Abstract
Age-related macular degeneration (AMD) is the primary cause of vision loss in elderly people of western European ancestry. Genetic, dietary, and environmental factors affect tissue concentrations of macular xanthophylls (MXs) within retinal cell types manifesting AMD pathology. In this article we review the history and state of science on the putative role of the MXs (lutein, zeaxanthin, and meso-zeaxanthin) in AMD and report findings on AMD-associated genes encoding enzymes, transporters, ligands, and receptors affecting or affected by MXs. We then use this context to discuss emerging research opportunities that offer promise for meaningful investigation and inference in the field.
INTRODUCTION
The dietary carotenoids lutein [(3R,3′R,6′R)-β,ϵ-carotene-3,3′-diol] and zeaxanthin [(3R,3′R)-β,β-carotene-3,3′-diol] are primary constituents of macular pigment (1, 2) and have been examined for their effect on health and disease of the retina for >200 y (3–5). These 2 nutrients and meso-zeaxanthin [(3R,3′S)-β, β-carotene-3,3′-diol], a metabolite of lutein (6), are known collectively as macular xanthophylls (MXs)5. Biological plausibility of MX-retinal disease relations exists because these compounds exhibit the following characteristics: 1) intake-dependent and -modifiable accretion to the retina, 2) preferential concentration and localization in retinal cells manifesting retinal pathology, and 3) biophysical and biochemical capacity to affect processes implicated in pathogenesis and progression of retinal diseases.
A number of large-scale human studies on age-related macular degeneration (AMD), the primary cause of irreversible retina-based visual impairment in elderly people of western European ancestry (7–9), showed AMD-nutrient relations with MX status and intake (10–17). Bird (18) and Ambati et al (19) provided details on the pathogenesis and pathophysiology of AMD. Atrophic and confluent degeneration of retinal pigmented epithelium (RPE) cells and adjacent photoreceptors is known as geographic atrophy (or “dry” AMD). An estimated 1.7 million (1.5%) US residents are living with advanced AMD (AAMD, ie, geographic atrophy and/or neovascular AMD). Choroidal neovascularization is the hallmark of “wet” or neovascular AAMD, a condition characterized by florid sub- and trans-RPE proliferation of new vessels from the choriocapillaris. A series of photographs (http://www.nei.nih.gov/photo/) and dynamic renderings of AMD progression (www.nei.nih.gov/photo/eyedis/VA04.mov) are available from the National Eye Institute.
In the primate retina, high concentrations of MXs exist within areas manifesting susceptibility to light damage and metabolic challenge. The laminar concentration and topographic distribution of MXs may explain how the human retina is typically capable of handling extreme physical and biochemical exposures without appreciable loss of function for ≥6 decades; even in people with AAMD, remarkable resilience of the MX-rich areas is sometimes seen. Bone et al (20) stated that “in the advanced form of AMD known as geographic atrophy, the foveal center, which contains the highest concentrations of lutein and zeaxanthin, tends to be spared until late in the course of the disease.” The phenomenon of foveal sparing in MX-dense regions has also been observed in a number of inherited macular diseases (21).
MODERN HISTORY OF RESEARCH ON THE ROLE OF MXs IN HEALTH AND DISEASE OF THE RETINA
At least 200 y of clinical observation and research on macular pigment preceded the 1980 report of Malinow et al (22) that described an absence of retinal MXs and associated RPE defects in macaque monkeys fed a MX-free diet across their life span. We refer the interested reader to an extensive scholarly review of the field published in 1981 by Nussbaum et al (3) and to works including early historical references (4, 5). A chronology of key findings from reports that applied biochemical analyses of retinal MXs is shown in Table 1, starting with the 1984 works by Snodderly et al (23, 24) on MX spatial distribution and absorption properties in the primate retina and subsequent work by Bone et al (1), which identified lutein and zeaxanthin as the major chromatographically separable components of macular pigment. The first evidence of MX-AMD relations in large-scale, well-phenotyped multicenter human cohorts emerged in the early 1990s from observational studies by Seddon et al (11), Mares-Perlman et al (36), and the Eye Disease Case-Control Study Research Group (37). Around this time, Bone et al (38) applied mass spectroscopic methods to determine the stereochemistry of macular pigment, thus providing information with which to model the biophysical properties of MXs in membranes. Conceptual frameworks for MX-AMD research have evolved from international congresses convening over the past 20 y (39–41) and from progressive reviews (4, 23, 24, 28, 42–50). Results from randomized, double-masked, placebo-controlled trials on MXs and visual function emerged in the mid-2000s (51, 52). In the largest of these, Richer et al (51) reported significant improvements in visual acuity among participants randomly assigned to receive 10 mg lutein/d for 12 mo. The Age-Related Eye Disease Study 2 (www.areds2.org) and the Carotenoids and Co-Oxidants in Age-Related Maculopathy Study (53) are large-scale trials designed to examine structural and functional retinal response to MXs in people with AMD.
TABLE 1.
Reports on biochemical analysis of MXs in primate retina1
| First author (reference) | Year | Sample | Key contributions |
| Snodderly (23) | 1984 | Monkey | MP absorbance spectra and localization in primate |
| Snodderly (24) | 1984 | Monkey | MP spatial distribution in primate retina |
| Bone (1) | 1985 | Human | L and Z identified as major constituents of MP |
| Bone (25) | 1988 | Human | L- and Z-specific distributions within the retina |
| Handelman (2) | 1988 | Human | MX quantities accurately estimated in retina |
| Handelman (26) | 1991 | Monkey | MX from fixed retina used for densitometry |
| Bone (27) | 1997 | Human | L:Z ratio first plotted with retinal eccentricity |
| Khachik (28) | 1997 | Primate | 3 major xanthophylls and 11 metabolites in retina |
| Bernstein (29) | 1998 | Human | MX in vivo imaging method validated on biochemistry |
| Sommerberg (30) | 1999 | Human | 25% of retinal carotenoids exist in rod outer segments |
| Rapp (31) | 2000 | Human | MXs exist in rod outer segments in perifoveal region |
| Bernstein (32) | 2001 | Human | Expanded analysis of MX concentration outside of retina |
| Bone (33) | 2001 | Human | Retinal MXs lower in people with AMD (vs AMD-free) |
| Johnson (6) | 2005 | Monkey | L is a dietary source of M-Z |
| Bhosale (34) | 2007 | Human | M-Z distribution varies with age |
| Bhosale (35) | 2007 | Human | MX concentrations higher in persons taking MX supplements |
AMD, age-related macular degeneration; L, lutein; MP, macular pigment; MX, macular xanthophyll (lutein, zeaxanthin, meso-zeaxanthin); M-Z, meso-zeaxanthin; Z, zeaxanthin.
CHEMICAL STRUCTURE OF MXs
MXs are dipolar dihydroxy carotenoids, existing as structural isomers and characterized by an internally symmetrical form with a conjugated polyene chain and 2 terminally hydroxylated ionone rings. Details on structures of MXs (C40H56O2) and their metabolites appear in Khachik et al (28) and Bernstein et al (54). Two- and three-dimensional renderings may be accessed at http://pubchem.ncbi.nlm.nih.gov/ (lutein, CID: 6433159; zeaxanthin, CID: 5280899).
CORE CONCEPTS TO GUIDE TRANSLATIONAL RESEARCH ON MX-AMD RELATIONS
Primates cannot synthesize lutein and zeaxanthin de novo (6, 22) and have adapted with a capacity for efficient MX uptake (26, 55), transport (56, 57), retention (20, 46, 58–60), and protection (49) in the retina; the efficient operation of these processes may be testimony to the physiologic significance of MXs in retinal health and disease. Biological plausibility for protective actions of MXs in AMD is supported by the following: 1) MX structure and natural biophysical properties (43), 2) specific accretion of MXs to the retina from a pool of ∼40 dietary (61) and ∼15 circulating (62, 63) carotenoids, and 3) specific laminar and topographic distribution (23, 24, 31, 32, 64) and unique membrane disposition of MXs (65). A number of unifying concepts, which are helpful in guiding the effort to determine how regulatory mechanisms and metabolic fate of MXs may affect MX-AMD associations, have emerged over the past 2.5 decades. These are as follows:
MX concentration is increased 1000- to 10,000-fold from the circulation to the retina (43, 54) via active transport mechanisms (43) involving specific binding proteins (19, 21, 22, 60, 61).
-
MXs are selectively concentrated (48) and specifically distributed in the retina with optical detection limits at linear distances out to 1.2–1.5 mm from the center of the fovea and biochemical signals quantifiable out to ∼4.0–5.8 mm from this same area (4). Noninvasive in vivo imaging technologies have been used to measure macular pigment optical density (MPOD; a quantitative estimate of the capacity of MXs to attenuate energy in the range of visual blue light), topographic distribution, and membrane disposition of MXs (reviewed in references 54 and 66). MX concentration is ∼1 mmol/L in the human fovea (44) and retinal concentrations can be 2 to 3 orders of magnitude higher than those in other tissues (43, 67). The average mass of lutein + zeaxanthin per unit retinal area is 1.33 ng/mm2 at the foveal center and 0.81 ng/mm2 at an eccentricity of 1.6–2.5 mm (25). The highest concentrations of MXs extend from the center of the fovea to ∼0.10 mm and decline exponentially thereafter. At 2.0 mm eccentricity from the foveal center, biochemical analyses show a 300-fold decrease in MX concentration; here, the in vivo optical detection signal for MXs is negligible. Peak MX concentration in all retinal layers exists at the foveola. The Henle fiber layer (a region in the outer plexiform layer of the central retina containing photoreceptor axons) is the most densely MX-concentrated area per unit area in the eye. In the parafovea, the next most concentrated area is the inner plexiform layer (a region occupied by a neuropil of cellular processes connecting interneurons and retinal ganglion cells). MXs are detectable in appreciable quantities in the outer retina within photoreceptor outer segments (30, 31), albeit in relatively lower concentrations than in the inner retina laminae. MX concentrations are relatively lower in the O2-rich region of the outer retina (photoreceptors and RPE) than they are in the low partial pressure of O2 environment of the inner retina (layer of Henle and inner plexiform layer); however, highly specific lutein-binding proteins are localized to the metabolically active photoreceptor inner segment [reviewed in Bernstein et al (54)].
