Abstract
There have been many advances in our knowledge about different aspects of P2Y receptor signaling since the last review published by our International Union of Pharmacology subcommittee. More receptor subtypes have been cloned and characterized and most orphan receptors deorphanized, so that it is now possible to provide a basis for a future subdivision of P2Y receptor subtypes. More is known about the functional elements of the P2Y receptor molecules and the signaling pathways involved, including interactions with ion channels. There have been substantial developments in the design of selective agonists and antagonists to some of the P2Y receptor subtypes. There are new findings about the mechanisms underlying nucleotide release and ectoenzymatic nucleotide breakdown. Interactions between P2Y receptors and receptors to other signaling molecules have been explored as well as P2Y-mediated control of gene transcription. The distribution and roles of P2Y receptor subtypes in many different cell types are better understood and P2Y receptor-related compounds are being explored for therapeutic purposes. These and other advances are discussed in the present review.
I. Brief Historical Background of Nucleotides and Their Receptors
The first description of the extracellular signaling by purines was by Drury and Szent-Györgyi (1929), and purinergic receptors were defined in 1976 (Burnstock, 1976). After an early hint (Spedding and Weetman, 1976), receptors for purines were subdivided into P1 (adenosine) and P2 (ATP and ADP) receptors (Burnstock, 1978), and later subdivision of P2 receptors into P2X and P2Y subtypes was made on the basis of pharmacology (Burnstock and Kennedy, 1985). It was recognized that some P2Y receptors responded to pyrimidines as well as purines (Seifert and Schultz, 1989). After cloning of P2 receptors and studies of transduction mechanisms in the early 1990s, the basis for subdivision into P2X and P2Y receptor families was confirmed and extended (Abbracchio and Burnstock, 1994) and seven subtypes of P2X receptors and eight subtypes of P2Y receptors are currently recognized (Ralevic and Burnstock, 1998; North, 2002; Burnstock, 2004).
II. Molecular Structure of P2Y Receptors
A. Nomenclature and Molecular History of P2Y Receptors
Regarding the currently used nomenclature, P2Y is used for functional mammalian receptor proteins and functional nonmammalian species. The lower case, p2y, is used for mammalian orphan receptors or functional nonmammalian receptors without a mammalian ortholog. The subscript number (1−n) following P2Y or p2y sequentially list proteins in their chronological order of cDNA cloning. The first P2 receptors were cloned in 1993 (Lustig et al., 1993; Webb et al., 1993). They corresponded to receptors previously characterized by pharmacological criteria: P2Y1 (formerly P2Y) and P2Y2 (formerly P2U). Since then several other subtypes were isolated by homology cloning and assigned a subscript on the basis of cloning chronology (P2Y4, P2Y6, and P2Y11). The long-awaited Gi-coupled ADP receptor (P2Y12) of platelets was finally isolated by expression cloning (Hollopeter et al., 2001), and P2Y13 and P2Y14 receptors were characterized during a systematic study of orphan receptors (Chambers et al., 2000; Communi et al., 2001a). As of today, there are eight accepted human P2Y receptors: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14 (Abbracchio et al., 2003) (Table 1; Fig. 1) (see also section IV.). The missing numbers represent either nonmammalian orthologs or receptors having some sequence homology to P2Y receptors but for which there is no functional evidence of responsiveness to nucleotides. In particular p2y3 (Webb et al., 1996a) may be a chicken ortholog of P2Y6 (Li et al., 1998), whereas p2y8 (Bogdanov et al., 1997) and tp2y (Boyer et al., 2000) could be the Xenopus and turkey orthologs of P2Y4, respectively. p2y7 (Akbar et al., 1996) is a leukotriene B4 receptor (Herold et al., 1997; Yokomizo et al., 1997); however, recently cross-reaction between agonists for some leukotriene receptors and some P2Y receptors has been found (see section IX.C.1), requiring further investigation. p2y5 (Webb et al., 1996b; Li et al., 1997) and p2y10 (Rao et al., 1999) must be considered as orphan receptors, although it has been reported (King and Townsend-Nicholson, 2000) that human p2y5 expressed in oocytes gives functional responses to ATP. p2y9 was reported to be a novel receptor for lysophosphatidic acid, distant from the Edg family (Noguchi et al., 2003). P2Y15 was recently introduced to designate the orphan receptor GPR80/GPR99 on the basis that it would be a receptor for adenosine 5′-monophospahte (AMP2) (Inbe et al., 2004), but it is now firmly established that it is actually a receptor for α-ketoglutarate (He et al., 2004; Qi et al., 2004; Suarez Gonzalez et al., 2004), as was also recently underlined in a report by the Subcommittee (Abbracchio et al., 2005).
TABLE 1.
Accepted human P2Y receptors
| Receptor | Chromosome (Human) | Agonist (Human) | Phenotype of Knockout Mice |
|---|---|---|---|
| P2Y1 | 3q24–25 | ADP | Inhibition of platelet aggregation |
| Increased bleeding time | |||
| Resistance to thromboembolism | |||
| P2Y2 | 11q13.5 | ATP = UTP | Abolition of chloride secretory response to ATP/UTP in airways |
| P2Y4 | Xq13 | UTP | Abolition of chloride secretory response to ATP/UTP in jejunum and colon |
| P2Y6 | 11q13.5 | UDP | No knockout mice are available |
| P2Y11 | 19p31 | ATP | No murine P2Y11 gene |
| P2Y12 | 3q21–25 | ADP | Inhibition of platelet aggregation |
| Increased bleeding time | |||
| Resistance to thromboembolism | |||
| P2Y13 | 3q24–25 | ADP | Available, no phenotype yet |
| P2Y14 | 3q24–25 | UDP-glucose | No knockout mice are available |
Fig. 1.

A phylogenetic tree (dendrogram) showing the relationships among the current members of the P2Y receptor family. The P2Y receptors can be divided into two subgroups, shown with green and blue backgrounds. Sequences were aligned using CLUSTALX, and the tree was built using the TREEVIEW software. Reprinted from Abbracchio et al. (2003) with permission from Elsevier.
B. Structural Aspects
In contrast with P2X receptors, P2Y receptor genes do not contain introns in the coding sequence, except for the P2Y11 receptor. Site-directed mutagenesis of the P2Y1 and P2Y2 receptors has shown that some positively charged residues in transmembrane domains (TM) 3, 6, and 7 are crucial for receptor activation by nucleotides (Erb et al., 1995; Jiang et al., 1997b) (Fig. 2) (section V.). They probably interact with the negative charges of the phosphate groups of nucleotides, since it is known that the receptor ligands are nucleotide species uncomplexed to magnesium or calcium. Actually, the eight P2Y receptors identified so far have a H-X-X-R/K motif in TM6. The P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors share a Y-Q/K-X-X-R motif in TM7, whereas another motif, K-E-X-X-L is found in P2Y12, P2Y13, and P2Y14 receptors (Abbracchio et al., 2003) (see also Fig. 2 and sections IV. and V.). More recently, for P2Y12, P2Y13, and P2Y14 receptors, one additional K residue in extracellular loop 2 has been suggested to be particularly important for nucleotide binding (Costanzi et al., 2004) (see also below; section V.).
Fig. 2.
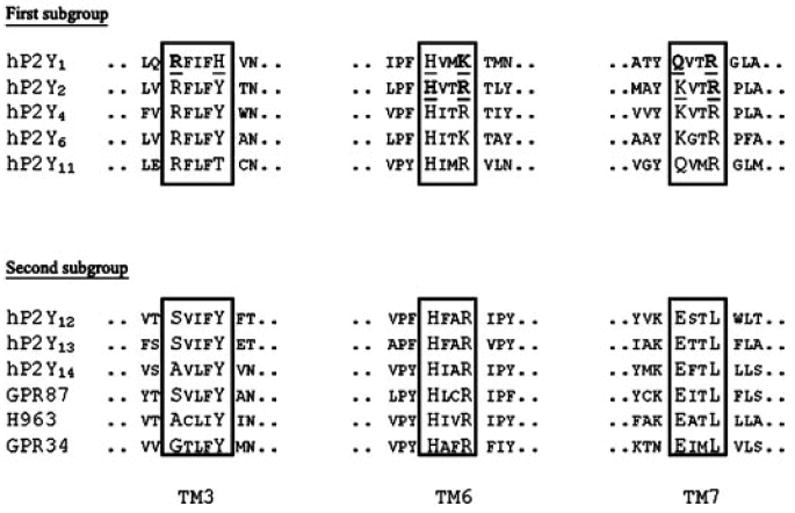
Conserved residues among P2Y receptors are shown in a larger size font. The residues that have been mutated in the studies of Erb et al. (1995) and Jiang et al. (1997b) are underlined. Those residues that are crucial in the activation of those receptors are in bold. The corresponding sequences of orphan receptors structurally related to P2Y receptors of the second subgroup are also displayed.
C. Orphan Receptors Related to P2Y Receptors
The bioinformatic analysis of the human genome has revealed the existence of approximately 800 G proteincoupled receptors (GPCRs) (Fredriksson et al., 2003), of which 367 would be receptors for endogenous ligands, the remaining ones being olfactory and other chemosensory receptors (Vassilatis et al., 2003). Among the 367 “endoGPCRs,” more than 150 remain orphans. Several of the currently available orphan GPCR sequences are structurally related to P2Y receptors (Lee et al., 2001; Wittenberger et al., 2001; Joost and Methner, 2002; Vassilatis et al., 2003): GPR87, H963, and GPR34. In particular, the sequence of those receptors contains the structural motifs in TM6 and TM7 described earlier (Fig. 2).
Human GPR87 has been shown to be highly expressed in placenta and thymus. The mouse ortholog has also been cloned and has been found to be expressed in brain and liver (Wittenberger et al., 2001).
Human H963 has been shown to be expressed in fibroblasts, human peripheral blood mononuclear cells, and T cells (S. Ferrario, D. Lecca, C. Volonté, and M. P. Abbracchio, unpublished data). Neither the mouse nor the rat orthologs have been cloned; however, a recent study has suggested that GPR34 is not a nucleotide receptor (Sugo et al., 2006).
Human GPR34 is expressed in brain, heart, placenta, small intestine, pancreas, spleen, thymus, kidney, and skeletal muscle (Schöneberg et al., 1999). The mouse ortholog has also been described, and prominent expression has been found in liver and testis (Schöneberg et al., 1999).
Despite active research in several laboratories, the ligands of these receptors have not been identified. For example, despite the demonstration that GPR34 and ADP-like receptors (e.g., P2Y12 and P2Y13) have a common evolutionary origin (Schulz and Schöneberg, 2003), in the inositol phosphate assay, Cos-7 cells coexpressing GPR34 and Gαqi4 did not show any response to ADP application (Schöneberg et al., 1999).
III. Second Messenger Systems and Ion Channels
A. Coupling to G Proteins and Intracellular Signaling Pathways
Coupling of the various P2Y receptors to specific G proteins was initially inferred from indirect evidence [measurement of intracellular levels of inositol phosphates, calcium, or cAMP and determination of pertussis toxin (PTX) sensitivity]. Direct evidence was recently obtained by measuring the effect of ADP on GTP hydrolysis in vesicles reconstituted with P2Y1 and either Gαqβ1γ2 or Gα11β1 γ2 (Waldo and Harden, 2004). Similar experiments demonstrated that P2Y12 couples to Gαi2 more effectively than to Gαi1 and Gαi3 and not at all to Gαo or Gαq (Bodor et al., 2003). One given P2Y receptor can couple to functionally distinct G proteins. For instance, in HEL cells, activation of phospholipase C (PLC) by the P2Y2 receptor is inhibited completely by a Gα16 antisense oligonucleotide but also partially by PTX (Baltensperger and Porzig, 1997). Similarly, in gastric smooth muscle cells, it appears that P2Y2 couples to PLC-β1 via Gαq/11 and to PLC-β3 via Gαi3β1γ2-derived βγ subunits (Murthy and Makhlouf, 1998). The P2Y2 receptor also has been shown to interact with αv integrin to promote Go-mediated chemotaxis in astrocytoma cells (Bagchi et al., 2005). The P2Y12 receptor activates phosphatidylinositol 3-kinase (PI3-K) via Gαi, but also RhoA and Rho kinase (Soulet et al., 2004). This action, which is insensitive to PTX, might be mediated by Gα12/13, recently shown to play a critical role in platelet activation (Moers et al., 2003). Coupling of the same P2Y receptor to distinct G proteins and signaling pathways provides the possibility of agonist-specific signaling involving distinct active conformations of the receptor. For instance, activation of the P2Y11 receptor by ATP leads to a rise in cAMP and in inositol trisphosphate (IP3) and cytosolic calcium, whereas activation by uridine 5′-triphosphate (UTP) has been reported to produce calcium mobilization without IP3 or cAMP increase (White et al., 2003). The P2Y13 receptor can simultaneously couple to G16, Gi, and, at high concentrations of ADP, to Gs (like other Gi-coupled receptors such as the α2-adrenergic receptor): these three signaling pathways are characterized by different ratios of ADP to 2-methylthio-ADP (2-MeSADP) potency, suggesting the existence of ligand-specific conformations (Marteau et al., 2003). The activation of several P2Y receptors is commonly associated with the stimulation of several mitogen-activated protein (MAP) kinases, in particular extracellular signal-regulated protein kinase (ERK) 1/2. According to the cell context and the particular subtype, other classes of the MAP kinases, protein kinase (PK) C, calcium, and PI3-K are found to be involved to a variable extent (Soltoff et al., 1998; Huwiler et al., 2000; Communi et al., 2001a; Santiago-Pérez et al., 2001; Sellers et al., 2001).
B. P2Y Receptor Coupling to Ion Channels
1. Significance
In recent years, GPCRs in neurons and other excitable cells have been found to modulate the activity of voltage-gated ion channels in the cell membrane through certain actions of activated G proteins. Such actions are now well established in closing (or in certain cases in opening or potentiating) various classes of K+ channels (Hille, 1994) and voltage-gated Ca2+ channels (Dolphin, 2003). Various voltage-gated Na+ channels also have been observed to be modulated in certain cases via GPCR actions (Cantrell and Catterall, 2001; Maurice et al., 2001; Mantegazza et al., 2005), but this has not been reported for P2Y receptors. Although GPCR downstream signaling can lead to indirect effects on ion channels via activation of protein kinases, some GPCR regulation of several types of ion channel is more specific and more direct. This action has been investigated so far for only a very small fraction of the GPCR class. For P2Y receptors, specific couplings to certain K+ and Ca2+ channels have been observed and analyzed. This coupling will be an important component of P2Y receptor signal transduction, but one that will usually be invisible in studies of second messenger or downstream pathways, since those channel interactions can occur in short timescales (down to ~100 ms) by a direct or quasi-direct pathway in the cell membrane.
Our consideration here of P2Y signaling through cell membrane channels is necessarily focused on cases in which the P2Y subtypes concerned have been identified. Thus, in a number of recent studies, the disturbing factors of enzymatic breakdown or interconversion of the nucleotides applied as agonists (Lazarowski et al., 2000) or the activation of adenosine receptors via such breakdown (Masino et al., 2002) have been experimentally minimized, allowing demonstration of ion channel responses upon activation of native P2Y receptors in brain neurons, with clear evidence for their identity (Wirkner et al., 2002; Khakh et al., 2003; Koizumi et al., 2003; Luthardt et al., 2003; Zhang et al., 2003b; Bowser and Khakh, 2004; Kawamura et al., 2004). That evidence shows that ATP (or UTP or their products ADP or UDP) present at synapses, plus ATP diffusing from astrocytes, activates P2Y receptors on distinct subsets of brain neurons, regulating their activities by the coupling of those receptors to specific ion channels, as detailed below.
Although ion channel couplings of P2Y receptors are primarily of importance in neurons, they have in a few cases been detected also in various other tissues, e.g., in cardiac muscle cells (Vassort, 2001). As yet that category has been little explored.
2. Approaches to Analysis of the Channel Interactions of Molecularly Identified P2Y Receptors
Some studies of channel coupling by P2Y receptors have been made by heterologous expression in commonly transfected host cell lines such as CHO or HEK293, or in the Xenopus oocyte. However, usually both the P2Y receptor and the identified ion channel under study must be introduced into them, and the final relationship and protein environment of those components may be far from that in any native neuron, in which individual GPCR types can be located specifically with their effectors in microdomains (Delmas et al., 2004).
The problems there can be minimized if a suitable neuronal host cell can be found. A number of requirements for this exist (e.g., endogenous P2 receptors to be insignificant therein), and all of those conditions have been found to be met in the superior cervical ganglion (SCG) cell from the sympathetic nervous system of the young rat or mouse (Brown et al., 2000a). This cell type is well equipped with endogenous ion channels of the types found in neurons generally (Ikeda, 1996; Filippov et al., 1997). Its size readily allows nuclear injection of a receptor cDNA, a route that favors normal processing and trafficking of the protein. Transfection difficulties with neurons are avoided, and recordings of the channel couplings can be made in each receptor-expressing cell, as reviewed below. Because single cells are constantly perfused with medium and subsequently with the (purified) agonist and the assay period is extremely short, the method avoids potential problems well known in other P2 receptor activity studies, i.e., accumulation of nucleotides released from cell populations or their acute release by cell disturbance, as well as losses of the added agonist by metabolism.
3. Voltage-Activated Channels Regulated by P2Y Receptors
Among the channels with which the SCG cell membrane is well endowed are two types of voltagegated channel that are important in receptor-based regulation of neuronal activity, the Ca2+ channel of the N-type and the M-current K+ channel. The M-current K+ channels are heteromers of subunits of the Kv7 family and are critical for setting the responsiveness of neurons to synaptic inputs (Selyanko et al., 2001, and references therein). Closing of the M-current K+ and/or N-type Ca2+ channels by action of certain P2Y receptors has been shown to occur.
a. Ca2+ channels
For Gi/o protein-coupled receptors in general, inhibition of the N-type Ca2+ current has been shown to occur through Gβγ subunits, acting directly on the channel (reviewed by Dolphin, 2003). This βγ action holds for the Gi-linked P2Y12 receptor in the SCG system, as shown by the demonstration that closure of the N-type Ca2+ channel via the P2Y12 receptor is fully sensitive to PTX and is totally abolished when Gα-transducin, a Gβγ-scavenging protein, is coexpressed (Simon et al., 2002). For the Gi/o-linked P2Y13 receptor, inhibition of voltage-gated Ca2+ channels would again be expected. Indeed, evidence has been obtained in HEK293 cells (into which the N-type Ca2+ channel had been introduced by transfection) to suggest that a native P2Y13 receptor is there and acts thus (Wirkner et al., 2004).
The action at N-type Ca2+ channels of activated P2Y1 and P2Y2 receptors (Table 1) is very similar to that of the endogenous M1 receptor (Gq/11-linked) in the same cells, with all three receptors showing a PTX-sensitive and a PTX-insensitive component. αq is presumably required for the latter component, as was established using knockout mice for the M1 inhibition of this channel (Haley et al., 2000), although those P2Y receptors could conceivably also use α11 in some other cells. The PTX-sensitive component requires (at least for M1; Haley et al., 2000) the Go protein. With P2Y1, in contrast, Gi/o does not act here (Table 2). However, with P2Y1,2,6 receptors, both the PTX-insensitive and the PTX-sensitive N-type Ca2+ channel responses are abolished when βγ subunits are sequestered by Gα-transducin (Simon et al., 2002; Filippov et al., 2004). Hence, the α and the βγ components of selected trimeric G protein(s) must operate together in this type of P2Y receptor action, as summarized in Table 2.
TABLE 2. Ion channel interactions of P2Y receptors.
Data derived from Filippov et al. (1998, 1999, 2000, 2003, 2004); Brown et al. (2000a); and Simon et al. (2002).
| P2Y | Ca2+ Channel Closure
|
K(M) Channela
|
GIRKa
|
|||||
|---|---|---|---|---|---|---|---|---|
| Whole Cell | Perf.a | PTX Blockb | G Proteinc | Closure | G Proteind | Activation | G Protein | |
| % | ||||||||
| 1 | Yese | ~50 | αq/11+βγ(Gq + Go) | Yese | αq/11 | Yese,f | βγ | |
| 2 | Yes | ~60 | αq/11+βγ(Gq + Go) | Yes | αq/11 | Yesf | βγ | |
| 4 | No | Weak | ~80a | βγ(Go)a | Yes | αq/11 | No | |
| 6 | Yes | Yes | ~0a | αq/11+βγ(Gq)a | Yes | αq/11 | No | |
| 12 | Yes | 100 | βγ(Gi/o)a | No | Yesf | βγ | ||
K(M) channel, M-current K+ channel; GIRK, G protein-activated inwardly rectifying K+channels.
Determined in perforated patch recording (Perf.), which avoids possible dialysis of some soluble cell components.
The percentage of the N-type Ca2+ current inhibition by P2Y action, which is blocked by PTX pre-treatment.
The G protein subunits which are proposed to act at N-type Ca2+ channels; in parentheses are the parent heterotrimeric G proteins deduced to provide the βγ subunits involved, this being noted only for the perforated patch state in which that is used; involvement of βγ (where tested) was stated by showing total prevention of channel closure by coexpressing excess Gα transducin.
The G protein subunit found to act at M-current K+ channels.
‘Yes’ denotes that the induced change occurs with agonist potencies similar to or greater than those known for other transductions of this receptor. ‘No’ denotes that the induced charge is essentially absent; ‘Weak’ denotes that the induced charge occurs but at greatly reduced agonist potency.
Highly sensitive to PTX pretreatment.
Higher potency of the agonists for a given subtype than in second messenger assay systems is generally observed in the channel transduction, which follows a direct route within the cell membrane; e.g., for 2-MeSADP at the P2Y1 receptor the EC50 value for Ca2+ channel closure is 0.57 ± 0.05 nM (Filippov et al., 2000). (All experimental values quoted in section III. are at 20–22°C). Adenosine triphosphates (e.g., 2-MeSATP and ATP) when in pure form also are agonists in this P2Y1 reaction, in conditions in which their agonistic diphosphates are excluded throughout; this has been controversial for P2Y1 receptors in some other expression systems, but the potency and efficacy of the triphosphates vary strongly with the density of this receptor subtype (see discussion and references in Filippov et al., 2000). The N-type Ca2+ channel closure via the P2Y12 receptor behaves likewise, with 2-MeSATP an agonist at P2Y12 receptors, too, with exceptional potency (Simon et al., 2002) (see also section VI.F. for further discussion). This behavior, compared with the variation of behavior seen with these agonists at P2Y12 receptors in different native situations (Barnard and Simon, 2001), again suggests a particularly strong dependence of the observed efficacy of these subtypes on receptor density in the membrane and on the directness of the transduction pathway involved.
Interestingly, the agonist selectivity of a P2Y receptor can also be changed in the channel interactions from that observed for it in transductions downstream. Thus, whereas the transfected P2Y6 receptor was reported to be UDP-selective and to have negligible action by UTP in its IP3 formation (Nicholas et al., 1996), both those nucleotides are strong agonists in the closure of the N-type Ca2+ channel and likewise for the M-current K+ channel response (Filippov et al., 1999). With impurity and metabolism of the UTP being excluded, the KA of rat P2Y6 for UTP in its channel activity is 20.1 ± 1.4 nM. A precedent for this phenomenon lies in the transduction dependence of relative agonist potencies described above for the P2Y13 receptor (section III.A.); it is interesting for the P2Y6 receptor because it indicates alternative binding site conformations for two native agonists, UTP and UDP.
b. The M-current K+ channel
The M-current can be inhibited through the activation of a number of GPCRs of the Gq/11-linked class (Brown and Yu, 2000) but not of other classes. The G protein subunit involved in GPCR-mediated closing of this channel by M1 muscarinic receptors in rat or mouse SCG neurons is Gαq, as shown both by anti-Gαq antibody depletion and by Gαq-gene knockout (Haley et al., 2000). For P2Y receptors, this parallel, together with the complete inability of the Gi/o-linked P2Y12 receptor to affect the M-current plus the PTX-insensitive closure of that channel by all four of the Gq/11-linked subtypes examined (Table 2), indicates that this action can be ascribed to a Gαq pathway also. Whether in other neurons Gα11 may also act thus is not yet excluded.
The pathway from Gαq-GTP to the M-type channel Kv7 proteins has been deduced to be, in general, via PLC-mediated depletion of phosphatidylinositol-4,5-bisphosphate (PIP2), removing its stabilizing interaction with those proteins and hence allowing channel closure (Ford et al., 2003; Zhang et al., 2003a; Suh et al., 2004; Winks et al., 2005). PIP2 thus acts as a second messenger, but one that is diffusible only within the membrane and acting negatively. Restoration of the membrane PIP2 occurs by a PI4-kinase and PI5-kinase pathway (Winks et al., 2005). The same PIP2/PI4-kinase mechanism has been supported for the closure of the N-type Ca2+ channel by similar observations on Gq-linked muscarinic M1 receptor action and its recovery (Gamper et al., 2004). We presume that a similar mechanism is operative for P2Y receptors of the Gq/11-linked class, but this has not as yet been specifically examined.
4. Activation or Inactivation of G Protein-Gated K+ Channels by P2Y Receptors
An entirely different class of K+ channels is specialized for interaction with GPCRs, i.e., the G protein-activated inward rectifiers (Kir3 or GIRK channels). Activations of Gi/o-linked receptors, but not of those linked to Gq/11, generally open these channels (Fernandez-Fernandez et al., 2001; Stanfield et al., 2002). In line with this, in the rat SCG neurons 2-MeSADP, acting at an introduced rat P2Y12 receptor, strongly activates a GIRK channel (EC50: 0.099 ± 0.008 nM) (Simon et al., 2002).
Very exceptionally, some P2Y receptors, which usually link to Gq/11, have also been found to efficiently activate GIRK channels. This case occurs with P2Y1 receptors (Simon et al., 2002), in which 2-MeSADP, for example, acts to open with high potency a GIRK channel in the SCG cell. Another instance has been seen in Xenopus oocytes, with the P2Y2 receptor (activated by ATP or by UTP) opening a GIRK channel, when both that and the receptor are implanted therein (Mosbacher et al., 1998; Mark et al., 2000). In these cases the normally Gαq/11-linked P2Y1 or P2Y2 receptors can display in neurons a channel-based transduction that is highly PTX-sensitive and dependent on Gαi. This action is fast and direct, through βγ subunits released in the cell membrane from Gαiβγ trimers and binding at the K+ channel (Simon et al., 2002; Filippov et al., 2004), i.e., as found with P2Y12. This mechanism of the P2Y1 receptor signal transduction via a K+ channel could be an indicator of an interaction of that receptor in neurons with a recruited GPCR-regulating component [e.g., a regulator of GPCR signaling (RGS) protein], since reconstituted P2Y1 protein gives no coupling to Gαi or Gαo (Waldo and Harden, 2004) (see section VI.A.).
A second, independent, type of interaction with GIRK channels can occur with P2Y receptors, namely the closing of an open GIRK channel. This slow inactivation phase is common in Gq/11-linked GPCRs, occurring in situ at GIRK channels already opened via endogenous Gi/o-linked receptors. It occurs with P2Y1 but not with P2Y12 receptors (Simon et al., 2002). This inactivation is found also with P2Y4 and P2Y6 in the neuron (Filippov et al., 2004) and with P2Y2 in oocyte expression (Mark et al., 2000). It is unaffected by PTX, with Gαq/11 being involved, as shown in the P2Y1 case with evidence that included blockade by sequestering the released αq/11 subunits using the coexpressed RGS2 protein, which is specific for that action (Filippov et al., 2004). Again, when investigated for P2Y1 receptors, depletion of PIP2 from the membrane and the liberation of IP3 in the cytosol can be seen to be correlated with the course of the GIRK current inactivation, when visualized by a sensor for them, PLCδ-PH, fluorescently tagged (Filippov et al., 2004). There appears to be a role for PIP2 in the GIRK inactivation by P2Y receptors, related to that proposed for it (see above) in the M-current K+ channel inactivation, but this requires further specification.
5. Conclusions on the Interactions with Identified Ion Channels
Five P2Y subtypes have been compared so far (Table 2). Clear differences are seen between the P2Y subtypes, with only P2Y1 and P2Y2 receptors showing a common behavior in the three transductions. These considerable variations support the conclusion that the channel couplings seen are not a general phenomenon produced by overloading with an exogenous receptor. Likewise, for all of these P2Y receptors, their maximum inhibition of the N-type Ca2+ current is well below 100% of the total N-type Ca2+ current recorded and is less than that attainable by test activations of the native α2-adrenergic or M1 muscarinic receptors in the same cell (see references cited in Table 2). There is also evidence for the coupling of some native P2Y receptors to such ion channels in brain neurons (section III.B.1.) and also in autonomic neurons and related cells (Ennion et al., 2004; Lechner et al., 2004, and references cited therein).
Some promiscuity between PTX-sensitive and PTX-insensitive G proteins is described here for these tranductions (Table 2). Such cross-reaction is already known for P2Y2 and P2Y4 receptors in other signaling pathways (see sections III.A. VI.B, and VI.C.). We noted its occurrence here, however, also with the P2Y1 receptor, in its coupling to a Ca2+ channel and to some but not all of its couplings to K+ channels. Indeed, the P2Y1-linked activation of the GIRK K+ channel described is almost entirely Gi/o-mediated, but none of that component is seen in its PLC-dependent transductions; this finding underlines the independence of the channel-coupling pathways in P2Y receptor signaling. The coupling to that channel is not, however, necessarily linked to a relaxation of selectivity for αq/11-containing heterotrimers, since the P2Y6 receptor, in contrast with the others, strictly maintains that selectivity in all of the couplings (Table 2) as well as in other transductions. Again, in the independent inactivation reaction of the GIRK channel all four of these P2Y receptors generally associated with Gq/11 linkage were seen here to fully maintain that selectivity.
Such ion channel responses are usually recorded in whole-cell patch-clamping, but this may permit diffusible intracellular cofactors to dialyze out. Most of the analyses of P2Y receptor coupling in the SCG cell discussed above were made instead in the more-laborious perforated-patch mode (using amphotericin B to form small membrane pores), to avoid that possibility. In the case of the P2Y6-mediated Ca2+ channel closure (Filippov et al., 1999), use of this configuration abolished a partial block by PTX (although for other subtypes it can remain). Hence a soluble component, possibly an RGS protein, may cooperate in the membrane-delimited reactions of the P2Y6 receptor to direct its G protein coupling.
The P2Y4 receptor has not so far been shown to occur in neurons, unlike P2Y1 and P2Y6 receptors; its mRNA is prominent in the brain ventricular system, cardiac and skeletal muscles, some smooth muscles, and some other peripheral sites but is undetectable in neurons (Webb et al., 1998). This may be why its coupling to the N-type Ca2+ channel of a neuron can be weak and readily lost (Table 2). Adaptation of such a P2Y subtype may evolve for a different signaling environment than that in neurons.
C. Other Potential Interactions with Ion Channels
Little is known of these as yet. One clue to some other interactions of the P2Y1 receptor comes from the recent finding (Fam et al., 2005) that it can bind strongly to the Na+/H+ exchanger regulatory factor 2 (NHERF-2) through the extreme C-terminal motif DTSL, which is specific to P2Y1 in this family. For comparison, binding of the related NHERF-1 to P2Y1 receptors (Hall et al., 1998) is negligible (Fam et al., 2005). The three membrane-located NHERF subtypes either activate or inhibit various Na+/H+ exchangers, but these actions are indirect since it is now known that NHERFs are actually scaffolding proteins, which can localize various exchangers in membrane microdomains with selected receptors and signaling intermediates, e.g., Gαq, Src, and certain isoenzymes of PLC and of PKC (Donowitz et al., 2005). For example, the NHERF-2 scaffold can link a cGMP-dependent protein kinase or a protein kinase A anchoring protein and hence protein kinase A, to modulate a tethered Na+/H+ exchanger by specific phosphorylations thereon (Cha et al., 2005; Donowitz et al., 2005).
