Abstract
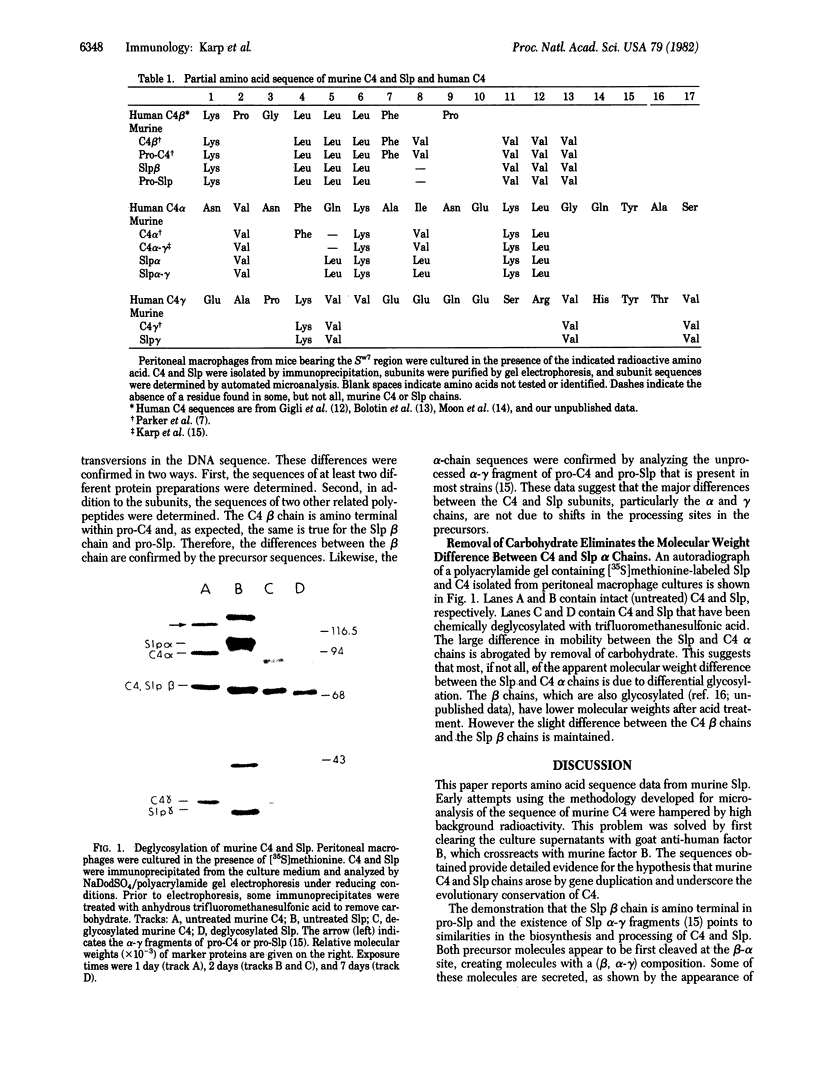
Limited primary sequence data have been obtained for all three subunits of the fourth component of murine complement (C4) and its related homologue, the sex-limited protein (Slp). These data show a high degree of NH2-terminal homology between C4 and Slp: four of the six residues identified for the alpha chain, seven of eight for the beta chain, and four of four for the gamma chain. This suggests that apparent molecular weight differences between C4 and Slp subunits are not, as previously suggested, due to a shift in the proteolytic processing sites in the pro-Slp polypeptide molecule. Chemical deglycosylation (apparently complete) of the C4 and Slp alpha chains with trifluoromethanesulfonic acid removes the molecular weight difference between them, suggesting that acquisition of extra glycosylation sites in the latter is responsible for this difference.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson J. P., McGinnis K., Brown L., Peterein J., Shreffler D. A murine C4 molecule with reduced hemolytic efficiency. J Exp Med. 1980 Feb 1;151(2):492–497. doi: 10.1084/jem.151.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotin C., Morris S., Tack B., Prahl J. Purification and structural analysis of the fourth component of human complement. Biochemistry. 1977 May 3;16(9):2008–2015. doi: 10.1021/bi00628a039. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Gigli I., von Zabern I., Porter R. R. The isolation and structure of C4, the fourth component of human complement. Biochem J. 1977 Sep 1;165(3):439–446. doi: 10.1042/bj1650439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T. H., Shreffler D. C. Characterization of a constitutive variant of the murine serum protein allotype, Slp. J Immunol. 1976 Nov;117(5 Pt 1):1507–1513. [PubMed] [Google Scholar]
- Karp D. R., Atkinson J. P., Shreffler D. C. Genetic variation in glycosylation of the fourth component of murine complement. Association with hemolytic activity. J Biol Chem. 1982 Jul 10;257(13):7330–7335. [PubMed] [Google Scholar]
- Karp D. R., Capra J. D., Atkinson J. P., Shreffler D. C. Structural and functional characterization of an incompletely processed form of murine C4 and Slp. J Immunol. 1982 May;128(5):2336–2341. [PubMed] [Google Scholar]
- Karp D. R., Parker K. L., Shreffler D. C., Capra J. D. Characterization of the murine C4 precursor (pro-C4): evidence that the carboxy-terminal subunit is the C4 gamma-chain. J Immunol. 1981 May;126(5):2060–2061. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moon K. E., Gorski J. P., Hugli T. E. Complete primary structure of human C4a anaphylatoxin. J Biol Chem. 1981 Aug 25;256(16):8685–8692. [PubMed] [Google Scholar]
- Parker K. L., Roos M. H., Shreffler D. C. Structural characterization of the murine fourth component of complement and sex-limited protein and their precursors: evidence for two loci in the S region of the H-2 complex. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5853–5857. doi: 10.1073/pnas.76.11.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K. L., Shreffler D. C., Capra J. D. Partial amino acid sequences of the murine fourth component of complement (C4): demonstration of homology with human C4 and identification of the amino-terminal subunit in pro-C4. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4275–4278. doi: 10.1073/pnas.77.7.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore H. C., Kubo J. K., Singh S. K., Klein J. The histocompatibility-2 system in wild mice. XI. Ss and Slp properties of wild-derived H-2 haplotypes. Immunogenetics. 1980;11(4):397–405. doi: 10.1007/BF01567806. [DOI] [PubMed] [Google Scholar]
- Passmore H. C., Shreffler D. C. A sex-limited serum protein variant in the mouse: inheritance and association with the H-2 region. Biochem Genet. 1970 Jun;4(3):351–365. doi: 10.1007/BF00485752. [DOI] [PubMed] [Google Scholar]
- Roos M. H., Atkinson J. P., Shreffler D. C. Molecular characterization of the Ss and Slp (C4) proteins of the mouse H-2 complex: subunit composition, chain size polymorphism, and an intracellular (PRO-Ss) precursor. J Immunol. 1978 Sep;121(3):1106–1115. [PubMed] [Google Scholar]
- Roos M. H., Kornfeld S., Shreffler D. C. Characterization of the oligosaccharide units of the fourth component of complement (Ss protein) synthesized by murine macrophages. J Immunol. 1980 Jun;124(6):2860–2863. [PubMed] [Google Scholar]
- Wiseman R. L., Fothergill J. E., Fothergill L. A. Replacement of asparagine by aspartic acid in hen ovalbumin and a difference in immunochemical reactivity. Biochem J. 1972 May;127(5):775–780. doi: 10.1042/bj1270775. [DOI] [PMC free article] [PubMed] [Google Scholar]