Abstract
Background: Although evidence from cohort studies has suggested that trans fatty acid (TFA) consumption may be associated with insulin resistance and diabetes, randomized placebo-controlled trials (RCTs) have yielded conflicting results.
Objective: In a meta-analysis, we combined all available RCTs that examined the role of TFA intake on glucose homeostasis.
Design: A systematic review of PubMed was performed, and a total of 7 RCTs were included in the meta-analysis. Primary outcomes were glucose and insulin concentrations. Secondary outcomes were total, LDL-, and HDL-cholesterol and triglyceride concentrations. The pooled effect size (ES) was calculated through fixed- and random-effects meta-analyses. The potential existence of publication bias was evaluated by using funnel-plot analysis. Metaregression analysis was performed to evaluate for potential dose-response relations between the ES of outcomes and TFA intake.
Results: Increased TFA intake did not result in significant changes in glucose or insulin concentrations. Increased TFA intake led to a significant increase in total and LDL-cholesterol [ES (95% CI): 0.28 (0.04, 0.51) and 0.36 (0.13, 0.60), respectively] and a significant decrease in HDL-cholesterol concentrations [ES (95% CI): −0.25 (−0.48, −0.01)]. Our analysis also showed the absence of publication bias and any dose-response relations between the ES and TFA intake.
Conclusions: Increased TFA intake does not result in changes in glucose, insulin, or triglyceride concentrations but leads to an increase in total and LDL-cholesterol and a decrease in HDL-cholesterol concentrations. There is no evidence to support a potential benefit of the reduction of dietary TFA intake on glucose homeostasis.
INTRODUCTION
trans Fatty acids (TFAs)4 are fatty acids that contain between 2 consecutive carbon atoms at least one double bond that is in the trans configuration. The average TFA intake in the United States is estimated to be ∼4 g/d. This intake corresponds to ∼2% of total energy intake (1). However, on an individual basis, it has been reported that a person could consume ≤50 g TFAs from a single high-fat meal or snack in the United States (2).
More so than other macronutrients, TFAs have been associated with cardiovascular mortality and morbidity (3). Evidence, which has been limited to cohort studies, has indicated that increased TFA consumption is associated with increased risk of coronary artery disease. Recommendations from the American Heart Association (4, 5) have led 13 local governments, one state, and Puerto Rico to implement a TFA ban (6, 7) and the Food and Drug Administration to require that trans fat content to be listed on the nutrition facts panel of foods and dietary supplements (8). However, to our knowledge, no randomized placebo-controlled trials (RCTs) have been published that showed a reduction in cardiovascular mortality associated with a reduction in the percentage of TFA intake (9).
The increased cardiometabolic risk associated with TFA consumption could be attributed to a TFA-induced unfavorable lipid profile, accentuation of systemic inflammation, endothelial dysfunction, or disruption of glucose homeostasis (3). Because long-term RCTs that evaluate whether the isoenergetic substitution of TFAs with other macronutrients modifies the risk of hard cardiovascular outcomes have been considered to be challenging to design and conduct, RCTs that analyze the effect of TFA intake on cardiovascular risk factors have been suggested to be a more acceptable alternative. In regard to the effect of TFAs on the lipid profile, there is convincing evidence at the level of meta-analyses of 13 and 60 studies, respectively, that increasing TFA intake leads to a more unfavorable lipid profile (10, 11). In the area of systemic inflammation and endothelial function, there is only one RCT study available, to our knowledge, that showed a 14.4% increase in circulating E-selectin after a high-TFA diet compared with a diet enriched with SFA (12). Finally, several RCTs that focused on glucose homeostasis have revealed conflicting results; however, to our knowledge, there is no meta-analysis that combined these RCTs to evaluate the effect of TFAs on variables of glucose homeostasis.
The purpose of this study was to perform a meta-analysis of all RCTs that evaluate the effect of TFA administration on variables of glucose homeostasis and insulin sensitivity, namely glucose and insulin concentrations. We also analyzed all studies for the existence of any potential publication bias, and we have performed a metaregression analysis to evaluate for the potential dose dependency between the dose of TFAs administered and changes in glucose and insulin concentrations. Finally, we performed a meta-analysis of the same RCTs that evaluate the effect of TFA administration on lipid concentrations to comparatively evaluate them in relation to the effects of TFAs on glycemia in the same studies.