When the endogenous P2Y1 receptor (as studied in C6 glioma cells) is linked through its tail to the NHERF-2 scaffold, the Ca2+ transients produced by its activation by 2-MeSADP become strongly prolonged (Fam et al., 2005). This will change the P2Y1 selectivity for the various calcium-sensitive signaling cascades and for ion channel interactions. Another interaction of the P2Y1 receptor is with the chloride channel of the cystic fibrosis transmembrane conductance regulator (CFTR); in renal epithelial cells, 2-MeSADP activation of native P2Y1 receptors stimulates the chloride channel activity of the CFTR. This is again an indirect action arising from the NHERF-2 colocalization of this P2Y subtype and the CFTR; expression of a dominant negative NHERF-2 mutant blocks the CFTR regulation through P2Y1, as does a blocker of the protein kinase A anchoring protein binding of PKA (Guerra et al., 2004). The evidence suggested that P2Y1 receptor-mediated PKC activation leads to potentiation of PKA and its action on the associated CFTR channels.
Additionally, a highly unusual mode of GPCR interaction with an ion channel has been suggested for several P2Y receptors by Lee et al. (2003b). A novel, unidentified, voltage-gated channel of the Xenopus oocyte, Tin, was reported to be activated and modulated after expression of human P2Y1,2,6,11, but not by P2Y4 nor by other Gq/11-linked GPCRs. It was deduced that this does involve a direct receptor binding to the channel. However, expression of Gα also activates this channel and the mechanism and physiological significance are at present unclear.
IV. Principles of P2Y Receptor Classification
As already outlined above, eight distinct mammalian P2Y receptors have been cloned and recognized so far: the P2Y1,2,4,6,11,12,13 and the recently reclassified P2Y14 receptors (Abbracchio et al., 2003). The missing numbers in the P2Y1–14 sequence represent GPCRs cloned from nonmammalian vertebrates or receptors for which a functional response to nucleotides has not yet been convincingly demonstrated.
Pharmacologically (Table 1) P2Y receptors can be broadly subdivided into 1) adenine nucleotide-preferring receptors mainly responding to ADP and ATP. This group includes human and rodent P2Y1, P2Y12, and P2Y13, and human P2Y11 (which has, however, been recently reported to also respond to UTP) (White et al., 2003); 2) uracil nucleotide-preferring receptors. This group includes human P2Y4 and P2Y6 responding to either UTP or UDP; 3) receptors of mixed selectivity (human and rodent P2Y2, rodent P2Y4 and, possibly, P2Y11); and 4) receptors responding solely to the sugar nucleotides UDP-glucose and UDP-galactose (P2Y14).
From a phylogenetic and structural (i.e., protein sequence) point of view, two distinct P2Y receptor subgroups characterized by a relatively high level of sequence divergence have been identified (Jacobson et al., 2002; Abbracchio et al., 2003). The first subgroup includes P2Y1,2,4,6,11 subtypes and the second subgroup encompasses the P2Y12,13,14 subtypes (see dendrogram in Fig. 1 reproduced from Abbracchio et al., 2003). Alignment of the deduced amino acid sequences of the cloned P2Y receptors has shown that the human members of this family are 21 to 48% identical (Table 3). The highest degree of sequence identity is found among the second subgroup of P2Y12,13,14. Interestingly, despite clear phylogenetic relationships with the first subgroup, the P2Y11 subtype seems to differ from all the others, for both sequence and pharmacological differences between species (e.g., canine versus human) and also based on its absence in the murine and rat genomes (Table 1). Thus, it might be hypothesized that this receptor differentiated from P2Y1 and subsequently underwent many modifications and insertions that led to a dissimilar receptor, despite the common origin. Cotranscription and intergenic splicing of the P2Y11 gene might be another evolutionary event accounting for its dissimilarity from the other P2Y receptors.
TABLE 3. Classification of P2Y receptors into two subsets.
For P2Y1,2,4,6, and 11 receptors, amino acid residues reported in bold in TM6 and TM7 have been shown to be important for ligand binding in mutagenesis studies (Erb et al., 1995; Jiang et al., 1997b; Boarder and Webb, 2001; Jacobson et al., 2002; see also section V.). For P2Y12,13, and 14 receptors, direct evidence for the functional importance of the reported motifs in TM6 and TM7 is currently lacking. However, in a patient with a congenital bleeding disorder, a R-Q substitution in TM6 of the P2Y12 receptor results in highly decreased receptor function (Cattaneo et al., 2003).
| Receptor | Percentage of Identity
|
Proposed Amino Acid Motifs for Receptor Function
|
IUPHAR Receptor Code | Primary G Protein Coupling/Second Messengera | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y1 | P2Y2 | P2Y4 | P2Y6 | P2Y11 | P2Y12 | P2Y13 | P2Y14 | TM6 | TM7 | |||
| P2Y1 | / | 38 | 44 | 46 | 32 | 24 | 24 | 27 | HXXK | QXXR | 2.1.NUCT0.01.000.00.00 | Gq/11; PLCβ activation |
| P2Y2 | / | 41 | 41 | 29 | 25 | 26 | 26 | HXXR | KXXR | 2.1.NUCT.02.000.00.00 | Gq/11; PLCβ activation | |
| P2Y4 | / | 43 | 32 | 25 | 26 | 28 | HXXR | KXXR | 2.1.NUCT.03.000.00.00 | Gq/11; PLCβ activation | ||
| P2Y6 | / | 34 | 24 | 24 | 23 | HXXK | KXXR | 2.1.NUCT.04.000.00.00 | Gq/11; PLCβ activation | |||
| P2Y11 | / | 22 | 21 | 23 | HXXR | QXXR | 2.1.NUCT.05.000.00.00 | Gq/11; PLCβ activation | ||||
| P2Y12 | / | 48 | 47 | HXXR | KEXXL | 2.1.NUCT.06.000.00.00 | Gi/o; inhibition of adenylate cyclase | |||||
| P2Y13 | / | 47 | HXXR | KEXXL | 2.1.NUCT.07.000.00.00 | Gi/o; inhibition of adenylate cyclase | ||||||
| P2Y14 | / | HXXR | KEXXL | 2.1.NUCT.08.000.00.00 | Gi/o; inhibition of adenylate cyclase | |||||||
The main transductional mechanisms are reported; see text for more details.
The two P2Y receptor subgroups highlighted above also differ in several other features. In particular, specific amino acid motifs in TM6 and TM7 have been previously proposed to be important for binding to extracellular nucleotides (Erb et al., 1995; Jiang et al., 1997b; Boarder and Webb, 2001; Jacobson et al., 2002). All human P2Y receptors share the typical TM6 H-X-X-R/K motif that might be important for agonist activity (Erb et al., 1995; Jiang et al., 1997b; Boarder and Webb, 2001; Jacobson et al., 2002) (Table 3; see also section V.). A Q/K-X-X-R defining motif in TM7 has also been proposed to participate in ligand binding for the P2Y1,2,4,6 and P2Y11 receptors. In P2Y12,13,14 receptors, this motif is substituted with K-E-X-X-L, which might affect ligand binding characteristics (Table 3). In humans, the genes of P2Y12,13,14 receptors cluster in the same region of chromosome 3, together with the gene encoding for the P2Y1 receptor; in this region; three additional still unidentified “orphan” GPCRs structurally related to the known P2Y receptors can also be found (Table 1). The mapping of the known genes of the P2Y receptors and the structurally related orphans in the human genome is detailed in Simon and Barnard (2003). Interestingly, two of these orphan receptors (i.e., GPR87 and H963) also show full conservation of the defining motifs in TM6 and TM7 typically found in P2Y12,13,14. Their functional characterization may eventually lead to inclusion in this P2Y receptor subgroup.
Finally, these two P2Y receptor subgroups also differ in their primary coupling to transductional G proteins. In particular, receptors in the first subgroup (i.e., P2Y1,2,4,6,11) all principally use Gq/G11 to activate the PLCβ/IP3 pathway and release intracellular calcium, whereas receptors in the second subgroup (i.e., P2Y12,13,14) almost exclusively couple to members of the Gi/o family of G proteins (Table 3; see also section III. and individual receptor subsections and individual receptor summary tables that appear at the end of the article). Secondary couplings have been also reported, especially for receptors of the first subgroup in heterologous expression systems (Simon et al., 2002; Burnstock, 2003; King and Townsend-Nicholson, 2003; Köttgen et al., 2003; White et al., 2003). For receptors of the second group, P2Y13 has been also reported to couple to Gα16 and stimulate PLC in recombinant systems over-expressing this G protein (Communi et al., 2001a; Marteau et al., 2003), whereas activation of the native P2Y14 receptor in astrocytes and microglia has been shown to increase intracellular calcium levels (Fumagalli et al., 2003; Bianco et al., 2005). Such “promiscuity” of G protein-coupling may depend on the indirect activation of additional G protein subtypes within protein complexes containing the P2Y receptor.
Thus, a division into two subgroups could be considered, based on 1) phylogenetic (i.e., sequence) similarity (Fig. 1; Table 3), the 2) presence of amino acid defining motifs proposed to be important for ligand binding (Table 3; see also section V.), and 3) selectivity of primary G protein coupling (Table 3). However, we prefer to wait to formally implement this subdivision until there is a more complete knowledge of these receptors, with some of the orphan P2Y-like receptors still waiting for deorphanization, the possibility of new receptors still to be discovered, and the place of the P2Y11 receptor still to be clearly resolved.
V. Agonists and Antagonists
A. Chemical Structure of Agonist and Antagonist Ligands
Most of the P2Y receptor subtypes are still lacking potent and selective synthetic agonists and antagonists. However, considerable progress in exploring structure-activity relationships (SARs) has been made for P2Y1 and P2Y12 receptors and to a lesser extent for the P2Y2 receptor. Radioligand binding studies have been successfully carried out at the P2Y1 and P2Y12 receptors, but so far not at any other P2Y receptor subtype. Here we describe the current state of molecular probes known for the P2Y receptors, categorized by the chemical class of the endogenous agonists.
1. ADP-Preferring P2Y Receptors: P2Y1, P2Y12, and P2Y13
ADP (1 in Fig. 3) is the endogenous agonist at the P2Y1, P2Y12, and P2Y13 receptors, and it interacts at these subtypes with generally greater affinity than does ATP (3) (Palmer et al., 1998; Boeynaems et al., 2003; Marteau et al., 2003). At P2Y1 receptors, derivatives of ADP tend to be full agonists (the EC50 of ADP is ~100 nM), whereas ATP appears to be a partial agonist. At P2Y12 receptors, ADP derivatives activate (the EC50 of ADP is ~100 nM) and 5′-triphosphate derivatives antagonize (Gachet and Hechler, 2005). At P2Y13 receptors, both ADP and ATP might act as full agonists, with EC50 values of ~100 nM. However, under some conditions, ATP can behave as a weak partial agonist, suggesting that, as described for the P2Y1 receptor, the activity of ATP in recombinant systems may vary according to the level of expression of the P2Y13 receptor (see also below).
Fig. 3.

Structures of adenine-derived nucleotide agonists of P2Y receptors.
Phosphate modifications among P2Y receptor ligands often serve to increase their stability toward ecto-nucleotidases. For example, the added stability of a terminal thiophosphate group resulted in its incorporation in some useful P2Y nucleotide agonists. One such analog, ADPβS (2), is a potent agonist of both P2Y1 (EC50 = 96 nM), P2Y12 (EC50 = 82 nM) (Jacobson et al., 2002), and P2Y13 receptors (EC50 = 42 nM) (Communi et al., 2001a). Although terminal thiophosphates are enzymatically more stable than the oxygen equivalents, they are subject to chemical oxidation reactions; thus, solutions of these compounds are prone to instability.
Figure 3 shows the structures of adenine-derived nucleotide agonists of P2Y receptors. The structure of the nucleobase of adenine nucleotides has been extensively probed for effects at P2Y receptors. 8-Aza and 1-deaza modifications are generally tolerated. A fluorescent adenine-modified derivative of ATP (5) behaved as a potent P2Y1 receptor agonist (Sharon et al., 2004). The 2-position of the adenine ring can accommodate a wide variety of substituents, with resultant activation of both P2Y1 and P2Y12 receptors. Long-chain and sterically bulky groups may be accommodated at the 2-position. In particular, 2-alkylthio ethers (Fischer et al., 1993; Brown et al., 2000b) appear to provide high potency at these subtypes when bonded to a variety of alkyl or alkylaryl groups. Notably, the smallest member of this class is 2-MeSADP [6, which is a potent agonist (EC50) at P2Y1 (6 nM), P2Y12 (1 nM), and P2Y13 (1 nM) receptors (Jacobson et al., 2002; Marteau et al., 2003); however, see also above and individual receptor subsection] and is highly selective in comparison with other P2Y receptor subtypes. For example, at the P2Y2 receptor compound 6 is inactive at 100 μM. The corresponding triphosphate, 2-MeSATP (7), is less potent and selective as a P2Y receptor agonist, since it also activates P2X receptors (King, 1998). The sterically bulky p-aminophenylethylthio analog (2-[2-(4-aminophenyl)ethylhio]adenosine 5′-triphosphate) (8), potently activated the P2Y1 receptor (EC50 = 1 nM) (Fischer et al., 1993). The SAR of alkynyl substitutions at the 2-position of P2Y1 receptor agonists has been explored (Cristalli et al., 2005).
Although AMP is inactive at the P2Y1 receptor, addition of a 2-thioether substituent as a receptor “anchor” causes AMP analogs to activate P2Y1 receptors. Among these derivatives, 2-hextylthioadenosine 5′monophosphate (9) was especially potent, with an EC50 of 59 nM at the turkey P2Y1 receptor (Boyer et al., 1996b). Certain 2-thioether derivatives of AMP derivatives also activate the P2Y12 receptor in C6 glioma cells in the micromolar range. The α-thio modification of AMP analogs (e.g., 10) increases potency at the P2Y1 receptor (Fischer et al., 1999). Such monophosphate derivatives have also been reported to inhibit ecto-nucleotidases, which complicates their use as P2Y receptor agonists.
A BH2 moiety may serve as a substitute for an ionized oxygen atom of the α-phosphate of ATP derivatives in promoting binding to the P2Y1 receptor binding site. Thus, 5′-(1-boranotriphosphate) derivatives such as the 2-methylthio derivative (11) have been found to potently activate the P2Y1 receptor (Nahum et al., 2002). Because the 1-boranotriphosphate moiety is chiral, it was possible to separate two stable isomers in this series. The more potent isomer of 11 displayed an EC50 of 2.6 nM at the rat P2Y1 receptor.
The ribose moiety of nucleotide derivatives was also modified, resulting in enhanced potency at the P2Y1 and P2Y12 receptors. Simple carbocyclic (cyclopentyl) analogs of ATP were found to enhance antagonist affinity at the P2Y12 receptor (see below) (van Giezen and Humphries, 2005). Similarly, at the P2Y1 receptor, carbocyclic and even acyclic substitutions of ribose were studied. In general, carbocyclics and ring-constrained nucleotide analogs were able to maintain agonism at the P2Y1 receptor, whereas acyclic derivatives proved to be exclusively antagonists (see below). A ring-expanded, yet nonglycosidic, dehydroanhydrohexitol analog MRS2255 (12) activated the P2Y1 receptor with an EC50 of 3.0 μM (Nandanan et al., 2000).
Among the more successful examples of the use of carbocyclic or sterically constrained carbocyclic substitution of the ribose moiety for P2Y receptor interactions are the “methanocarba” analogs (Nandanan et al., 2000; Kim et al., 2002). These analogs incorporate a conformationally fixed bicyclic ring system, consisting of fused cyclopentane and cyclopropane rings, in place of the ribose moiety. Depending on the position of fusion, the resulting nucleotides may adopt one of two conformations: (N), north, or (S), south. Correlation of ring geometry with the biological activities helped define the conformational requirements of the ribose moiety in P2Y receptor binding and led to pharmacological probes of unusual selectivity and affinity. For example, the two isomeric methanocarba equivalents of ATP indicated a strong preference (ratio of potency >100-fold) at the P2Y1 receptor for the (N)-isomer 13 over the (S)-isomer 14 (Kim et al., 2002).
Combination of this ring system with other favorable modifications of ADP or ATP resulted in large qualitative differences from the native nucleotides in receptor activation. For example, whereas β,γ-methylene ATP (β,γ-meATP) is a weak partial agonist at the human P2Y1 receptor, the corresponding (N)-methanocarba-β,γ-meATP (15) was a full agonist with an EC50 of 158 nM (Ravi et al., 2002). MRS2365 (16), the most potent known agonist of the human P2Y1 receptor, with an EC50 of 0.4 nM, induces platelet shape change without aggregation (Chhatriwala et al., 2004). In addition, the high selectivity of 16 for the P2Y1 receptor in comparison to its inactivity at P2Y12 and P2Y13 receptors was striking, in contrast to the relatively nonselective 2-MeSADP (2). Thus, the P2Y12 and P2Y13 receptors have very different conformational preferences within the ribose-binding region than does the P2Y1 receptor. At P2Y13 receptors, under optimal experimental conditions, ATP (2) and 2-MeSATP (5) are equipotent as agonists.
Figure 4 shows the structures of nucleotide-based antagonists of P2Y receptors. A successful approach to the development of potent and selective P2Y1 receptor antagonists was made possible by the observation by Boyer et al. (1996a) that naturally occurring adenosine bisphosphate derivatives such as A3P5P (17) act as partial agonists or antagonists of the receptor. Thus, the splitting and repositioning of the phosphate groups of the 5′-diphosphate group of ADP to separate ribose positions (5′- and either 3′- or 2′-) reduces efficacy at the P2Y1 receptor. Removal of the 2′-hydroxyl group and addition of the potency-enhancing N6-methyl group resulted in MRS2179 (18), and the corresponding 2-chloro analog MRS2216 (19), which became full antagonists at the P2Y1 receptor with IC50 values of 300 and 100 nM, respectively (Nandanan et al., 1999; Brown et al., 2000b). The SAR of alkyl, thioether, and other substitutions at the 2-position of bisphosphate antagonists has been explored (Nandanan et al., 1999, Raboisson et al., 2002a). Raboission et al. (2002b) synthesized a C-nucleotide bisphosphate derivative (20) that antagonized P2Y1 receptors. In addition, the adenine N9 nitrogen is not essential in P2Y1 receptor interaction, and similarly the N1 nitrogen was found to be unnecessary through the evaluation of 1-deaza analogs (Nandanan et al., 1999).
Fig. 4.

Structures of nucleotide-based antagonists of P2Y receptors.
Upon introduction of the conformationally preferred (N)-methanocarbo ring system into this series of bisphosphate nucleotide antagonists, the P2Y1 receptor affinity was further enhanced. Thus, MRS2279 (21), the (N)-methanocarbo equivalent of the riboside (19), and the corresponding 2-iodo derivative MRS2500 (22) demonstrated high affinity in competitive antagonism at the human, turkey, rat, and mouse P2Y1 receptors (Nandanan et al., 2000; Boyer et al., 2002; Waldo et al., 2002; Cattaneo et al., 2004). The Ki value of MRS2500 was 0.78 nM, as determined in inhibition binding experiments at the human P2Y1 receptor (Ohno et al., 2004), and the compound was highly specific for this subtype. MRS2279 or related antagonists were demonstrated to be inactive at P2Y2,4,6,11,12,13 and P2X2,3,4,7 receptors (Boyer et al., 2002; Marteau at al., 2003). Weak antagonism by MRS2279 of the rat P2X1 receptor expressed in Xenopus oocytes was observed (Brown et al., 2000b). However, the potency of many of the known P2 receptor antagonists is magnified in this assay; thus, MRS2279 may still be considered highly selective for the P2Y1 receptor. In platelet studies, for example, antagonism of the P2X1 receptor by this compound and related P2Y1 receptor antagonists is not observed (Baurand et al., 2001). The Ki value of MRS2500 at the P2Y1 receptors was found to be 0.79 nM, and it was consistently potent in inhibiting the ADP-induced aggregation of human and rat platelets. [33P]MRS2179, [3H]MRS2279, and [32P]MRS2500 were prepared and shown to be effective as radioligand probes in platelets and in other tissue (Baurand et al., 2001; Waldo et al., 2002; Houston et al., 2005). A novel ring system was incorporated in a carbocyclic locked nucleic acid derivative MRS2584 (23), which acted as a P2Y1 receptor antagonist with a binding Ki of 23 nM (Ohno et al., 2004).
In addition to the approach of rigidifying the ribose moiety in a conformation that approximates the conformation preferred in receptor binding, the opposite approach, which used a flexible acyclic ribose equivalent, also produced bisphosphate antagonists of the P2Y1 receptor of intermediate affinity. The acyclic nucleotide analog MRS2298 (24) represents a bisphosphate structure that was optimized for affinity at the P2Y1 receptor, with an IC50 in inhibition of PLC of 200 nM (Cattaneo et al., 2004). A related analog containing the metabolically stable phosphonate linkage, MRS2496 (25), displayed an IC50 at the rat platelet P2Y1 receptor of 0.68 μM (Xu et al., 2002a).
Extensive structure-activity studies of ATP derivatives as antagonists of the platelet P2Y12 receptor resulted in high-affinity, selective antagonists of interest as antithrombotic agents. Several such 5′-triphosphate derivatives, including AR-C67085MX (26) and ARC69931MX (27) (Ingall et al., 1999), were used in clinical trials, with the recognition that triphosphate derivatives would not be suitable for oral administration. In this series, it was also possible to substitute the unwieldy triphosphate group with uncharged moieties such as short alcohols or esters, thus proving that a highly anionic moiety is not needed for recognition by the P2Y12 receptor. This discovery led to compounds such as the carbocyclic nucleoside derivative AZD6140 (28), which is an orally active P2Y12 receptor antagonist of nanomolar affinity that inhibits platelet aggregation up to 8 h after administration (Springthorpe, 2003). The presence of the 3,4-difluorophenyl group limits the metabolism of compound 28.
Analogs of ADP having neutral, hydrophobic substitutions at the ribose 2′- and 3′-hydroxyl groups and at the adenine NH2 position were found to antagonize the P2Y12 receptor. One such analog is INS49266 (29a), which displayed a KB of 361 nM in the inhibition of ADP-induced platelet aggregation (Douglass et al., 2002). The agonist potencies of compound 29a at P2Y1 and P2Y2 receptors were >10 and 14 μM, respectively. A related analog in this series, INS50589 (29b), has entered clinical trials as a platelet aggregation inhibitor with a rapid onset and offset mechanism of action that is intended for intravenous administration.
The acyclic template used in MRS2298 (24) has been adapted to antagonists of the P2Y12 receptor (Xu et al., 2002a). Upon replacement of the two phosphate groups with hydrophobic esters, such as in the dipivaloate MRS2395 (30), the selectivity shifted entirely from the P2Y1 receptor to the P2Y12 receptor. MRS2395 displayed an IC50 of 3.6 μM in the inhibition of ADP-induced aggregation of rat platelets by antagonizing the P2Y12 receptor.
The action of the successful antithrombotic drug clopidogrel (31 in Fig. 5) is also dependent on the P2Y12 receptor present on platelets. Clopidogrel (31) produces a metabolite (32), which acts as an irreversible P2Y12 receptor antagonist (Savi et al., 2000). CS-747 (33) (also known as prasugrel or LY640315) is acting with a similar mechanism (Sugidachi et al., 2001; Niitsu et al., 2005). CT50547 (34) is a P2Y12 antagonist characterized by a non-nucleotide (and not highly charged) structure (Scarborough et al., 2001). Recently, a novel P2Y12 receptor antagonist structure in the form of a pyrazolidine-3,5-dione derivative (35) was presented (Fretz et al., 2005). The search for more drug-like (nonphosphate-containing or uncharged), and structurally novel antagonists of the P2Y1 receptor has so far had limited success. A dipeptide conjugate of adenosine, which bore carboxylate groups, was found to antagonize hP2Y1 receptor responses with a KB value of 4.0 μM (Sak et al., 2000). Metabolites of the hypolipidemic drug nafenopin may act as P2Y1 receptor antagonists, including a coenzyme A conjugate that displayed a KB value of 58 nM. A cyclic depsipeptide YM-254890 (36), a fermentation product of a Chromobacterium isolated from soil, inhibited the ADP-induced aggregation of platelets with an IC50 of 31 nM and was inactive at P2Y12 receptors (Uemura et al., 2006). The mechanism was found to be inhibition of Gαq rather than direct interaction with P2Y1 receptors.
Fig. 5.

Structures of non-nucleotide antagonists of P2Y receptors.
Figure 5 shows the structures of non-nucleotide antagonists of P2Y receptors. Known non-nucleotide antagonists of P2 receptors were also modified in an effort to achieve P2Y1 receptor selectivity. Many of the classic antagonists of P2 receptors are highly negatively charged polycyclic compounds and tend to block certain P2Y as well as P2X responses. Although no antagonists are totally nonselective with respect to the entire P2 superfamily (such an antagonist would be a useful tool), these polyanions are the least selective and often have activities beyond the P2 receptors. For example, the nonselective P2X/P2Y antagonist Reactive blue 2 (37) and its derivatives (such as Acid Blue 80, Acid Blue 129, and Acid Violet 34) have been shown to block action at P2Y1 receptors; however, high potency and selectivity have not been achieved (Brown and Brown, 2002; Jacobson et al., 2002). The polysulfonate suramin (38) and its derivatives, in addition to displaying trypanocidal drug properties, are relatively nonselective P2 antagonists with, in general, reversibility upon washout (King, 1998). Within the P2Y family, suramin has been characterized as an antagonist of P2Y2 receptors (Wildman et al., 2003) and P2Y11 (Communi et al., 1999b) receptors. Derivatives of the pyridoxal phosphate derivative PPADS (39) have been shown to antagonize P2Y1 receptor effects in a competitive fashion, although at μM concentrations (Lambrecht et al., 2002). Extensive SAR manipulations within these families have not resulted in antagonists of nanomolar affinity.
A variety of structurally diverse, non-nucleotide antagonists of the P2Y1 receptor have been demonstrated to be noncompetitive inhibitors. For example, pyridyl isatogen tosylate (41), which has been explored as a possible allosteric modulator of the receptor (King et al., 1996), was found to be a P2Y1-selective antagonist in recombinant P2Y receptor systems (Gao et al., 2004). It reduced the maximal effect of 2-MeSADP in stimulation of PLC, with an IC50 of 0.14 μM, but had no effect on the binding of [3H]MRS2279.
Two nucleotide 5′-triphosphate derivatives (26 and 27) were shown to potently antagonize the P2Y13 receptor (Kim et al., 2005), but in a noncompetitive manner. The following compounds were found to antagonize action at the P2Y13 receptor (IC50): suramin (38) (2.3 μM), PPADS (39) (11.7 μM), Ap4A (0.216 μM), and ARC69931MX (27) (0.004 μM) (Marteau et al., 2003). Recently, a derivative of PPADS, MRS2211 (40), was shown to selectively antagonize the human P2Y13 receptor (Kim et al., 2005). The antagonism of MRS 2211 of agonist-induced PLC was competitive with a pA2 value of 6.3.
2. ATP-Preferring P2Y Receptor: P2Y11
At P2Y11 receptors, ATP is the preferred native ligand (Communi et al., 1999b), and ATPγS (4) is a more potent agonist than ATP. Selective antagonists of the P2Y11 receptors are under development (Ullmann et al., 2005). The P2Y12 antagonist AR-C67085MX (26) acts as a potent agonist at the P2Y11 receptor (Communi et al., 1999b).
3. UTP-Recognizing P2Y Receptors: P2Y2 and P2Y4
The P2Y2 receptor is activated nearly equipotently by UTP (47 in Fig. 6) and ATP (3) but is not activated by the corresponding 5′-diphosphates, i.e., UDP (46) and ADP (1). The P2Y4 receptor is primarily activated by uracil nucleotides, depending on species. In the rat, ATP is also a P2Y4 agonist, but in humans it acts as a P2Y4 antagonist. Uridine β-thiodiphosphate (UDPβS, 48) and the γ-thiophosphate (UTPγS, 49) are selective agonists for P2Y6 and P2Y2/P2Y4 receptors, respectively (Malmsjö et al., 2000). Numerous substitutions of the uracil ring of UTP have been reported to reduce potency at the P2Y2 receptor (Müller, 2002). The 5-bromo derivative of UDP (50) retains potency at the P2Y6 receptor.
Fig. 6.

Structures of uracil-derived nucleotide agonists of P2Y receptors.
The adenine dinucleotide Ap4A is a potent agonist at the rat P2Y4 receptor and is less potent than ATP at the P2Y2 receptor. Other uracil dinucleotides, such as INS365 (Up4U, 51), also potently activate the P2Y2 receptor (Shaver et al., 2005). The dependence of potency at various P2Y receptors on the number of bridging phosphate units in the dinucleotide series indicates an optimum at the tetraphosphate. Newer-generation P2Y2 receptor agonists, such as INS37217 (Up4dC, 52), have been reported (Pendergast et al., 2001; Yerxa et al., 2002). INS37217 is less prone to enzymatic hydrolysis than more common dinucleotide agonists. P2Y2 receptor agonists are of clinical interest for the treatment of pulmonary and ophthalmic diseases and possibly cancer.
Ribose substitution with the (N)-methanocarba ring system has been shown to preserve the potency of both adenine and uracil nucleotides at the P2Y2 receptor and UTP (e.g., MRS2341, 53) at the P2Y4 receptor (Kim et al., 2002). However, inclusion of the same (N)-methanocarba ring system in the corresponding 5′-diphosphate prevented activation of the P2Y6 receptor. Therefore, enzymatic cleavage of compound 53 to the diphosphate does not have complicating actions at the P2Y6 subtype.
Figure 6 shows the structures of uracil-derived nucleotide agonists of P2Y receptors. Suramin (38) is a weak antagonist at the P2Y2 receptor with an IC50 of 48 μM (Müller, 2002). A family of selective, heterocyclic antagonists of the P2Y2 receptor containing a thiouracil moiety, including AR-C126313 (42) and the related aminotetrazole derivative AR-C118925 (43), has been reported (Meghani, 2002). Reactive Blue 2 (37) at a concentration of 100 μM effectively blocks rat P2Y4 receptors but only partially blocks human P2Y4 receptors. ATP antagonizes the human but not rat P2Y4 receptor (Kennedy et al., 2000).
Flavonoids have been identified as a new lead for the design of P2Y2 receptor antagonists (Kaulich et al., 2003). Tangeretin (44) is a potent, noncompetitive antagonist with an IC50 of 12 μM.
4. UDP-Preferring P2Y Receptor: P2Y6
UDP derivatives activate the P2Y6 receptor more potently than the corresponding 5′-triphosphates (Malmsjö et al., 2000; Müller, 2002); thus, UDP is a selective agonist at this subtype. The β-thiodiphosphate (48) was shown to be more potent than UDP in activation of the P2Y6 receptor and more stable to degradation. INS48823 is a potent P2Y6 agonist (Korcok et al., 2005).
Various diisothiocyanate derivatives were found to be potent insurmountable (and possibly irreversible by virtue of the reactive isothiocyanate groups) antagonists of human P2Y6 as well as of other P2Y receptors (Mamedova et al., 2004). A 1,4-di-(phenylthioureido)butane derivative (MRS2578, 45) selectively inhibited UDP-induced PLC activity through both human (IC50 = 37 nM) and rat (IC50 = 98 nM) P2Y6 receptors expressed in 1321N1 human astrocytes and was inactive at human P2Y1, P2Y2, P2Y4, and P2Y11 receptors. Limitations of using these isothiocyanate derivatives as P2Y antagonists include the pharmacological irreversibility, relative instability of the compounds in aqueous medium, and hydrophobicity and consequent low aqueous solubility.
