Abstract
Objectives: The objective of this study was to assess the existing theoretical and empirical literature examining the link between "local production" of pharmaceuticals and medical devices and increased local access to these products. Our preliminary hypothesis is that studies showing a robust relationship between local production and access to medical products are sparse, at best.
Methods: An extensive literature search was conducted using a wide variety of databases and search terms intending to capture as many different aspects of this issue as possible. The results of the search were reviewed and categorized according to their relevance to the research question. The literature was also reviewed to determine the rigor used to examine the effects of local production and what implications these experiences hold for other developing countries.
Results: Literature addressing the benefits of local production and the link between it and access to medical products is sparse, mainly descriptive and lacking empirical evidence. Of the literature we reviewed that addressed comparative economics and strategic planning of multinational and domestic firms, there are few dealing with emerging markets and lower-middle income countries and even fewer that compare local biomedical producers with multinational corporations in terms of a reasonable metric. What comparisons exist mainly relate to prices of local versus foreign/multinational produced medicines.
Conclusions: An assessment of the existing theoretical and empirical literature examining the link between "local production" of pharmaceuticals and medical devices and increased local access to these products reveals a paucity of literature explicitly dealing with this issue. Of the literature that does exist, methods used to date are insufficient to prove a robust relationship between local production of medical products and access to these products. There are mixed messages from various studies, and although the studies may correctly depict specific situations in specific countries with reference to specific products, such evidence cannot be generalized. Our review strongly supports the need for further research in understanding the dynamic link between local production and access to medical products
Keywords: Pharmaceutical Policy, Industrial Policy, Access to Medicines, Pharmaceuticals
Introduction
Local production (LP) of essential medical technologies is at the interface of industrial/economic development policy and public health policy. From an industrial policy perspective, generating assured quality products by having a competitive pharmaceutical/ medical device industry with sufficient economies of scale wouldbe desirable for low and middle income countries (LMICs) [1]. Clearly, countries such as India, Brazil, and others have proven that this is possible for medicines [2-6]. It is not clear whether it is possible for other LMICs to successfully repeat these efforts due to the need for major investments in human resources, financing and infrastructure to support innovation.
This question has been receiving much high-level attention in recent years with work funded by various governmental and non-governmental agencies including the United Kingdom (UK) Department for International Development- DFID [7], the American Enterprise Institute [8], the German Development Institute [9], the World Bank [1,10], Deutsche Gesellschaft für Internationale Zusammenarbeit GmbH, GIZ [11-14], the African Union [15] and the Southern Africa Development Council [16].
We further note the Global Strategy and Plan of Action on Public Health, Innovation and Intellectual Property (GSPA-PHI) of the World Health Organization (WHO) that includes a mandate to support development cooperation, partnerships, and networks to build and improve transfer of technology related to health innovation [17]. The WHO, in partnership with the United Nations Conference on Trade and Development (UNCTAD) and the International Centre for Trade and Sustainable Development (ICTSD), and with funding by the European Union (EU), is undertaking a project on improving access to medical products in developing countries through local production and related technology transfer [18].
From a public health perspective, understanding how changes in LP capacity will impact access to medical products is of great significance. We pose this as a question: "Does local production of medical products have beneficial impact on the resulting access to these products?" Such beneficial impact might, in principle, manifest itself as greater availability and/or lower prices for locally produced products, as opposed to imported products.
In this paper, we present results of a systematic literature review, summarizing existing theoretical and empirical work on LP of pharmaceutical products in LMICs, and its potential impact on access to medicines in LMICs. We assess to what extent the linkages between LP and access to medical products are explored in such studies; critically analyze whether the methods employed in the literature are sufficient to suggest a robust relationship between local production and access; and evaluate whether results obtained could be directly applied to local production conditions in developing and least developed country contexts.
Methodology
What do we mean by "local production"?
It is important to define what we understand by the term local production. Some "local" manufacturers are subsidiaries of multinational corporations (MNCs) and some are locally owned small-scale manufacturers serving a portion of the domestic market [19]. We use a jurisdictional, not an ownership definition. If production takes place in-country to produce biomedical products, this is "local production". For pharmaceuticals, "production" can be primary (manufacture of active pharmaceutical ingredients (APIs) and intermediates from basic substances), secondary (production of finished dosage forms from raw materials and excipients or tertiary (packaging andlabelling finished products or repackaging finished products). For vaccines, technology is specific for each inactivated or live attenuated vaccine product and may include isolating viral particles, producing "seed" viruses, bulk manufacture, and assembling polyvalent vaccines. For medical devices, the "device" component can be simple to complex, e.g. a bed to a Magnetic Resonance Imagery (MRI) machine [20].
What are "low- and middle- income" countries?
United Nations categorizations provide no established convention for the designation of "developed" and "developing" countries. The World Bank classifies countries according to income and this does not necessarily reflect development status. Significantly, all the World Bank low- and middle-income countries are considered to be "developing" under the United Nations classification.
For this review, we classify LMICs according to the widely usedWorld Bank system [21] which divides countries according to 2009 Gross National Income (GNI) per capita (calculated using the World Bank Atlas method): low income, $995 or less ; lower- middle income, $996 - $3,945; upper-middle income, $3,946- $12,195. All other countries according to the World Bank scheme, are considered "developed"/high income countries (GNI per capita $12,196 or more. Middle-income countries such as Brazil, India, Mexico, South Africa and Taiwan have been called "emerging markets" using other classification systems.
What is "access to medicines"?
In the context of local production, "access" includes: (a) lower prices (thus greater affordability); (b) greater availability through the presence of locally made products and local distribution networks. In principle, these penetrate rural markets better than MNC produced products; (c) local adaptation of existing products by local firms through incremental innovation efforts; (d) new forms of innovative medicines and products developed locally and tailored to the local population(s).
Search strategies
The primary objective of this review was to identify operational or implementation/analytical studies identifying empirically robust links between LP and access to biomedical products in LMICs. The kind of robust evidence that would satisfy our primary objectives can be summarized in Table 1.
