Abstract
Interest in vitamin A as a regulator of immune function goes back to the early 1900s. Recently, several lines of evidence have converged to show that retinoic acid (RA), a major oxidative metabolite of vitamin A, plays a key role in the differentiation of T cell subsets, the migration of T cells into tissues, and the proper development of T cell–dependent antibody responses. This review discusses evidence from experimental studies that RA promotes the differentiation of regulatory T cells, which help to suppress inflammatory reactions, and plays a significant role in normal mucosal immunity by modulating T cell activation and regulating cell trafficking. RA also promotes antibody responses to T cell–dependent antigens. Conversely, in a state of vitamin A deficiency, inflammatory T cell reactions may be inadequately opposed and therefore become dominant. Although data from human studies are still needed, the framework now developed from studies in mice and rat models suggests that adequate vitamin A status, whether derived from ingestion of preformed retinol or β-carotene, is important for maintaining a proper balance of well-regulated T cell functions and for preventing excessive or prolonged inflammatory reactions.
INTRODUCTION
The idea that vitamin A is important for immunity goes back to the early 20th century when Edward Mellanby and his colleague Harry Green reported on vitamin A and β-carotene as “anti-infective” agents (1, 2). Mellanby later recollected that while they were conducting studies on bone health in dogs fed diets lacking in fat-soluble vitamins A and D, they noted the development of bronchopulmonary infections, which they believed were unrelated to the status of the bones, and thus independent of a deficiency of the antirachitic factor, vitamin D (3). Green and Mellanby then undertook studies in rats and reported in 1929–1930 that vitamin A conferred protection against infection (1), as did β-carotene (2).
By the turn of the 21st century, several randomized clinical trials of vitamin A intervention (reviewed in references 4–8) had shown that correcting a deficiency of vitamin A in at-risk populations can improve overall outcomes in terms of reduced morbidity and mortality. In young children, reductions in deaths from measles and diarrheal disease are believed to underlie much of this effect (4, 9–11). Today, dietary vitamin A, acting through its active metabolite, retinoic acid (RA)5, is recognized as an essential factor for normal immune system development and regulation. Whereas many immune cell types are involved in nearly every disease process, T lymphocytes are central to intestinal mucosal immunity. In the past few years, substantial progress has been made in understanding the roles of vitamin A in the regulation of T cell–dependent responses. This review highlights new information regarding vitamin A and RA in T cell differentiation, regulation of immune responses in the intestine, and the antibody response.
RA IN THE REGULATION OF T CELL DIFFERENTIATION
T cells orchestrate a wide variety of immune responses. For a mature but naive T cell to become an effector T cell, the naive cell must receive multiple signals and integrate them effectively. Signals are derived by cell-cell contacts with antigen-presenting cells, including dendritic cells (DCs), macrophages, and B cells, which are themselves regulated in part by vitamin A and RA (12), and by signals delivered by cytokines present in the cell's immediate environment that bind to receptors on the T cell surface and initiate signal transduction events. The concept that T helper (Th) cells can undergo a process of stable differentiation along 2 distinct pathways, leading alternatively to Th1 and Th2 cells, was proposed by Mosmann et al (13) and Mosmann and Coffman (14) in the late 1980s as a framework for understanding discrete patterns of cytokine secretion observed in cloned, activated CD4+ T cells. Further studies showed that when uncommitted CD4+ T cells are activated through the T cell receptor in a microenvironment rich in the proinflammatory cytokine IL-12, which is produced by activated DCs and macrophages in response to infection or inflammation, as well as the cytokine interferon γ, produced by natural killer cells and T cells, they become polarized into “Th1 cells.” The Th1 cells themselves become producers of interferon γ as their “signature cytokine.” Alternatively, when uncommitted CD4+ T cells are activated in a microenvironment rich in IL-4, a cytokine produced by a variety of cell types, they become polarized into “Th2 cells.” These cells then produce IL-4 as their signature cytokine, along with IL-5 and other cytokines. The concept of stably differentiated effector Th1 and Th2 cell subsets stimulated great interest in understanding how pathogens and host environmental factors, including micronutrients, interact to regulate T cell activation and differentiation. Vitamin A deficiency was shown to result in an environment conducive to the differentiation of naive precursor CD4+ T cells into interferon γ–secreting Th1 cells (15, 16). Conversely, vitamin A and RA generally promote differentiation toward Th2 cells and the production of IL-4 and IL-5 (17–21) or increase the ratio of Th2 cytokines relative to Th1 cytokines by reducing the Th1 response (22).
