Abstract
Background: Despite the benefits of exclusive breastfeeding (EBF), exposure to HIV from breast milk has relegated EBF to an option only when formula feeding is not affordable, feasible, safe, and sustainable. Mixed feeding remains the norm in sub-Saharan Africa.
Objective: We evaluated whether the duration of EBF was associated with mortality and HIV infection in children followed to ≤5 y of age.
Methods: A total of 690 mother-infant pairs from the Trial of Vitamins with information on infant feeding, HIV status, and at least one visit in the first year were included in the analysis. The duration of EBF was defined in months as a time-varying covariate at each follow-up visit. Associations of the duration of EBF with mortality, HIV infection, and HIV infection or death were estimated by using Cox proportional hazards models and Kaplan-Meier survival curves.
Results: A 1-mo increase in EBF was associated with a 49% reduction in early infant mortality in the first 6 mo of life (RR: 0.51; 95% CI: 0.28, 0.93) and a nonsignificant 15% reduction in risk of HIV infection or death (RR: 0.85; 95% CI: 0.71, 1.01; P = 0.07) over the first 5 y of life. EBF was not associated with HIV infection (RR: 0.93; 95% CI: 0.76, 1.15).
Conclusion: Longer EBF by HIV-positive mothers was associated with reduced mortality in the first 6 mo of life without increased HIV infection, which makes EBF the best option for women who cannot sustain exclusive formula feeding. This trial was registered at clinicaltrials.gov as NCT00197743.
INTRODUCTION
Exclusive breastfeeding (EBF)4 provides infants nourishment at a lower risk of contamination than does the mixing and storage of formula with water, especially in poor households (1). Growth factors in breast milk are known to improve the maturation of the infant's gastrointestinal system while maintaining mucosal integrity that limits the passage of pathogens and antigens from the lumen (2, 3). Secretory immunoglobulin A antibodies, chemokines, complement, and other immune factors in breast milk (3–7) protect against viral as well as bacterial infections. Protective effects of breastfeeding also occur from other substances that may augment the immune system and growth such as lactoferrin, lysozymes, nucleotides, and oligosaccharides (6, 7).
The WHO recommends that women breastfeed their infants exclusively in the first 6 mo of life to maximize the benefits of breastfeeding (8). With consideration of risk of mother-to-child transmission of HIV through breast milk (9, 10) and the benefits of breastfeeding, the WHO adopted new guidelines in 2010 that provided the following 3 choices to HIV-positive mothers: 1) EBF for the first 6 mo of life, introduction of appropriate complementary foods thereafter, and the continuation of breastfeeding for the first 12 mo of life; 2) formula feeding, if acceptable and affordable and if safe, clean water is available and home conditions support such feeding; and 3) the use of expressed heat-treated breast milk in specific circumstances (11). The WHO recommends that all HIV-positive mothers receive antiretrovirals, at least during breastfeeding if not lifelong (11). The social stigma of HIV infection, economic conditions, and beliefs about breast-milk quality and transmission of HIV through breast milk govern infant-feeding decisions (12). A lack of sustained access to safe, clean water and strong cultural norms that surround breastfeeding make adherence to nonbreastfeeding options extremely difficult. Therefore, most women end up practicing a mixture of non-EBF along with formula or animal-milk feeding, thereby exposing the child to the health risks of all of the choices provided by the WHO (13). EBF has been shown to be associated with lower mortality and HIV infection. In an intervention cohort study in South Africa, the cumulative 3-mo mortality in infants given replacement feeds was higher at 15.1% (range: 7.63–28.73%) than at 6.1% (range: 4.74–7.92%) in exclusively breastfed infants (HR: 2.06; 95% CI: 1.00, 4.27; P = 0.051) (14). Infants who also received solids with breastfeeding were 11 times more likely to acquire HIV infection than exclusively breastfed infants (HR: 10.87; 95% CI: 1.51, 78.00; P = 0.018). The more widespread availability of antiretroviral therapy (ART) has made breastfeeding safer for longer in mothers who receive ART, which has led to recommendations for longer periods of breastfeeding for women who receive ART (11).
We conducted a secondary analysis of infants born to HIV-infected, ART-naive women enrolled in a trial of vitamins in Tanzania to examine whether the duration of EBF is associated with child mortality, HIV infection, or HIV infection or death (either HIV infection or death).
SUBJECTS AND METHODS
The Trial of Vitamins enrolled HIV-infected pregnant women who were residents of Dar es Salaam, Tanzania, and between 12 and 27 wk of gestation from April 1995 to July 1997. The trial aimed to examine the effects of oral supplementation of multivitamins on HIV-disease progression in women, mother-to-child transmission of HIV-1, and morbidity and mortality outcomes in women and children. Enrolled women and infants born to them were followed up at monthly intervals after delivery at study clinics. During these visits, women were asked about infant-feeding practices in the previous month or since the last clinic visit. Information obtained and recorded included current breastfeeding status and frequency, any other food or liquid introduced, age at weaning, and age of introduction of the following foods: cow milk, formula, fruit juices, or solids in the past month. Detailed descriptions of the trial design and follow-up procedures have been published (15–18). EBF was defined as the feeding of breast milk to a child without the introduction of any other liquid, milk, or solid (19). The duration of EBF was defined as the time in months from birth to the time when the mother first reported having given the child any other foods in addition to breast milk or medications. Only singleton children were included in the analysis because feeding patterns in twins are significantly different and few twins are both exclusively breastfed.
Information collected at enrollment provided data on a number of socioeconomic and demographic characteristics. These included the age of parents, their occupation and education, the mother's marital status, family income and size, the number of children living in the household, and obstetric history. During monthly visits, study nurses performed anthropometric measurements on women and their children. Weight, height, and midupper arm circumference (MUAC) were measured by using a standard set of regularly calibrated instruments and procedures (15–18).