We based our literature search strategy on a single working hypothesis: studies showing a robust relationship between LP and access to medical products are sparse or even non-existent.
Issues related to local production of medical products are often unlikely to be labelled as such, since "local production" is not a common term in academic research. Because of its cross-cutting nature, the "local production" literature is likely scattered in writings on innovation capacity, science and technology, industrial and pharmaceutical policy, intellectual property analysis and sometimes, health economics.
We carried out a literature search using key words and theirsynonyms, "local, national, regional, domestic" and "production, manufacturing" and "pharmaceutical, medicine, diagnostic" in various combinations and searched in the title and/or abstract. Each database, however, has a unique set of keywords and search terms. This is why the search terms vary among the various databases, although the overall strategy remains the same (See Appendix 1). Specifically, MeSH terms were used for PUBMED and major subject headings used for EMBASE, CSA/ PAIS, and POPLINE. The search strategies were meant to capture both "high income" countries (e.g., U.S., Europe, Canada, Japan, New Zealand, Australia and the like) and "low- and middle- income" countries. In addition, there is a large amount of literature comparing MNCs and local producers in various countries with regard to finances, Foreign Direct Investment (FDI), and labour productivity that spans across sectors. "Local production" is not an economic term, so a further search was done for literature on comparative economics between domestic and foreign manufacturers in terms of business performance. The databases were searched using combinations of terms such as "comparison, foreign, multinational, domestic, local, performance, price, pharmaceutical, emerging market". PUBMED search terms are in Appendix 1.
The specific databases used were: AfricaWide Information, PUBMED (including the "Health Services" Subcategory, CINAHL, EMBASE, Thomson Reuters (formerly ISI) Web of Science, EconLit, CSA International Bibliography of Social Science, International Network of Rational Use of Medicines (INRUD), PAIS International, POPLINE (One Source), and Google Scholar®. References from PUBMED (including the "Health Services" Subcategory, CINAHL, EMBASE, Thomson Reuters (formerly ISI) Web of Science, EconLit, CSA International Bibliography of Social Science, PAIS International, and POPLINE were placedin EndNote®bibliographic software files. We reviewed these EndNote®files and searched within all articles with abstracts for terms "local, national, regional, domestic" and "production, manufacturing" and "pharmaceutical, medicine, diagnostic" in various combinations. We read each of the resulting abstracts or full-length articles (if available) and then applied the "screening"criteria of Table 1.
To search for so-called gray literature, we reviewed the following websites and any associated databases for literature dealing with both local production and access: OECD, the World Bank, the World Health Organization (WHO), Pan American Health Organization (PAHO), the Medicines Transparency Alliance (MeTA), UNIDO/GTZ, UNDP, LEXISNEXIS, e-medicine archives, Google®, Google Scholar®. We then applied the "screening" criteria of Table 1 to the result.
For the Google®searches, we also looked for specific countries: Argentina, Ghana, Nigeria, Brazil, Egypt, Jordan, South Africa, Thailand, Bangladesh, Philippines, Tanzania, Mexico, and India. We reviewed all articles up to the first 20 "hits". The most relevant of the first 20 articles (based on whether it was concerned with both local production and access) were then searched for all hyperlinked "related articles". We repeated this search twice, once for "medicines" and again for "diagnostics" (See Appendix 2). For all Google®based searches that were not country specific, the total number of initial "hits" was enormous so we limited ourselves to reviewing the first 100 references and applied the screening criteria of Table 1.
Table 1. Criteria for robust evidence regarding LP and access
| Criteria | Explanation |
| Study objective | Define the relationship between LP and 'access' to biomedical products (medicines and/or diagnostics) |
| Study design | Interrupted time series analysis, and/or Repeated measures studies, and/or Controlled or uncontrolled before (-LP) and after (+LP) studies and/or One-time descriptive comparisons of local and foreignmade products. |
| Study sites | Low and middle income countries Public and/or private health care institutions and/or pharmaceutical retail sector and/or public or private biomedical manufacturing site(s) |
| Preferred Study outcomes |
Demonstrating a causal or strongly inferential link between LP of a medical product and improved/modified/ enhanced access to that product |
Results
We found a total of 154 relevant references and based on theTable 1 screening tool, we narrowed this down to a total of 20 (See Tables 2-4). See Appendix 1 and Appendix 2 for more information on search terms for these references.
We have identified two themes of the literature that are relevant:
The business and economics literature on the comparative economics and strategic planning of multinational and domestic firms. Of this literature, there are few references on emerging markets or LMICs and even fewer with regard to comparing local and MNC pharmaceutical producers.
The sparse and descriptive literature on the benefits of local production.
Theme 1: Comparing the "behavior" of domestic and foreign producers (MNCs) in-country
There is an extensive literature showing that MNCs and local firms are different, based on the fact that MNCs are relatively more intensive in research and development (R&D) and advertising assets than non-MNCs [22-25]. The theoretical literature attempts to explain the existence of MNCs in foreign markets when they are at a disadvantage relative to local firms with respect to knowledge of domestic markets. Theories focus on explaining how MNCs overcome these disadvantages by possessing proprietary, knowledge-based and generally intangible assets related to production techniques and processes, marketing networks and/or management ability.
We have identified literature on the comparative behavior ofMNCs and local pharmaceutical and chemical producers (Table2). The study on India is not directed at "access" specifically but at structural and functional properties of domestic firms versus MNCs [27]. The comparative study on Bangladesh asserts that local producers have a distinct cost advantage over MNCs but there is no data in the paper to support this [28].