Subsequent molecular studies have shown that the differentiation of Th0 cells into either Th1 or Th2 cells is orchestrated by specific transcription factors. These factors act as master regulators that drive the expression of key genes that, in turn, determine the phenotype of the resulting differentiated T cells. For Th1 cells, the master regulator is T-bet, which, together with another transcription factor, runt-related transcription factor 3, drives the expression of the IFNγ gene and is required for Th1 cell differentiation, while at the same time silencing the IL-4 gene (23). For Th2 cells, the master transcriptional regulator is GATA3, which drives expression of the IL-4 gene and is required for Th2 cell differentiation. Epigenetic changes further stabilize the polarized state of these T cell subsets (24). The cytokines produced by committed Th1 and Th2 cells act in an autocrine-paracrine manner to further regulate local immune responses. In experimental studies, animals or isolated naive T cells treated with appropriate stimuli in the presence of RA have generally expressed higher levels of Th2-associated genes and produced higher amounts of Th2 cytokines, such as IL-4 (19, 20). Alternatively, the relative amount of IL-4 compared with interferon γ or IL-12 may also be elevated as a result of downregulation of Th1 cytokines, as shown in RA-treated immunized mice (22), which also exhibited reduced expression of the Th1 factors T-bet and interferon regulatory factor 1 (22). In a mouse model of autoimmune insulitis (type 1 diabetes), RA reduced the T cell expression of T-bet as well as Signal Transducers and Activators of Transcription 4, a transducer of IL-12 signals. However, the induction of T-regulatory (Treg) cells, discussed below, was also required for suppression of the islet-infiltrating T cells responsible for this autoimmune disorder (25).
Several additional Th cell lineages have now been identified, and others have been proposed (26). A third major subset, the Th17 cell (27, 28), has emerged as playing a major role in the inflammatory response, especially at mucosal barriers where these cells are most abundant and where they function as effector cells against intracellular pathogens (27). Th17 cells also play an important role in the pathology of autoimmune and allergic disorders, and therefore it is important for multiple reasons to understand what controls their differentiation. Th17 cells are induced when T cells are activated in the presence of transforming growth factor β (TGF-β) combined with IL-6, which provides a distinct proinflammatory signal. Th17 cells produce IL-17 as their signature cytokine, as well as IL-23, IL-22, IL-6, TNF-α, and chemokines (27, 29), which help recruit neutrophils to sites where the Th17 cells were initially activated. The Th17 cell master regulators are 2 orphan nuclear receptors, retinoid orphan receptor (ROR) γt and RORα, named for their homology to the nuclear retinoid receptor proteins (28, 30). RA has been shown to oppose Th17 cell commitment (29) whereas, as noted below, RA induces regulatory T cells that are critical for maintaining immune homeostasis and for preventing the induction of autoimmune T cells.
Whereas the rapid induction of effector T cell responses, discussed above, is important for host defense, Th1 and especially Th17 cells can cause excessive activation and tissue damage when their effects are prolonged or are not appropriately attenuated. Another subset of CD4+ Th cells, Treg cells, which also are prominent at mucosal surfaces (27), typically perform a suppressive function. Although Treg cells are functionally and phenotypically diverse, they produce cytokines that modulate inflammatory responses, such as IL-10. These cells are indispensable for self-tolerance and have been shown to suppress autoimmune disorders (31). Whereas “natural” Treg cells develop in the thymus, Treg cells can also develop in nonthymic tissues in response to infection or inflammation; this subset is referred to as induced Treg (iTreg) cells (31, 32) or adaptive Treg cells (33). As noted later, one crucial role for iTreg cells is in the maintenance of intestinal homeostasis, which is required for tolerance to commensal bacteria and food proteins. iTreg cells are marked by their expression of the Forkhead/winged helix family transcription factor forkhead box P3 (Foxp3), which functions as their master regulator and is essential for iTreg formation (28, 29, 31). Where does vitamin A fit in? Within the past few years, it has become recognized that RA is one of the critical factors that provides signals for the differentiation of iTreg cells (33–36). In the presence of TGF-β and adequate amounts of RA, but low IL-6, iTreg differentiation is favored. Moreover, Foxp3 can directly bind to and inhibit the transcriptional activity of RORα and RORγt through protein-protein interactions (27), aiding the induction of iTreg cells. RA also suppresses Th17 cell development by increasing TGF-β signaling and reducing the level of expression of the IL-6 receptor (35). The relative increase in iTreg and the inhibition of Th17 development can shift the balance of these cells toward more effective immune homeostasis. Overall, the balance between RORγt+ Th17 and Foxp3+ iTreg cells and their ability to cross-regulate one another (27, 28, 34) have emerged as crucial factors for normal mucosal immunity.
