Abstract
The beneficial effects of a high intake of tomatoes and tomato products on the risk of certain chronic diseases have been presented in many epidemiologic studies, with the suggestion that lycopene (a major carotenoid in tomatoes) is a micronutrient with important health benefits. Within the past few years, we have gained greater knowledge of the metabolism of lycopene and the biological effects of lycopene derivatives. In particular, the characterization and study of β-carotene 9′,10′-oxygenase has shown that this enzyme can catalyze the excentric cleavage of both provitamin and non–provitamin A carotenoids to form apo-10′-carotenoids, including apo-10′-lycopenoids from lycopene. This raised an important question of whether the effect of lycopene on various cellular functions and signaling pathways is a result of the direct actions of intact lycopene or its derivatives. Several reports, including our own, support the notion that the biological activities of lycopene can be mediated by apo-10′-lycopenoids. More research is clearly needed to identify and characterize additional lycopene metabolites and their biological activities, which will potentially provide invaluable insights into the mechanisms underlying the effects of lycopene in humans.
INTRODUCTION
The most abundant carotenoids in human plasma include β-carotene, α-carotene, β-cryptoxanthin, lutein, zeaxanthin, and lycopene. These 6 major carotenoids account for ∼70% of all carotenoids identified in human plasma and tissues. Carotenoids are divided into 2 major groups: xanthophylls, which are oxygenated carotenoids that include lutein, zeaxanthin, and β-cryptoxanthin, and carotenes, which are hydrocarbon carotenoids that are either cyclized, such as α-carotene and β-carotene, or linear, such as lycopene (1). More recently, lycopene has attracted considerable attention because of its association with a decreased risk of certain chronic diseases, including cardiovascular diseases and cancers (2). Considerable efforts have been expended to identify its biological and physiochemical properties. Relative to β-carotene, lycopene has the same molecular mass and chemical formula, yet lycopene is an open-polyene chain lacking the β-ionone ring structure. Whereas the metabolism of β-carotene has been extensively studied, the absorption, transport, metabolism, and biological activities of lycopene remain undefined. Within the past few years we have gained greater knowledge of the biological effects of lycopene and its derivatives (3, 4); apo-lycopenoids, which are lycopene derivatives (5), are formed when the carbon skeleton is shortened by the removal of fragments from one or both ends of the lycopene with the position of the point of cleavage indicated, eg, apo-10′-lycopenal from lycopene. The cleavage of lycopene with a long-chain of conjugated double bonds by autooxidation, radical-mediated oxidation, and singlet oxygen has been documented; however, the importance of such products remains poorly understood (6). Recently, the characterization and study of β-carotene 9′,10′-oxygenase (BCO2)6 have shown that this enzyme can catalyze the excentric cleavage of both provitamin and non–provitamin A carotenoids to form apo-10′-carotenoids including apo-10′-lycopenoids from lycopene (7, 8). Importantly, these metabolites possess specific and nonspecific biological activities in both in vitro and in vivo systems (9). In this review, the metabolic pathway of lycopene and the potential biological actions of lycopene metabolites will be highlighted.
LYCOPENE ABSORPTION AND TRANSPORT
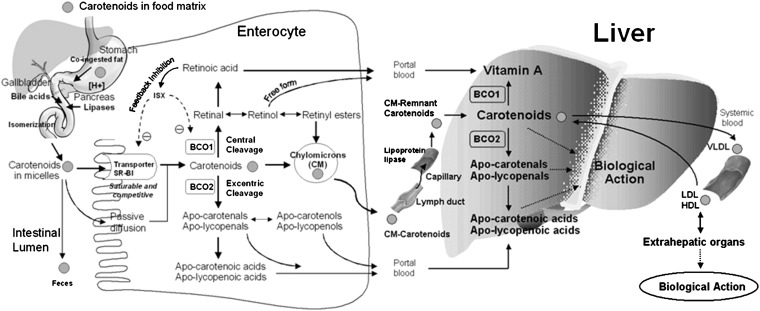
Humans absorb a significant portion of intact lycopene directly, and it circulates through and accumulates in their plasma, liver, and peripheral tissues. The half-life of plasma carotenoids ranges from ≤12 d for β-carotene, α-carotene, and cryptoxanthin to 12–33 d for lycopene and 33–61 d for zeaxanthin and lutein (10). In terms of absorption, β-carotene, not lycopene and other carotenoids, has been the most extensively studied. Although further investigation of the absorption of individual carotenoids and their cis isomers is needed, the factors that influence the absorption of β-carotene may affect lycopene similarly (Figure 1). Similar to other carotenoids, tomato carotenoids, including lycopene, phytoene, and phytofluene, are embedded in their food matrix and cannot be absorbed efficiently. Food processing and cooking, which causes the mechanical breakdown of the food matrix and the release of carotenoids, can improve their intestinal absorption. After release from the food matrix, ingested carotenoids must be emulsified and solubilized into micelles before they are absorbed into the intestinal mucosa (Figure 1). Although previous research assumed that the process of carotenoid absorption occurs via passive diffusion, recent studies indicate the involvement of an active process for the uptake of carotenoids via the scavenger receptor class B type 1 protein (SR-B1) transporter (11). SR-B1 is found in the small intestine of humans as well as the in the liver, adrenals, ovaries, placenta, kidneys, prostate, and brain. Therefore, SR-B1 may be partially responsible for the transport of carotenoids from lipoprotein to tissues and from tissues to lipoproteins (12). A diet-responsive regulatory network involving the intestine-specific homeobox (ISX) transcription factor is shown to regulate intestinal β-carotene uptake and vitamin A production via a negative feedback regulatory mechanism (11). ISX repressed both expression of intestinal β-carotene 15,15′-oxygenase (BCO1) (13) and SR-B1 (14), which facilitates the absorption of dietary lipids and carotenoids (15). Because ISX is under the control of retinoic acid and retinoic acid receptor (RAR)–dependent mechanisms, during vitamin A insufficiency both BCO1 and SR-B1 expression are induced to increase absorption and conversion of β-carotene to vitamin A (Figure 1). Cleavage of β-carotene by BCO1 produces retinal, which can be further oxidized to retinoic acid. Retinoic acid induces the expression of the ISX transcription factor and then represses expression of both BCO1 and SR-B1, which completes the dietary feedback mechanism (Figure 1). Another protein, CD36, a surface membrane glycoprotein in the duodenum and jejunum involved in the uptake of long-chain fatty acids and oxidized LDLs, might also play a role in the movement of carotenoids into cells. A recent study has shown that CD36 is involved in lycopene and lutein uptake by adipose tissue (16).
FIGURE 1.
Simplified schematic illustration of absorption, metabolism, and transport of carotenoids. Adapted with permission from reference 3. BCO1, β-carotene 15,15′-oxygenase; BCO2, β-carotene-9′,10′-oxygenase; ISX, intestine-specific homeobox; SR-B1, scavenger receptor class B type 1.
After carotenoids are taken up by the mucosa of the small intestine, they are either cleaved by BCO1 and/or BCO2 into vitamin A and other metabolites or packaged into chylomicrons and secreted into the lymphatic system for transport to the liver and other peripheral tissues (Figure 1). Some polar metabolites can be directly transported into the liver via the portal blood system (17). It seems that the differential absorption of carotenoids and their metabolites into lymph or portal blood is dependent on the polarity of the metabolites involved. Chylomicrons in the bloodstream are partially degraded by lipoprotein lipase, which leaves chylomicron remnants that are quickly taken up by the liver (Figure 1). Some carotenoids may be released from these lipoproteins and taken up directly by extrahepatic tissues. In the fed state, the liver stores or secretes the carotenoids in VLDL and LDL. In the fasting state, plasma carotenes are found mainly in LDL. Xanthophylls (lutein, zeaxanthin, and β-cryptoxanthin) are located mainly in both LDL and HDL, with small amounts located in VLDL. LDL transport accounts for ∼55%, HDL for 31%, and VLDL for 14% of total blood carotenoids. Specific factors that regulate tissue uptake, recycling of carotenoids back to the liver, and excretion are not yet understood (18).
