Abstract
Background: Neuronal processes that underlie the subjective experience of satiety after a meal are not well defined.
Objective: We investigated how satiety alters the perception of and neural response to visual food cues.
Design: Normal-weight participants (10 men, 13 women) underwent 2 fMRI scans while viewing images of high-calorie food that was previously rated as incompatible with weight loss and “fattening” and low-calorie, “nonfattening” food. After a fasting fMRI scan, participants ate a standardized breakfast and underwent reimaging at a randomly assigned time 15–300 min after breakfast to vary the degree of satiety. Measures of subjective appetite, food appeal, and ad libitum food intake (measured after the second fMRI scan) were correlated with activation by “fattening” (compared with “nonfattening”) food cues in a priori regions of interest.
Results: Greater hunger correlated with higher appeal ratings of “fattening” (r = 0.46, P = 0.03) but not “nonfattening” (r = −0.20, P = 0.37) foods. Fasting amygdalar activation was negatively associated with fullness (left: r = −0.52; right: r = −0.58; both P ≤ 0.01), whereas postbreakfast fullness was positively correlated with activation in the dorsal striatum (right: r = 0.44; left: r = 0.45; both P < 0.05). After breakfast, participants with greater activation in 4 regions—medial orbital frontal cortex (r = 0.49, P < 0.05), left amygdala (r = 0.49, P < 0.05), left insula (r = 0.47, P < 0.05), and nucleus accumbens (right: r = 0.57, P < 0.01; left: r = 0.43, P < 0.05)—chose buffet foods with higher fat content.
Conclusions: Postmeal satiety is shown in regional brain activation by images of high-calorie foods. Regions including the amygdala, nucleus accumbens, and dorsal striatum may alter perception of, and reduce motivation to consume, energy-rich foods, ultimately driving food choice. This trial was registered at clinicaltrials.gov as NCT01631045.
INTRODUCTION
The consumption of a meal reduces appetite through the perception of satiety. Thus, the process of eating must generate signals to the brain that alter our subjective sense of hunger, decrease food reward, and change our perception of food in the environment, resulting in a transient reduction in food intake. Peripheral mechanisms that mediate satiation (which leads to meal termination) and satiety (transient, meal-induced decrease of appetite) include gastric distention and the release of satiety signals (peptides) into circulation from the gut (1, 2). Many satiety signals act through vagal afferents that project to the hindbrain (1) and have been shown to both reduce food intake (3–5) and interact with upstream brain regions (6–9). Less is known about how these brain regions alter perceptions of food during meal-induced satiety to reduce further food intake.
Visual evaluation of food is likely integral to this process, because humans readily recognize and categorize food cues in their environment (10–12) as a first step to food consumption. fMRI studies provide ample evidence that brain regions involved in object recognition, attention, reward processing, and executive decision making respond differentially to foods compared with nonfood objects (13–15), and food cues may even evoke responses at a subconscious level (16). Moreover, key brain areas involved in the control of eating selectively respond to photographs of high-calorie (14, 17, 18) or “fattening” food (15), and these responses are altered by physiologic stimuli. Both fasting (19, 20) and the orexigenic hormone ghrelin (6) enhance activation by visual food cues in the amygdala, ventral striatum (nucleus accumbens), orbital frontal cortex (OFC)5, and insular cortex, whereas the anorexigenic hormone leptin (7) and gut-derived satiety signals (8) have the opposite effect. Findings are less straightforward in the dorsal striatum (caudate and putamen), where leptin administration has been shown to enhance (21) and reduce (7) activation by food cues. It remains unclear, however, whether normal meal-induced satiety alters brain responses to food cues to inhibit food intake, perhaps by reducing perceived food appeal.
We therefore measured responses to visual food cues in the brain regions discussed above at randomly assigned time intervals after a meal to manipulate participants’ proximity to the meal and therefore their experience of satiety. We expected food appeal to vary in relation to subjective satiety and that participants who rated food as less appealing would eat less. We further hypothesized that the magnitude of responses to the high-calorie visual food cues within brain reward regions would reflect participants’ perception of satiety and predict their subsequent food intake or food choice.
SUBJECTS AND METHODS
Participants
Ten male and 13 female healthy, normal-weight participants aged 18–50 y were recruited at the University of Washington by posted flyers, newspaper advertisements, and websites. Additional screening took place by telephone and during an in-person visit for measurement of height and weight and completion of questionnaires on medical history and eating behavior. Exclusion criteria were as follows: BMI (in kg/m2) <18.5 or >24.9 at the time of screening; current dieting for weight loss; a behavior pattern of restrained eating [Restraint Scale score >12 (22) or Three-Factor Eating Questionnaire (TFEQ) cognitive restraint scale score >10 (23)]; a history of eating disorders (24), prior obesity, or weight-loss surgery; chronic health conditions, including diabetes; use of medications that alter appetite (eg, atypical antipsychotic medications); pregnancy or use of oral contraceptives or estrogen replacement; recreational drug use or heavy alcohol use (25) of ≥2 drinks/d for women and ≥3 drinks/d for men; food allergies or inability to consume study foods; current smoking; and any contraindication to MRI, such as implanted metal or claustrophobia. One eligible participant completed study procedures but was subsequently excluded from all analyses for unusable fMRI data resulting from scanner artifact, which left a final sample size of 23. All study procedures were approved by the University of Washington Human Subjects Committee, and all participants provided written informed consent.
Study procedures
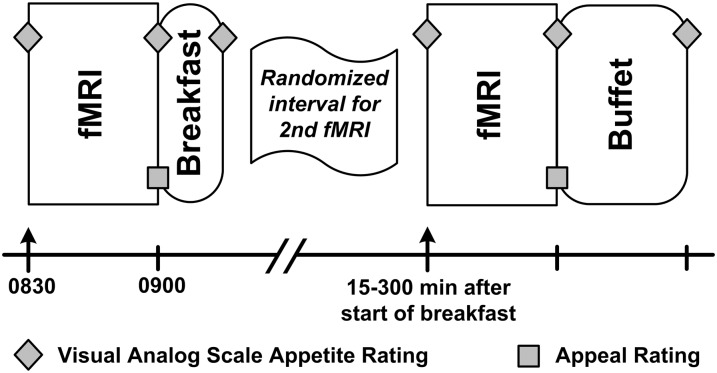
Participants began fasting at 2130 h on the night before the study visit. They arrived at the University of Washington Clinical Research Center the next morning at 0800 h and completed all study procedures on the same day. All participants underwent 2 fMRI scans (Figure 1): a fasting, prebreakfast scan at 0830 h and a postbreakfast scan at a subsequent, randomly assigned time point. At 0900 h, all participants were given a standardized breakfast (15% protein, 35% fat, 50% carbohydrate), consisting of egg and cheese on an English muffin served with orange juice. The breakfast was titrated to represent 20% of estimated daily caloric requirements, calculated by the Mifflin-St Jeor equation (26) and an activity factor. Participants had 15 min to complete this meal. After breakfast, the protocol varied to produce varying degrees of satiety. Each participant underwent a second, postbreakfast fMRI scan at a time that was randomly assigned to be 15 (n = 2), 30 (n = 2), 60 (n = 4), 120 (n = 3), 180 (n = 3), 240 (n = 4), or 300 (n = 4) min after the start of the standardized breakfast. Thus, some participants were scanned immediately after eating breakfast, whereas others waited for up to 5 h (in a supervised room with all food cues removed) before their postbreakfast scan. The actual time elapsed between the start of breakfast and the end of the postbreakfast fMRI acquisition was recorded for each participant and used for analyses. Random assignment to the 15- and 30-min time points was halted at n = 4 participants because the actual time intervals were identical for the 2 groups. After the second scan and subsequent food appeal ratings were completed, each participant was taken to a private room, where he or she had 30 min to select and consume food at the ad libitum buffet (described below). Participants were not informed that the buffet was part of study procedures or that their food consumption at the buffet was being monitored until a subsequent debriefing session that concluded the study.