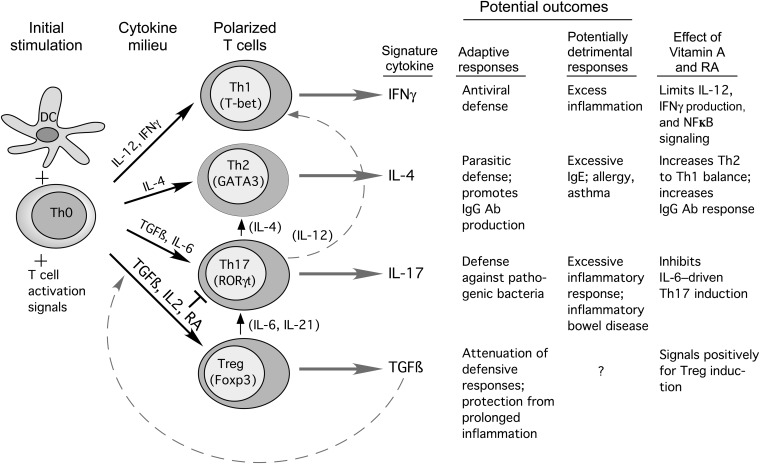
A current model of T cell development and its regulation by RA is shown in Figure 1. Whereas early models suggested that Th cell differentiation resulted in stable, terminally differentiated T cell subsets, there is now increasing evidence for T cell plasticity, ie, the potential for one type of differentiated T cell to develop some of the characteristics of another (26, 37). Plasticity is supported by studies of the epigenetic modification of chromatin, which have shown, for example, that the Foxp3 promoter in some iTreg cells is only partially demethylated, whereas in contrast the Foxp3 promoter in natural thymic Treg cells is fully demethylated, which is indicative of their full commitment to Foxp3 expression (24). Similarly, some iTreg cell histones possess “bivalent” modifications, such lysine methylation patterns, which suggest that the gene is poised for further modification of its expression, rather than having reached a fixed, terminal state (24). Th cell plasticity is thought to be relatively high in the early stages of CD4+ T cell differentiation toward a given Th subset and then reduced as the T cells are further stimulated with polarizing cytokines and become more strongly committed to their particular subset type (26). It has been stated that plasticity of the T cell compartment allows the immune response to be tailored to the local milieu (28). However, the detection of Th cells that express both Foxp3 and RORγt has added complexity and may suggest a kind of “hybrid” T cell or an intermediate stage in T cell differentiation in mucosal tissues. It could also be that iTreg cells can take on some of the characteristics of Th17 cells, albeit with lower IL-17 gene expression, depending on the amount of IL-6 or other factors, including IL-1β, that are present in the local environment (27, 28, 37, 38). In this regard, the ability of the local environment to provide an adequate supply of RA to promote iTreg maintenance, or to be conducive to the conversion of Th17 cells into iTreg cells, could be very important. In current models, RA and IL-6 [and also IL-1β in human T cells (27, 28)] function as binary switches that, on top of the necessary signals provided by TGF-β for both of these differentiation pathways, direct naive T cells along the iTreg pathway or Th17 pathway, respectively (27–29). Whereas there is evidence that iTreg cells can convert to Th17-producing cells, evidence for the reverse is lacking (37). The “reprogramming” or “redifferentiation” of iTreg cells into effector Th cells other than Th17 cells has also been reported (37, 39). Fate mapping studies (40) have shown that a substantial number of IL-4– and interferon γ–producing cells had once expressed Foxp3, which also suggests plasticity over time. It has been cautioned that most studies of T cell plasticity have been conducted at the population level, and thus the programs of differentiation of individual cells need further clarification (37).
FIGURE 1.
Model of T cell differentiation, after contact with antigen-activated DCs, from uncommitted naive T cells into different T cell subsets that produce different cytokines and thus promote different functional activities. Black arrows indicate the cytokines that mainly drive the differentiation to specific subsets. RA together with TGF-β promotes the differentiation of Treg cells, which generally are antiinflammatory, whereas RA also decreases Th1 activity and often promotes Th2 functions (see text). Upward arrows show proposed “plasticity” or interconversion among some of the T cell subsets (see references 26, 37, and 39 for detailed reviews). The signature cytokines and potential outcomes, which also are determined by the environment and type of pathogen or antigen encountered, are listed. Effects of vitamin A or RA differ contextually but generally limit Th1 and Th17 processes while increasing Th2 and Treg-mediated processes. Ab, antibody; DC, dendritic cells; Fox, forkhead; IFNγ, interferon γ; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; RA, retinoic acid; RORγt, retinoid orphan receptor γt; TGFβ, transforming growth factor β; Th, T helper; Treg, T-regulatory.
VITAMIN A IS A NECESSARY FACTOR IN THE HOMING OF T CELLS AND B CELLS IN THE INTESTINE
One way in which a deficiency of vitamin A may increase the risk of morbidity and mortality is through an impaired response to diarrheal infections (4, 7). Vitamin A is necessary for maintaining intestinal integrity (41), regulating mucin gene expression (42), and normal production of intestinal IgA (43). Studies on the status of T cells in the intestine of vitamin A–deficient mice showed that the submucosal lamina propria region was nearly devoid of CD4+ and CD8+ T cells (44). A lack of lamina propria T cells would likely impair the immune response of the intestine to pathogens that have breeched the epithelium. Next, the expression of chemokine (C-C motif) receptor (CCR) 9 and α4β7 integrin on the T cell surface was examined in vitamin A–deficient mice because previous work had shown that the cell-surface chemokine receptor CCR9, involved in T cell migration, and the integrin family adhesion molecule α4β7, which binds to MAdCAM-1 (mucosal addressin cell adhesion molecule 1), are important for appropriate migration of gut-homing T cells. Both molecules were reduced significantly in vitamin A–deficient mice (44). In contrast, RA and an analog of RA that functions as a retinoid nuclear receptor ligand increased the expression of CCR9 and increased T cell chemotactic activity. It was also shown that the mesenteric lymph nodes (MLNs) express the gene for retinal dehydrogenase Raldh1a2 (RALDH2) and that 3H-labeled retinol was metabolized to RA by MLN cells ex vivo (44). These studies provided the first line of evidence that RA is essential for “imprinting” gut-homing specificity on T cells activated by intestinal DCs and suggested that MLN DCs are a source of RA that drives T cell differentiation toward the gut-homing phenotype (45).
