Abstract
Background: The effectiveness of commercial weight-loss programs consisting of very-low-calorie diets (VLCDs) and low-calorie diets (LCDs) is unclear.
Objective: The aim of the study was to quantify weight loss and dropout during a commercial weight-loss program in Sweden (Itrim; cost: $1300/€1000; all participants paid their own fee).
Design: This observational cohort study linked commercial weight-loss data with National Health Care Registers. Weight loss was induced with a 500-kcal liquid-formula VLCD [n = 3773; BMI (in kg/m2): 34 ± 5 (mean ± SD); 80% women; 45 ± 12 y of age (mean ± SD)], a 1200–1500-kcal formula and food-combination LCD (n = 4588; BMI: 30 ± 4; 86% women; 50 ± 11 y of age), and a 1500–1800-kcal/d restricted normal-food diet (n = 676; BMI: 29 ± 5; 81% women; 51 ± 12 y of age). Maintenance strategies included exercise and a calorie-restricted diet. Weight loss was analyzed by using an intention-to-treat analysis (baseline substitution).
Results: After 1 y, mean (±SD) weight changes were −11.4 ± 9.1 kg with the VLCD (18% dropout), −6.8 ± 6.4 kg with the LCD (23% dropout), and −5.1 ± 5.9 kg with the restricted normal-food diet (26% dropout). In an adjusted analysis, the VLCD group lost 2.8 kg (95% CI: 2.5, 3.2) and 3.8 kg (95% CI: 3.2, 4.5) more than did the LCD and restricted normal-food groups, respectively. A high baseline BMI and rapid initial weight loss were both independently associated with greater 1-y weight loss (P < 0.001). Younger age and low initial weight loss predicted an increased dropout rate (P < 0.001). Treatment of depression (OR: 1.4; 95% CI: 1.1, 1.9) and psychosis (OR: 2.6; 95% CI: 1.1, 6.3) were associated with an increased dropout rate in the VLCD group.
Conclusion: A commercial weight-loss program, particularly one using a VLCD, was effective at reducing body weight in self-selected, self-paying adults.
See corresponding editorial on page 949.
INTRODUCTION
Because most US and European adults are overweight or obese (1, 2), health services are struggling to cope with the large number of individuals in need of weight loss. Bariatric surgery induces large weight losses and reduces type 2 diabetes and mortality (3–6) but is generally restricted to severely obese individuals with comorbidity, whereas antiobesity drugs are struggling to gain Food and Drug Administration approval (7). Many overweight and obese individuals, therefore, find their options limited to commercial weight-loss programs, most of which have not been scientifically evaluated (8, 9).
Weight Watchers and Jenny Craig are 2 commercial weight-loss operators whose programs have been evaluated in long-term (≥1 y) randomized controlled trials (10, 11). A recent randomized trial found that a commercial weight-loss program (Weight Watchers) was twice as effective as standard care at reducing body weight after 2 y (−4.0 compared with −1.8 kg, intention-to-treat analysis with baseline carried forward) (12).
Research on commercial weight loss is still scarce, however, and there is a need to quantify the effectiveness of commercial weight-loss diets, especially in real-life settings. The aim was to evaluate weight loss and the dropout rate after 1 y of a commercial weight-loss program in Sweden, where weight loss was induced with a 500-kcal very-low-calorie diet (VLCD)4, a 1200–1500-kcal low-calorie diet (LCD), or a 1500–1800-kcal restricted normal-food diet followed by a diet and exercise maintenance program.
SUBJECTS AND METHODS
Recruitment
Participants were consecutively enrolled customers (n = 9037) from the commercial weight-loss company Itrim in Sweden from 1 January 2006 to 31 May 2009 (see Figure S1 under “Supplemental data” in the online issue). Data were collected from 28 centers across Sweden. All customers were enrolled in the Itrim weight-loss program. The regional ethics review board in Stockholm approved the study (registration numbers 2010/151–31/5 and 2010/1059–31/1).
Weight-loss programs
The 1-y weight-loss program consisted of an initial 3-mo weight-loss phase followed by a 9-mo weight-maintenance phase. At the start of the weight-loss phase, participants and their designated health coaches discussed and decided on an appropriate weight-loss diet that was largely based on baseline BMI, desired weight loss, and personal preference:
1) VLCD: consisted of a liquid-based formula diet of 500 kcal/d for 6 to 10 wk (Itrim; 125 kcal/sachet, 4 sachets/d, each sachet contained 13 g protein, 15 g carbohydrates, 2 g fat, and 3 g fiber; approved as the sole-source VLCD by the Swedish National Food Agency) followed by a 2-wk gradual introduction of normal food. Early introduction of normal food (6 as opposed to the full 10 wk) occurred when the participant was either satisfied with the achieved weight loss or had reached a normal-weight BMI (in kg/m2) of ≤25.
2) LCD: consisted of 2 calorie-restricted normal-food meals and 2 formula-diet meal-replacement sachets providing a total caloric intake of ∼1200–1500 kcal/d depending on body size and exercise levels.
3) Restricted normal-food diet: consisted mainly of food with high protein, low-glycemic-index carbohydrates, low fat intake, low energy density, and a high fiber content, providing a caloric intake of ∼1500–1800 kcal/d depending on body size and exercise levels.
After the weight-loss phase, all 3 groups entered the same 9-mo weight-maintenance program, which included an exercise program (circuit training, with a mix of aerobic and strength-training workout stations, at the center 2–3 times/wk for 30 to 45 min; physically active transport to and from work; and use of a Yamax SW-200 pedometer to encourage walking), dietary advice, and behavioral changes. Dietary advice included the use of restricted portions sizes and consumption of a diet rich in protein, with a low glycemic index, and with a low overall energy density (eg, consumption of vegetables and water as opposed to caloric beverages).