Table 2. Summary of literature on comparative behavior of MNCs and local pharmaceutical/chemical producers
| Country | Analytical Method | Conclusion(s) | Reference |
| Turkey | Surveys | Comparison of the product structure of MNCs and that of local firms. No significant difference between them in terms of the products that they produce and market. The author could NOT conclude that the presence of local firms in the Turkish pharmaceutical industry had been beneficial because; "...all the negative aspects of pharmaceutical production and exchange which the critics have attributed solely to MNCs have been similarly reproduced by local firms in the pharmaceutical industry in Turkey. " Local firms and MNCs were equally involved in overpricing activities. The available evidence indicated that MNCs overpriced to an even higher extent than local firms. | [26] |
| India | Firm-level data from National Statistics Office: Econometric study | Domestic firms, most of which are controlled by family based structures, enjoy higher efficiencies (operating profit margins, net profit margins, fixed asset turnover, working capital, inventory holding period, and many others) than affiliates of MNCs | [27] |
| Bangladesh | Stock exchange data/Econometric study | Domestic production's cost advantage over large MNCs gives local products a price advantage. MNCs have more advantageous infrastructures, technology, finances and administration | [28] |
Theme 2: Benefits of local production of medical products
Competitive costing
In principle, a dedicated local production facility could be competitive against the lowest cost international producers on the basis of improved process technology, continuous (as opposed to batch) processing, and better economies of scale. The extent of the cost saving depends on which products are being manufactured and what processing steps are required. Table 3 summarizes the evidence gathered from our review on this topic.
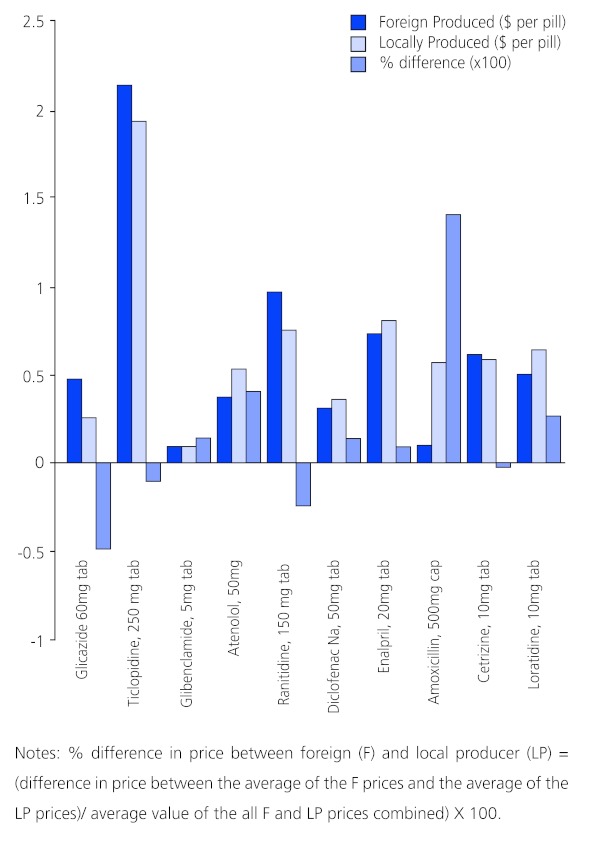
Figure 1 (opposite) is adapted from Table 1 of reference 33. The solid dark blue bars show the average price of the listed foreign- produced generic medicines ($ per pill: Y axis) of Germany, Cyprus, India, Canada, Italy, and the bars to their right are the average price ($ per pill: Y axis) of the Malaysian generic counterpart. The light blue bars are the percentage (x100) difference in price between the foreign and locally-produced generics. The foreign generic version was more expensive than the locally-produced generic version in just 4 of the 10medicines (glicazide 60 mg, ticlopidine 250 mg, ranitidine 150 mg and cetirizine 10mg). The locally-produced generic versions of atenolol, loratidine and amoxicillin were significantly more expensive than the foreign-produced versions.
Figure 1.
Table 3. Summary of literature on cost of locally produced and imported medicines.
| Country | Analytical Method | Conclusion(s) | Reference |
| Tanzania | Survey | Nearly half (46%) of various tracer medicines were locally made; only injectable, some chronic illness medicines, and one antibiotic were solely available as imports. No significant differences existed between prices of medicines from the three main countries of origin (India, Kenya, Tanzania), suggesting competitive pricing with no apparent advantage given to the Tanzanian products | [29,39] |
| Tanzania | Survey | Local production supplies approximately 30% of private and public markets. Various "tracer medicines" were widely available in shops and non-government facilities. Of these medicines, 66% were locally made (compare the 46% figure cited above by ref. 30) and "¡few significant price differentials by country of origin for the most widely distributed medicines among ¡ tracer drugs". | [30] |
| Brazil | Time series | As of 2006, prices for Brazil's locally produced generics were generally much higher than corresponding global prices. These prices have risen in Brazil while declining globally. The estimated "excess" costs of Brazil's locally produced generics totaled US$110 million from 2001 to 2005. | [31] |
| Various sub-Saharan African countries | Economic modeling | Domestic production of a variety of medicines may have a "modest" impact on medicine affordability. "Modest", defined as between a 1-26% reduction in ex works price. This price reduction was found to be very sensitive to increase in API prices or a loss of (or failure to reach) market share and this could "easily" negate price reductions. | [7] |
| India | Economic modelingi | "Significant" additional expenditure that the representative Indian consumer would need to incur in the face of the domestic product withdrawal(s) and assumed to be an impact on "access" due to " ¡differences in the marketing and distribution networks, domestic products being more readily available to Indian consumers than products produced by foreign subsidiaries." In absolute terms, without any price regulation, the prices of foreign patented products would rise between 100% and 400% when local production ceased. | [32] |
| Malaysia | Survey | Some local generics were more expensive than imported generic medicines. Retail markups for both were assumed identical and local producers may not be "efficiently producing affordable medicines" and are passing the high costs on to the consumer (See Figure 1, below). | [33] |
| Bangladesh | Survey | Pricing differentiation of 35 essential medicines between local producers and multinational pharmaceutical companies showed that only two products (Aspirin 300 mg, Chlorpromazine 25 mg) out of 35 essential medicine products had locally-produced unit prices higher than the corresponding MNC products. The prices of various locally produced dosages of ibuprofen and paracetamol were only slightly less than the MNC versions. The majority of locally produced anti-infectives were less expensive than their MNC counterparts. Five essential medicine products for chronic conditions (Atenolol 50 mg, Glibenclamide, Amitriptyline, Griseofulvin and Salbutamol) had exactly the same prices for locally produced and MNC-produced. | [34] |
| Vietnam | Survey | Locally produced HIV/AIDS medicines l (anti-retrovirals: ARVs) are priced considerably lower than imported ARVs currently on the Vietnamese market, but they are five to seven times higher than the current best offer on the international market. | [35] |
| Vietnam | Survey | Locally produced drugs are "less expensive than those imported from the West, Malaysia and Thailand" but this statement is not supported by any data. | [36] |
| Palestine | Survey | Although only at a single Palestinian pharmacy, locally produced pharmaceuticals were significantly cheaper than their foreign counterparts. | [37] |
| Palestine | Survey | Survey Analysis of 34 single and 6 combination antibiotic preparations of local and foreign firms (including those marketed by Israel) showed that in all cases the "¡ price difference was in favor of the locally manufactured products, as all the prices of local antibiotics are less than imported ones." (no data presented) | [38] |
iThe basic counterfactual scenarios all involve the withdrawal of one or more of the locally produced product groups from the market in the face of patent protection. The idea is that if patents for, e.g., ciprofloxacin, had been recognized in India, not all domestic products containing ciprofloxacin would be present in the market. That would leave only the foreign ciprofloxacin product group in the market.