The distribution of total MXs within retinal laminae varies with retinal eccentricity (25, 27, 44). With increasing distance from the fovea, pigment concentrations decrease most rapidly in inner retina layers. At ∼0.4 mm from the foveal center, the distribution of MXs shows a relatively balanced distribution, with approximately the same density of MXs in the nerve fiber layer as in most other layers. The distribution of specific MXs varies with retinal eccentricity; zeaxanthin and meso-zeaxanthin dominate in the fovea with concentrations declining more rapidly than those of lutein as the distance from the fovea increases (25, 27). The lutein:zeaxanthin ratio at 0–5° is ∼1.0:1.5; at 5–19°, ∼1.5:1.0; and at 19–38°, ∼2.0:1.0 (a 1° angular subtense in the retina represents ∼0.29 mm of retinal extent]. Meso-zeaxanthin is virtually absent in the human food supply and plasma; it is present at similar concentrations to zeaxanthin in the foveola and has negligible signal outside of the fovea (55). The relatively lower concentration of lutein within the central retina has led to speculation that meso-zeaxanthin may be metabolized from oxidized lutein via a cone-photoreceptor–specific enzyme (25, 27, 28, 44). In 2005 Johnson et al (6) identified lutein as a dietary precursor of meso-zeaxanthin.
There is substantial interindividual variation in global (4) and local (68–70) topographic macular pigment density (59, 71–73). Sharifzedah et al classified 5 major patterns in the distribution of macular pigment in elderly people with resonance Raman (74) and 2 wavelength autofluorescence (75) imaging techniques: very low foveal MPOD existed in 10% of those studied; 1 in 5 persons showed a slightly enhanced foveal MPOD with extension of MXs to eccentric regions; 1 in 3 persons expressed a “sole, sharp, central distribution” of MPOD; 20% of persons showed a dense foveal MPOD with a ring of pigment surrounding this area; and 1 in 10 persons expressed a “uniform, laterally extended distribution” of MPOD.
Membrane orientation and localization of MXs affects cytoarchitectural stability, light filtering, and the capacity to modulate oxidative stress in the retina (43, 65).
- MXs show a capacity to affect processes implicated in AMD pathogenesis, because they have been shown to
- interact with key molecules in signal transduction cascades (76, 89–91) inhibiting cell growth and stimulating differentiation (92–94), transactivating nuclear receptors (92), antagonizing nuclear receptor activation (95), influencing expression of connexin genes (96, 97) acting in adhesion complexes to maintain cellular homeostasis (98), and binding immunomodulatory lipocalin proteins (76, 99).
-
5) Biochemical analyses (1, 2, 25, 27, 35) and in vivo imaging studies (54, 66) indicate that genetic (100, 101), dietary (54, 102, 103), and environmental (102) factors can affect MX tissue concentrations within retinal layers and cell types manifesting pathology in AMD (4, 30, 31, 49, 54, 64). Points germane to these issues are as follows:
Serum and plasma MX concentrations vary directly with dietary MX intake (104–112) and supplement use (113, 114), according to most reports.
Neuringer et al (103) and Johnson et al (6) used biochemical and in vivo imaging methods to analyze retinal tissue in rhesus monkeys receiving an MX intervention to provide direct evidence that 1) MX supplementation late in life is capable of strongly increasing retinal MX concentrations, even after “nutritional deprivation”, and 2) lutein is a dietary source of meso-zeaxanthin. This in vivo primate model also was used to examine the effect of MX intake on the retinal vulnerability to acute photochemical damage induced by small-spot exposures of coherent light at 476 nm (115). Rhesus monkeys were fed a lifelong MX-free diet, and at the start of the study had no MXs detected biochemically in serum or adipose tissue or by reflectometry in the retina. The absence of retinal MX was confirmed biochemically in post mortem samples (6). Dietary supplementation with either pure lutein or zeaxanthin for 6–24 mo brought retinal MXs to concentrations similar to or higher than those of animals receiving dietary MXs across their life spans (6). After ∼6 mo of MX supplementation, the animals showed reduced amounts of ophthalmoscopically determined damage within the fovea, compared with measurements made before supplementation (115). The degree of foveal damage in the supplemented animals was similar to that of animals fed since birth on a standard laboratory-based diet containing MXs and significantly less than that of a comparison group maintained on the MX-free diet. Furthermore, in both the standard diet controls and the supplemented animals, the foveal region showed less damage than the parafoveal region located at 1.5 mm (∼6°) eccentricity, outside the area of detectable MPOD, whereas before supplementation no such foveal photoprotection was found. No differences due to MX status or supplementation were seen in the parafoveal region beyond the area of dense macular pigment.
Biochemical measurement of retinal response to MX intake exists from post mortem human studies. Bhosale et al (35) reported 3-fold higher retinal MX concentrations in ∼20% of eye donors from their 131-person cohort aged ≥48 y. Retrospective surveys on MX intake for this high-MX-retinal-status group indicated frequent daily use of high-dose MX supplements in 82% of people; the remaining 18% had a history of consuming MX-rich diets. Surveys on a random sample of 20 eye donors with retinal MX concentrations within expected limits indicated that none of these people regularly took MX supplements or consumed foods concentrated in MXs.
Increased MX intake (11–13, 16, 17) and status (33, 36, 37, 45) are inversely associated with advanced AMD. As with work on MX status-AMD relations, equivocal findings exist for endpoints restricted to early or intermediate AMD.
THE PROMISE OF MOLECULAR GENETICS FOR EXAMINING THE EFFECT OF MXs ON AMD
AMD is a complex disease with a strong hereditary component (116). Aspects of retinal MX absorption, distribution, metabolism, and excretion are genetically determined, as shown by studies in twins (117, 118) and first-degree family members (119–121). Notably, retinal MX status profiles have shown stronger relations among monozygotic than dizygotic twins (101). Findings from a large cohort of married people indicate that interspouse relations exist for dietary intake and serum concentrations of MXs (100), but not for MPOD (a proxy for retinal MX status). Work in the field examining the molecular genetics of AMD has focused on both DNA sequence variation in genes encoding constituents of the complement system and those involved in HDL transport and metabolism. The link between MX status, complement genes, and AMD (122) has been examined in a cross-sectional sample of 302 healthy adults (123); carriers of AMD-associated complement factor H and age-related maculopathy susceptibility 2 (ARMS2) variants had lower MPOD values than their peers. An axis of AMD-HDL-MX status relations may also exist, because MXs are carried on HDL (124) and interact with numerous receptors and transporters affecting cholesterol metabolism.
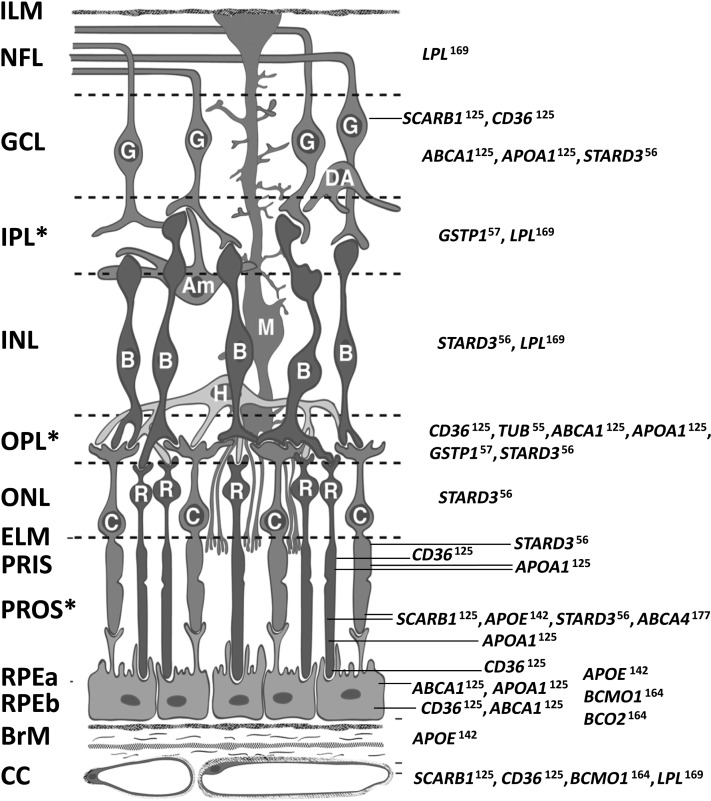
Key references on relations of AMD with variants in genes encoding receptors, targets, transporters, enzymes, and hormones affecting or affected by MXs, their metabolites, and cofactors are shown in Table 2; findings are presented in the context of information on MX status relations with these same genes. Findings from immunolocalization studies on constituents of this MX gene set in primate retina are summarized in Figure 1 (55, 57, 125, 142, 164, 169). Information on gene expression for these genes exists at the Retina Central database (University of Regensburg, http://www.retinacentral.org/) and the NEIBank (http://neibank.nei.nih.gov/index.shtml). We are currently applying the evidence base presented in Table 2 and Figure 1 to guide investigations designed to identify core elements of a “molecular phenotype” (a pattern gene regulation/expression and DNA variation) representing individual capacity to use transporters, receptors, enzymes, and hormones targeting or affected by MXs in ways that may reduce risk of AMD incidence or progression. Although we have not yet identified any single sequence variant explaining a proportion of variance in AMD risk comparable to those of the complement pathway genes, there is now informative work examining putative AMD-associated single-nucleotide polymorphisms present in genes encoding proteins involved in MX transport (121, 131, 137, 146, 148–154, 156–161), binding/capture (141, 162, 163), cleavage (158, 160), and diseases associated with lower MX status (177–184). Projects examining the associations of MX-related genes with retinal pathophysiology in in vivo models have supported inferences on AMD relations with variants in MX transport genes (128, 130, 136, 143).