Every month, enrolled women and children were evaluated and treated by a study clinician for any health problems or illnesses that were shown. Clinicians also staged HIV disease in mothers and examined them for breast conditions such as mastitis, breast discharge, and cracked nipples. Staging of HIV was performed by using criteria described by the WHO (20). Hemoglobin, erythrocyte sedimentation rate, HIV status, absolute counts of T cell subsets (CD4+ and CD8+), and total lymphocytes were measured at 6 mo of age and at 3-mo intervals in children who were followed up. CD4+ cell counts in the infant were also measured at birth. Children whose peripheral blood sample tested positive on a polymerase chain reaction test at any age before 18 mo were considered HIV positive. Subsequent samples after 18 mo of age were analyzed by using an enzyme-linked immunosorbent assay. Positive results were confirmed by using Western blot tests before a child was labeled HIV positive.
Survival duration was defined as the time from birth to either the end of follow-up (≤60 mo), the last time the child was known to be alive, or the date of death. Infant mortality was defined as death between birth and 12 mo of age. The duration to HIV infection or death was the duration for which a child was alive and HIV free. Children were eligible for this analysis only if they were HIV negative at birth.
Ethics
The Research and Publications Committee of Muhimbili University College of Health Sciences, the Ethical Committee of the National AIDS Control Program of the Tanzanian Ministry of Health, and the Institutional Review Board of the Harvard School of Public Health approved the study protocol. Informed consent was obtained from caregivers.
Statistics
Cox proportional hazards regression models were used to examine the relation between EBF and child mortality, HIV infection, and HIV infection or death (21). A data structure with one record per child visit was used to facilitate the handling of time-varying covariates. EBF was entered into the model as a time-varying continuous variable (duration of EBF in months) during the 0–5-mo period, and as a fixed continuous variable thereafter because no children were exclusively breastfed after 6 mo. Children were censored at the end of 60 mo if they were event free. Analyses were run for the entire study period and for the following periods: 0–5, 6–11, 12–23, and 24–60 mo of age. For inclusion in the model, we considered potential confounders and independent risk factors for outcomes from a list of candidate variables. Potential confounders were categorized as previously reported for this trial (15–18), maternal HIV disease stage by WHO criteria (20), and maternal blood CD4+ counts as ≥350 and <350 cells/μL. Variables were included in multivariate models if the estimated risk ratio for EBF changed by ≥10% compared with in the saturated main-effects model (22). Confounders that were identified were applied to the respective models for each endpoint. RRs and their corresponding 95% CIs are reported. Kaplan-Meier survival curves were generated for EBF (23).
We performed secondary analyses to examine whether the associations of EBF with the outcomes were modified by the time-varying HIV status of the children, maternal baseline CD4+ cell counts, CD8+ cell counts, and lymphocyte counts. Likelihood-ratio tests were used to assess the interactions examined. All statistical analyses were performed with the statistical software package SAS 9.1 (SAS Institute Inc). Significance tests were 2-sided, and differences were considered significant at P < 0.05.
RESULTS
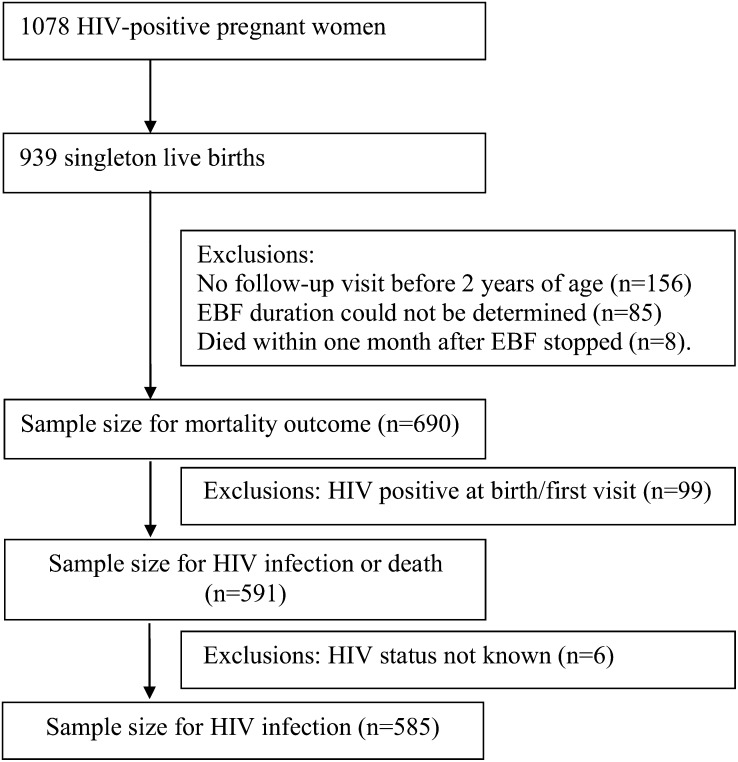
From a total of 939 singleton live births to HIV-infected women in the study conducted between April 1995 and July 1997, we excluded children in whom the duration of breastfeeding could not be determined (85 children with no follow-up visits ever or after 3 mo from birth); the first follow-up visit was after 2 y of age, which made the duration of breastfeeding unreliable (156 children); or a time sequence between EBF and death could not be established (8 children who died within 1 mo after EBF was stopped) (Figure 1). Information was available from 690 children to examine the association between EBF and mortality. Ninety-nine children who were HIV positive at the time of the first follow-up visit were excluded from the analysis of EBF and HIV infection or death. In addition, 6 more children whose HIV status was not known were excluded from the analysis of EBF and HIV infection.
