Abstract
Objectives: Developing countries face considerable problems in both accessing and properly utilizing essential medicines. One challenge to achieving these goals in resource-poor settings is a limited knowledge base as to what works to improve the selection, access and use of essential medicines including; ways to ensure affordable prices, increase sustainable financing, and strengthen reliable supply systems that are relevant to these settings. The objective of this study was to search the existing evidence base on access to medicine issues in developing countries and to assess publication patterns regarding the nature of topics studied, areas where gaps of information exist and the general trends in publications in this area.
Methods: A PubMed search was conducted to retrieve publications on access to medicines in developing countries between 1999-2008. Our search strategy builds and expands on a search strategy developed for a Cochrane review to include a wider range of topics related to access to medicines and pharmaceutical policy. Retrieved articles were categorized by research topics, year of publication, study area, and country of residence of corresponding author to establish patterns in publications with respect to these categories over the past 10 years.
Results: Medicine selection, intellectual property rights, and monitoring and quality assurance were among the top topics studied over the last 10 years. Corresponding authors residing in high-income countries represented around 50% of all publications relative to low-income (18%) and middle-income countries (32%). Although an increasing trend in the number of publications per year was found, the increase was relatively small and variable over a 10-year period.
Conclusions: There are few peer-reviewed publications on access to medicines in developing countries with an average of only 76 publications per year over the past 10 years. Increasing the local evidence base as to what works to improve access to medicines in resource poor countries, particularly to the poor, is of the utmost priority to accelerating the achievement of global medicine goals.
Keywords: Bibliometrics, Access to Medicines, Essential Medicines, Drug Utilization
Background
Thirty-two years have passed since the World Health Organization (WHO) created the first Model Essential Medicines List (EML) in1977 as a standard for countries to select medicines and to create their own lists of essential medicines1. Increasing access to these essential medicines is crucial to preventing millions of deaths a year [2]. As of 2008, at least four out of every five countries in the world had a national EML in place [1]. Medicines account for20 – 60% of health spending in developing countries, and upto 90% of people in developing countries have to pay for their medicines out-of-pocket [3]. Individuals affected by these high payments are disproportionately poor and medicines remain unaffordable for many [2,3,4].
In 1978, the Declaration of Alma Ata identified the quality, rational use and provision of essential medicines as one of the eight key components of primary health care [1]. During the 1985 Conference of Experts on Rational Use of Drugs in Nairobi, the modern definition of rational use of medicines was promulgated. This definition states, “rational use of drugs requires that patients receive medications appropriate to their clinical needs, in doses that meet their own individual requirements for an adequate period of time, and at the lowest cost to them and their community [5].” In both 1988 and 2003, the WHO published Guidelines for Developing National Drug Policies to help member countries create and improve their own national policies and to increase access to medicines. Despite all of these landmarks, the problems of limited access to and the irrational use of medicines still persist, especially in the developing world and for the world’s poorest citizens. One factor could be the limited knowledge base in resource-poor settings [6]. These countries are not well equipped to apply existing knowledge to create effective mechanisms for improving the selection and use of essential medicines, ensuring affordable prices, increasing sustainable financing, and strengthening reliable supply systems [7]. A solid and diverse evidence base is key to informed policy making [1,8].
Recently, four Cochrane reviews were published on pharmaceutical policy, dealing primarily with pricing, financial incentives for prescribers, rational use and the effects of caps and co-payments on rational use [9-12]. However, very few of the papers considered in these reviews were from developing countries.
In August 2008, the United Kingdom’s Department for International Development (DfID) proposed the establishment of a global Access to Medicines Research Network (ATM RN) involving both high-income and developing country research institutions [13]. The purpose of the ATM RN is to address the issue of poor people’s access to essential medicines by producing policy research on the topic, specifically focusing on medicine availability, quality and utilization. One of the main priorities of this network is to promote the generation and use of new research on pharmaceutical policy relevant to developing countries and to build capacity of developing country researchers to set priorities for and undertake research relevant to their own settings [13].
The objective of this study was to perform a bibliometric analysis of publications related to access to medicines policies in the published literature to assess the state of access to medicines research in developing countries. Bibliometrics look at publication patterns in research areas using quantitative analysis and statistics to analyze citation data. An examination of the nature of existing evidence through analysis of the data by topic and study region occurred. Publication patterns were examined to explore changes in the output of and capacity for research in developing countries over the past 10 years. These findings will serve as evidence based guide for the proposed ATM Research Network and in setting priorities and building capacity for research related to pharmaceutical policy in developing countries in the future.