TABLE 2.
Studies that examined relations of genes with MX status and AMD1
| Relation |
|||||||
| Gene-MX status |
Gene-AMD |
||||||
| Action on MXs (reference) | Gene symbol | In vitro | In/ex vivo | Human | In vitro | In/ex vivo | Human |
| 1. Uptake, transport, and accretion of MXs | |||||||
| MX transport in intestine, choroid, RPE | CD36 (125) | ARPE-19 (126) | — | (127) | (128) | CD36−/− (128–130) | (131) |
| MX transport in intestine, choroid, RPE | SCARB1 (125) | ARPE-19 (126) | Fly (132–134) | (135) | — | SCARB−/− (136) | (137) |
| MX accretion/transport in OPL (137) | Tubulins (55) | — | Primate (179) | (140) | — | — | (141) |
| MX transport in circulation | APOE (142) | — | ApoE−/− (143) | MPOD (144, 145) | — | — | (146–154) |
| MX transport /binding on HDL-C | ABCA1 (125) | — | WHAM−/− (124) | — | — | Human (155) | (156–160) |
| MX transport /binding on HDL-C | APOA1 (125) | — | WHAM−/− (124) | — | — | — | (161) |
| Specific zeaxanthin uptake in retina | GSTP1 (57) | — | — | Retina (55, 57) | — | — | (162, 163) |
| Specific lutein uptake in retina | STARD3 (56) | — | Human (56) | Retina (55, 56) | — | — | — |
| 2. Cleavage of MXs | |||||||
| 15,15′ (symmetric) cleavage | BCMO1 (164) | — | — | (165), MPOD (127) | — | — | — |
| 9′,10′ (eccentric) cleavage | BCO2 (264) | (91)2 | (166–169) | – | — | — | — |
| Synthesis/degradation of MX-rich HDL-C | LPL (169) | — | — | Serum (170) | — | — | (158, 160) |
| 3. Antioxidant potential | |||||||
| Structural integrity of DNA | POLB, POLL | (171) | — | — | — | — | — |
| 4. Effect on adaptive cellular response | |||||||
| Adhesion complex signaling | Connexins | (96) | — | — | (172) | — | — |
| Proliferation/apoptosis | CDK2, CCND1 | (91)2 | — | — | – | — | — |
| 5. Interference with growth factors | IGF genes | (91)2 | — | — | (173) | — | (174, 175)3 |
| 6. Diseases associated with low MX status | |||||||
| Sjögren-Larsson syndrome | ALDH3A2 | — | — | Retina (176) | — | — | — |
| Stargardt disease 1/cone-rod dystrophy | ABCA4 (177) | — | — | (21) | — | — | (177–184) |
Numbers in parentheses correspond to reference numbers. Full names for genes represented by symbols are available from http://www.ncbi.nlm.nih.gov/gene. References in the “Gene symbol” column refer to immunolocalization studies in primate retina. AMD, age-related macular degeneration; ApoE, apolipoprotein E; ARPE-19, human retinal pigment epithelium immortalized cell line; CD36, CD36 molecule (thrombospondin receptor); HDL-C, HDL cholesterol; MPOD, macular pigment optical density; MX, macular xanthophyll; OPL, outer plexiform layer; RPE, retinal pigmented epithelium; SCARB, scavenger receptor class B; WHAM, Wisconsin hypoalpha mutant; −/−, double knockout.
Evidence based on tests with lycopene.
Retinopathy endpoint.
FIGURE 1.
Distribution of proteins affecting or affected by macular xanthophylls in primate retina. Full names for genes are available from http://www.ncbi.nlm.nih.gov/gene. Superscripts on gene symbols refer to reference numbers of immunolocalization studies containing micrographs. Am, amacrine cell; B, bipolar cell; BrM, Bruch's membrane; C, cone photoreceptors; CC, retinal choroid layer; DA, displaced amacrine cell; ELM, external limiting membrane; G, ganglion cell; GCL, ganglion cell layer; H, horizontal cell; ILM, inner limiting membrane; INL, inner nuclear layer; IPL, inner plexiform layer (interneurons); M, Müller cell; NFL, nerve fiber layer; ONL, outer nuclear layer; OPL, outer plexiform layer; PRIS, photoreceptor inner segments; PROS, photoreceptor outer segments; R, rod photoreceptors; RPEa, retinal pigmented epithelium apical area; RPEb, retinal pigmented epithelium basal area. *Retinal layers with macular xanthophyll concentrations. The schematic was created by D Fisher and reproduced with permission from reference 187.
KNOWLEDGE GAPS AND RESEARCH OPPORTUNITIES
Findings on the biochemical and biophysical actions of MXs in primates may be integrated with bioinformatic data on MX-related genes and proteins to investigate the putative actions of these factors in AMD pathogenesis and pathophysiology. We see promising opportunities for meaningful advances in the field with the following:
Ultrastructural localization studies on MX-affected proteins in the retina. Although specific binding proteins for lutein and zeaxanthin exist in the human and monkey retina (19, 21, 22, 25, 67), sites of subcellular MX localization are still unknown (65).
Broader analysis of genetic variation and regulation in DNA sequence encoding proteins responsible for MX binding in the retina. Genotyping efforts for STARD3 and GSTP1 have not been comprehensive. Exome-focused sequencing and analysis of regulatory elements (microRNA and transcription factor binding sites, histone methylation and acetylation marks) are likely to yield informative results. We provide a list of single-nucleotide polymorphisms capable of producing deleterious peptide shifts in STARD3 and GSTP1 (see the supplemental material under “Supplemental data” in the online issue).
Development of model systems (in vivo animal models and targeted mutagenesis in human retinal cells) based on findings from
• natural models of metabolic MX insufficiency (ALDH3A2 and ABCA4 genes)
• findings from efforts discussed in number 2
Integrated systems-based approaches to examine metabolic fate of MXs, their precursors, and metabolites. AMD is a polygenic disease, and we must reasonably examine relations of gene variation, regulation, and expression in dynamic biological systems. We give the example for GSPT1 and the protein encoded by the Fanconi anemia complementation group C (FANCC, 9q22.3). The FANCC gene product increases catalytic activity of GSTP1 during apoptosis by preserving the structure of bonds in GSTP1 that normally sustain disruptions in disulfide structure with exposure to oxidizing agents (158). Direct interaction of FANCC and GSTP1 has been shown in a model by using the in vitro coimmunoprecipitation paradigm (186). We have observed a number of coinherited AMD-associated variants in FANCC in a number of our large-scale cohorts (rs356666, P ≤ 0.004; rs356677, P ≤ 0.001; rs356669, P ≤ 0.0005). rs356669 exists in a binding domain for MafK and MafF transcription factors; this relation may provide leads in the study of GSTP1 activation in AMD. Although P values are not in the range of those reported for complement system genes, the biological plausibility of these findings in the context of the evidence base supports the rationale to extend investigations in future studies.
Concepts discussed in this article provide a foundation for planning mechanistic, translational, and applied clinical research projects for investigation of MX-AMD relations. To make meaningful inferences from such efforts, multidisciplinary teams must work to 1) develop a mechanism- and system-centered evidence base on the molecular genetics of enzymes, transporters, ligands, and receptors affecting or affected by MXs and influencing MX uptake, transport, retention, and protection in the neural and vascular retina, and 2) apply this evidence base in the context of the science on AMD pathogenesis and pathophysiology.
Acknowledgments
The authors’ responsibilities were as follows—JPS and MN: conceived, designed, and wrote the manuscript; and JPS had primary responsiblity for final content. Both authors read and approved the final manuscript. Neither of the authors had a conflict of interest.
Footnotes
Abbreviations used: AAMD, advanced age-related macular degeneration; AMD, age-related macular degeneration; MPOD, macular pigment optical density; MX, macular xanthophyll; RPE, retinal pigmented epithelium.