FIGURE 1.
Flowchart of infants available for analysis. EBF, exclusive breastfeeding.
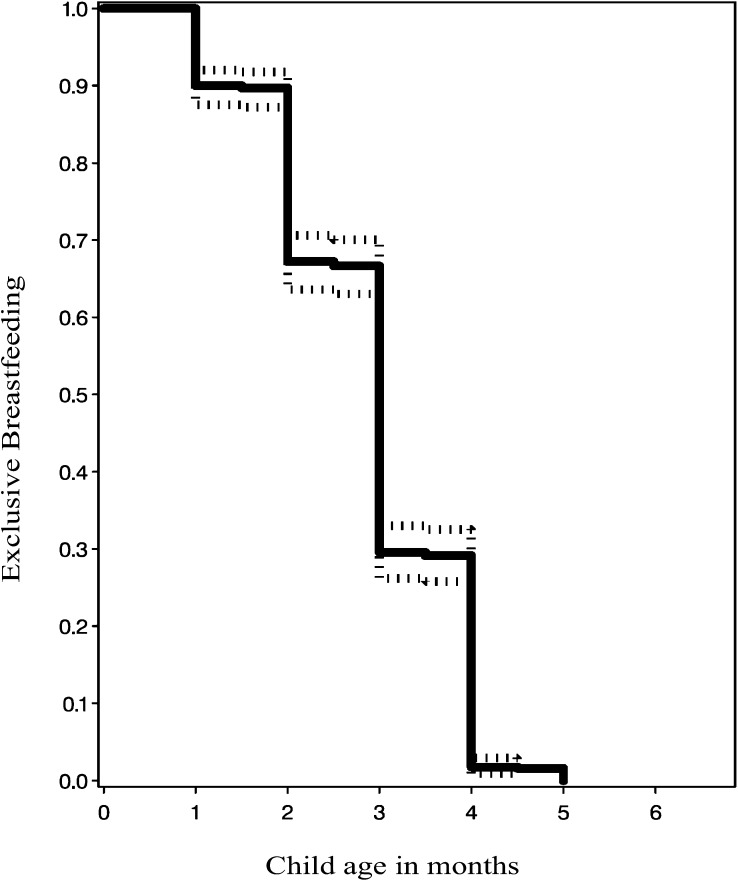
Only 30% of the children were exclusively breastfed for ≥3 mo; other liquids or solids were introduced before 3 mo in the remaining 70% of children (Figure 2). The distribution of maternal and paternal sociodemographic characteristics, maternal indicators of nutritional, immunological status and HIV stage, obstetric history, and child HIV and nutritional status are shown in Table 1. In the remaining sections, we present results on the associations between EBF and child mortality, HIV infection, and HIV infection or death.
FIGURE 2.
Rates (95% confidence bounds) of exclusive breastfeeding by age in children born to HIV-infected mothers (estimated by using Kaplan-Meier survival curves).
TABLE 1.
Social and clinical characteristics of HIV-infected mothers and children born to them1
| Characteristic | Value |
| Maternal age (n) | 690 |
| <20 y [n (%)] | 80 (12) |
| 20–24 y [n (%)] | 272 (39) |
| 25–29 y [n (%)] | 222 (32) |
| ≥30 y [n (%)] | 116 (17) |
| Maternal education (n) | 690 |
| 0–8 y [n (%)] | 618 (90) |
| ≥9 y [n (%)] | 72 (10) |
| Maternal occupation (n) | 666 |
| Housewife [n (%)] | 487 (73) |
| Other [n (%)] | 179 (27) |
| Partner education (n) | 611 |
| 0–8 y [n (%)] | 397 (65) |
| ≥9 y [n (%)] | 214 (35) |
| Maternal BMI 6 wk after delivery (n) | 604 |
| <18.5 kg/m2 [n (%)] | 54 (9) |
| 18.5–24.99 kg/m2 [n (%)] | 447 (74) |
| ≥25 kg/m2 [n (%)] | 103 (17) |
| Maternal baseline hemoglobin (n) | 682 |
| <8.5 g/dL [n (%)] | 175 (26) |
| ≥8.5 g/dL [n (%)] | 507 (74) |
| Maternal WHO HIV disease stage (n) | 689 |
| I [n (%)] | 559 (81) |
| II [n (%)] | 125 (18) |
| III [n (%)] | 4 (<1) |
| IV [n (%)] | 1 (<1) |
| Maternal baseline CD4+ T cell count (n) | 655 |
| 0–199 cells/μL [n (%)] | 68 (10) |
| 200–499 cells/μL [n (%)] | 382 (58) |
| ≥500 cells/μL [n (%)] | 205 (31) |
| Maternal baseline CD8+ T cell count (n) | 690 |
| <565 cells/μL [n (%)] | 225 (33) |
| ≥565 cells/μL [n (%)] | 475 (67) |
| Maternal baseline lymphocyte count (n) | 690 |
| <1340 cells/μL [n (%)] | 189 (27) |
| ≥1340 cells/μL [n (%)] | 501 (73) |
| Maternal viral load (n) | 315 |
| <50,000 copies/mL [n (%)] | 169 (54) |
| ≥50,000 copies/mL [n (%)] | 146 (46) |
| Child sex (n) | 688 |
| M [n (%)] | 353 (51) |
| F [n (%)] | 335 (49) |
| Child birth weight (n) | 635 |
| <2500 g [n (%)] | 52 (8) |
| ≥2500 g [n (%)] | 583 (92) |
| Child HIV status at 3 mo (n) | 684 |
| Not infected [n (%)] | 551 (81) |
| Infected [n (%)] | 133 (19) |
| Child MUAC2 at 3 mo (n) | 431 |
| <13 cm [n (%)] | 147 (34) |
| ≥13 cm [n (%)] | 284 (66) |
Women were pregnant and between 12 and 27 wk of gestation at enrollment. Percentages may not total to 100% because of rounding.