METHODS
Literature search and study selection
A systematic literature review of PubMed (http://www.ncbi.nlm.nih.gov/pubmed) was performed to identify RCTs of interest (see Figure 1 under “Supplemental data” in the online issue). In addition, we performed a literature search within each one of the journals in which we identified relevant studies. We used the following key words either alone or in combination: trans fatty acids, TFAs, insulin resistance, insulin sensitivity, glucose tolerance, glucose, insulin, metabolic syndrome, and diabetes mellitus. Only original articles were included in the analysis. Selection criteria were the randomized, placebo-controlled or crossover design of the study. Studies were also required to describe the effect of the TFA consumption on glucose and insulin concentrations, composition of the control and TFA-enriched diet, percentage of total energy intake attributed to TFAs, and duration of the study. However, review articles were used to identify additional relevant studies. Individual authors were contacted via e-mail when additional information about their study was required for the purposes of our analysis. The purpose of this meta-analysis was to evaluate the collective evidence from interventional RCTs; thus, only such studies were included in the analysis. Studies in which the TFA intake was not quantified were excluded from the analysis. Our primary outcomes were insulin (in μIU/mL) and glucose (in mmol/L) concentrations. Secondary outcomes were triglyceride concentrations and total, LDL, and HDL cholesterol (in mmol/L) in the same studies that we analyzed for insulin and glucose. TFA intake was expressed as the percentage of total energy intake. Two authors (KNA and SMK) independently performed the literature search, evaluation of trials, and data extraction.
FIGURE 1.
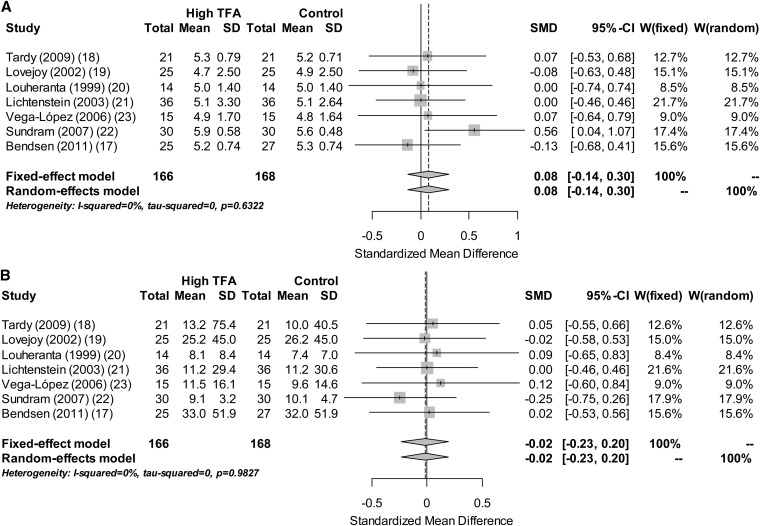
Forest plots that summarize the results of the meta-analysis. Gray boxes denote the effect sizes of studies, and the size of each box is proportional to the weight given to each study. The whiskers bilateral to each gray box represent the 95% CI of the effect size of each study, and diamonds at the bottom of the graph represent the inverse-variance pooled-effect sizes of the meta-analysis, with the first diamond representing the results from the fixed-effect model and the second diamond representing the results from the random-effects model. The effect size is expressed as the SMD. On the left side of the Forest plot, the sample size and mean glucose (A) and insulin (B) concentrations and their SDs in the High TFA and Control groups of each study are shown. On the right side of the Forest plot, W(fixed) and W(random) represent the weight that was given to each study in the fixed- and random-effect meta-analysis model, respectively. SMD, standardized mean difference; TFA, trans fatty acid.