Behavioral changes were facilitated by using a structured support program, which included weekly 1-h group sessions during the weight-loss phase and every 4 wk during the weight-maintenance phase (20 in total). Each session was supervised by a company-trained health coach, who provided encouragement to participants throughout the program. Each group session covered a specific topic, such as health benefits of weight loss, healthy eating strategies, finding realistic eating and exercise routines, and stress management. There were also 30-min face-to-face counseling sessions at baseline and 3, 6, and 12 mo. Self-monitoring was facilitated through diaries, including diet and exercise plans, and graphs for plotting weight, waist circumference, planned and completed circuit training sessions, and steps per day.
Specific restrictions with use of a VLCD
Although participants were free to choose their method of weight loss, the company used criteria for use of VLCDs, consistent with the VLCD recommendations of the European Union Scientific Cooperation Task Report (13). First, individuals were required to have a BMI ≥30 or a BMI ≥27 with elevated waist circumference (≥102 cm for men and ≥88 cm for women). In addition, unless they had signed approval from their physician, individuals with any of the following conditions were barred from following a VLCD: insulin-treated diabetes, gallstones, gout, cancer during the past 2 y, cardiovascular disease (CVD) during the past 3 mo, pregnancy, breastfeeding, catabolic disease, kidney disease, anorexia nervosa, or bulimia.
Cost
The cost for attending the 1-y weight-loss program (including the exercise program) was ∼9000 Swedish krona (∼US$1300, €1000), excluding the liquid diets. At the start, all participants either paid for the whole 12-mo period or committed to paying the whole amount in monthly installments. All participants paid their own fees.
Observational data on weight loss and dropout
The primary outcome variable was weight loss after 1 y. Data on body weight and waist circumference were collected at baseline and 3, 6, and 12 mo. Body weight was measured with the TBF-300 bioelectrical impedance scales (Tanita Corporation). Waist circumference was measured at the point midway between the iliac crest and the lower rib (exhaled). Height was measured with a wall-mounted stadiometer. Dropout was defined as missing data on body weight during 10–14 mo from baseline, including body weight measured at group sessions, and the 1-y follow-up.
Linkage with data from National Health Care Registers
Data on history of comorbidities during the past 5 y were collected from the Swedish National Patient Register, which includes virtually all inpatient and nonprimary outpatient care visits (14). Data on hospital visits for malignancies (International Classification of Disease, Tenth Revision code C00-C97) and CVD (code I00-I99) were also collected. Data on diabetes treated with insulin [Anatomic Therapeutic Chemical classification system (ATC) code A10A] or oral antidiabetic drugs (ATC A10B) were collected from the Prescribed Drug Register. Drug dispensation data were retrieved from the Prescribed Drug Register during the 6 mo preceding the start of the program. We also collected data on dispensations of antihypertensive drugs (ATC C02, C03, C07, C08, and C09), lipid-lowering agents (ATC C10AA, C10AB, C10AC, C10AD, C10B, and C10AX), antipsychotics (N05A), antidepressants (ATC N06A), and the antiobesity drugs orlistat (A08AB01) and sibutramine (A08AA10). Finally, we collected data from the Causes of Death Register (for a data linkage outline, see Online Supplementary Material under “Supplemental data” in the online issue).
Statistical analyses
Weight loss was primarily analyzed with an intention-to-treat analysis by using baseline observation carried forward when follow-up data were missing. In sensitivity analyses, we also used last observation carried forward, multiple imputation (age, sex, and baseline value were used to predict missing values at 1 y), and a completers-only analysis to see whether our findings were affected by the choice of data-imputation method.
When quantifying the within-group effects of the 3 weight-loss diets, we used a paired sample's t test. Between-group comparisons were performed by using ANCOVA with adjustment for age, sex, baseline body weight, center, calendar year, drug dispensations, and comorbidities. Multiple linear regression was used to study predictors of weight loss, and we used multivariable logistic regression to identify predictors of dropout.
When analyzing dropout within groups, we excluded data from the restricted normal-food group because of insufficient power. Because we wanted to study the potential influence of initial weight loss on dropout, we excluded participants who dropped out before the 3-mo follow-up. A 2-sided P value <0.05 was considered statistically significant. All data were analyzed with SPSS version 20 and SAS statistical software (version 9.3; SAS Institute Inc).
RESULTS
The mean age of the participants was 48 ± 12 y (range: 18–81 y), BMI was 32 ± 5 (range: 18–68), and waist circumference was 105 ± 13 cm (72–164); 83% of the participants were women, 4% were normal weight, 38% were overweight, 37% were class I obese, 15% were class II obese, and 6% were class III obese (Table 1).
TABLE 1.