FIGURE 1.
Schematic of the study protocol. Participants (n = 23) underwent a fasting fMRI scanning session beginning at 0830 h followed by a standardized breakfast with 20% of their estimated daily caloric needs. Each participant was randomly assigned to a second fMRI session time (15, 30, 60, 120, 180, 240, or 300 min after the start of their standardized breakfast) to vary satiety across participants. Immediately after the second fMRI scanning session, participants were presented with an ad libitum buffet. Participants completed serial visual analog scale appetite ratings; appeal ratings followed each fMRI scanning session.
Measures
Body weight and eating behavior
Weight and height were measured, and BMI was calculated [weight/(height squared)]. To identify individuals with chronic dieting or weight concerns who may have been less likely to eat according to their appetite, we administered the Revised Restraint Scale (22) and the TFEQ (23). The Revised Restraint Scale is a 10-item self-report questionnaire designed to identify individuals with chronic dieting and weight concerns (22). The TFEQ is a 52-item questionnaire with 3 subscales that assess different aspects of eating behavior (hunger, disinhibition, and cognitive restraint) (23).
Food appeal ratings
Immediately after each fMRI scan, participants viewed 42 photographs of “fattening” and “nonfattening” foods. These photographs were a subset of the stimuli presented during the fMRI scans and are described in depth below (see Food cue images). Participants were instructed to mark the number that “best describes how appealing the food shown in the photograph appears to you right now” on a 10-point Likert scale ranging from “not at all” to “extremely” appealing.
Visual analog scale appetite ratings
Questions on hunger, fullness, satisfaction, and prospective food consumption assessed subjective appetite every 30 min. Assessments were based on a validated rating system that uses a 0–100-mm visual analog scale (VAS) (27). Pre- and post-fMRI ratings were averaged to obtain the “during fMRI” rating. For missing values (n = 2), the single pre- or post-fMRI rating was used.
Ad libitum caloric intake
Objective satiety was measured by using an ad libitum buffet meal. The buffet consisted of a wide variety of foods appropriate to morning or midday meals. Foods differed in caloric and macronutrient content as well as in hedonic appeal (eg, bagels, turkey, ham, fruit, pastries) and were presented in amounts that widely exceeded each participant's estimated energy requirements (∼5000 kcal provided). All uneaten foods were weighed to determine the total kilocalories and macronutrient percentages of food consumed (ProNutra; Viocare Technologies).
Food cue images
Selection and validation of study images were completed in separate studies and have been described previously (15, 28). In brief, adults evaluated a set of photographs of food and rated whether the foods depicted were acceptable or unacceptable to eat while dieting to lose weight. Foods in the latter category were universally characterized by high caloric content and were usually high in fat, sugar, or both. In a separate study, these food images were presented to another group of adults, who categorized 98% as “fattening” (28). Foods depicted included candy, desserts, pastries, and high-fat savory foods such as pizza, hamburgers, and French fries. Thus, the “fattening” food cues consisted of images of foods that are commonly regarded as incompatible with weight loss and are typically high in calories. The “nonfattening” food cues represented foods that were deemed compatible with weight loss. These depicted low-calorie foods, including fruit, vegetables, salads, low-fat meats (eg, chicken breast), and seafood (see Supplementary Figure 1 under “Supplemental data” in the online issue for examples). Adults in the separate study rated 100% of these food images as “nonfattening” (28). Food images that elicited conflicting responses regarding their compatibility with weight loss (eg, breads) were excluded from the paradigm.
Imaging paradigm
Each fMRI session in the current study included a distinct set of 13 blocks of 10 photographs each. Nonfood blocks (n = 7) were alternated with fattening (n = 3) and nonfattening (n = 3) food blocks. The order of blocks was counterbalanced such that for one-half of participants (n = 12) the first food block contained fattening food images, whereas for the other half the first food block contained nonfattening images. Nonfood images consisted of common, recognizable large and small objects such as furniture, sundries, toiletries, and electronics.
All images were commercial-quality stock photographs obtained from websites (eg, www.iStockphoto.com) or donated for research use (Great American Stock Photo). All photographs were matched for size (600 × 400 dpi), quality, and visual interest and were group-matched for luminosity [F(2, 257) = 0.00, P = 0.99]. Each photograph was projected for 2.4 s on a screen that was easily viewed in a mirror by the participant while in the fMRI scanner.
Before each scan, to ensure that they focused on the images, participants were told that they would be tested on the photographs they had seen. After each scan, they were given a memory test consisting of images viewed in the scanner mixed with distracter images not previously shown. They were asked to indicate whether they had seen each image while in the scanner, and the percentage of correct responses was calculated. Thirty-two pictures were included (16 nonfoods, 8 fattening foods, 8 nonfattening foods) with 50% in each category as distracter images. Participants scored equivalently on both tests (mean prebreakfast percentage correct = 83.3% ± 9.1% compared with postbreakfast percentage correct = 83.3% ± 9.7%), showing no order effects on attention to the task.
Image acquisition and processing
All scans were acquired on a 3-tesla (-T) Philips Achieva MR System (version 1.5; Philips Medical Systems) with dual Quasar gradients (80 mT/m at a slew rate of 110 mT · m−1 · s−1 or 40 mT/m at a slew rate of 220 mT · m−1 · s−1) by using a Philips 8-channel SENSE head coil. Time series data, consisting of 130 T2*-weighted axial oblique volumes (whole-brain coverage, 40 slices, 3.44 × 3.44 × 3.5-mm voxels) were acquired by using a single-shot echo-planar imaging (EPI) sequence [repetition time (TR) = 2000 ms, echo time (TE) = 21 ms]. A B0 field map with the same slice coverage and orientation was also obtained by using a fast field echo sequence (TR = 210 ms, minimum TE = 2.3 ms, Δ TE 1.0 ms, flip angle = 30°) to facilitate geometric distortion correction of EPI data. In addition, a high-resolution 3-dimensional T1-weighted sagittal structural scan (MPRAGE sequence, TR/TE = 7.7/3.6 ms, θ = 8°, SENSE factor = 1, matrix = 256 × 256 × 200, 0.86 × 0.8 × 1-mm voxels) was acquired for anatomic coregistration of the fMRI data. The acquisition protocol was identical for the pre- and postbreakfast scans.
fMRI data processing was performed primarily by using the Oxford Centre for Functional MRI of the Brain (FMRIB) Software Library (www.fmrib.ox.ac.uk/fsl). The following pre–statistical processing steps were applied: motion correction with MCFLIRT (29), field map–based EPI unwarping with PRELUDE+FUGUE (30, 31), nonbrain removal with the Brain Extraction Tool (32), grand-mean intensity normalization of the entire 4-dimensional data set by a single multiplicative factor, high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with σ = 45.0 s), and 3-dimensional denoising by using a wavelet-based hierachical approach (33). The time series statistical analysis was performed with FMRIB's Improved Linear Model with local autocorrelation correction (34). The regression model included covariates for the fattening and nonfattening stimulus conditions, as well as nuisance covariates (average signal time courses in white matter and cerebrospinal fluid and motion parameter estimates). To allow for variation in the BOLD response over time, each block of fattening and nonfattening visual stimuli was modeled separately by using a boxcar convolved with a gamma function and its temporal derivative. Condition effects were estimated from the average response across blocks for our contrast of interest (fattening compared with nonfattening). Condition effects for 2 additional contrasts (fattening compared with object and nonfattening compared with object) were considered secondary outcomes and were used to aid our interpretation of findings.