Methodology
A bibliometric search was performed in PubMed, building on a search strategy developed in a Cochrane review on pharmaceutical policy and expanded to include a wider range oftopics related to this area [12]. The targeted search included studies published between 1999-2008. However in examining trends we also explored any recent change in trends, i.e., between 2005 and 2008 that may be linked to the various initiatives to improve research for health around this period, e.g., the Mexico Summit for Health Research and efforts to accelerate progress towards achieving the millennium development goals for health.
Articles were selected if they met the following criteria:
Focus on one or more of the following themes:
-
Medicines regulation and classification (licensing) policies
Drug monitoring
Medicines selection
Medicines pricing policies
Medicines intellectual property/patent policies
Medicines marketing policies
Medicines information
Prescribing policies
Medicines utilization or medicines use
Medicines insurance policy and medicines financing
Medicine reform/policy
Access to Medicines
Medicines supply management
Publications concerning developing countries
Publication date: 1999 to 2008
Limited to human subjects
All languages were includedStudies on substance abuse or poisoning were excluded.
Retrieved articles and methods for analysis
A total of 761 publications were retrieved for the period between 1999-2008; 350 were published between 2005-2008. The retrieved citations were downloaded from the PubMed database and analysed using Reference Manager and Excel software. Extracted data included author names, publication title and abstract, publication date, affiliation and country of residence of corresponding author, study area, main theme with respect to the categories listed above, and journal.
Three publications from 1999-2008 were eliminated because they were published in 2009, this occurred because they were published electronically in 2008. Only 535 publications had data on country of residence of corresponding author.
The retrieved articles were further analyzed to categorize the country of residence of corresponding author according to World Bank country income classification and the World Bank and the geographical regions [14,15]. A more detailed reportincluding the search strategy is available from the WHO [16].
We also analyzed the frequency of publications by topic for the retrieved articles. This was done in two stages. The first following the detailed topics listed above for the selection criteria and the second using a more general categorization involving fewer overarching content areas. The aggregated categories included procurement, quality assurance and references to pharmacists. It should be noted that the proportion of articles published by theme does not add up to 100% as some articles are classified under more than one theme.
Results
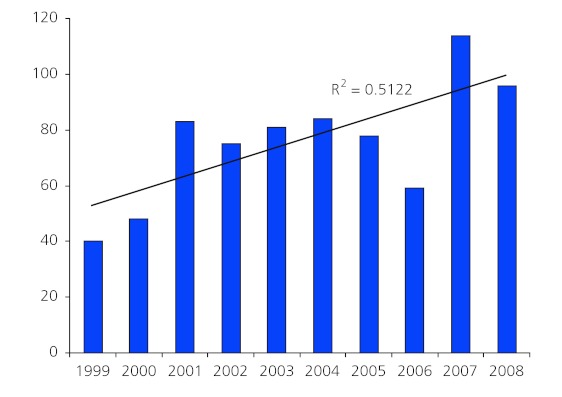
Table 1 shows the distribution of articles per year. Although there was a general increasing trend in the number of publications per year, there was some fluctuation between years.
The overall correlation reflecting the association between the number of publications and year was 0.715 p< 0.05. The R-squared value 0.51 suggests a steady and significant increase over the 10 years.
We cannot explain the increase in publications between 2006 and 2007. Further analysis revealed that authors residing in Brazil tripled their previous contribution of four papers in 2002 to 12 in 2007, which may explain some of this difference. However this was not sustained in 2008, when a total of 96 papers were published.
The top aggregated themes for both time periods were selection, intellectual property, monitoring, regulation and quality assurance, access, and insurance and financing. Monitoring moved from fourth most common theme in 1999-2004 to the most important theme from 2004-2008. Since several themes might have been addressed in the same paper, this analysis only gives a general description of the distribution and does not add up to 100%, see Table 2.
Despite a limited increase in studies related to pharmaceutical policy and reform, these papers ranked 7th for themes studied in 2005-2008. The bottom four themes of pharmacists, prescribing/utilization, information and marketing remained the same for both time periods.
On average, 50% of corresponding authors resided in high- income countries both during the whole 10-year period (1999-2008) and the last four years (2005-2008). Corresponding authors residing in low-income countries represented 18% and 19%, on average, of the total number of papers over the past10 years and four years respectively, see Figure 2.
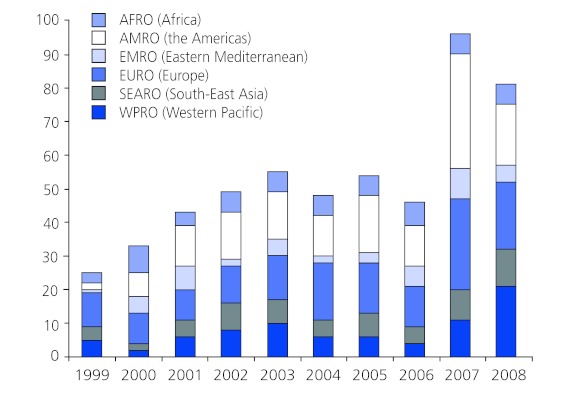
Figure 3 shows the contribution of the EURO and AMRO WHO regions, who combined represent on average around 50% of the total publications per year during the analysis period. The next biggest contributors were authors residing in the Western Pacific region with around 14% of publications per year on average. EMRO country authors only contributed a total of 45 articles (8.5%) for the entire time period of 1999-2008, the lowest of all WHO regions.