Further studies (46) have shown that the iTreg cells formed in the presence of RA are more effective in vivo in suppressing an acute small intestinal inflammation, but they were not more effective in a model of chronic colitis. In other studies, the gut-homing T cells formed in the presence of RA were, after transfer in vivo, more effective than untreated cells in suppressing an acute small intestinal inflammation, but they were not more effective in a model of chronic colitis (47). Thus, high amounts of certain cytokines may overcome the regulatory properties of T cells that are induced by RA. Others have reported finding that the conditions of vitamin A deficiency and vitamin A excess resulted in the induction of different subsets of Foxp3+ T cells, yet, surprisingly, both sets were effective in reversing an intestinal inflammation (48). The differentiation of intestinal mucosal Treg cell responses is initiated by a subset of CD103+ DCs and dependent on TGF-β and RA (49). These results newly identify RA as a cofactor in Treg cell generation, providing a mechanism via which functionally specialized gut-associated lymphoid tissue DCs can extend the repertoire of Treg cells focused on the intestine.
Other studies that have compared MLN cells and cells from peripheral tissues (spleen, cutaneous lymph nodes, or other sites) have also indicated that MLN cells express higher levels of genes likely to be involved in RA metabolism (50). In a study of human CD8+ T cells stimulated with DCs from various sites, T cells cultured with MLN DCs in the presence of RA strongly expressed α4β7 integrin and CCR9 proteins on their surface, whereas incubation with the retinoid receptor antagonist LE540 completely blocked this expression (51). Furthermore, treatment of activated murine CD8+ T cells in vitro with DCs from different tissue sources, with or without RA, resulted in much higher gut-homing of T cells activated in the presence of MLN DCs and RA (51). However, the increased expression of α4β7 and CCR9 seems to require continued treatment with RA, because cells that were first stimulated in the presence of RA and later restimulated in its absence were less effective in a gut-homing migration assay (51). These cells therefore might not be fully imprinted but rather plastic in their phenotype.
Oral tolerance to foreign antigens requires a form of immune suppression. The relation between vitamin A and oral tolerance has been investigated in only a few studies, and the results at present are intriguing but complex. Chang et al (52) reported no differences between vitamin A–adequate and vitamin A–deficient mice in response to oral challenge with ovalbumin, a nonself protein, followed by systemic immunization with the same protein. It was reported that depletion of CCR7+ langerin+ DCs, which were shown to express RALDH2, also resulted in reduced oral tolerance to ovalbumin. This suggests these DCs are important for the immune suppression that is necessary for tolerance to ingested antigens. MLN DCs from vitamin A–deficient mice, when cocultured with T cells, generated fewer Foxp3+ iTreg cells and more IL-17–producing T cells, compared with cocultures with MLN DCs from control mice (52). Another study used mice carrying an IL-15 transgene in their DCs, as a model of celiac disease (gluten enteropathy) in humans in which IL-15 expression is elevated, and evaluated whether RA exerted an anti- or proinflammatory effect on isolated cells and in intact mice (53). In the presence of IL-15, RA produced a more inflammatory response, shown by fewer Foxp3+ iTreg cells and a higher production of IL-12p70 and interferon γ, indicative of an inflammatory Th1 cell response. Both RA and an agonist selective for binding to the nuclear receptor RAR increased c-Jun terminal kinase signaling, which is also consistent with a proinflammatory response. In contrast, the elimination of the RARα receptor prevented the expression of interferon γ and IL-12p70 proteins in DCs cultured ex vivo. RA also synergized with IL-15 in intact mice to increase the proinflammatory response to gliadin feeding (53). The results of these 2 studies suggest that whether RA promotes or impairs tolerance to exogenous proteins in the intestine may depend exquisitely on the cytokine environment. It can also be inferred that when RA is used for therapeutic purposes, it should be used cautiously in subjects with various inflammatory bowel conditions and sensitivities to dietary antigens.