5. UDP-Sugar-Preferring P2Y Receptor: P2Y14
The most recently cloned receptor, P2Y14, responds to UDP-glucose (54) and has a sequence more similar to the P2Y12 and P2Y13 receptors than to the other P2Y subtypes (Abbracchio et al., 2003). The P2Y14 receptor is also activated by UDP-galactose (55). It is the only known P2Y subtype to be activated by nucleotide sugars. The SAR at this subtype has not yet been explored. Antagonists of the P2Y14 receptor are still unknown.
B. Molecular Modeling Studies
The two distinct subgroups of P2Y receptors were successfully modeled by homology modeling, with the high-resolution structure of bovine rhodopsin serving as a template (Moro and Jacobson, 2002). The putative TM binding site and other regions of the human P2Y1 receptor have been extensively studied by means of mutagenesis (Fig. 2). To ascertain which residues of the P2Y1 receptor are involved in ligand recognition, individual residues of the TMs and extracellular loops were mutated to Ala and other amino acids (see also Table 4).
TABLE 4. Analysis of the pharmacological effects and structural role of individual residues of the human P2Y1 receptor, deduced from site-directed mutagenesis, molecular modeling, and homology to other GPCRs.
Where applicable, the data indicate the effects of single amino acid replacement on the activation of PLC by mutant human P2Y1 receptors transiently expressed in COS-7 cells and results of molecular modeling of the docked complex of 2-MeSADP or ATP within the wild-type hP2Y1 receptor (see Moro and Jacobson, 2002; Costanzi et al., 2004; and references therein).
| Site | Mutation (if Performed) | Positiona | Putative Interactions, Pharmacological Role | Potency Loss (Ratio for 2-MeSADP)a |
|---|---|---|---|---|
| TM1 | ||||
| Y58 | 1.39 | H-bond donor to S314b,c,d | N.A. | |
| N69 | 1.50 | H-bond donor to S317c,d | N.A. | |
| TM2 | ||||
| S87 | 2.40 | H-bond acceptor from Y324c,d | N.A. | |
| N92 | 2.45 | H-bond acceptor from W176c,d | N.A. | |
| D97 | 2.50 | NA+ bindingc | N.A. | |
| Y110 | 2.63 | Agonist effecte | N.A. | |
| TM3 | ||||
| R128 | A | 3.29 | Counterion to α-phosphated,f | >50,000 |
| F131 | A | 3.32 | Modulatory toward agonist, but not antagonistf | 8 |
| H132 | A | 3.33 | Modulatoryg | 7 |
| Y136 | A | 3.37 | Modulatoryg | 6 |
| S138 | 3.39 | H-bond acceptor from N316c,d | N.A. | |
| H148 | 3.49 | H-bond acceptor from R255c,d | N.A. | |
| TM4 | ||||
| S172 | 4.46 | H-bond acceptor from N92c,d | N.A. | |
| W176 | 4.50 | H-bond donor to N92c,d | N.A. | |
| TM5 | ||||
| T221 | A | 5.42 | Small effectf | 7 |
| T222 | A | 5.43 | Proximity to γ-phosphatef | 5 |
| F226 | A | 5.47 | Binding of antagonistsf | 9 |
| TM6 | ||||
| R255 | 6.30 | H-bond donor to H148c,d,h | N.A. | |
| Y273 | A | 6.48 | Receptor activationd,i | >3,000 |
| F | 6.48 | Receptor activation rescuedd | 2 | |
| H277 | A | 6.52 | Effect of agonist but not antagonistf | 45 |
| K280 | A | 6.55 | Counterion to β-phosphate of ADP; action of PPADSd,e,j | 810 |
| TM7 | ||||
| Y306 | A | 7.35 | Modulatory toward agonist effects onlyd | 5 |
| F | 7.35 | Modulatoryd,g | 6 | |
| Q307 | A | 7.36 | H-bond acceptor from exocyclic NH of ADPd,f | 210 |
| R310 | A | 7.39 | Counterion to α-phosphated,f | >50,000 |
| K | 7.39 | Rescue of agonist (partial) and antagonist (full) effectsf | 190 | |
| S314 | A | 7.43 | Activation, H-bond donor to adenine N1 or N3;d,f H-bond acceptor from Y58b | >50,000 |
| T | 7.43 | Rescue of agonist effectf | 5 | |
| N316 | 7.45 | H-bond donor to S138c,d | N.A. | |
| S317 | A | 7.46 | H-bond acceptor from N69; no pharmacological effectd,f | 0.7 |
| D320 | 7.49 | Receptor activationc,k | N.A. | |
| Y324 | 7.53 | H-bond donor to S87c,d | N.A. | |
| Extracellular and cytoplasmic regions | ||||
| C42 | A | N-terminal | Disulfide with C296f | 22,000 |
| T47 | N-terminal | Meta-binding sitec,f | N.A. | |
| C124 | A | EL1 | Disulfide with C202 | >50,000 |
| K125 | A | EL1 | No effectf | 3 |
| L157 | IL2 | G protein interactionc,l | N.A. | |
| R195 | A | EL2 | No effectf | 2 |
| K196 | A | EL2 | No effectf | 1 |
| K198 | A | EL2 | Modulatoryf | 8 |
| Y203 | A | EL2 | Agonist effectsd,m | >3,000 |
| F | EL2 | Rescue of agonist effectsd | 5 | |
| D204 | A | EL2 | Meta-binding sitef | 30 |
| N | EL2 | Unable to rescue functionf | 270 | |
| E | EL2 | Unable to rescue functionf | 66 | |
| D208 | A | EL2 | No effectf | 2 |
| E209 | A | EL2 | Meta-binding site; H-bonding to ribosef | 7800 |
| D | EL2 | H-bonding rescuedf | 1 | |
| Q | EL2 | H-bonding rescuedf | 4 | |
| R | EL2 | H-bonding rescuedf | 2 | |
| R212 | A | EL2 | Small effectf | 4 |
| R285 | A | EL3 | Small effectf | 4 |
| R287 | A | EL3 | Meta-binding site; H-bonding to E209f | 6700 |
| K | EL3 | Charge rescue (partial) of agonist effectsf | 34 | |
| Q | EL3 | Partial rescuef | 240 | |
| E | EL3 | Disrupts E209 interactionf | >50,000 | |
| D289 | A | EL3 | No effectf | 2 |
| C296 | A | EL3 | Disulfide with C42f | 2300 |
| D300 | A | EL3 | Agonist effectsf | 45 |
| R301 | A | EL3 | No effectf | 1 |
| D329 | C-terminal | Beginning of H8c,d,n | N.A. | |
| R333 | C-terminal | Required for Gq couplingo | N.A. | |
| R334 | C-terminal | Required for Gq couplingo | N.A. | |
| K341 | C-terminal | End of H8c,d,n | N.A. |
N.A. not applicable; EL extracellular loop; IL intracellular loop.
Residue identifier of format X.YZ refers to the TM X and residue YZ with respect to a highly conserved amino acid in each TM numbered 50 (Ballesteros et al., 2001; Costanzi et al., 2004); potency ratio shown is EC50 at mutant receptor divided by EC50 at the wild-type; the EC50 of 2-MeSADP at the wild-type human P2Y1 receptors was reported as 2.2 ± 0.5f or 3 ± 1d nM.
Applies only to ground state of receptor.
Not mutated, prediction from modeling only.
Moro and Jacobson (2002) and references therein.
Modulatory toward effects of agonist and nucleotide antagonist.
Analogous to E(6.30) of the β2 adrenergic receptor involved in an ‘ionic lock’ with R(3.50) but no electrostatic interaction with V(3.50) of the P2Y1 receptor is possible; for the P2Y1 receptor, a similarly placed interaction is predicted between R(6.30) and H(3.49).
Mutation precludes only activation by agonist, but not binding of agonist or antagonist.
Analogous to the N of the NPxxxY motif involved in activation of thyroid-stimulating hormone (Claeysen et al., 2002) and other receptors.
Mutation precludes only activation and binding of agonist, but not binding of antagonist.
An eighth helical region (cytoplasmic), by analogy to rhodopsin; a putative palmitoylation site (Cys) in this region is predicted for P2Y4, P2Y12, and P2Y14, but not P2Y1 receptors.
Recent computational models of all of the P2Y receptors were derived from a multiple-sequence alignment based on a combined manual and automatic approach, which takes into account not only the primary structure of the proteins but also the three-dimensional information deducible from the secondary and tertiary structures of the template (Costanzi et al., 2004). The receptors display the general motif of a single-polypeptide chain forming seven helical TMs, which are connected by three extracellular and three intracellular loops. The ends of the chain form an extracellular amino-terminal region and a cytoplasmic carboxyl-terminal region, as shown for the P2Y1 and P2Y12 receptors (Fig. 7). According to the models, at the cytoplasmic end of TM7 both the receptors fold at an angle of ~90° to form a helical segment that is homologous to H8 in rhodopsin and runs parallel to the plane of the cell membrane (Hoffmann et al., 1999).
Fig. 7.

Theoretical structures of the putative nucleotide binding sites of P2Y1 (A) and P2Y12 (B) receptors based on mutagenesis and molecular modeling experiments as described in Costanzi et al. (2004).
Figure 7 shows the theoretical structures of the putative nucleotide binding sites of P2Y1 (A) and P2Y12 (B) receptors, based on mutagenesis and molecular modeling experiments as described in Costanzi et al. (2004). The large figures show the binding sites as viewed from the plane of the plasma membrane with docked nucleotide ligands (the antagonists MRS2500 for P2Y1 and AZD6140 for P2Y12). Key residues found to interact with the ligand in the human P2Y1 and P2Y12 receptors are indicated. To the left of each detailed structure is a smaller three-dimensional representation of the receptor including seven TMs (color coded: cyan, TM1; orange, TM2; green, TM3; magenta, TM4; blue, TM5; red, TM6; gray, TM7) and the connecting loops. The orientation of the entire receptor relative to the membrane is the same as for each detailed binding site model.
Table 4 summarizes the effects and structural role of specific amino acid residues of the human P2Y1 receptor, deduced from site-directed mutagenesis, molecular modeling, and homology to other GPCRs. Ligand docking modeling was performed on the P2Y1 and P2Y12 receptor models. The results suggested that ADP binds to the P2Y1 and P2Y12 receptors on the exofacial side of the cavity delimited by TM1, TM2, TM3, TM6, and TM7 and capped with extracellular loop 2 (Fig. 7). Two different sets of three basic amino acids for each of the two subgroups are involved in coordination of the phosphate moiety of agonists (Costanzi et al., 2004). Molecular recognition in the P2Y1receptor of non-nucleotide antagonists, such as derivatives of PPADS (compound 39), was also studied.
A cluster of positively charged amino acid side chains in TMs 3, 6, and 7 was proposed to form the counterions to the negatively charged 5′-di- or triphosphate moiety at the P2Y1 receptor. Site-directed mutagenesis validated this prediction and further indicated several uncharged hydrophilic residues that may coordinate the nucleobase. Thus, the agonist 2-MeSADP (compound 6) was inactive at R128(3.29)A and R310(7.39)A and S314(7.43)A mutant P2Y1 receptors and had a markedly reduced potency at K280(6.55)A and Q307(7.36)A mutant P2Y1 receptors.
In the P2Y12 subgroup, the role of R3.29 in TM3 seemed to be fulfilled by a Lys residue in extracellular loop 2, whereas the residue R7.39 in TM7 seemed to be substituted by K7.35, located within the same TM but at a distance of four residues, i.e., one helical turn in the exofacial direction. Only R6.55 was common to the essential cationic residues of the two subclasses of P2Y receptors (Costanzi et al., 2004).
VI. P2Y Receptor Subtypes
The history of each currently recognized P2Y receptor subtype and available information on molecular structure, coupling to G proteins, transductional mechanisms, response to agonist/antagonist ligands, tissue distribution and function are reported in the following (additional information, including the official International Union of Pharmacology alphanumerical code, previous names, gene bank accession numbers, chromosomal localization, availability of radioligands, and knockout or knockin animals, are also schematically reported in the individual tables for each subtype).
A. P2Y1
Human (Ayyanathan et al., 1996; Janssens et al., 1996; Léon et al., 1996; Schacter et al., 1996), rat (Tokuyama et al., 1995), mouse (Tokuyama et al., 1995), cow (Henderson et al., 1995), chick (Webb et al., 1993), turkey (Filtz et al., 1994), and Xenopus (Cheng et al., 2003) P2Y1 receptors have been cloned and characterized. In most species, ADP is a more potent agonist than ATP and their 2-methylthio derivatives are more potent than the parent compounds. UTP, UDP, CTP, and GTP are inactive (Waldo et al., 2002; Waldo and Harden, 2004). At present, the most potent agonist known is the N-methanocarba analog of 2-MeSADP, MRS2365 (Chhatriwala et al., 2004). ATP is, in fact, a partial agonist at the P2Y1 receptor (Palmer et al., 1998) and so at low levels of receptor expression will act as an antagonist (Léon et al., 1997; Hechler et al., 1998b). Extracellular acidification and alkalinization do not appear to modify the activity of ATP (King et al., 1996). The first antagonists to display selectivity for the P2Y1 receptor were A3P5P and A3P5PS (Boyer et al., 1996a), but these have been superseded by the highly selective and more potent MRS2179 (Boyer et al., 1998; Camaioni et al., 1998) and MRS2279 (Boyer et al., 2002). More recent studies showed that modification of both MRS2179 (Mathieu et al., 2004) and MRS2279 (Kim et al., 2003a; Cattaneo et al., 2004) at the 2-position of the adenine moiety further increases antagonist potency. The increased potency and selectivity of MRS2279 has been used in binding studies showing that [3H]MRS2279 bound specifically to the human P2Y1 receptor, with a Kd of 3.8 nM. The binding was displaced by 2-MeSADP > ADP = 2-MeSATP > ATP and by MRS2279 = MRS2179 > A3P5P (Waldo et al., 2002; Waldo and Harden, 2004).
Site-directed mutagenesis studies on the human P2Y1 receptor have produced a binding pocket model in which amino acid residues in TM3, 6 and 7 are critical determinants in the binding of ATP and other nucleotide derivatives (Jiang et al., 1997b; Moro et al., 1998). Arginine 128 (TM3) and lysine 280 (TM6) are proposed to interact with the α and β phosphate groups of ATP, arginine 310 (TM7) also with the β phosphate, and threonine 222 (TM5) with the γ phosphate. Additionally, glutamine 307 (TM7) and serine 314 (TM7) may interact with the adenine ring. Alanine scanning mutagenesis studies revealed that four cysteine residues in the extracellular loops, which are conserved in P2Y receptors, are essential for proper trafficking of the human P2Y1 receptor to the cell surface (Hoffmann et al., 1999). These studies also showed that several charged residues in the extracellular loops are essential for P2Y1 activation.
A recent study on the purified and reconstituted human P2Y1 receptor showed that it couples to Gαq and Gα11 but not to Gαi1, Gαi2, Gαi3, or Gαo (Waldo and Harden, 2004). This is consistent with a large body of studies showing that activation of this receptor evokes an increase in intracellular IP3 levels and the release of intracellular Ca2+ stores, in a PTX-insensitive manner.
Northern blotting and RT-PCR revealed P2Y1 receptor mRNA in most human tissues, including the brain, heart, placenta, lungs, liver, skeletal muscle, kidneys, pancreas, and various blood cells (Ayyanathan et al., 1996; Janssens et al., 1996; Léon et al., 1996). Two mRNA bands of 7.0 to 7.5 and 4.4 kb, whose expression pattern varied with the tissue examined, were seen (Ayyanathan et al., 1996; Léon et al., 1996). Quantitative RT-PCR indicated that expression was highest in the brain, prostate gland, and placenta and was also detected at varying levels in the pituitary gland, lymphocytes, spleen, heart, lung, liver, kidney, stomach, intestine, skeletal muscle, adipose tissue, and pancreas (Moore et al., 2001). In the study within the brain, P2Y1 mRNA was highest in the nucleus accumbens, putamen, caudate nucleus, and striatum, with lower levels seen in the hippocampus, parahippocampal gyrus, globus pallidus, cingulate gyrus, and hypothalamus. Histochemical studies with a specific antibody also showed widespread distribution of the P2Y1 receptor throughout the brain, with notable staining in the cerebral cortex, cerebellar cortex, hippocampus, caudate nucleus, putamen, globus pallidus, subthalamic nucleus, red nucleus, and midbrain (Moore et al., 2000a). In addition, in post-mortem brain sections from persons with Alzheimer’s disease, the P2Y1-like immunoreactivity in the hippocampus and entorhinal cortex was localized to neurofibrillary tangles, neuritic plaques, and neuropil threads, which are characteristic Alzheimer’s structures (Moore et al., 2000b). Western blots showed bands of 45, 90 and 180 kDa in the smooth muscle cells of the human left internal mammary artery and of 90 and 180 kDa in human umbilical vein endothelial cells (Wang et al., 2002).
A similar pattern of mRNA distribution is seen in rat tissues by Northern blotting (Tokuyama et al., 1995; Nakamura and Strittmatter, 1996). Mapping of the P2Y1 mRNA by in situ hybridization has been performed across the chick brain (Webb et al., 1998), showing an abundant expression in many regions; at the cellular level this was located in many of the cell bodies of neurons, as well as in astrocytes. In rat brain, there is prominent P2Y1-like immunoreactivity in neurons in Purkinje cells, the cerebellar cortex and hippocampus (Moran-Jiminez and Matute, 2000), ventral tegmentum (Krügel et al., 2001), midbrain, brainstem, and medulla (Fong et al., 2002). Glial cells in the brain also stain positive (Moran-Jiminez and Matute, 2000; Fong et al., 2002; Weick et al., 2003; Franke et al., 2004). The receptor is also expressed in sensory neurons (Nakamura and Strittmatter, 1996; Fong et al., 2002; Ruan and Burnstock, 2003; Gerevich et al., 2004), consistent with a potential role for P2Y1 receptors in sensory reception. The presence of P2Y1-like immunoreactivity (Fong et al., 2002) and mRNA (Buvinic et al., 2002; Kaiser and Buxton, 2002) in rat arterial endothelial cells is also consistent with the well-characterized vasodilatory activity of P2Y1 receptors. P2Y1-like immunoreactivity is also present in rat uterus epithelial cells (Slater et al., 2002), the pancreas (Coutinho-Silva et al., 2003), and glomeruli of rat kidney (Bailey et al., 2004). Northern blot analysis in chick embryos during the first 10 days of development showed that expression of P2Y1 mRNA varied in a regulated manner in the limb buds, mesonephros, somites, brain, and facial primordia (Meyer et al., 1999). This suggests that the receptor may play a role in the development of each of these systems.
P2Y1 receptor knockout mice have been generated by homologous recombination in two separate laboratories, and the reported phenotypes are identical (Fabre et al., 1999; Léon et al., 1999a) (see also Section.VII.J.). These mice are viable with no apparent abnormalities affecting their development, survival, and reproduction. Platelet counts and morphology are normal. In contrast, platelet shape change and aggregation to usual concentrations of ADP are completely abolished in these mice, whereas the ability of ADP to inhibit cAMP formation is maintained (Léon et al., 1999a). At higher concentrations of ADP (>10 μM), aggregation is observed without shape change, which is entirely due to P2Y12 (Kauffenstein et al., 2001). Their bleeding time is mildly prolonged. These mice display resistance to systemic thromboembolism and to localized arterial thrombosis (Léon et al., 1999b, 2001; Lenain et al., 2003). Conversely, transgenic mice overexpressing the P2Y1 receptor specifically in the megakaryocytic/platelet lineage have also been generated using the promoter of the tissue-specific platelet factor 4 gene (Hechler et al., 2003). This led to a phenotype of platelet hyper-reactivity in vitro. Moreover, overexpression of the P2Y1 receptor enabled ADP to induce granule secretion, unlike in wild-type platelets, which suggests that the level of P2Y1 expression is critical for this event and that the weak responses of normal platelets to ADP are due to a limited number of P2Y1 receptors rather than to activation of a specific transduction pathway. In addition, transgenic mice display a shortened bleeding time and an increased sensitivity to in vivo platelet aggregation induced by infusion of a mixture of collagen and adrenaline (Hechler et al., 2003).
The P2Y1 receptor is broadly expressed throughout the body. Thus, its gene knockout could have phenotypic consequences other than the sole hemostasis system. This is indeed the case with glucose homeostasis, since it has been found that the knockout mice display higher glucose levels as well as higher weight than wild-type mice (Léon et al., 2005)
For more information on the P2Y1 receptor, see Table 5.
TABLE 5. P2Y1 receptor (previously known as P2Y purinoceptor 1).
2.1:NUCT:1:P2Y1.
| TM | aa | AC | Chr. Map | References | |
|---|---|---|---|---|---|
| Human | 7 | 373 | NM_002563 | 3q25.2 | Ayyanathan et al. (1996); Léon et al. (1996) |
| Rat | 7 | 373 | NM_012800 | 2q31 | Tokuyama et al. (1995) |
| Mouse | 7 | 373 | NM_008772 | 3 | Tokuyama et al. (1995) |
| Functional assays | Measure of calcium fluxes in Jurkat T lymphocytes expressed the cloned P2Y1 receptor (Léon et al., 1997); human blood platelets; rat and rabbit mesenteric artery; turkey platelets |
| Agonist potencies | Ki values for P2Y1 receptor agonists for inhibition of [3H]MRS2279 binding to purified P2Y1 receptor: 2-MeSADP (Ki = 0.0099) > ADP (Ki = 0.92) > ATPγS (Ki = 1.33) > 2-MeSATP (Ki = 1.87) > ADPβS (Ki = 2.42) > ATP (Ki = 17.7) (Waldo and Harden, 2004) |
| Antagonist potencies | MRS2179 (pKB = 6.75 (Moro et al., 1998) (selective); MRS2279 (pKB = 8.10) (Boyer et al., 2002) (selective); reactive blue 2 (Lambrecht et al., 2002); PPADS (pKB = 5) (Guo et al., 2002); suramin (pKB = 5.5) (Ralevic and Burnstock, 1998); A3P5PS (pA2 = 6.5) (Boyer et al., 1996) |
| Radioligand assays | Equilibrium binding of [3H]MRS2279 to P2Y1 receptor expressed in membranes (Waldo et al., 2002; Waldo and Harden, 2004); binding studies with [33P]MRS2179 (Baurand et al., 2001) |
| Radioligands | [3H]MRS2279; [33P]MRS2179 |
| Transduction mechanism | Gq/G11; PI hydrolisis (PLC activation) and increasing of calcium in expression systems |
| Distribution | Brain, placenta, prostate, heart, skeletal muscle, platelets, neuronal tissue; P2Y1 mRNA could not be detected in cartilage and bone; it was expressed at barely detectable levels in liver, kidney, stomach, lymphocytes, and bone marrow (Moore et al., 2001) |
| Tissue function | Endothelium-dependent relaxation; smooth muscle relaxation (Ralevic and Burnstock, 1998); role in ADP-induced intracellular calcium mobilization, platelet shape change, aggregation, and TXA2 generation (Hechler et al., 1998a,b); mitogenic action in rat aorta smooth muscle (Ralevic and Burnstock, 1998); inhibition of N-type Ca2+ channels (Filippov et al., 2000); role of the receptor in nucleotide-mediated calcium signaling in astrocytes (Fumagalli et al., 2003); possible role in the modulation of neuro-neural signaling transmission (von Kügelgen and Wetter, 2000); role in osteoclastic bone resorption (Hoebertz et al., 2002) |
| Phenotypes | P2Y1-null mice* |
| Comments | In all species, the receptor is selective for adenine nucleotide;* these mice were viable with no apparent abnormalities that affected their development and survival; platelet count in these animals was identical to that of wild-type mice, but they were unable to aggregate in response to usual concentrations of ADP, whereas high concentrations of ADP induced platelet aggregation without shape change; P2Y1-null mice had no spontaneous bleeding tendency but were resistant to thromboembolism induced by intravenous injection of ADP (Fabre et al., 1999; Léon et al., 1999a); molecular modeling study of the human P2Y1 receptor supports the idea that ATP binding to at least two distinct domains of the P2Y1 receptor, both outside and within the TM core; the two disulfide bridges present in the human P2Y1 receptor play a major role in the structure and stability of the receptor, to constrain the loops within the receptor, specifically stretching the extracellular loop 2 over the opening of the TM cleft and thus defining the path of access to the binding site (Moro et al., 2002); MRS2179 is the first P2Y1 receptor antagonist with antithrombotic action (Baurand and Gachet, 2003) |
aa, amino acids; AC, accession; chr., chromosome; MRS2179, 2′-deoxy-N6-methyladenosine-3′,5′-bisphosphate; MRS2279, N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate; A3P5PS, adenosine-3′-phosphate-5′-phosphosulfate; A3P5P, adenosine 3′, 5′-diphosphate; A2P5P, adenosine 2′, 5′-diphosphate; ATP-α-B, 5′-O-(1-boranotriphosphate).
B. P2Y2
P2Y2 receptors, previously known as P2U, have been cloned and pharmacologically characterized from human, rat, mouse, canine, and porcine cells or tissues (Lustig et al., 1993; Parr et al., 1994; Bowler et al., 1995; Rice et al., 1995; Chen et al., 1996; Zambon et al., 2000; Shen et al., 2004). P2Y2 receptors are fully activated by equivalent concentrations of ATP and UTP, whereas ADP and UDP are less effective agonists (Lustig et al., 1993; Parr et al., 1994; Lazarowski et al., 1995a). An exception is the porcine P2Y2 receptor, which is relatively insensitive to ATP (Shen et al., 2004). UTPγS has been shown to be a potent hydrolysis resistant agonist of P2Y2 receptors (Lazarowski et al., 1995b). Suramin acts as a competitive antagonist of human and rat P2Y2 receptors (Charlton et al., 1996; Lambrecht et al., 2002; Wildman et al., 2003). P2Y2 receptors can directly couple to PLCβ1 via Gαq/11 protein to mediate the production of IP3 and diacylglycerol (DAG), second messengers for calcium release from intracellular stores and PKC activation, respectively. Coupling of P2Y2 receptors to other G protein subtypes has been reported (Baltensperger and Porzig, 1997; Murthy and Makhlouf, 1998; Weisman et al., 1998; Bagchi et al., 2005). The P2Y2 receptor is partially sensitive to PTX, a Gi/Go protein inhibitor, and studies indicate that access to Go protein is dependent upon association of the P2Y2 receptor with αVβ3/β5 integrins (Erb et al., 2001) and regulates nucleotide-induced chemotaxis (Bagchi et al., 2005). Expression of P2Y2 receptor mRNA has been detected in human skeletal muscle, heart, brain, spleen, lymphocytes, macrophages, bone marrow, and lung, with lower expression levels detected in liver, stomach, and pancreas (Moore et al., 2001). Functional P2Y2 receptors are expressed in epithelial, smooth muscle, and endothelial cells and in leukocytes, cardiomyocytes, osteoblasts, and cells derived from the peripheral and central nervous system, including Schwann cells, rat cortical neurons, oligodendrocytes, dorsal horn and cortical astrocytes, immortalized astrocytes, astrocytoma cells, and NG108-15 neuroblastoma × glioma hybrid cells (Bowler et al., 1995; Ho et al., 1995; Kirischuk et al., 1995; Rice et al., 1995; Berti-Mattera et al., 1996; Kim et al., 1996; Kunapuli and Daniel, 1998; Weisman et al., 1999; Pillois et al., 2002; Seye et al., 2002; Gendron et al., 2003; Kumari et al., 2003).
Site-directed mutagenesis of the P2Y2 receptor has been used to demonstrate that replacement of positively charged amino acids in TM helices 6 and 7 with neutral amino acids decreases the potencies of ATP and UTP, suggesting that these domains play a role in binding the negatively charged moieties of nucleotide agonists (Erb et al., 1995) (see above). The P2Y2 receptor undergoes agonist-induced desensitization in several cell types (Wilkinson et al., 1994; Garrad et al., 1998; Clarke et al., 1999; Otero et al., 2000; Velázquez et al., 2000; Santiago-Pérez et al., 2001), and mutagenesis studies indicate that deletion of structural motifs in the intracellular C-terminal domain of the P2Y2 receptor that contain putative phosphorylation sites for GPCR kinase diminishes agonist-induced desensitization and internalization of the P2Y2 receptor (Garrad et al., 1998). The P2Y2 receptor also contains the consensus integrin-binding motif, Arg-Gly-Asp (RGD) in its first extracellular loop that facilitates P2Y2 receptor colocalization with αVβ3/β5 integrins when the P2Y2 receptor is expressed in human 1321N1 astrocytoma cells that are devoid of endogenous G protein-coupled P2Y receptors (Erb et al., 2001). A mutant P2Y2 receptor in which the RGD motif was replaced with Arg-Gly-Glu, a sequence that does not have high affinity for integrins, exhibited an EC50 for nucleotide-induced calcium mobilization that was ~1000-fold greater than that for the wild-type P2Y2 receptor (Erb et al., 2001). The αVβ3/β5 integrins are known to regulate angiogenesis and inflammatory responses including cell proliferation, migration, adhesion, and infiltration (Zhang et al., 2002b; Hutchings et al., 2003; Kannan, 2003; Li et al., 2003; Pidgeon et al., 2003), responses also mediated by P2Y2 receptor activation (Wilden et al., 1998; Seye et al., 2002; Greig et al., 2003a,b; Schafer et al., 2003; Bagchi et al., 2005; Kaczmarek et al., 2005), suggesting that nucleotides may transactivate integrin signaling pathways by virtue of P2Y2 receptor binding to integrins. The RGD sequence in the P2Y2 receptor also has been shown to play an integrin-independent role in targeting of the receptor to the apical membrane of Madin-Darby canine kidney cells (Qi et al., 2005).
P2Y2 receptor activation increases the synthesis and/or release of arachidonic acid (AA), prostaglandins and nitric oxide (NO) (Lustig et al., 1992; Pearson et al., 1992a,b; Xing et al., 1999; Xu et al., 2002b, 2003; Welch et al., 2003). In primary murine astrocytes, P2Y2 receptors mediate the activation of calcium-dependent and calcium-independent PKCs and ERK1/2 that can activate cytosolic phospholipase A2, leading to production of AA (Gendron et al., 2003; Xu et al., 2003), the precursor of eicosanoids, prostaglandins, and leukotrienes (Balsinde et al., 2002). Activation of P2Y2 receptors in isolated UTP- or ATP-perfused rat hearts induces pronounced vasodilatation (Godecke et al., 1996), consistent with the role of P2Y2 receptors in relaxation of smooth muscle through the endothelium-dependent release of NO and prostacyclin (Lustig et al., 1992; Pearson et al., 1992a,b). P2Y2 receptor expression in smooth muscle cells is up-regulated by agents that mediate inflammation, including interleukin (IL)-1β, interferon-γ, and tumor necrosis factor-α (Hou et al., 1999, 2000) and P2Y2 receptor up-regulation has been shown to promote nucleotide-induced activation of PKC, cyclooxygenase, and MAPK (Koshiba et al., 1997; Turner et al., 1998; Seye et al., 2002). In addition to ERK1/2, P2Y2 receptor activation can induce the phosphorylation of the stress-activated kinases JNK and p38 (Gendron et al., 2003). P2Y2 receptor activation also induces p38- and ERK1/2-dependent up-regulation of genes that regulate cell survival in human astrocytoma cells (i.e., Bcl-2 and Bcl-xl) and genes that regulate neurite outgrowth in PC-12 cells (Chorna et al., 2004). Human neutrophil P2Y2 receptors have been shown to regulate neutrophil degranulation induced by fibrinogen, independent of AA metabolites (Meshki et al., 2004), and P2Y2 receptors have been suggested to play a role in the wound healing process (Burrell et al., 2003; Greig et al., 2003a,b).