Statistical analysis
The statistical analysis was performed with R2.13 software (R Development Core Team) (13) by using the meta package (14). We performed both fixed- and random-effects inverse-variance meta-analyses through the metacont routine (15). The effect size (ES) was expressed as standardized mean difference, and Hedges’ g was used to pool variances for the standardization. We performed a meta-analysis on 7 RCTs that presented data regarding the effect of TFAs on insulin and glucose concentrations. With the use of the same RCTs, we also meta-analyzed results regarding the effect of TFAs on triglycerides and total, LDL, and HDL cholesterol. The heterogeneity of outcome variables was evaluated by using the I2 statistic. In a secondary analysis, we also analyzed for the existence of a potential publication bias, which was defined as the tendency of authors and editors to handle studies in which the experimental results achieved statistical significance more favorably than in studies in which the results failed to reach significance, which would ultimately introduce bias into the overall published literature (16). The existence of a potential publication bias was evaluated with funnel plots. In a funnel plot, the x-axis represents the ES of each study, whereas the y axis represents the SE of the ES of the corresponding study. The solid vertical line represents the pooled ES as estimated by the meta-analysis. Individual studies should be located symmetrically on the 2 sides of the vertical line. Funnel-plot asymmetry was evaluated by using the Egger's test for small-study effects through the metabias routine. We evaluated for potential dose-response relations by using a random-effects metaregression with aggregate-level data in which ES was regressed on the dose of TFAs.
RESULTS
Study characteristics
The PubMed search yielded 925 records that were subsequently screened. Review articles, editorials, viewpoints, commentaries, and in vitro and in vivo animal studies were excluded from our meta-analysis. Studies that did not provide data on the effect of TFA consumption on glucose or insulin were also excluded. Ultimately, only 7 studies qualified for the meta-analysis because they were double-blinded RCTs that reported glucose and insulin concentrations and featured the consistency of both the control and experimental diets (see Figure 1 under “Supplemental data” in the online issue). A total of 208 subjects were included in the analysis, and the average sample size of each study was 30 subjects (range: 14–63 subjects). All studies, except the studies by Bendsen et al (17) and Tardy et al (18), had a crossover design, which enabled for the control of potential confounding variables. The intake of TFAs ranged from 2.59% to 7.8% of total energy intake, whereas the duration of the intervention was 4–6 wk except in the study of Bendsen et al (17), in which it was 16 wk. The participants of 4 of the 7 studies analyzed were healthy individuals (19–22). The study of Vega-López et al (23) evaluated hyperlipidemic subjects who were but otherwise healthy, Tardy et al (18) evaluated obese individuals, and Bendsen et al (17) evaluated overweight postmenopausal women. The studies of Lovejoy et al (19) and Luheranta et al (20) had a washout period, between the different diets, of ≥2 wk. The studies of Lichtenstein et al (21), Vega-López et al (23), and Sundram et al (22) did not clearly mention the duration of the washout period. A summary of study characteristics and the control diet for each study is presented in Table 1.
TABLE 1.
Characteristics of studies included in the meta-analysis1
| Glucose |
Insulin |
||||||||||
| Study | Year | Design | Sample size | Subjects | Duration | Control diet | TFA intake | Control group | High-TFA group | Control group | High-TFA group |
| n | wk | % of total energy intake | mmol/L | μIU/mL | |||||||
| Tardy et al (18) | 2009 | RCT | 63 | O | 4 | Isoenergetic substitution of TFAs with cis MUFAs | 2.59 | 5.22 ± 0.012 | 5.28 ± 0.01 | 10.00 ± 0.64 | 13.20 ± 1.19 |