OTHER ACTIONS OF VITAMIN A AND RA ON T CELL–DEPENDENT IMMUNITY
RA is also an important factor in the response to immunization, which requires collaboration among antigen-presenting cells, T cells, and B cells. Retinoids apparently have opposing effects on T cell and B cell proliferation, limiting the former but stimulating the latter (21, 54–57). Several reports have shown that vitamin A deficiency results in a poor response to immunization, with generally low antibody responses to immunization with T cell–dependent antigens (58, 59). The IgA response was lower and the IgG response elevated in a mouse model of viral infection, but nonetheless both responses were dissimilar to the normal response of vitamin A–adequate mice (60). In various animal models, vitamin A deficiency causes abnormalities in nearly all of the lymphocyte populations examined (61–63). RA affects numerous B cell processes, both in isolated B cells in vitro and in intact animals, and it interacts with other costimulatory signals, such as cytokines and adjuvants, in a manner that is additive or synergistic (21). Several aspects of the B cell response that are influenced by all-trans-RA are as follows: 1) germline-immunoglobulin gene transcription (56); 2) expression of coreceptor molecules necessary for B and T cell activation (22); 3) rate of cell proliferation as noted above; 4) expression of enzymes such as activation-induced cytidine deaminase (56, 57), an enzyme essential for antibody diversification by class switching and somatic hypermutation (64); 5) formation of the germinal center in which class switching and affinity maturation of antibodies take place (65); and 6) expression of B cell surface markers indicative of plasma cell development (57). In studies conducted in adult and neonatal mice, the antibody titers produced after immunization with tetanus toxoid, a classical T cell–dependent antigen, were higher when RA was administered orally at the time of priming (first immunization). Moreover, the recall response to a second immunization several weeks later was much higher in animals that had received RA with their initial antigen priming (22, 66). This may represent another form of “imprinting,” in which memory T and B cells that developed in the presence of RA during the initial response to immunization continue to express differentiated characteristics that permit them to produce a more robust secondary antibody response when they are later stimulated with antigen alone. The results of these studies are relevant to understanding the role of vitamin A in the humoral antibody response to immunization, which is the hallmark of successful vaccination.
THE FIELD CONTINUES TO MOVE AHEAD RAPIDLY
The keen interest in retinoids and their ability to interact with growth factors and cytokines to regulate immune cell differentiation continues, and several interesting reports have appeared since this conference. A few highlights include a study that showed that TGF-β and RA induce the expression of a microRNA, miR-10a, in Treg cells, and that an outcome of this induction is reduction of the transcriptional repressor Bcl-6 and the corepressor N-Cor, which functions with a number of nuclear receptors (67). The expression of miR-10a was shown to maintain the pool of inducible Treg cells and to limit the production of Th17 cells. It also was reported recently that CYP26b1, a cytochrome P450 implicated in RA catabolism, is expressed in antigen-experienced CD44+ T cells. Whereas RA induced CYP26b1 and limited the RA-induced expression of the gut-homing molecule CCR9, the presence of TGF-β limited the expression of CYP26b1 (68). This would be expected to maintain or prolong higher RA concentrations in the cells, although RA concentrations have yet to be measured experimentally. Overall, new pathways are beginning to come into focus through which the RA signal is regulated, which may in turn influence the balance of T cell types and their tissue-homing ability. Other articles not cited here have also contributed recently to the understanding of regulatory lymphocyte populations, and more are expected in the future.
QUESTIONS FOR FUTURE RESEARCH
It is now understood that vitamin A through RA can alter the levels of expression of several hundred genes (50), thus regulating numerous physiologic processes. The discovery that vitamin A has a major impact on intestinal immunity adds to this understanding, and it also suggests important questions for future research. Vitamin A deficiency is an extreme condition, but marginal vitamin A status is relevant in many parts of the world. Does the immune system adapt to marginal vitamin A status, and if so how? Is marginal vitamin A deficiency a risk factor for poor response to infection, or poor tolerance to commensal organisms, or allergic responses to food proteins? As noted elsewhere in this supplement, vitamin A supplementation programs for children and women have been promoted by the WHO and instituted in numerous participating countries (69). Does a large, bolus dose of vitamin A differ in its effect from that of a continuous, adequate dietary intake of vitamin A? Systemic immunity is also affected by vitamin A status, and similar questions about the sources of vitamin A and metabolic processes that generate RA in immune system microenvironments are also relevant. Recent studies have begun to shed light on RA synthesis and degradation pathways in immune system cells (44, 70). As of yet, few studies have addressed vitamin A and immunity in the very young or the elderly, and yet infants, young children, and the elderly are the populations most vulnerable to infections and may have low vitamin A status. β-Carotene metabolism may also play a role, and it is interesting to note that duodenal infusion of β-carotene results in higher concentrations of RA in the portal vein (71). This suggests that the mesenteric-splanchnic region may be a significant source of RA in the immediate postprandial period. RA is also a drug, and the impact of pharmacologic doses of RA on intestinal immune function needs to be further evaluated.
Other potential physiologic sources of vitamin A for RA production, both in the intestine and periphery, could include plasma retinol bound to retinol-binding protein (RBP) and chylomicron remnants. If RBP is involved, are there receptors for RBP, such as Stra6 (stimulated by retinoic acid 6) (72), on gut immune cells? Are there chylomicron remnant receptors on antigen-presenting cells that intercept newly absorbed chylomicron retinyl esters as a source of retinol for production of RA, as suggested by studies of bone marrow and blood leukocytes (73)? A challenge for the future is to better understand the connections between vitamin A nutrition, vitamin A metabolism, and immune function in contexts that are relevant to human nutrition. Future experimental studies that combine good animal models studied under conditions of dietary control with broad-based, systems-based informatics approaches are likely to better define the complex influence that vitamin A and RA have on immune system regulation, homeostasis, and response to infectious disease.