Reliability of supply
Local production in-country would improve security of supply and extend procurement options, although proving this empirically would be difficult. In Tanzania, the government procurement agency obtains supplies through one large annual tender [39] . (See Table 4)
Table 4. Summary of literature on presumed benefits of local production of medical products.
| Potential Benefit of LP | Country | Analytical Method | Conclusion(s) | Reference |
| Reliable supply | Tanzania | Survey | In Tanzania, there are several competing supply chains: 1. Delivery chain of mostly ARV and Tuberculosis (TB) medicines from only international firms to facilities treating free at point of use. 2. Supply chain from local firms and Indian importers to public/NGO facilities for out-ofpocket payment. 3. Private market without a controlled supply chain, selling both subsidized imports and local and imported commercial supplies. The ARV/TB supply chain excludes local suppliers. The supply chain for public/NGO facilities tends to encourage local suppliers, and could lead to "...upgrading of local industrial capabilities and employment", although the validity of this assertion was not analyzed. |
[40] |
| Improved quality standards | Seven African countries | Survey/chemical analyses of a pilot study to assess the quality of chloroquine syrup (CQS) or tablets (CQT) | There were quality failures of 56% (27/48) among locally made products, compared to 47.2% (17/36) for foreign products for CQT active ingredient content, and 28% (7/25) versus 13% (3/23) for CQS active ingredient content. | [41] |
| Kenya | Cross-sectional laboratory analysis and survey of pharmaceutical companies in Nairobi | Private pharmacies stocked few of the locally manufactured products due to "low doctor and/or patient acceptance." Varying factors contributed to poor availability and acceptability of some locally manufactured products in Kenya. | [42] | |
| Developing innovation capacity | Uganda | Survey; case studies | Ugandan pharmaceutical companies upgraded their technology by a combination of upstream vertical linkages to suppliers, their existing linkages downstream in the chain as importers and retailers of pharmaceuticals for the domestic market, and by the government policies. The Ugandan companies have upgraded by importing finished technologies and knowledge, not by learning production methods. Production is at a low level technologically and has not increased the companies' technological capabilities. | [43] |
| Developing human capital | Tanzania | Survey of a single company whose staff comprised mainly of Indian and British expatriates | Tanzanian staff was in the minority and that this was "... a major problem." The company would prefer to employ Tanzanian staff, but the competency needed for pharmaceutical production is simply not available in the country. In total the company employs 800 people in Tanzania. The Tanzanian employees are unskilled and work in the packaging area, whereas the Indian and British staff is skilled. | [12] |
Improved quality standards
In principle, local production with regular surveillance on quality control issues in conjunction with health authorities could lead to improved quality standards without compromising on cost (See Table 4).
Foreign import savings
Local production may, to an extent, offset the very large import deficit and foreign exchange exposure that is almost inevitable for some medicines that are produced primarily by MNCs (e.g., ARVs). We could find no literature fitting our criteria to support this for LMICs.
Development of further innovation capacity
Many policy makers in LMICs have competed rigorously in attracting foreign direct investment (FDI). A common justification for this incentive-based competition is the argument that FDI provides not only capital and additional employment but also new knowledge to recipient economies. In LMICs, dependence on foreign production explains the large number of studies emphasizing the importance of accessing and absorbing international knowledge for acquiring competitiveness and fostering economic growth in these countries, and in particular the important role that international knowledge spillovers could play in that process. The literature is vast [44]. See Table 4 for the evidence supporting the role of local production as a means of furthering innovation in medical products.
Creation of enhanced export capacity
In principle, a local producer could also become a significant exporter. Although the initial intention of a "local producer" is most likely to develop as a local supplier of a highly strategic or niche product, ultimately this could assist in building a regional production capacity which would benefit, for instance, the entire African continent. From a macroeconomic view, this may help improve any trade imbalance. But this will also depend on the products themselves, their patent cover and the scope of any voluntary license agreements which may cover patented products. We found no direct evidence fitting our criteria to support the link between LP and increased exports e.g. Sub-Saharan Africa (see Table 1).
Development of human capital
Most of the essential skills for a successful biomedicine manufacturing sector may already be well developed in certain countries (e.g., India, Thailand, South Africa) within academic institutions (organic chemistry, chemical engineering, mechanical engineering, pharmacology, etc). At the same time, it may be that experienced local professionals with knowledge of pharmaceutical manufacturing within an industrial environment are very limited (See Table 4).
Discussion
Absence of evidence is not evidence of absence. There are surely observable links between local production and access to medical products in LMICs. We infer from the literature that the link between local production and price, if such a link exists, should be observable and measurable. Further, the link between local production and accessibility should be similarly observable. Nonetheless, we have not seen rigorous evidence for either of these links in the literature we have reviewed. In short, the direct evidence in LMICs is too weak to answer the question of whether or not local production of medical products has a salutary effect on the resulting access to these products. There is a preponderance of case studies and descriptive surveys.