| Lovejoy et al (19) | 2002 | RCT-X | 25 | NO | 4 | Isoenergetic substitution of TFAs with oleic acid | 9.0 | 4.90 ± 0.10 | 4.70 ± 0.10 | 26.2 ± 1.80 | 25.20 ± 1.8 |
| Louheranta et al (20) | 1999 | RCT-X | 14 | NO | 4 | Isoenergetic substitution of TFAs with cis MUFAs | 5.10 | 5.00 ± 0.10 | 5.00 ± 0.10 | 7.40 ± 0.50 | 8.10 ± 0.6 |
| Lichtenstein et al (21) | 2003 | RCT-X | 36 | NO | 5 | Isoenergetic substitution of soybean oil with sticky margarine | 7.83 | 5.06 ± 0.07 | 5.06 ± 0.09 | 11.20 ± 0.85 | 11.20 ± 0.81 |
| Vega-López et al (23) | 2006 | RCT-X | 15 | HL | 5 | Isoenergetic substitution of TFAs with cis PUFAs | 4.15 | 4.81 ± 0.10 | 4.94 ± 0.11 | 9.60 ± 0.97 | 11.50 ± 1.07 |
| Sundram et al (22) | 2007 | RCT-X | 30 | NO | 4 | Isoenergetic substitution of partially hydrogenated soybean oil with palm olein | 3.20 | 5.60 ± 0.01 | 5.90 ± 0.01 | 10.10 ± 0.15 | 9.10 ± 0.10 |
| Bendsen et al (17) | 2011 | RCT | 27C, 25T | OW, PM | 16 | Isoenergetic substitution of TFAs with oleic and palmitic acids | 7.00 | 5.30 ± 0.02 | 5.20 ± 0.02 | 32.00 ± 2.07 | 33.00 ± 2.07 |
For all studies that had a crossover design, the sample size refers to the number of subjects who went through each phase of the study (ie, all subjects who completed a low–TFA-intake arm and a high–TFA-intake arm. For RCTs, the numbers of subjects who completed the low– and high–TFA-intake arms are given separately. C, control diet; HL, hyperlipidemic; NO, nonobese; O, obese; OW, overweight; PM, postmenopausal; RCT, randomized placebo-controlled trial; RCT-X, randomized placebo-controlled trial with crossover design; T, diet enriched with TFAs; TFA, trans fatty acid.
Mean ± SEM (all such values).
Pooled effects of TFA administration
A meta-analysis of the 7 published RCTs revealed no effect of TFA consumption on glucose [ES (95% CI): 0.08 (−0.14, 0.29)] or insulin [ES (95% CI): −0.02 (−0.23, 0.19)] concentrations (Figure 1). Both primary outcomes were homogenous as evaluated with the I2 statistic (I2 = 0.0%; P > 0.5 for both insulin and glucose). A meta-analysis of the secondary outcomes reveals that increased TFA intake was associated with a significant increase in total and LDL cholesterol [ES (95% CI): 0.28 (0.04, 0.51) and 0.36 (0.13, 0.60), respectively] and a significant decrease in HDL cholesterol [ES (95% CI): −0.25 (−0.48, −0.01)]. There was a trend for triglyceride concentrations to increase with increased TFA intake; however, this result failed to reach significance [ES (95% CI): 0.12 (−0.11, 0.36)]. Similar to the primary outcomes, the between-studies heterogeneity of secondary outcomes was nonsignificant (I2 = 0.0%; P > 0.7 for all secondary outcomes). Glucose and insulin concentrations did not change when we analyzed the data by excluding the study of Bendsen et al (17), which had the longest duration [ES (95% CI) for glucose: 0.12 (−0.11, 0.35); ES (95% CI) for insulin: −0.03 (−0.26, 0.21)] and when we analyzed data only from studies that evaluated lean individuals [ES (95% CI) for glucose: 0.13 (−0.14, 0.41); ES (95% CI) for insulin: −0.06 (−0.33, 0.20)].
Publication-bias analysis
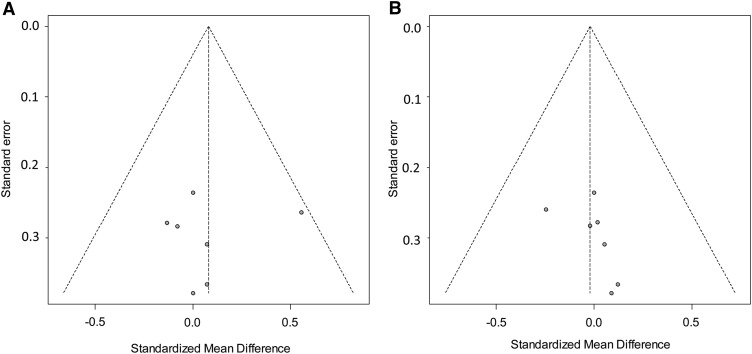
From inspection of the funnel plots, we concluded that, overall, the plots seemed symmetric for both primary and the secondary outcomes. Because the funnel plots of HDL cholesterol and triglycerides seemed slightly asymmetric to the right (which suggested a potential publication bias), we quantified the potential asymmetry in both our primary and secondary outcomes by using the Egger's test for small-study effects (24). The Egger's test performs a linear regression of the intervention effect estimates on their SEs, weighting by the inverse variance of the intervention effect estimate and testing the null hypothesis of no small-study effect (ie, no funnel plot asymmetry). Our analysis failed to reject the null hypothesis for both our primary and secondary outcomes (all P > 0.1), which suggested the absence of a publication bias from the literature (Figure 2).