Participants’ fMRI data were registered to their high-resolution structural scans by using a boundary-based registration procedure (35). The high-resolution structural scans were then registered to the Montreal Neurological Institute template space (ICBM152) by using FMRIB's linear image registration tool (29), with further refinement by using FMRIB's nonlinear image registration tool (36). For each participant, the derived transformations were concatenated and applied to the statistical images to allow for group-level analyses.
We used a region of interest (ROI) approach that combined a priori anatomic areas of interest with a functional criterion based on a minimum level of responsiveness to visual food cues. Within a set of hypothesized anatomic areas chosen on the basis of known responsiveness to food cues (14, 15) or physiologic satiety signals (8, 9), ROIs were functionally defined as voxels exhibiting a significantly greater BOLD response to fattening food compared with nonfood object stimuli in the prebreakfast scan (P < 0.05, uncorrected) by using a mixed-effects model. To create ROIs, these functionally defined areas were combined with anatomic areas on the basis of the Harvard-Oxford probabilistic atlas (37) with a minimum probability criterion of 25%. Anatomic areas included the frontal medial cortex to define the medial OFC region, the right/left frontal orbital cortex to define the lateral OFC region, right/left amygdala, right/left nucleus accumbens, right/left operculum, right/left dorsal striatum (caudate/putamen), and right/left insula. The resulting extent and location of all combined ROIs are presented in Supplementary Figure 2 under “Supplemental data” in the online issue. Voxel-wise participant-level parameter estimates for each contrast were first averaged within the combined ROIs before use as the dependent measure in correlational analyses. This technique represents an unbiased approach to test a priori hypotheses and avoids problems of circularity, whereby the nonindependence of ROI definitions and analyses inflates correlations based on extracted ROI values (38).
Statistical analysis
Means and SDs were calculated to describe participant characteristics. Group differences were compared by using unpaired Student's t test or paired t tests for within-participant comparisons. To ensure effective randomization, participants were grouped on the basis of their randomly assigned time of the second fMRI, and ANOVA was used to test for baseline differences in our subjective measures of satiety. For all other analyses of time, the actual time elapsed between the start of breakfast and the end of the postbreakfast scan was used instead of the randomized time point because of variation (range: 2–24 min) between the randomized, preassigned time and the actual time of image acquisition.
Three participants (2 men, 1 woman) consumed <20% of estimated daily intake during the standardized breakfast. Because these participants were not matched for caloric intake, they were excluded from all analyses of time elapsed after eating (resulting in n = 20 for these analyses). However, data from these participants were included in prebreakfast analyses and in analyses of the relation of subjective and objective satiety to caloric intake, appeal ratings, and fMRI activation, which were examined independently of time and amount of breakfast consumed.
Pearson's correlation coefficients were calculated among continuous variables, and linear regression models were used to determine significant associations (P < 0.05). Covariates with nonnormal distributions were transformed by using a Box-Cox transformation procedure before use in regression models. Predictors of buffet intake were entered into a model in a stepwise manner by using multiple linear regression; Wald tests were used to determine significant independent predictors. All statistical analyses were performed with GraphPad Prism (version 5.04; GraphPad Software), Stata 9 (StataCorp LP), or a regress program for the |STAT package (http://hcibib.org/perlman/stat/).
RESULTS
Participant characteristics
Participants had a mean BMI of 22.1 ± 1.7 (range: 19–25.1) and a mean age of 26.5 ± 6.6 y (range: 18–44 y) (Table 1). There were no differences by sex in other demographic characteristics, time elapsed between breakfast and postbreakfast fMRI, or VAS hunger and fullness ratings pre- or postbreakfast (data not shown). However, women rated nonfattening foods as significantly more appealing than did men but only in the fasted condition (7.1 ± 1.3 compared with 5.7 ± 1.7; P < 0.05; postbreakfast: 6.5 ± 1.2 compared with 5.8 ± 1.1; P = 0.21). When examined on the basis of their randomly scheduled fMRI sessions, groups did not differ at baseline in terms of hunger [F(6) = 2.12, P = 0.11], fullness [F(6) = 0.35, P = 0.90], or ratings of the appeal of fattening [F(6) = 0.48, P = 0.48] or nonfattening [F(6) = 0.97, P = 0.48] food images.
TABLE 1.
Characteristics of participants (n = 23)1
| Values | |
| BMI (kg/m2) | 22.1 ± 1.7 |
| Age (y) | 26.5 ± 6.6 |
| Time elapsed since meal (min) | 156 ± 100 |
| Fasted VAS scores (mm) | |
| Hunger | 69 ± 15 |
| Fullness | 17 ± 16 |
| Prospective food consumption | 71 ± 19 |
| Satisfied | 19 ± 13 |
| Postbreakfast VAS scores (mm) | |
| Hunger | 25 ± 19 |
| Fullness | 61 ± 21 |
| Prospective food consumption | 36 ± 20 |
| Satisfied | 59 ± 26 |
| Restraint Scale score | 7.3 ± 2.8 |
| TFEQ score | |
| Cognitive restraint | 6.2 ± 2.2 |
| Disinhibition | 3.5 ± 2.2 |
| Hunger | 4.1 ± 3.0 |
| Estimated daily energy needs (kcal) | 2610 ± 549 |
| Standardized meal (kcal) | 500 ± 87 |
| Ad libitum buffet (kcal) | 1092 ± 462 |
| Buffet fat intake (%) | 34 ± 9 |
| Buffet carbohydrate intake (%) | 52 ± 11 |
| Buffet protein intake (%) | 13 ± 4 |
| Mean appeal rating of fattening food images (fasting) | 5.7 ± 1.6 |
| Mean appeal rating of nonfattening food images (fasting) | 6.5 ± 1.7 |
n = 10 men and 13 women. TFEQ, Three-Factor Eating Questionnaire; VAS, visual analog scale.
Effect of the standardized breakfast on satiety
During fasting, participants’ VAS hunger ratings were significantly higher than their fullness ratings (Table 1; P < 0.0001), whereas immediately after the standardized breakfast their subjective hunger and prospective food intake ratings significantly decreased, and their fullness and satisfaction ratings significantly increased (Table 1; P < 0.0001 for all). These data suggest that the standardized breakfast sufficiently induced satiety and that the experience of postprandial satiety was effectively measured by the VAS.
After the standardized breakfast, we induced variability in satiety by varying the interval between breakfast and the ad libitum buffet. Consequently, total intake at the buffet ranged from 71 to 2109 kcal, which represented ∼3–72% of estimated daily caloric needs. Macronutrient consumption at the buffet ranged from 7% to 46% of calories from fat and from 39% to 90% from carbohydrate.