LYCOPENE METABOLISM
Carotenoids such as β-carotene, α-carotene, and β-cryptoxanthin are cleaved symmetrically at their central double bond by BCO1 (19–21) and are present in several mouse and human tissues (eg, liver, kidney, intestinal tract, and testis) (22, 23). Purified recombinant human BCO1 enzyme cleaves β-carotene in vitro with a Michaelis constant (Km) and Vmax of 7 μmol/L and 10 nmol retinal/mg × min, respectively (24). Retinal formed from β-carotene can be subsequently reduced to retinol or oxidized further to form retinoic acid (Figure 1). Non–provitamin A carotenoids, such as lycopene, were cleaved by purified recombinant murine and ferret BCO1 with much lower or no activity (22, 25, 26). Four conserved histidines and one conserved glutamate residue are essential for the catalytic mechanism of BCO1, presumably for the coordination of the iron cofactor required for catalytic activity (27). Chicken BCO1 showed substrate specificity toward a broad array of carotenoid substrates, including α-carotene, β-carotene, γ-carotene, β-cryptoxanthin, apo-4′-carotenal, and apo-8′-carotenal (26). In light of this evidence, it appears that the presence of at least one unsubstituted β-ionone ring is sufficient for catalytic cleavage of the central carbon 15,15′ double bond.
Building on evidence that the excentric cleavage of β-carotene leads to a series of homologous carbonyl cleavage products (28–30), the existence of this pathway was confirmed by the molecular identification of BCO2 in mice, humans, zebra fish, and ferrets (7, 8). BCO2 shares overall sequence homology with BCO1 and the same conserved pattern of histidine residues and glutamate residues presumably involved in binding the iron cofactor in both proteins (7, 27). BCO2, a mitochondrial enzyme (31), is highly expressed in the liver and testis, and at lower amounts in the kidney, lung, heart, spleen, prostate, intestine, stomach, colon, and brain (7, 8). Recombinant ferret BCO2 cleaves all-trans β-carotene to form β-apo-10′-carotenal in a pH- and time-dependent linear manner with a pH optimum of between 8.0 and 8.5. The reaction exhibited Michaelis-Mention kinetics, with an estimated Km of 3.5 ± 1.1 μmol/L for β-carotene and a Vmax of 32.2 ± 2.9 pmol β-apo-10′-carotenal · mg−1 · h−1. β-Apo-carotenals can be cleaved further by BCO1 to produce retinol and retinoic acid (32, 33) or oxidized to their corresponding apo-β-carotenoic acids (Figure 1). Apo-β-carotenoic acids may then undergo a process similar to β-oxidation of fatty acids, until the further oxidation is blocked by the methyl group at the C13 position (34). This shortening produces retinoic acid from β-carotene (34). β-Apo-12′-carotenal and β-apo-10′-carotenal were isolated from ferret intestinal mucosa after perfusion of β-carotene in vivo (35, 36) and β-apo-8′-carotenal was detected in humans given an oral dose of all-trans [10,10′,11,11′-14C]-β-carotene (37). More recently, underlycopenoids, including apo-6-, apo-8′-, apo-10′-, apo-12′-, and apo-14′-lycopenal, were detected in the plasma of humans who had consumed tomato juice (38) Although the exact contribution of BCO2 in vitamin A biosynthesis remains unknown (39), recent data show that the mutation in the bovine BCO2 gene results in increased adipose, serum, and milk β-carotene concentrations and decreased liver retinol (40, 41).
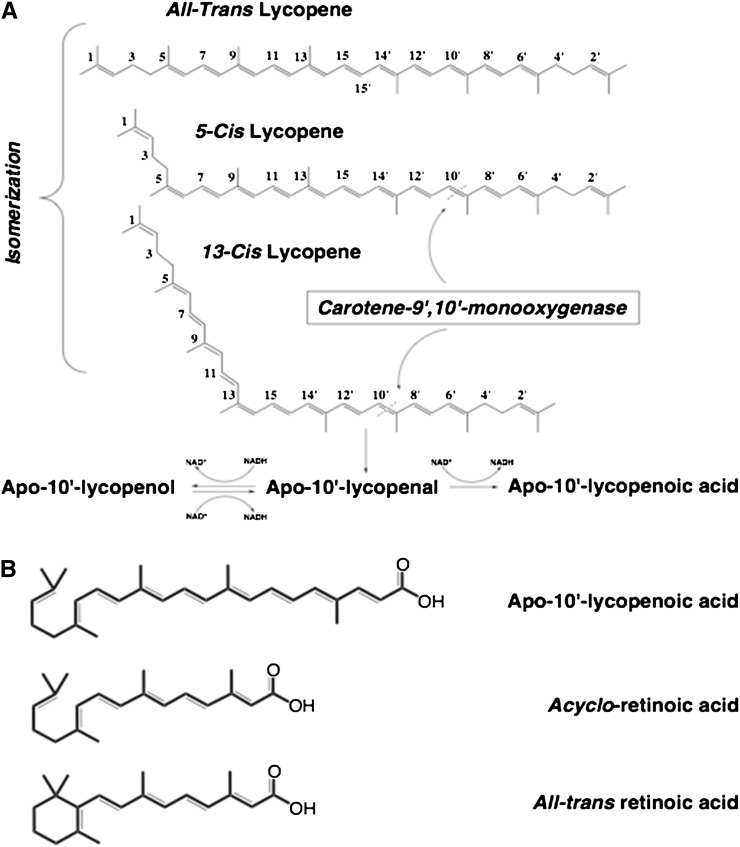
Whereas BCO1 catalyzes the cleavage of provitamin A carotenoids with much greater activity than non–provitamin A carotenoids, the activity of BCO2 is higher toward non–provitamin A carotenoids, such as cis-lycopene isomers, lutein, and zeaxanthin, than toward β-carotene as a substrate (8, 42). Interestingly, the recombinant ferret BCO2 catalyzes the excentric cleavage of cis-lycopene isomers effectively but not all-trans lycopene at the 9′,10′ double bond (Figure 2) (8). In addition, cis-lycopene may act as a better substrate than all-trans β-carotene for the ferret BCO2 (8, 43). The mechanism whereby ferret BCO2 preferentially cleaves the 5-cis and 13-cis isomers of lycopene into apo-10′-lycopenal but not all-trans lycopene is currently unknown. One possible explanation is that the chemical structure of cis isomers of lycopene could mimic the ring structure of the β-carotene molecule and fit into the substrate-enzyme binding pocket. Although this hypothesis warrants further investigation, the observation that supplementation of all-trans lycopene results in a significant increase in cis-lycopene tissue concentration in ferrets underlies the significance of this observation (44, 45). Recently, it has been shown that the non–provitamin A carotenoids including lutein and zeaxanthin are preferentially cleaved over provitamin A carotenoids, indicating a key role of BCO2 in non–provitamin A carotenoid metabolism (8, 42). This provides strong biochemical evidence supporting the recent genetic evidence that accumulation of the xanthophylls lutein and zeaxathin in adipose tissue and skin are due to mutations in the BCO2 gene (46, 47). Recent animal genetic reports have provided evidence of broad substrate specificity of BCO2. Bovine BCO2 was shown to contain a single nucleotide polymorphism resulting in a truncated and presumably nonfunctional BCO2 protein (40, 41). In Norwegian white sheep (Ovis aries), a nonsense mutation in the BCO2 gene was significantly associated with a yellow adipose phenotype (46). In chickens, a yellow skin phenotype is associated with a single nucleotide polymorphism in the BCO2 gene (47). The decrease in skin BCO2 leads to the yellow skin pigmentation of domestic chickens, suggesting a decreased ability to cleave the xanthophylls lutein and zeaxanthin, which are the major accumulated carotenoids in chicken skin (48). Considering the possible beneficial effects of lycopene, lutein, and zeaxanthin in human health, enzymatic cleavage of non–provitamin A carotenoids by BCO2 represents a new avenue of research regarding vertebrate carotenoid metabolism and biological function.