Acknowledgments
The author did not declare any conflicts of interest.
Footnotes
Abbreviations used: CCR, chemokine (C-C motif) receptor; DC, dendritic cell; Foxp3, forkhead box P3; iTreg, induced T-regulatory; MLN, mesenteric lymph node; RA, retinoic acid; RBP, retinol-binding protein; ROR, retinoid orphan receptor; TGF-β, transforming growth factor β; Th, T helper; Treg, T-regulatory.
REFERENCES
- 1.Green HN, Mellanby E. Vitamin A as an anti-infective agent. BMJ 1928;2:691–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green HN, Mellanby E. Carotene and vitamin A: the anti-infective action of carotene. Br J Exp Pathol 1930;11:81–9 [Google Scholar]
- 3.Mellanby E. Nutrition and disease: the interaction of clinical and experimental work. Edinburgh, United Kingdom: Oliver and Boyd, 1934 [Google Scholar]
- 4.Beaton GH, Martorell R, Aronson KA, Edmonston B. McCabe, G, Ross, AC, Harvey, B. Vitamin A supplementation and child morbidity and mortality in developing countries. Food Nutr Bull 1994;15(4):282–9 [Google Scholar]
- 5.Semba RD. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc Nutr Soc 1999;58:719–27 [DOI] [PubMed] [Google Scholar]
- 6.Semba RD. The vitamin A and mortality paradigm: past, present, and future. Scand J Nutr 2001;45:46–50 [Google Scholar]
- 7.Bhutta ZA, Ahmed T, Black R, Cousens S, Dewey K, Giugliani E, Haider BA, Kirkwood B, Morris SS, Sachdev HP, et al. ; Maternal and Child Undernutrition Study Group What works? Interventions for maternal and child undernutrition and survival. Lancet 2008;371:417–40 [DOI] [PubMed] [Google Scholar]
- 8.Sommer A, Vyas KS. A global clinical view on vitamin A and carotenoids. Am J Clin Nutr 2012;96:1204S–6S [DOI] [PubMed] [Google Scholar]
- 9.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr 2001;21:167–92 [DOI] [PubMed] [Google Scholar]
- 10.Villamor E, Fawzi WW. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev 2005;18:446–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Progress in reducing global measles deaths: 1999-2004. Wkly Epidemiol Rec 2006;81:90–4 [PubMed] [Google Scholar]
- 12.Duriancik DM, Lackey DE, Hoag KA. Vitamin A as a regulator of antigen presenting cells. J Nutr 2010;140:1395–9 [DOI] [PubMed] [Google Scholar]
- 13.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986;136:2348–57 [PubMed] [Google Scholar]
- 14.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989;17:145–73 [DOI] [PubMed] [Google Scholar]
- 15.Cantorna MT, Nashold FE, Hayes CE. Vitamin A deficiency results in a priming environment conducive for Th1 cell development. Eur J Immunol 1995;25:1673–9 [DOI] [PubMed] [Google Scholar]
- 16.Smith SM, Levy NL, Hayes CE. Impaired immunity in vitamin A deficient mice. J Nutr 1987;117:857–65 [DOI] [PubMed] [Google Scholar]
- 17.Hoag KA, Nashold FE, Goverman J, Hayes CE. Retinoic acid enhances the T helper 2 cell development that is essential for robust antibody responses through its action on antigen-presenting cells. J Nutr 2002;132:3736–9 [DOI] [PubMed] [Google Scholar]
- 18.Stephensen CB, Rasooly R, Jiang X, Ceddia MA, Weaver CT, Chandraratna RA, Bucy RP. Vitamin A enhances in vitro Th2 development via retinoid X receptor pathway. J Immunol 2002;168:4495–503 [DOI] [PubMed] [Google Scholar]
- 19.Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress T(h)1 development and enhance T(h)2 development via retinoic acid receptors. Int Immunol 2003;15:1017–25 [DOI] [PubMed] [Google Scholar]
- 20.Stephensen CB, Jiang X, Freytag T. Vitamin A deficiency increases the in vivo development of IL-10-positive Th2 cells and decreases development of Th1 cells in mice. J Nutr 2004;134:2660–6 [DOI] [PubMed] [Google Scholar]
- 21.Ross AC, Chen QYM. Augmentation of antibody responses by retinoic acid and costimulatory molecules. Semin Immunol 2009;21:42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Y, Chen Q, Ross AC. Retinoic acid and polyriboinosinic:polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J Immunol 2005;174:7961–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol 2007;8:145–53 [DOI] [PubMed] [Google Scholar]
- 24.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol 2009;9:91–105 [DOI] [PubMed] [Google Scholar]
- 25.Van YH, Lee WH, Ortiz S, Lee MH, Qin HJ, Liu CP. All-trans retinoic acid inhibits type 1 diabetes by T regulatory (Treg)-dependent suppression of interferon-gamma-producing T-cells without affecting Th17 cells. Diabetes 2009;58:24–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Locksley RM. Nine lives: plasticity among T helper cell subsets. J Exp Med 2009;206:1643–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weaver CT, Hatton RD. Interplay between the Th17 and Treg cell lineages: a (co)evolutionary perspective. Nat Rev Immunol 2009;9:883–9 [DOI] [PubMed] [Google Scholar]