Baseline characteristics (n = 9037) of participants in a commercial weight-loss program including a VLCD (500 kcal/d), an LCD (1200–1500 kcal/d), or a restricted normal-food diet (1500–1800 kcal/d)1
| VLCD (n = 3773) | LCD (n = 4588) | Restricted normal-food diet (n = 676) | P | |
| Age (y) | 45 ± 12 (18–77)2 | 50 ± 11 (18–81) | 51 ± 12 (19–78) | <0.00134 |
| Female sex (%) | 80 | 86 | 81 | <0.00135 |
| Body weight (kg) | 98 ± 17 (64–195) | 85 ± 14 (54–190) | 81 ± 16 (51–166) | <0.0013–5 |
| Body weight, completers only (kg) | 98 ± 17 (64–188) | 86 ± 14 (54–190) | 82 ± 17 (51–166) | <0.0013–5 |
| BMI (%) | 34 ± 5 (22–58) | 30 ± 4 (21–68) | 29 ± 5 (18–54) | <0.0013–5 |
| <25 kg/m2 | 0.4 | 4.8 | 21.6 | |
| 25–29 kg/m2 | 16.9 | 53.0 | 49.4 | |
| 30–34 kg/m2 | 47.9 | 30.3 | 19.1 | |
| 35–39 kg/m2 | 23.5 | 9.3 | 6.8 | |
| ≥40 kg/m2 | 11.3 | 2.6 | 3.1 | |
| Waist circumference (cm) | 110 ± 12 (78–164) | 101 ± 11 (72–160) | 98 ± 13 (72–145) | <0.0013–5 |
| Waist circumference, completers only (cm) | 110 ± 12 (78–155) | 102 ± 11 (72–160) | 98 ± 13 (74–145) | <0.0013–5 |
| Waist circumference ≥102/88 cm (%)6 | 92 | 83 | 68 | |
| BMI ≥30 kg/m2 or waist circumference ≥102/88 cm (%)6 | 98 | 88 | 74 | |
| Comorbidities and drugs (%)7 | ||||
| History of CVD | 7.9 | 8.0 | 10.9 | 0.02645 |
| History of cancer | 1.6 | 2.6 | 3.7 | <0.0013–5 |
| Antiobesity drugs | ||||
| Orlistat | 1.0 | 0.5 | 1.0 | 0.0383 |
| Sibutramine | 1.7 | 0.8 | 0.9 | <0.00134 |
| Diabetes | 1.9 | 2.4 | 7.2 | <0.00145 |
| Insulin | 0.4 | 0.8 | 4.3 | <0.0013–5 |
| Oral antidiabetes | 1.8 | 2.0 | 5.8 | <0.00145 |
| Antidyslipidemia drugs | 5.8 | 7.9 | 9.2 | <0.0013–5 |
| Antihypertension drugs | 16.4 | 19.7 | 21.0 | <0.0013–5 |
| Antidepression drugs | 11.6 | 13.2 | 12.0 | 0.095 |
| Antipsychosis drugs | 0.7 | 1.1 | 0.9 | 0.22 |
CVD, cardiovascular disease; LCD, low-calorie diet; VLCD, very-low-calorie diet.
Mean ± SD; range in parentheses (all such values).
VLCD compared with LCD.
VLCD compared with restricted normal-food diet.
LCD compared with restricted normal-food diet.
Waist circumference ≥102 cm for men, ≥88 cm for women.
Drug use was assessed during the period 6 mo before baseline through register linkage with the Prescribed Drug Register, whereas comorbidity data (except for diabetes) were retrieved from the National Patient Register during the past 5 y. Because diabetes is, to a large extent, treated in primary care, it was defined as use of either insulin or oral antidiabetics. Group differences were analyzed by using ANOVA with a Scheffe post hoc test.
During the 5 y preceding baseline, 8% of all participants had hospital visits listing diagnoses for CVD and 2% with cancer. During the 6-mo period preceding baseline, 18% were dispensed antihypertensives, 12% antidepressants, 7% lipid-lowering drugs, 2% oral antidiabetics, 1% insulin, and 1% antipsychotics.
Weight loss after 1 y: intention-to-treat analysis
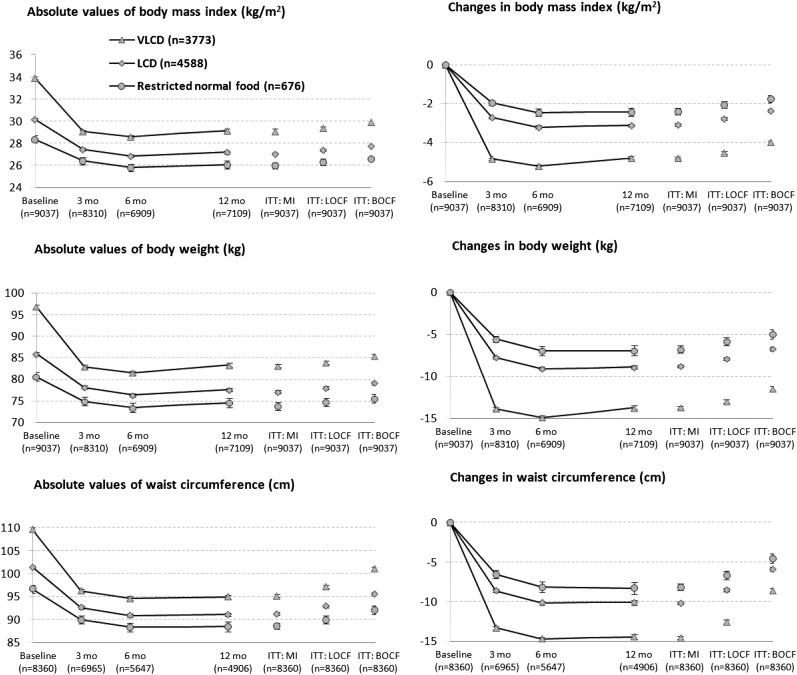
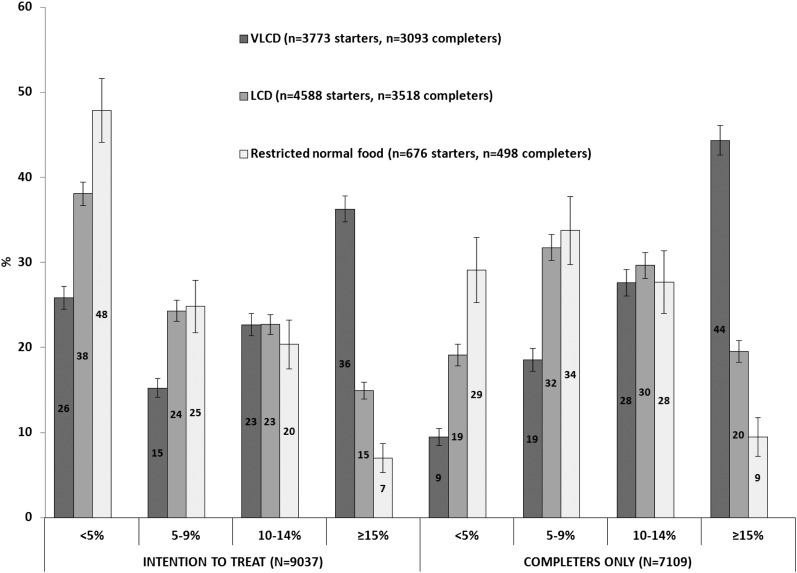
The unadjusted mean (±SD) weight change in the 3 weight-loss groups combined was −8.6 ± 8.0 kg (−9.5% of baseline body weight). Within-group weight changes were −11.4 ± 9.1 kg with the VLCD, −6.8 ± 6.4 kg with the LCD, and −5.1 ± 5.9 kg with the restricted normal-food diet (P < 0.001 for all within-group changes; Tables 2 and 3). BMI, percentage weight loss, and waist circumference were likewise reduced in a dose-response relation according to caloric intake (Figure 1). A higher proportion of participants in the VLCD group also lost ≥15% body weight, and there were fewer participants who lost <5% of body weight (Figure 2).