FIGURE 2.
A, B: Proposed metabolic pathway and chemical structures of lycopene found in human plasma and tissues. Cleavage of cis-lycopene by BCO2 may occur at either the 9,10 or 9′,10′ double bond to produce apo-10′-lycopenal, which can be oxidized to apo-10′-lycopenoic acid or reduced to apo-10′-lycopenol. Adapted with permission from reference 43. BCO2, β-carotene 9′,10′-oxygenase.
However, the identification of enzymatic metabolites of lycopene in vivo is challenging. Previously, Khachik et al (49) identified a group of lycopene oxidative products, 2,6-cyclolycopene-1,5-diol A and B, in human serum and many other tissues, including prostate, lung, and colon. Recently, a series of apo-lycopenals, including apo-10′-lycopenal, have been identified in human plasma (38), although it is unknown whether these metabolites are enzymatic cleavage products or the products of chemical oxidative cleavage of lycopene. In animal studies, among several metabolites detected in lung tissue of these lycopene-supplemented ferrets, one was identified as apo-10′-lycopenol, with a concentration of 8 ± 3 pmol/g wet-weight lung tissue (8). However, no apo-10′-lycopenal was detected, which suggested that apo-10′-lycopenal, the primary cleavage product, might be an intermediate compound and could be either reduced to apo-10′-lycopenol or oxidized to apo-10′-lycopenoic acid. This was supported by the conversion of apo-10′-lycopenal into apo-10′-lycopenoic acid in the presence of NAD+, and to both apo-10′-lycopenoic acid and apo-10′-lycopenol in the presence of NADH (8) (Figure 2). Gajic et al (50) reported the detection of apo-8′- and apo-12′-lycopenal as well as other unidentified polar metabolites of lycopene in the liver of rats given lycopene-enriched food. Interestingly, a recent study indicated that apo-10′- and apo-14′-lycopenoic acid have a remarkable ability to upregulate BCO2 expression (51). In addition, the expression of BCO2 mRNA in ferret lung was upregulated 4-fold by lycopene supplementation compared with animals not receiving lycopene supplementation (8). These results show that lycopene can be converted to apo-10′-lycopenoids, which can regulate BCO2 function in mammalian tissues both in vitro and in vivo. These observations also raise the question of whether apo-10′-lycopenoids have important biological functions related to human health (see below).
BIOLOGICAL FUNCTIONS OF LYCOPENE AND ITS METABOLITES
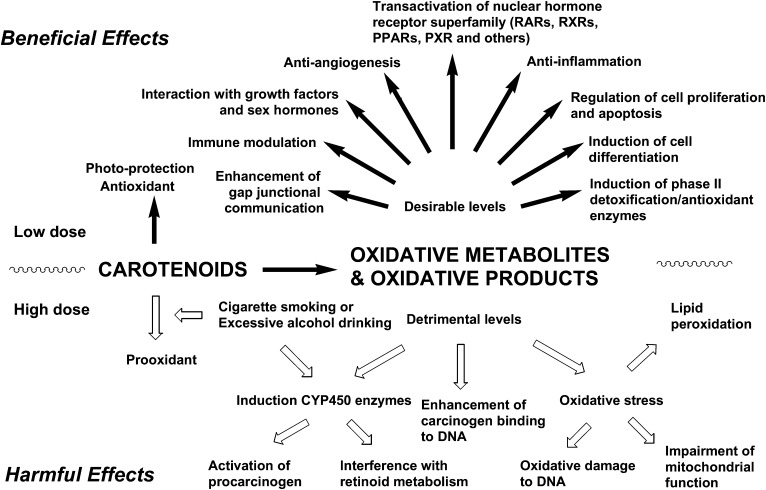
Because plasma values of carotenoids are biomarkers for the consumption of diets rich in fruit and vegetables, which contain other potentially bioactive nutrients, an association does not necessarily prove that lycopene is the active compound. To show these molecular effects in human systems, which involve multiple genetic and epigenetic events, is even more challenging. On the other hand, although it has not been confirmed whether or not lycopene is an important food component with health benefits, many human epidemiologic, cell culture, and animal model studies provide strong evidence that lycopene and its metabolites are active in several biological activities (Figure 3). Because there are several recent reviews on lycopene metabolism and biological function (12, 52, 53), recent studies on the biological activities of lycopene metabolites will briefly be reviewed.
FIGURE 3.
Schematic illustration of potential biological effects, both beneficial and harmful, attributed to carotenoids and their metabolites to human health. Although small quantities of carotenoid metabolites may offer protection against chronic diseases and certain cancers, larger amounts may be harmful, especially when coupled with a highly oxidative environment (eg, the lungs of cigarette smokers or liver of excessive alcohol drinkers). Adapted with permission from reference 3. PPAR, peroxisome proliferator-activated receptor; PXR, pregnane X receptor; RAR, retinoic acid receptor; RXR, retinoid X receptor.
INDUCTION OF ANTIOXIDANT/PHASE II DETOXIFYING ENZYMES
Free radicals can cause cellular damage by reacting with proteins, lipids, carbohydrates, and DNA and may be involved in the etiology of human diseases including cancer, cardiovascular disease, and age-related diseases. Whereas the initial impetus for studying the benefits of lycopene in chronic disease prevention was its antioxidant capacity, significant advances have been made in the understanding of the action of lycopene cleavage products with regard to modulation of antioxidant/detoxifying phase II enzymes via nuclear factor E2–related factor 2 (Nrf2) signaling. The transcription factor Nrf2, a key regulator of the cellular response to oxidative stress in multiple tissue and cell types, is a primary factor in the induction of antioxidant/phase II detoxifying enzymes (54, 55). Phase II enzymes have important detoxifying and antioxidant properties in combating reactive oxygen species and foreign substances (xenobiotics), including potential carcinogens. Induction of phase II detoxify/antioxidant enzymes is mediated through cis-regulatory DNA sequences known as antioxidant response elements (AREs), which are located in the promoter or enhancer region of the gene. The major ARE transcription factor Nrf2 is a primary agent in the induction of antioxidant and detoxifying enzymes, such as heme oxygenase-1 (HO-1), glutathione S-transferases, and NAD(P)H:quinone oxidoreductase. Under normal conditions, most of the Nrf2 is sequestered in the cytoplasm by Kelch-like erythroid Cap'n'Collar homolog-associated protein 1, and only residual nuclear Nrf2 binds to the ARE to drive basal activities. Exposure to certain carotenoids leads to the dissociation of the Nrf2–Kelch-like erythroid Cap'n'Collar homolog-associated protein 1 complex in the cytoplasm and the translocation of Nrf2 into the nucleus (56, 57). The nuclear accumulation of Nrf2 subsequently activates target genes of phase II/antioxidant enzymes. Non–provitamin A carotenoids including lycopene, lutein, canthaxanthin, and astaxanthin are shown to induce several phase II enzymes both in vivo and in vitro (58, 59). Because of its critical roles in the detoxification and antioxidant process during carcinogenesis, Nrf2 has been recognized as a potential molecular target for cancer prevention.