REFERENCES
- 1.Bone RA, Landrum JT, Tarsis SL. Preliminary identification of the human macular pigment. Vision Res 1985;25(11):1531–5 [DOI] [PubMed] [Google Scholar]
- 2.Handelman GJ, Dratz EA, Reay CC, van Kuijk JG. Carotenoids in the human macula and whole retina. Invest Ophthalmol Vis Sci 1988;29(6):850–5 [PubMed] [Google Scholar]
- 3.Nussbaum JJ, Pruett RC, Delori FC. Historic perspectives. Macular yellow pigment. The first 200 years. Retina 1981;1(4):296–310 [PubMed] [Google Scholar]
- 4.Beatty S, Boulton M, Henson D, Koh HH, Murray IJ. Macular pigment and age related macular degeneration. British Journal of Ophthalmology 1999;83(7):867–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitehead AJ, Mares JA, Danis RP. Macular pigment: a review of current knowledge. Arch Ophthalmol 2006;124(7):1038–45 [DOI] [PubMed] [Google Scholar]
- 6.Johnson EJ, Neuringer M, Russell RM, Schalch W, Snodderly DM. Nutritional manipulation of primate retinas, III: Effects of lutein or zeaxanthin supplementation on adipose tissue and retina of xanthophyll-free monkeys. Invest Ophthalmol Vis Sci 2005;46(2):692–702 [DOI] [PubMed] [Google Scholar]
- 7.Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122(4):564–72 [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Chou CF, Klein BE, Zhang X, Meuer SM, Saaddine JB. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol 2011;129(1):75–80 [DOI] [PubMed] [Google Scholar]
- 9.Congdon N, O'Colmain B, Klaver CCW, Klein R, Muoz B, Friedman D, Kempen J, Taylor H, Mitchell P. Causes and prevalence of visual impairment among adults in the United States. Archives of ophthalmology 2004;122(4):477–85 [DOI] [PubMed] [Google Scholar]
- 10.Risk factors for neovascular age-related macular degeneration The Eye Disease Case-Control Study Group. Arch Ophthalmol 1992;110(12):1701–8 [DOI] [PubMed] [Google Scholar]
- 11.Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Miller DT, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. Journal of the American Medical Association 1994;272(18):1413–20 [PubMed] [Google Scholar]
- 12.Snellen EL, Verbeek AL, Van Den Hoogen GW, Cruysberg JR, Hoyng CB. Neovascular age-related macular degeneration and its relationship to antioxidant intake. Acta Ophthalmol Scand 2002;80(4):368–71 [DOI] [PubMed] [Google Scholar]
- 13.SanGiovanni JP, Chew EY, Clemons TE, Ferris FL, 3rd, Gensler G, Lindblad AS, Milton RC, Seddon JM, Sperduto RD. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol 2007;125(9):1225–32 [DOI] [PubMed] [Google Scholar]
- 14.Gale CR, Hall NF, Phillips DI, Martyn CN. Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest Ophthalmol Vis Sci 2003;44(6):2461–5 [DOI] [PubMed] [Google Scholar]
- 15.Delcourt C, Carriere I, Delage M, Barberger-Gateau P, Schalch W. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA Study. Invest Ophthalmol Vis Sci 2006;47(6):2329–35 [DOI] [PubMed] [Google Scholar]
- 16.Cho E, Hankinson SE, Rosner B, Willett WC, Colditz GA. Prospective study of lutein/zeaxanthin intake and risk of age-related macular degeneration. Am J Clin Nutr 2008;87(6):1837–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan JS, Wang JJ, Flood V, Rochtchina E, Smith W, Mitchell P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology 2008;115(2):334–41 [DOI] [PubMed] [Google Scholar]
- 18.Bird AC. Therapeutic targets in age-related macular disease. J Clin Invest 2010;120(9):3033–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol 2003;48(3):257–93 [DOI] [PubMed] [Google Scholar]
- 20.Bone RA, Landrum JT, Guerra LH, Ruiz CA. Lutein and zeaxanthin dietary supplements raise macular pigment density and serum concentrations of these carotenoids in humans. J Nutr 2003;133(4):992–8 [DOI] [PubMed] [Google Scholar]
- 21.Aleman TS, Cideciyan AV, Windsor EA, Schwartz SB, Swider M, Chico JD, Sumaroka A, Pantelyat AY, Duncan KG, Gardner LM, et al. Macular pigment and lutein supplementation in ABCA4-associated retinal degenerations. Invest Ophthalmol Vis Sci 2007;48(3):1319–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malinow MR, Feeney-Burns L, Peterson LH, Klein ML, Neuringer M. Diet-related macular anomalies in monkeys. Invest Ophthalmol Vis Sci 1980;19(8):857–63 [PubMed] [Google Scholar]
- 23.Snodderly DM, Brown PK, Delori FC, Auran JD. The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Invest Ophthalmol Vis Sci 1984;25(6):660–73 [PubMed] [Google Scholar]
- 24.Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci 1984;25(6):674–85 [PubMed] [Google Scholar]
- 25.Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci 1988;29(6):843–9 [PubMed] [Google Scholar]
- 26.Handelman GJ, Snodderly DM, Krinsky NI, Russett MD, Adler AJ. Biological control of primate macular pigment. Biochemical and densitometric studies. Invest Ophthalmol Vis Sci 1991;32(2):257–67 [PubMed] [Google Scholar]
- 27.Bone RA, Landrum JT, Friedes LM, Gomez CM, Kilburn MD, Menendez E, Vidal I, Wang W. Distribution of lutein and zeaxanthin stereoisomers in the human retina. Exp Eye Res 1997;64(2):211–8 [DOI] [PubMed] [Google Scholar]
- 28.Khachik F, Bernstein PS, Garland DL. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci 1997;38(9):1802–11 [PubMed] [Google Scholar]
- 29.Bernstein PS, Yoshida MD, Katz NB, McClane RW, Gellermann W. Raman detection of macular carotenoid pigments in intact human retina. Invest Ophthalmol Vis Sci 1998;39(11):2003–11 [PubMed] [Google Scholar]
- 30.Sommerburg OG, Siems WG, Hurst JS, Lewis JW, Kliger DS, van Kuijk FJ. Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr Eye Res 1999;19(6):491–5 [DOI] [PubMed] [Google Scholar]
- 31.Rapp LM, Maple SS, Choi JH. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest Ophthalmol Vis Sci 2000;41(5):1200–9 [PubMed] [Google Scholar]
- 32.Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res 2001;72(3):215–23 [DOI] [PubMed] [Google Scholar]
- 33.Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, Twaroska EE. Macular pigment in donor eyes with and without AMD: a case-control study. Invest Ophthalmol Vis Sci 2001;42(1):235–40 [PubMed] [Google Scholar]
- 34.Bhosale P, Zhao da Y, Serban B, Bernstein PS. Identification of 3-methoxyzeaxanthin as a novel age-related carotenoid metabolite in the human macula. Invest Ophthalmol Vis Sci 2007;48(4):1435–40 [DOI] [PubMed] [Google Scholar]
- 35.Bhosale P, Zhao da Y, Bernstein PS. HPLC measurement of ocular carotenoid levels in human donor eyes in the lutein supplementation era. Invest Ophthalmol Vis Sci 2007;48(2):543–9 [DOI] [PubMed] [Google Scholar]
- 36.Mares-Perlman JA, Brady WE, Klein R, Klein BE, Bowen P, Stacewicz-Sapuntzakis M, Palta M. Serum antioxidants and age-related macular degeneration in a population-based case-control study. Arch Ophthalmol 1995;113(12):1518–23 [DOI] [PubMed] [Google Scholar]
- 37.Antioxidant status and neovascular age-related macular degeneration Eye Disease Case-Control Study Group. Arch Ophthalmol 1993;111(1):104–9 [DOI] [PubMed] [Google Scholar]
- 38.Bone RA, Landrum JT, Hime GW, Cains A, Zamor J. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci 1993;34(6):2033–40 [PubMed] [Google Scholar]
- 39. The Gordon Research Conference on Carotenoids ( http://www.grc.org/conferences.aspx?id=0000309) [Google Scholar]
- 40. The International Carotenoid Society ( http://www.carotenoidsociety.org/meetings-and-conferences) [Google Scholar]
- 41. The Oxygen Club of California ( http://www.oxyclubcalifornia.org/OCC/past_OCC_meetings.php) [Google Scholar]
- 42.Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press, 2000 [PubMed] [Google Scholar]
- 43.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr 2003;23:171–201 [DOI] [PubMed] [Google Scholar]
- 44.Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys 2001;385(1):28–40 [DOI] [PubMed] [Google Scholar]
- 45.Landrum JT, Bone RA, Kilburn MD. The macular pigment: a possible role in protection from age-related macular degeneration. Adv Pharmacol 1997;38:537–56 [DOI] [PubMed] [Google Scholar]
- 46.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 2000;45(2):115–34 [DOI] [PubMed] [Google Scholar]
- 47.Schalch W, Dayhaw-Barker P, Barker FM., II The Carotenoids of the Human Retina. Edtion ed. In: Taylor A, ed. Nutritional and Environmental Influences on the Eye. Washington, D.C.: CRC Press, 1999:215–50. [Google Scholar]