Two key points emerge from this work.
The vast majority of pricing surveys observed do not distinguish the price of "local" versus "foreign" producers on a product- by-product basis. An important first step in development of this literature would be if even a few of the comprehensive analyses of price, accessibility and affordability performed by the WHO and Health Action International (HAI) were repeated using distinctions between local- and foreign-made identical products [45-49] .
There is an almost complete absence of rigorous information on the link between LP and access to medical devices. Modern technology is producing an abundance of medical devices at a rate that soon makes the latest device obsolete. Furthermore, there is an extreme diversity in the medical device arena in terms of types of devices, degrees of complexity, applications, usage, users and categories. Just as with pharmaceuticals, research in medical devices can be mismatched with actual public health needs. Furthermore, almost all medical devices present in developing countries have been designed for use in industrialized countries. Whether or not local production of medical devices can contribute to improved access to devices is an open question.
In retrospect, there are several reasonable explanations for the apparent lack of published evidence in general. First and foremost, many of the complexities of investigating the link between LP and access to medical products are simply not susceptible to formal academic analysis. For the most part in many LMICs, relevant data sets are limited and are of doubtful quality [10]. While there is excellent long term data primarily compiled by international pharmaceutical market research audit companies, beyond the OECD such data is sparse [10].
Second, the relationship between LP and access to medical products is extremely dynamic. The literature provides a retrospective view but the business of developing policy, of technology transfer and of manufacturing a product for marketwill not wait for academicsI. The most useful information may indeed be available directly in-country and in real time.
Third, notwithstanding some national policies in LMICs that support local production, "access to medicines" is not the primary reason for building a local factory. At present, the business and industry pressures to build a local producer in an LMIC will still render health policy concerns of secondary importance. It could be that links between LP and access have not been explored because it is harder to make access a particular concern for an individual firm, and at the collective level, accountability is hard to enforce (since it cannot be broken up for each and every firm)II.
We cannot state unequivocally that the references found here are the only potentially useful and reliable sources of information on this subject. Although we attempted to create a systematic search strategy, one could certainly find additional documents using a less efficient free form search. It is almost certainly true that this search strategy has not covered the entire literature, given its cross-cutting nature. However, what is presented here covers sufficient ground to serve as a starting point. In our view, we can say with confidence that while some details have been missed in our search strategy, overall, this is the general sense of the literature at the present time.
Going further, if we are going create a more robust evidentiary framework for the linkage between LP and "access", we need better monitoring and evaluation. In principle, it is possible to create longitudinal data or cross-sectional time series data, where the same subjects (e.g., several local and MNC producers) are observed at multiple time periods. One can imagine a nationally representative sample of local producers and /or MNC subsidiaries and/or a sample of pharmacies, clinics and the like, each member of the panel being surveyed repeatedly over multiple years for various phenomena. Realistically, there is likely to be very poor access to firm- and/or plant-level data. The lack of good data may make it impossible to sort out the various influences that are involved over time. For example, one might observe in a region dominated by local producers a time series that shows higher prices than an adjacent "control" region dominated by MNC producers, this may result from the fact that foreign MNCs are more capital and technology intensive and that this price difference would disappear if differences in capital intensity could be controlled for.
An interrupted time series may be useful in studying the linkage between LP and access [50-51]. In this analysis, the effect of an intervention on an outcome variable can assume a variety of forms over time. In this case, the intervention is made by someone other than the researcher and it is not normally made for experimental purposes and would be considered a naturalexperiment. If available, one creates a time series beginningfrom well before the intervention and continuing through and after it. For instance, prior to, during, and after a major financial investment and/or a policy change and/or a new factory going "on line", one could look at: 1. product-by-product price comparisons of various local vs. MNC products; or 2. market share surveys of availability of local vs. MNC-produced generics/ brand names on a product-by-product basis from the same sites; or 3. repeated surveys of patterns of medicine distribution of a suite of local producers vs. importers/in-country MNCs . The limiting factors are again the existence of data on medicine production, or price or access/affordability, volume market share and the like.
Conclusions
This appears to be the first such review of the literature that attempts to answer the question regarding the kinds of evidence linking LP and access to medical products. Our conclusions appear to support our preliminary working hypothesis that studies showing a robust relationship between LP and access to medical products are sparse at best.
Although "local production" is being actively pursued in many LMICs, the link between local production and access to medical products remains implicit in most cases. The extent to which local production for medical products and new investments in this area in developing countries are aligned with those countries' public health needs is an important question and requires close examination and policy attention. Even if such policies are aligned, how can the link between local production and access to medicines be supported by good evidence? In this regard, we hope that this document contributes towards beginning an evidence-base linking industrial and health policy.
Authors' contribution
WAK carried out the study, developed the search strategy, searched relevant databases, reviewed the literature and wrote the article. LSR and MV developed the search strategy, searched relevant databases, reviewed the literature and wrote an early draft of the abstract.
Conflict of interest
The authors have declared that no conflict of interest exists.
Appendix 1: Search terms used for databases and number of references identified
The search terms for PUBMED were as follows:
(domestic[All Fields] AND ("economics"[MeSH Terms] OR "economics"[All Fields] OR "production"[All Fields])) AND ("pharmacy"[MeSH Terms] OR "pharmacy"[All Fields] OR "pharmaceutical"[All Fields] OR "dosage forms"[MeSH Terms] OR ("dosage"[All Fields] AND "forms"[All Fields]) OR "dosage forms"[All Fields])
"medicine industry"[Mesh] AND "medicine"[Mesh]
(Medicine[ti] OR Pharmaceutical[ti] OR Diagnostic[ti] OR"Medicines, Essential/supply and distribution"[MAJR]) OR "Medicines, Essential/economics"[MeSH Terms]) AND (Production[tiab] OR Manufacture[tiab]) AND (Local[tiab] OR regional[tiab] OR national[tiab] OR domestic[tiab]) NOT (("cells"[MeSH Terms] OR "cells"[All Fields] OR "cell"[All Fields]) NOT clinical[All Fields])
Limits - Humans
Search Terms to find "Developing Countries"
"Developing Countries"[Mesh] OR Africa[Mesh] or "Africa South of the Sahara"[Mesh] or Asia[Mesh] or "South America" [Mesh] or "Central America"[Mesh] OR Africa[tiab] or Asia[tiab] or "South America"[tiab] or "Latin America"[tiab] or "Central America"[tiab]
Table A1.