The first evidence was that an ethanolic extract containing lycopene and unidentified hydrophilic oxidative derivatives was shown to induce phase II enzymes and activate ARE-driven reporter gene activity with a similar potency to lycopene (56), although those chemically produced oxidative derivatives have not been found in mammalian tissues. Further evidence has been obtained recently to show that apo-10′-lycopenal, apo-10′-lycopenol, and apo-10′-lycopenoic acid were all effective in activating the Nrf2-mediated induction of HO-1 (60). HO-1, one of the major targets of Nrf2 regulation, is a rate-limiting enzyme in the degradation of heme to produce biliverdin (and its break-down product bilirubin), which behaves as a potent antioxidant by scavenging free radicals and plays an important antiinflammatory role in a number of chronic inflammatory diseases including alcohol-related inflammation (61, 62). Carbon monoxide, another break-down product of heme by HO-1, has strong antiinflammatory effects (61, 62). Work with BEAS-2B human bronchial epithelial cells has shown a dose-dependent and time-dependent increase in the accumulation of nuclear Nrf2 protein, as well as induced mRNA expression of HO-1, after apo-10′-lycopenoic acid treatment (60). In addition, pretreatment of BEAS-2B cells with apo-10′-lycopenoic acid resulted in a dose-dependent inhibition of both endogenous reactive oxygen species production and H2O2-induced oxidative damage, as measured by release of lactate dehydrogenase (60). These in vitro studies provide a mechanistic understanding for the chemopreventive effect of lycopene metabolites against carcinogen-induced cancer development in animal models in vivo (57). However, lycopene supplementation at a higher dose significantly induced hepatic phase I enzyme cytochrome P4502E1 protein and the incidence of inflammatory foci in alcohol-fed rats (63). These data indicate an interaction between chronic alcohol ingestion and lycopene supplementation and suggest a need for caution among individuals consuming high amounts of both alcohol and lycopene. Because induction of phase II detoxifying or antioxidant genes by dietary carotenoids represents an important cellular defense in response to oxidative and electrophilic insults, more research is clearly needed to identify and characterize additional carotenoid metabolites and their biological activities.
INTERACTION WITH RETINOID RECEPTORS
Provitamin A carotenoids can serve as direct precursors for all-trans and 9-cis retinoic acid (64, 65), which are ligands for RAR and retinoid X receptor. β-Carotene was able to maintain normal tissue levels of retinoic acid and inhibit the activation of mitogen-activated protein kinase pathways, cell proliferation, and phosphorylation of p53 (66). Certain excentric cleavage metabolites, such as β-apo-carotenoic acid, but not β-apo-13-carotenone, can also induce RARβ expression and transactivate the RARβ2 promoter via primary metabolism to the potent RAR ligand, all-trans retinoic acid (67). Therefore, the molecular mode of the action of provitamin A carotenoids is likely to be mediated by retinoic acid through transcriptional activation of a series of genes (68).
Discovery of the excentric cleavage of carotenoids heightens interest in carotenoid cleavage products and their possible biological interaction with nuclear receptors. The production of apo-carotenoids and apo-lycopenals is shown in several studies (37, 42, 69, 70). Without being converted into retinoids, the nonvolatile apo-carotenoids and apo-lycopenoids can inhibit cell growth (57, 71–73), stimulate differentiation (74), transactivate nuclear receptors (57), or antagonize nuclear receptor activation (69, 75). The volatile apo-carotenoid β-ionone is also shown to inhibit cell proliferation and induce apoptosis both in vitro (76–78) and in vivo (79). Interestingly, β-cryptoxanthin dose-dependently increases retinoic acid response element (RARE)–dependent promoter activity of retinoic acid receptor β in cells cotransfected with an RAR expression vector (80), and this transactivation activity of β-cryptoxanthin is shown to be due to its binding and activating RAR receptors directly without its conversion into retinoids (81). It is possible that beyond participating in known retinoid signaling pathways, carotenoids and their metabolites are able to interact directly with transcription factors without their conversion into retinoids.
Among identified lycopene metabolites, acycloretinoic acid has been shown to inhibit cell proliferation (82–84), induce apoptosis (85), and enhance gap junction communication (86). As an analog of retinoic acid, the ability of acycloretinoic acid to activate RAR was first examined by Ben-Dor et al (83) and Stahl et al (86). Although acycloretinoic acid is able to activate RARE-driven luciferase gene transcription, the required concentration is much higher than that of all-trans retinoic acid, which suggests that acycloretinoic acid is a weak activator of RARs (83, 86). Because of the similarity in chemical structures among apo-10′-lycopenoic acid, acycloretinoic acid, and all-trans retinoic acid (Figure 2), we questioned whether apo-10′-lycopenoic acid is an activator of RARs. We showed that treatment with 3–5 μmol apo-10′-lycopenoic acid/L significantly increased the mRNA level of RARβ, which is a transcriptional target of RARs (87), in lung cells (normal human bronchial epithelial, BEAS-2B, and A549 cells) (57). We then constructed a reporter vector containing the RARβ promoter fragment in the promoter region of luciferase gene. We showed that apo-10′-lycopenoic acid treatment increased the luciferase activity of HeLa cells transfected with this reporter vector. When the RARE in RARβ promoter was mutated, the ability of apo-10′-lycopenoic acid to transactivate RARβ promoter was abolished. These results suggest that apo-10′-lycopenoic acid can transactivate RARs and that activation of RARs may account for the growth inhibitory effect of apo-10′-lycopenoic acid (57).
MODULATION OF HORMONES AND GROWTH FACTORS
Steroid hormones (such as androgens and estrogen) and insulin-like growth factor (IGF) signaling systems may play a role in the biological action of carotenoids, in particular lycopene (88). Lycopene reduced the expression of 5α-reductase-1 in rat prostate tumors (89). Lycopene, phytoene, and phytofluene inhibited the estrogen-induced transactivation of the estrogen response element bound by the nuclear estrogen receptors ERα and ERβ (90). The IGF signaling system may also play a role in the biological action of lycopene (91). It has been suggested that the IGF signaling system may play a critical role in the biological action of lycopene (91, 92). By binding to membrane IGF-1 receptor, IGFs activate intracellular phosphatidylinositol 3′-kinase/Akt/protein kinase B and Ras/Raf/mitogen-activated protein kinase pathways, which regulate various biological processes such as cell-cycle progression, survival, and transformation (93). Lycopene treatment was shown to inhibit IGF-1–stimulated insulin receptor substrate 1 phosphorylation and cyclin D1 expression, block IGF-1–stimulated cell-cycle progression (91, 94), and increase membrane-associated IGF binding proteins (IGFBPs) in MCF-7 breast cancer cells (91), suggesting that lycopene may inhibit cell proliferation by acting on IGF-1 signaling pathway.
In circulation, IGFs are sequestered by a family of binding proteins (IGFBP-1–IGFBP-6), which regulate the availability of IGFs to bind to the IGF receptors (93). Consistent with in vitro studies that showed lycopene as a modulator of IGF signaling (91, 92), higher dietary intake of lycopene has been associated with lower circulating concentrations of IGF-1 (95) and higher concentrations of IGFBP-3 (96) in epidemiologic studies. In addition, the modulation of IGF-1 signaling is suggested to play an important role in lung carcinogenesis (97–99). Ferrets (Mustela putorious furo) offer an excellent model for mimicking the conditions of carotenoid intervention studies in humans because ferrets and humans are similar in terms of lycopene absorption, tissue distribution and concentrations, and metabolism (100). By using the ferret as a model, we found that plasma concentrations of IGF-1 were not affected by either lycopene supplementation or cigarette smoke exposure (44). Interestingly, cigarette smoke exposure decreased ferret plasma concentrations of IGFBP-3, whereas ferrets supplemented with both doses of lycopene had higher concentrations of IGFBP-3, regardless of cigarette smoke exposure. Furthermore, the ratio of IGF-I to IGFBP-3 was significantly decreased in ferrets supplemented with lycopene and exposed to smoke than in those exposed to smoke alone (44). The increased plasma IGFBP-3 by lycopene supplementation was associated with the inhibition of cigarette smoke–induced lung squamous metaplasia, decreased proliferating cellular nuclear antigen and phosphorylated BAD protein concentrations and the restoration of cleaved caspase-3 concentrations in lung tissue (44), which suggested the inhibition of proliferation and induction of apoptosis. Recently, we observed that apo-10′-lycopenoic acid treatment significantly induced hepatic sirtuin 1 (101) and IGFBP-3 expression in several cancer cell lines including lung BEAS-2B cells, liver THLE-2 cells, and prostate PC-3 cells (F Lian, KQ Hu, CA Peach, XD Wang, unpublished data, 2011). These results support the notion that lycopene metabolites may affect IGF signaling by the modulation of IGFBP-3 expression and play a role in the prevention of lung tumor development.