- 48.Schalch W. Carotenoids in the retina–a review of their possible role in preventing or limiting damage caused by light and oxygen. Exs 1992;62:280–98 [DOI] [PubMed] [Google Scholar]
- 49.Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr 1995;62(6 Suppl):1448S-61S [DOI] [PubMed] [Google Scholar]
- 50.Mares-Perlman JA, Millen AE, Ficek TL, Hankinson SE. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. Overview. J Nutr 2002;132(3):518S–24S [DOI] [PubMed] [Google Scholar]
- 51.Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, Pei K, Tsipursky M, Nyland J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry 2004;75(4):216–30 [DOI] [PubMed] [Google Scholar]
- 52.Bartlett HE, Eperjesi F. Effect of lutein and antioxidant dietary supplementation on contrast sensitivity in age-related macular disease: a randomized controlled trial. Eur J Clin Nutr 2007;61(9):1121–7 [DOI] [PubMed] [Google Scholar]
- 53.Neelam K, Hogg RE, Stevenson MR, Johnston E, Anderson R, Beatty S, Chakravarthy U. Carotenoids and co-antioxidants in age-related maculopathy: design and methods. Ophthalmic Epidemiol 2008;15(6):389–401 [DOI] [PubMed] [Google Scholar]
- 54.Bernstein PS, Delori FC, Richer S, van Kuijk FJ, Wenzel AJ. The value of measurement of macular carotenoid pigment optical densities and distributions in age-related macular degeneration and other retinal disorders. Vision Res 2010;50(7):716–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li B, Vachali P, Bernstein PS. Human ocular carotenoid-binding proteins. Photochem Photobiol Sci 2010;9(11):1418–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li B, Vachali P, Frederick JM, Bernstein PS. Identification of StARD3 as a Lutein-Binding Protein in the Macula of the Primate Retina. Biochemistry 2011;50(13):2541–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhosale P, Larson AJ, Frederick JM, Southwick K, Thulin CD, Bernstein PS. Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J Biol Chem 2004;279(47):49447–54 [DOI] [PubMed] [Google Scholar]
- 58.Hammond BR, Jr, Johnson EJ, Russell RM, Krinsky NI, Yeum KJ, Edwards RB, Snodderly DM. Dietary modification of human macular pigment density. Invest Ophthalmol Vis Sci 1997;38(9):1795–801 [PubMed] [Google Scholar]
- 59.Hammond BR, Jr, Wooten BR, Snodderly DM. Individual variations in the spatial profile of human macular pigment. J Opt Soc Am A Opt Image Sci Vis 1997;14(6):1187–96 [DOI] [PubMed] [Google Scholar]
- 60.Zeimer M, Hense HW, Heimes B, Austermann U, Fobker M, Pauleikhoff D. [The macular pigment: short- and intermediate-term changes of macular pigment optical density following supplementation with lutein and zeaxanthin and co-antioxidants. The LUNA Study]. Ophthalmologe 2009;106(1):29–36 [DOI] [PubMed] [Google Scholar]
- 61.Khachik F, Beecher GR, Goli MB, Lusby WR. Separation and quantitation of carotenoids in foods. Methods Enzymol 1992;213:347–59 [DOI] [PubMed] [Google Scholar]
- 62.Khachik F, Beecher GR, Goli MB, Lusby WR, Daitch CE. Separation and quantification of carotenoids in human plasma. Methods Enzymol 1992;213:205–19 [DOI] [PubMed] [Google Scholar]
- 63.Khachik F, Spangler CJ, Smith JC, Jr, Canfield LM, Steck A, Pfander H. Identification, quantification, and relative concentrations of carotenoids and their metabolites in human milk and serum. Anal Chem 1997;69(10):1873–81 [DOI] [PubMed] [Google Scholar]
- 64.Sommerburg O, Siems WG, van Kuijk FJ. Localization of carotenoids in different eye tissues. Biofactors 2000;11(1-2):3–6 [DOI] [PubMed] [Google Scholar]
- 65.Subczynski WK, Wisniewska A, Widomska J. Location of macular xanthophylls in the most vulnerable regions of photoreceptor outer-segment membranes. Arch Biochem Biophys 2010;504(1):61–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beatty S, van Kuijk FJ, Chakravarthy U. Macular pigment and age-related macular degeneration: longitudinal data and better techniques of measurement are needed. Invest Ophthalmol Vis Sci 2008;49(3):843–5 [DOI] [PubMed] [Google Scholar]
- 67.Kaplan LA, Lau JM, Stein EA. Carotenoid composition, concentrations, and relationships in various human organs. Clin Physiol Biochem 1990;8(1):1–10 [PubMed] [Google Scholar]
- 68.Nolan JM, Stringham JM, Beatty S, Snodderly DM. Spatial profile of macular pigment and its relationship to foveal architecture. Invest Ophthalmol Vis Sci 2008;49(5):2134–42 [DOI] [PubMed] [Google Scholar]
- 69.Delori FC, Goger DG, Keilhauer C, Salvetti P, Staurenghi G. Bimodal spatial distribution of macular pigment: evidence of a gender relationship. J Opt Soc Am A Opt Image Sci Vis 2006;23(3):521–38 [DOI] [PubMed] [Google Scholar]
- 70.Berendschot TT, van Norren D. Macular pigment shows ringlike structures. Invest Ophthalmol Vis Sci 2006;47(2):709–14 [DOI] [PubMed] [Google Scholar]
- 71.Werner JS, Donnelly SK, Kliegl R. Aging and human macular pigment density. Appended with translations from the work of Max Schultze and Ewald Hering. Vision Res 1987;27(2):257–68 [DOI] [PubMed] [Google Scholar]
- 72.Pease PL, Adams AJ, Nuccio E. Optical density of human macular pigment. Vision Res 1987;27(5):705–10 [DOI] [PubMed] [Google Scholar]
- 73.Hammond BR, Jr, Fuld K. Interocular differences in macular pigment density. Invest Ophthalmol Vis Sci 1992;33(2):350–5 [PubMed] [Google Scholar]
- 74.Sharifzadeh M, Zhao DY, Bernstein PS, Gellermann W. Resonance Raman imaging of macular pigment distributions in the human retina. J Opt Soc Am A Opt Image Sci Vis 2008;25(4):947–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharifzadeh M, Bernstein PS, Gellermann W. Nonmydriatic fluorescence-based quantitative imaging of human macular pigment distributions. J Opt Soc Am A Opt Image Sci Vis 2006;23(10):2373–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Demmig-Adams B, Adams WW., 3rd Antioxidants in photosynthesis and human nutrition. Science 2002;298(5601):2149–53 [DOI] [PubMed] [Google Scholar]
- 77.Wisniewska A, Subczynski WK. Distribution of macular xanthophylls between domains in a model of photoreceptor outer segment membranes. Free Radic Biol Med 2006;41(8):1257–65 [DOI] [PubMed] [Google Scholar]
- 78.Boulton M, Rózanowska M, Rózanowski B. Retinal photodamage. Journal of Photochemistry and Photobiology B: Biology 2001;64(2-3):144–61 [DOI] [PubMed] [Google Scholar]
- 79.Ham WT, Jr, Mueller HA, Ruffolo JJ, Jr, Millen JE, Cleary SF, Guerry RK, Guerry D., 3rd Basic mechanisms underlying the production of photochemical lesions in the mammalian retina. Curr Eye Res 1984;3(1):165–74 [DOI] [PubMed] [Google Scholar]
- 80.Kim SR, Nakanishi K, Itagaki Y, Sparrow JR. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp Eye Res 2006;82(5):828–39 [DOI] [PubMed] [Google Scholar]
- 81.Sujak A, Gabrielska J, Grudzinski W, Borc R, Mazurek P, Gruszecki WI. Lutein and zeaxanthin as protectors of lipid membranes against oxidative damage: the structural aspects. Arch Biochem Biophys 1999;371(2):301–7 [DOI] [PubMed] [Google Scholar]
- 82.Trevithick-Sutton CC, Foote CS, Collins M, Trevithick JR. The retinal carotenoids zeaxanthin and lutein scavenge superoxide and hydroxyl radicals: a chemiluminescence and ESR study. Mol Vis 2006;12:1127–35 [PubMed] [Google Scholar]
- 83.Bhosale P, Bernstein PS. Synergistic effects of zeaxanthin and its binding protein in the prevention of lipid membrane oxidation. Biochim Biophys Acta 2005;1740(2):116–21 [DOI] [PubMed] [Google Scholar]
- 84.Wrona M, Rozanowska M, Sarna T. Zeaxanthin in combination with ascorbic acid or alpha-tocopherol protects ARPE-19 cells against photosensitized peroxidation of lipids. Free Radic Biol Med 2004;36(9):1094–101 [DOI] [PubMed] [Google Scholar]
- 85.Siems WG, Sommerburg O, van Kuijk FJ. Lycopene and beta-carotene decompose more rapidly than lutein and zeaxanthin upon exposure to various pro-oxidants in vitro. Biofactors 1999;10(2-3):105–13 [DOI] [PubMed] [Google Scholar]
- 86.Cantrell A, McGarvey DJ, Truscott TG, Rancan F, Bohm F. Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Arch Biochem Biophys 2003;412(1):47–54 [DOI] [PubMed] [Google Scholar]
- 87.Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys 2001;385(1):20–7 [DOI] [PubMed] [Google Scholar]