TABLE 2.
Changes in absolute and percentage body weight and waist circumference in participants after 1 y in a commercial weight-loss program (n = 9037) including a VLCD (500 kcal/d), an LCD (1200–1500 kcal/d), or a restricted normal-food diet (1500–1800 kcal/d)1
| Within-group changes |
|||
| VLCD (n = 3773) | LCD (n = 4588) | Restricted normal-food diet (n = 676) | |
| Intention to treat (n = 9037)2 | |||
| Body weight (kg) | −11.5 (−11.7, −11.2) | −6.8 (−7.0, −6.6) | −5.0 (−5.6, −4.5) |
| Body weight (%) | −11.7 (−11.9, −11.4) | −7.7 (−7.9, −7.5) | −6.0 (−6.5, −5.4) |
| Waist circumference (cm) | −8.6 (−8.9, −8.3) | −5.9 (−6.1, −5.6) | −4.5 (−5.2, −3.9) |
| Completers only (n = 7109) | |||
| Body weight (kg) | −13.8 (−14.0, −13.5) | −8.9 (−9.2, −8.7) | −7.0 (−7.6, −6.4) |
| Body weight (%) | −14.0 (−14.2, −13.8) | −10.1 (−10.4, −9.9) | −8.3 (−8.9, −7.7) |
| Waist circumference (cm) | −14.4 (−14.8, −14.1) | −10.1 (−10.4, −9.8) | −8.3 (−9.1, −7.5) |
All values are estimated marginal means; 95% CIs in parentheses. ANCOVA was conducted, and the values were adjusted for age, sex, center, calendar year, history of cardiovascular disease and cancer during the past 5 y, and dispensation of drugs for obesity, diabetes, hypertension, dyslipidemia, depression, and psychosis during the 6 mo preceding baseline. LCD, low-calorie diet; VLCD, very-low-calorie diet.
With baseline substitution.
TABLE 3.
Adjusted between-group differences in absolute and relative body weight and waist circumference in participants after 1 y in a commercial weight-loss program (n = 9037) including a VLCD (500 kcal/d), an LCD (1200–1500 kcal/d), or a restricted normal-food diet (1500–1800 kcal/d)1
| Between-group differences |
|||
| VLCD compared with LCD | VLCD compared withrestricted normal-food diet | LCD compared withrestricted normal-food diet | |
| Intention to treat (n = 9037)2 | |||
| Body weight (kg) | −2.8 (−3.2, −2.5) | −3.8 (−4.5, −3.2) | −1.0 (−1.6, −0.4) |
| Body weight (%) | −3.0 (−3.4, −2.7) | −4.3 (−4.9, −3.7) | −1.3 (−1.9, −0.7) |
| Waist circumference (cm) | −3.0 (−3.4, −2.5) | −4.1 (−5.0, −3.3) | −1.1 (−1.9, −0.3) |
| Completers only (n = 7109) | |||
| Body weight (kg) | −3.0 (−3.3, −2.6) | −3.9 (−4.5, −3.3) | −1.0 (−1.5, −0.4) |
| Body weight (%) | −2.9 (−3.3, −2.6) | −4.3 (−5.0, −3.7) | −1.4 (−2.0, −0.8) |
| Waist circumference (cm) | −2.1 (−2.5, −1.7) | −3.1 (−3.8, −2.4) | −1.0 (−1.7, −0.4) |
All values are estimated marginal means; 95% CIs in parentheses. ANCOVA was conducted, and the values were adjusted for age, sex, baseline BMI, center, calendar year, history of cardiovascular disease and cancer during the past 5 y, and dispensation of drugs for obesity, diabetes, hypertension, dyslipidemia, depression, and psychosis during the 6 mo preceding baseline. All between-group differences were P < 0.05. LCD, low-calorie diet; VLCD, very-low-calorie diet.
With baseline substitution.
FIGURE 1.
Absolute values for and changes in BMI, body weight, and waist circumference during a 12-mo commercial weight-loss program including a VLCD (500 kcal/d), an LCD (1200–1500 kcal/d), or a restricted normal-food diet (1500–1800 kcal/d). ANCOVA was conducted, and the data are estimated marginal means adjusted for age, sex, center, calendar year, history of cardiovascular disease and cancer, and dispensation of drugs for obesity, diabetes, hypertension, dyslipidemia, depression, or psychosis. Error bars represent 95% CIs. ITT: BOCF, intention to treat with use of baseline observation carried forward; ITT: LOCF, intention to treat with use of last observation carried forward; ITT: MI, intention to treat with use of multiple imputation; LCD, low-calorie diet; VLCD, very-low-calorie diet.