CHEMOPREVENTIVE EFFECTS
Previously, it has been shown that a mixture of lycopene oxidative products were able to inhibit the growth of HL-60 human promyelocytic leukemia cells (82) and induce ARE-dependent transcription of phase II xenobiotic-metabolizing enzymes (56), suggesting that lycopene metabolites may be biologically active components against initiation, promotion, or progression stages of carcinogenesis. In addition to acycloretinoic acid as mentioned above, the biological activities of other lycopene metabolites have also been investigated. For example, Aust et al (102) showed that 2,7,11-trimethyl-tetradecahexaene-1,14-dial, a metabolite of lycopene formed by a fragmentation at the 5,6 and 12′,11′ positions, is able to enhance gap-junction communication, whereas Zhang et al (103) showed that (E,E,E)-4-methyl-8-oxo-2,4,6-nonatrienal, the product of oxidative cleavages at the 5,6- and 13,14 double bonds of lycopene induced apoptosis, downregulated Bcl-2 and Bcl-XL, and activated caspase cascades in HL-60 cells. However, the physiologic roles of these lycopene products remain unknown because none of these metabolites have been detected in biological systems. Because we have detected apo-10′-lycopenol, a derivative of BCO2 cleavage of lycopene, in ferret lungs after lycopene supplementation (8, 42), we investigated the biological activity of apo-10′-lycopenoic acid, as well as its potential chemopreventive effect on lung carcinogenesis (57). We have shown that apo-10′-lycopenoic acid significantly decreased lung cancer cells in the S phase and increased cells in the G1 phase and was associated with decreased cyclin E and increased p21 and p27 expression. In addition, the lower sensitivity of A549 cells to apo-10′-lycopenoic acid treatment, as compared with NHBE and BEAS-2B cells, suggests that apo-10′-lycopenoic acid may be a better agent for cancer prevention than cancer therapy (57). This was further confirmed by an in vivo study to determine whether apo-10′-lycopenoic acid could inhibit lung tumor development in the A/J mouse model of lung cancer. Supplementation with apo-10′-lycopenoic acid decreased tumor number in a dose-dependent manner from an average of 16 tumors/mouse in the 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone injection–alone group, to an average of 10, 7, and 5 tumors/mouse in groups injected with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and supplemented with apo-10′-lycopenoic acid at doses of 10, 40, and 120 mg/kg, respectively, which represents declines of 32.7%, 53.6%, and 65.4% in tumor number compared with the nonsupplemented group (57). A recent study showed that apo-12′-lycopenal reduced the proliferation of androgen-independent DU145 prostate cancer cells, in part by inhibiting normal cell-cycle progression (104). In summary, both in vitro and in vitro studies have shown that apo-10′-lycopenoic acid, an enzymatic metabolite of lycopene, is a potential chemopreventive agent against lung cancer. It will be interesting to investigate if lycopene metabolites may, at least in part, mediate the chemopreventive effect of lycopene on other cancers.
CONCLUSIONS
There is evidence from both epidemiologic studies and animal and cell culture studies that lycopene has multifaceted biological actions. These data have led to an increased effort to better understand the role of lycopene and its derivatives in the process of chronic diseases. In particular, the characterization and study of BCO2 has shown that this enzyme can catalyze the excentric cleavage of both provitamin and non–provitamin A carotenoids to form apo-10′-carotenoids, including apo-10′-lycopenoids from lycopene. Enzymatic kinetic analysis indicates that the non–provitamin A carotenoids including lycopene are preferentially cleaved over provitamin A carotenoids, indicating a key role of BCO2 in non–provitamin A carotenoid metabolism. Several in vivo and in vitro reports suggested that the biological activities of lycopene can be mediated, in part, by lycopene metabolites. More research is clearly needed to identify and characterize additional lycopene metabolites and their biological activities, which will potentially provide invaluable insight into the mechanisms underlying the beneficial effects of lycopene in humans, particularly in terms of chronic disease prevention. As our understanding of carotenoid metabolism, molecular biological properties, and their interaction with genetic and epigenetic factors improves, greater insight will be achieved into the role and application of carotenoids and their metabolites in human health and disease.
Acknowledgments
The author had no conflicts of interest.
Footnotes
Abbreviations used: ARE, antioxidant response element; BCO1, β-carotene 15,15′-oxygenase; BCO2, β-carotene-9′,10′-oxygenase; HO-1, heme oxygenase-1; IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein; ISX, intestine-specific homeobox; Nrf2, nuclear factor E2–related factor 2; RAR, retinoic acid receptor; RARE, retinoic acid response element; SR-B1, scavenger receptor class B type 1.
REFERENCES
- 1.Britton G, Liaaen-Jensen S, Pfander H. Carotenoids handbook. Basel, Switzerland: Birkhaumluser Verlag, 2004.
- 2.Story EN, Kopec RE, Schwartz SJ, Harris GK. An update on the health effects of tomato lycopene. Annu Rev Food Sci Technol 2010;1:189–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang XD. Carotenoids. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern nutrition in health and disease. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2012:427–37.
- 4.Mein J, Wang XD. Oxidative metabolites of lycopene and their biological functions : Landrum JT. Carotenoids. Boca Raton, FL: Taylor and Francis Group, 2010:417–35 [Google Scholar]
- 5.Lindshield BL, Canene-Adams K, Erdman JW., Jr Lycopenoids: are lycopene metabolites bioactive? Arch Biochem Biophys 2007;458:136–40 [DOI] [PubMed] [Google Scholar]
- 6.Caris-Veyrat C. Formation of carotenoid oxygenaed cleavage products : Landrum JT. ed. Carotenoids Boca Raton, FL: Taylor and Francis Group, 2010:215–28 [Google Scholar]
- 7.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem 2001;276:14110–6 [DOI] [PubMed] [Google Scholar]
- 8.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem 2006;281:19327–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang XD. Biological activities of carotenoid metabolites. In: Britton G, Liaaen-Jensen S, Pfander H, eds. Carotenoids. Basel, Switzerland: Birkhäuser Verlag, 2009:383–408.