- 28.Ziegler SF, Buckner JH. FOXP3 and the regulation of Treg/Th17 differentiation. Microbes Infect 2009;11:594–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol 2010;40:1830–5 [DOI] [PubMed] [Google Scholar]
- 30.Eberl G, Littman DR. The role of the nuclear hormone receptor RORgammat in the development of lymph nodes and Peyer's patches. Immunol Rev 2003;195:81–90 [DOI] [PubMed] [Google Scholar]
- 31.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010;10:490–500 [DOI] [PubMed] [Google Scholar]
- 32.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation induced by retinoic acid. Science 2007;317:256–60 [DOI] [PubMed] [Google Scholar]
- 33.Benson MJ, Pino-Lagos K, Rosemblatt N, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation and homing in the face of high levels of co-stimulation. J Exp Med 2007;204:1765–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mucida D, Park Y, Cheroutre H. From the diet to the nucleus: vitamin A and TGF-β join efforts at the mucosal interface of the intestine. Semin Immunol 2009;21:14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL23 receptor expression. J Immunol 2008;181:2277–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takaki H, Ichiyama K, Koga K, Chinen T, Takaesu G, Sugiyama Y, Kato S, Yoshimura A, Kobayashi T. STAT6 inhibits TGF-β1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. J Biol Chem 2008;283:14955–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou L, Chong MMW, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity 2009;30:646–55 [DOI] [PubMed] [Google Scholar]
- 38.Koenen HJ, Smeets RL, Vink PM, van Rijsses E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood 2008;112:2340–52 [DOI] [PubMed] [Google Scholar]
- 39.Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh T-Y, Watford WT, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity 2009;30:155–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, Bluestone JA. Selective miRNA disruption of T reg cells leads to uncontrolled autoimmunity. J Exp Med 2008;205:1983–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziegler TR, Evans ME, Fernández-Estívariz C, Jones DP. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr 2003;23:229–61 [DOI] [PubMed] [Google Scholar]
- 42.Gray T, Koo JS, Nettesheim P. Regulation of mucous differentiation and mucin gene expression in the tracheobronchial epithelium. Toxicology 2001;60:35–46 [DOI] [PubMed] [Google Scholar]
- 43.Gangopadhyay NN, Moldoveanu Z, Stephensen CB. Vitamin A deficiency has different effects on immunoglobulin A production and transport during influenza A infection in BALB/c mice. J Nutr 1996;126:2960–7 [DOI] [PubMed] [Google Scholar]
- 44.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2004;21:527–38 [DOI] [PubMed] [Google Scholar]
- 45.Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin Immunol 2009;21:8–13 [DOI] [PubMed] [Google Scholar]
- 46.Menning A, Loddenkemper C, Westendorf AM, Szilagyi B, Buer J, Siewert C, Hamann A, Huehn J. Retinoic acid-induced gut tropism improves the protective capacity of Treg in acute but not in chronic gut inflammation. Eur J Immunol 2010;40(9):2539–48 [DOI] [PubMed] [Google Scholar]
- 47.Menning A, Loddenkemper C, Westendorf AM, Szilagyi B, Buer J, Siewert C, Hamann A, Huehn J. Retinoic acid-induced gut tropism improves the protective capacity of Treg in acute but not in chronic gut inflammation. Eur J Immunol 2010;40:2539–48 [DOI] [PubMed] [Google Scholar]
- 48.Kang SG, Wang C, Matsumoto S, Kim CH. High and low vitamin A therapies induce distinct FoxP3+ T-cell subsets and effectively control intestinal inflammation. Gastroenterology 2009;137:1391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coombes JL, Siddiqui KRR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β- and retinoic acid-dependent mechanism. J Exp Med 2007;204:1757–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol 2006;66:606–30 [DOI] [PubMed] [Google Scholar]
- 51.Eksteen B, Mora JR, Haughton EL, Henderson NC, Lee-Turner L, Villablanca EJ, Curbishley SM, Aspinall AI, von Andrian UH, Adams DH. Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology 2009;137:320–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang S-Y, Cha H-R, Chang J-H, Ko H-J, Yang H, Malissen B, Iwata M, Kweon M-N. Lack of retinoic acid leads to increased langerin-expressing dendritic cells in gut-associated lymphoid tissue. Gastroenterology 2010;138:1468–78 [DOI] [PubMed] [Google Scholar]