Studies have indicated that P2Y2 receptor-mediated MAPK activation in rat-1 fibroblasts and PC12 cells is dependent upon transactivation of the epidermal growth factor receptor (EGFR) via a Src/Pyk2-dependent pathway (Soltoff, 1998; Soltoff et al., 1998). In contrast, embryonic fibroblasts derived from Src−/−, Pyk2−/−, or Src−/−Pyk2−/− mice have been used to demonstrate that Src and Pyk2 were essential for GPCR-mediated transactivation of the EGFR but not for GPCR-mediated MAPK activation (Andreev et al., 2001). Gβγ subunits have been shown to regulate Src-mediated transactivation of growth factor receptors (Luttrell et al., 1997), which may represent a common pathway whereby GPCRs stimulate cell proliferation. Other studies have identified two SH3-binding domains (i.e., PXXP motifs in which P is proline and X is any amino acid) in the intracellular carboxyl-terminal tail of the human P2Y2 receptor that are necessary for transactivation of epidermal or platelet-derived growth factor (PDGF) receptors by ATP or UTP (Liu et al., 2004). Deletion of these SH3-binding domains inhibited nucleotide-induced P2Y2 receptor colocalization with EGFR and UTP-induced EGFR transactivation (i.e., phosphorylation) but did not suppress ERK1/2 activation when the mutant receptors were expressed in 1321N1 astrocytoma cells (Liu et al., 2004), most likely because of the ability of the P2Y2 receptor to also activate Src and ERK1/2 via PLC and integrin signaling pathways (Erb et al., 2001). Furthermore, Src coimmunoprecipitated with the P2Y2 receptor in UTP-treated cells expressing the wild-type P2Y2 receptor, but not the SH3-binding domain deletion mutant (Liu et al., 2004), strongly suggesting that activation of the P2Y2 receptor promotes Src binding.
Activation of P2Y2 receptors causes proliferation and/or migration of human epidermal keratinocytes, lung epithelial tumor cells, glioma cells, and smooth muscle cells (Wilden et al., 1998; Tu et al., 2000; Seye et al., 2002; Schafer et al., 2003, Greig et al., 2003b). P2Y2 receptor activation also can induce cell cycle progression in smooth muscle cells from G1 to S and M phases (Malam-Souley et al., 1996; Miyagi et al., 1996). HeLa cell proliferation in response to P2Y2 receptor activation is associated with the PI3-K- and ERK1/2-dependent expression of the early response protein c-fos (Muscella et al., 2003). Consistent with a role for P2Y2 receptors in cell proliferation, P2Y2 receptor mRNA expression is down-regulated during cell differentiation (Martin et al., 1997). The P2Y2 receptor is up-regulated in thymocytes as an immediate early gene response (Koshiba et al., 1997) and in injured and stressed tissues (Turner et al., 1997; Seye et al., 2002), and nucleotides induce ERK-dependent astrocyte proliferation under a variety of conditions including stretch- or stab-induced injury (Neary et al., 1994, 1996, 1999, 2003; Franke et al., 1999). Up-regulation of P2Y2 receptors occurs in arteries after balloon angioplasty (Seye et al., 1997), in rat submandibular gland cells after short-term culture or upon duct ligation in vivo (Turner et al., 1997, 1998), and in a mouse model of Sjögren’s syndrome (Schrader et al., 2004). Placement of a vascular collar around rabbit carotid arteries increases expression of P2Y2 receptors in smooth muscle and endothelium that is associated with neointimal hyperplasia, monocyte infiltration, and smooth muscle cell expression of the proliferative protein osteopontin, responses that were enhanced by application of the P2Y2 receptor agonist UTP (Seye et al., 2002). In addition, P2Y2 receptor up-regulation in endothelial cells increases the binding of monocytes to endothelial cells due to P2Y2 receptor-mediated increases in the endothelial expression of vascular cell adhesion molecule-1 (Seye et al., 2003), a process that promotes vascular inflammation in atherosclerosis. Up-regulation of vascular cell adhesion molecule-1 was shown to be dependent on P2Y2 receptor-mediated transactivation of vascular endothelial growth factor receptor-2 (KDR/Flk-1), a response that was inhibited by deletion of the SH3 binding motifs from the P2Y2 receptor, demonstrating a mechanism whereby P2Y2 receptors can cause inflammatory responses (Seye et al., 2004).
P2Y2 receptor activation increases Cl− secretion and inhibits Na+ absorption in epithelial cells, which has potential relevance for the treatment of cystic fibrosis, a disease that is caused by genetic defects in the gene for the CFTR, a major epithelial anion channel (Clarke and Boucher, 1992; Parr et al., 1994; Clarke et al., 2000; Kellerman et al., 2002). The synthetic P2Y2 receptor agonist dCp4U (INS37217) has been used to promote chloride and water secretion in tracheal epithelium and increase ciliary beat frequency and mucin release in human airway epithelium (Yerxa et al., 2002) and to stimulate subretinal fluid reabsorption in a rabbit model of retinal detachment (Meyer et al., 2002).
A P2Y2 receptor knockout mouse that is defective in nucleotide-stimulated ion secretion in airway epithelial cells has been produced, confirming a role for the P2Y2 receptor in the regulation of epithelial transmembrane ion transport (Cressman et al., 1999). In addition, P2Y2 receptors have been shown to inhibit bone formation by osteoblasts (Hoebertz et al., 2002), and N-type calcium currents in neurons (Filippov et al., 1997; Brown et al., 2000a). P2Y2 receptors also can induce α-secretase-dependent amyloid precursor protein processing in astrocytoma cells, suggesting a neuroprotective role (Camden et al., 2005). Collectively, studies with the P2Y2 receptor and its signaling pathways have elucidated potential pharmacological targets in atherosclerosis, inflammation, cystic fibrosis, eye disease, osteoporosis, cancer, and neurodegenerative disorders.
For more information on the P2Y2 receptor, see Table 6.
TABLE 6. P2Y2 receptor (previously known as P2U, P2U purinoceptor 1, and P2Y purinoceptor 2).
2.1:NUCT:2:P2Y2.
| TM | aa | AC | Chr. Map | References | |
|---|---|---|---|---|---|
| Human* | 7 | 377 | NM_176072; NM_002564; NM_176071 | 11q13.5–q14.1 | Parr et al. (1994) |
| Rat | 7 | 374 | NM_017255 | 1q32 | Chen et al. (1996) |
| Mouse | 7 | 373 | NM_008773 | 7 | Lustig et al. (1993) |
| Functional assays | Rat coronary artery (Godecke et al., 1996); rat salivary glands (Turner et al 1997); human airway epithelium (Clarke et al., 1992); murine gallbladder epithelium (Clarke et al., 1999); current measurement in microinjected rat superior cervical sympathetic neurons (Brown, 2000); calcium imaging in HT29 and Colo320 DM colorectal carcinoma cells (Burnstock and Knight, 2004); HeLa cells (Muscella, 2003); A6 cells (Burnstock and Knight, 2004); HEK 293 cells (Schacter et al., 1996); studies of IP3 accumulation and intracellular calcium mobilization in 1321N1 astrocytoma cells (Parr et al., 1994; Lazarowski et al., 1995a); [3H]inositol polyphosphate metabolism in rat aortic smooth muscle cells (Kumari, 2003); human coronary artery endothelial cells (Seye, 2003); intracellular calcium signaling, electrophysiology, and fluid transport in human fetal, rat, and rabbit retina (Meyer, 2002); Ca2+ mobilization in HL-60 human neutrophil cell line (Meshki, 2004) |
| Ligand | Action | Selectivity | Endogenous | References |
|
| ||||
| UTP | Agonist | No | Yes | Lazarowski et al. (1995b) |
| UTPγS | Agonist | No | No | Lazarowski et al. (1995b) |
| ATP | Agonist | No | Yes | Lazarowski et al. (1995b) |
| 5BrUTP | Agonist | No | No | Lazarowski et al. (1995b) |
| 2ClATP | Agonist | No | No | Lazarowski et al. (1995b) |
| dCp4U (INS37217) | Agonist | No | No | Kellerman et al. (2002) |
| Up4U (INS365) | Agonist | No | No | Pendergast, et al. (2001) |
| Agonist potencies | IP3: UTP (EC50 0.14 ± 0.02 μM) > ATP (EC50 0.23 ± 0.01 μM) > Ap4A (EC50 0.72 ± 0.02 μM) > ATPγS (EC50 1.72 ± 0.15 μM) > 5BrUTP (EC50 2.06 ± 0.04 μM); Ca2+: dCp4U (INS37217) (EC50 0.22 μM); Up4U (INS365) (EC50 0,1 μM) (Lazarowski et al., 1995; Pendergast et al., 2001; Yerxa et al., 2002) |
| Antagonist potencies | Suramin; PPADS insensitive (Charlton et al., 1996) |
| Radioligand assays | None |
| Radioligands | None |
| Transduction mechanism | Gq/G11, PLCβ, and possibly Gi/Go; PI hydrolysis (PLCβ activation) and elevated Ca2+ in expression systems; activation of ICl/Ca in Xenopus oocytes; transactivation of integrin and growth factor receptors (Soltoff, 1998; Erb et al., 2001; Seye et al., 2003; Liu et al., 2004) |
| Distribution | Quantitative RT-PCR: skeletal muscle, heart, and some brain regions; at more moderate levels spleen, lymphocytes, macrophages, bone marrow, and lung; lowest levels of mRNA in liver, stomach, and pancreas (Moore et al., 2001); vascular smooth muscle and endothelial cells (Ralevic and Burnstock, 1998) |
| Tissue function | Epithelial cell Cl− secretion (Clarke, 1992; Parr, 1994); pulmonary surfactant secretion (Rice, 1995); receptors on endothelial cells mediate NO release and subsequent vasodilatation (Ralevic and Burnstock, 1998); receptors on smooth muscle in some blood vessels mediate vasoconstriction (Ralevic and Burnstock, 1998); growth inhibition and apoptosis in human colorectal carcinoma cells (Burnstock and Knight, 2004); role in bone remodeling (Hoebertz et al., 2002); monocyte recruitment by vascular endothelium (Seye et al., 2003); proliferation of HeLa cells induced by c-Fos (Muscella, 2003); proliferation of lung cancer cells (Schafer, 2003); role in dry eye disease (Yerxa et al., 2002); regulation of proliferation in human keratinocyte (Burrel, 2003; Greig et al., 2003b); role in neutrophil degranulation (Meshki, 2004); growth of the arterial cell wall (Seye et al., 2002) |
| Phenotypes | Reduction of chloride secretion in airway epithelia P2Y2 knockout mice (Cressman et al., 1999) |
| Comments | The human P2Y2 receptor is activated by nucleotide triphosphates but not diphosphates (Nicholas et al., 1996) and is PPADS-insensitive (Charlton et al., 1996); P2Y2 receptor signaling is partially inhibited by PTX, indicating some involvement of Gi in receptor coupling (Parr et al., 1994)—in addition, native and recombinant forms of rat P2Y2 couple to PTX-sensitive Gi/o (Filippov et al., 1997; Mosbacher et al., 1998); Go activation by P2Y2 receptors requires integrin receptor transactivation (Erb et al., 2001); rabbit P2Y2 couples to Gi/Go isoforms (Murthy et al., 1998); *three transcript variants that encode the same protein have been identified for this gene (Parr et al., 1994); results of preclinical research suggest that P2Y2 receptor agonists inhibit Na absorption, restore Cl conductance, and rehydrate the cystic fibrosis airway surface (Kellermann, 2002); dCp4U (INS37217), a metabolically stable and potent P2Y2 agonist given for inhalation, has been evaluated in patients with cystic fibrosis lung disease (Kellerman et al., 2002); an RGD sequence in the P2Y2 receptor interacts with aVb3 integrins (Erb et al., 2001); Src homology 3 binding domains in the P2Y2 receptor activate Src and transactivate growth factor receptors (Erb et al., 2001; Seye et al., 2004); the C-terminal domain of the P2Y2 receptor mediates receptor desensitization and internalization (Garrad et al., 1998) |
aa, amino acids; AC, accession; chr., chromosome.
C. P2Y4
Human (Communi et al., 1995; Nguyen et al., 1995; Stam et al., 1996), rat (Bogdanov et al., 1998b; Webb et al., 1998), and mouse (Lazarowski et al., 2001b; Suarez-Huerta et al., 2001) P2Y4 receptors have been cloned and characterized (Communi et al., 2005b). UTP is the most potent activator of the recombinant human P2Y4 receptor (Nicholas et al., 1996). GTP and ITP are approximately 10 times less potent than UTP (Communi et al., 1996a). ATP behaves as a competitive antagonist (Kennedy et al., 2000). Up4U (INS365) (Pendergast et al., 2001) and dCp4U (INS37217) (Yerxa et al., 2002) are agonists of the human P2Y4 receptor, whereas Ap4A is inactive. On the contrary, the recombinant rat and mouse P2Y4 receptors are activated equipotently by ATP and UTP and also with a lower potency by ITP, GTP and CTP (Bogdanov et al., 1998b; Webb et al., 1998). Pharmacological discrimination between rodent P2Y4 and P2Y2 receptors is thus difficult. No selective antagonist is available. Extracellular acidification enhanced the potency of ATP and UTP at rat P2Y4, but not at rat P2Y2 (Wildman et al., 2003). Zn2+ inhibited the ATP response at the rat P2Y4 receptor but had no effect on rat P2Y2 (Wildman et al., 2003). A study of chimeric human/rat P2Y4 receptors showed that the structural determinants of agonism versus antagonism by ATP are located in the N-terminal domain and the second extracellular loop. Mutational analysis revealed that three residues in the second extracellular loop contribute to impart agonist property to ATP: Asn-177, Ile-183, and Leu-190 (Herold et al., 2004). Although their sequence is only distantly related to mammalian P2Y4, Xenopus p2y8 (Bogdanov et al., 1997) and a turkey p2y (Boyer et al., 2000) could represent orthologs. They are activated with similar potencies by ATP, UTP, GTP, ITP, and CTP. The tp2y receptor is coupled to the stimulation of PLC and inhibition of adenylyl cyclase, indicating a dual coupling to Gq/11 and Gi/o.
In the absence of reconstitution data, there is no hard molecular evidence of P2Y4 receptor interaction with specific G proteins. The IP3 response to UTP of the recombinant human P2Y4 receptor is partially inhibited by PTX, at early times after agonist addition but not later (Communi et al., 1996a). This finding suggests that the P2Y4 receptor couples mainly to a Gq/11 protein and accessorily to a Gi/o protein. In sympathetic neurons injected with P2Y4 cDNA, UTP inhibited the N-type Ca2+ current. This inhibition was relieved by PTX and by expression of the βγ subunits of transducin (Filippov et al., 2003). These observations are consistent with the involvement of βγ subunits released from Gi/o proteins in the function of the P2Y4 receptor. In the same model, UTP inhibited the M-type K+ current in a PTX-insensitive way.
Among human organs, P2Y4 mRNA was detected in placenta by Northern blotting (Communi et al., 1995) and was most abundant in the intestine, according to TaqMan quantitative RT-PCR (Moore et al., 2001). RT-PCR revealed the presence of P2Y4 message in human umbilical vein endothelial cells, peripheral blood leukocytes (Jin et al., 1998b), fetal cardiomyocytes (Bogdanov et al., 1998a), and various cell lines derived from the human lung (Communi et al., 1999a). In the airway submucosal cell line 6CFSMEo−, an IP3 response to UTP but not to ATP suggests a functional expression of the P2Y4 receptor (Communi et al., 1999a). In the rat, the expression of P2Y4 message in heart and brain was much higher in neonates than in adults (Webb et al., 1998; Cheung et al., 2003). In in situ hybridization on the adult rat brain neuronal P2Y4 receptor mRNA was not detectable, it being present in the ventricular/choroid plexus system and strongly in the pineal gland, as well as apparently in astrocytes (Webb et al., 1998). In mouse, RT-PCR revealed that P2Y4 message was the most abundant in stomach, liver, and intestine (Suarez-Huerta et al., 2001). Several histochemical studies have been performed using commercially available anti-rat P2Y4 polyclonal rabbit antibodies, the specificity of which remains questionable (as is illustrated by Tung et al., 2004). Those studies suggest expression of the P2Y4 receptor in rat dorsal root and trigeminal ganglia neurons (Ruan and Burnstock, 2003) and in the proximal convoluted tubular epithelium of the rat kidney (Turner et al., 2003). A combination of RT-PCR, Western blotting, immunohistochemistry, and pharmacology suggests that the P2Y4 receptor is expressed and plays a role in the inner ear of rat, guinea pig, and gerbil (organ of Corti, stria vascularis marginal cells, and vestibular dark cells) (Teixeira et al., 2000; Sage and Marcus, 2002; Parker et al., 2003). These data are consistent with the hypothesis that nucleotides could play a role in the adaptation of the cochlea to noise exposure (Housley et al., 2002). Noise would increase the release of ATP and UTP in the scala media, leading to the inhibition of K+ release at the apical surface of the stria vascularis, presumably via activation of P2Y4 receptors (Marcus and Scofield, 2001). In conclusion, a major site of P2Y4 expression appears to be the intestine. Indeed RT-PCR has revealed that P2Y4 message was particularly abundant in the human and murine intestine. This is consistent with the loss of the chloride secretory response to apical UTP in the jejunal epithelium of P2Y4-null mice (Robaye et al., 2003).
P2Y4-null mice have apparently normal behavior, growth, and reproduction (Robaye et al., 2003). The proportion of genotypes is consistent with X-linked Mendelian transmission. The chloride secretory response of the jejunal epithelium to apical UTP and ATP, measured in Ussing chambers, is abolished in P2Y4-null mice. At the basolateral side, both P2Y4 and P2Y2 receptors are involved (Ghanem et al., 2005). In the colon, the epithelial secretion of chloride in response to UTP is mediated exclusively by the P2Y4 subtype (Ghanem et al., 2005), whereas both P2Y2 and P2Y4 are involved in the UTP-induced secretion of potassium (Matos et al., 2005). Thus, in the intestine, the epithelial response to nucleotides is mediated mainly by the P2Y4 receptor, but with a contribution of the P2Y2 subtype, whereas in airways it involves mainly the P2Y2 receptor (Cressman et al., 1999).
For more information on the P2Y4 receptor, see Table 7.
TABLE 7. P2Y4 receptor (previously known as P2Y purinoceptor 4 and uridine nucleotide receptor).
2.1:NUCT:3:P2Y4
| TM | aa | AC | Chr. Map | References | |
|---|---|---|---|---|---|
| Human | 7 | 365 | NM_002565 | Xq13 | Nguyen et al. (1995) |
| Rat | 7 | 361 | NM_031680 | Xq31 | Bogdanov et al. (1998b) |
| Mouse | 7 | 361 | NM_020621 | X | Lazarowski et al. (2001) |
| Functional assays | Calcium mobilization in 1321N1 astrocytoma cells that express recombinant receptor (Communi et al., 1995; Nguyen et al., 1995) |
| Ligand | Action | Selectivity | Endogenous | References |
|
| ||||
| UTP | Agonist | No | Yes | Communi et al. (1995) |
| UTPγS | Agonist | No | No | Jacobson et al. (2002) |
| 5BrUTP | Agonist | No | No | Nguyen et al. (1995) |
| UDP | Partial agonist | No | Yes | Nguyen et al. (1995) |
| ITP | Partial agonist | No | No | Communi et al. (1995) |
| ATP* | Agonist/antagonist | No | Yes | Herold et al. (2004) |
| PPADS | Antagonist | No | No | Charlton et al. (1996) |
| Reactive blue 2 | Antagonist | No | No | Brown et al. (2002) |
| Agonist potencies | Human: UTP (EC50 = 2.5 μM) > ITP (33 μM) (Communi et al., 1996a); rat: ITP (EC50 = 1.4 μM) = ATP (1.8 μM) = UTP (2.6 μM) (Bogdanov et al., 1998b) |
| Antagonist potencies | Human: PPADS (25% reduction at 100 μM) (Charlton et al., 1996); ATP is a competitive antagonist (Kennedy et al., 2000); rat: reactive blue 2 (IC50 = 21 μM) (Bogdanov et al., 1998b) |
| Radioligand assays | None |
| Radioligands | None |
| Transduction mechanism | Gq/G11 and possibly GI; PI hydrolysis (PLCβ activation) and elevated [Ca2+]i in expression systems; activation of ICl/Ca in Xenopus oocytes (by increased [Ca2+]i); inhibition of N-type Ca2+ and M-type K+ channels (Filippov et al., 2003) |
| Distribution | Quantitative RT-PCR: high levels of mRNA in intestine, pituitary, and brain; low levels in liver and bone marrow (Moore et al., 2001); monocyte and lymphocytes (Jin et al., 1998b) |
| Tissue Function | Endothelial cell receptors mediate NO release and subsequent vasodilatation (Burnstock et al., 2002); mitogenic actions on vascular smooth muscle cells (Burnstock et al., 2002); regulation of epithelial chloride transport in jejunum (Robaye, 2003) |
| Phenotypes | Loss of nucleotide regulation of epithelial Cl− transport in the jejunum of null mice (Robaye et al., 2003) |
| Comments | When expressed in a mammalian cell line, this receptor was activated specifically by UTP > UDP but not by ATP and ADP (Communi et al., 1995; Nguyen et al., 1995); competitive; a recent study shows that ATP is a potent antagonist at the hP2Y4, whereas it is a full agonist at the rat P2Y4 (Herold et al 2004)*; rat P2Y4 is not selective for uridine nucleotides and instead shows an agonist potency order of ATP = UTP; ADP, ATPγS, 2-MeSATP, and UDP are partial agonists (Bogdanov et al., 1998b; Webb et al., 1998); both human and rat P2Y4 are suramin-insensitive (Charlton et al. 1996); UTP regulates ion transport in jejunum (Cressman et al., 1999)—the disappearance of the jejunal Cl− secretory response to UTP and ATP in P2Y4-null mice demonstrates the involvement of the P2Y4 receptor; P2Y4 receptors might be considered a potential pharmacotherapeutic target for cystic fibrosis (Robaye et al., 2003) |
aa, amino acids; AC, accession; chr., chromosome.
D. P2Y6
The mouse (Lazarowski et al., 2001b), rat (Chang et al., 1995; Nicholas et al., 1996), and human (Communi et al., 1996b) P2Y6 receptors are UDP receptors. The p2y3 receptor is the avian ortholog of the mammalian P2Y6 receptor and also displays selectivity for UDP (Webb et al., 1996a; Li et al., 1998). At the human P2Y6 receptor, the rank order of potency of various nucleotides is as follows: UDP > UTP > ADP > 2-MeSATP ≫ ATP (Communi et al., 1996b). Adenine dinucleotides have little effect on P2Y6, whereas diuridine triphosphate is a selective agonist of P2Y6 (Pendergast et al., 2001). No selective competitive antagonist is available. Some aryl diisothiocyanate derivatives behave as potent insurmountable antagonists and exhibit selectivity for P2Y6 compared with P2Y1, P2Y2, P2Y4, and P2Y11 receptors (Mamedova et al., 2004).
In the absence of reconstitution data, there is no hard molecular evidence of P2Y6 interaction with specific G proteins. The IP3 response to UDP of the recombinant P2Y6 receptor is insensitive to PTX inhibition, suggesting a coupling to Gq/11 (Chang et al., 1995; Robaye et al., 1997). A P2Y6-mediated increase in cAMP has been reported, but it is probably an indirect effect mediated by prostaglandins, since it was at least partially inhibited by indomethacin (Köttgen et al., 2003). A unique feature of the P2Y6 receptor compared with other P2Y subtypes is its slow desensitization and internalization (Robaye et al., 1997; Brinson and Harden, 2001). This can be explained by the short C-terminal sequence of P2Y6 that contains a single threonine and misses the Ser333 and Ser334 that play a key role in the UTP-dependent phosphorylation, desensitization, and internalization of P2Y4 (Brinson and Harden, 2001).
Northern blotting has revealed a rather wide tissue distribution of P2Y6 mRNA. In particular, the P2Y6 transcript has been found in human spleen, thymus, placenta, intestine, and blood leukocytes (Communi et al., 1996b) and in rat lung, spleen, stomach, intestine, and aorta (Chang et al., 1995). Consistent with those initial observations, the expression and potential role of the P2Y6 receptor has been documented in placenta (Somers et al., 1999), vascular smooth muscle, epithelia, and immune cells. P2Y6 message is present in smooth muscle cells cultured from the rat aorta (Chang et al., 1995; Hou et al., 2002). UDP acts as a growth factor for these cells (Hou et al., 2002). In P2X1-deficient mice, the vasoconstrictor effect of ATP on mesenteric arteries is abolished (Vial and Evans, 2002), but a contractile effect of UTP and UDP is maintained and probably involves the P2Y6 receptor, although the pharmacological profile of that response, characterized by the equipotency of UTP and UDP, is atypical. UDP and UDPβS induced the contraction of other vessels such as rat (Malmsjö et al., 2003a) and human (Malmsjö et al., 2003b) cerebral arteries. The expression of P2Y6 receptors on the basolateral side of rat colonic epithelial cells was demonstrated by immunochemistry and is involved in a sustained stimulatory effect of UDP on chloride secretion (Köttgen et al., 2003). In mouse gallbladder, apical UDP stimulates secretion of chloride, an effect that is maintained in P2Y2-null mice and is likely to involve the P2Y6 receptor (Cressman et al., 1999; Lazarowski et al., 2001b). Expression of the P2Y6 receptor has also been demonstrated by RT-PCR and immunochemistry in human nasal epithelial cells (Kim et al., 2004), in which apical UDP stimulates chloride secretion (Lazarowski et al., 1997c). In human monocytic THP-1 cells, UDP stimulates the production of IL-8 (Warny et al., 2001). Furthermore, endogenous UDP released from those cells contributes to the IL-8 secretion induced by lipopolysaccharide (LPS), which is indeed partially decreased by P2Y6 antisense oligonucleotides. UDP also acts on murine and human dendritic cells, where it increases cytosolic calcium, induces chemotaxis, and stimulates the release of chemokine CXCL8 (Marriott et al., 1999; Idzko et al., 2004). Northern blots revealed the expression of P2Y6 mRNA in activated but not resting human CD4+ and CD8+ T cells (Somers et al., 1998). This expression was associated with a UDP-induced rise of [Ca2+]i in the activated T cells only. In situ hybridization showed the presence of P2Y6 mRNA in T cells infiltrating the lesions of patients with inflammatory bowel disease (IBD).
For more information on the P2Y6 receptor, see Table 8.
TABLE 8. P2Y6 receptor (previously known as P2Y purinoceptor 6, pyrimidinoceptor, and uridine nucleotide receptor).
2.1:NUCT:4:P2Y6
| TM | aa | AC | Chr. Map | References | |
|---|---|---|---|---|---|
| Human* | 7 | 328 | NM_176797; NM_176798; NM_176796; NM_004154 | 11q13.5 (Somers et al., 1997) | Communi et al. (1996b) |
| Rat | 7 | 328 | NM_057124 | 1Q32 | Chang et al. (1995) |
| Mouse | 7 | 328 | AF298899 | N.A. | Lazarowski et al. (2001b) |
| Functional assays | Formation of IP3 in 1321N1 cells that stably express recombinant receptor (Communi et al., 1996); rat C6–2B glioma cells that natively express the receptor (Nicholas et al., 1996) | |||
| Ligand | Action | Selectivity | Endogenous | References |
|
| ||||
| UDP | Agonist | No | Yes | Communi et al. (1996b) |
| UDPβS | Agonist | Yes | No | Jacobson et al. (2002) |
| 5BrUTP | Weak agonist | No | No | Communi et al. (1996b) |
| UTP | Weak agonist | No | Yes | Communi et al. (1996b) |
| ADP | Weak agonist | No | Yes | Communi et al. (1996b) |
| 2-MeSATP | Weak agonist | No | No | Communi et al. (1996b) |
| PPADS | Antagonist | No | No | Robaye et al. (1997) |
| Reactive blue 2 | Antagonist | No | No | Robaye et al. (1997) |
| MRS2567 | Antagonist | Yes | No | Mamedova et al. (2004) |
| MRS2578 | Antagonist | Yes | No | Mamedova et al. (2004) |
| MRS2575 | Antagonist | Yes | No | Mamedova et al. (2004) |
| Agonist potencies | IP3 formation: UDP (EC50 = 300 nM) > 5BrUTP (EC50 = 800 nM) > UTP (EC50 = 6 μM) > ADP (EC50 = 30uM) ≫ 2-MeSATP (EC50 = 100 μM) (Communi et al., 1996b) |
| Antagonist potencies | Reactive blue 2 > PPADS > suramin (Robaye et al., 1997) |
| Radioligand assays | None |
| Radioligands | None |
| Transduction mechanism | Gq/G11; PI hydrolysis (PLCβ activation) and elevated [Ca2+]i in expression systems |
| Distribution | Northern blot: placenta, spleen, thymus, intestine (Communi et al., 1996b); vascular smooth muscle, lung (Ralevic and Burnstock, 1998); RT-PCR: human bone, two osteoblastic cell lines, brain-derived cell lines (Burnstock and Knight, 2004); quantitative RT-PCR: spleen, placenta, kidney (high levels), lung, intestine, adipose, bone, heart (moderate expression), and some brain regions (Moore et al., 2001) |
| Tissue function | Regulation of chemokine production and release in monocytes (Warny et al., 2001); NaCl secretion in colonic epithelial cells (Burnstock and Knight, 2004); role in proliferation of lung epithelial tumor cells (Schafer et al., 2003); role in mediating contractions of human cerebral arteries (Malmsjö et al., 2003b); interaction with the TNF-α-related signals to prevent apoptotic cell death (Kim et al., 2003b) |
| Phenotypes | None |
| Comments | Four transcript variants that encode the same isoform have been identified for this gene*; a pseudogene was also described (Burnstock and Knight, 2004); rat and mouse homologs have also been cloned (Chang et al., 1995; Lazarowski et al., 2001b); rat, mouse, and human P2Y6 receptors are UDP-selective, and UTP is less potent, whereas ATP is a very weak partial agonist; the P2Y6 receptor has a pharmacological profile very similar to the UDP receptor on rat C6–2B glioma cells (Lazarowski et al., 1994b); rat P2Y6 potently inhibited N-type Ca channels when expressed in sympathetic neurons, and receptor transcripts were reported to be present in brain tissues (Filippov et al., 1999) |
aa, amino acids; AC, accession; chr., chromosome.
E. P2Y11
Among P2Y receptors, the human P2Y11 has a unique profile: 1) it is the only P2Y receptor gene that contains an intron in the coding sequence; 2) the potency of its natural agonist ATP is relatively low; and 3) it is dually coupled to PLC and adenylyl cyclase stimulation. There does not seem to be a rodent ortholog of P2Y11, confirming that this receptor subtype is quite different from the other members of the family. At the recombinant human P2Y11 receptor, the rank order of potency with which nucleotides increase either cAMP or IP3 is ARC67085MX ≥ ATPγS ≈ BzATP > dATP > ATP > ADP (Communi et al., 1997, 1999b; Qi et al., 2001a). UTP, GTP, CTP, TTP, ITP, and dinucleotides (Ap4A, Ap5A, and Ap6A) were completely inactive (although UTP has been claimed to be an agonist in some studies; see below and White et al., 2003). The EC50 of ATP is in the 5 to 100 μM range, whereas in the same expression systems (i.e., 1321N1 or CHO cells), the EC50 characterizing the activation of the other P2Y subtypes by their respective ligands is in the 10 to 500 nM range. Suramin behaves as a competitive antagonist of the human (h) P2Y11 receptor with a Ki close to 1 μM (Communi et al., 1999b). The dog P2Y11 receptor, the sequence of which shares only 70% amino acid identity with hP2Y11, has a completely different profile characterized by a greater potency of diphosphates compared with triphosphates (Zambon et al., 2001). Indeed, the rank order of agonist potency is as follows: 2-MeSADP > ADPβS > ADP > ATP. A mutational analysis revealed that the change of Arg-265, located at the junction between TM6 and the third extracellular loop in the hP2Y11, to Gln in cP2Y11 is at least partially responsible for the diphosphate selectivity of the canine receptor (Qi et al., 2001b). Although the existence of a functional P2Y11 receptor in Xenopus embryos was recently reported (C. Drew, unpublished data), an ortholog gene could not be detected in the murine genome, and there is no evidence of a functional P2Y11 receptor in rat or mouse.