Effect of time on subjective appetite and food appeal
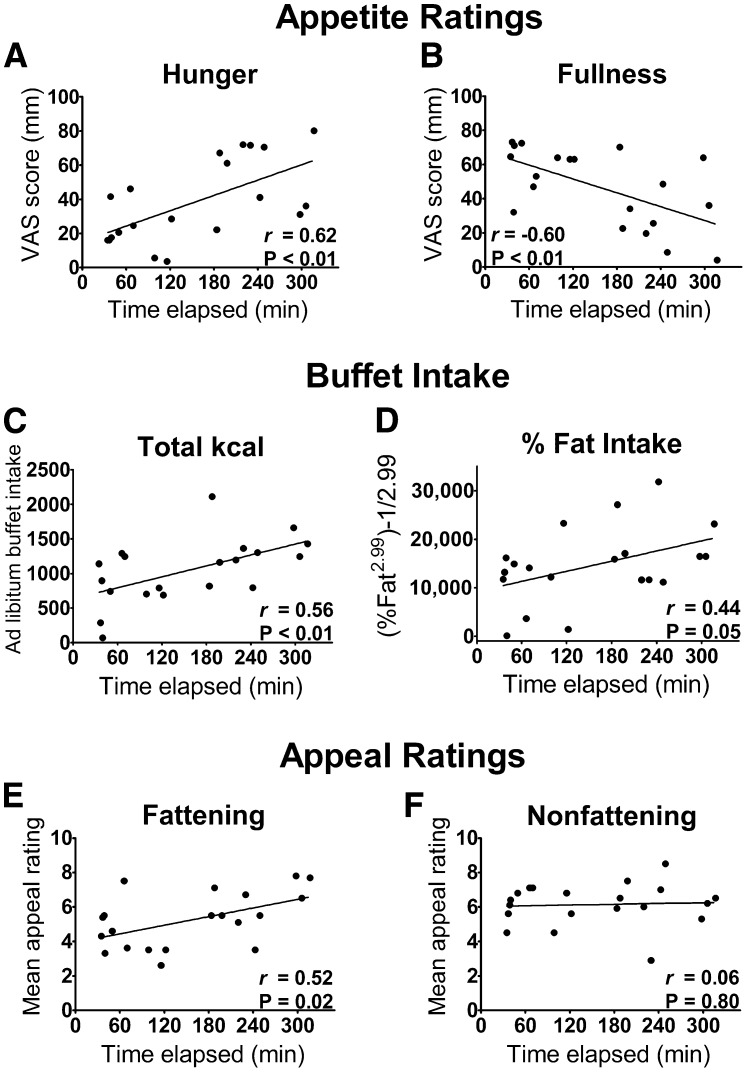
As expected, the interval between the standardized breakfast and the postbreakfast fMRI scan was positively associated with the degree of subjective hunger at the time of postbreakfast assessments and was negatively associated with fullness scores (Figure 2, A and B). Relations were in the same direction, but were not significant, for measures of prospective food consumption and satisfaction (r = 0.35, P = 0.14, and r = −0.39, P = 0.09, respectively). Time elapsed since breakfast was also positively correlated with the quantity of kilocalories consumed at the buffet (Figure 2C). These relations were characterized by considerable interindividual variation, especially among those for whom the buffet meal began ∼30–60 min after the standardized breakfast.
FIGURE 2.
Time elapsed since breakfast is related to subjective and objective measures of satiety. A, B: VAS appetite ratings; C, D: measures of food intake at the ad libitum buffet; E, F: subjective ratings of the appeal of “fattening” compared with “nonfattening” foods. Fattening food images depicted high-calorie foods that were previously rated as incompatible with weight loss and considered fattening. Pearson's correlation coefficients are presented with P values derived from linear regression (n = 20; 3 participants were excluded for consuming <20% of estimated needs at the standardized breakfast). VAS, visual analog scale.
Whereas nonfattening foods were rated as similarly appealing regardless of the amount of time elapsed since participants had last eaten, images of fattening foods were rated as more appealing when longer times had elapsed since the standardized breakfast (Figure 2, E and F). Longer intervals between breakfast and the ad libitum buffet were also associated with a higher proportion of kilocalories consumed from fat at the buffet (P < 0.05; Figure 2D) and a trend for a lower proportion from carbohydrates (r = −0.39, P = 0.09).
Relation of satiety to food appeal
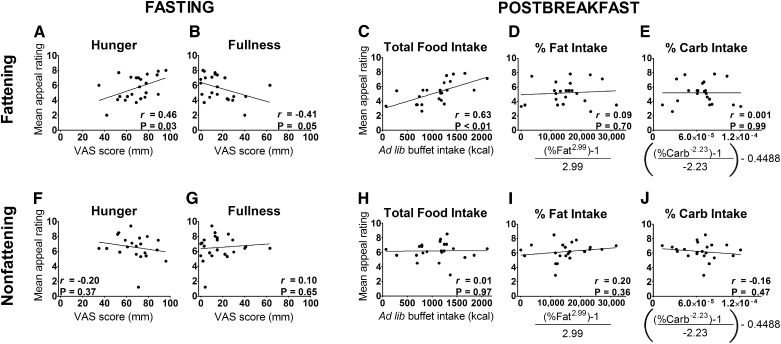
When fasted, hungrier participants reported a greater appeal of the fattening foods, whereas the reverse was true for participants who were more full (Figure 3, A and B). Similar findings were obtained for prospective food consumption (r = 0.42, P < 0.05) and satisfaction (r = −0.52, P = 0.01). We found no significant relations between the appeal of the nonfattening foods and these appetite measures (Figure 3, F and G; prospective food consumption: r = −0.07, P = 0.7; satisfied: r = 0.08, P = 0.7). In the postbreakfast state, fattening foods were again rated as more appealing by individuals who were hungrier (r = 0.59, P < 0.01), whereas fullness was unrelated to the appeal of fattening foods (r = −0.27, P = 0.22). The appeal of nonfattening foods did not vary with subjective appetite in the postbreakfast state (hunger: r = 0.17, P = 0.45; fullness: r = −0.22, P = 0.32; prospective food consumption: r = −0.07, P = 0.7; satisfied: r = 0.08, P = 0.7). Higher appeal ratings of fattening food obtained immediately before the ad libitum buffet predicted consumption of more kilocalories during the buffet (Figure 3C), but ratings of nonfattening food were unrelated to total buffet intake (Figure 3H). Appeal ratings were unrelated to macronutrient consumption at the buffet (Figure 3, D, E, I, and J). The above findings were unchanged in models that adjusted for sex (data not shown).
FIGURE 3.
A–J: Ratings of food appeal and their correlation with measures of subjective and objective appetite. Appeal ratings were obtained immediately after the first fMRI scan (fasting) and second fMRI scan (postbreakfast). “Fattening” food images depicted high-calorie foods that were previously rated as incompatible with weight loss and considered fattening. In the fasted state, participants’ (n = 23) appeal ratings for fattening food, but not for nonfattening food, were positively associated with hunger and negatively associated with fullness. Postbreakfast appeal for fattening food, but not for nonfattening food, predicted the number of calories consumed at an ad libitum buffet but not food choice. Percentage fat (range: 7–46%) and carbohydrate (range: 39–90%) intake data were transformed by using a Box-Cox transformation. Pearson's correlation coefficients are presented with P values derived from linear regression. Ad lib, ad libitum; Carb, carbohydrate; VAS, visual analog scale.