| Database(s) | Serch tem key words for database(s) | Number of initial "hits" | Number relevant | Number after "screening" |
| LEXIS NEXIS | Local, production, pharmaceutical, medicine diagnostic | 997 | 0 | 0 |
| GOOGLE/GOOGLE SCHOLAR | Local, innovation, pharmaceutical, medicine, diagnostic, access | 1000 | 51 | 0 |
| AfricaWide Information CINAHL |
Local production pharmaceutical, medicine diagnostic | 0 0 |
||
| OECD PUBMED HEALTH SERVICES SUBSET OF PUBMED |
Local production Drug Industry {MeSH} AND Medicine {MeSH} Local production |
68 2057 4 |
0 26 0 |
0 0 0 |
| POPLINE ECONLIT ECONLIT |
medicine / pharmac* / diagnostic & production / manufacture medicine / pharmac* / diagnostic & production / manufacture Comparative AND (foreign OR multinational) AND (domestic OR local) AND performance OR price AND "pharmaceutical" |
21 32 1127 |
3 9 27 |
0 3 6 |
| CSA ISI Web of Knowledge CSA |
Local production pharmaceutical medicine diagnostic Local production pharmaceutical medicine diagnostic Comparative AND (foreign OR multinational) AND (domestic OR local) AND performance OR price Same as immediately above AND "pharmaceutical" |
13 429 818 38 |
3 8 13 |
0 2 3 |
| BioOne Abstracts and Indexes PAIS International Worldwide Political Science Abstracts |
(local or domestic or national) and AB=production and AB=(pharmaceutic* or medicine or diagnostic) local or domestic or national) and AB=production and AB=(pharmaceutic* or medicine or diagnostic) local or domestic or national) and AB=production and AB=(pharmaceutic* or medicine or diagnostic) |
12 12 8 |
3 6 5 |
0 0 1 |
| International Bibliography of the Social Sciences | AB=(local or national or domestic) and AB=production and KW=(medicine or pharmaceu*) |
22 | 0 | 0 |
AB: Abstract; KW: keywords
Appendix 2: Search term used for Google Scholar®country specific searches
Table A2.
| Database | Search term key words for database(s) |
| GOOGLE SCHOLAR COUNTRY SPECIFIC | I. Specific country AND pharmaceutical AND with the exact phrase: "production" AND with at least one of these words: "local domestic national regional diagnostic" II. Specific country AND diagnostic AND with the exact phrase: "production" AND with at least one of the words: "local domestic national regional pharmaceutical" |
Funding Statement
This report was commissioned by the Department of Public Health Innovation and Intellectual Property of the World Health Organization with funding from the European Union under EUContribution Agreement PP-AP/2008/172-129 December 2008.
Footnotes
I The dynamic nature of this can be illustrated by the United States. Medicine shortages in the United States have been growing in number, driven by many factors such as shortage of raw materials, manufacturing delays, business decisions to manufacture another product, a tendency by hospitals and wholesalers to order medicines on demand rather than stockpile supplies [52, 53].
II We note, however, the Access to Medicines Index (http://www.accesstomedicineindex.org/) which ranks 27 MNCs, comprising 20 originators and seven generics manufacturers. The ranking is based on 106 indicators that measure activities across four strategic and seven technical areas, including pricing, patenting and philanthropy.
References
- 1.Kaplan W, Laing R. Local production of pharmaceuticals: industrial policy and access to medicines: an overview of key concepts, issues and opportunities for future research. Health, Nutrition and Population Discussion Paper. Washington DC: World Bank; 2005. http://siteresources. worldbank.org/HEALTHNUTRITIONANDPOPULATION/Resources/281627-1095698140167/KaplanLocalProductionFinal.pdf. [Google Scholar]
- 2.Galvão Jane. Access to antiretroviral drugs in Brazil. Lancet. 2002;360(9348):1862–1865. doi: 10.1016/S0140-6736(02)11775-2. http://www.nlm.nih.gov/medlineplus/hivaidsmedicines.html. [DOI] [PubMed] [Google Scholar]
- 3.do Lago Regina Ferro, Costa Nilson do Ros¨¢rio. Antiretroviral manufacturers and the challenge of universal access to drugs through the Brazilian National STD/AIDS Program. Cad Saude Publica. 2009;25(10):2273–2284. doi: 10.1590/s0102-311x2009001000017. http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S0102-311X2009001000017&lng=en&nrm=iso&tlng=en. [DOI] [PubMed] [Google Scholar]
- 4.Ford Nathan, Wilson David, Costa Chaves Gabriela, Lotrowska Michel, Kijtiwatchakul Kannikar. Sustaining access to antiretroviral therapy in the less-developed world: lessons from Brazil and Thailand. AIDS. 2007;21(Suppl 4):21–29. doi: 10.1097/01.aids.0000279703.78685.a6. http://www.nlm.nih.gov/medlineplus/hivaidsmedicines.html. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri S. The WTO and India's pharmaceuticals industry: patent protection, TRIPS, and developing countries. New York, New Delhi: Oxford University Press; 2005. [Google Scholar]
- 6.Chaudhuri S. The gap between successful innovation and access to its benefits: Indian pharmaceuticals. Euro J Dev Res. 2007;19:49–65. [Google Scholar]
- 7.Guimier J-M. The evidence base for domestic production and greater access to medicines. London: DFID Heath Systems Resource Centre; 2004. http://www.hlsp.org/LinkClick.aspx?fileticket=-wnRCJ0AhQs%3D&tabid=1643. [Google Scholar]
- 8.Bate R. Local Pharmaceutical production in developing countries. Campaign for fighting diseases. London WC2E 8HA UK: International Policy Press; 2008. http://www.libinst.ch/publikationen/LI-LocalPharmaceuticalProduction.pdf. [Google Scholar]