The hP2Y11 gene differs from other P2Y genes by the presence in the coding sequence of a 1.9-kb intron that separates an exon encoding the first six amino acid residues from a second exon encoding the remaining part of the protein (Communi et al., 2001b). The hP2Y11 gene is adjacent to the gene encoding the human ortholog of Ssf1, a nuclear protein playing an important role in Saccharomyces cerevisiae mating. Chimeric mRNA resulting from the cotranscription and intergenic splicing of the two genes is ubiquitously present in human organs. However, the fusion protein could only be detected after recombinant overexpression. Only half a dozen cases of intergenic splicing have been described in mammalian cells, and they are likely to represent an evolutionary tool to create new function via fusion of preexisting protein domains (Finta et al., 2002).
Activation of recombinant hP2Y11 or cP2Y11 receptors leads to an increase of both cAMP and IP3, presumably via the dual activation of Gs and Gq/11 (Communi et al., 1997, 1999b). Indeed, the use of various pharmacological tools (inhibition of PLC or prostaglandin synthesis, chelation of intracellular calcium, and down-regulation of PKC) has demonstrated that the cAMP increase is not merely an indirect consequence of rises in IP3, [Ca2+]i, and PKC activity (Suh et al., 2000; Qi et al., 2001a). However, PKC activation plays some role and amplifies the stimulation of adenylyl cyclase. In addition, it has been claimed that UTP acts via the hP2Y11 receptor to induce an IP3-independent Ca2+ mobilization that is sensitive to PTX inhibition, whereas the effect of ATP is not (White et al., 2003). This finding illustrates the fact that different agonists can recruit distinct signaling pathways via the same receptor.
Northern blotting has revealed a significant expression of P2Y11 mRNA in human spleen and to a lesser extent intestine and liver (Communi et al., 1997, 2001b). According to a quantitative RT-PCR study, P2Y11 message is most abundant in human brain and pituitary (Moore et al., 2001). It is also present in B lymphocytes from patients with chronic lymphocytic leukemia (Conigrave et al., 2001) as well as in human HL-60 (Communi et al., 1997, 2000) and NB4 (van der Weyden et al., 2000b) promyelocytic leukemia cells. In the HL-60 cell line, P2Y11 message is up-regulated by all the agents that induce granulocytic differentiation, such as retinoic acid and granulocyte colony-stimulating factor (Communi et al., 2000). ATP has been shown to induce the differentiation of HL-60 cells into neutrophil-like cells (Jiang et al., 1997a). This action was associated with a rise in cAMP and the rank order potency of various nucleotides is consistent with that of recombinant P2Y11 (Conigrave et al., 1998). P2Y11 mRNA is also expressed in human monocyte-derived dendritic cells, in which ATP induces a semimaturation state characterized by increased surface expression of costimulatory molecules, inhibition of the production of proinflammatory cytokines such as IL-12, stimulation of IL-10 production, and modification of the repertoire of chemokine and chemokine receptor expression, resulting in a change of migratory behavior (Wilkin et al., 2001, 2002; La Sala et al., 2002; Schnurr et al., 2003). Semimature dendritic cells can orient CD4+ T lymphocytes toward a Th2 rather than a Th1 response or induce tolerance.
For more information on the P2Y11 receptor, see Table 9.
TABLE 9. P2Y11 receptor (previously known as P2Y).
2.1:NUCT:5:P2Y11
| TM | aa | AC | Chr. Map | References | |
|---|---|---|---|---|---|
| Human | 7 | 374 | NM _002566* | 19p13.2 | Communi et al. (1997) |
| Functional assays | cAMP measurement in CHO-K1 cells stably expressing the P2Y11 receptor; IP3 measurement in 1321N1 astrocytoma cells stably expressing the P2Y11 receptor (Communi et al., 1997) |
| Ligand | Action | Selectivity | Endogenous | References |
|
| ||||
| ATP | Agonist | No | Yes | Communi et al. (1999b) |
| ADP | Agonist | No | Yes | Communi et al. (1999b) |
| ATPγS | Agonist | No | No | Communi et al. (1999b) |
| BzATP | Agonist | No | No | Communi et al. (1999b) |
| dATP | Agonist | No | No | Communi et al. (1999b) |
| ADPβS | Agonist | No | No | Communi et al. (1999b) |
| 2-MeSATP | Agonist | No | No | Communi et al. (1999b) |
| AR-C67085 | Agonist | No | No | Communi et al. (1999b) |
| ADPγS | Partial agonist | No | No | Communi et al. (1999b) |
| AMPαS | Partial agonist | No | No | Communi et al. (1999b) |
| A3P5PS | Partial agonist | No | No | Communi et al. (1999b) |
| Suramin | Antagonist | No | No | Communi et al. (1999b) |
| Reactive blue 2 | Antagonist | No | No | Communi et al. (1999b) |
| UTP | Agonist | No | Yes | White et al. (2003) |
| Agonist potencies** | cAMP: ATPγS (EC50 3.4 ± 0.3 μM) > BzATP (EC50 7.2 ± 0.5 μM) > dATP (EC50 8.9 ± 0.6 μM) > ATP (EC50 17.4 ± 6.1 μM) > ADPβS (EC50 29.7 ± 2.7 μM) > 2-MeSATP (EC50 50 ± 4 μM) (Communi et al., 1999b); IP3: BzATP (EC50 10.5 ± 0.3 μM) > ATPγS (EC50 13.5 ± 2.7 μM) > dATP (EC50 16.3 ± 0.7 μM) > ATP (EC50 65 ± 12 μM) > ADPβS (EC50 174 ± 28 μM) > 2-MeSATP (EC50 210 ± 6 μM) (Communi et al., 1999b) |
| Antagonist potencies | PI hydrolysis: suramin (IC50 1 μM), reactive blue 2 (IC50 9 μM); cAMP: suramin (IC50 16 μM) (Communi et al., 1999b) |
| Radioligand assays | None |
| Radioligands | None |
| Transduction mechanism | Gq/G11 and Gs (see “Comments”); PI hydrolysis (PLCβ activation) and elevated [Ca2+]i in expression systems; adenylate cyclase stimulation and elevated cAMP levels (Qi et al., 2001a) |
| Distribution | Brain, spleen, lymphocytes, intestine; all other tissues expressed more moderate levels of P2Y11 mRNA, with lowest levels detected in liver, cartilage, and bone (Moore et al., 2001) |
| Tissue function | Role in maturation, migration of dendritic cells (Wilkin et al., 2001; Schnurr et al., 2003), and in granulocytic differentiation (Communi et al., 2000); secretory role in pancreatic duct epithelial cells (Nguyen et al., 2001) |
| Phenotypes | None |
| Comments | In P2Y11 gene, an intron was found (Communi et al., 1997)*; P2Y11 couples to Gq and Gs, and the pharmacological profile of the GPCR is slightly different for each transduction pathway (PLCβ/IP3 and adenylate cyclase/cAMP) (Communi et al., 1999b; Qi et al., 2001a); the receptor does not activate PLA2 or PLD (Communi et al., 1999b); the P2Y12 antagonist ARC67085MX is an agonist here; a chimeric mRNA due to intergenic splicing between the P2Y11 and Ssf1 genes has been found in many mammalian cells— the function of this fusion protein is unknown (Communi et al., 2001); a canine (Qi et al., 2001a; Zambon et al., 2001) but no mouse and rat orthologs have been cloned; agonist potencies are species-dependent (Qi et al., 2001a; Zambon et al., 2001)** |
aa, amino acids; AC, accession; chr., chromosome.
F. P2Y12
The human (Hollopeter et al., 2001; Savi et al., 2001; Zhang et al., 2001), rat (Hollopeter et al., 2001), and mouse (Foster et al., 2001) P2Y12 receptors have been identified and characterized. ADP is the natural agonist of this receptor, whereas conflicting results were reported concerning the effects of ATP and its triphosphate analogs. For diphosphates, the rank order of agonist potency in all cases reported is 2-MeSADP ≫ ADP > ADPβS. Concerning ATP and its analogs, they were found to be agonists either in native P2Y12-expressing cells (Simon et al., 2001; Unterberger et al., 2002) or in some heterologously transfected cells (Takasaki et al., 2001; Zhang et al., 2001; Simon et al., 2002). However, in platelets there have been reports over a long period that ATP and a wide range of its triphosphate analogs behave as antagonists of ADP-induced adenylyl cyclase inhibition (reviewed by Gachet, 2001). This has recently been confirmed: ATP and its triphosphate analogs are antagonists of the P2Y12 receptor both in human and mouse platelets, provided care is taken to remove contaminants and to prevent enzymatic production of ADP or 2-MeSADP (Kauffenstein et al., 2004). There are currently, therefore, two alternative interpretations extant of the action of adenosine triphosphates on the P2Y12 receptor (either native or recombinant). One (Kauffenstein et al., 2004) is that, both in the platelet and in nucleated (nonplatelet) cells, these agents are antagonists: an apparent agonist action of triphosphates would then be due in all cases to a secondary introduction of diphosphate, as just noted. The second (Barnard and Simon, 2001; Simon et al., 2002) is that the triphosphates are intrinsically agonistic at the P2Y12 receptor generally, but that they behave as apparent antagonists in the platelet, due to the much lower receptor density there (or possibly to an absence in the platelet of some modifying component involved in nucleated cells). The latter interpretation has been made also (see section VI.A.) for a parallel difference in adenosine triphosphate behavior of the P2Y1 receptor in the platelet and in most other cell types tested.
Behavior of ATP and ATPγS rather similar to that reported for these agents at P2Y12 in the platelet can be obtained in a system reconstituted with purified P2Y12 receptor protein (see section III.A.), in which enzymatic degradation or interconversion of the nucleotides is not possible (Bodor et al., 2003). There, also, one of the same explanations just given for the platelet case could hold, e.g., the effective density of functional P2Y12 receptors in the vesicle surface may be too low for those agents to show intrinsic agonism. The general dichotomy discussed here need not apply to all adenosine triphosphates: ATPγS may behave at P2Y12 receptors in general as an antagonist, that action being shown clearly in transfected 1321 N1 cells as well as in the platelet (Kauffenstein et al., 2004) (ATPγS has not yet been tested with full precautions elsewhere).
A clear agonist action of adenosine triphosphates of P2Y12 receptors other than in platelets was measured for adenylate cyclase inhibition in the cases noted above (Simon et al., 2001, 2002; Unterberger et al., 2002) with all of the precautions used by Kauffenstein et al. (2004) and with a demonstration of negligible loss of ATP occurring during the assay. Furthermore, in the direct transduction by the P2Y12 receptor in neurons through the N-type Ca2+ channel (see section III.B.3.), in which a fast application of pure 2-MeSATP is made with constant perfusion flow, in contact with only a low cell density and with an assay time of 50 ms, 2-MeSATP (EC50 0.042 nM) is a definite agonist, 2.5 times more potent than 2-MeSADP (Simon et al., 2002). Clearly this effect cannot be due to an artifactual introduction of the diphosphate. In conclusion, the situation concerning the agonist selectivity of the P2Y12 receptor is controversial, may vary with the cell type, and is at present left open here.
The P2Y12 receptor is mostly expressed in the megakaryocyte/platelet lineage in which it is the molecular target of the active metabolite of the antiplatelet drug clopidogrel (Savi and Herbert, 2005; see also below). This metabolite covalently binds cysteine residues of the extracellular loops resulting in inhibition of ligand binding (Savi et al., 2001). Ticlopidine and clopidogrel are efficient antithrombotic drugs of the thienopyridine family of compounds. A third antithrombotic thienopyridine, CS-747 or prasugrel, is currently under clinical evaluation (Niitsu et al., 2005). Potent direct competitive P2Y12 antagonists also exist, including the ARC69931MX compound named cangrelor as well as other AR-C compounds, which are all ATP analogs (Ingall et al., 1999). Of these, AZD6140 is a nonphosphorylated and orally active compound currently under clinical evaluation (Peters and Robbie, 2004).
In addition to the platelet lineage, the P2Y12 receptor has also been shown to be expressed in subregions of the brain (Hollopeter et al., 2001), but its function there is not yet known. Glial cells (Fumagalli et al., 2003; Sasaki et al., 2003; Bianco et al., 2005), brain capillary endothelial cells (Simon et al., 2001), smooth muscle cells (Wihlborg et al., 2004), and chromaffin cells (Ennion et al., 2004) express P2Y12 receptors. The precise role in these locations is still under study.
The platelet P2Y12 receptor is coupled to Gαi2 (see also section VII.J.), as was first been shown by photolabeling with radiolabeled GTP (Ohlmann et al., 1995) and confirmed in Gαi2-deficient mouse platelets (Jantzen et al., 2001) as well as in reconstituted systems (Bodor et al., 2003). In the latter case, Gαi2 was found to be the preferred Gα subunit, whereas Gαi1 and Gαi3 were poorly effective. No coupling with Gz was observed.
P2Y12 knockout mice have been generated (Foster et al., 2001; André et al., 2003; see also section VII.J.), which display the phenotype of clopidogrel-treated animals, i.e., prolonged bleeding time, inhibition of platelet aggregation to ADP, and resistance to arterial thrombosis in various models (Conley and Delaney, 2003). In humans, molecular defects of this receptor exist, which result in hemorrhagic syndromes. Four families of patients have been described so far with essentially the same phenotype. Among these, three have a defect in receptor expression (Cattaneo et al., 1992, 1997; Nurden et al., 1995), whereas in one family, a mutant form of the receptor is expressed with defective function (for a recent review, see Cattaneo, 2005; Cattaneo et al., 2003).
For more information on the P2Y12 receptor, see Table 10.
TABLE 10. P2Y12 receptor [previously known as SP1999, P2T(AC), and P2Y(AC)].
2.1:NUCT:6:P2Y12
| TM | aa | AC | Chr. Map | References | |
|---|---|---|---|---|---|
| Human | 7 | 342 | NM_022788*; NM_176876 | 3q24–25 | Hollopeter et al. (2001) |
| Rat | 7 | 347 | NM_022800 | 2q31 | Hollopeter et al. (2001) |
| Mouse | 7 | 343 | NM_027571 | 2 |
| Functional assays | cAMP measurement in CHO cells and CHO cells stably transfected with hP2Y12 (Hollopeter et al., 2001; Zhang et al., 2001); FLIPR assay in CHO-dihydrofolate reductase-deficient transiently or stably transfected with hP2Y12 (Zhang et al., 2001); cAMP measurement in platelets (Cattaneo et al., 2003) |
| Ligand | Action | Selectivity | Endogenous | References |
|
| ||||
| ATP** | Partial agonist | No | Yes | Waldo and Harden (2004) |
| 2-MeSADP | Agonist | No | No | Zhang et al. (2001) |
| 2-MeSATP | Agonist | No | No | Zhang et al. (2001) |
| ADP | Agonist | No | Yes | Zhang et al. (2001) |
| ADPβS | Agonist | No | No | Zhang et al., (2001) |
| ATPγS | Agonist | No | No | Zhang et al. (2001) |
| 2ClATP | Agonist | No | No | Zhang et al. (2001) |
| Reactive blue 2 | Antagonist | No | No | Zhang et al. (2001) |
| Suramin | Antagonist | No | No | Zhang et al. (2001) |
| Ticlopidine | Antagonist | No | No | Savi and Herbert (2005) |
| Clopidogrel | Antagonist | No | No | Savi and Herbert (2005) |
| CS-747 | Antagonist | No | No | Savi and Herbert (2005) |
| AR-C66096MX | Antagonist | No | No | Gachet (2005) |
| AR-M69931MX | Antagonist | No | No | Gachet (2005) |
| AR-C67085MX | Antagonist | No | No | Gachet (2005) |
| Agonist potencies | cAMP assays: 2-MeSATP (EC50 3.4 ± 0.5 nM) = 2-MeSADP (EC50 14.1 ± 2 nM) > ADP (EC50 60.7 ± 10 nM) > ATPγS (EC50 110 ± 100 nM) > ADPβS (EC50 191 ± 20 nM) > 2ClATP (EC50 636 ± 100 nM) (Zhang et al., 2001); FLIPR assay: 2-MeSADP (EC50 2.3 ± 0.3 nM) = 2-MeSATP (EC50 3.4 ± 0.3 nM) > ADP (EC50 74 ± 10 nM) = ADPβS (EC50 89 ± 15 nM) > 2ClATP (EC50 470 ± 100 nM) > ATPγS (EC50 1200 ± 200 nM) (Zhang et al., 2001) |
| Antagonist potencies | Reactive blue 2 (Ki = 1.3 μM), suramin (Ki = 3.6 μM); AR-C66096, AR-C69931MX, tioclopidine, clopidogrel [prodrug that produces a metabolite that irreversibly binds to P2Y12 (Savi et al., 2000; Gachet et al., 2001, 2005)]; no effect of PPADS |
| Radioligand assays | Binding of [33P]2-MeSADP (Cattaneo et al., 1997) |
| Radioligands | [33P]2-MeSADP (Cattaneo et al., 1997) |
| Transduction mechanism | Gαi, inhibition of adenylate cyclase |
| Distribution | Brain (in particular glial cells), spinal cord, platelets (Zhang et al., 2001; Sasaki et al., 2003) |
| Tissue function | Role in dense granule secretion (Daniel et al., 1998), fibrinogen-receptor activation, and thrombus formation (Gachet, 2005); role in the recruitment of the platelets to the site of injury and in the enhancement of the efficiency of platelet activation by other agonists, such as thrombin and thromboxane A2 (Gachet, 2005); this receptor is defective in several patients with blood coagulation disorders (Cattaneo et al., 2000, 2003; Hollopeter et al., 2001) |
| Phenotypes | P2Y12-null mice*** |
| Comments | Two transcript variants that encode the same isoform have been identified for this gene*; ATP is a partial agonist**; when P2Y12 expression is low, as is seen in platelets, it acts as an antagonist, whereas at high expression levels, as seen with recombinant receptors, it is an agonist (Waldo and Harden, 2004); radiation hybrid analysis mapped the P2Y12 gene on 3q24–3q25 (Hollopeter et al., 2001), a region that also includes the P2Y1, P2Y13, and P2Y14 receptors; in a patient with congenital bleeding, Cattaneo et al. (2003) found compound heterozygosity for two mutations in the P2Y12 gene: neither mutation interfered with receptor surface expression but both altered function—in particular, these patients generally have normal platelet shape change responses to ADP but have impaired abilities to inhibit adenylate cyclase activity (Cattaneo et al., 1992); P2Y12 knockout mice seem normal, but they exhibit highly prolonged bleeding times, and their platelets aggregate poorly in responses to ADP and display a reduced sensitivity to thrombin and collagen (André et al., 2003)***; clinical studies using clopidogrel demonstrate a significantly reduced risk of peripheral artery disease, myocardial infarction, ischemic stroke, or vascular death compared with aspirin therapy; phase II studies of intravenous AR-C69931MX in patients with acute coronary syndromes show that this agent has a rapid onset of action, rapidly achieving steady-state inhibition of platelet aggregation with a half-life of only a few minutes (Gachet, 2005) |
aa, amino acids; AC, accession; chr., chromosome; FLIPR, fluorometric imaging plate reader.
G. P2Y13
The human (Communi et al., 2001a; Zhang et al., 2002a), mouse (Zhang et al., 2002a), and rat (Fumagalli et al., 2004) P2Y13 receptors have been identified and characterized (see also Communi et al., 2005a). ADP and Ap3A are naturally occurring agonists of the P2Y13 receptor. IDP is also a potent agonist of the murine P2Y13 receptor, but is 10-fold less potent than ADP on the human one (Zhang et al., 2002a). Ap4A, Ap5A, and Ap6A are inactive. When contaminating ADP was enzymatically removed and testing was performed over a short period, ATP behaved as a weak partial agonist (Marteau et al., 2003). As described for the P2Y1 receptor, the activity of ATP may vary according to the level of expression of the P2Y13 receptor in different recombinant systems. The relative potencies of ADP and 2-MeSADP differed according to assays used. 2-MeSADP was more potent than ADP in competing with [33P]2-MeSADP on intact 1321N1 cells expressing hP2Y13 and in stimulating binding of GTPγ[35S] to membranes of the same cells, whereas ADP was more potent than 2-MeSADP on the rat P2Y13 (Fumagalli et al., 2004). In CHO-K1 cells expressing hP2Y13, ADP and 2-MeSADP produced an equipotent inhibition of cAMP accumulation. These discrepancies suggest that the P2Y13 receptor might exist in multiple active conformations characterized by differences in affinity for 2-MeSADP versus ADP, kinetics, and preference for G proteins. The antiplatelet and antithrombotic action of clopidogrel is mediated by an active metabolite. That metabolite has been shown to inhibit the binding of [33P]2-MeSADP to hP2Y12 with an IC50 of 100 nM (Savi et al., 2001), but it had no effect on hP2Y13 up to 2 μM (Marteau et al., 2003). Cangrelor (AR-C69931MX) is currently in development as an antiplatelet and antithrombotic agent. It is an ATP derivative that inhibits platelet aggregation by ADP at nanomolar concentrations (Ingall et al., 1999). It was previously believed to be a selective antagonist of the hP2Y12 receptor (IC50 = 2.4 nM) (Takasaki et al., 2001), but in the same range of nanomolar concentrations, it is also an antagonist of human and rat P2Y13 receptors (Marteau et al., 2003; Fumagalli et al., 2004). Two other P2Y12 antagonists, Ap4A and 2-MeSAMP, are also antagonists of the P2Y13 receptor (Marteau et al., 2003).
The effects of ADP mediated by the recombinant P2Y13 receptor were all inhibited by PTX: increased binding of GTPγ[35S], inhibition of cAMP formation, ERK1/2 phosphorylation, and accumulation of IP3 in cells coexpressing Gα16 (Communi et al., 2001a; Marteau et al., 2003). This finding suggests that the P2Y13 receptor is primarily coupled to a Gi/o protein. The only exception was the increased cAMP formation observed at high ADP concentrations, and that presumably results from promiscuous coupling to Gs, a phenomenon observed with other recombinant Gi/o-coupled receptors, such as the α2-adrenergic receptor (Communi et al., 2001a). More direct evidence for the coupling to Gi/o derives from the measurement of [Ca2+]i increases in HEK cells coexpressing various chimeric G proteins (Zhang et al., 2002a). A significant stimulation by ADP was obtained in cells expressing either Gαq/i or Gαq/i3, that is, Gαq in which the five C-terminal residues have been replaced by the corresponding sequence in either Gαi1/2 or Gαi3, respectively.
P2Y13 mRNA was amplified by RT-PCR in several human organs. Signals were the most intense in spleen and brain (Communi et al., 2001a). In dot blot analysis, the spleen gave the most intense positive signal, followed by placenta, liver, bone marrow, lung, and various brain regions (Zhang et al., 2002a). Quantitative RT-PCR revealed a significant expression in human monocytes, T cells, and dendritic cells derived from blood monocytes or bone marrow, but not in human platelets (Zhang et al., 2002a; Wang et al., 2004a). Northern blots were positive for murine spleen, brain, liver, and heart. In the rat, again the RT-PCR signal was the most intense in the spleen (Fumagalli et al., 2004).
P2Y13-null mice have been generated recently (A. Ben Addi and B. Robaye, unpublished data). No phenotype has been characterized so far.
For more information on the P2Y13 receptor, see Table 11.
TABLE 11. P2Y13 receptor (previously known as GPR86, GPR94, and SP174).
2.1:NUCT:7:P2Y13
| TM | aa | AC | Chr. Map | References | |
|---|---|---|---|---|---|
| Human | 7 | 333 | NM_176894 | 3q24 | Communi et al. (2001a) |
| Rat | 7 | 336 | AY639875 | 2 | Fumagalli et al. (2004) |
| Mouse | 7 | 337 | NM_028808 | 2 | Zhang et al. (2002a) |
| Functional Assays | Inositol phosphate measurement in 1321N1 astrocytoma cells that express recombinant receptor (Communi et al., 2001a); cAMP assays on stably transfected CHO-K1 cells (Communi et al., 2001a); binding of [33P]2-MeSADP in 1321N1 astrocytoma cells that express recombinant receptor (Marteau et al., 2003); assays of GTPγ[35S] binding to membranes of 1321N1 that express recombinant receptor (Marteau et al., 2003; Fumagalli et al., 2004) |
| Ligand | Action | Selectivity | Endogenous | References |
|
| ||||
| 2-MeSADP | Agonist | No | No | Zhang et al. (2002a); Marteau et al. (2003) |
| ADPβS | Agonist | No | No | Zhang et al. (2002a); Marteau et al. (2003) |
| 2-MeSATP | Agonist | No | No | Zhang et al. (2002a); Marteau et al. (2003) |
| ADP | Agonist | No | Yes | Zhang et al. (2002a); Marteau et al. (2003) |
| Ap3A | Agonist | No | No | Zhang et al. (2002a); Marteau et al. (2003) |
| ATP | Agonist | No | Yes | Zhang et al. (2002a); Marteau et al. (2003) |
| IDP | Agonist | No | No | Zhang et al. (2002a); Marteau et al. (2003) |
| PPADS | Antagonist | No | No | Marteau et al. (2003) |
| Suramin | Antagonist | No | No | Marteau et al. (2003) |
| AR-C67085MX | Antagonist | No | No | Marteau et al. (2003) |
| AR-C69931MX | Antagonist | No | No | Marteau et al. (2003) |
| Agonist potencies | Fluorimetric imaging assay: 2-MeSADP (EC50 19 ± 5 nM) = ADPβS (EC50 31 ± 15 nM) = 2-MeSATP (EC50 32 ± 8 nM) > ADP (EC50 60 ± 26 nM) > Ap3A (EC50 72 ± 9 nM) > ATP (EC50 261 ± 48 nM) > IDP (EC50 552 ± 64 nM) (Zhang et al., 2002a) |
| Antagonist potencies | AR-C69931MX > PPADS > suramin; MRS2179 inactive (Marteau et al., 2003) |
| Radioligand assays | Binding of [33P]2-MeSADP (Marteau et al., 2003) |
| Radioligands | [33P]2-MeSADP (Marteau et al., 2003) |
| Transduction mechanism | Gi and G16 (Communi et al., 2001) |
| Distribution | Spleen, brain regions, liver, pancreas, bone marrow, heart, peripheral leukocyte (Communi et al., 2001a; Zhang et al., 2002a) |
| Tissue function | No precise evidence but presence in both immune system and brain implies roles |
| Phenotypes | None |
| Comments | This receptor clusters in the same region of the human chromosome 3q24–3q25, where some of the cloned P2Y receptors (P2Y1, P2Y12, and P2Y14) and several orphan GPCRs are also present (GPR87, GPR91, and H963) (Abbracchio et al., 2003); activated by diphosphate adenine nucleotides and not by uridine nucleotides; cloning and pharmacological characterization of the P2Y13 receptor from rat tissues has been recently described: by using the [35S]GTPγS binding assay in 1321N1 cells that express the rat receptor, ADP was found to be more potent than 2-MeSADP (Fumagalli et al., 2004) |
aa, amino acids; AC, accession; chr., chromosome.
H. P2Y14
From a phylogenetic and structural point of view, the P2Y14 receptor (previously known as GPR105 or UDPglucose receptor) lies with the P2Y12 and P2Y13 receptors in the second main branch of the P2Y receptor family and is 47% identical to these receptors. The gene for this receptor has been found in human chromosome 3q24–3q25 where a cluster of other related GPCRs, consisting of P2Y1-P2Y12, and P2Y13 receptors and the orphan receptors GPR87, GPR91, and H963 have been found (Abbracchio et al., 2003).
The P2Y14 receptor is activated by UDP-glucose as well as UDP-galactose, UDP-glucuronic acid, and UDP-N-acetylglucosamine but not by uridine or adenine nucleotides (Chambers et al., 2000; Harden, 2004). Of these endogenous ligands, to date only UDP-glucose has been shown to be released extracellularly by a variety of cell lines (Lazarowski et al., 2003b). At present, no selective antagonists are available, although it has to be underlined that the currently available P2 receptor antagonists have not been tested on this receptor. No radioligand binding assay is available for quantification of P2Y14 receptor binding sites.
The complete sequences of the rat (VTR-15-20; Charlton et al., 1997; Freeman et al., 2001) and mouse orthologs (Freeman et al., 2001) have also been described. The rat and mouse orthologs show 80 and 83% amino acid identity, respectively, with human P2Y14 and show similar agonist pharmacology (Freeman et al., 2001).
The P2Y14 receptor couples to the Gi/o family of G proteins. In particular, data obtained on the recombinant receptor in HEK-293 cells show coupling to Gα subunits of the Gi/o family (Gα16, Gαqo5, and Gαqi5), but not to Gs family or to endogenous Gq/11 proteins (Moore et al., 2003). Stimulation of native P2Y14 receptors in primary rat cortical astrocytes as well as in murine N9 and rat primary microglial cells results in transient intracellular calcium increases (Fumagalli et al., 2003; Bianco et al., 2005), although the mechanisms at the basis of this transductional effect are not known yet.
P2Y14 mRNA is widely distributed in the human body, with moderate to high levels observed in placenta, adipose tissue, stomach, intestine, selected brain regions (e.g., corpus striatum, cerebellum, caudate nucleus, hippocampus, and hypothalamus), spleen, lung, heart, bone marrow, and thymus. RT-PCR also revealed expression in brain glial cells and prominent expression in neutrophils, lymphocytes and megakaryocytic cells (Chambers et al., 2000; Moore et al., 2003). P2Y14 receptor mRNA was high in immature monocyte-derived and low in mamature monocyte-derived dendritic cells, suggesting a role for the receptor and its agonists in dendritic cell activation (Skelton et al., 2003).
Antiserum to a sequence in the first extracellular domain of P2Y14 was used to isolate a population of hematopoietic cells restricted to bone marrow. Conditioned media from bone marrow stroma induce receptor activation and chemotaxis, suggesting a role for P2Y14 as a chemoattractant receptor (Lee et al., 2003a). Antibody against the carboxyl terminus of the P2Y14 receptor was used to demonstrate broad distribution in postmortem human brain, with glial cells as the primary site of expression, suggesting a role for this receptor in neuroimmune function (Moore et al., 2003). In the rat, P2Y14, which is abundantly expressed in brain, has been reported to be regulated by immunological challenge (Charlton et al., 1997; Moore et al., 2003), suggesting that this receptor may link the humoral and nervous system responses to infection and inflammation. Consistent with this result, exposure of murine N9 cells to LPS resulted in a highly significant increase of receptor function, as suggested by the increase in the percentage of cells responding to UDP-glucose with intracellular calcium transients (Bianco et al., 2005).
For more information on the P2Y14 receptor, see Table 12.
TABLE 12. P2Y14 receptor (previously known as GPR105, KIAA0001, and UDP-glucose receptor).