FIGURE 2.
Categories of percentage weight loss at 1 y in a commercial weight-loss program including a VLCD, an LCD, or a restricted normal-food diet. The analyses were conducted as both intention to treat with baseline substitution and completers only. Error bars represent 95% CIs. LCD, low-calorie diet; VLCD, very-low-calorie diet.
After adjustment for covariates (baseline body weight, age, sex, calendar year, center, comorbidities, and drug dispensations), the VLCD group lost 2.8 kg (95% CI: 2.5, 3.2) more than did the LCD group and 3.8 kg (95% CI: 3.2, 4.5) more than did the restricted normal-food diet. The LCD group lost 1.0 kg (95% CI: 0.4, 1.6) more than did the restricted normal-food diet group.
Weight loss after 1 y: completers-only analysis
In the completers-only analysis, the unadjusted mean (±SD) weight change for all 3 weight-loss groups was −10.9 ± 7.5 kg, equivalent to 12.0% of baseline body weight. Within-group, unadjusted weight changes were −13.9 ± 8.1 kg for the VLCD group, −8.8 ± 5.9 kg for the LCD group, and −6.9 ± 5.9 kg for the restricted normal-food group.
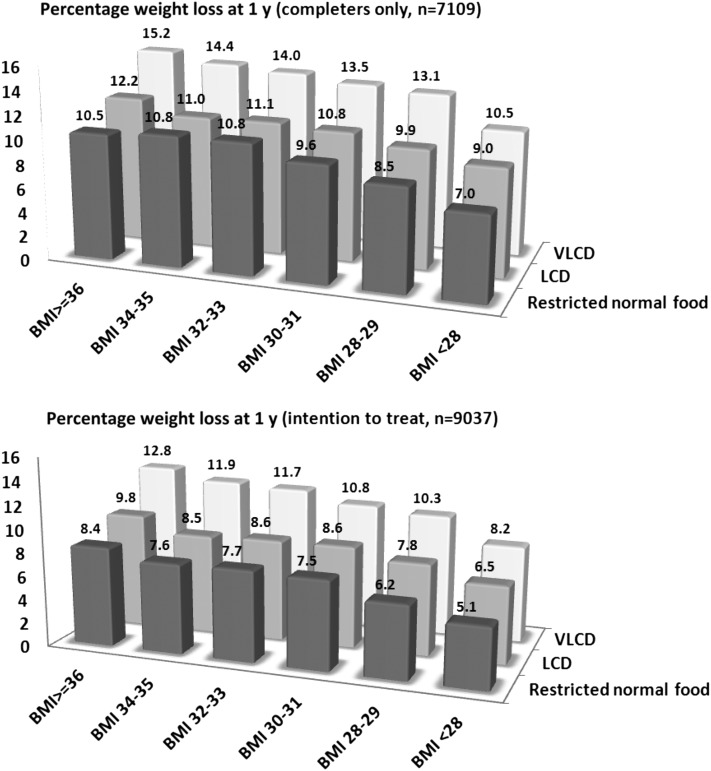
By using linear regression we found that higher baseline body weight (Figure 3), greater initial weight loss, and female sex were associated with increased weight loss after 1 y across all weight-loss groups (P < 0.01–0.001; see Table S1 under “Supplemental data” in the online issue). A history of treatment with the previously available antiobesity drug sibutramine predicted a smaller weight loss after 1 y in the VLCD and LCD groups (P = 0.026 and P = 0.008, respectively).
FIGURE 3.
Independent effects of baseline BMI (in kg/m2) and a VLCD (500 kcal/d; n = 3773), an LCD (1200–1500 kcal/d; n = 4588), and a restricted normal-food diet (1500–1800 kcal/d; n = 676) at predicting percentage weight loss after 1 y in a commercial weight-loss program. ANCOVA was conducted with adjustment for age, sex, center, calendar year, history of cardiovascular disease and cancer, and dispensation of drugs for obesity, diabetes, hypertension, dyslipidemia, depression, and psychosis. Data are estimated marginal means. LCD, low-calorie diet; VLCD, very-low-calorie diet.
Dropout after 1 y
Crude dropout rates were 18% in the VLCD group, 23% in the LCD group, and 26% in the restricted normal-food group. In multivariable analysis, with adjustment for age, sex, baseline BMI, center, calendar year, and comorbidities and drug dispensations, and with the use of the VLCD group as reference, dropout was significantly higher in the LCD group (OR: 1.43; 95% CI: 1.27, 1.62; P < 0.001) and restricted normal-food group (OR: 1.66; 95% CI: 1.37, 2.01; P < 0.001).
Within the VLCD group, younger age (<40 y) and low initial weight loss (<5%) after 3 mo were associated with an increased dropout rate (Table 4). Drug dispensations for depression and psychosis were also associated with an increased risk of dropout. Within the LCD group, predictors of dropout were younger age (<40 y), a low BMI at baseline (<30), and a low initial weight loss (<5% of baseline body weight). Sex, history of treatment of CVD or cancer, or drug dispensation for hypertension, dyslipidemia, or diabetes were not associated with dropout.
TABLE 4.