The top eight countries of residence of corresponding authors in 2008 were the United States, the United Kingdom, Brazil, China, India, the Republic of Korea, Nigeria and Japan, (Table3). The following six countries all contributed two publications in 2008 (Australia, Egypt, Malaysia, the Netherlands, Sweden and Switzerland). Out of the 46 total publications representing the top eight countries in 2008, 35% were from corresponding authors residing in developing countries (China, India and Nigeria). Korea went from 1to 2 articles a year for only four of the previous nine years to six articles in 2008.
Table 1. Number of publications per year, 1999-2008
| Year | Number of Publications |
| 1999 | 40 |
| 2000 | 48 |
| 2001 | 83 |
| 2002 | 75 |
| 2003 | 81 |
| 2004 | 84 |
| 2005 | 78 |
| 2006 | 59 |
| 2007 | 114 |
| 2008 | 96 |
Table 2. Publication topics and numbers per year grouping
| 1999-2004 | No. | 2005-2008 | No. |
| Selection | 135 | Monitoring | 106 |
| Intellectual Property | 88 | Selection | 98 |
| Regulation & Quality Assurance | 84 | Regulation & Quality Assurance | 62 |
| Monitoring | 70 | Intellectual Property | 46 |
| Isurance and Financing | 39 | Access | 35 |
| Access | 24 | Procurement & Distribution | 25 |
| Procurement & Distribution | 21 | Policy/Reform | 17 |
| Medicine Supply | 13 | Insurance and Financing | 16 |
| Policy/Reform | 9 | Medicine Supply | 12 |
| Pharmacists | 8 | Pharmacists | 11 |
| Prescribing & Utilization | 8 | Prescribing & Utilization | 11 |
| Information | 4 | Information | 6 |
| Marketing | 3 | Marketing | 2 |
Table 3. Top eight countries of residence of corresponding author, 2008
| Country | Nunber of Publications |
| United States of America | 8 |
| United Kindom | 7 |
| Brazil | 6 |
| China | 6 |
| India | 6 |
| Republic of Korea | 6 |
| Nigeria | 4 |
| Japan | 3 |
Figure 1.Number of publications per year, 1999 – 2008.
Figure 2. Country of residence of corresponding authors by World Bank region.
Figure 3. Country of residence of corresponding authors by World Health Organization region.
Discussion
Publications over time
While the number of publications has increased over the period of review, there is considerable variation over the last decade. These findings highlight the importance of examining the content and geographical origin of publications over a series of years rather than assuming that a cross-sectional examination at a point in time is generalizable over a number of years.
Topics
Insurance and financing has dropped off in terms of relative interest in recent years. This is concerning when one considers that in developing countries, medicines account for 20-60% of overall health care spending compared with less than 15% in most high-income countries [2]. The increased focus on drug monitoring including adverse drug reaction and pharmacovigilance in the developing world are heartening since there seems to be a greater interest in whether or not the medicines are working effectively.
Publications related to Intellectual property (IP) were high in both time periods, since IP continues to create interest and further policy debate. Publications on prescribing and utilization remained low over the 10-year period. There is a need for greater attention to the patterns of prescribing and utilization of drugs to provide important data sources if policy planning is to take place in the future. Such data will contribute to a better understanding of how to best prioritize medications in low and moderate-income countries.
Country of Origin of Corresponding Author
As predicted, high-income countries contributed most to research on access to medicines relative to middle and low- income countries. The EURO and AMRO regions were the highest contributors to the literature on access to medicines. Developing country authors have recently contributed to a higher share of publications related to access to medicines in their respective countries, which shows increased interest and capacity to undertake this type of research from developing country researchers.
Limitations
One of the main limitations of this study was limiting our search to only the PubMed search engine and the journals covered by this database. In addition this search does not include studies published in the grey literature, sometimes the most common form of publication in the developing world, but which is often difficult to access. However, since the objective of this study was to search for the nature of the evidence and its distribution among topics and study areas, we believe that this analysis provides a fair representation of the general trends regarding the evidence. This study also provides a baseline with which to compare future studies.
Conclusion
Access to essential affordable medicines is a Millennium Development Goal. There is a need for increased capacity of developing country researchers to perform research and take the lead in choosing questions relevant to them, study the issues and publish to share their knowledge. The evidence base on medicines pricing, quality, affordability and the impact of policies in developing countries should be strengthened to better inform policy.