- 88.Krinsky NI, Yeum KJ. Carotenoid-radical interactions. Biochem Biophys Res Commun 2003;305(3):754–60 [DOI] [PubMed] [Google Scholar]
- 89.Krinsky NI. Effects of carotenoids in cellular and animal systems. Am J Clin Nutr 1991;53(1 Suppl):238S-46S [DOI] [PubMed] [Google Scholar]
- 90.Krinsky NI. Mechanism of action of biological antioxidants. Proc Soc Exp Biol Med 1992;200(2):248–54 [DOI] [PubMed] [Google Scholar]
- 91.Mein JR, Lian F, Wang XD. Biological activity of lycopene metabolites: implications for cancer prevention. Nutr Rev 2008;66(12):667–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lian F, Smith DE, Ernst H, Russell RM, Wang XD. Apo-10'-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis 2007;28(7):1567–74 [DOI] [PubMed] [Google Scholar]
- 93.Prakash P, Liu C, Hu KQ, Krinsky NI, Russell RM, Wang XD. Beta-carotene and beta-apo-14'-carotenoic acid prevent the reduction of retinoic acid receptor beta in benzo[a]pyrene-treated normal human bronchial epithelial cells. J Nutr 2004;134(3):667–73 [DOI] [PubMed] [Google Scholar]
- 94.Tibaduiza EC, Fleet JC, Russell RM, Krinsky NI. Excentric cleavage products of beta-carotene inhibit estrogen receptor positive and negative breast tumor cell growth in vitro and inhibit activator protein-1-mediated transcriptional activation. J Nutr 2002;132(6):1368–75 [DOI] [PubMed] [Google Scholar]
- 95.Ziouzenkova O, Orasanu G, Sukhova G, Lau E, Berger JP, Tang G, Krinsky NI, Dolnikowski GG, Plutzky J. Asymmetric cleavage of beta-carotene yields a transcriptional repressor of retinoid X receptor and peroxisome proliferator-activated receptor responses. Mol Endocrinol 2007;21(1):77–88 [DOI] [PubMed] [Google Scholar]
- 96.Zhang LX, Cooney RV, Bertram JS. Carotenoids enhance gap junctional communication and inhibit lipid peroxidation in C3H/10T1/2 cells: relationship to their cancer chemopreventive action. Carcinogenesis 1991;12(11):2109–14 [DOI] [PubMed] [Google Scholar]
- 97.Basu HN, Del Vecchio AJ, Flider F, Orfhoefer FT. Nutritional and potentential disease prevention properties of carotenoids. Journal of the American Oil Chemists Society 2001;78:665–75 [Google Scholar]
- 98.Dbouk HA, Mroue RM, El-Sabban ME, Talhouk RS. Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun Signal 2009;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bugos RC, Hieber AD, Yamamoto HY. Xanthophyll cycle enzymes are members of the lipocalin family, the first identified from plants. J Biol Chem 1998;273(25):15321–4 [DOI] [PubMed] [Google Scholar]
- 100.Wenzel AJ, Sheehan JP, Burke JD, Lefsrud MG, Curran-Celentano J. Dietary intake and serum concentrations of lutein and zeaxanthin, but not macular pigment optical density, are related in spouses. Nutrition Research 2007;27(8):462–9 [Google Scholar]
- 101.Liew SH, Gilbert CE, Spector TD, Mellerio J, Marshall J, van Kuijk FJ, Beatty S, Fitzke F, Hammond CJ. Heritability of macular pigment: a twin study. Invest Ophthalmol Vis Sci 2005;46(12):4430–6 [DOI] [PubMed] [Google Scholar]
- 102.Mares JA, LaRowe TL, Snodderly DM, Moeller SM, Gruber MJ, Klein ML, Wooten BR, Johnson EJ, Chappell RJ. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study, an ancillary study of the Women's Health Initiative. Am J Clin Nutr 2006;84(5):1107–22 [DOI] [PubMed] [Google Scholar]
- 103.Neuringer M, Sandstrom MM, Johnson EJ, Snodderly DM. Nutritional manipulation of primate retinas, I: effects of lutein or zeaxanthin supplements on serum and macular pigment in xanthophyll-free rhesus monkeys. Invest Ophthalmol Vis Sci 2004;45(9):3234–43 [DOI] [PubMed] [Google Scholar]
- 104.Hammond BR, Jr, Curran-Celentano J, Judd S, Fuld K, Krinsky NI, Wooten BR, Snodderly DM. Sex differences in macular pigment optical density: relation to plasma carotenoid concentrations and dietary patterns. Vision Res 1996;36(13):2001–12 [DOI] [PubMed] [Google Scholar]
- 105.Carroll YL, Corridan BM, Morrissey PA. Carotenoids in young and elderly healthy humans: dietary intakes, biochemical status and diet-plasma relationships. Eur J Clin Nutr 1999;53(8):644–53 [DOI] [PubMed] [Google Scholar]
- 106.Bone RA, Landrum JT, Dixon Z, Chen Y, Llerena CM. Lutein and zeaxanthin in the eyes, serum and diet of human subjects. Exp Eye Res 2000;71(3):239–45 [DOI] [PubMed] [Google Scholar]
- 107.Brady WE, Mares-Perlman JA, Bowen P, Stacewicz-Sapuntzakis M. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J Nutr 1996;126(1):129–37 [DOI] [PubMed] [Google Scholar]
- 108.Ciulla TA, Curran-Celantano J, Cooper DA, Hammond BR, Jr, Danis RP, Pratt LM, Riccardi KA, Filloon TG. Macular pigment optical density in a midwestern sample. Ophthalmology 2001;108(4):730–7 [DOI] [PubMed] [Google Scholar]
- 109.Hammond BR, Jr, Ciulla TA, Snodderly DM. Macular pigment density is reduced in obese subjects. Invest Ophthalmol Vis Sci 2002;43(1):47–50 [PubMed] [Google Scholar]
- 110.Nolan J, O'Donovan O, Kavanagh H, Stack J, Harrison M, Muldoon A, Mellerio J, Beatty S. Macular pigment and percentage of body fat. Invest Ophthalmol Vis Sci 2004;45(11):3940–50 [DOI] [PubMed] [Google Scholar]
- 111.Rock CL, Thornquist MD, Neuhouser ML, Kristal AR, Neumark-Sztainer D, Cooper DA, Patterson RE, Cheskin LJ. Diet and lifestyle correlates of lutein in the blood and diet. J Nutr 2002;132(3):525S–30S [DOI] [PubMed] [Google Scholar]
- 112.Burke JD, Curran-Celentano J, Wenzel AJ. Diet and serum carotenoid concentrations affect macular pigment optical density in adults 45 years and older. J Nutr 2005;135(5):1208–14 [DOI] [PubMed] [Google Scholar]
- 113.Rosenthal JM, Kim J, de Monasterio F, Thompson DJ, Bone RA, Landrum JT, de Moura FF, Khachik F, Chen H, Schleicher RL, et al. Dose-ranging study of lutein supplementation in persons aged 60 years or older. Invest Ophthalmol Vis Sci 2006;47(12):5227–33 [DOI] [PubMed] [Google Scholar]
- 114.Huang LL, Coleman HR, Kim J, de Monasterio F, Wong WT, Schleicher RL, Ferris FL, 3rd, Chew EY. Oral supplementation of lutein/zeaxanthin and omega-3 long chain polyunsaturated fatty acids in persons aged 60 years or older, with or without AMD. Invest Ophthalmol Vis Sci 2008;49(9):3864–9 [DOI] [PubMed] [Google Scholar]
- 115.Barker FM, 2nd, Snodderly DM, Johnson EJ, Schalch W, Koepcke W, Gerss J, Neuringer M. Nutritional Manipulation of Primate Retinas. V: Effects of Lutein, Zeaxanthin and n–3 Fatty Acids on Retinal Sensitivity to Blue Light Damage. Invest Ophthalmol Vis Sci 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Swaroop A, Chew EY, Rickman CB, Abecasis GR. Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu Rev Genomics Hum Genet 2009;10:19–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Klein ML, Mauldin WM, Stoumbos VD. Heredity and age-related macular degeneration. Observations in monozygotic twins. Arch Ophthalmol 1994;112(7):932–7 [DOI] [PubMed] [Google Scholar]
- 118.Seddon JM, Cote J, Page WF, Aggen SH, Neale MC. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol 2005;123(3):321–7 [DOI] [PubMed] [Google Scholar]
- 119.Heiba IM, Elston RC, Klein BE, Klein R. Sibling correlations and segregation analysis of age-related maculopathy: the Beaver Dam Eye Study. Genet Epidemiol 1994;11(1):51–67 [DOI] [PubMed] [Google Scholar]
- 120.Seddon JM, Ajani UA, Mitchell BD. Familial aggregation of age-related maculopathy. Am J Ophthalmol 1997;123(2):199–206 [DOI] [PubMed] [Google Scholar]
- 121.Klaver CC, Wolfs RC, Assink JJ, van Duijn CM, Hofman A, de Jong PT. Genetic risk of age-related maculopathy. Population-based familial aggregation study. Arch Ophthalmol 1998;116(12):1646–51 [DOI] [PubMed] [Google Scholar]
- 122.Ho L, van Leeuwen R, Witteman JC, van Duijn CM, Uitterlinden AG, Hofman A, de Jong PT, Vingerling JR, Klaver CC. Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and omega-3 fatty acids: the Rotterdam study. Arch Ophthalmol 2011;129(6):758–66 [DOI] [PubMed] [Google Scholar]
- 123.Loane E, Nolan JM, McKay GJ, Beatty S. The association between macular pigment optical density and CFH, ARMS2, C2/BF, and C3 genotype. Exp Eye Res 2011 [DOI] [PubMed] [Google Scholar]
- 124.Connor WE, Duell PB, Kean R, Wang Y. The prime role of HDL to transport lutein into the retina: evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter. Invest Ophthalmol Vis Sci 2007;48(9):4226–31 [DOI] [PubMed] [Google Scholar]