FIGURE 2.
Funnel plots for the detection of potential publication bias. The x axis represents effect size as expressed with the standardized mean difference, and the y axis represents the SE of the standardized mean difference. The vertical dotted line represents the pooled-effect size as calculated from the meta-analysis. The oblique dotted lines represent the 95% confidence limits around the summary treatment effect for each SE on the vertical axis. These show the expected distribution of studies in the absence of heterogeneity or of selection biases. Because these lines are not strict 95% limits, we consider them pseudo–95% confidence limits.
Dose-response relation
A random-effect metaregression analysis yielded no significant dose-response relation for any of the primary and secondary outcomes (all P > 0.3; data not shown).
DISCUSSION
This meta-analysis of RCTs showed that an increase in TFA intake from 2.59% to 7.8% of total energy intake did not lead to any significant change in circulating glucose and insulin concentrations. The metaregression analysis also revealed the absence of any dose-response relation between the dose of TFA intake and any effect on glucose and insulin concentrations. Our analysis also showed that there was no evident publication bias in the literature. The negative results of this meta-analysis of the currently available interventional clinical studies that evaluated the effect of TFA consumption on variables of insulinemia and glycemia remained unchanged irrespective of whether all studies were included or only short-term studies were included and indicated that the effect of TFAs was apparently null.
We analyzed all RCTs on normal subjects and showed no difference (ie, a null effect of TFAs). The 2 randomized crossover studies of TFA administration in obese, hyperlipidemic patients with diabetes yielded controversial results. Vega-López et al (23) showed that an increase of TFA intake to 4.15% of total energy intake resulted in a 25–28% increase in the HOMA-IR index and, in a different study, it was described that the substitution of TFAs with cis MUFAs or SFAs for 6 wk led to a significant reduction in the postprandial insulin response (25). The only study in hyperlipidemic individuals yielded a significant increase in the HOMA-IR index (23). Thus, additional studies, particularly RCTs, are needed to evaluate this particular population and to determine whether hyperlipidemic individuals are more prone to insulin resistance induced by an increased TFA intake.
We also analyzed separately RCTs with short-term duration (4–6 wk) and showed null results (ie, no difference from the meta-analysis of all 7 studies). There was only one recent dietary intervention study in overweight but otherwise healthy postmenopausal females, in which Bendsen et al (17) showed that the isocaloric substitution of ∼7% of total energy intake TFAs with a control oil, which mostly consisted of oleic and palmitic acids, for 16 wk did not have any significant effect on insulin sensitivity or β cell function. The aforementioned study is in agreement with a previous study in premenopausal overweight females, in whom a reduction of TFA intake from 4.86–5.58 to 0.54 g/d TFAs for 4 wk did not improve insulin sensitivity (18). Similarly, clinical studies conducted in healthy, euglycemic, normal-weight individuals, with similar sample size, design, and duration, failed to show any significant change in insulin sensitivity with 5–9% of total energy intake TFA consumption compared with cis MUFA or SFA consumption (19–21). The doses of TFAs used in these studies ranged from 3% to 8% of total energy intake, which were doses considerably higher than those consumed by average individuals in the United States (1). This meta-analysis of the described RCTs showed that a reduction in TFA intake does not lead to any significant change in circulating glucose and insulin concentrations, which suggests that there is no association between the reduction in TFA intake and an improvement in insulin sensitivity.
Additional information regarding TFA intake and glucose homeostasis has been derived from observational prospective cohorts (26–29) and case-control studies (30, 31), again with conflicting results. Results from these studies could not be combined in a meta-analysis because most of the data required for such a task could not be recovered; however, the lack of concordance in the results of the cohort studies described in the current article is in agreement with the results of the short-term RCTs used in this meta-analysis. A definitive answer regarding the question of whether TFAs are causally associated with the development of insulin resistance and diabetes mellitus could be derived from RCTs that evaluate whether a decrease in TFA intake from habitual to very low could lead to a decrease in the incidence of diabetes mellitus; however, to our knowledge, no such trials have been published.