The recent initiative to create an ATM research network with a particular focus on developing countries and other similar initiatives will hopefully address some of these knowledge gaps. The ATM Research Network could catalyse collaborative opportunities for and investments in more relevant research for the developing world, as well as increase the capacity to undertake and use evidence from this research to improve access to essential medicines in developing countries. Creating and fostering cooperation and future integration between developing and developed world institutions is an important strategy to build this capacity and to achieve a sustainable solution for improving access to medicines in developing countries through informed policy-making.
This study provides the first bibliometric analysis of publications in the access to medicines field. The findings presented in this paper provide a solid basis for setting priorities for research on access to medicines in developing countries and for monitoring the progress in the knowledge base over time. In addition, the search strategy developed for this analysis will assist policy makers in identifying evaluations for different pharmaceutical policy options and will inform future studies interested in both the development of the field as well as in gathering the latest evidence on available interventions.
Acknowledgments
This work was conducted in Geneva and Boston as an internship activity through the Pharmaceutical Assessment, Management and Policy program at Boston University School of Public Health and the WHO Geneva.
References
- 1.Essential Medicines List (EML) 30 years of vital health care. Geneva: World Health Organization; 2008. http://www.who.int/mediacentre/factsheets/fs325/en/index.html. [Google Scholar]
- 2.WHO Medicines Strategy 2008-2013. Geneva: World Health Organization; 2008. http://www.who.int/medicines/areas/policy/medstrategy_consultation/en/index.html. [Google Scholar]
- 3.Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373(9659):240–9. doi: 10.1016/S0140-6736(08)61762-6. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(08)61762-6/abstract. [DOI] [PubMed] [Google Scholar]
- 4.MDG Gap Task Force. Millennium development goal 8. Delivering on the global partnership for achieving millennium development goals. Geneva: 2008. http://www.who.int/medicines/mdg/MDG8EnglishWeb.pdf. [Google Scholar]
- 5.The rational use of drugs: report of the Conference of Experts, Nairobi. Geneva: World Health Organization; 1987. [Google Scholar]
- 6.Harris E. Building scientific capacity in developing countries. EMBO Report. 2004;5(1):7–11. doi: 10.1038/sj.embor.7400058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Report of the Task Force on HIV/ AIDS, Malaria, TB, and Access to Essential Medicines, Working Group on Access to Essential Medicines. Sterling, VA: Earthscan; 2005. UN Millennium Project. Prescription for Healthy Development: Increasing Access to Medicines. [Google Scholar]
- 8.Bennett Sara, Adam Taghreed, Zarowsky Christina, Tangcharoensathien Viroj, Ranson Kent, Evans Tim, Mills Anne, Alliance STAC. From Mexico to Mali: progress in health policy and systems research. Lancet. 2008;372(9649):1571–8. doi: 10.1016/S0140-6736(08)61658-X. http://www.scholaruniverse.com/ncbi-linkout?id=18984191. [DOI] [PubMed] [Google Scholar]
- 9.Aaserud M. Pharmaceutical policies: effects of reference pricing, other pricing, and purchasing policies. Cochrane Database Syst Rev. 2006;2 doi: 10.1002/14651858.CD005979. Art. No.: CD005979. [DOI] [PubMed] [Google Scholar]
- 10.Austvoll-Dahlgren A. Pharmaceutical policies: effects of cap and co-payment on rational drug use. Cochrane Database Syst Rev. 2008;1 doi: 10.1002/14651858.CD007017. Art. No.: CD007017. [DOI] [PubMed] [Google Scholar]
- 11.Sturm H. Pharmaceutical policies: effects of financial incentives for prescribers. Cochrane Database Syst Rev. 2007;3 doi: 10.1002/14651858.CD006731. Art. No.: CD006731. [DOI] [PubMed] [Google Scholar]
- 12.Aaserud M. Pharmaceutical policies: effects on rational drug use, an overview of 13 reviews (Protocol) Cochrane Database Syst Rev. 2006;2 doi: 10.1002/14651858.CD007017. Art. No.: CD006731. [DOI] [PubMed] [Google Scholar]
- 13.Access to Medicines Research Network On-line Consultation: Summary of Responses. DfID; 2008. http://www.dfid.gov.uk/Documents/consultations/ATMRN-consult-summary.pdf. [Google Scholar]
- 14.Country Groups. World Bank; 2009. http://go.worldbank.org/D7SN0B8YU0. [Google Scholar]
- 15.WHO – its people and offices. WHO; 2009. http://www.who.int/about/structure/en/index.html. [Google Scholar]
- 16.Ritz L. Access to medicines Publications in Developing Countries. A Bibliometric Study and its Implications for the Access to Medicines Research Network. document forthcoming.