- 10.Rock CL, Swendseid ME, Jacob RA, McKee RW. Plasma carotenoid levels in human subjects fed a low carotenoid diet. J Nutr 1992;122:96–100 [DOI] [PubMed] [Google Scholar]
- 11.Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal beta,beta-carotene absorption and vitamin A production. FASEB J 2010;24:1656–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Lintig J. Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu Rev Nutr 2010;30:35–56 [DOI] [PubMed] [Google Scholar]
- 13.Seino Y, Miki T, Kiyonari H, Abe T, Fujimoto W, Kimura K, Takeuchi A, Takahashi Y, Oiso Y, Iwanaga T, et al. ISX participates in the maintenance of vitamin A metabolism by regulation of beta-carotene 15,15′-monooxygenase (Bcmo1) expression. J Biol Chem 2008;283:4905–11 [DOI] [PubMed] [Google Scholar]
- 14.Choi S, Koo S. Efficient syntheses of the keto-carotenoids canthaxanthin, astaxanthin, and astacene. J Org Chem 2005;70:3328–31 [DOI] [PubMed] [Google Scholar]
- 15.During A, Harrison EH. Intestinal absorption and metabolism of carotenoids: insights from cell culture. Arch Biochem Biophys 2004;430:77–88 [DOI] [PubMed] [Google Scholar]
- 16.Moussa M, Gouranton E, Gleize B, Yazidi CE, Niot I, Besnard P, Borel P, Landrier JF. CD36 is involved in lycopene and lutein uptake by adipocytes and adipose tissue cultures. Mol Nutr Food Res 2011;55:578–84 [DOI] [PubMed] [Google Scholar]
- 17.Wang XD, Krinsky NI, Marini RP, Tang G, Yu J, Hurley R, Fox JG, Russell RM. Intestinal uptake and lymphatic absorption of beta-carotene in ferrets: a model for human beta-carotene metabolism. Am J Physiol 1992;263:G480–6 [DOI] [PubMed] [Google Scholar]
- 18.Canene-Adams K, Erdman JW. Absorption, transport, distribution in tissues and bioavailability : Britton G, Liaaen-Jensen S, Pfander H. eds. Carotenoids Basel, Switzerland: Birkhäuser Verlag, 2009:115–48 [Google Scholar]
- 19.von Lintig J, Vogt K. Filling the gap in vitamin A research: molecular identification of an enzyme cleaving beta-carotene to retinal. J Biol Chem 2000;275:11915–20 [DOI] [PubMed] [Google Scholar]
- 20.Leuenberger MG, Engeloch-Jarret C, Woggon WD. The reaction mechanism of the enzyme-catalyzed central cleavage of beta-carotene to retinal. Angew Chem Int Ed Engl 2001;40:2613–7 [DOI] [PubMed] [Google Scholar]
- 21.Wyss A, Wirtz GM, Woggon WD, Brugger R, Wyss M, Friedlein A, Riss G, Bachmann H, Hunziker W. Expression pattern and localization of beta,beta-carotene 15,15'-dioxygenase in different tissues. Biochem J 2001;354:521–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redmond TM, Gentleman S, Duncan T, Yu S, Wiggert B, Gantt E, Cunningham FX., Jr Identification, expression, and substrate specificity of a mammalian beta-carotene 15,15'-dioxygenase. J Biol Chem 2001;276:6560–5 [DOI] [PubMed] [Google Scholar]
- 23.Lindqvist A, He YG, Andersson S. Cell type-specific expression of β-carotene 9′,10′-monooxygenase in human tissues. J Histochem Cytochem 2005;53:1403–12 [DOI] [PubMed] [Google Scholar]
- 24.Lindqvist A, Dreja K, Sward K, Hellstrand P. Effects of oxygen tension on energetics of cultured vascular smooth muscle. Am J Physiol Heart Circ Physiol 2002;283:H110–7 [DOI] [PubMed] [Google Scholar]
- 25.Lindqvist A, Andersson S. Biochemical properties of purified recombinant human beta-carotene 15,15′-monooxygenase. J Biol Chem 2002;277:23942–8 [DOI] [PubMed] [Google Scholar]
- 26.Kim YS, Oh DK. Biotransformation of carotenoids to retinal by carotenoid 15,15′-oxygenase. Appl Microbiol Biotechnol 2010;88:807–16 [DOI] [PubMed] [Google Scholar]
- 27.Poliakov E, Gentleman S, Cunningham FX, Jr, Miller-Ihli NJ, Redmond TM. Key role of conserved histidines in recombinant mouse beta-carotene 15,15′-monooxygenase-1 activity. J Biol Chem 2005;280:29217–23 [DOI] [PubMed] [Google Scholar]
- 28.Ganguly J, Sastry PS. Mechanism of conversion of beta-carotene into vitamin A—central cleavage versus random cleavage. World Rev Nutr Diet 1985;45:199–220 [PubMed] [Google Scholar]
- 29.Wang XD, Tang GW, Fox JG, Krinsky NI, Russell RM. Enzymatic conversion of beta-carotene into beta-apo-carotenals and retinoids by human, monkey, ferret, and rat tissues. Arch Biochem Biophys 1991;285:8–16 [DOI] [PubMed] [Google Scholar]
- 30.Tang GW, Wang XD, Russell RM, Krinsky NI. Characterization of beta-apo-13-carotenone and beta-apo-14′-carotenal as enzymatic products of the excentric cleavage of beta-carotene. Biochemistry 1991;30:9829–34 [DOI] [PubMed] [Google Scholar]
- 31.Amengual J, Lobo GP, Golczak M, Li HN, Klimova T, Hoppel CL, Wyss A, Palczewski K, von Lintig J. A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J 2011;25:948–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lakshmanan MR, Pope JL, Olson JA. The specificity of a partially purified carotenoid cleavage enzyme of rabbit intestine. Biochem Biophys Res Commun 1968;33:347–52 [DOI] [PubMed] [Google Scholar]
- 33.Liu C, Wang XD, Russell RM. Biosynthesis of retinoic acid from beta-apo-14′-carotenal in ferret in vivo. J Nutr Biochem 1997;8:652–7 [Google Scholar]
- 34.Wang XD, Russell RM, Liu C, Stickel F, Smith DE, Krinsky NI. Beta-oxidation in rabbit liver in vitro and in the perfused ferret liver contributes to retinoic acid biosynthesis from beta-apocarotenoic acids. J Biol Chem 1996;271:26490–8 [PubMed] [Google Scholar]
- 35.Wang XD, Marini RP, Hebuterne X, Fox JG, Krinsky NI, Russell RM. Vitamin E enhances the lymphatic transport of beta-carotene and its conversion to vitamin A in the ferret. Gastroenterology 1995;108:719–26 [DOI] [PubMed] [Google Scholar]
- 36.Hébuterne X, Wang XD, Johnson EJ, Krinsky NI, Russell RM. Intestinal absorption and metabolism of 9-cis-beta-carotene in vivo: biosynthesis of 9-cis-retinoic acid. J Lipid Res 1995;36:1264–73 [PubMed] [Google Scholar]
- 37.Ho CC, de Moura FF, Kim SH, Clifford AJ. Excentral cleavage of beta-carotene in vivo in a healthy man. Am J Clin Nutr 2007;85:770–7 [DOI] [PubMed] [Google Scholar]
- 38.Kopec RE, Riedl KM, Harrison EH, Curley RW, Jr, Hruszkewycz DP, Clinton SK, Schwartz SJ. Identification and quantification of apo-lycopenals in fruits, vegetables, and human plasma. J Agric Food Chem 2010;58:3290–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hessel S, Eichinger A, Isken A, Amengual J, Hunzelmann S, Hoeller U, Elste V, Hunziker W, Goralczyk R, Oberhauser V, et al. CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J Biol Chem 2007;282:33553–61 [DOI] [PubMed] [Google Scholar]
- 40.Berry SD, Davis SR, Beattie EM, Thomas NL, Burrett AK, Ward HE, Stanfield AM, Biswas M, Ankersmit-Udy AE, Oxley PE, et al. Mutation in bovine beta-carotene oxygenase 2 affects milk color. Genetics 2009;182:923–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian B, Sun Z, Shen S, Wang H, Jiao J, Wang L, Hu Y, Hua Y. Effects of carotenoids from Deinococcus radiodurans on protein oxidation. Lett Appl Microbiol 2009;49:689–94 [DOI] [PubMed] [Google Scholar]