- 125.Tserentsoodol N, Gordiyenko NV, Pascual I, Lee JW, Fliesler SJ, Rodriguez IR. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol Vis 2006;12:1319–33 [PubMed] [Google Scholar]
- 126.During A, Doraiswamy S, Harrison EH. Xanthophylls are preferentially taken up compared with beta-carotene by retinal cells via a SRBI-dependent mechanism. J Lipid Res 2008;49(8):1715–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Borel P, de Edelenyi FS, Vincent-Baudry S, Malezet-Desmoulin C, Margotat A, Lyan B, Gorrand JM, Meunier N, Drouault-Holowacz S, Bieuvelet S. Genetic variants in BCMO1 and CD36 are associated with plasma lutein concentrations and macular pigment optical density in humans. Ann Med 2011;43(1):47–59 [DOI] [PubMed] [Google Scholar]
- 128.Houssier M, Raoul W, Lavalette S, Keller N, Guillonneau X, Baragatti B, Jonet L, Jeanny JC, Behar-Cohen F, Coceani F, et al. CD36 deficiency leads to choroidal involution via COX2 down-regulation in rodents. PLoS Med 2008;5(2):e39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun M, Finnemann SC, Febbraio M, Shan L, Annangudi SP, Podrez EA, Hoppe G, Darrow R, Organisciak DT, Salomon RG, et al. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: a potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. J Biol Chem 2006;281(7):4222–30 doi: 10.1074/jbc.M509769200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Picard E, Houssier M, Bujold K, Sapieha P, Lubell W, Dorfman A, Racine J, Hardy P, Febbraio M, Lachapelle P, et al. CD36 plays an important role in the clearance of oxLDL and associated age-dependent sub-retinal deposits. Aging (Albany NY) 2010;2(12):981–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kondo N, Honda S, Kuno S, Negi A. Positive association of common variants in CD36 with neovascular age-related macular degeneration. Aging (Albany NY) 2009;1(2):266–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Voolstra O, Kiefer C, Hoehne M, Welsch R, Vogt K, von Lintig J. The Drosophila class B scavenger receptor NinaD-I is a cell surface receptor mediating carotenoid transport for visual chromophore synthesis. Biochemistry 2006;45(45):13429–37 [DOI] [PubMed] [Google Scholar]
- 133.Kiefer C, Sumser E, Wernet MF, Von Lintig J. A class B scavenger receptor mediates the cellular uptake of carotenoids in Drosophila. Proc Natl Acad Sci U S A 2002;99(16):10581–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Giovannucci DR, Stephenson RS. Identification and distribution of dietary precursors of the Drosophila visual pigment chromophore: analysis of carotenoids in wild type and ninaD mutants by HPLC. Vision Res 1999;39(2):219–29 [DOI] [PubMed] [Google Scholar]
- 135.Constantineau J, Greason E, West M, Filbin M, Kieft JS, Carletti MZ, Christenson LK, Rodriguez A. A synonymous variant in scavenger receptor, class B, type I gene is associated with lower SR-BI protein expression and function. Atherosclerosis 2010;210(1):177–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Provost AC, Vede L, Bigot K, Keller N, Tailleux A, Jais JP, Savoldelli M, Ameqrane I, Lacassagne E, Legeais JM, et al. Morphologic and electroretinographic phenotype of SR-BI knockout mice after a long-term atherogenic diet. Invest Ophthalmol Vis Sci 2009;50(8):3931–42 [DOI] [PubMed] [Google Scholar]
- 137.Zerbib J, Seddon JM, Richard F, Reynolds R, Leveziel N, Benlian P, Borel P, Feingold J, Munnich A, Soubrane G, et al. rs5888 variant of SCARB1 gene is a possible susceptibility factor for age-related macular degeneration. PLoS One 2009;4(10):e7341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bernstein PS, Balashov NA, Tsong ED, Rando RR. Retinal tubulin binds macular carotenoids. Invest Ophthalmol Vis Sci 1997;38(1):167–75 [PubMed] [Google Scholar]
- 139.Crabtree DV, Ojima I, Geng X, Adler AJ. Tubulins in the primate retina: evidence that xanthophylls may be endogenous ligands for the paclitaxel-binding site. Bioorg Med Chem 2001;9(8):1967–76 [DOI] [PubMed] [Google Scholar]
- 140.Yemelyanov AY, Katz NB, Bernstein PS. Ligand-binding characterization of xanthophyll carotenoids to solubilized membrane proteins derived from human retina. Exp Eye Res 2001;72(4):381–92 [DOI] [PubMed] [Google Scholar]
- 141.Machalinska A, Klos P, Safranow K, Dziedziejko V, Rudnicki M, Paczkowska E, Karczewicz D, Machalinski B. Neural stem/progenitor cells circulating in peripheral blood of patients with neovascular form of AMD: a novel view on pathophysiology. Graefes Arch Clin Exp Ophthalmol 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Anderson DH, Ozaki S, Nealon M, Neitz J, Mullins RF, Hageman GS, Johnson LV. Local cellular sources of apolipoprotein E in the human retina and retinal pigmented epithelium: implications for the process of drusen formation. Am J Ophthalmol 2001;131(6):767–81 [DOI] [PubMed] [Google Scholar]
- 143.Fernandez-Robredo P, Recalde S, Arnaiz G, Salinas-Alaman A, Sadaba LM, Moreno-Orduna M, Garcia-Layana A. Effect of zeaxanthin and antioxidant supplementation on vascular endothelial growth factor (VEGF) expression in apolipoprotein-E deficient mice. Curr Eye Res 2009;34(7):543–52 [DOI] [PubMed] [Google Scholar]
- 144.Loane E, Nolan JM, Beatty S. The respective relationships between lipoprotein profile, macular pigment optical density, and serum concentrations of lutein and zeaxanthin. Invest Ophthalmol Vis Sci 2010;51(11):5897–905 [DOI] [PubMed] [Google Scholar]
- 145.Loane E, McKay GJ, Nolan JM, Beatty S. Apolipoprotein E genotype is associated with macular pigment optical density. Invest Ophthalmol Vis Sci 2010;51(5):2636–43 [DOI] [PubMed] [Google Scholar]
- 146.Souied EH, Benlian P, Amouyel P, Feingold J, Lagarde JP, Munnich A, Kaplan J, Coscas G, Soubrane G. The epsilon4 allele of the apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. Am J Ophthalmol 1998;125(3):353–9 [DOI] [PubMed] [Google Scholar]
- 147.Klaver CC, Kliffen M, van Duijn CM, Hofman A, Cruts M, Grobbee DE, van Broeckhoven C, de Jong PT. Genetic association of apolipoprotein E with age-related macular degeneration. Am J Hum Genet 1998;63(1):200–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zareparsi S, Reddick AC, Branham KE, Moore KB, Jessup L, Thoms S, Smith-Wheelock M, Yashar BM, Swaroop A. Association of apolipoprotein E alleles with susceptibility to age-related macular degeneration in a large cohort from a single center. Invest Ophthalmol Vis Sci 2004;45(5):1306–10 [DOI] [PubMed] [Google Scholar]
- 149.Baird PN, Guida E, Chu DT, Vu HT, Guymer RH. The epsilon2 and epsilon4 alleles of the apolipoprotein gene are associated with age-related macular degeneration. Invest Ophthalmol Vis Sci 2004;45(5):1311–5 [DOI] [PubMed] [Google Scholar]
- 150.Francis PJ, Hamon SC, Ott J, Weleber RG, Klein ML. Polymorphisms in C2, CFB and C3 are associated with progression to advanced age related macular degeneration associated with visual loss. J Med Genet 2009;46(5):300–7 [DOI] [PubMed] [Google Scholar]
- 151.Tikellis G, Sun C, Gorin MB, Klein R, Klein BE, Larsen EK, Siscovick DS, Hubbard LD, Wong TY. Apolipoprotein e gene and age-related maculopathy in older individuals: the cardiovascular health study. Arch Ophthalmol 2007;125(1):68–73 [DOI] [PubMed] [Google Scholar]
- 152.Wong TY, Shankar A, Klein R, Bray MS, Couper DJ, Klein BE, Sharrett AR, Folsom AR. Apolipoprotein E gene and early age-related maculopathy: the Atherosclerosis Risk in Communities Study. Ophthalmology 2006;113(2):255–9 [DOI] [PubMed] [Google Scholar]
- 153.Zareparsi S, Buraczynska M, Branham KE, Shah S, Eng D, Li M, Pawar H, Yashar BM, Moroi SE, Lichter PR, et al. Toll-like receptor 4 variant D299G is associated with susceptibility to age-related macular degeneration. Hum Mol Genet 2005;14(11):1449–55 [DOI] [PubMed] [Google Scholar]