2.1:NUCT:8:P2Y14
| TM | aa | AC | Chr. Map | References | |
|---|---|---|---|---|---|
| Human | 7 | 338 | NM_014879 | 3q24–25 | Chambers et al. (2000); Abbracchio et al. (2003) |
| Rat | 7 | 338 | NM_133577 | 2q31 | Charlton et al. (1997) |
| Mouse | 7 | 305 | NM_133200 | 2 |
| Functional assays | Intracellular calcium assays in HEK 293 cells that express recombinant receptor (Chambers et al., 2000) |
| Ligand | Action | Selectivity | Endogenous | References |
|
| ||||
| UDP-glucose | Agonist | Yes | Yes | Chambers et al. (2000) |
| UDP-galactose | Agonist | Yes | Yes | Chambers et al. (2000) |
| UDP-glucuronic acid | Agonist | Yes | No | Chambers et al. (2000) |
| UDP-N-acetylglucosamine | Agonist | Yes | Yes | Chambers et al. (2000) |
| Agonist potencies | UDP-glucose (EC50 80 ± 31 nM) > UDP-galactose (EC50 124 ± 17 nM) > UDP-glucuronic acid (EC50 370 ± 33 nM) > UDP-N-acetylglucosamine (EC50 710 ± 27 nM) [stable HEK 293 cell line expressing both recombinant Gα16 and the human receptor (Chambers et al., 2000)] |
| Antagonist potencies | No antagonists tested |
| Radioligand assays | None |
| Radioligands | None |
| Transduction mechanism | Gi/Go (Chambers et al., 2000)—in particular, data show a coupling to Gα subunits (Gα16, Gαqo5, and Gαqi5) of the Gi/o family but not members of the Gs family or to endogenous Gq/11 proteins in HEK 293 (Moore et al., 2003) |
| Distribution | Placenta, adipose tissue, stomach, intestine, some brain regions, spleen, lung, heart, bone marrow, brain glia, and peripheral immune cells (Chambers et al., 2000; Moore et al., 2003) |
| Tissue function | Role as chemoattractant receptor in bone marrow hematopoietic stem cells (Lee et al., 2003a); role in neuroimmune function (Moore et al., 2003); role in dendritic cells activation (Skelton et al., 2003) |
| Phenotypes | None |
| Comments | The P2Y14 receptor, from a phylogenetic and structural point of view, lies with the P2Y12 and P2Y13 receptors in the second main branch of the P2Y family—the gene for this receptor has been found in human chromosome 3q24–3q25, where a cluster of other related GPCRs, consisting of P2Y1, P2Y12, and P2Y13 receptors and of orphan GPR87, GPR91, and H963 have been found (Abbracchio et al., 2003); the complete sequences of the rat (VTR 15-20; Charlton et al., 1997; Freeman et al., 2001); and mouse orthologs (Freeman et al., 2001); of the human UDP-glucose receptor have also been described; rat P2Y14, which is abundantly expressed in brain, has been reported to be regulated by immunologic challenge (Charlton et al., 1997; Moore et al., 2003), suggesting that this receptor may link the humoral and nervous system responses to infection and inflammation; rat primary astrocytes and murine microglia have been recently reported to respond to UDP-glucose with increases of intracellular calcium concentrations, highlighting a role of this receptors in glial cells (Fumagalli et al., 2003; Bianco et al., 2005) |
aa, amino acids; AC, accession; chr., chromosome.
VII. Receptor Distribution and Function
A recent article reviews at depth the cellular distribution and functions of P2 receptor subtypes in different systems and cell types based on studies of subtype mRNA and proteins as well as functional data (Burnstock and Knight, 2004). Additional information can also be found above in individual P2Y receptor subsections and in individual summary tables. A general review of P2Y receptor distribution and function in mammalian organs and systems is presented below. (For references, see Burnstock and Knight, 2004.)
A. Excitable Cells, Nerves, Glial Cells, and Muscle
P2Y1 receptors are widespread in many regions of the brain, whereas the P2Y2 receptors have been localized on pyramidal neurons in the hippocampus and prefrontal cortex, on supraoptic magnocellular neurosecretory neurons in the hypothalamus, and on neurons in the dorsal horn of the spinal cord. In addition, mRNA but not protein has been reported for P2Y4 and P2Y6 receptor subtypes in the cerebellum and hippocampus, whereas P2Y12 receptor mRNA has also been described in the cerebellum and P2Y13 in the cortex. In the periphery, P2Y1,2,4,6 receptors on subpopulations of sympathetic neurons, P2Y2 and P2Y4 receptors in intracardiac ganglia, and P2Y1 and P2Y2 receptors on sensory neurons (although P2Y4 and P2Y6 mRNA have also been reported) have been described, whereas P2Y1 receptors appear to be the dominant subtype on enteric neurons.
P2Y1,2,4,6 functional receptors have been found on astrocytes in the central nervous system (Neary et al., 1994, 1996, 1999, 2003; Bolego et al., 1997; Centemeri et al., 1997; Fumagalli et al., 2003) and also on microglia where functional P2Y12 receptors have also been identified (for review, see Abbracchio and Verderio, 2006). P2Y1 and P2Y2 receptors have been located in Schwann cells and oligodendrocytes, in which functional P2Y12 receptors also appear to be present. P2Y2 (and/or P2Y4) receptors are expressed on enteric glial cells. There is also emerging evidence for P2Y receptors on stem cells.
All cloned P2Y receptors have been found in both healthy and failing human hearts (Banfi et al., 2005) to support functional roles in myocardial function (Vassort, 2001). Whereas the dominant P2 receptor subtype on smooth muscle is P2X1, P2Y receptor subtypes are also present, notably P2Y1 and/or P2Y2 receptors on visceral smooth muscle and P2Y1,2,4,6 receptors on vascular smooth muscle. P2Y1 receptors are present in developing skeletal muscle with evidence for P2Y2 receptors on the myotube C2C12 cell line.
B. Immune Cells
ATP stimulates production of prostaglandin and has mitogenic actions on thymocytes largely via P2Y2 receptors, although P2Y1 receptor mRNA is also present. P2Y2,6,11,13 receptor mRNAs have been identified in whole spleen.
P2Y2 receptors are the dominant subtype in macrophages, although the presence of P2Y1,4,12 receptors on alveolar macrophages and P2Y11 receptors on human macrophages has also been noted. ATP and UTP acting via P2Y1 and P2Y2 receptors promote adhesion of neutrophils to endothelial cells.
P2Y1,2,4,6,11 receptor mRNA have been identified with RT-PCR in both eosinophils and lymphocytes. Several P2Y subtypes are expressed in human monocyte–derived dendritic cells, in particular the P2Y11 receptor that mediates the semimaturation of these cells in response to ATP (Wilkin et al., 2001; La Sala et al., 2002; Schnurr et al., 2003).
P2Y1/2/4/6 receptors have been identified in monocytes, and UDP leads to production of IL-8 (Warny et al., 2001). ATP action via P2Y1 and P2Y2 receptors leads to degranulation and release of histamine from mast cells as well as cell migration and chemoattraction.
C. Endocrine, Adipose, and Exocrine Cells
The P2Y2 receptors are dominant in the anterior pituitary in which they modulate prolactin release, and P2Y1 receptors have been identified in the pineal gland. P2Y2 receptors mediate regulation of aldosterone secretion in the adrenal gland. In the thyroid gland, P2Y2,4,6 receptors mediate cell proliferation, whereas P2Y2 receptors are involved in insulin release from pancreatic islet β cells.
P2Y2 receptors are present in testicular Sertoli and in Leydig cells involved in estradiol and testosterone secretion. P2Y2 receptors are expressed in ovary and placenta. P2Y2 receptors mediate antagonism of estradiol and progesterone secretion from granulosa cells in the ovary.
Brown adipocytes express P2Y1,2,4 receptors involved in regulation of lipogenesis. P2Y2 receptors involved in regulation of ionic balance are dominant in salivary and lachrymal glands.
In sweat glands and exocrine pancreas, P2Y1,2,4 receptors are present. They have been implicated in regulation of secretion.
D. Gut, Liver, and Biliary System
P2Y1 and P2Y11 receptors appear to mediate the purinergic component of nonadrenergic, noncholinergic relaxation of smooth muscle in the gastrointestinal tract, whereas P2Y1,2,4,6 receptors on gut epithelial cells may mediate ion secretion, although P2Y4 seems to play a major role, as demonstrated in knockout mice. P2Y1 receptors have also been identified in intrinsic enteric neurons in both myenteric and submucosal plexuses. P2Y1,2,4,6,13 receptors have been described in hepatocytes and may serve to regulate gluconeogenesis and glycolysis. P2Y2,4,6 receptors are present on bile duct epithelium; in addition, P2Y1 receptor mRNA is present in gallbladder epithelium. UTP has been show to stimulate Cl− secretion and modulate bile release.
E. Kidney and Bladder
P2Y receptors are richly expressed in all regions of the kidney tubule and glomerulus. P2Y1,4,6 receptors in the proximal convoluted tubule and loop of Henle are involved in reabsorption of water, ions, and nutrients. The P2Y2 receptor is dominant in the distal convoluted tubule concerned with secretion of ions, acids, and toxins whereas P2Y1,2,4,6 receptors appear to be involved in transport of water and ions in the collecting duct. In the glomerulus, P2Y1,2,4,11,12 subtypes are all expressed on mesangial cells, P2Y1,2,6 on podocytes, and P2Y2 on endothelial cells. In rat bladder, ATP exerted an immediate and transient contraction, followed by a slower sustained relaxation, which was completely abolished by the G protein blocking agent, guanosine 5′-O-(2-thiodiphosphate), suggesting a functional role for P2Y receptors (Bolego et al., 1995). Evidence for purinergic functional regulation of human urinary bladder, which may have potential therapeutic implications for human incontinence, has been also provided (Palea et al., 1993).
F. Lung
P2Y2 and P2Y6 receptors on cultured smooth muscle cells from the lung induce an increase and a decrease in smooth muscle proliferation, respectively. P2Y2 receptors are the predominant receptor subtype in lung epithelial cells and are involved in mucin secretion and mucociliary clearance. In addition, P2Y1 and P2Y6 receptor subtypes have been identified on respiratory epithelium cell lines.
G. Bone and Cartilage
P2Y2 receptors on osteoblasts mediate inhibition of bone formation, whereas ADP acting via P2Y1 receptors on osteoclasts increase bone resorption. Chondrocytes express P2Y1 and P2Y2 receptors, which mediate cartilage resorption and prostaglandin production.
H. Skin
P2 receptors play a major role in cell turnover of keratinocytes in stratified epithelium. P2Y1 and P2Y2 receptors modulate cell proliferation in basal cells.
I. Endothelial Cells
P2Y1 and/or P2Y2 receptors are dominant on vascular endothelial cells, where they mediate release of NO and subsequent vasodilatation as well as cell proliferation and the expression of adhesion proteins for monocytes. In some blood vessels, P2Y4,6,11,12 receptors have also been found.
J. Special Senses
In the eye, P2Y2 receptors mediate trophic events in the retina and cornea, whereas P2Y1 receptors have been implicated in regulation of fluid secretion in the ciliary body. In the inner ear, P2Y receptors have been implicated in cochlear function, particularly P2Y2 and P2Y4 receptors in vestibular dark cells and stria vascularis marginal cells. P2Y receptors also appear to be involved in olfactory function, especially P2Y2 and P2Y6 receptors on nasal epithelium.
K. Platelets
Platelets express three nucleotide receptors: the P2X1 cation channels activated by ATP, and two GPCRs, P2Y1 and P2Y12, both activated by ADP, which perhaps represent the most studied P2Y receptors in a native system (see also above). Each of these receptors has a selective role during platelet activation (Hechler et al., 2005), which has implications for their role in thrombosis (Gachet and Hechler, 2005). We will not expand here on the role of the P2X1 receptor, which is not negligible, being involved in platelet activation by collagen and in the thrombosis of small arteries (Hechler et al., 2003; Mahaut-Smith et al., 2004). However, its deficiency does not induce a defect in normal hemostasis and does not modify the platelet responses to ADP. ADP-induced platelet aggregation is under the control of P2Y1 and P2Y12. Coactivation of both receptors is necessary for normal ADP-induced platelet aggregation since separate inhibition of each of them by selective antagonists results in dramatic inhibition of aggregation (Jin and Kunapuli, 1998; Gachet, 2001; Hechler et al., 2005).
1. The P2Y1 Receptor Initiates Platelet Activation and Aggregation
The presence and role of the P2Y1 receptor in platelets, initially suggested by the detection of P2Y1 mRNA in both megakaryoblastic cells and platelets (Léon et al., 1997), was confirmed using selective P2Y1 antagonists (Léon et al., 1997; Hechler et al., 1998b) and by studies in P2Y1 receptor-deficient mice (Fabre et al., 1999; Léon et al., 1999a). There are ~150 P2Y1 receptors/platelet (Baurand et al., 2001; Baurand and Gachet, 2003), a number that is very low compared, for instance, with thromboxane prostanoid receptors or with the thrombin receptor PAR-1 (1000–2000 receptors/platelet). The P2Y1 receptor coupled to Gαq triggers calcium mobilization from internal stores, which results in platelet shape change and weak and transient aggregation in response to ADP (Hechler et al., 1998a, b; Jin et al., 1998a; Savi et al., 1998). In addition, the P2Y1 receptor participates in aggregation induced by collagen, as shown by the reduced amplitude of aggregation and the prolongation of the lag phase from the addition of collagen to the onset of aggregation in P2Y1 knockout platelets (Léon et al., 1999a). P2Y1 receptors also play a key role in collagen-induced shape change when thromboxane (TX) A2 formation is prevented (Mangin et al., 2004). Examination of the morphological changes during platelet aggregation indicates that the P2Y1 receptor is involved in the centralization of platelet granules induced by ADP and the formation of filopodia in platelets activated with low concentrations of agonists such as TXA2 or thrombin (Eckly et al., 2001). Overall, the P2Y1 receptor mediates weak responses to ADP and has a crucial role in the early steps of platelet activation induced by ADP or collagen.
2. The P2Y12 Receptor Completes and Amplifies Platelet Activation and Aggregation
Several lines of evidence suggested the presence of a second platelet ADP receptor coupled to Gi2 and responsible for adenylyl cyclase inhibition, well before its identification (Gachet, 2001). Indeed, molecules such as clopidogrel or the ATP analogs of the AR-C series selectively inhibit the Gi-coupled ADP response without any impact on the P2Y1 receptor-mediated effects (Hechler et al., 1998a; Jin et al., 1998a; Savi et al., 1998), whereas P2Y1 receptor antagonists inhibit ADP-induced platelet aggregation without inhibiting the effect of ADP on adenylyl cyclase activity (Daniel et al., 1998; Hechler et al., 1998b; Savi et al., 1998). In addition, the P2Y1 receptor is normal, at the genetic and pharmacological levels, in a patient with congenital impairment of platelet responses to ADP (Cattaneo et al., 1992; Léon et al., 1999b). Lastly, in P2Y1 receptor-deficient mice, ADP is still able to inhibit platelet adenylyl cyclase activity (Fabre et al., 1999; Léon et al., 1999a). The requirement of this receptor to complete aggregation to ADP was confirmed by the generation of P2Y12 receptor-deficient mice, which display a defect in platelet aggregation in response to ADP, although shape change is conserved (Foster et al., 2001; André et al., 2003). Numerous studies established the key role of this receptor not only for completion of aggregation in response to ADP but also for the ADPdependent amplification of platelet aggregation induced by other agents acting at the Gαq-coupled serotonin receptor 5HT2A (Savi et al., 1998) or the Gαq and G12/13-coupled TXA2 and PAR-1 receptors (Dorsam et al., 2002). Similarly, the P2Y12 receptor is an important cofactor of platelet aggregation and secretion induced by cross-linking of the FcγRIIa receptor with specific antibodies (Gratacap et al., 2000), or when platelets are activated by collagen through the GPVI/tyrosine kinase/PLC-γ2 pathway (Nieswandt et al., 2001). The P2Y12 receptor is also involved in potentiation of platelet secretion (Cattaneo et al., 1997, 2000) and mediates the stabilization of platelet aggregates induced by thrombin (Trumel et al., 1999) or TXA2 (Eckly et al., 2001).
The P2Y12 receptor is coupled to the Gαi2 G protein subunit (Ohlmann et al., 1995; Bodor et al., 2003). Downstream of Gαi2, several signaling pathways are involved in amplification mechanisms. First, inhibition of cAMP production, although not sufficient to trigger platelet aggregation, has a facilitating effect on activation (Haslam, 1973) at least by inhibition of the cAMP-dependent PKA-mediated phosphorylation of vasodilator- stimulated phosphoprotein (VASP). VASP is an actin regulatory protein and a negative modulator of αIIbβ3 integrin activation. Thus, levels of VASP phosphorylation/dephosphorylation reflect the P2Y12 inhibition/activation state, which might constitute a sensitive marker to identify patients insufficiently protected by clopidogrel treatment (Schwarz et al., 1999; Aleil et al., 2004). Second, P2Y12 stimulates PI3-K activity, which is important to sustain aggregation (Trumel et al., 1999; Kauffenstein et al., 2001). In addition, P2Y12 is known to activate the small GTPase RapIb through a PI3-K dependent mechanism (Lova et al., 2002, 2003; Woulfe et al., 2002; Larson et al., 2003). These multiple pathways explain why P2Y12 plays such a central role in hemostasis and thrombosis (Conley and Delaney, 2003). Thus, overall, the P2Y12 receptor appears to be responsible for most of the cofactor role of ADP in amplification of platelet activation induced by low concentrations of agonists such as TXA2, thrombin, collagen, chemokines, or immune complexes.
The P2Y1 and P2Y12 receptors are differentially involved in the procoagulant activity of platelets. Both receptors are indirectly involved through platelet P-selectin exposure and formation of platelet-leukocyte conjugates, leading to leukocyte tissue factor exposure, whereas the P2Y12 receptor is also directly involved in the procoagulant activity of platelets through phosphatidylserine exposure at the surface of platelets (Léon et al., 2001, 2003). An interesting feature of these receptors is their regulation after activation. Once activated by a first application of ADP, platelets become unresponsive to a second stimulation with ADP. This so-called refractory state of platelets to ADP has been recently shown to be caused by desensitization of the P2Y1 receptor with a resultant loss of shape change and aggregation (Baurand et al., 2000), whereas the P2Y12 receptor remains functional. Further studies established that the P2Y1 and P2Y12 receptors are differentially regulated and relocated upon activation, the P2Y1 receptor after clathrin-dependent internalization, whereas the P2Y12 receptor mainly stays at the plasma membrane (Baurand et al., 2005). This action may be of major consequence in vivo, since even in platelets refractory to stimulation by ADP, the P2Y12 receptor would be able to ensure platelet reactivity at sites of injury, thus preventing loss of the hemostatic function. In summary, both P2Y1 and P2Y12 receptors are necessary for normal hemostasis and both play a key role in arterial thrombosis. P2Y12 receptors targeting antithrombotic drugs already exist, and new compounds have been developed and are under clinical evaluation.
For P2Y1, studies with knockout mice as well as pharmacological inhibition with antagonists such as MRS2179 and more recently MRS2500 clearly demonstrated its relevance as a possible target for new antiplatelet drugs (Gachet and Hechler, 2005). P2Y12 receptor-deficient mice have been generated. They are viable, fertile, and display no spontaneous bleeding or other overt abnormalities (Foster et al., 2001; André et al., 2003). They display prolonged bleeding time compared with wild-type as well as P2Y1 receptor-deficient mice. Platelet aggregation in response to ADP is inhibited, but shape change is conserved. Finally, these mice are insensitive to clopidogrel treatment (Foster et al., 2001), confirming that the P2Y12 receptor is the target of this drug. These mice have been used in several models of arterial thrombosis and were shown to be resistant to thrombosis. The role of the P2Y12 receptor in the stabilization of the thrombus was confirmed in these mice. Whether other phenotypic modification could be observed has not yet been reported.
VIII. Source of Naturally Occurring Ligands and Mechanisms of Transport and Breakdown
ATP is released into the extracellular space as an autocrine/paracrine molecule in response to neuronal stimulation, platelet aggregation, stress, mechanical stimulation and a number of receptor agonists. As previously indicated, known P2 receptors are stimulated by ATP, ADP, UTP, UDP, or UDP-glucose, and concentrations of these nucleotides in the extracellular space ranging from 0.1 to 10 μM are required for activity.
The dynamic regulation of extracellular nucleotides manifested by the balance between release, catabolism, and interconversion in the extracellular space has imposed a layer of complexity to the understanding and quantification of the pharmacologically active nucleotides. Additional confounding factors such as cell lysis or tissue damage contribute to the complexity by releasing large quantities of intracellular nucleotides. In addition, most of the measurements of extracellular levels of ATP have been performed in the bulk phase of the extracellular environment of the cells, and, therefore, these measurements largely underestimate the true concentration of extracellular nucleotides at the receptor level in the pericellular environment of the cells. Attempts to overcome some of these difficulties have been addressed by the development of sensitive assays capable of estimating subnanomolar concentrations of ATP and UTP (Levitt et al., 1984; Chen et al., 1994; Yang et al., 1994; Taylor et al., 1998; Beigi et al., 1999; Lazarowski and Harden, 1999; Huang et al., 2001; Lazarowski et al., 2003a) and by the development of specific biosensors such as the attachment of the luciferase enzyme to the surface of the cell (Beigi et al., 1999; Joseph et al., 2003), and by the use of nucleotide receptors to “sense” the pericellular nucleotide concentrations (Homolya et al., 2000; Sorensen and Novak, 2001; Coco et al., 2003; Hayashi et al., 2004). These techniques have allowed the assessment of nucleotide concentrations at the pericellular level and have demonstrated that the measurements of nucleotides in the bulk phase could underestimate the values at the receptor level by at least 20-fold (Joseph et al., 2003).
Millimolar concentrations of ATP exist in the cytoplasm of all cell types. However, because of the net negative charge of the molecule, ATP and other nucleotides do not permeate the cell membrane and therefore, require specialized transport mechanisms to reach the extracellular space and to act on the target receptors. Most of the available information on extracellular nucleotides refers to ATP and the products of its catabolism; however, the other agonist of P2Y receptors, UTP (and probably other nucleotides as well), is also released to the extracellular space by mechanisms similar to those for ATP. Several forms of nucleotide release have been proposed to date, as summarized below.
A. Basal Unstimulated Nucleotide Release
1. Constitutive Release of ATP
Extracellular nucleotides have been detected in multiple cell types under unstimulated conditions (Lazarowski et al., 2000, 2001a; Ostrom et al., 2000; Joseph et al., 2003). Stress or mechanical stimulation of many cell types results in a large elevation in the concentration of extracellular nucleotides, followed by a rapid decrease to a constant level in resting conditions that ranges between 0.5 and 10 nM in most cells. Lazarowski et al. (2000, 2001a) and Donaldson et al. (2000) demonstrated that resting levels of ATP and UTP are maintained by a steady-state equilibrium between release and metabolism. This observation suggests that a constitutive unstimulated release of ATP and UTP exists in many cells. Consistent with this observation, the resting levels of ATP increased gradually by inhibition of the metabolism of ATP with decreasing the temperature of primary cultures of endothelial cells (Schwiebert et al., 2002) or by treatment of BAC1.2F5, 1321N1, PC12, and C6 cells with the ecto-ATPase inhibitor α,β-meATP (Beigi and Dubyak, 2000; Joseph et al., 2004).
Now that the tools have been developed to assess low levels of extracellular nucleotides including UTP, UDP, and UDP-glucose, along with sensitive “biosensors” and methodology to monitor the levels of ATP in the pericellular space, the physiological relevance of the resting levels of extracellular nucleotides can be explored. For example, Ostrom et al. (2000) demonstrated in MDCK cells that basal levels of ATP acting on P2Y receptors and thus promoting the formation of prostaglandins is the key determinant of the “set point” of the cAMP signal transducing pathway, illustrating the relevance of constitutive release of ATP on the autocrine/paracrine regulation of other signal transduction mechanisms (Ostrom et al., 2000).
Another example of the physiological relevance of constitutive release of extracellular ATP is observed in the airway epithelium. This steady state modulates some of the important functions of CFTR via activation of A2B receptors, including regulation of the airway surface liquid volume to afford efficient cilia movement and optimal viscoelastic properties of the mucus layer to maintain efficient transport and clearance of the airways (Lazarowski et al., 2004).
2. Constitutive Release of UDP-Glucose
The identification of UDP-glucose as the endogenous ligand of an orphan GPCR (Chambers et al., 2000; Freeman et al., 2001) that has been incorporated into the family of P2Y receptors on the basis of the sequence homology and structural motifs (Abbracchio et al., 2003) prompted the development of a sensitive and selective assay for UDP-glucose (Lazarowski et al., 2003b). This assay was subsequently used for the assessment of extracellular levels of the receptor agonist and to explore the potential mechanisms of release of UDP-glucose into the extracellular space. Mechanical stimulation of 1321N1 astrocytoma cells resulted in an increase of extracellular levels of ATP and a modest increase of UDP-glucose. In marked contrast with ATP, the levels of UDP-glucose remained elevated for an extended period of time. Under resting conditions at steady state, the levels of UDP-glucose are severalfold higher than those of ATP or UDP. UDP-glucose is released in primary cultures of human airway epithelial cells, Calu-3, COS-7, CHO-K1, and C6 glioma cells with resting levels similar to or higher than those of ATP. These studies suggest that UDP-glucose, is released constitutively in a manner similar to ATP, and it is metabolized at a slower rate than ATP in all cells studied.
UDP-glucose participates in the quality control of protein synthesis in the lumen of the endoplasmic reticulum where it is actively transported by an UDP-glucose/UMP antiporter. The authors speculate that UDP-glucose transported to the endoplasmic reticulum lumen could be released from cells as cargo as part of the constitutive pathway that delivers glycoproteins to the plasma membrane (Lazarowski et al., 2003b, 2004).
B. ATP Release by Excitable and Secretory Tissues
Exocytosis of ATP-containing vesicles occurs in neurons, platelets, adrenal medullary chromaffin cells, neuroendocrine cells, and mast cells. Neuronal release of nucleotides was first identified 50 years ago by Holton and Holton (1954) and is now one of the best-understood sources of extracellular nucleotides (Bodin and Burnstock, 2001a). ATP and probably other nucleotides are stored at high concentrations in dense granules in platelets, synaptic vesicles in neurons, and chromaffin granules in adrenal glands. The concentration of nucleotides in these vesicles could reach up to 150 to 1000 mM. Stimulation of these cells results in the calcium-dependent fusion of these vesicles with the plasma membrane and the release of vesicular contents into the extracellular space. The specific mechanisms of exocytotic granule release have been extensively investigated (Sperlágh and Vizi, 1996).
C. ATP Release by Nonexcitatory Cells
Extracellular nucleotides are released by multiple nonexcitatory cell types such as endothelial and epithelial cells, smooth muscle cells, fibroblasts, astrocytes, red blood cells, lymphocytes, monocytes, and a number of transformed cell lines. The release of nucleotides by nonexcitatory cells is not completely understood and involves the participation of multiple mechanisms. No evidence for the existence of specialized ATP-containing vesicles in most of these cells has been demonstrated, and, therefore, alternative mechanisms for the release of nucleotides have been postulated. These mechanisms are outlined below in the context of 1) stress/hypoxia/mechanical stimulation, 2) vesicular trafficking, and 3) agonist-promoted stimulation.
1. Stress/Hypoxia/Mechanical Stimulation
Evidence for non-neuronal, nonlytic release of ATP has been documented from a large number of tissues under a variety of physiological and experimental conditions. One of the first reports of the physiological non-neuronal release of ATP dates from 1969 by Forrester and Lind, who demonstrated the presence of ATP in the venous effluent of a forearm subjected to a sustained contraction.
Release of ATP and UTP induced by mechanical stimulation has also been documented in vitro in cell lines expressing P2Y receptors. Normal cell culture manipulation and/or medium changes resulted in marked P2Y receptor-dependent accumulation of inositol phosphates in cells expressing the recombinant P2Y1, P2Y2, and P2Y4 receptors, but not in nontransfected cells (Filtz et al., 1994; Parr et al., 1994; Lazarowski et al., 1997b). The effect of medium changes and cell manipulation were eliminated by treatment of cells with apyrase. Because no evidence of cell lysis was observed in these and other studies, these observations indicate that mechanical stimulation results in the nonlytic release of sufficient concentrations of ATP and UTP to effectively trigger the signaling of nucleotide receptors.
One of the most relevant responses to physiological mechanical stimulation occurs in epithelial cells. The bladder is subjected to marked changes in tension and pressure during normal filling and voiding. Burnstock (1999) proposed that the distension of the bladder and other visceral tissues such as ureter, vagina, and gallbladder leads to the release of ATP in the vicinity of the epithelium, where sensory nerve endings containing P2X receptors (P2X3 or P2X2/3) are stimulated to convey nociceptive stimuli to the central nervous system (Bodin and Burnstock, 2001a).
Several studies have demonstrated that perfusion of endothelial cells at high shear force leads to the release in a nonlytic fashion of significant amounts of nucleotides to the extracellular space, and hypoxia potentiates the release of nucleotides induced by shear force (Bodin and Burnstock, 1995). Hypoxia induces ATP release from pulmonary artery adventitial fibroblasts and endothelial cells (Gerasimovskaya et al., 2002), and the resulting autocrine activation of P2 receptors appears to be involved in the proliferation of fibroblasts under hypoxic conditions. Under hypoxia, erythrocytes release ATP that induces vasodilatation in skeletal muscle and pulmonary artery (Sprague et al., 2001; Gonzalez-Alonso et al., 2002).
Extracellular nucleotides play an important role in the regulation of the function of airway epithelial cells. Activation of P2Y2 receptors located in the apical membrane results in calcium-activated chloride channel activity important in the regulation of fluid secretion, mucociliary clearance, and the regulation of the volume of the periciliary liquid, enabling efficient cilia movement and mucus layer transport (Schwiebert and Kishore, 2001; Leipziger, 2003).
The lack of nerve endings in airway epithelial cells, led to the suggestion that the epithelial cell itself is the main source of extracellular nucleotides. This topic has been extensively studied during the past few years and evidence suggests that ATP and UTP release from epithelial cells occurs preferentially at the luminal face (Lazarowski et al., 2004).
Several mechanisms for the release of ATP in response to nonlytic stimuli have been contemplated: transport by ATP binding cassette (ABC) proteins, stretch-activated channels, and voltage-activated channels and release via vesicular trafficking (Bodin and Burnstock, 2001b; Knight et al., 2002; Lazarowski et al., 2003a).
a. ATP binding cassette proteins
The CFTR and the multidrug resistance (MDR) gene product, P-glycoprotein, have been suggested to participate in the transport of ATP into the extracellular space (Abraham et al., 1993; Reisin et al., 1994; Schwiebert et al., 1995). However, further studies did not confirm that CFTR functions as an ATP transport protein (Li et al., 1996; Reddy et al., 1996; Grygorczyk and Hanrahan, 1997; Watt et al., 1998). An alternative hypothesis to explain the conflicting results has been proposed, suggesting that CFTR and the MDR1 gene product participate in the release of ATP by regulating the activity of alternative nucleotide transporters associated with the ABC protein (Jiang et al., 1998; Sugita et al., 1998; Hazama et al., 2000; Braunstein et al., 2001; Roman et al., 2001). Evidence against a direct role of CFTR on ATP release has been demonstrated in ocular ciliary epithelial cells (Mitchell et al., 1998).
b. Stretch and voltage-activated Cl− channels
Cell volume regulation represents a unique opportunity to study a physiological mechanism of nucleotide release by epithelial cells. ATP release stimulated by treatment with hypo-osmotic solutions has been demonstrated in a number of cell types including epithelial and endothelial cells, suggesting that a mechanosensitive ATP channel tightly associated with CFTR is implicated in the efflux of ATP. These conclusions were based on the pharmacological effects of gadolinium (Gd3+), a nonselective inhibitor of stretch-activated channels (Braunstein et al., 2001). Further studies by Boudreault and Grygorczyk (2002) caution that these observations could be produced by interference of Gd3+ with the luciferin-luciferase bioluminescence assay used in these studies. Consistent with this conclusion, Knight et al. (2002), failed to demonstrate an effect of Gd3+ (or glibenclamide) on ATP release during nonlytic distension of the ureter. In contrast, these authors found that monensin and brefeldine A inhibited the distension-evoked ATP release, suggesting that nucleotide efflux from the ureter epithelium occurs by vesicular exocytosis (Knight et al., 2002).