- 42.Mein JR, Dolnikowski GG, Ernst H, Russell RM, Wang XD. Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin and beta-cryptoxanthin by ferret carotene-9′,10′-monooxygenase. Arch Biochem Biophys 2011;506:109–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mein JR, Lian F, Wang XD. Biological activity of lycopene metabolites: implications for cancer prevention. Nutr Rev 2008;66:667–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boileau AC, Merchen NR, Wasson K, Atkinson CA, Erdman JW., Jr cis-Lycopene is more bioavailable than trans-lycopene in vitro and in vivo in lymph-cannulated ferrets. J Nutr 1999;129:1176–81 [DOI] [PubMed] [Google Scholar]
- 45.Liu C, Lian F, Smith DE, Russell RM, Wang XD. Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets. Cancer Res 2003;63:3138–44 [PubMed] [Google Scholar]
- 46.Våge DI, Boman IA. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries). BMC Genet 2010;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eriksson J, Larson G, Gunnarsson U, Bed'hom B, Tixier-Boichard M, Stromstedt L, Wright D, Jungerius A, Vereijken A, Randi E, et al. Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet 2008;4:e1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castañeda MP, Hirschler EM, Sams AR. Skin pigmentation evaluation in broilers fed natural and synthetic pigments. Poult Sci 2005;84:143–7 [DOI] [PubMed] [Google Scholar]
- 49.Khachik F. Chemical and metabolic oxidation of carotenoids : Packer L, Kraemer K, Obermuller-Jevic U, Sies H. eds. Carotenoids and retinoids: molecular aspects and health issues Champaign, IL: AOCS Press, 2005:61–75 [Google Scholar]
- 50.Gajic M, Zaripheh S, Sun F, Erdman JW., Jr Apo-8′-lycopenal and apo-12′-lycopenal are metabolic products of lycopene in rat liver. J Nutr 2006;136:1552–7 [DOI] [PubMed] [Google Scholar]
- 51.Reynaud E, Aydemir G, Ruhl R, Dangles O, Caris-Veyrat C. Organic synthesis of new putative lycopene metabolites and preliminary investigation of their cell-signaling effects. J Agric Food Chem 2011;59:1457–63 [DOI] [PubMed] [Google Scholar]
- 52.Palozza P, Parrone N, Catalano A, Simone R. Tomato lycopene and inflammatory cascade: basic interactions and clinical implications. Curr Med Chem 2010;17:2547–63 [DOI] [PubMed] [Google Scholar]
- 53.Kelkel M, Schumacher M, Dicato M, Diederich M. Antioxidant and anti-proliferative properties of lycopene. Free Radic Res 2011;45:925–40 [DOI] [PubMed] [Google Scholar]
- 54.Kensler TW, Wakabayashi N. Nrf2: friend or foe for chemoprevention? Carcinogenesis 2010;31:90–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW. When NRF2 talks, who's listening? Antioxid Redox Signal 2010;13:1649–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ben-Dor A, Steiner M, Gheber L, Danilenko M, Dubi N, Linnewiel K, Zick A, Sharoni Y, Levy J. Carotenoids activate the antioxidant response element transcription system. Mol Cancer Ther 2005;4:177–86 [PubMed] [Google Scholar]
- 57.Lian F, Smith DE, Ernst H, Russell RM, Wang XD. Apo-10′-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis 2007;28:1567–74 [DOI] [PubMed] [Google Scholar]
- 58.Gradelet S, Astorg P, Leclerc J, Chevalier J, Vernevaut MF, Siess MH. Effects of canthaxanthin, astaxanthin, lycopene and lutein on liver xenobiotic-metabolizing enzymes in the rat. Xenobiotica 1996;26:49–63 [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int J Cancer 2010;126:1788–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lian F, Wang XD. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int J Cancer 2008;123:1262–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mandal P, Park PH, McMullen MR, Pratt BT, Nagy LE. The anti-inflammatory effects of adiponectin are mediated via a heme oxygenase-1-dependent pathway in rat Kupffer cells. Hepatology 2010;51:1420–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mandal P, Pritchard MT, Nagy LE. Anti-inflammatory pathways and alcoholic liver disease: role of an adiponectin/interleukin-10/heme oxygenase-1 pathway. World J Gastroenterol 2010;16:1330–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.World Health Organization International classification of diseases: manual of the international statistical classification of diseases, injuries, and causes of death., Vol. 1, 9th rev. Geneva, Switzerland: World Health Organization, 1977 [Google Scholar]
- 64.Napoli JL, Race KR. Biogenesis of retinoic acid from beta-carotene: differences between the metabolism of beta-carotene and retinal. J Biol Chem 1988;263:17372–7 [PubMed] [Google Scholar]
- 65.Wang XD, Krinsky NI, Benotti PN, Russell RM. Biosynthesis of 9-cis-retinoic acid from 9-cis-β-carotene in human intestinal mucosa in vitro. Arch Biochem Biophys 1994;313:150–5 [DOI] [PubMed] [Google Scholar]
- 66.Kim Y, Lian F, Yeum KJ, Chongviriyaphan N, Choi SW, Russell RM, Wang XD. The effects of combined antioxidant (beta-carotene, alpha-tocopherol and ascorbic acid) supplementation on antioxidant capacity, DNA single-strand breaks and levels of insulin-like growth factor-1/IGF-binding protein 3 in the ferret model of lung cancer. Int J Cancer 2007;120:1847–54 [DOI] [PubMed] [Google Scholar]
- 67.Prakash P, Liu C, Hu KQ, Krinsky NI, Russell RM, Wang XD. Beta-carotene and beta-apo-14'-carotenoic acid prevent the reduction of retinoic acid receptor beta in benzo[a]pyrene-treated normal human bronchial epithelial cells. J Nutr 2004;134:667–73 [DOI] [PubMed] [Google Scholar]
- 68.Wang XD. Carotenoid oxidative/degradative products and their biological activities : Krinsky NI, Mayne ST, Sies H. eds. Carotenoids in health and disease New York, NY: Marcel Dekker, 2004:313–35 [Google Scholar]
- 69.Eroglu A, Hruszkewycz DP, Curley RW, Jr, Harrison EH. The eccentric cleavage product of beta-carotene, beta-apo-13-carotenone, functions as an antagonist of RXRalpha. Arch Biochem Biophys 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shmarakov I, Fleshman MK, D'Ambrosio DN, Piantedosi R, Riedl KM, Schwartz SJ, Curley RW, Jr, von Lintig J, Rubin LP, Harrison EH, et al. Hepatic stellate cells are an important cellular site for beta-carotene conversion to retinoid. Arch Biochem Biophys 2010;504:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki T, Matsui M, Murayama A. Biological activity of (all-E)-beta-apo-12′-carotenoic acid and the geometrical isomers on human acute promyelocytic leukemia cell line HL-60. J Nutr Sci Vitaminol (Tokyo) 1995;41:575–85 [DOI] [PubMed] [Google Scholar]
- 72.Tibaduiza EC, Fleet JC, Russell RM, Krinsky NI. Excentric cleavage products of beta-carotene inhibit estrogen receptor positive and negative breast tumor cell growth in vitro and inhibit activator protein-1-mediated transcriptional activation. J Nutr 2002;132:1368–75 [DOI] [PubMed] [Google Scholar]
- 73.Linnewiel K, Ernst H, Caris-Veyrat C, Ben-Dor A, Kampf A, Salman H, Danilenko M, Levy J, Sharoni Y. Structure activity relationship of carotenoid derivatives in activation of the electrophile/antioxidant response element transcription system. Free Radic Biol Med 2009;47:659–67 [DOI] [PubMed] [Google Scholar]
- 74.Winum JY, Kamal M, Defacque H, Commes T, Chavis C, Lucas M, Marti J, Montero JL. Synthesis and biological activities of higher homologues of retinoic acid. Farmaco 1997;52:39–42 [PubMed] [Google Scholar]