The analysis of our secondary outcomes showed that there was a significant increase in circulating total and LDL-cholesterol concentrations and a decrease in the HDL-cholesterol concentrations with increased TFA intake, which was consistent with what has been reported in the literature in meta analyses of 13 and 60 studies, respectively (10, 11). In a meta-analysis of 60 RCTs, Mensink et al (11) showed that increased TFA intake led to a significant increase in the total:HDL cholesterol ratio, whereas in a different meta-analysis of 13 RCTs, Mozaffarian et al (10) calculated that the isocaloric replacement of TFAs with SFAs, cis MUFAs, or cis PUFAs improves the lipid profile by significantly decreasing triglyceride concentrations, the total:HDL cholesterol ratio, and the apolipoprotein B:apolipoprotein A ratio. We failed to demonstrate any significant effect of TFA intake on triglyceride concentrations. Dietary intervention studies have shown that increased TFA consumption is associated with an unfavorable lipid profile characterized by increased triglyceride and LDL- and total cholesterol concentrations and decreased HDL-cholesterol and apolipoprotein A-I concentrations (32–34). In addition, Mauger et al (35) showed that an increased TFA intake leads to an increase in circulating apolipoprotein B particles and a decrease in apolipoprotein A particles. In a different meta-analysis that focused on the effects of TFAs on lipid and lipoprotein profiles, 1% of total energy intake substitution of TFAs with SFA, cis MUFAs, or cis PUFAs improved the apolipoprotein B:apolipoprotein A-I ratio and also significantly decreased lipoprotein(a) concentrations (10).
The strength of this study was that it is the first meta-analysis, to our knowledge, to evaluate the effect of TFA intake on variables of insulin sensitivity and glycemia. We controlled for heterogeneity of the different TFA doses across the various studies with the metaregression approach. The studies included in this meta-analysis were all RCTs that could provide reliable inference about causality. Limitations of this study included the relatively short duration of the studies that have been performed to date; such duration, however, would have been more than adequate to show changes in glycemia or insulinemia and was adequate to show a change in total, LDL-, and HDL-cholesterol concentrations. Short-term studies may not be able to detect delayed effects of TFAs on insulin resistance. It takes a considerable period of time, at least a number of months, for adipose tissue stores to stabilize and reflect a new diet. During the postabsorptive period, it is these stored TFAs that largely circulate and presumably have their acute metabolic effects that could increase over time.
Another limitation of the study was the finite range of the TFA doses used in the studies published to date, but the latter contributed to the homogeneity of our study. In any case, these doses were considerably higher than the average TFA intake in the United States. Thus far, an inherent limitation of all of the studies is the fact that TFA intake was increased in RCTs and not decreased as recommended by public health recommendations. Although we could not detect a dose-response effect in our study, there is a distinct possibility that a threshold may exist below which an additional reduction may have no effect on cardiovascular risk. This possibility remains to be tested in future RCTs that would test a decreased compared with an average dose of TFAs. These studies are needed to fully justify the public health interventions that were recently implemented at the state and national levels.
In conclusion, to our knowledge, this is the first meta-analysis to evaluate RCTs that evaluated the effect of TFAs on variables of insulin resistance and showed that there is no significant effect of a decreased TFA intake on insulin sensitivity or hyperglycemia. In addition, to our knowledge, there is no randomized, controlled evidence that demonstrates that a reduction of TFA intake below the average 2% of total energy intake TFAs in the American diet could lead to any improvement in terms of insulin resistance and glycemia or overt diabetes mellitus and/or hard cardiovascular endpoints, systemic inflammatory status, endothelial function, or visceral adiposity. The performance of RCTs that evaluate the aforementioned hypothesis could prove to be extremely useful in the establishment of scientific evidence and the potent guidance of public health recommendations and policies.