Multivariable-adjusted ORs (95% CIs) of dropouts at 12 mo during a commercial weight-loss program including a VLCD (500 kcal/d) or an LCD (1200–1500 kcal/d)1
| VLCD (n = 532 dropouts) |
LCD (n = 818 dropouts) |
|||
| OR (95% CI) | P | OR (95% CI) | P | |
| Male sex | 0.84 (0.65, 1.09) | 0.18 | 0.97 (0.76, 1.24) | 0.81 |
| Age | ||||
| ≥60 y | Reference | Reference | ||
| 50–59 y | 1.13 (0.71, 1.81) | 0.61 | 1.24 (0.97, 1.59) | 0.092 |
| 40–49 y | 2.47 (1.57, 3.90) | <0.001 | 1.67 (1.28, 2.17) | <0.001 |
| <40 y | 4.12 (2.61, 6.50) | <0.001 | 2.68 (2.04, 3.51) | <0.001 |
| P-trend | <0.001 | <0.001 | ||
| Baseline BMI | ||||
| ≥40 kg/m2 | Reference | Reference | ||
| 35–39 kg/m2 | 1.17 (0.81, 1.68) | 0.42 | 2.58 (1.29, 5.15) | 0.007 |
| 30–34 kg/m2 | 1.46 (1.04, 2.05) | 0.027 | 2.50 (1.29, 4.84) | 0.007 |
| <30 kg/m2 | 1.35 (0.92, 1.98) | 0.13 | 3.02 (1.57, 5.81) | 0.001 |
| P-trend | 0.082 | 0.003 | ||
| Weight loss at 3 mo | ||||
| ≥15% | Reference | Reference | ||
| 10–14% | 1.50 (1.19, 1.88) | 0.001 | 1.39 (0.92, 2.11) | 0.12 |
| 5–9% | 2.49 (1.91, 3.24) | <0.001 | 2.51 (1.67, 3.76) | <0.001 |
| <5% | 3.41 (2.33, 5.00) | <0.001 | 4.99 (3.27, 7.63) | <0.001 |
| P-trend | <0.001 | <0.001 | ||
| Comorbidities and drug use | ||||
| History of cancer | 1.24 (0.57, 2.73) | 0.59 | 0.98 (0.58, 1.64) | 0.98 |
| History of CVD | 1.04 (0.70, 1.55) | 0.82 | 1.09 (0.80, 1.48) | 0.60 |
| Antihypertension drugs | 1.00 (0.71, 1.42) | 1.00 | 1.09 (0.85, 1.38) | 0.50 |
| Antidyslipidemia drugs | 0.88 (0.51, 1.55) | 0.66 | 0.89 (0.63, 1.25) | 0.63 |
| Antidiabetes drugs | 1.31 (0.62, 2.74) | 0.48 | 0.92 (0.52, 1.62) | 0.76 |
| Antidepression drugs | 1.44 (1.08, 1.91) | 0.013 | 1.24 (0.99, 1.55) | 0.065 |
| Antipsychosis drugs | 2.63 (1.09, 6.32) | 0.031 | 0.55 (0.23, 1.33) | 0.18 |
| Antiobesity drugs | 0.73 (0.32, 1.67) | 0.49 | 1.25 (0.55, 2.84) | 0.60 |
Drug use was assessed during the period 6 mo before baseline through register linkage with the Prescribed Drug Register, whereas comorbidity data (except for diabetes) were retrieved from the National Patient Register during the past 5 y. Because diabetes is, to a large extent, treated in primary care, it was defined as use of either insulin or oral antidiabetics. We were not able to analyze the restricted normal-food diet group because of insufficient power and likewise for the antiobesity drug orlistat (only sibutramine was analyzed). There were 3466 participants with complete data on dropout predictors in the VLCD group (91.9%), of whom 532 dropped out, and 4163 in the LCD group (90.7%), of whom 818 dropped out. ORs were quantified by using multivariable logistic regression. CVD, cardiovascular disease; LCD, low-calorie diet; VLCD, very-low-calorie diet.
Sensitivity analysis
To minimize the influence of choice of method for handling missing data, we carried out sensitivity analysis using last observation carried forward, a completers-only analysis, and multiple imputation. None of the 3 alternative data-imputation methods produced results that differed materially from the main analysis (Figure 1).
DISCUSSION
Main findings
Weight loss was largest in the 500-kcal VLCD group, followed by the 1200–1500-kcal LCD group, and finally the 1500–1800 restricted normal-food group, which demonstrated a linear, dose-response relation between energy intake and reduced body weight during commercial weight loss. The differences in effectiveness between weight-loss methods were similar when we analyzed waist circumference and percentage body weight as outcome variables. Moreover, the effects of weight-loss method were independent of covariate adjustment and the method for handling missing data.
Baseline body weight and rapid initial weight loss were also independently associated with greater weight loss after 12 mo, whereas treatment of CVD, cancer, hypertension, dyslipidemia, depression, diabetes, or psychosis did not influence weight loss. Dropout was lowest in the VLCD group, followed by the LCD group, and finally the restricted normal-food group. Younger age and low BMI (the latter for the LCD group only) were associated with increased dropout. Previous treatment of depression and psychosis was also associated with increased dropout in the VLCD group.
Potential mechanisms for the observed effects
The finding that weight loss was determined in a linear dose-response relation according to energy intake suggests that the laws of thermodynamics were the major determinants of weight loss. Moreover, there was no difference in exercise programs between weight-loss groups [Itrim also operates an exercise-only program, with a mean (±SD) weight and waist reduction of 1.6 ± 4.3 kg and 2.4 ± 5.2 cm after 1 y; completers-only analysis, n = 570, data not shown].
Greater initial weight loss during the first 3 mo was also associated with improved 1-y weight loss and reduced the dropout rate, indicating that a good start promotes long-term compliance, possibly through increased motivation (15). The markedly increased risk of dropout associated with younger age needs to be studied further.