- 75.Ziouzenkova O, Orasanu G, Sukhova G, Lau E, Berger JP, Tang G, Krinsky NI, Dolnikowski GG, Plutzky J. Asymmetric cleavage of beta-carotene yields a transcriptional repressor of retinoid X receptor and peroxisome proliferator-activated receptor responses. Mol Endocrinol 2007;21:77–88 [DOI] [PubMed] [Google Scholar]
- 76.Duncan RE, Lau D, El-Sohemy A, Archer MC. Geraniol and beta-ionone inhibit proliferation, cell cycle progression, and cyclin-dependent kinase 2 activity in MCF-7 breast cancer cells independent of effects on HMG-CoA reductase activity. Biochem Pharmacol 2004;68:1739–47 [DOI] [PubMed] [Google Scholar]
- 77.Liu JR, Dong HW, Sun XR, Wang Q, Sun WG, Parry JW, Liu Q, Han XH, Sun CH, Chen BQ, et al. Effects of beta-ionone on mammary carcinogenesis and antioxidant status in rats treated with DMBA. Nutr Cancer 2010;62:58–65 [DOI] [PubMed] [Google Scholar]
- 78.Jung M, Mo H, Elson CE. Synthesis and biological activity of beta-ionone-derived alcohols for cancer chemoprevention. Anticancer Res 1998;18:189–92 [PubMed] [Google Scholar]
- 79.Liu JR, Sun XR, Dong HW, Sun CH, Sun WG, Chen BQ, Song YQ, Yang BF. Beta-ionone suppresses mammary carcinogenesis, proliferative activity and induces apoptosis in the mammary gland of the Sprague-Dawley rat. Int J Cancer 2008;122:2689–98 [DOI] [PubMed] [Google Scholar]
- 80.Lian F, Hu KQ, Russell RM, Wang XD. Beta-cryptoxanthin suppresses the growth of immortalized human bronchial epithelial cells and non-small-cell lung cancer cells and up-regulates retinoic acid receptor beta expression. Int J Cancer 2006;119:2084–9 [DOI] [PubMed] [Google Scholar]
- 81.Matsumoto A, Mizukami H, Mizuno S, Umegaki K, Nishikawa J, Shudo K, Kagechika H, Inoue M. Beta-cryptoxanthin, a novel natural RAR ligand, induces ATP-binding cassette transporters in macrophages. Biochem Pharmacol 2007;74:256–64 [DOI] [PubMed] [Google Scholar]
- 82.Nara E, Hayashi H, Kotake M, Miyashita K, Nagao A. Acyclic carotenoids and their oxidation mixtures inhibit the growth of HL-60 human promyelocytic leukemia cells. Nutr Cancer 2001;39:273–83 [DOI] [PubMed] [Google Scholar]
- 83.Ben-Dor A, Nahum A, Danilenko M, Giat Y, Stahl W, Martin HD, Emmerich T, Noy N, Levy J, Sharoni Y. Effects of acyclo-retinoic acid and lycopene on activation of the retinoic acid receptor and proliferation of mammary cancer cells. Arch Biochem Biophys 2001;391:295–302 [DOI] [PubMed] [Google Scholar]
- 84.Kotake-Nara E, Kushiro M, Zhang H, Sugawara T, Miyashita K, Nagao A. Carotenoids affect proliferation of human prostate cancer cells. J Nutr 2001;131:3303–6 [DOI] [PubMed] [Google Scholar]
- 85.Kotake-Nara E, Kim SJ, Kobori M, Miyashita K, Nagao A. Acyclo-retinoic acid induces apoptosis in human prostate cancer cells. Anticancer Res 2002;22:689–95 [PubMed] [Google Scholar]
- 86.Stahl W, von Laar J, Martin HD, Emmerich T, Sies H. Stimulation of gap junctional communication: comparison of acyclo-retinoic acid and lycopene. Arch Biochem Biophys 2000;373:271–4 [DOI] [PubMed] [Google Scholar]
- 87.de Thé H, Vivanco-Ruiz MM, Tiollais P, Stunnenberg H, Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature 1990;343:177–80 [DOI] [PubMed] [Google Scholar]
- 88.Herzog A, Siler U, Spitzer V, Seifert N, Denelavas A, Hunziker PB, Hunziker W, Goralczyk R, Wertz K. Lycopene reduced gene expression of steroid targets and inflammatory markers in normal rat prostate. FASEB J 2005;19:272–4 [DOI] [PubMed] [Google Scholar]
- 89.Siler U, Barella L, Spitzer V, Schnorr J, Lein M, Goralczyk R, Wertz K. Lycopene and vitamin E interfere with autocrine/paracrine loops in the Dunning prostate cancer model. FASEB J 2004;18:1019–21 [DOI] [PubMed] [Google Scholar]
- 90.Hirsch K, Atzmon A, Danilenko M, Levy J, Sharoni Y. Lycopene and other carotenoids inhibit estrogenic activity of 17beta-estradiol and genistein in cancer cells. Breast Cancer Res Treat 2007;104:221–30 [DOI] [PubMed] [Google Scholar]
- 91.Karas M, Amir H, Fishman D, Danilenko M, Segal S, Nahum A, Koifmann A, Giat Y, Levy J, Sharoni Y. Lycopene interferes with cell cycle progression and insulin-like growth factor I signaling in mammary cancer cells. Nutr Cancer 2000;36:101–11 [DOI] [PubMed] [Google Scholar]
- 92.Levy J, Bosin E, Feldman B, Giat Y, Miinster A, Danilenko M, Sharoni Y. Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene. Nutr Cancer 1995;24:257–66 [DOI] [PubMed] [Google Scholar]
- 93.Jones JI, Clemmons D. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 1995;16:3–34 [DOI] [PubMed] [Google Scholar]
- 94.Nahum A, Zeller L, Danilenko M, Prall OW, Watts CK, Sutherland RL, Levy J, Sharoni Y. Lycopene inhibition of IGF-induced cancer cell growth depends on the level of cyclin D1. Eur J Nutr 2006;45:275–82 [DOI] [PubMed] [Google Scholar]
- 95.Mucci LA, Tamimi R, Lagiou P, Trichopoulou A, Benetou V, Spanos E, Trichopoulos D. Are dietary influences on the risk of prostate cancer mediated through the insulin-like growth factor system? BJU Int 2001;87:814–20 [DOI] [PubMed] [Google Scholar]
- 96.Holmes MD, Pollak MN, Hankinson SE. Lifestyle correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev 2002;11:862–7 [PubMed] [Google Scholar]
- 97.Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J Natl Cancer Inst 1999;91:151–6 [DOI] [PubMed] [Google Scholar]
- 98.London SJ, Yuan JM, Travlos GS, Gao YT, Wilson RE, Ross RK, Yu MC. Insulin-like growth factor I, IGF-binding protein 3, and lung cancer risk in a prospective study of men in China. J Natl Cancer Inst 2002;94:749–54 [DOI] [PubMed] [Google Scholar]
- 99.Wakai K, Ito Y, Suzuki K, Tamakoshi A, Seki N, Ando M, Ozasa K, Watanabe Y, Kondo T, Nishino Y, et al. Serum insulin-like growth factors, insulin-like growth factor-binding protein-3, and risk of lung cancer death: a case-control study nested in the Japan Collaborative Cohort (JACC) Study. Jpn J Cancer Res 2002;93:1279–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang XD. Can smoke-exposed ferrets be utilized to unravel the mechanisms of action of lycopene? J Nutr 2005;135:2053S–6S [DOI] [PubMed] [Google Scholar]
- 101.Chung J, Koo K, Lian F, Hu KQ, Ernst H, Wang XD. Apo-10′-lycopenoic acid, a lycopene metabolite, increases sirtuin 1 mRNA and protein levels and decreases hepatic fat accumulation in ob/ob mice. J Nutr 2012;142:405–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aust O, Ale-Agha N, Zhang L, Wollersen H, Sies H, Stahl W. Lycopene oxidation product enhances gap junctional communication. Food Chem Toxicol 2003;41:1399–407 [DOI] [PubMed] [Google Scholar]
- 103.Zhang H, Kotake-Nara E, Ono H, Nagao A. A novel cleavage product formed by autoxidation of lycopene induces apoptosis in HL-60 cells. Free Radic Biol Med 2003;35:1653–63 [DOI] [PubMed] [Google Scholar]
- 104.Ford NA, Elsen AC, Zuniga K, Lindshield BL, Erdman JW., Jr Lycopene and apo-12′-lycopenal reduce cell proliferation and alter cell cycle progression in human prostate cancer cells. Nutr Cancer 2011;63:256–63 [DOI] [PubMed] [Google Scholar]