Acknowledgments
The authors’ responsibilities were as follows—KNA and SMK: reviewed the literature, screened the records, assessed the quality of the studies, and extracted data; KNA: performed the statistical analysis and wrote the manuscript; CSM: wrote the manuscript and supervised the study; and all authors: reviewed and took responsibility for the content of the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: ES, effect size; RCT, randomized placebo-controlled trial; TFA, trans fatty acid.
REFERENCES
- 1.Harnack L, Lee S, Schakel SF, Duval S, Luepker RV, Arnett DK. Trends in the trans-fatty acid composition of the diet in a metropolitan area: the Minnesota Heart Survey. J Am Diet Assoc 2003;103:1160–6 [DOI] [PubMed] [Google Scholar]
- 2.Stender S, Astrup A, Dyerberg J. Ruminant and industrially produced trans fatty acids: health aspects. Food Nutr Res 2008;52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Micha R, Mozaffarian D. Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nat Rev Endocrinol 2009;5:335–44 [DOI] [PubMed] [Google Scholar]
- 4.Eckel RH, Borra S, Lichtenstein AH, Yin-Piazza SY. Understanding the complexity of trans fatty acid reduction in the American diet: American Heart Association Trans Fat Conference 2006: report of the Trans Fat Conference Planning Group. Circulation 2007;115:2231–46 [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation 2006;114:82–96 [DOI] [PubMed] [Google Scholar]
- 6. CSPI: Center for Science in the Public Interest. Available from: http://www.cspinet.org/transfat/ (cited 23 June 2012)
- 7. NCSL: National Conference of State Legislatures. Trans fat and menu labeling legislation. Available from: http://www.ncsl.org/default.aspx?tabid=14362 (cited 23 June 2012)
- 8.Miyake H, Furukawa J, Kurahashi T, Fujisawa M. Serum level of clusterin and its density in men with prostate cancer as novel biomarkers reflecting disease extension. Urology 2010;75:454–9 [DOI] [PubMed] [Google Scholar]
- 9.Aronis KN, Joseph RJ, Blackburn GL, Mantzoros C. trans. -Fatty acids, insulin resistance/diabetes, and cardiovascular disease risk: should policy decisions be based on observational cohort studies, or should we be waiting for results from randomized placebo-controlled trials? Metabolism 2011;60:901–5. [DOI] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Clarke R. Quantitative effects on cardiovascular risk factors and coronary heart disease risk of replacing partially hydrogenated vegetable oils with other fats and oils. Eur J Clin Nutr 2009;63(suppl 2):S22–33. [DOI] [PubMed] [Google Scholar]
- 11.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146–55 [DOI] [PubMed] [Google Scholar]
- 12.Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr 2004;79:969–73 [DOI] [PubMed] [Google Scholar]
- 13.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2011. ISBN 3-900051-07-0. Available from: http://www.R-project.org/
- 14.Schwarzer G. meta: meta-analysis with R. R package version 1.6-1, 2010. Available from: http://CRAN.R-project.org/package=meta. [Google Scholar]
- 15.Harris R, Bradburn M, Deeks J, Harbord R, Altman D, Sterne J. metan: fixed- and random-effects meta-analysis. Stata Journal 2008;8:3–28 [Google Scholar]
- 16.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet 1991;337:867–72 [DOI] [PubMed] [Google Scholar]
- 17.Bendsen NT, Haugaard SB, Larsen TM, Chabanova E, Stender S, Astrup A. Effect of trans-fatty acid intake on insulin sensitivity and intramuscular lipids–a randomized trial in overweight postmenopausal women. Metabolism 2011;60:906–13 [DOI] [PubMed] [Google Scholar]
- 18.Tardy AL, Lambert-Porcheron S, Malpuech-Brugere C, Giraudet C, Rigaudiere JP, Laillet B, Leruyet P, Peyraud JL, Boirie Y, Laville M, et al. Dairy and industrial sources of trans fat do not impair peripheral insulin sensitivity in overweight women. Am J Clin Nutr 2009;90:88–94 [DOI] [PubMed] [Google Scholar]