Relation of satiety to regional brain activation by fattening food cues
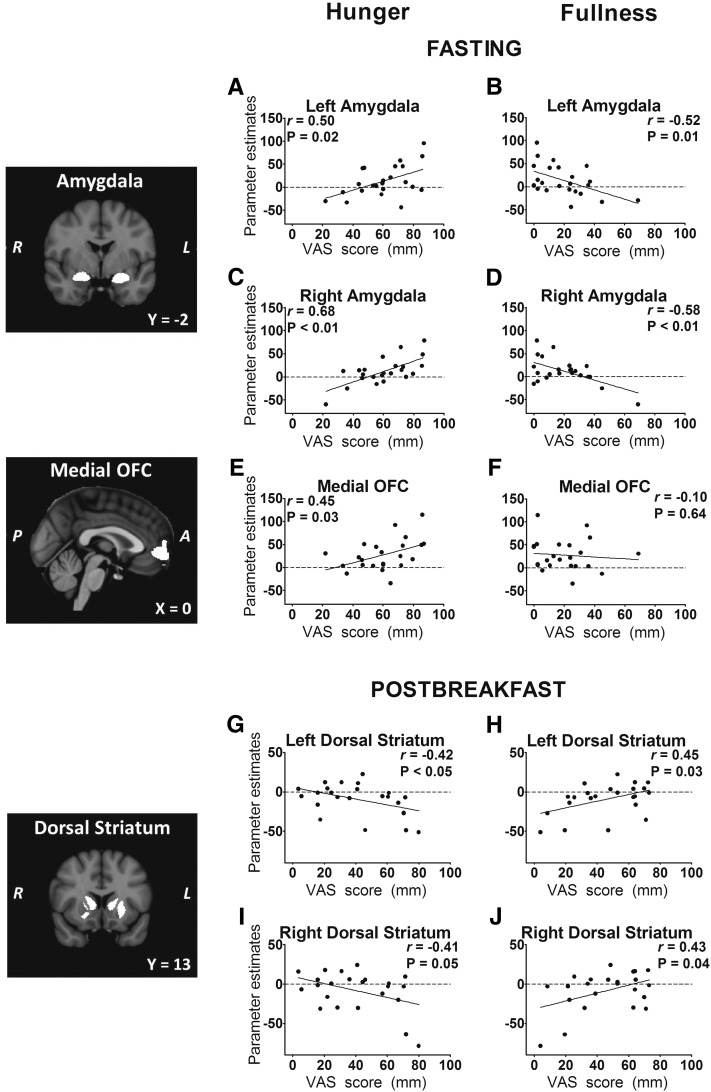
Correlational analyses that examine measures of subjective and objective satiety in relation to fMRI-assessed activation in brain ROIs are shown in Table 2. In the fasted state, bilateral activation in the amygdala by fattening food images was positively associated with hunger scores and negatively associated with fullness scores (Figure 4, A–D). Activation by fattening food cues in the medial OFC was also associated with higher hunger ratings (Figure 4E). During the postbreakfast fMRI scans, responses in the bilateral dorsal striatum showed an association between fullness and greater activation by fattening food cues and between hunger and less activation (Figure 4, G–J). In the right amygdala, activation by fattening food cues (as compared with nonfood objects) was again greater in participants who reported more hunger and less fullness (r = 0.56, P < 0.01, and r = −0.46, P = 0.03, respectively).
TABLE 2.
Pearson's correlation coefficients for behavioral measures compared with activation in a priori regions of interest1
| Regions of interest |
|||||||||||||
| Nucleus accumbens |
Amygdala |
Dorsal striatum |
Insula |
Operculum |
OFC |
||||||||
| Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left lateral | Right lateral | Medial | |
| Fasting2 | |||||||||||||
| VAS hunger | −0.27 | −0.21 | 0.50* | 0.68** | 0.36 | 0.25 | 0.13 | 0.16 | 0.35 | 0.25 | 0.35 | 0.21 | 0.45* |
| VAS fullness | 0.17 | 0.20 | −0.52* | −0.58** | −0.22 | 0.05 | −0.21 | −0.07 | −0.14 | −0.05 | −0.36 | −0.12 | −0.10 |
| Postbreakfast | |||||||||||||
| VAS hunger | −0.05 | −0.14 | −0.06 | 0.15 | −0.42* | −0.41# | −0.08 | 0.15 | −0.10 | −0.16 | −0.03 | −0.03 | −0.02 |
| VAS fullness | −0.04 | −0.07 | −0.05 | −0.17 | 0.45* | 0.43* | 0.01 | −0.22 | 0.06 | 0.17 | −0.09 | −0.004 | −0.10 |
| Buffet intake (kcal) | −0.03 | 0.09 | 0.02 | 0.03 | −0.10 | −0.03 | −0.17 | 0.13 | −0.11 | −0.01 | 0.03 | 0.07 | 0.10 |
| Fat intake (%) | 0.43* | 0.57** | 0.49* | 0.33 | 0.34 | 0.16 | 0.47* | 0.34 | 0.26 | 0.02 | 0.32 | 0.36 | 0.49* |
| Carbohydrate intake (%) | −0.48* | −0.54** | −0.46* | −0.32 | −0.26 | −0.12 | −0.41# | −0.28 | −0.22 | 0.04 | −0.33 | −0.31 | −0.46* |
Activation was determined by calculating each participants’ (n = 23) individual mean parameter estimate for the contrast of fattening > nonfattening food cues within each region of interest. These values were correlated with behavioral measures (Pearson's), and simple linear regression was used to determine P values. *P < 0.05, #P = 0.05, **P < 0.01. OFC, orbital frontal cortex; VAS, visual analog scale.
Fasting data include the average VAS score (hunger and fullness calculated from measures taken directly before and after the first fMRI scan). Postbreakfast data include average VAS scores (hunger and fullness calculated from measures taken directly before and after the second fMRI scan) and ad libitum food intake at a buffet, including total kilocalories consumed as well as percentages of total kilocalories from fat and carbohydrate intake.
FIGURE 4.
A–J: Activation by fattening food cues is a marker of subjective appetite in the amygdala, medial OFC, and dorsal striatum. Left panels: coronal sections of bilateral amygdala and bilateral dorsal striatum ROIs and sagittal section of the medial OFC ROI. Right panels: plots of individual mean ROI parameter estimates (n = 23) for the contrast fattening > nonfattening foods compared with average VAS appetite ratings for the fasting and postbreakfast fMRI scan. “Fattening” food images depicted high-calorie foods that were previously rated as incompatible with weight loss and considered fattening. Pearson's correlation coefficients are presented with P values derived from linear regression. A, anterior; L, left; OFC, orbital frontal cortex; P, posterior; R, right; ROI, region of interest; VAS, visual analog scale.