MUAC, midupper arm circumference.
A total of 113 children (17%) died within the first 2 y, and a total of 142 children (20%) died during the first 5 y of life. Univariate and multivariate adjusted associations (adjusted for mother's social support, maternal time-varying BMI, maternal CD4 counts, child sex, delivery type, child time-varying HIV status, and child MUAC) between EBF and child mortality at different risk periods are presented in Table 2. Risk of early infant mortality (0–5 mo) was inversely associated with the duration of EBF (RR: 0.51; 95% CI: 0.28, 0.93) after maternal social support, time-varying BMI, CD4 counts, child sex, delivery type, time-varying HIV status, and MUAC were controlled for. In the multivariate analysis, the duration of EBF was not associated with a significant reduction in risk of mortality between 6 and 11 mo (RR: 0.98; 95% CI: 0.70, 1.36), 12 and 23 mo (RR: 0.85; 95% CI: 0.64, 1.12), or 24 and 60 mo (RR: 0.84; 95% CI: 0.59, 1.21) of age.
TABLE 2.
Duration of exclusive breastfeeding in relation to child mortality, HIV infection, and HIV infection or death during the first 5 y of life in children born to HIV-infected mothers1
| Univariate |
Multivariate |
|||||
| Outcome | n | Total n | RR (95% CI) | P | RR (95% CI) | P |
| Mortality2 | ||||||
| 0–5 mo | 16 | 662 | 0.58 (0.35, 0.95) | 0.03 | 0.51 (0.28, 0.93) | 0.03 |
| 6–11 mo | 43 | 625 | 0.91 (0.67, 1.24) | 0.56 | 0.98 (0.70, 1.36) | 0.89 |
| 12–23 mo | 54 | 557 | 0.87 (0.67, 1.14) | 0.31 | 0.85 (0.64, 1.12) | 0.24 |
| 24–60 mo | 29 | 477 | 0.82 (0.58, 1.17) | 0.28 | 0.84 (0.59, 1.21) | 0.37 |
| 0–60 mo | 142 | 690 | 0.87 (0.74, 1.03) | 0.10 | 0.87 (0.73, 1.03) | 0.11 |
| HIV infection3 | ||||||
| 0–5 mo | 47 | 560 | 0.75 (0.53, 1.06) | 0.10 | 0.75 (0.52, 1.10) | 0.14 |
| 6–11 mo | 19 | 493 | 0.84 (0.53, 1.32) | 0.45 | 0.82 (0.49, 1.38) | 0.46 |
| 12–23 mo | 39 | 439 | 0.78 (0.56, 1.07) | 0.13 | 0.72 (0.50, 1.02) | 0.06 |
| 24–60 mo | 4 | 369 | 1.07 (0.38, 2.99) | 0.90 | —4 | |
| 0–60 mo | 109 | 585 | 0.92 (0.76, 1.12) | 0.41 | 0.93 (0.76, 1.15) | 0.52 |
| HIV infection or death5 | ||||||
| 0–5 mo | 50 | 564 | 0.75 (0.54, 1.05) | 0.10 | 0.74 (0.51, 1.07) | 0.11 |
| 6–11 mo | 33 | 497 | 0.73 (0.52, 1.03) | 0.07 | 0.69 (0.47, 1.02) | 0.06 |
| 12–23 mo | 50 | 439 | 0.74 (0.56, 0.97) | 0.03 | 0.68 (0.50, 0.93) | 0.02 |
| 24–60 mo | 10 | 370 | 0.74 (0.40, 1.38) | 0.34 | 0.77 (0.38, 1.54) | 0.46 |
| 0–60 mo | 143 | 591 | 0.82 (0.70, 0.98) | 0.03 | 0.85 (0.71, 1.01) | 0.07 |
Time-varying Cox proportional hazard regression models were used to estimate RRs and 95% CIs. Five months includes infants <6 mo of age (similarly for each age group).
Adjusted for maternal social support, maternal time-varying BMI, maternal CD4 counts, child sex, delivery type, child time-varying HIV status, and child midupper arm circumference.
Excludes children who were HIV positive at the first visit after birth (n = 99) and children in whom HIV status could not be determined (n = 6). Adjusted for number of living children given birth by the index mother, maternal social support, maternal time-varying BMI, maternal CD4 counts, maternal hemoglobin concentrations, maternal viral load, child birth weight, and child time-varying midupper arm circumference.
RR could not be estimated in the multivariate model because of the small number of events in this age group.
Excludes children who were HIV positive at the first visit after birth (n = 99). Adjusted for number of living children given birth by the index mother, maternal social support, maternal time-varying BMI, maternal CD4 counts, maternal hemoglobin concentrations, maternal viral load, child birth weight, and child time-varying midupper arm circumference.
EBF was not associated with HIV infection in any age interval from 0 to 60 mo in the univariate analysis. There was no association between risk of HIV infection and duration of EBF in the ages between 0 and 5 mo (RR: 0.75; 95% CI: 0.52, 1.10), 6 and 11 mo (RR: 0.82; 95% CI: 0.49, 1.38) (Table 2), and 12 and 23 mo (RR: 0.72; 95% CI: 0.50, 1.02) in the multivariate analysis (adjusted for the number of living children born to the index mother, maternal social support, maternal time-varying BMI, maternal CD4 counts, maternal hemoglobin concentrations, maternal viral load, child birth weight, and child time-varying MUAC). Only 4 children became HIV positive between 24 and 60 mo of age, and none were among the children who were exclusively breastfed. Overall, from 0 to 60 mo of age, EBF was not associated with increased risk of HIV infection (RR: 0.93; 95% CI: 0.76, 1.15).