- 19.Lovejoy JC, Smith SR, Champagne CM, Most MM, Lefevre M, DeLany JP, Denkins YM, Rood JC, Veldhuis J, Bray GA. Effects of diets enriched in saturated (palmitic), monounsaturated (oleic), or trans (elaidic) fatty acids on insulin sensitivity and substrate oxidation in healthy adults. Diabetes Care 2002;25:1283–8 [DOI] [PubMed] [Google Scholar]
- 20.Louheranta AM, Turpeinen AK, Vidgren HM, Schwab US, Uusitupa MI. A high-trans fatty acid diet and insulin sensitivity in young healthy women. Metabolism 1999;48:870–5 [DOI] [PubMed] [Google Scholar]
- 21.Lichtenstein AH, Erkkila AT, Lamarche B, Schwab US, Jalbert SM, Ausman LM. Influence of hydrogenated fat and butter on CVD risk factors: remnant-like particles, glucose and insulin, blood pressure and C-reactive protein. Atherosclerosis 2003;171:97–107 [DOI] [PubMed] [Google Scholar]
- 22.Sundram K, Karupaiah T, Hayes KC. Stearic acid-rich interesterified fat and trans-rich fat raise the LDL/HDL ratio and plasma glucose relative to palm olein in humans. Nutr Metab (Lond) 2007;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vega-López S, Ausman LM, Jalbert SM, Erkkila AT, Lichtenstein AH. Palm and partially hydrogenated soybean oils adversely alter lipoprotein profiles compared with soybean and canola oils in moderately hyperlipidemic subjects. Am J Clin Nutr 2006;84:54–62 [DOI] [PubMed] [Google Scholar]
- 24.Harbord RM, Harris RJ, Sterne JAC. Updated tests for small-study effects in meta-analyses. Stata Journal 2009;9:197–210 [Google Scholar]
- 25.Christiansen E, Schnider S, Palmvig B, Tauber-Lassen E, Pedersen O. Intake of a diet high in trans monounsaturated fatty acids or saturated fatty acids. Effects on postprandial insulinemia and glycemia in obese patients with NIDDM. Diabetes Care 1997;20:881–7 [DOI] [PubMed] [Google Scholar]
- 26.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790–7 [DOI] [PubMed] [Google Scholar]
- 27.Meyer KA, Kushi LH, Jacobs DR, Jr, Folsom AR. Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care 2001;24:1528–35 [DOI] [PubMed] [Google Scholar]
- 28.van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002;25:417–24 [DOI] [PubMed] [Google Scholar]
- 29.Papantoniou K, Fito M, Covas MI, Munoz D, Schroder H. trans. Fatty acid consumption, lifestyle and type 2 diabetes prevalence in a Spanish population. Eur J Nutr 2010;49:357–64 [DOI] [PubMed] [Google Scholar]
- 30.Esmaillzadeh A, Azadbakht L. Consumption of hydrogenated versus nonhydrogenated vegetable oils and risk of insulin resistance and the metabolic syndrome among Iranian adult women. Diabetes Care 2008;31:223–6 [DOI] [PubMed] [Google Scholar]
- 31.Hodge AM, English DR, O'Dea K, Giles GG. Dietary patterns and diabetes incidence in the Melbourne Collaborative Cohort Study. Am J Epidemiol 2007;165:603–10. [DOI] [PubMed] [Google Scholar]
- 32.Mensink RP, Katan MB. Effect of dietary trans fatty acids on high-density and low-density lipoprotein cholesterol levels in healthy subjects. N Engl J Med 1990;323:439–45 [DOI] [PubMed] [Google Scholar]
- 33.Zock PL, Katan MB. Hydrogenation alternatives: effects of trans fatty acids and stearic acid versus linoleic acid on serum lipids and lipoproteins in humans. J Lipid Res 1992;33:399–410 [PubMed] [Google Scholar]
- 34.Wood R, Kubena K, O'Brien B, Tseng S, Martin G. Effect of butter, mono- and polyunsaturated fatty acid-enriched butter, trans fatty acid margarine, and zero trans fatty acid margarine on serum lipids and lipoproteins in healthy men. J Lipid Res 1993;34:1–11 [PubMed] [Google Scholar]
- 35.Mauger JF, Lichtenstein AH, Ausman LM, Jalbert SM, Jauhiainen M, Ehnholm C, Lamarche B. Effect of different forms of dietary hydrogenated fats on LDL particle size. Am J Clin Nutr 2003;78:370–5 [DOI] [PubMed] [Google Scholar]