- 9.Liebig K. Auswirkungen des internationalen patentregimes auf die medikamentenproduktion und den zugang zu medikamenten in LDC's. German Development Institute Bonn, Germany; 2006. http://www.unido.org/fileadmin/user_media/Services/PSD/BEP/DIE-GDI.pdf. [Google Scholar]
- 10.Attridge CJ, Preker A. Improving access to medicines in developing countries. Health, Nutrition and Population Discussion Paper. Washington, D.C: World Bank; 2005. http://siteresources.worldbank.org/HEALTHNUTRITIONANDPOPULATION/ Resources/281627-1095698140167/AttridgeImprovingAccessFinal.pdf. [Google Scholar]
- 11.Harper J, Gyansa-Lutterordt M. The viability of pharmaceutical manufacturing in Ghana to address priority endemic diseases in the West Africa sub-region. Deutsche Gesellschaft für Technische Zusammenarbeit GmbH, Eschborn; 2007. http://www.unido.org/fileadmin/user_media/Services/PSD/BEP/002_en- viability-pharmaceutical-manufacturing-ghana-2007.pdf. [Google Scholar]
- 12.Losse K. The viability of local pharmaceutical production in Tanzania. Deutsche Gesellschaft für Technische Zusammenarbeit GmbH, Eschborn; 2005. http://www.unido.org/fileadmin/user_media/Services/PSD/BEP/Tanzania.pdf. [Google Scholar]
- 13.Pokorski da Cunha U. Study on the viability of high quality drugs manufacturing in Bangladesh. eutsche Gesellschaft für Technische Zusammenarbeit GmbH, Eschborn; 2007. http://www.unido.org/fileadmin/user_media/Services/PSD/BEP/en-high- quality-drugs-bangladesh-2007.pdf. [Google Scholar]
- 14.Chiwandamira DP, Kamanzi D. Analysis of legal aspects of local pharmaceutical production in Rwanda. Deutsche Gesellschaft für Technische Zusammenarbeit GmbH, Eschborn; http://www.unido.org/fileadmin/user_media/Services/PSD/BEP/Rwanda.pdf. [Google Scholar]
- 15.Pharmaceutical manufacturing plan for Africa. African Union; 2007. http://www.unido.org/fileadmin/user_media/Services/PSD/BEP/Pharmaceutical%20manufacturing%20plan%20 for%20Africa-English.pdf. [Google Scholar]
- 16.SADC pharmaceutical business plan 2007-2013. SADC Secretariat; 2007. http://www.unido.org/fileadmin/user_media/Services/PSD/BEP/SADC%20PHARMACEUTICAL%20BUSINESS%20PLAN%20-APPROVED%20PLAN.pdf. [Google Scholar]
- 17.WHO global strategy and plan of action on public health, innovation and intellectual property: the contribution of Switzerland. Swiss Confederation; 2011. www.deza.admin.ch/ressources/resource_en_200711.pdf. [Google Scholar]
- 18.WHO project on improving access to medicines in developing countries through local production and related technology transfer. Geneva, Switzerland: World Health Organization; 2011. http://www.who.int/phi/implementation/TotLCProject.pdf. [Google Scholar]
- 19.Mercurio B. & Pol'y. Asian J WTO & Int'l Health L. & Pol'y 2009. 2009;27:1–29. [Google Scholar]
- 20.Medical devices: managing the mismatch. Geneva, Switzerland: World Health Organization; 2010. http://whqlibdoc.who.int/publications/2010/9789241564045_eng.pdf. [Google Scholar]
- 21.How we classify countries. Washington, DC, USA: The World Bank Group; http://data.worldbank.org/ about/country-classifications. [Google Scholar]
- 22.Dunning JH. The eclectic paradigm of international production: a restatement and some possible extensions. J Int'l Bus Studies. 1988;19:1–31. [Google Scholar]
- 23.Dunning JH, Lundan SM. Multinational enterprises and the global economy. UK and Massachusetts: Edward Elgar Publishing; 2008. [Google Scholar]
- 24.Markusen JR, Maskus KE. Discriminating among alternative theories of the multinational enterprise. 1991. http://www.columbia.edu/~dew35/PDF%20files/Markusen%20and%20Maskus%20Text1.pdf. [Google Scholar]
- 25.Caves RE. Multinational enterprise and economic analysis. New York: Cambridge University Press; 2007. [Google Scholar]
- 26.Kirim AS. Transnational corporations and local capital: comparative conduct and performance in the Turkish pharmaceutical industry. World Dev. 1986;14:503–521. http://www.sciencedirect.com/science/article/pii/0305750X86900665. [Google Scholar]
- 27.Saranga H, Phani BV. Determinants of operational efficiencies in the Indian pharmaceutical industry. Int'l Trans in Op Res. 2009;16:109–130. doi: 10.1111/j.1475-3995.2009.00668.x. http://onlinelibrary.wiley.com/doi/10.1111/j.1475-3995.2009.00668.x/pdf. [DOI] [Google Scholar]
- 28.Ahmed S. Bangladesh: Independent University; 2008. Financial performance and characteristics of pharmaceutical and chemical industry in Bangladesh: multinational versus domestic corporations. www.sb.iub.edu.bd/Internship_Report_by_ Shoeb_Ahmed.pdf. [Google Scholar]
- 29.Mackintosh M, Mujinja PGM. Pricing and competition in essential medicines markets: the supply chain to Tanzania and the role of NGOs. IKD Working Paper. 2008;32 Open University Research Centre on Innovation Knowledge and Development http://www.open.ac.uk/ikd/projects_lowcostdrugs.shtml. [Google Scholar]
- 30.Chaudhuri S. Indian generics producers, access to essential medicines and local production in Africa: an argument with reference to Tanzania. Euro J Development Res. 2010;22:451–468. [Google Scholar]
- 31.Nunn AS. East Afr Med. PLoS Med. 2007;4:1804–1817. doi: 10.1371/journal. pmed.0040305. http://medicine.plosjournalsorg/perlserv/?request=get-document&doi=10.1371/journal. pmed.0040305&ct=1. [DOI] [Google Scholar]