Comparisons with other commercial weight-loss programs
Weight Watchers and Jenny Craig have both evaluated their programs in randomized trials. Weight Watchers promotes a balanced, hypoenergetic, normal-food diet according to healthy-eating principles (12). In an intention-to-treat analysis with baseline carried forward, mean weight loss after 1 y was 4.1 kg (within-group analysis) and the dropout rate was 42% (12).
Jenny Craig initially uses prepackaged meals with a low fat and low energy content, typically 1200–2000 kcal/d, and then gradually reintroduces a greater proportion of normal food. In an intention-to-treat analysis with baseline carried forward, mean weight loss after 1 y was 6.6 kg (within group), and the dropout rate was 9% (16). In the completers-only analysis, weight loss was 7.3 kg—almost identical to the 7.0 kg lost by completers in the restricted normal-food group in the current study. The greater weight losses seen in the current study suggest that liquid low-calorie formula diets that promote rapid initial weight loss can improve both weight loss and the dropout rate in commercial weight-loss programs.
However, differences in study protocols, participants, and payment complicate direct comparisons of the effectiveness of the Itrim program with Weight Watchers, Jenny Craig, and other commercial weight-loss programs. Observational studies, such as the current analysis, are likely to benefit from highly motivated, self-selected, and self-paying participants. Similarly, randomized trials may benefit from having a controlled environment and added visits with study personnel, doctors, and nurses.
Concerns with use of a VLCD
VLCDs have been associated with adverse events, such as gallstone formation and sudden death (17–19), which have contributed to stricter rules in the United States (where VLCD programs must be managed by a physician) than in Europe (8). Rapid weight loss, whether by VLCD or bariatric surgery, increases the risk of developing gallstones, and clinical recommendations advise physicians to inform patients about this risk (13, 17, 20). Gallstone formation has mainly been associated with VLCDs containing low amounts of fat (∼1 g/d) (21–25), and a higher fat content (12–30 g/d) seems to reduce gallstone formation (26–29).
Another concern with VLCDs is weight regain. Although initial weight loss predicted lower body weight at follow-up in the current study and in others (30), rapid weight loss was also associated with greater regain during the weight-loss maintenance phase in all 3 weight-loss groups (see Figure S2 under “Supplemental data” in the online issue). Responsible use of VLCDs requires, at a minumum, a weight-loss maintenance program, transparency about the substantial efforts required to maintain weight loss (9, 31–34) and risk of regain, and exclusion of nonobese individuals.
Strengths and limitations of the study
First, it was not possible to randomly assign participants to different weight-loss methods, meaning that between-group comparisons are affected by selection bias and baseline differences. We tried to minimize confounding from baseline differences by using percentage weight loss in addition to absolute weight loss, and we performed a BMI-stratified analysis (Figure 3). We also adjusted for baseline body weight in between-group analyses. Our study was aided by a large sample size and the use of 3 different energy-intake methods. The data were furthermore collected in consecutively enrolled participants in a real-life setting, as opposed to data collected in a controlled research setting, where more strict inclusion criteria can limit external validity. We also supplemented the observational data on effectiveness with data from national health registers, which allowed us to describe the medical history of our participants and the influence of such data on effectiveness.
Conclusion
Both weight-loss and dropout rates were more favorable for the 500-kcal/d VLCD group than for the 1200–1500-kcal/d LCD group and the 1500–1800-kcal/d restricted normal-food group, which suggests an approximately linear dose-response relation between initial caloric intake and long-term effectiveness during commercial weight loss.
Acknowledgments
The authors’ responsibilities were as follows—EH: study conception and design, data acquisition, drafting of the manuscript, analysis and interpretation of the data, statistical analysis, critical revision of the manuscript for important intellectual content, and primary responsibility for final the content; KJ: data acquisition, statistical analysis, and revision of the manuscript for important intellectual content; JE: statistics and critical revision of the manuscript for important intellectual content; JS: study conception and design, interpretation of the data, critical revision of the manuscript for important intellectual content, and supervision; MN: data acquisition, study conception and design, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, and supervision; and CM: study conception and design, critical revision of the manuscript for important intellectual content, and supervision. All authors read and approved the final manuscript. The funding source (Itrim International) collected all data related to the program's effectiveness but was not involved in the design or conduct of the study; the management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript. EH has received consultancy fees from Itrim and was employed part-time by Itrim as program director during 2006–2008. MN, JS, and CM are paid members of Itrim's Scientific Advisory Board. KJ and JE had no conflicts of interest.
Footnotes
Abbreviations used: ATC, Anatomic Therapeutic Chemical classification system; CVD, cardiovascular disease; LCD, low-calorie diet; VLCD, very-low-calorie diet.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–41 [DOI] [PubMed] [Google Scholar]
- 2.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011;377:557–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753–61 [DOI] [PubMed] [Google Scholar]
- 4.Bolen SD, Chang HY, Weiner JP, Richards TM, Shore AD, Goodwin SM, Johns RA, Magnuson TH, Clark JM. Clinical outcomes after bariatric surgery: a five-year matched cohort analysis in seven US states. Obes Surg 2012;22:749–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–93 [DOI] [PubMed] [Google Scholar]
- 6.Sjöström L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–52 [DOI] [PubMed] [Google Scholar]
- 7.Dvorak RV, Sharma AM, Astrup A. Anti-obesity drugs: to be or not to be? Obes Rev 2010;11:833–4 [DOI] [PubMed] [Google Scholar]
- 8.Tsai AG, Wadden TA. Systematic review: an evaluation of major commercial weight loss programs in the United States. Ann Intern Med 2005;142:56–66 [DOI] [PubMed] [Google Scholar]
- 9.Freedhoff Y, Sharma AM. “Lose 40 pounds in 4 weeks”: regulating commercial weight-loss programs. CMAJ 2009;180:367–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heshka S, Anderson JW, Atkinson RL, Greenway FL, Hill JO, Phinney SD, Kolotkin RL, Miller-Kovach K, Pi-Sunyer FX. Weight loss with self-help compared with a structured commercial program: a randomized trial. JAMA 2003;289:1792–8 [DOI] [PubMed] [Google Scholar]
- 11.Rock CL, Flatt SW, Sherwood NE, Karanja N, Pakiz B, Thomson CA. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women: a randomized controlled trial. JAMA 2010;304:1803–10 [DOI] [PubMed] [Google Scholar]
- 12.Jebb SA, Ahern AL, Olson AD, Aston LM, Holzapfel C, Stoll J, Amann-Gassner U, Simpson AE, Fuller NR, Pearson S, et al. Primary care referral to a commercial provider for weight loss treatment versus standard care: a randomised controlled trial. Lancet 2011;378:1485–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. SCOOP-VLCD. SCOOP-VLCD Task 7.3. Scientific Co-operation on Questions Relating to Food: Directorate-General Health and Consumer Protection. European Union, 2002.