HIV infection or death was not associated with EBF in univariate and multivariate analyses during the risk periods of 0–5, 6–11, or 24–60 mo of age (Table 2). However, an increase in duration of EBF by 1 mo was associated with a 32% reduction in HIV infection or death (RR: 0.68; 95% CI: 0.50, 0.93) within the period between 12–23 mo of age.
The time-varying HIV status of the child and maternal baseline CD4+, CD8+, and lymphocyte counts were assessed as potential effect modifiers of the associations between the duration of EBF and mortality (Table 3). Although an every-month increase in duration of EBF was associated with a 38% reduction in child mortality (95% CI: 0.43, 0.91; P = 0.01) in HIV-negative children, the duration of EBF was not associated with mortality in children who were HIV positive (RR: 0.95; 95% CI: 0.79, 1.15; P = 0.63; P-interaction = 0.06). We observed a borderline association between the duration of EBF and reduced child mortality (RR: 0.80; 95% CI: 0.63, 1.00; P = 0.05) born to mothers with counts of ≥565 CD8+ cells/μL at baseline but not in children born to women with baseline counts <565 CD8+ cells/μL (P-interaction = 0.06). We did not observe interactions by amounts of maternal CD4+ and total lymphocyte counts.
TABLE 3.
Associations between exclusive breastfeeding and mortality within strata of potential effect modifiers
| Child mortality (0–60 mo of age) |
||||
| Potential modifier | n | RR (95% CI)1 | P1 | P-interaction2 |
| Time-varying child HIV status | ||||
| Negative | 28 | 0.62 (0.43, 0.91) | 0.01 | 0.06 |
| Positive | 106 | 0.95 (0.79, 1.15) | 0.63 | — |
| Maternal baseline CD4+ cell count | ||||
| <350 cells/μL | 63 | 0.82 (0.64, 1.05) | 0.12 | 0.31 |
| ≥350 cells/μL | 61 | 0.97 (0.75, 1.26) | 0.84 | — |
| Maternal baseline CD8+ cell count | ||||
| <565 cells/μL | 39 | 1.20 (0.85, 1.71) | 0.30 | 0.06 |
| ≥565 cells/μL | 85 | 0.80 (0.63, 1.00) | 0.05 | — |
| Maternal baseline lymphocyte count | ||||
| <1340 lymphocytes/μL | 37 | 1.05 (0.72, 1.55) | 0.79 | 0.27 |
| ≥1340 lymphocytes/μL | 94 | 0.83 (0.68, 1.03) | 0.08 | — |
Values were obtained by using adjusted Cox proportional hazards regression models. Covariates were adjusted as indicated in Table 2.
P values for interaction between exclusive breastfeeding and potential modifiers were obtained by using likelihood-ratio tests.
We further examined whether maternal immunologic status, represented by CD4+, CD8+, and lymphocyte counts, modifies the associations of EBF duration with HIV infection and HIV infection or death (Table 4). Although P-interaction values were not significant, a longer duration of EBF was associated with lower risk of HIV infection and lower risk of HIV infection or death in infants born to women with counts of ≥350 CD4+ cells/μL, ≥565 CD8+ cells/μL, or ≥1340 lymphocytes/μL at baseline but not in children born to women with counts of <350 CD4+ cells/μL, <565 CD8+ cells/μL, or ≤1340 lymphocytes/μL at baseline.
TABLE 4.
Association of exclusive breastfeeding with HIV infection and HIV infection or death within strata of potential modifiers
| HIV infection (0–60 mo of age) |
HIV infection or death (0–60 mo of age) |
|||||||
| Potential modifier | n | RR (95% CI)1 | P1 | P-interaction2 | n | RR (95% CI)1 | P1 | P-interaction2 |
| Maternal baseline CD4+ cell count | ||||||||
| <350 cells/μL | 48 | 0.86 (0.59, 1.24) | 0.42 | 0.42 | 65 | 0.87 (0.64, 1.19) | 0.39 | 0.10 |
| ≥350 cells/μL | 61 | 0.74 (0.55, 1.00) | 0.05 | — | 76 | 0.64 (0.50, 0.83) | <0.001 | — |
| Maternal baseline CD8+ cell count | ||||||||
| <565 cells/μL | 35 | 0.84 (0.57, 1.25) | 0.39 | 0.35 | 48 | 0.85 (0.61, 1.19) | 0.35 | 0.10 |
| ≥565 cells/μL | 74 | 0.75 (0.58, 0.98) | 0.04 | — | 93 | 0.67 (0.53, 0.85) | <0.001 | — |
| Maternal baseline lymphocyte count | ||||||||
| <1340 lymphocytes/μL | 28 | 0.92 (0.55, 1.54) | 0.75 | 0.18 | 39 | 0.73 (0.48, 1.12) | 0.15 | 0.32 |
| ≥1340 lymphocytes/μL | 81 | 0.73 (0.57, 0.93) | 0.01 | — | 102 | 0.71 (0.57, 0.88) | 0.002 | — |
Values were obtained by using adjusted Cox proportional hazards regression models. Covariates were adjusted as indicated in Table 2.
P values for interaction between exclusive breastfeeding and potential modifiers were obtained by using likelihood-ratio tests.