Another potential mechanism for the regulation of cell volume is by the electrogenic translocation of ATP via an anion channel independent of CFTR. Recent studies have provided evidence for an ATP-conducting maxianion channel involved in volume regulation (Sabirov et al., 2001; Dutta et al., 2002; Bell et al., 2003b). This channel has been named the volume (and voltage)-dependent ATP conductive large conductance anion channel (see Sabirov and Okada, 2004). This channel has a large unitary conductance and a permeability ratio (PATP/PCl) of 0.14. This permeability, although apparently low, is sufficient to produce physiologically significant ATP efflux in the presence of high intracellular Cl− concentrations. Further studies by these investigators indicated that this channel fits the pharmacological profile of swelling-induced ATP release, namely, sensitivity to Gd3+, 4-acetamido-4′-isothiocyanostilbene-2,2′-disulfonate, and 5-nitro-2-(3-phenylpropylamino)benzoic acid, and insensitivity to phloretin, niflumic acid, and glibenclamide (Sabirov and Okada, 2004).
Activation of this ATP-conducting maxi-anion channel has been demonstrated in macula densa cells in response to an increase in tubular NaCl concentration. Under these conditions, ATP is released at relevant physiological concentrations from the basolateral membrane of macula densa cells in close proximity to the mesangial cells that express P2Y2 receptors (Bell et al., 2003a).
A cell-to-cell paracrine signal transduction mediated by ATP and this anion channel plays a central role in stress sensory transduction for cell volume regulation and tubuloglomerular feedback in the kidney. The molecular entity of this channel has not been established; however, a candidate molecule with similar electrophysiological properties is the mitochondrial voltage-dependent anion channel (VDAC-1) porin.
Recently, Okada et al. (2004) reported that a splice variant of mitochondrial VDAC-1 is expressed in airway cells and demonstrated that VDAC-1 contributes to the release of ATP in murine cells in response to hypoosmotic challenge. However, the authors also observed that cells from VDAC-1 knockout mice exhibit significant release of ATP, suggesting that additional ATP transport molecules play a role in volume regulation in murine cells (Okada et al., 2004).
2. Nucleotide Release via Vesicular Trafficking
An alternative mechanism for the release of nucleotides into the extracellular space via vesicular trafficking has been proposed (Burnstock, 1999; Bodin and Burnstock, 2001b). Distension of the guinea pig ureter by increasing the intraluminal pressure resulted in the release of ATP in a pressure-dependent manner. Treatment with different drugs added to the perfusion medium was used to determine the possible mechanism of ATP release. Monensin, an inhibitor of vesicular formation from the Golgi apparatus, and brefeldin A, a drug that disrupts the vesicular traffic between the endoplasmic reticulum and the Golgi apparatus, inhibited the distensionevoked ATP release by the urothelium. Drugs that inhibit ABC proteins or nucleoside and nucleotide transporters had no effect on the release of ATP, suggesting that the release of ATP from the ureter epithelium is mediated by vesicular exocytosis (Knight et al., 2002).
3. Agonist-Promoted ATP Release
Activation of signaling pathways by some receptor agonists can result in the specific release of ATP at sufficient concentrations to elicit a physiological response in the same cell or in a neighboring cell. Receptor-mediated ATP release has been observed in aortic endothelial and smooth muscle cells treated with thrombin (Pearson and Gordon, 1979). Bradykinin, acetylcholine (ACh), and serotonin also promote release of ATP by endothelial cells and bradykinin and phenylephrine promote release from MDCK cells (Yang et al., 1994; Ostrom et al., 2000).
Increases of extracellular levels of ATP have been reported in response to treatment with ADP in coronary endothelial cells (Buxton et al., 2001) and with UTP in COS-7 and HEK-293 cells (Ostrom et al., 2000); however, in both cases the appearance of ATP could result from NDPK-dependent phosphorylation of added ADP in the first case and from phosphorylation of endogenous ADP in the second case. The intrinsic mechanisms for the agonist-mediated ATP release have not been elucidated and are probably related to the activation of the specific signaling pathways promoted by these agonists. In MDCK, COS-7, and HEK293 cells, the Ca2+ mobilizing hormones, bradykinin and phenylephrine, but not the cAMP-elevating forskolin and prostaglandin E1 promoted the release of ATP, suggesting that a Ca2+-dependent event downstream of Gq and PLC was involved in the release of ATP (Ostrom et al., 2000). Joseph et al. (2003) recently demonstrated that the release of ATP by thrombin was only partially dependent on Ca2+. This observation, together with the evidence for the participation of PI3-K (Feranchak et al., 1998, 1999) and Rho or Rho kinase in the release of ATP (Koyama et al., 2001) suggest that, in the case of thrombin, ATP release could be mediated by G12-dependent activation of Rho. Because Rho and PI3-K are involved in the regulation of vesicle traffic and cytoskeleton organization, an interpretation of the effects of thrombin described above is that the mechanism of ATP release could involve vesicle transport (Lazarowski et al., 2003a).
Pathogenic bacteria (Shigella and Escherichia coli) induce the release of ATP from gut epithelial cells (Crane et al., 2002; Tran Van Nhieu et al., 2003). The released ATP correlates with bacterial invasion and spreading, but also promotes secretory diarrhea. The release of ATP by bacteria is partially due to cell lysis but also involves specific mechanisms of recognition, e.g., the ligation of host glycolipid asialo-GM1 by the flagellin of Pseudomonas aeruginosa flagellum (McNamara et al., 2001).
D. ATP Release by Tissue Damage
Cell lysis or tissue damage results in the release of the intracellular nucleotide contents into the extracellular space providing nucleotide concentrations sufficient to activate nucleotide receptors. This source of extracellular nucleotides accounts for only some of the known physiological actions of ATP, including transmission of nociceptive signals and onset of inflammation.
E. Extracellular Nucleotide Metabolism
The physiological effect of extracellularly released nucleotides depends mainly on the particular receptor subtype(s) expressed in the tissue. In addition, an important modulatory effect of the physiological response is imposed by the presence of soluble and membrane-bound ecto-nucleotidases that rapidly metabolize the nucleotides with the potential to terminate or initiate receptorspecific signaling cascades.
Extracellular ecto-nucleotidases are expressed in most if not in all cell types. Several families of ectoenzymes participate in the degradation and interconversion of extracellular nucleotides. The most prominent enzyme classes are the ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDase) family, the ecto-nucleotide pyrophosphatase/phosphodiesterase (E-NPP) family, ecto-5′-nucleotidase, alkaline phosphatase, the nucleotide-converting enzyme ecto-NDPK, and adenylate kinase. Ecto-nucleotidases exist as membrane-associated forms or soluble forms. Some of the soluble ectonucleotidases are produced by the proteolytic cleavage of the extracellular domains of membrane-bound forms or by the release from a glycosylphosphatidylinositol-anchoring phospholipid. The characterization of the ectonucleotidases has uncovered significant overlapping substrate specificities and tissue expression (Zimmermann, 2000; Goding et al., 2003).
1. Ecto-Nucleoside Triphosphate Diphosphohydrolases
E-NTPDases previously known as ectoapyrases, NTPases, or E-ATPases can hydrolyze both nucleoside triphosphates (NTPs) and nucleoside diphosphates (NDPs). Eight members of this family (E-NTPDases 1–8) have been described. E-NTPDases 1, 2, 3, and 8 are cell surface-associated, whereas E-NTPDases 4, 5, 6, and 7 are intracellular enzymes. E-NTPDases 1, 2, 3, 4, 7, and 8 have N-and C-terminal transmembrane domains, whereas E-NTPDases 5 and 6 have only a single N-terminal transmembrane domain that can be cleaved, releasing the extracellular domain as soluble protein.
E-NTPDase 1, previously known as apyrase or CD39, hydrolyzes both NTPs and NDPs with a 1:1 selectivity ratio. E-NTPase 2 also known as ecto-ATPase or CD39L1, selectively hydrolyzes NTPs over NDPs with a selectivity ratio of 1:0.03 and E-NTPDase 3, known as ecto-ATPDase, CD39L3, and HB6, has an intermediate selectivity ratio of 1:0.3. Recently, a new member of this family, E-NTPDase 8, has been described. This enzyme hydrolyzes adenine and uridine nucleotides with low apparent Km values (Bigonnesse et al., 2004). All members of this family share five highly conserved sequence domains, known as apyrase conserved domains that are essential for the catalytic activity (Handa and Guidotti, 1996; Vasconcelos et al., 1996; Zimmermann, 2000).
2. Ecto-Nucleotide Phosphophosphates/Phosphodiesterases
The E-NPP family comprises five members. E-NPP1–3 are transmembrane metalloenzymes with a structure consisting of a short N-terminal intracellular domain, a single transmembrane domain, and a large extracellular domain containing the catalytic active site. Soluble forms of these enzymes have been found and are presumably originated by proteolytic cleavage of the extracellular domains of the membrane-bound forms. E-NPP1–3 are classified as alkaline ecto-nucleotide pyrophosphatase/phosphodiesterase I and catalyze the hydrolysis of pyrophosphate and phosphodiester bonds. Therefore, these enzymes hydrolyze ATP into AMP and PPi; 3′,5′-cAMP into AMP; ADP into AMP and Pi and NAD+ into AMP and nicotinamide mononucleotide. E-NPP isoenzymes can hydrolyze purine and pyrimidine nucleotides, as well as dinucleotides and UDP-sugars (see below). Genes encoding putative E-NPP4 and 5 have been identified in searches of databases. The gene products of these enzymes have not been characterized (Goding et al., 2003).
Different investigators have independently characterized members of the E-NPP family, and several names have been given to the same protein. E-NPP1 is also known as PC-1 (Van Driel et al., 1985; Van Driel and Goding, 1987), E-NPP2 is also known as PD-Iα and autotaxin, and E-NPP3 is known as PD-Iβ, B10, and gp130RB13-6 (Van Driel et al., 1985; Van Driel and Goding, 1987; Stracke et al., 1992; Narita et al., 1994; Deissler et al., 1995; Jin-Hua et al., 1997; Scott et al., 1997). PD-Iα and autotaxin are splice variants of the same gene (Murata et al., 1994; Kawagoe et al., 1995), whereas PD-Iβ, B10, and gp130RB13-6 correspond to the same protein.
In addition to their role in the metabolism of extracellular nucleotides, E-NPP isoenzymes have other, probably related, physiological roles including nucleotide recycling, regulation of pyrophosphate levels, bone mineralization, and cell motility (see Goding et al., 2003). Recently, the substrate selectivity for E-NPP2 has been expanded with the observation that this enzyme, but not E-NPP1 or E-NPP3, hydrolyzes lysophosphatidylcholine into lysophosphatidic acid, linking the activity of the enzyme with tumor cell growth and motility (Tokumura et al., 2002; Umezu-Goto et al., 2002).
3. Hydrolysis of UDP-Glucose
UDP-glucose, the natural agonist of the P2Y14 receptor, and other nucleotide sugars are also substrates of E-NPP isoenzymes. Lazarowski et al. (2003b) demonstrated that cultures of 1321N1 human astrocytoma cells hydrolyzed UDP-glucose into UMP and glucose 1-phosphate. Similar findings were observed in COS-7, Calu-3, and C6 cells (E. Lazarowski, personal communication). Whether UDPglucose is hydrolyzed by an E-NPP isoenzyme or a novel enzymatic activity requires further investigation.
4. Hydrolysis of Diadenosine Polyphosphates
Diadenosine polyphosphates via stimulation of P2Y (and P2X) receptors regulate a large variety of physiological functions, such as synaptic transmission (Miras-Portugal et al., 1999, 2003), platelet aggregation (Zamecnik et al., 1992), vascular tone (Ralevic et al., 2001), and intraocular pressure (Pintor et al., 2004). Diadenosine polyphosphates are stored and released into the extracellular space from chromaffin cells, neurons, and platelets among other cell types. Upon release, diadenosine polyphosphates are rapidly hydrolyzed, presumably by members of the E-NPP family. Studies of the hydrolysis of dinucleoside polyphosphates conducted with adrenal, endothelial, airway epithelial, blood cell, and synaptic membranes (Lüthje and Ogilvie, 1988; Ramos and Rotllan, 1995; Mateo et al., 1997a,b; Picher and Boucher, 2000), demonstrated an asymmetric hydrolysis of the dinucleotide polyphosphates into AMP and Apn-1. Identical degradation products from several dinucleoside polyphosphates were found in a recent study by Vollmayer et al. (2003) using E-NPP1, E-NPP2, and E-NPP3, suggesting that these enzymes are the major candidates for the hydrolysis of dinucleotides.
5. 5′-Nucleotidase
Ecto-5′-nucleotidase is a glycosylphosphatidylinositol-anchored enzyme also known as CD73. This enzyme converts nucleoside monophosphates into the corresponding nucleoside and inorganic phosphate. 5′-Nucleotidase is the major enzyme involved in the formation of adenosine in the extracellular space (Zimmermann, 1992, 1996b). A soluble form of 5′-nucleotidase released from the glycosylphosphatidylinositol anchor has been described.
6. Nucleoside Diphosphokinase
Extracellular nucleotides are also substrates of enzymes that participate in the interconversion and preservation of high-energy nucleotides. Extracellular NDPK activity has been described in 1321N1 astrocytoma and C6 glioma cells, as well as in airway epithelial cells. This enzyme catalyzes the reversible transphosphorylation of nucleoside diphosphates into nucleoside triphosphates such as ATP + UDP ⇄ ADP + UTP (Lazarowski et al., 1997a, 2000; Grobben et al., 1999; Yegutkin et al., 2000, 2001, 2002).
The presence of this transphosphorylation activity in a tissue or cell line could be higher than that of the ecto-nucleotidase activity, as in the case of 1321N1 or C6 cells, and this condition could lead to erroneous interpretation of the effects of administration of exogenous nucleotides to these cells. For example, the addition of UTP in a system with high levels of endogenous ADP and NDPK could result in the formation of sufficient ATP to elicit a pharmacological response that could be misinterpreted as being produced by UTP. The molecular nature of this enzymatic activity and its tissue distribution remain to be elucidated.
7. Alkaline Phosphatase
This enzymatic activity alone can catalyze the complete sequential hydrolysis of a nucleoside triphosphate to the corresponding nucleoside. Alkaline phosphatase can also hydrolyze PPi (Zimmermann, 1996a, 2000). The presence of this enzyme was described recently in the mucosal surface of human airway epithelium (Picher et al., 2004). Alkaline phosphatase activity and mRNA expression were elevated 3-to 6-fold above normal in the airway epithelium of patients with chronic airway diseases such as primary ciliary dyskinesia, cystic fibrosis, and α1-antitrypsin deficiency (Picher et al., 2004).
8. Adenylate Kinase
Adenylate kinase catalyzes the conversion of two ADP molecules into ATP + AMP in a reversible manner. In contrast with alkaline phosphatase and NDPK activities, the substrate selectivity for adenylate kinase is restricted only to adenine nucleotides. This transphosphorylation activity plays a role in the metabolism of extracellular nucleotides by counter-acting the stepwise nucleotide breakdown by E-NTPDases, E-NPPs, and 5′-nucleotide. Ecto-adenylate kinase activity has been documented in endothelial cells (Yegutkin et al., 2001, 2002), human airway epithelial cells (Donaldson et al., 2002; Picher and Boucher, 2003), and rat brain synaptosomes (Nagy et al., 1989).
The mechanisms of nucleotide release, hydrolysis, and interconversion are not fully understood. The recognition of the molecular basis of nucleotide release and the catalytic mechanism of hydrolysis is essential for the understanding of nucleotide receptor signaling and its role under normal physiological and pathological conditions.
A better understanding of the regulation of these processes will prove useful in the broadening of potential therapeutic approaches to modulate extracellular nucleotide signaling and their multiple physiological consequences.
IX. Interactions between P2Y and Other Receptors
A. Modes of Interaction between G Protein-Coupled Receptors
Individual GPCRs were long considered to preferentially activate specific intracellular signaling pathways and so act in a linear fashion to produce a change in cellular activity. However, it is now clear that they can also modulate the signals initiated by another GPCR to potentiate or inhibit the activity of these receptors. Such interactions can take place at the level of the GPCR themselves, through the formation of oligomers, or downstream of the receptor through the action of second messengers. The former process is commonly referred to as receptor dimerization and is characterized by the appearance of a physical receptor complex as demonstrated by coimmunoprecipitation or other methods, which displays novel pharmacological properties and/or interactions with second messenger systems. The latter process, known as receptor cross-talk, can have either positive or negative effects and serves to integrate coincident signals from multiple types of receptors, which are not physically associated.
B. Receptor Dimerization
Until recently, individual GPCRs were assumed to exist and function as monomers. However, in the mid-1990s it started to become clear that many GPCRs can coalesce into functional dimers or oligomers. Indeed, such aggregation may be the rule rather than the exception and may be essential for the correct trafficking and membrane expression of GPCRs (Bouvier, 2001; Milligan, 2004). At present, it is difficult to differentiate experimentally between dimers and oligomers and so the term dimer tends to be used. Within a receptor family, individual subtypes can form homodimers or different subtypes can form heterodimers. An increasing number of combinations are also being described for receptors from totally different families. As GPCRs are already major therapeutic targets, dimerization has important implications for the development of new drugs.
There is also evidence that the human P2Y2 receptor forms homodimers. Expression in cell lines of constructs in which the receptor was tagged at the C terminus with the cyan or yellow variants of green fluorescent protein produced functional receptors that were activated by UTP and which coupled to endogenous G proteins to induce release of Ca2+ stores (Kotevic et al., 2005). When both variants were coexpressed in the same cells, fluorescence resonance energy transfer (FRET) imaging indicated that they were in close proximity, i.e., they formed constitutive receptor complexes. It was notable that UTP did not change the FRET signal, indicating that binding of an agonist does not induce dissociation of the oligomeric complex. Further studies are required to determine whether other P2Y subtypes similarly form homodimers.
To date, the interaction between the rat P2Y1 receptor and adenosine A1 receptor is the only example of dimerization involving P2Y receptors with non-P2Y receptors that has been characterized extensively. Yoshioka et al. (2001) coexpressed the rat P2Y1 and adenosine A1 receptors in HEK293 cells. Initial experiments showed that these receptors coimmunoprecipitated in Western blots of whole cell membrane lysates, indicating that they formed a heteromeric complex. Coexpressing the P2Y1 receptor did not alter surface expression of the A1 receptor, but it did inhibit the binding of radiolabeled A1 agonists and antagonists in membrane preparations. This change was not seen in a mixture of membranes from cells expressing each receptor individually. Additionally, the binding of an A1 agonist was displaced by the P2Y1 agonist ADPβS and P2Y1 antagonist MRS2179 in cotransfected cells, but not in cells expressing the A1 receptor only. These data again indicate formation of a heteromeric complex.
A1 receptors couple to Gi and so mediate depression of intracellular cAMP levels, whereas P2Y1 receptors interact with Gq/11 and have no effect on cAMP. ADPβS inhibited cAMP production in cotransfected cells only, an effect that was antagonized by the A1 antagonist 8-cyclopentyl-1,3-dipropylxanthine, but not by MRS2179, and abolished by pertussis toxin. Thus, ADPβS appears to have acted via the A1 receptor ligand-binding site, i.e., the P2Y1/A1 dimer has novel pharmacological properties compared with the parent receptors. Interestingly, although ADPβS induced inositol phosphate synthesis, the A1 agonist cyclopentyl adenosine (CPA) did not. Thus, dimerization did not lead to a complete change in pharmacological properties in this case.
Using confocal laser microscopy to study the subcellular distribution of the P2Y1 and A1 receptors, Yoshioka et al. (2001) showed that both were expressed mainly near the plasma membrane of HEK293 cells. Furthermore, there was a strong overlap in their distribution in individual cells. This was confirmed in a subsequent study using the biophysical technique of bioluminescence resonance energy transfer (Yoshioka et al., 2002b). In the absence of agonists, the receptors showed a homogeneous colocalization across the cells. Addition of ADPβS and CPA together, but not alone, induced an increase in the bioluminescence resonance energy transfer ratio over 10 min. Thus, although the receptors have a constitutive association, their coactivation increased the association. This association was also seen with native receptors in central neurons. Using confocal laser microscopy and double immunofluorescence, Yoshioka et al. (2002a) demonstrated that the P2Y1 and A1 receptors colocalized in neurons of the rat cortex, hippocampus, and cerebellum. A direct association was then shown by their coimmunoprecipitation in membrane extracts from these regions.
Together, these studies clearly indicate that the rat P2Y1 and A1 receptors physically interact to form a functional dimer with novel pharmacological properties. The structural requirements for this interaction are not known at present. The physiological roles of the P2Y1/A1 dimer also remain to be determined, although Nakata et al. (2003) have pointed out that its pharmacological properties resemble those of a presynaptic receptor that mediates inhibition of neurotransmitter release in some tissues. Finally, it is still not known whether other P2Y subtypes also form functional heterodimers, but this is likely to be the case as Yoshioka et al. (2001) reported that the rat P2Y2 receptor also coimmunoprecipitated with the A1 receptor when they were coexpressed in HEK293 cells. Thus, the formation of oligomers by P2Y receptors is likely to be widespread and to greatly increase the diversity of purinergic signaling.
Indeed, homodimers of the human P2Y1 receptor have recently been shown to exist [Choi et al., 2005a (http://www.pa2online.org/abstracts/Vol3Issue2abst010P.pdf)]. Expressed receptors were labeled in culture, and FRET confocal microscopy was applied. This method can be used to report specifically on the receptors located at the cell membrane and measure the percentage dimerized. Constitutive P2Y1 homodimers were found; dimerization was increased by exposure to agonist to 80 to 90%. An obligate determinant of the dimer formation resides in the tail of the sequence. P2Y1 receptor agonist-induced internalization is preceded by the dimerization [Choi et al., 2005b (http://www.pa2online.org/abstracts/Vol3Issue2abst121P.pdf)].
C. Receptor Cross-Talk
The ability of GPCRs to modulate the signals initiated by other GPCRs, receptor tyrosine kinases and ligandgated ion channels is widespread and has been studied extensively (Selbie and Hill, 1998; Hur and Kim, 2002). A variety of mechanisms can underlie these interactions, and here we will discuss a few examples of P2Y receptor-mediated cross-talk with these three different types of receptors.
1. G Protein-Coupled Receptors
A common form of cross-talk is for two types of receptors to produce a greater than additive, or synergistic, change in the level of second messengers. Thus, coactivation of a variety of Gi-coupled receptors and Gq/11-coupled P2Y receptors induces a synergistic rise in intracellular IP3 and Ca2+ levels (Gerwins and Fredholm, 1992; Megson et al., 1995; Selbie et al., 1997; Werry et al., 2002) and release of AA (Felder et al., 1991; Selbie et al., 1997). For example, the DDT1 MF-2 smooth muscle cell line expresses native A1 and P2Y2 or P2Y4 receptors and at concentrations that had little or no effect on IP3 levels, the A1 agonist CPA potentiated the response to ATP in a concentration-dependent manner (Gerwins and Fredholm, 1992). Similar effects were seen when the downstream effect of IP3, a rise in intracellular [Ca2+], was measured. Low concentrations of CPA and ATP or UTP applied individually had little effect, but when administered together the increase in [Ca2+] was nearly maximal. A subsequent study demonstrated that PKC translocation from the cytoplasm to the plasma membrane was also potentiated in these cells (Fredholm et al., 2003). Recombinant human CXCR2 chemokine receptors expressed in HEK293 cells and the native P2Y1 and P2Y2 receptors also display cross-talk (Werry et al., 2002). The CXCR2 receptor couples to Gi and so its agonist IL-8 has no effect on intracellular Ca2+ levels when applied alone. However, after prestimulation of cells with ATP or UTP, IL-8 induced a substantial [Ca2+] elevation. Prestimulation of P2Y1 and P2Y2 receptors also induced the appearance of a Ca2+ response to subsequent stimulation of the endogenous, Gs-coupled β-adrenoceptor. Activation of Gq/11-coupled P2Y receptors also leads to synthesis of DAG and stimulation of PKC, which can induce the release of AA. This arm of the Gq/11-activated signaling cascade can also cross-talk with Gi-coupled receptors. Expression and activation of recombinant m2, α2, and D2 receptors in CHO-K1 cells did not induce AA release per se, but each augmented the response mediated by ATP acting at an endogenous P2Y receptor (Felder et al., 1991).
The cross-talk between the P2Y2 and CXCR2 receptors involved an enhancement of PLC activity (Werry et al., 2003) and that between neuropeptide Y1 and P2Y receptors in CHO-K1 cells was inhibited by βγ subunit scavengers (Selbie et al., 1997). Thus, the most likely possibility is that G protein βγ subunits generated by Gi-coupled receptors interact with G protein α subunits generated by the P2Y agonists at the level of PLCβ, thus increasing the production of IP3 and DAG, with consequent increases in Ca2+ release and PKC activation.
P2Y receptors have also been reported to inhibit effects evoked by non-P2Y GPCR. Sphingosine-1-phosphate acts via sphingosine-1-phosphate receptors in renal mesangial cells to activate the MAP kinase pathway and induce mitogenesis. Activation of endogenous P2Y receptors by ATP or UTP inhibits these actions (Xin et al., 2004). The nucleotide-mediated inhibition was reversed by inhibitors of protein kinase C. In rat C6 glioma, β-adrenoceptors mediate a decrease in the activity of protein kinase B and induce cellular differentiation (Van Kolen and Slegers, 2004). Costimulation of the native P2Y12 receptors reversed the inhibition of protein kinase B to activation, abolished the differentiation, and induced cell proliferation. These effects were Gi- and phosphatidylinositol 3-kinase-dependent. Thus, P2Y receptors may be important modulators of cell growth and differentiation induced by other GPCR agonists.
Recently, two types of cross talk between P2Y receptors and receptors for cysteinyl-leukotrienes (CysLT) have been described. The first is a positive action, as found in human mast cells, where UDP at nanomolar levels activated the CysLT1 and CysLT2 receptors to stimulate Ca2+ mobilization and cytokine production (Mellor et al., 2001, 2002, 2003). ATP and UTP were ineffective, and the UDP preference suggests an interaction with the P2Y6 receptor. The nature and mechanism of this interaction are at present not determined (Kanaoka and Boyce, 2004). The second type is a negative cross-talk between P2Y and CysLT1 receptors both in human monocyte/macrophage-like U937 cells (which constitutively express P2Y2 and P2Y6 receptors), and in COS-7 cells transiently expressing the CysLT1 receptor (Capra et al., 2005). In U937 cells, activation of P2Y receptors with ATP or UDP induced CysLT1 receptor heterologous desensitization. Conversely, activation of the CysLT1 receptor by cysteinyl-leukotrienes had no effect on P2Y receptor responses, suggesting that the latter have a hierarchy in producing desensitizing signals. Although cysteinyl-leukotriene-induced homologous desensitization of the CysLT1 receptor was followed by receptor internalization, ATP/UDP-induced CysLT1 receptor desensitization was unable to cause receptor internalization. Moreover, at variance with homologous desensitization, P2Y receptor-induced regulation of the CysLT1 receptor was dependent upon PKC, suggesting that P2Y receptors may mediate PKC-dependent phosphorylation of the CysLT1 receptor. This study demonstrates, for the first time, that CysLT1 receptor desensitization and trafficking are differentially regulated by its cognate ligand or by extracellular nucleotides. Moreover, because both cysteinyl-leukotrienes and nucleotides accumulate at sites of inflammation, this receptor cross-talk may represent a mechanism to fine tune the inflammatory response, i.e., a feedback mechanism by which extracellular nucleotides protect cells from the vast increase of inflammatory mediators characteristic of several pathological processes. The high degree of functional interaction between P2Y and CysLT receptors is also confirmed by the demonstration that montelukast and pranlukast, two well-known selective CysLT1 receptor antagonists (Brink et al., 2003), functionally interact with P2Y receptor signaling pathways by inhibiting nucleotide-induced calcium mobilization in a relatively nonsubtype-specific manner (Mamedova et al., 2005).
2. Receptor Tyrosine Kinases
P2Y receptors can modulate the activity of receptor tyrosine kinases. For example, LPS acts through a member of this family to activate IκB kinase, which controls the activity of the transcription factor NF-κB. In murine J774 macrophages, UTP, acting via P2Y6 receptors, slightly induced IκB kinase activation and greatly potentiated the effect of LPS (Chen and Lin, 2001). The release of intracellular Ca2+ stores and activation of calmodulin-dependent kinase by UTP played a major role in this synergistic interaction. P2Y receptors can also act to inhibit receptor tyrosine kinase signaling. The growth hormone PDGF acts via a receptor tyrosine kinase to increase human vascular smooth muscle cell proliferation, as measured by [3H]thymidine incorporation into DNA and an increase in cell number (White et al., 2000). UTP and UDP had no effect on [3H]thymidine incorporation on their own, but significantly reduced the response to PDGF. Similarly, the nucleotides had no effect on cell number per se, but the increase induced by PDGF over 7 days was abolished. Interestingly, ATP potentiated the [3H]thymidine incorporation elicited by PDGF. The mechanism underlying these excitatory and inhibitory effects of P2Y receptors is not known, but the authors demonstrated clearly that changes in Ca2+ levels or p42/p44 MAPK activity were not involved. The fact that different nucleotide agonists had opposing effects also indicates that the roles of P2Y receptors in cell proliferation can be complex. P2Y6 receptors have also been reported to protect against apoptosis induced by the receptor tyrosine kinase agonist tumor necrosis factor α in 1321N1 astrocytoma cells (Kim et al., 2003b). Interestingly, activation of the P2Y4 receptor was ineffective, again indicating that the interactions of P2Y receptors with receptor tyrosine kinases can be complex.
3. Ligand-Gated Cation Channels
P2X receptors are ligand-gated cation channels and are often expressed in the same cells as P2Y receptors. Thus, there is a great scope for bidirectional cross talk between these two families of nucleotide-sensitive receptors. When ATP is applied to Xenopus oocytes expressing the recombinant human P2X1 receptor, it induces a transient inward current (Vial et al., 2004). Repeated application at 5-min intervals caused rundown of this response, which was reversed by coexpression and coadministration of the P2Y1 receptor, plus ADP, or the P2Y2 receptor, plus UTP. This effect was mediated by activation of a protein kinase but did not involve direct phosphorylation of the P2X1 receptor. Rather, an accessory protein appeared to be the target.
Cross-talk can also take place in the opposite direction, e.g., P2X1 receptors have been shown to modulate P2Y receptor activity. P2X1 receptors are Ca2+ permeable and in megakaryocytes, the P2X1 agonist α,β-meATP induced a rapid and transient Ca2+ influx (Vial et al., 2002). In contrast, ADP, acting via P2Y1 receptors, evoked a slower, larger, and more maintained Ca2+ influx. The response seen when the two agonists were coapplied was markedly accelerated and the peak amplitude was potentiated, suggesting that the P2X1 receptor may have a priming role in activation of P2Y1 receptors during platelet stimulation.
P2Y receptors also interact with non-nucleotide ligand-gated cation channels. The TRPV1 receptor is the target for capsaicin and is coexpressed with P2Y1 receptors in a population of nociceptive sensory nerves (Kennedy et al., 2003). ATP, 2-MeSATP, and ADP shifted the capsaicin concentration-response curve 2-fold to the left, with no change in maximum response, and decreased the threshold for activation by heat from 42 to 35°C (Tominaga et al., 2001). The potentiation was inhibited by the PKC inhibitor calphostin and mimicked and then occluded by the PKC activator PMA. Thus, phosphorylation of TRPV1 receptors by P2Y1 receptor-activated PKC can modulate the sensitivity of sensory neurons to noxious stimuli.