- 32.Chaudhuri S. Amer Econ Rev. Estimating the effects of global patent protection in pharmaceuticals: A case study of quinolones in India. Amer Econ Rev 2006; 2006. Estimating the effects of global patent protection in pharmaceuticals: A case study of quinolones in India; pp. 1477–1514. [DOI] [PubMed] [Google Scholar]
- 33.Shafie AA, Hassali MA. East Afr Med. J Generic Med. 2008;6:35–42. [Google Scholar]
- 34.Chowdury N, Kabir ER. Per pill price differences across therapeutic categories: a study of the essential drug brands marketed by multinational and local pharmaceutical companies in Bangladesh. East Afr Med. 2009;1:220–226. http://www.academicjournals.org/ajmm. [Google Scholar]
- 35.Kuanpoth J. Patents and access to antiretroviral medicines in Vietnam after World Trade Organization accession. J World Intell Prop. 2007;10:201–224. [Google Scholar]
- 36.Simonet D. The Vietnamese pharmaceutical market: a comparison of foreign entry strategies. Int J Bus and Emerging Mkts. 2008;1:61–79. [Google Scholar]
- 37.Sweileh WM. Substitution of foreign prescribed medicines by community pharmacies in Palestine: a legal and pharmaco- economic analysis. An-Najah Univ J Nat Sci. 2003;17:35–41. http://www.najah.edu/page/2147. [Google Scholar]
- 38.Sweileh W. Antibiotic drug cost variations in Palestine: physicians and patients dilemma. An-Najah Univ J Nat Sci. 2004;18:73–79. [Google Scholar]
- 39.Chaudhuri S. Indian generic companies, affordability of drugs and local production in Africa with special reference to Tanzania. IKD Working Paper. 2008;37 Milton Keynes, Open University Research Centre on Innovation Knowledge and Development http://www.open.ac.uk/ikd/documents/working-papers/ikd-working-paper-37.pdf. [Google Scholar]
- 40.Mackintosh M. Sant'Anna School of Advanced Studies. Vol. 1. Pisa, Italy: 2011. Essential medicines supply chains and inequality: the need for new indicators from pharma and beyond. http://www.innovation-equity.eu/file_upload/maureen- mackintosh_presentation.pdf. [Google Scholar]
- 41.Maponga C, Ondari C. WHO/EDM/PAR/2003.4. Geneva: World Health Organization; 2004. The quality of antimalarials: a study in selected African countries. http://whqlibdoc.who.int/hq/2003/WHO_EDM_PAR_2003.4.pdf. [Google Scholar]
- 42.Orwa JA. Influence of manufacturing practices on quality of pharmaceutical products manufactured in Kenya. East Afr Med. 2004;81:287–292. doi: 10.4314/eamj.v81i6.9177. http://www.ajol.info/index.php/eamj/article/viewFile/9177/2097. [DOI] [PubMed] [Google Scholar]
- 43.Haakonsson SJ. Learning by importing in global value chains: upgrading and south to south strategies in the Ugandan pharmaceutical industry. Dev Southern Africa. 2009;26:499–515. [Google Scholar]
- 44.Rodrik D, editor. Handbook of development economics. Vol. 5. New York and London: Elsevier; 2009. [Google Scholar]
- 45.Niëns Laurens M, Cameron Alexandra, Van de Poel Ellen, Ewen Margaret, Brouwer Werner B F, Laing Richard. Quantifying the impoverishing effects of purchasing medicines: a cross-country comparison of the affordability of medicines in the developing world. PLoS Med. 2010;7(8) doi: 10.1371/journal.pmed.1000333. http://ukpmc.ac.uk/abstract/MED/20824175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Mourik Maaike S M, Cameron Alexandra, Ewen Marg, Laing Richard O. Availability, price and affordability of cardiovascular medicines: a comparison across 36 countries using WHO/HAI data. BMC Cardiovasc Disord. 2010;10(25) doi: 10.1186/1471-2261-10-25. http://ukpmc.ac.uk/abstract/MED/20534118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kotwani A. Availability, price and affordability of asthma medicines in five Indian states. Int J Tuberc Lung Dis. 2011;13(5):574–579. http://www.haiweb.org/medicineprices/news/index.html. [PubMed] [Google Scholar]
- 48.WHO Eastern Mediterranean Survey. Medicine prices, availability, affordability and price components: a synthesis report of medicine price surveys undertaken in selected countries of the WHO Eastern Mediterranean Region. WHO EMRO / HAI; http:// www.emro.who.int/dsaf/dsa964.pdf. [Google Scholar]
- 49.Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2008;373(9659):240–249. doi: 10.1016/S0140-6736(08)61762-6. http://www.haiweb.org/medicineprices/news/31122008/MedPrices%20-%20Word2.pdf. [DOI] [PubMed] [Google Scholar]
- 50.Soumerai Stephen B, Zhang Fang, Ross-Degnan Dennis, Ball Daniel E, LeCates Robert F, Law Michael R, Hughes Tom E, Chapman Daniel, Adams Alyce S. Use of atypical antipsychotic drugs for schizophrenia in Maine Medicaid following a policy change. Health Aff. 2008;27(3):185–195. doi: 10.1377/hlthaff.27.3.w185. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Fang, Wagner Anita K, Soumerai Stephen B, Ross-Degnan Dennis. Methods for estimating confidence intervals in interrupted time series analyses of health interventions. J Clin Epidemiol. 2009;62(2):143–148. doi: 10.1016/j.jclinepi.2008.08.007. http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@rn+81-81-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ledford H. Drug shortage slows clinical trials: US researchers faced with cancer-drug shortfall struggle to keep trials on track. Nature News. 2011. http://www.nature.com/news/2011/111003/full/news.2011.570.htm. [DOI]
- 53.American Society of Health System Pharmacists. Drug Shortages-Current Drugs. http://www.ashp.org/DrugShortages/Current/