- 53.DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, Marietta EV, Kasarda DD, Waldmann TA, Murray JA, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature 2011;471:220–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ertesvåg A, Naderi S, Blomhoff HK. Regulation of B cell proliferation and differentiation by retinoic acid. Semin Immunol 2009;21:36–41 [DOI] [PubMed] [Google Scholar]
- 55.Naderi S, Blomhoff HK. Retinoic acid prevents phosphorylation of pRB in normal human B lymphocytes: regulation of cyclin E, cyclin A, and p21(Cip1). Blood 1999;94:1348–58 [PubMed] [Google Scholar]
- 56.Chen Q, Ross AC. Vitamin A and immune function: retinoic acid modulates population dynamics in antigen receptor and CD38-stimulated splenic B cells. Proc Natl Acad Sci USA 2005;102:14142–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Q, Ross AC. Retinoic acid promotes mouse splenic B cell surface IgG expression and maturation stimulated by CD40 and IL-4. Cell Immunol 2007;249:37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pasatiempo AM, Kinoshita M, Taylor CE, Ross AC. Antibody production in vitamin A-depleted rats is impaired after immunization with bacterial polysaccharide or protein antigens. FASEB J 1990;4:2518–27 [DOI] [PubMed] [Google Scholar]
- 59.Sankaranarayanan S, Ma Y, Bryson MS, Li N-Q, Ross AC. Neonatal-age treatment with vitamin A delays postweaning vitamin A deficiency and increases the antibody response to T-cell dependent antigens in young adult rats fed a vitamin A-deficient diet. J Nutr 2007;137:1229–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stephensen CB, Moldoveanu Z, Gangopadhyay NN. Vitamin A deficiency diminishes the salivary immunoglobulin A response and enhances the serum immunoglobulin G response to influenza A virus infection in BALB/c mice. J Nutr 1996;126:94–102 [DOI] [PubMed] [Google Scholar]
- 61.Twining SS, Schulte DP, Wilson PM, Fish BL, Moulder JE. Vitamin A deficiency alters rat neutrophil function. J Nutr 1997;127:558–65 [DOI] [PubMed] [Google Scholar]
- 62.Zhao Z, Ross AC. Retinoic acid repletion restores the number of leukocytes and their subsets and stimulates natural cytotoxicity in vitamin A–deficient rats. J Nutr 1995;125:2064–73 [DOI] [PubMed] [Google Scholar]
- 63.Kuwata T, Wang I-M, Tamura T, Ponnamperuma RM, Levine R, Holmes KL, Morse HC, III, DeLuca LM, Ozato K. Vitamin A deficiency in mice causes a systemic expansion of myeloid cells. Blood 2000;95:3349–56 [PubMed] [Google Scholar]
- 64.Cattoretti G, Buttner M, Shaknovich R, Kremmer E, Alobeid B, Niedobitek G. Nuclear and cytoplasmic AID in extrafollicular and germinal center B cells. Blood 2006;107:3967–75 [DOI] [PubMed] [Google Scholar]
- 65.Ma Y, Ross AC. Toll-like receptor 3 ligand and retinoic acid enhance germinal center formation and increase the tetanus toxoid vaccine response. Clin Vaccine Immunol 2009;16:1476–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma Y, Ross AC. The anti-tetanus immune response of neonatal mice is augmented by retinoic acid combined with polyriboinosinic:polyribocytidylic acid. Proc Natl Acad Sci USA 2005;102:13556–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takahashi H, Kanno T, Nakayamada S, Kirahar K, Sciumé G, Muljo SA, Kuchen S, Casellas R, Wei L, Kanno Y, et al. TGF-β and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nat Immunol 2012;13:587–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takeuchi H, Yokoto A, Ohoka Y, Iwata M. Cyp26b1 regulates retinoic acid-dependent signals in T cells and its expression is inhibited by transforming growth factor β. PLoS ONE 2011;6:e16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Expanded Programme on Immunization. EPI Global Advisory Group Meeting E-G. Programmes for the control of vitamin A deficiency: the role of the EPI in new initiatives for the 1990's. World Health Organization 1988;October:1–23.
- 70.Molenaar R, Knippenberg M, Goverse G, Olivier BJ, deVos AF, O'Toole T, Mebius RE. Expression of retinaldehyde dehydrogenase enzymes in mucosal dendritic cells and gut-draining lymph node stromal cells is controlled by dietary vitamin A. J Immunol 2011;186:1934–42 [DOI] [PubMed] [Google Scholar]
- 71.Wang XD, Russell RM, Marini RP, Tang G, Dolnikowski GG, Fox JG, Krinsky NI. Intestinal perfusion of beta-carotene in the ferret raises retinoic acid level in portal blood. Biochim Biophys Acta 1993;1167:159–64 [DOI] [PubMed] [Google Scholar]
- 72.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 2007;315:820–5 [DOI] [PubMed] [Google Scholar]
- 73.Skrede B, Olafsdottir AE, Blomhoff R, Norum KR. Retinol and retinyl esters in rabbit bone marrow and blood leukocytes. Scand J Clin Lab Invest 1993;53:515–9 [DOI] [PubMed] [Google Scholar]