- 154.McKay GJ, Patterson CC, Chakravarthy U, Dasari S, Klaver CC, Vingerling JR, Ho L, de Jong PT, Fletcher AE, Young IS, et al. Evidence of association of APOE with age-related macular degeneration - a pooled analysis of 15 studies. Hum Mutat 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Duncan KG, Hosseini K, Bailey KR, Yang H, Lowe RJ, Matthes MT, Kane JP, LaVail MM, Schwartz DM, Duncan JL. Expression of reverse cholesterol transport proteins ATP-binding cassette A1 (ABCA1) and scavenger receptor BI (SR-BI) in the retina and retinal pigment epithelium. Br J Ophthalmol 2009;93(8):1116–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yu Y, Reynolds R, Fagerness J, Rosner B, Daly MJ, Seddon JM. Association of variants in the LIPC and ABCA1 genes with intermediate and large drusen and advanced age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52(7):4663–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yu Y, Bhangale TR, Fagerness J, Ripke S, Thorleifsson G, Tan PL, Souied EH, Richardson AJ, Merriam JE, Buitendijk GH, et al. Common variants near FRK/COL10A1 and VEGFA are associated with advanced age-related macular degeneration. Hum Mol Genet 2011;20(18):3699–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fauser S, Smailhodzic D, Caramoy A, van de Ven JP, Kirchhof B, Hoyng CB, Klevering BJ, Liakopoulos S, den Hollander AI. Evaluation of Serum Lipid Concentrations and Genetic Variants at High-Density Lipoprotein Metabolism Loci and TIMP3 in Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 2011;52(8):5525–8 [DOI] [PubMed] [Google Scholar]
- 159.Neale BM, Fagerness J, Reynolds R, Sobrin L, Parker M, Raychaudhuri S, Tan PL, Oh EC, Merriam JE, Souied E, et al. Genome-wide association study of advanced age-related macular degeneration identifies a role of the hepatic lipase gene (LIPC). Proc Natl Acad Sci U S A 2010;107(16):7395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen W, Stambolian D, Edwards AO, Branham KE, Othman M, Jakobsdottir J, Tosakulwong N, Pericak-Vance MA, Campochiaro PA, Klein ML, et al. Genetic variants near TIMP3 and high-density lipoprotein-associated loci influence susceptibility to age-related macular degeneration. Proc Natl Acad Sci U S A 2010;107(16):7401–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Colak E, Kosanovic-Jakovic N, Zoric L, Radosavljevic A, Stankovic S, Majkic-Singh N. The association of lipoprotein parameters and C-reactive protein in patients with age-related macular degeneration. Ophthalmic Res 2011;46(3):125–32 [DOI] [PubMed] [Google Scholar]
- 162.Oz O, Aras Ates N, Tamer L, Yildirim O, Adiguzel U. Glutathione S-transferase M1, T1, and P1 gene polymorphism in exudative age-related macular degeneration: a preliminary report. Eur J Ophthalmol 2006;16(1):105–10 [DOI] [PubMed] [Google Scholar]
- 163.Guven M, Gorgun E, Unal M, Yenerel M, Batar B, Kucumen B, Dinc UA, Guven GS, Ulus T, Yuksel A. Glutathione S-transferase M1, GSTT1 and GSTP1 genetic polymorphisms and the risk of age-related macular degeneration. Ophthalmic Res 2011;46(1):31–7 [DOI] [PubMed] [Google Scholar]
- 164.Lindqvist A, He YG, Andersson S. Cell type-specific expression of beta-carotene 9',10'-monooxygenase in human tissues. J Histochem Cytochem 2005;53(11):1403–12 [DOI] [PubMed] [Google Scholar]
- 165.Ferrucci L, Perry JR, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, et al. Common variation in the beta-carotene 15,15'-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet 2009;84(2):123–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Castaneda MP, Hirschler EM, Sams AR. Skin pigmentation evaluation in broilers fed natural and synthetic pigments. Poult Sci 2005;84(1):143–7 [DOI] [PubMed] [Google Scholar]
- 167.Eriksson J, Larson G, Gunnarsson U, Bed'hom B, Tixier-Boichard M, Stromstedt L, Wright D, Jungerius A, Vereijken A, Randi E, et al. Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet 2008;4(2):e1000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vage DI, Boman IA. A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries). BMC Genet 2010;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Casaroli-Marano RP, Peinado-Onsurbe J, Reina M, Staels B, Auwerx J, Vilaro S. Lipoprotein lipase in highly vascularized structures of the eye. J Lipid Res 1996;37(5):1037–44 [PubMed] [Google Scholar]
- 170.Herbeth B, Gueguen S, Leroy P, Siest G, Visvikis-Siest S. The lipoprotein lipase serine 447 stop polymorphism is associated with altered serum carotenoid concentrations in the Stanislas Family Study. J Am Coll Nutr 2007;26(6):655–62 [DOI] [PubMed] [Google Scholar]
- 171.Horie S, Okuda C, Yamashita T, Watanabe K, Kuramochi K, Hosokawa M, Takeuchi T, Kakuda M, Miyashita K, Sugawara F, et al. Purified canola lutein selectively inhibits specific isoforms of mammalian DNA polymerases and reduces inflammatory response. Lipids 2010;45(8):713–21 [DOI] [PubMed] [Google Scholar]
- 172.Kojima A, Nakahama K, Ohno-Matsui K, Shimada N, Mori K, Iseki S, Sato T, Mochizuki M, Morita I. Connexin 43 contributes to differentiation of retinal pigment epithelial cells via cyclic AMP signaling. Biochem Biophys Res Commun 2008;366(2):532–8 [DOI] [PubMed] [Google Scholar]
- 173.Lambooij AC, van Wely KH, Lindenbergh-Kortleve DJ, Kuijpers RW, Kliffen M, Mooy CM. Insulin-like growth factor-I and its receptor in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 2003;44(5):2192–8 [DOI] [PubMed] [Google Scholar]
- 174.Smith LE. IGF-1 and retinopathy of prematurity in the preterm infant. Biol Neonate 2005;88(3):237–44 [DOI] [PubMed] [Google Scholar]
- 175.Whitmire W, Al-Gayyar MM, Abdelsaid M, Yousufzai BK, El-Remessy AB. Alteration of growth factors and neuronal death in diabetic retinopathy: what we have learned so far. Mol Vis 2011;17:300–8 [PMC free article] [PubMed] [Google Scholar]
- 176.van der Veen RL, Fuijkschot J, Willemsen MA, Cruysberg JR, Berendschot TT, Theelen T. Patients with Sjogren-Larsson syndrome lack macular pigment. Ophthalmology 2010;117(5):966–71 [DOI] [PubMed] [Google Scholar]
- 177.Molday LL, Rabin AR, Molday RS. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat Genet 2000;25(3):257–8 [DOI] [PubMed] [Google Scholar]
- 178.Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science 1997;277(5333):1805–7 [DOI] [PubMed] [Google Scholar]
- 179.Rizzo WB, Carney G. Sjogren-Larsson syndrome: diversity of mutations and polymorphisms in the fatty aldehyde dehydrogenase gene (ALDH3A2). Hum Mutat 2005;26(1):1–10 [DOI] [PubMed] [Google Scholar]
- 180.Allikmets R, Seddon JM, Bernstein PS, Hutchinson A, Atkinson A, Sharma S, Gerrard B, Li W, Metzker ML, Wadelius C, et al. Evaluation of the Best disease gene in patients with age-related macular degeneration and other maculopathies. Hum Genet 1999;104(6):449–53 [DOI] [PubMed] [Google Scholar]
- 181.Bernstein PS, Leppert M, Singh N, Dean M, Lewis RA, Lupski JR, Allikmets R, Seddon JM. Genotype-phenotype analysis of ABCR variants in macular degeneration probands and siblings. Invest Ophthalmol Vis Sci 2002;43(2):466–73 [PubMed] [Google Scholar]
- 182.De La Paz MA, Guy VK, Abou-Donia S, Heinis R, Bracken B, Vance JM, Gilbert JR, Gass JD, Haines JL, Pericak-Vance MA. Analysis of the Stargardt disease gene (ABCR) in age-related macular degeneration. Ophthalmology 1999;106(8):1531–6 [DOI] [PubMed] [Google Scholar]
- 183.Shroyer NF, Lewis RA, Allikmets R, Singh N, Dean M, Leppert M, Lupski JR. The rod photoreceptor ATP-binding cassette transporter gene, ABCR, and retinal disease: from monogenic to multifactorial. Vision Res 1999;39(15):2537–44 [DOI] [PubMed] [Google Scholar]
- 184.Brion M, Sanchez-Salorio M, Corton M, de la Fuente M, Pazos B, Othman M, Swaroop A, Abecasis G, Sobrino B, Carracedo A. Genetic association study of age-related macular degeneration in the Spanish population. Acta Ophthalmol 2010;89(1):e12–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cumming RC, Lightfoot J, Beard K, Youssoufian H, O'Brien PJ, Buchwald M. Fanconi anemia group C protein prevents apoptosis in hematopoietic cells through redox regulation of GSTP1. Nat Med 2001;7(7):814–20 [DOI] [PubMed] [Google Scholar]
- 186.Reuter TY, Medhurst AL, Waisfisz Q, Zhi Y, Herterich S, Hoehn H, Gross HJ, Joenje H, Hoatlin ME, Mathew CG, et al. Yeast two-hybrid screens imply involvement of Fanconi anemia proteins in transcription regulation, cell signaling, oxidative metabolism, and cellular transport. Exp Cell Res 2003;289(2):211–21 [DOI] [PubMed] [Google Scholar]
- 187.Zheng W, Reem RE, Omarova S, Huang S, Dipatre PL, Charvet CD, Curcio CA, Pikuleva IA. Spatial distribution of the pathways of cholesterol homeostasis in human retina. PLoS One 2012;7(5):e37926 doi: 10.1371/journal.pone.0037926 [DOI] [PMC free article] [PubMed] [Google Scholar]