatin as induction therapy for resectable IB–IIIA non-small cell lung cancer. J Clin Oncol. 2007;25(Suppl):Abstr.18045. [Google Scholar]
- 56.Lau SC, Rosa DD, Jayson G. Technology evaluation: VEGF Trap (cancer), Regeneron/sanofi-aventis. Curr Opin Mol Ther. 2005;7:493–501. [PubMed] [Google Scholar]
- 57.Adis International Limited. Aflibercept: AVE 0005, AVE 005, AVE0005, VEGF Trap – Regeneron, VEGF Trap (R1R2), VEGF Trap-Eye. Drugs R D. 2008;9:261–9. doi: 10.2165/00126839-200809040-00006. [DOI] [PubMed] [Google Scholar]