Predictors of total caloric intake and food choice
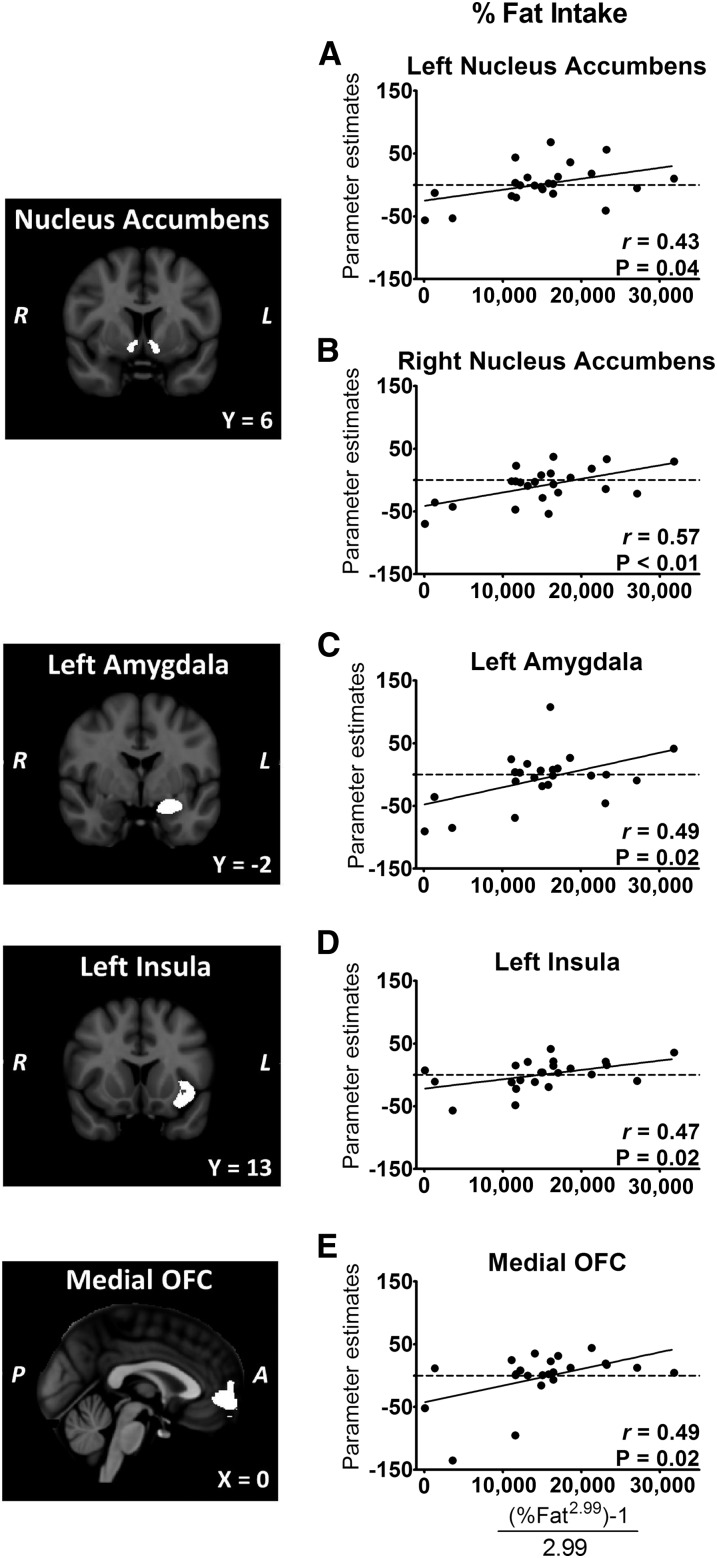
As anticipated, subjective and objective measures of appetite were related. Greater subjective hunger immediately before the ad libitum buffet was associated with consumption of more kilocalories at the buffet (r = 0.66, P < 0.001), whereas greater fullness was associated with consumption of fewer kilocalories (r = −0.59, P = 0.003). However, we found no significant correlations between activation in any ROI and total kilocalories consumed at the buffet (Table 2). Several ROIs showed prospective relations such that activation by fattening food cues was associated with a greater consumption of kilocalories from fat at the buffet (Figure 5). These included the medial OFC, left amygdala, left insula, and bilateral nucleus accumbens. These regions were reciprocally associated with carbohydrate intake (Table 2), because increases in percentage of fat consumed were strongly associated with decreases in percentage of carbohydrate consumed (r = −0.94, P < 0.0001). Thus, activation in these brain areas was related to food choice during the buffet meal but not to total caloric intake.
FIGURE 5.
A–E: Greater activation by fattening food cues predicts increased intake of calories from fat. Participants (n = 23) underwent fMRI scans at randomly assigned times after a standardized breakfast, followed by ad libitum intake of foods at a buffet. Left panels: coronal sections of bilateral nucleus accumbens, left amygdala, and left insula ROIs and sagittal section of the medial OFC ROI. Right panels: plots of individual mean ROI parameter estimates for the contrast fattening > nonfattening foods compared with percentage of fat intake. “Fattening” food images depicted high-calorie foods that were previously rated as incompatible with weight loss and considered fattening. Percentage fat intake data (range: 7–46%) were transformed by using a Box-Cox transformation. A, anterior; L, left; OFC, orbital frontal cortex; P, posterior; R, right; ROI, region of interest.
We used multiple linear regression modeling to identify independent predictors of total kilocalories consumed at the ad libitum buffet. As noted above, VAS ratings, appeal ratings for fattening food, and time elapsed since breakfast were associated with buffet intake, but fMRI markers were not. In the initial univariate model, VAS fullness predicted 35% of the variance in buffet intake (β = −12.6; 95% CI: −20.5, −4.7; P = 0.003). Appeal ratings for fattening food were also a significant independent predictor of buffet intake (β = 156; 95% CI: 61, 251; P = 0.007). Together, VAS fullness and appeal ratings explained 59% of the variance in ad libitum caloric intake. Time elapsed after breakfast was not an independent predictor of intake after adjustment for fullness and appeal ratings, nor were sex or BMI.
We also used multiple linear regression to examine whether activation by fattening food cues in the bilateral nucleus accumbens was associated with percentage of fat consumed at the buffet after other potential explanatory factors were accounted for. In univariate models, nucleus accumbens activation on the left side predicted 18% and the right predicted 32% of the variance in fat intake. In all models of the right and left nucleus accumbens, activation remained a significant (P < 0.05 or less) predictor of fat intake at the buffet, even after adjustment for key variables such as time elapsed after breakfast, sex, BMI, VAS ratings of hunger and fullness, and subjective appeal of fattening food.
DISCUSSION
Our behavioral findings suggest that satiety is characterized by a reduction in the subjective appeal of highly palatable, energy-rich food, with no change in the appeal of less palatable, lower calorie food. As meal-induced satiety wanes, the subjective appeal of these “fattening” foods increases, with associated increases in the quantity of kilocalories ingested at an ad libitum buffet. We found that fMRI-assessed activation by fattening food cues in the amygdala, medial OFC, and dorsal striatum were biomarkers of subjective satiety after a meal. In addition, whereas brain responses to food cues did not predict total caloric intake at the buffet, brain activation by fattening food cues in the amygdala, insula, medial OFC, and nucleus accumbens was related to food choice. Specifically, greater activation by fattening food cues predicted consumption of foods that were higher in fat at the buffet. Together, these findings suggest that postmeal satiety is reflected in regional brain activation by fattening food cues, thereby affecting perception of, and reducing motivation to consume, highly palatable, energy-rich foods.
Subjective satiety and “fattening” food
Among normal-weight subjects, postmeal satiety was characterized by reduced appeal of “fattening” foods. The foods depicted in the images used for appeal ratings and as fMRI stimuli are called “fattening” because they are commonly perceived as incompatible with weight loss, commonly described as “fattening” (as validated in a prior study), and universally characterized by higher caloric content and palatability (15, 28). Because ratings of fattening food images independently predicted total caloric intake at varying times after a standardized meal, we suggest that appeal ratings for these types of foods are a marker of satiety with relevance to human eating behavior. Thus, a meal reduces—whereas fasting, hunger, and prolonged time after eating increase—the appeal of exactly the kind of food that is typically avoided by people who try to lose weight.