DISCUSSION
In children born to HIV-infected women, we showed that every additional month of EBF was associated with a 49% reduction in mortality from birth to 6 mo of age. The associations between the duration of EBF and child mortality and HIV infection or death, until 5 y of life in this study, were of borderline significance. The duration of EBF was not associated with risk of acquiring HIV during the first 5 y of life.
This analysis confirmed similar findings reported previously in HIV-negative and -positive populations (14, 24–31). A community-based, nested case-control study conducted in rural Tanzania showed that mixed feeding in the first 3 mo of life compared with EBF was associated with 40% increased risk of mortality in children <5 y of age (24). However, the study did not report the HIV status of children and their mothers. Contrary to our findings, a cohort study from South Africa in infants born to HIV-infected women reported higher mortality in HIV-infected infants who were exclusively breastfed (19%) than in infants who were mixed fed (13%), and no deaths were observed in children who were exclusively formula fed (0%) (25). Risk of infant mortality in EBF compared with mixed feeding groups was not significant. This study in South Africa was limited by its small sample size (n = 133) with 17 deaths that occurred in HIV-infected children (7 children were in the EBF group, and 10 children were in the mixed-feeding group). A number of studies since 1997 have reported lower risks of HIV infection in infants of HIV-infected women who were exclusively breastfed than in infants who received a mixture of breast milk and top feeding (formula or animal milk) (26–31). However, in our study, risk of HIV infection was not different between exclusively breastfed and mixed-fed infants. Variations in associations between EBF and mortality or HIV infection compared with mixed or formula feeding depend on access to clean water and hygiene in infant-feeding practices. All of these studies followed infants to ≤24 mo of age (and most studies followed infants to 6–11 mo of age), whereas infants in our cohort were followed to ≤60 mo of age by which time more HIV-related deaths would have occurred. This analysis demonstrates that EBF by HIV-positive mothers in Tanzania provides survival benefits over the first 6 mo without increased mortality over follow-up until 5 y of age. Few infants were exclusively formula fed, and thus, EBF was not compared with exclusive formula feeding.
HIV (or the AIDS virus) is shed in breast milk, which exposes and puts the infant at risk of HIV infection (9, 10). What is less well known is whether immune factors and antiviral substances in breast milk may also play a role in protecting the infant from HIV infection (32, 33). In addition, breast milk may improve mucosal integrity by making the intestinal mucosa less permeable to the HIV virus (34). Women who breastfeed their children are also less likely to suffer from breast and nipple conditions that increase the HIV viral load in breast milk (35, 36). It has been well documented that breastfeeding leads to a remarkable reduction in morbidity and mortality from childhood infectious diseases such as diarrhea and respiratory illnesses (37–39). These infections still account for the highest proportions of mortality in infants and children <5 y of age in Africa, Asia, and other less developed and developing regions of the world (40). It is likely that EBF reduces mortality, especially in the first 6 mo of life, by reducing the incidence of the most common childhood infections as has been reported previously from follow-up of the children included in this analyses (41). In these infants, EBF was associated with reductions in risk of cough (51%), cough and fever (56%), cough and difficulty breathing or chest retraction or refusal to feed (69%), acute diarrheal episodes, fever, and outpatient visits during the first 6 mo of life (41).
Women in this study were not assigned to a type of feeding (they chose to exclusively breastfeed or not) or the duration of EBF if they exclusively breastfed. The underlying reasons that influence women to make a feeding choice could confound risk of dying or being infected by HIV. More than 25% women-child pairs could not be included in this analysis because outcomes occurred before the first available visit or information on EBF was unavailable.
Formula feeding continues to remain an expensive and impractical option for many African mothers (42). When a free or unrestricted supply of formula milk powder is unavailable from governments or household finances, it is often excessively diluted with unsafe water and becomes a vehicle for infections as well as being nutritionally inadequate. In such a scenario, exclusive formula feeding, with the complete exclusion of breast milk, is difficult. In fact, not a single mother was able to maintain exclusive formula feeding in our study. Also, mixed messages with different recommendations for HIV-infected and uninfected mothers can be confusing and may have a bearing on EBF rates and durations in HIV-uninfected mothers. ART was not available in Tanzania at the time of this trial, and none of the participants received any ART. As more and more mothers receive ART, their immunological status (CD4+, lymphocyte counts, and AIDS stages) is likely to be better. We have shown that risk of infant mortality can be greatly reduced by EBF, and these associations between EBF and childhood outcomes may be better in mothers with better lymphocyte counts (total and CD4+). In 2010, the WHO revised their recommendations for infant feeding in infants born to HIV-positive mothers (11). EBF is now recommended for the first 6 mo of life except when formula feeding is acceptable, feasible, affordable, sustainable, and safe. A word of caution may be added for women with advanced disease (low lymphocyte or CD4+ counts) against breastfeeding, especially if they are not on ART. Our results add to other recently published results that support the breastfeeding recommendations of the WHO for all mothers irrespective of HIV status in Africa, except when an uninterrupted availability of formula and safe water can be ensured. Also, these results from children who were followed up for 5 y allay fears that increased mortality later in childhood from mother-to-child transmission of HIV may offset the benefits of EBF in infancy. In addition, the increasing availability of ART for HIV and AIDS should reduce risk of mother-to-child transmission through breastfeeding and make EBF safer for longer in HIV-infected mothers as now recommended by the WHO (11, 43). Although economic progress and political will are necessary to make safe water available, interventions that can make a choice of EBF in HIV-positive women safer for the infant could be evaluated. Early during the HIV epidemic, messages against EBF to HIV-positive mothers may have contributed to many infants being deprived of the benefits of EBF in the absence of safe alternatives and better interventions to reduce infant mortality from infectious diseases. Nevertheless, as access to safe water and quality formula feeding become available along with reductions in infections, disease mortality, and morbidity during childhood, these recommendations on breastfeeding will need to be revisited.