- 14.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Astrup A, Rossner S. Lessons from obesity management programmes: greater initial weight loss improves long-term maintenance. Obes Rev 2000;1:17–9 [DOI] [PubMed] [Google Scholar]
- 16.Rock CL, Pakiz B, Flatt SW, Quintana EL. Randomized trial of a multifaceted commercial weight loss program. Obesity (Silver Spring) 2007;15:939–49 [DOI] [PubMed] [Google Scholar]
- 17.National Task Force on the Prevention and Treatment of Obesity, NIH Very low-calorie diets. JAMA 1993;270:967–74 [PubMed] [Google Scholar]
- 18.Saris WH. Very-low-calorie diets and sustained weight loss. Obes Res 2001;9(suppl 4):295S–301S [DOI] [PubMed] [Google Scholar]
- 19.Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and meta-analysis. Obesity (Silver Spring) 2006;14:1283–93 [DOI] [PubMed] [Google Scholar]
- 20.Everhart JE. Contributions of obesity and weight loss to gallstone disease. Ann Intern Med 1993;119:1029–35 [DOI] [PubMed] [Google Scholar]
- 21.Broomfield PH, Chopra R, Sheinbaum RC, Bonorris GG, Silverman A, Schoenfield LJ, Marks JW. Effects of ursodeoxycholic acid and aspirin on the formation of lithogenic bile and gallstones during loss of weight. N Engl J Med 1988;319:1567–72 [DOI] [PubMed] [Google Scholar]
- 22.Kamrath RO, Plummer LJ, Sadur CN, Adler MA, Strader WJ, Young RL, Weinstein RL. Cholelithiasis in patients treated with a very-low-calorie diet. Am J Clin Nutr 1992;56:255S–7S [DOI] [PubMed] [Google Scholar]
- 23.Liddle RA, Goldstein RB, Saxton J. Gallstone formation during weight-reduction dieting. Arch Intern Med 1989;149:1750–3 [PubMed] [Google Scholar]
- 24.Shiffman ML, Kaplan GD, Brinkman-Kaplan V, Vickers FF. Prophylaxis against gallstone formation with ursodeoxycholic acid in patients participating in a very-low-calorie diet program. Ann Intern Med 1995;122:899–905 [DOI] [PubMed] [Google Scholar]
- 25.Yang H, Petersen GM, Roth MP, Schoenfield LJ, Marks JW. Risk factors for gallstone formation during rapid loss of weight. Dig Dis Sci 1992;37:912–8 [DOI] [PubMed] [Google Scholar]
- 26.Festi D, Colecchia A, Orsini M, Sangermano A, Sottili S, Simoni P, Mazzella G, Villanova N, Bazzoli F, Lapenna D, et al. Gallbladder motility and gallstone formation in obese patients following very low calorie diets. Use it (fat) to lose it (well). Int J Obes Relat Metab Disord 1998;22:592–600 [DOI] [PubMed] [Google Scholar]
- 27.Gebhard RL, Prigge WF, Ansel HJ, Schlasner L, Ketover SR, Sande D, Holtmeier K, Peterson FJ. The role of gallbladder emptying in gallstone formation during diet-induced rapid weight loss. Hepatology 1996;24:544–8 [DOI] [PubMed] [Google Scholar]
- 28.Hoy MK, Heshka S, Allison DB, Grasset E, Blank R, Abiri M, Heymsfield SB. Reduced risk of liver-function-test abnormalities and new gallstone formation with weight loss on 3350-kJ (800-kcal) formula diets. Am J Clin Nutr 1994;60:249–54 [DOI] [PubMed] [Google Scholar]
- 29.Spirt BA, Graves LW, Weinstock R, Bartlett SJ, Wadden TA. Gallstone formation in obese women treated by a low-calorie diet. Int J Obes Relat Metab Disord 1995;19:593–5 [PubMed] [Google Scholar]
- 30.Wadden TA, Neiberg RH, Wing RR, Clark JM, Delahanty LM, Hill JO, Krakoff J, Otto A, Ryan DH, Vitolins MZ. Four-year weight losses in the Look AHEAD study: factors associated with long-term success. Obesity (Silver Spring) 2011;19:1987–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klem ML, Wing RR, Lang W, McGuire MT, Hill JO. Does weight loss maintenance become easier over time? Obes Res 2000;8:438–44 [DOI] [PubMed] [Google Scholar]
- 32.Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr 1997;66:239–46 [DOI] [PubMed] [Google Scholar]
- 33.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 2005;82:222S–5S [DOI] [PubMed] [Google Scholar]
- 34.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med 2006;355:1563–71 [DOI] [PubMed] [Google Scholar]