Subjective satiety associated with brain activation by food cues
Our findings support a model in which reduced activation of the amygdala and the medial OFC by fattening food cues is a biomarker of subjective satiety. Anatomically, ascending pathways from hindbrain satiety centers synapse in the central nucleus of the amygdala (39), among other centers, and connect to striatal regions that direct motivated behavior and motor action (40). Functionally, the amygdala plays a key role in encoding the motivational and emotional value of stimuli and in focusing attention on emotional stimuli to engage behaviors that meet the needs of individual organisms (41–43). Thus, when energy needs are reduced after a meal, the effect of food cues to increase amygdalar activity is diminished or absent (42, 43), resulting in the devaluation of these cues through satiation. Furthermore, the amygdala and orbital frontoinsular cortex are part of a distributed “salience network” that directs attention toward the most homeostatically relevant environmental stimuli (44). Recent findings suggest that the anterior cingulate cortex, a region that we did not examine in the current study, may participate with the amygdala and medial OFC to heighten responses to energy-dense food stimuli during hunger (45).
Conversely, activation in the dorsal striatum increases along with perceptions of greater fullness after a meal. The dorsal striatum, like many brain regions, participates in diverse and sometimes opposing functions (46), which perhaps explains conflicting findings in the literature (6, 7, 21, 47), such as greater activation by food cues in individuals with anorexia nervosa (48) and with obesity (47), as compared with controls. Dorsal striatal functions include both permissive aspects of motor feeding (49) and general motor inhibition (50–52). Increases in dorsal striatal activity after a meal could reflect top-down attentional control (46, 53) and directly inhibit the amygdala and medial OFC, thereby suppressing further food intake. However, recent findings localize dopamine-driven response inhibition (52) to the caudate and putamen, emphasizing their role in suppression of motor action in general and opening the possibility for a similar role in meal termination. This hypothesis is consistent with our finding that dorsal striatal activation was associated with fullness after breakfast but not while fasting. On this reasoning, findings that taste stimuli fail to stimulate dorsal striatal responses in obesity (54) may reflect impaired satiety.
Brain activation, food consumption, and food choice
A key finding of this study is that activation by fattening food cues in the nucleus accumbens, left amygdala, left insula, and medial OFC—regions known to mediate reward learning and motivated behavior (40, 43, 55, 56)—was associated with macronutrient choice at an ad libitum buffet. In particular, activation in the nucleus accumbens was related to the amount of fat consumed at the buffet but not to subjective satiety. Thus, suppression of activation by fattening food cues in the nucleus accumbens may shape food choices by dampening motivation to consume particular foods. Both the accumbens and the amygdala contain opioid pathways that potently stimulate intake of highly palatable or energy-dense foods (40, 57). This effect is sensitive to leptin and insulin (hormones that convey information on body fat stores to the brain), because both hormones suppress opioid-stimulated sucrose consumption in rodents (58), whereas nasal administration of postprandial insulin enhances satiety and reduces intake of palatable snacks in humans (59). Fasting produces the opposite effect, increasing preferential consumption of a high-fat diet in rodents (60) and activation by high-calorie food cues in humans (20). Thus, along with prior studies, the current findings suggest that responses to high-calorie food cues may be physiologically regulated to alter food choice in response to homeostatic rather than simply hedonic inputs.
Limitations
Other authors have distinguished between “liking” and “wanting” foods (61), but we were unable to separate these factors in our study. In addition, “fattening” is a subjective construct that does not provide a precise description of nutrient composition. Our findings nonetheless indicate that this construct identifies a category of food with considerable relevance to human appetite regulation. Moreover, we studied both men and women, and prior studies have noted sex differences in brain responses to satiation (62). However, the men and women behaved very similarly in our study, perhaps because we used strict criteria to exclude individuals with major concerns about eating or dieting, or with substantial cognitive restraint, because these are sex-related attitudes that can influence brain responses (28). We did not control for menstrual phase among women, which may have increased variation in response to food cues (63), nor for potential sex differences in brain volume. In addition, brain centers such as the amygdala, dorsal striatum, and nucleus accumbens have substantial functional heterogeneity, which complicates interpretation of results over large anatomic areas. A key strength of this study is the use of a combined functional and anatomic strategy to define ROIs, which were activated by fattening food cues in the fasted state. However, because these ROIs were also applied to the postprandial state, it is possible that we inadvertently omitted areas that became responsive to fattening food cues only after a meal.
Conclusions
During satiety, brain systems alter our perception of environmental food cues, thereby suppressing food intake and altering food choices. Our findings suggest that this response is specific to “fattening” food cues—ie, depictions of energy-dense, highly palatable foods that are commonly regarded as incompatible with weight loss. Although we studied normal-weight participants, our results may have implications for understanding obesity pathogenesis and treatment. We showed that the normal experience of satiety after a meal is characterized by reductions in the subjective appeal of, and the regional brain response to, fattening food cues, accompanied by increases in the dorsal striatal response. Deficiencies in this process could unify findings of hyperresponsiveness to food cues (17, 47, 64) with findings of impaired satiety (65) in obese individuals, especially in those with increased genetic risk (66). Future work could include connectivity analyses to link the identified brain regions into functional networks involved in satiety processing and should examine the impact of meal composition (67), gene variants, and metabolic factors (eg, insulin resistance) on satiety processing in the brain.
Acknowledgments
The authors’ responsibilities were as follows—EAS, MWS, and TG: designed the experiment; AS and VT: conducted the research; EAS, SM, and SJM: analyzed the data and performed the statistical analyses; EAS and SJM: wrote the manuscript; and EAS: had primary responsibility for the final content. All authors read and approved the final manuscript. None of the authors had any conflicts of interest to report.
Footnotes
Abbreviations used: EPI, echo-planar imaging; FMRIB, Oxford Centre for Functional MRI of the Brain; OFC, orbital frontal cortex; ROI, region of interest; TE, echo time; TFEQ, Three-Factor Eating Questionnaire; TR, repetition time; VAS, visual analog scale.
REFERENCES
- 1.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest 2007;117:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson KA, Bloom SR. Appetite and hedonism: gut hormones and the brain. Endocrinol Metab Clin North Am 2010;39:729–43 [DOI] [PubMed] [Google Scholar]
- 3.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 2002;418:650–4 [DOI] [PubMed] [Google Scholar]
- 4.Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP. C-terminal octapeptide of cholecystokinin decreases food intake in man. Am J Clin Nutr 1981;34:154–60 [DOI] [PubMed] [Google Scholar]
- 5.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998;101:515–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 2008;7:400–9 [DOI] [PubMed] [Google Scholar]
- 7.Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science 2007;317:1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Silva A, Salem V, Long CJ, Makwana A, Newbould RD, Rabiner EA, Ghatei MA, Bloom SR, Matthews PM, Beaver JD, et al. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab 2011;14:700–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 2007;450:106–9 [DOI] [PubMed] [Google Scholar]
- 10.Nijs IM, Muris P, Euser AS, Franken IH. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite 2010;54:243–54 [DOI] [PubMed] [Google Scholar]
- 11.Stockburger J, Schmalzle R, Flaisch T, Bublatzky F, Schupp HT. The impact of hunger on food cue processing: an event-related brain potential study. Neuroimage 2009;47:1819–29 [DOI] [PubMed] [Google Scholar]
- 12.Toepel U, Knebel JF, Hudry J, le Coutre J, Murray MM. The brain tracks the energetic value in food images. Neuroimage 2009;44:967–74 [DOI] [PubMed] [Google Scholar]
- 13.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage 2003;19:1381–94 [DOI] [PubMed] [Google Scholar]
- 14.van der Laan LN, de Ridder DT, Viergever MA, Smeets PA. The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage 2011;55:296–303 [DOI] [PubMed] [Google Scholar]
- 15.Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 2009;33:653–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks SJ, O'Daly OG, Uher R, Schioth HB, Treasure J, Campbell IC. Subliminal food images compromise superior working memory performance in women with restricting anorexia nervosa. Conscious Cogn 2012;21:751–63 [DOI] [PubMed] [Google Scholar]
- 17.Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008;41:636–47 [DOI] [PubMed] [Google Scholar]
- 18.Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR. Effects of overfeeding on the neuronal response to visual food cues. Am J Clin Nutr 2007;86:965–71 [DOI] [PubMed] [Google Scholar]
- 19.Führer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring) 2008;16:945–50 [DOI] [PubMed] [Google Scholar]
- 20.Goldstone AP, de Hernandez CGP, Beaver JD, Muhammed K, Croese C, Bell G, Durighel G, Hughes E, Waldman AD, Frost G, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci 2009;30:1625–35 [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest 2008;118:2583–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herman CP, Polivy J. Restrained eating. In: Stunkard AJ, ed. Obesity. Philadelphia, PA: WB Saunders, 1980:208–25.