Acknowledgments
We thank the mothers and children and field teams, including nurses, midwives, supervisors, laboratory staff, and administrative staff, who made the study possible and Muhimbili Medical Centre, Muhimbili University College of Health Sciences, and the National AIDS Control Program in Dar es Salaam for their institutional support. We thank Annamaria Kiure for her initial work on the manuscript.
The authors’ responsibilities were as follows—WWF, DS, CD, and KP: designed the research; WWF, GM, and SA: conducted the research; EL, UCMN, and DS: analyzed data; WWF and UCMN: wrote the manuscript and had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: ART, antiretroviral therapy; EBF, exclusive breastfeeding; MUAC, midupper arm circumference.
REFERENCES
- 1.Jelliffe DB, Jelliffe EF. Nutrition and human milk. Postgrad Med 1976;60:153–6 [DOI] [PubMed] [Google Scholar]
- 2.Goldman AS. Modulation of the gastrointestinal tract of infants by human milk. Interfaces and interactions. An evolutionary perspective. J Nutr 2000;130:426S–31S [DOI] [PubMed] [Google Scholar]
- 3.Walker WA. The dynamic effects of breastfeeding on intestinal development and host defense. Adv Exp Med Biol 2004;554:155–70 [DOI] [PubMed] [Google Scholar]
- 4.Hanson L, Silfverdal SA, Strömbäck L, Erling V, Zaman S, Olcén P, Telemo E. The immunological role of breast feeding. Pediatr Allergy Immunol 2001;12(suppl 14):15–9 [DOI] [PubMed] [Google Scholar]
- 5.Hanson LA. Session 1: feeding and infant development breast-feeding and immune function. Proc Nutr Soc 2007;66:384–96 [DOI] [PubMed] [Google Scholar]
- 6.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr 2005;25:37–58 [DOI] [PubMed] [Google Scholar]
- 7.Goldman AS, Goldblum RM, Hanson LA. Anti-inflammatory systems in human milk. Adv Exp Med Biol 1990;262:69–76 [DOI] [PubMed] [Google Scholar]
- 8. WHO. Global strategy on infant and young child feeding. Geneva, Switzerland: WHO, 2003. Available from: http://www.who.int/child_adolescent_health/documents/9241562218/en/index.html (cited 5 August 2011)
- 9.Thiry L, Sprecher-Goldberger S, Jonckheer T, Levy J, Van de Perre P, Henrivaux P, Cogniaux-LeClerc J, Clumeck N. Isolation of AIDS virus from cell-free breast milk of three healthy virus carriers. Lancet 1985;2:891–2 [DOI] [PubMed] [Google Scholar]
- 10.Dunn DT, Newell ML, Ades AE, Peckham CS. Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet 1992;340:585–8 [DOI] [PubMed] [Google Scholar]
- 11.WHO. Guidelines on HIV and infant feeding. Principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva, Switzerland: WHO, 2010. Available from: http://www.who.int/child_adolescent_health/documents/9789241599535/en/index.html (cited 5 August 2011)
- 12.Leshabari SC, Blystad A, Moland KM. Difficult choices: infant feeding experiences of HIV-positive mothers in northern Tanzania. SAHARA J 2007;4:544–55 [DOI] [PubMed] [Google Scholar]
- 13.Young SL, Israel-Ballard KA, Dantzer EA, Ngonyani MM, Nyambo MT, Ash DM, Chantry CJ. Infant feeding practices among HIV-positive women in Dar es Salaam, Tanzania, indicate a need for more intensive infant feeding counselling. Public Health Nutr 2010;13:2027–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coovadia HM, Rollins NC, Bland RM, Little K, Coutsoudis A, Bennish ML, Newell ML. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet 2007;369:1107–16 [DOI] [PubMed] [Google Scholar]
- 15.Fawzi WW, Msamanga GI, Spiegelman D, Urassa EJ, McGrath N, Mwakagile D, Antelman G, Mbise R, Herrera G, Kapiga S, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet 1998;351:1477–82 [DOI] [PubMed] [Google Scholar]
- 16.Fawzi WW, Msamanga G, Hunter D, Urassa E, Renjifo B, Mwakagile D, Hertzmark E, Coley J, Garland M, Kapiga S, et al. Randomized trial of vitamin supplements in relation to vertical transmission of HIV-1 in Tanzania. J Acquir Immune Defic Syndr 2000;23:246–54 [DOI] [PubMed] [Google Scholar]
- 17.Fawzi WW, Msamanga GI, Wei R, Spiegelman D, Antelman G, Villamor E, Manji K, Hunter D. Effect of providing vitamin supplements to human immunodeficiency virus-infected, lactating mothers on the child's morbidity and CD4+ cell counts. Clin Infect Dis 2003;36:1053–62 [DOI] [PubMed] [Google Scholar]
- 18.Antelman G, Msamanga GI, Spiegelman D, Urassa EJ, Narh R, Hunter DJ, Fawzi WW. Nutritional factors and infectious disease contribute to anemia among pregnant women with human immunodeficiency virus in Tanzania. J Nutr 2000;130:1950–7 [DOI] [PubMed] [Google Scholar]
- 19.WHO. Breastfeeding and replacement feeding practices in the context of mother-to-child transmission of HIV. An assessment tool for research. Geneva, Switzerland: WHO, 2001. Available from: http://www.who.int/child_adolescent_health/documents/cah_01_21/en/index.html (cited 5 August 2011)
- 20.WHO International Collaborating Group for the Study of the WHO Staging System. Proposed World Health Organization staging system for HIV infection and disease: preliminary testing by an international collaborative cross-sectional study. AIDS 1993;7:711–8 [PubMed] [Google Scholar]