- 23.Stunkard AJ, Messick S. The Three-Factor Eating Questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985;29:71–83 [DOI] [PubMed] [Google Scholar]
- 24.Keski-Rahkonen A, Sihvola E, Raevuori A, Kaukoranta J, Bulik CM, Hoek HW, Rissanen A, Kaprio J. Reliability of self-reported eating disorders: Optimizing population screening. Int J Eat Disord 2006;39:754–62 [DOI] [PubMed] [Google Scholar]
- 25.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998;158:1789–95 [DOI] [PubMed] [Google Scholar]
- 26.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–7 [DOI] [PubMed] [Google Scholar]
- 27.Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord 2000;24:38–48 [DOI] [PubMed] [Google Scholar]
- 28.Schur EA, Kleinhans NM, Goldberg J, Buchwald DS, Polivy J, Del Parigi A, Maravilla KR. Acquired differences in brain responses among monozygotic twins discordant for restrained eating. Physiol Behav 2012;105:560–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17:825–41 [DOI] [PubMed] [Google Scholar]
- 30.Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med 2003;49:193–7 [DOI] [PubMed] [Google Scholar]
- 31.Jenkinson M, Wilson JL, Jezzard P. Perturbation method for magnetic field calculations of nonconductive objects. Magn Reson Med 2004;52:471–7 [DOI] [PubMed] [Google Scholar]
- 32.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khullar S, Michael A, Correa N, Adali T, Baum SA, Calhoun VD. Wavelet-based fMRI analysis: 3-D denoising, signal separation, and validation metrics. Neuroimage 2011;54:2867–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 2001;14:1370–86 [DOI] [PubMed] [Google Scholar]
- 35.Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 2009;48:63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka spatial normalization. FMRIB technical report TR07JA2. Available from: www.fmrib.ox.ac.uk/analysis/techrep (cited 2007)
- 37.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–80 [DOI] [PubMed] [Google Scholar]
- 38.Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci 2009;4:274–90 [DOI] [PubMed] [Google Scholar]
- 39.McDonald AJ, Jackson TR. Amygdaloid connections with posterior insular and temporal cortical areas in the rat. J Comp Neurol 1987;262:59–77 [DOI] [PubMed] [Google Scholar]
- 40.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 2005;86:773–95 [DOI] [PubMed] [Google Scholar]
- 41.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 2005;48:175–87 [DOI] [PubMed] [Google Scholar]
- 42.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci 2001;115:493–500 [DOI] [PubMed] [Google Scholar]
- 43.Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science 2003;301:1104–7 [DOI] [PubMed] [Google Scholar]
- 44.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benedict C, Brooks SJ, O'Daly OG, Almen MS, Morell A, Aberg K, Gingnell M, Schultes B, Hallschmid M, Broman JE, et al. Acute sleep deprivation enhances the brain's response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab 2012;97:E443–7 [DOI] [PubMed] [Google Scholar]
- 46.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci 2007;27:8161–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 2007;37:410–21 [DOI] [PubMed] [Google Scholar]
- 48.Brooks SJ, O'Daly OG, Uher R, Friederich HC, Giampietro V, Brammer M, Williams SC, Schioth HB, Treasure J, Campbell IC. Differential neural responses to food images in women with bulimia versus anorexia nervosa. PLoS ONE 2011;6:e22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci 2008;1129:35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zandbelt BB, Vink M. On the role of the striatum in response inhibition. PLoS ONE 2010;5:e13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vink M, Kahn RS, Raemaekers M, van den Heuvel M, Boersma M, Ramsey NF. Function of striatum beyond inhibition and execution of motor responses. Hum Brain Mapp 2005;25:336–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, Brown AK, Monterosso JR, Aron AR, Mandelkern MA, et al. Striatal dopamine D2/D3 receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. J Neurosci 2012;32:7316–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci 2000;3:284–91 [DOI] [PubMed] [Google Scholar]
- 54.Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 2008;322:449–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rolls ET. Taste, olfactory and food texture reward processing in the brain and obesity. Int J Obes (Lond) 2011;35:550–61 [DOI] [PubMed] [Google Scholar]
- 56.Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Ann N Y Acad Sci 1999;877:523–47 [DOI] [PubMed] [Google Scholar]
- 57.Kim EM, Quinn JG, Levine AS, O'Hare E. A bi-directional mu-opioid-opioid connection between the nucleus of the accumbens shell and the central nucleus of the amygdala in the rat. Brain Res 2004;1029:135–9 [DOI] [PubMed] [Google Scholar]
- 58.Figlewicz DP, MacDonald Naleid A, Sipols AJ. Modulation of food reward by adiposity signals. Physiol Behav 2007;91:473–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hallschmid M, Higgs S, Thienel M, Ott V, Lehnert H. Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes 2012;61:782–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith BK, Berthoud HR, York DA, Bray GA. Differential effects of baseline macronutrient preferences on macronutrient selection after galanin, NPY, and an overnight fast. Peptides 1997;18:207–11 [DOI] [PubMed] [Google Scholar]
- 61.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci 2003;26:507–13 [DOI] [PubMed] [Google Scholar]
- 62.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, Nievelstein RA, van der Grond J. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr 2006;83:1297–305 [DOI] [PubMed] [Google Scholar]
- 63.Frank TC, Kim GL, Krzemien A, Van Vugt DA. Effect of menstrual cycle phase on corticolimbic brain activation by visual food cues. Brain Res 2010;1363:81–92 [DOI] [PubMed] [Google Scholar]
- 64.Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci 2012;32:5549–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gautier JF, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M, Ravussin E, Reiman EM, Tataranni PA. Differential brain responses to satiation in obese and lean men. Diabetes 2000;49:838–46 [DOI] [PubMed] [Google Scholar]
- 66.Wardle J, Carnell S, Haworth CM, Farooqi IS, O'Rahilly S, Plomin R. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab 2008;93:3640–3 [DOI] [PubMed] [Google Scholar]
- 67.Rolls BJ. The relationship between dietary energy density and energy intake. Physiol Behav 2009;97:609–15 [DOI] [PMC free article] [PubMed] [Google Scholar]