- 21.Cox DR. Regression models and life tables. J R Stat Soc B 1972;34:187–220 [Google Scholar]
- 22.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health 1989;79:340–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc 1958;53:457–81 [Google Scholar]
- 24.Armstrong Schellenberg JR, Nathan R, Abdulla S, Mukasa O, Marchant TJ, Tanner M, Lengeler C. Risk factors for child mortality in rural Tanzania. Trop Med Int Health 2002;7:506–11 [DOI] [PubMed] [Google Scholar]
- 25.Bobat R, Moodley D, Coutsoudis A, Coovadia H. Breastfeeding by HIV-1-infected women and outcome in their infants: a cohort study from Durban, South Africa. AIDS 1997;11:1627–33 [DOI] [PubMed] [Google Scholar]
- 26.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, Ndinya-Achola J, Bwayo J, Onyango FE, Hughes J, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomised clinical trial. JAMA 2000;283:1167–74 [DOI] [PubMed] [Google Scholar]
- 27.Coutsoudis A, Pillay K, Spooner E, Kuhn L, Coovadia HM. Influence of infant-feeding patterns on early mother-to-child transmission of HIV-1 in Durban, South Africa: a prospective cohort study. Lancet 1999;354:471–6 [DOI] [PubMed] [Google Scholar]
- 28.Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, Moulton LH, Ward BJ, Humphrey JH. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases ‘HIV infection or death’. AIDS 2005;19:699–708 [DOI] [PubMed] [Google Scholar]
- 29.Miotti PG, Taha TE, Kumwenda NI, Broadhead R, Mtimavalye LA, Van der Hoeven L, Chiphangwi JD, Liomba G, Biggar RJ. HIV transmission through breastfeeding: a study in Malawi. JAMA 1999;282:744–9 [DOI] [PubMed] [Google Scholar]
- 30.Kuhn L, Sinkala M, Kankasa C, Semrau K, Kasonde P, Scott N, Mwiya M, Vwalika C, Walter J, Tsai WY, et al. High uptake of exclusive breastfeeding and reduced early post-natal HIV transmission. PLoS ONE 2007;2:e1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becquet R, Ekouevi DK, Menan H, Amani-Bosse C, Bequet L, Viho I, Dabis F, Timite-Konan M, Leroy V. Early mixed feeding and breastfeeding beyond 6 months increase the risk of postnatal HIV transmission: ANRS 1201/1202 Ditrame Plus, Abidjan, Côte d'Ivoire. Prev Med 2008;47:27–33 [DOI] [PubMed] [Google Scholar]
- 32.Farquhar C, VanCott TC, Mbori-Ngacha DA, Horani L, Bosire RK, Kreiss JK, Richardson BA, John-Stewart GC. Salivary secretory leukocyte protease inhibitor is associated with reduced transmission of human immunodeficiency virus type 1 through breast milk. J Infect Dis 2002;186:1173–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saeland E, de Jong MA, Nabatov AA, Kalay H, Geijtenbeek TB, van Kooyk Y. MUC1 in human milk blocks transmission of human immunodeficiency virus from dendritic cells to T cells. Mol Immunol 2009;46:2309–16 [DOI] [PubMed] [Google Scholar]
- 34.Smith MM, Kuhn L. Exclusive breast-feeding: does it have the potential to reduce breast-feeding transmission of HIV-1? Nutr Rev 2000;58:333–40 [DOI] [PubMed] [Google Scholar]
- 35.Semba RD, Kumwenda N, Hoover DR, Taha TE, Quinn TC, Mtimavalye L, Biggar RJ, Broadhead R, Miotti PG, Sokoll LJ, et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis 1999;180:93–8 [DOI] [PubMed] [Google Scholar]
- 36.Willumsen JF, Filteau SM, Coutsoudis A, Newell ML, Rollins NC, Coovadia HM, Tomkins AM. Breastmilk RNA viral load in HIV-infected South African women: effects of subclinical mastitis and infant feeding. AIDS 2003;17:407–14 [DOI] [PubMed] [Google Scholar]
- 37.Dewey KG, Heinig MJ, Nommsen-Rivers LA. Differences in morbidity between breastfed and formula-fed infants. J Pediatr 1995;126:696–702 [DOI] [PubMed] [Google Scholar]
- 38.WHO Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Lancet 2000;355:451–5. (Published erratum appears in Lancet 2000;355:1104.) [PubMed] [Google Scholar]
- 39.Arifeen S, Black RE, Antelman G, Baqui A, Caulfield L, Becker S. Exclusive breastfeeding reduces acute respiratory infection and diarrhea deaths among infants in Dhaka slums. Pediatrics 2001;108:E67. [DOI] [PubMed] [Google Scholar]
- 40.Black RE, Morris SS, Bryce J. Where and why are 10 million children dying every year? Lancet 2003;361:2226–34 [DOI] [PubMed] [Google Scholar]
- 41.Mwiru RS, Spiegelman D, Duggan C, Peterson K, Liu E, Msamanga G, Aboud S, Fawzi WW. Relationship of exclusive breast-feeding to infections and growth of Tanzanian children born to HIV-infected women. Public Health Nutr 2011;14:1251–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coutsoudis A, Goga AE, Rollins N, Coovadia HM; Child Health Group Free formula milk for infants of HIV-infected women: blessing or curse? Health Policy Plan 2002;17:154–60 [DOI] [PubMed] [Google Scholar]
- 43.Coovadia HM, Coutsoudis A, Rollins NC. Antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med 2008;359:1846; author reply 1848. [PubMed] [Google Scholar]