X. Gene Activation Regulated by P2Y Receptors
A. Scope of the Gene Activations
GPCRs possess the important capability of maintaining signal transduction processes over extended periods where needed, allowing some transductions to proceed to the level of nuclear gene regulation and protein synthesis. Nearly all the cases for which this is known involve Gs-linked receptors, through their activation of the cAMP response element-binding transcription factor (CREB). Only one Gs-linked P2Y receptor is known: P2Y11 (see section VI.E.); its responses in dendritic cells of the immune system (Wilkin et al., 2001; Marteau et al., 2004, 2005) suggest that it is likely there to promote transcription through the CREB pathway, since the activation of P2Y11 led to production of specific cytokines via a cAMP increase. Furthermore, another study (van der Weyden et al., 2000a) made in renin-expressing cells has shown that activation of the P2Y11 receptor therein increased renin mRNA and protein 3-fold over 36 h and gave strong luciferase-reporter activity when linked to a renin gene construct. This response was established to be through the CREB pathway, since the P2Y11 activation stimulated CREB phosphorylation and its effects were blocked by mutation at the cAMP response element within the renin gene promoter.
There is a small amount of definitive information on gene transcription control by identified Gq- or Gi/o-linked P2Y receptors. One such case is represented by the P2Y2 receptor. When stably expressed in 1321N1 cells, this receptor was found to signal through the p38 MAPK cascade to phosphorylate CREB, which then mediated cis-activation of target genes, including the antiapoptotic bcl-2 and bcl-xl genes (Chorna et al., 2004). UTP incubation also up-regulated expression of a range of genes for neurotrophins and neuropeptides and induced proliferation of the astrocytoma cells. It is interesting that P2Y1 behaves differently from P2Y2 in the same cells. In a study performed with the P2Y1 receptor therein (Sellers et al., 2001), agonist treatment led, via a different intracellular cascade, to the activation instead of the transcription factor Elk-1. The outcome of that transduction route was apoptosis and inhibition of cell proliferation. In another study performed on rat astrocytes, activation of a native PTX-sensitive P2Y-like receptor increased the binding to DNA of the activator protein-1 and NF-κB transcription factors (Brambilla et al., 2003). The outcome in that case was the de novo synthesis of cyclooxygenase-2 (confirmed at the protein level), whose gene transcription has been shown to occur through these factors (Brambilla et al., 1999, 2000, 2002, 2003), followed by elongation of astrocytic processes, an index of reactive astrogliosis.
B. Synaptically Released ATP Can Act in the Control of Gene Transcription
At many synapses, ATP is stored and released with another transmitter throughout life; hence, it has the potential to serve as an additional nerve-released trophic factor, which would imply its regulation of gene transcription. This possibility has recently been explored using initially the neuromuscular junction (NMJ) of skeletal muscles; this type of synapse has the greatest accessibility and uniformity in its population and is currently the best understood of all types in structure, operation, and synaptogenesis. ATP is costored in vesicles of the nerve terminals with ACh and is coreleased quantally with it (at a ratio of approximately 1 ATP to 6 ACh) (Silinsky and Redman, 1996). Also, UTP is present in those vesicles at ~10% of the ATP content (Zimmermann, 1994).
Functional postsynaptic P2Y1 and P2Y2 receptors colocalized at the NMJs with the nicotinic ACh receptors (AChRs) have been demonstrated in mammalian, chicken, and amphibian muscles (Choi et al., 2001; Tsim et al., 2003; Tung et al., 2004). There is earlier phenotypic evidence that muscle AChR and acetylcholinesterase (AChE) have some controls of their expression and clustering at the NMJ in common (Sanes and Lichtman, 1999); hence, the genes of both those effectors have been studied (Choi et al., 2001, 2003), using differentiated myotubes when the P2Y receptors are present throughout the cell surface before being clustered at nerve contact when in vivo. Exposure to 2-MeSADP or to UTP each produces an activation of the genes of the multiple subunits of the AChR and also of the AChE catalytic subunit gene. Properties of the gene activations mediated by the native P2Y1 and P2Y2 receptors in the muscle cells (Choi et al., 2001; Tung et al., 2004) are 1) a strong increase (up to 550%) in each mRNA (confirmed at the protein level where tested), related to the concentration of the P2Y agonists; 2) a dose-dependent agonistinduced increase in the activity of a promoter-luciferase reporter construct for each of the subunit genes; 3) approximate equivalence for the P2Y1 and P2Y2 receptor activities in stimulating the promoter, for each gene (one example is shown in Fig. 8C); 4) a total block of the P2Y1-coupled action at the gene promoters by the specific P2Y1 antagonist MRS2179 (Fig. 8C) or by suramin for both receptors; and 5) a great increase in the 2-MeSADP-activated promoter activities of the AChR genes when the P2Y1 receptor protein content was boosted by a transfection of its cDNA.
Fig. 8.

Examples of analysis of gene activations controlled through P2Y receptor action. A, promoter region of the gene for the catalytic subunit of AChE. The first exon is noncoding, within the 5′-untranslated region. Promoter sites lie on either side of that, illustrated for four of the transcription factors active in muscle cells. Shown are two of the four predicted sites there for the transcription factor Elk-1 (Elk-1 [1] and Elk-1 [3]), which were identified as specific Elk-1 binding sites by gel mobility shift analyses on extracts of myotube nuclei. DNA sequences at those sites were inserted upstream in a luciferase reporter vector for assays as in B. B, two reporter constructs controlled by the specific binding sites for Elk-1 from the AChE gene promoter are used to show that Elk-1 is activated when the P2Y1 or P2Y2 receptors in a muscle cell are stimulated; hence, Elk-1 binds to activate transcription of the AChE gene there. Mouse myotubes transfected with either construct were incubated at 37°C with the agonists shown, or with none (Basal), or with the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA), which produces a nonspecific activation of AChE synthesis in muscle cells. Both Elk-1 sites are seen to have receptor-stimulated promoter activity (and when combined in tandem they are synergistic: not shown). Elk-1 is activated by specific phosphorylation through P2Y1 (2-MeSADP) or P2Y2 (UTP) action. The effect of ATP is significantly higher than effects for the other two (with all three at a saturating concentration), since it acts at both subtypes. C, similar analysis performed using the promoter region of the ε subunit of the nicotinic AChR gene. Both the P2Y1 and P2Y2 receptors act through Elk-1 in activating the multiple genes for the AChR in muscle cells (α, δ, and ε subunit genes have been tested). Note that in confirmation, the P2Y1-specific antagonist MRS2179 blocks this action by 2-MeSADP but not that by UTP (data from Choi et al., 2003; Tung et al., 2004).
Examination of several potential transcription factors for this action has identified Elk-1 as a candidate, which becomes maximally phosphorylated and nuclear-localized when myotubes are exposed for 30 min to 2-MeSADP or UTP. Four potential DNA binding sequences for Elk-1 were identified in the first intron of the AChE gene (Fig. 8A). Two were proven to be active by gel mobility shift assays, and each of these sites was used in the reporter assay, replacing the entire 2.2-kb DNA of the promoter-containing region by a 20-nucleotide Elk-1 binding site. Each again showed AChE gene promoter activity dependent on P2Y1 or P2Y2 receptor activation on the myotube (Fig. 8B). This was enhanced when the two sites were combined. In confirmation of their activity in the native gene, when the entire 2.2-kb promoter-containing DNA was mutated at six bases in those two sites, its P2Y1/P2Y2-mediated promoter activity was abolished.
The significance of the location at the synapse of two P2Y receptors that signal to the transcription of genes encoding effectors of the postsynaptic machinery should be considered in the context that a few subsynaptic nuclei at each junction become transcriptionally specialized during skeletal muscle development to sustain the local synthesis of the operational proteins of the junction, including the postsynaptic AChR and AChE (Duclert and Changeux, 1995). The P2Y1 and P2Y2 receptors at the synapse are thus part of a local circuit allowing efficient genetic control. ATP would not be the sole regulator of the expression of the AChR and AChE genes at the NMJ, since the neurally released trophic protein neuregulin is another major one, which also has its receptor, the ErbB protein, at the postsynaptic NMJ membrane (Fischbach and Rosen, 1997) and signals through a transduction cascade to activate a specific transcription factor in the nearby subsynaptic nuclei (Schaeffer et al., 2001). There is some evidence for an equivalent cascade route for the P2Y1 receptor there (Choi et al., 2003), but this is not known fully yet, nor for the P2Y2 receptor at this location. The primary action of neuregulin is in the development of the NMJ, although it later contributes to the maintenance of the NMJ (Buonanno and Fischbach, 2001). It is a protein secreted in low amount, and it may be that the constant large quantity of ATP released is also needed for an additional role in the maintenance, specifically, of synaptic AChRs and AChE.
In conclusion, for some P2Y receptors a contribution to gene transcription control over a longer time frame may commonly be a role. Synapses elsewhere should be examined for a situation similar to that at the NMJ. In short-term phenotypic analyses, it may be difficult to recognize all of the functions of those receptors. Cases in which a P2Y gene knockout seems to have no obvious effect, other than where that is shown to be by compensation by another subtype, may be in this category.
XI. Potential Therapeutic Applications
There is increasing interest in the expression of P2Y receptors in pathological conditions and in the therapeutic potential of P2Y receptor-related compounds (see also reviews by Burnstock and Williams, 2000; Boeynaems et al., 2001, 2005a,b; Yerxa, 2001; Burnstock, 2002, 2006; Jacobson et al., 2002; Ralevic and Burnstock, 2003; Gachet, 2005).
Based on the firmly established role of P2Y1 and P2Y12 receptors in platelet aggregation (see above), one of the most interesting therapeutic applications is currently represented by the use of antagonists of these receptors as antithrombotic agents in the prevention of recurrent stroke and heart attacks. Thus, both P2Y1 and P2Y12 receptors constitute targets for antithrombotic therapy, and compounds with a dual action might also be of interest. However, the agents currently on the market (ticlopidine and clopidogrel) or known to be in development (prasugrel, cangrelor, and AZD6140) all target the P2Y12 receptor. The thienopyridines (ticlopidine, clopidogrel, and prasugrel) inactivate the P2Y12 receptor irreversibly via covalent binding of an active metabolite generated in the liver. The other compounds are competitive antagonists. Cangrelor (AR-C69931MX), an ATP derivative, is suitable for intravenous perfusion, whereas AZD6140 is in clinical development as an orally active agent (Boeynaems et al., 2005b). Human myocardium expresses a full panel of both P2Y and P2X receptors (Banfi et al., 2005), and it has also been suggested recently that antagonists to P2Y2, P2Y6, and P2Y11 receptors could be beneficial in the treatment of hypertension and congestive heart failure (Balogh et al., 2005). P2Y2 receptor up-regulation has been reported to contribute to neointimal hyperplasia and inflammation of rabbit carotid arteries through the increased proliferation of smooth muscle cells and the recruitment of monocytes via the endothelium-dependent up-regulation of vascular cell adhesion molecule-1 (Seye et al., 2002, 2003, 2004). These findings may open up further therapeutic possibilities in the cardiovascular field, in particular, in preventing and treating atherosclerosis.
P2Y receptors appear to be involved in insulin release from pancreatic β cells. Thus, they are a candidate target for the design of innovative antidiabetic drugs (Solini et al., 2003).
Purinergic signaling plays major roles in different activities in the gut. For example, T cells are thought to play a primary role in the induction of epithelial cell damage in IBD, and the P2Y6 receptor was found to be highly expressed in the T cells infiltrating bowel involved in IBD but absent in T cells in the unaffected bowel. P2Y6 receptors appear to be involved in monocytic release of inflammatory cytokines. P2Y receptors on smooth muscle and ATP production in myenteric plexus increase in postoperative ileus, probably contributing to delayed colonic transport (Wang et al., 2004b).
P2Y2 receptors are present in both quiescent and activated hepatic stellate cells. Quiescent cells express P2Y2 and P2Y4 receptors whereas activated cells express P2Y6 receptors (Dranoff et al., 2004).
P2Y1, P2Y2, and P2Y11 receptors located on immune and inflammatory cells play a pivotal role in inflammation and immunomodulation (Schnurr et al., 2003; Luttikhuizen et al., 2004). Multiple P2Y receptors on osteoblasts, osteoclasts, and chondrocytes are being considered as potential targets for the development of therapeutics to inhibit bone resorption in diseases such as rheumatoid arthritis, osteoporosis, tumor-induced osteolysis, and periodontitis (Dixon and Sims, 2000).
There is a substantial presence of P2Y receptors in the kidney, in different regions of the nephron, in the glomerulus, and in the renal vascular system. P2Y receptors mediate renin secretion, and P2Y receptor-related compounds are being explored for the treatment of chronic renal failure and transplantation-induced erythrocytosis (Jackson, 2001). In polycystic kidney disease, there is an increase in expression of P2Y2 and P2Y6 receptors and P2Y antagonists and inhibitors of ATP release are being explored as therapeutic agents (Turner et al., 2004)
P2Y receptor antagonists have been proposed as potential neuroprotective agents in the brain, by modulation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-induced currents and by excessive activation of glutamate receptor systems being implicated in cell death associated with stroke, epileptic seizures, and neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (Zona et al., 2000). Endogenous ATP has been claimed to be involved in the regulation of anxiety via stimulation of P2Y1 receptors in the dorsomedial hypothalamus in rats (Kittner et al., 2003).
Multiple P2X and P2Y receptor subtypes are expressed by astrocytes, oligodendrocytes, and microglia (see Abbracchio and Verderio, 2006). It has also been claimed that P2Y2 receptors activate neuroprotective mechanisms in astrocytic cells (Chorna et al., 2004). P2Y receptors mediate reactive astrogliosis via induction of cyclooxygenase-2, and P2Y receptor antagonists might counteract excessive cyclooxygenase-2 activation in both acute and chronic neurological disease (Brambilla et al., 1999, 2002; Brambilla and Abbracchio, 2003). It has been reported that ATP continually modulates the cerebellar circuit by increasing the inhibitory input to Purkinje neurons probably via P2Y2 and/or P2Y4 (and possibly P2X5) receptors, thus decreasing the main cerebellar output activity that contributes to locomotor coordination (Brockhaus et al., 2004).
The anticancer activity of adenine nucleotides has been known since 1983. Intraperitoneal injection of ATP into tumor-bearing mice results in significant anticancer activity against several fast-growing, aggressive carcinomas (Agteresch et al., 2003). The subtypes of P2 receptors involved are largely unresolved, but P2Y1 and P2Y2 receptors are considered as targets for tumor cell antiproliferation.
ATP is clearly involved in the initiation of pain in nociceptive sensory nerves, and although the main focus of attention has been P2X3 receptors, there is increasing evidence for a role of P2Y receptors in the pain pathways. P2Y1 and P2X3 receptors are colocalized in a subpopulation of dorsal root sensory neurons (Ruan and Burnstock, 2003) and up-regulation of P2Y1 receptor expression occurs after transection of sciatic nerves (Xiao et al., 2002). Analgesic effects of intrathecal administration of P2Y receptor agonists UTP and UDP in normal rat and neuropathic rat pain models have been reported, suggesting that P2Y2 (and/or P2Y4) and P2Y6 receptors produce inhibitory effects on spinal pain transmission (Okada et al., 2002).
In airway epithelial cells, activation of P2Y2 receptors stimulates the secretion of chloride via outwardly rectifying chloride channels and independently from the CFTR. This constitutes the rationale for the development of aerosolized P2Y2 receptor agonists for improving clearance of secretions from the bronchi in the treatment of cystic fibrosis (Bennett et al., 1996), chronic bronchitis (Olivier et al., 1996), chronic obstructive pulmonary disease, and sputum expectoration in smokers (Yerxa, 2001). ATP and UTP stimulate P2Y2 receptormediated surfactant secretion and transepithelial chloride secretion in type II alveolar cells, but there are abnormalities in this mechanism in cystic fibrosis. In view of the bronchoconstrictor action of adenosine, uracil nucleotides were preferred to adenine nucleotides, and dinucleotides were selected because of their longer halflife: first Up4U (INS365) and later Up4dC (INS37217) (Yerxa et al., 2002). Various clinical trials have been performed or planned in cystic fibrosis, chronic bronchitis, the common cold, and allergic rhinitis. However, in ophthalmology the first convincing results have been obtained. P2Y2 receptors stimulate chloride and water secretion from conjunctival epithelial cells, leading to a better lubrication of the ocular surface. In a phase III clinical trial, Up4U drops improved corneal lesions over a 6- to 24-week period in patients suffering from dry eye syndrome (Nichols et al., 2004). A new drug application for Up4U (diquafosol) in that indication has been submitted to the U.S. Food and Drug Administration.
Purinergic signaling is widespread in the eye, and novel therapeutic strategies are being developed for glaucoma, dry eye, and retinal detachment (Pintor et al., 2003). P2Y receptors on human corneal epithelial cells appear to play a critical role in the injury repair process (Klepeis et al., 2004). In the auditory system, ATP, acting via P2Y receptors, depresses sound-evoked compound action potentials in the auditory nerve (Sueta et al., 2003).
ATP potently relaxes cavernosal smooth muscle via P2Y receptors that are present both on smooth muscle and endothelial cells, suggesting that P2Y receptor-mediated purinergic signaling may be involved in the pathophysiology of erectile dysfunction (Gür and Öztürk, 2000). ATP stimulates a biphasic change in transepithelial conductance in the human uterine cervix, phase 1 mediated by the P2Y2 receptor and phase II by the P2X4 receptor (Gorodeski, 2002), which has potential for therapeutic manipulation of fertility and contraception.
Tables 5 through 12 summarize the P2Y receptor subtypes discussed in this article.
Acknowledgments
We thank Dr. Stefano Costanzi, Molecular Recognition Section, Laboratory of Bioorganic Chemistry, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD, for providing Fig. 7, and Dr. Davide Lecca, Department of Pharmaceutical Science, University of Milan, Milan, Italy, for bioinformatics help in the preparation of the P2Y receptor sheets.
This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, and partly by the Italian Ministry of Education (Project of National Research Interest PRIN-COFIN 2002 and 2004 and FIRB RB4U09ZEN to M.P.A.).
Footnotes
Abbreviations: AMP, adenosine 5′-monophosphate; TM, transmembrane domain; GPCR, G protein-coupled receptor; PTX, pertussis toxin; PLC, phospholipase C; PI3-K, phosphatidylinositol 3-kinase; IP3, inositol triphosphate; UTP, uridine 5′-triphosphate; 2-MeSADP, 2-methylthio-ADP; MAP, mitogen-activated protein; ERK, extracellular signal-regulated protein kinase; PK, protein kinase; UDP, uridine 5′-diphosphate; CHO, Chinese hamster ovary; SCG, superior cervical ganglion; 2-MeSATP, 2-methylthio-ATP; PIP2, phosphatidylinositol-4,5-bisphosphate; PI, phosphatidylinositol; GIRK, G protein-activated inward rectifier, Kir3; RGS, regulator of GPCR signaling; NHERF, Na+/H+ exchanger regulatory factor; CFTR, cystic fibrosis transmembrane conductance regulator; SAR, structure-activity relationship; ADPβS, adenosine 5′-O-(2-thiophosphate); MRS2255, 1,5-anhydro-2-(adenin-9-yl)-2,3-dideoxy-d-arabino-hexitol-6-triphosphate tetraammonium salt; β,γ-meATP, β,γ-methylene ATP; MRS2365, (1′S, 2′R, 3′S, 4′R,5′S)-4-[(6-amino-2-methylthio-9H-purin-9-yl-1-diphosphoryloxymethyl]bicyclo[3.1.0]hexane-2,3-diol; A3P5P, adenosine 3′,5′-diphosphate; MRS2179, 2′-deoxy-N6-methyladenosine-3′, 5′-bisphosphate; MRS2216, 2′-deoxy-2-chloro-N6-methyladenosine-3′,5′-biphosphate; MRS2279, (N)-methanocarba-N6-methyl-2chloro-2′-deoxyadenosine-3′,5′bisphosphate; MRS2500, 2-iodo-N6-methyl-(N)-methanocarba-20-deosyadenosine-30,50-bisphosphate; MRS2584, (1′S, 4′R,6′S,7′S)-phosphoric acid mono-[6-(2-iodo-6-methylaminopurin-9-yl)-4-phosphonooxymethyl-2-oxabicyclo[2.2.1]hept-7-yl] ester; MRS-2298, 2-[2-(2-chloro-6-methylamino-purin-9-yl)methyl]propane-1,3-bisoxy(diammoniumphosphate); AR-C67085MX, 2-propylthio-d-β,γ-dichloromethylene-ATP; AR-C69931MX, N6-[2-(methylthioethyl)-2-(3,3,3-trifluoropropylthio)-β,γ-dichloromethyl ATP, cangrelor; AZD-6140, 3-[{7-[2–3,4-difluoro-phenyl)-cyclopropryamino]-5-propylsulfanyl[1,2,3]triazolo[4,5-d]pyrimidine-3-yl}-5-(2-hydroxymethoxy)-cyclopentane-1,2-diol; INS49266, 6-phenylurea-2′,3′-phenylacetaldehyde acetal ATP; INS50589, ((2S,3aR,4R,6R,6aR)-4-(6-(3-ethylureido)-9H-purin-9-yl)-tetrahydro-2-styrylfuro[3,4-d][1,3]dioxol-6-yl)methyl dihydrogen phosphate; MRS2395, 2,2-dimethyl-propionic acid 3-(2-chloro-6-methylaminopurin-9-yl)-2-(2,2-dimethyl-propionyloxymethyl)-propyl ester; PPADS, pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid; Ap4A, diadenosine tetraphosphate; MRS2211, 3,5-dietylo-2-methyl-4-(trans-2-(4-nitrophenyl)vinyl-6-phenyl-1,4-dihydropyridine-3,5-dicarboxilate; ATPγS, adenosine-5′-O-(3-thio)triphosphate; UDPβS, uridine β-thiodiphosphate; UTPγS, uridine-5′-O-(2-thio)triphosphate; INS365, Up4U, P1,P4-di(uridine 5′-)tetraphosphate (tetrasodium salt); INS-37217, Up4dC, P1-(uridine 5′)-P4- (2′-deoxycytidine 5′)tetraphosphate (tetrasodium salt); MRS2341, (1′S,2′R,3′S,4′R,5′S)-4-(2,4(H,3H)-dioxopyrimidin-1-yl)-1 (riphosphoryloxymethyl)bicyclo[3.1.0]-hexane-2,3-diol; AR-C126313, 5-(7-chloro-4H-1-thia-3-aza-benzo[f]-4-yl)-3-methyl-6-thioxo-piperadin-2-one; AR-C118925, 5-((3,4-dihydro-2-oxo-5–2,8-dimethyl-dibenzo[2,3:6,7]cyclohept-4-enyl-4-thioxopyrimidin-1(2H)-yl)methyl)-N-(1H-tetrazol-5-yl)furan-2-carboxamide; MRS2578, 1,4-di-[(3-isothio-cyanato phenyl)-thioureido]butane; A3P5PS, adenosine 3′-phosphate 5′phosphosulfate; RT-PCR, reverse transcriptionpolymerase chain reaction; kb, kilobase(s); DAG, diacylglycerol; RGD, Arg-Gly-Asp; AA, arachidonic acid; NO, nitric oxide; IL, interleukin; MAPK, mitogen-activated protein kinase; JNK, c-Jun NH2-terminal kinase; EGFR, epidermal growth factor receptor; SH3, Src homology 3; PDGF, platelet-derived growth factor; LPS, lipopolysaccharide; IBD, inflammatory bowel disease; Ap5A, diadenosine pentaphosphate; Ap6A, diadenosine hexaphosphate; h, human; CS-47, prasugrel (LY640315); Ap3A, diadenosine triphosphate; GTPγS, guanosine 5′-O-(3-thio)triphosphate; TX, thromboxane; VASP, vasodilator-stimulated phosphoprotein; MDCK, Madin-Darby kidney cell; ABC, ATP-binding cassette; MDR, multidrug resistance; VDAC-1, voltage-dependent anion channel-1; ACh, acetylcholine; NDPK, nucleoside diphosphokinase; E-NTPDase, ecto-nucleotide triphosphate diphosphohydrolase; ENPP, ecto-nucleotide phosphophosphate/phosphodiesterase; NTP, nucleoside triphosphate; NDP, nucleoside diphosphate; FRET, fluorescence resonance energy transfer; CPA, cyclopentyl adenosine; CysLT, cysteinyl leukotriene receptor; IκB, inhibitor of nuclear factor (NF)-κB; PMA, phorbol 12-myristate 13-acetate; CREB, cAMP response element- binding tran-scription factor; NMJ, neuromuscular junction; AChR, acetylcholine receptor; AChE, acetylcholinesterase.
References
- Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, et al. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbracchio MP, Burnstock G, Boeynaems J-M, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, et al. The recently deorphanized GPR80 (GPR99) proposed to be the P2Y15 receptor is not a genuine P2Y receptor. Trends Pharmacol Sci. 2005;26:8–9. doi: 10.1016/j.tips.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Abbracchio MP, Verderio C. Pathophysiological roles of P2 receptors in glial cells. Novartis Found Symp. 2006;276:91–103. [PubMed] [Google Scholar]
- Abraham EH, Prat AG, Gerweck L, Seneveratne T, Arceci RJ, Kramer R, Guidotti G, Cantiello HF. The multidrug resistance (mdr1) gene product functions as an ATP channel. Proc Natl Acad Sci USA. 1993;90:312–316. doi: 10.1073/pnas.90.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agteresch HJ, van Rooijen MHC, van den Berg JWO, Minderman-Voortman GJ, Wilson JHP, Dagnelie PC. Growth inhibition of lung cancer cells by adenosine 5-triphosphate. Drug Dev Res. 2003;60:196–203. [Google Scholar]
- Akbar GK, Dasari VR, Webb TE, Ayyanathan K, Pillarisetti K, Sandhu AK, Athwal RS, Daniel JL, Ashby B, Barnard EA, et al. Molecular cloning of a novel P2 purinoceptor from human erythroleukemia cells. J Biol Chem. 1996;271:18363–18367. doi: 10.1074/jbc.271.31.18363. [DOI] [PubMed] [Google Scholar]
- Aleil B, Ravanat C, Cazenave J, Rochoux G, Heitz A, Gachet C. Flow cytometric analysis of intra-platelet VASP phosphorylation for the detection of clopidogrel resistance in patients with ischemic cardiovascular diseases. J Thromb Haemost. 2004;36:17–19. doi: 10.1111/j.1538-7836.2004.01063.x. [DOI] [PubMed] [Google Scholar]
- André P, Delaney SM, LaRocca T, Vincent D, DeGuzman F, Jurek M, Koller B, Phillips DR, Conley PB. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Investig. 2003;112:398–406. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev J, Galisteo ML, Kranenburg O, Logan SK, Chiu ES, Okigaki M, Cary LA, Moolenaar WH, Schlessinger J. Src and Pyk2 mediate G-protein-coupled receptor activation of epidermal growth factor receptor (EGFR) but are not required for coupling to the mitogen-activated protein kinase (MAPK) signaling cascade. J Biol Chem. 2001;276:20130–20135. doi: 10.1074/jbc.M102307200. [DOI] [PubMed] [Google Scholar]
- Ayyanathan K, Webb TE, Sandhu AK, Athwal RS, Barnard EA, Kunapuli SP. Cloning and chromosomal localization of the human P2Y1 purinoceptor. Biochem Biophys Res Commun. 1996;218:783–788. doi: 10.1006/bbrc.1996.0139. [DOI] [PubMed] [Google Scholar]
- Bagchi S, Liao Z, Gonzalez FA, Chorna NE, Seye CI, Weisman GA, Erb L. The P2Y2 nucleotide receptor requires interaction with αv integrins to communicate with Go and stimulate chemotaxis. J Biol Chem. 2005;280:39058–39066. doi: 10.1074/jbc.M504819200. [DOI] [PubMed] [Google Scholar]
- Bailey MA, Turner CM, Hus-Citharel A, Marchetti J, Imbert-Teboul M, Milner P, Burnstock G, Unwin RJ. P2Y receptors present in the native and isolated rat glomerulus. Nephron Physiol. 2004;96:79–90. doi: 10.1159/000076753. [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Shi L, Javitch JA. Structural mimicry in G protein-coupled receptors: implications of the high-resolution structure of rhodopsin for structure-function analysis of rhodopsin-like receptors. Mol Pharmacol. 2001;60:1–19. [PubMed] [Google Scholar]
- Balogh J, Wihlborg AK, Isackson H, Joshi BV, Jacobson KA, Arner A, Erlinge D. Phospholipase C and cAMP-dependent positive inotropic effects of ATP in mouse cardiomyocytes via P2Y11-like receptors. J Mol Cell Cardiol. 2005;39:223–230. doi: 10.1016/j.yjmcc.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsinde J, Winstead MV, Dennis EA. Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett. 2002;531:2–6. doi: 10.1016/s0014-5793(02)03413-0. [DOI] [PubMed] [Google Scholar]
- Baltensperger K, Porzig H. The P2U purinoceptor obligatorily engages the heterotrimeric G protein G16 to mobilize intracellular Ca2+ in human erythroleukemia cells. J Biol Chem. 1997;272:10151–10159. doi: 10.1074/jbc.272.15.10151. [DOI] [PubMed] [Google Scholar]
- Banfi C, Ferrario S, De Vincenti O, Ceruti S, Fumagalli M, Mazzola A, D’Ambrosi N, Volonté C, Fratto P, Vitali E, et al. P2 receptors in human heart: upregulation of P2X6 in patients undergoing heart transplantation, interaction with TNFα and potential role in myocardial cell death. J Mol Cell Cardiol. 2005;39:929–939. doi: 10.1016/j.yjmcc.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Simon J. An elusive receptor is finally caught: P2Y12, an important drug target in platelets. Trends Pharmacol Sci. 2001;22:388–391. doi: 10.1016/s0165-6147(00)01759-4. [DOI] [PubMed] [Google Scholar]
- Baurand A, Eckly A, Bari N, Leon C, Hechler B, Cazenave JP, Gachet C. Desensitization of the platelet aggregation response to ADP: differential down-regulation of the P2Y1 and P2cyc receptors. Thromb Haemost. 2000;84:484–491. [PubMed] [Google Scholar]
- Baurand A, Eckly A, Hechler B, Kauffenstein G, Galzi JL, Cazenave JP, Leon C, Gachet C. Differential regulation and relocation of the platelet P2Y receptors following activation: a way to avoid loss of haemostatic properties? Mol Pharmacol. 2005;67:721–733. doi: 10.1124/mol.104.004846. [DOI] [PubMed] [Google Scholar]
- Baurand A, Gachet C. The P2Y1 receptor as a target for new antithrombotic drugs: a review of the P2Y1 antagonist MRS-2179. Cardiovasc Drug Rev. 2003;21:67–76. doi: 10.1111/j.1527-3466.2003.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Baurand A, Raboisson P, Freund M, Leon C, Cazenave JP, Bourguignon JJ, Gachet C. Inhibition of platelet function by administration of MRS2179, a P2Y1 receptor antagonist. Eur J Pharmacol. 2001;412:213–221. doi: 10.1016/s0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]
- Beigi RD, Dubyak GR. Endotoxin activation of macrophages does not induce ATP release and autocrine stimulation of P2 nucleotide receptors. J Immunol. 2000;165:7189–7198. doi: 10.4049/jimmunol.165.12.7189. [DOI] [PubMed] [Google Scholar]
- Beigi R, Kobatake E, Aizawa M, Dubyak GR. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol. 1999;276:C267–C278. doi: 10.1152/ajpcell.1999.276.1.C267. [DOI] [PubMed] [Google Scholar]
- Bell PD, Lapointe JY, Peti-Peterdi J. Macula densa cell signaling. Annu Rev Physiol. 2003a;65:481–500. doi: 10.1146/annurev.physiol.65.050102.085730. [DOI] [PubMed] [Google Scholar]
- Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA. 2003b;100:4322–4327. doi: 10.1073/pnas.0736323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JS. Novel platelet inhibitors. Annu Rev Med. 2001;52:161–184. doi: 10.1146/annurev.med.52.1.161. [DOI] [PubMed] [Google Scholar]
- Bennett WD, Olivier KN, Zeman KL, Hohneker KW, Boucher RC, Knowles MR. Effect of uridine 5′-triphosphate plus amiloride on mucociliary clearance in adult cystic fibrosis. Am J Respir Crit Care Med. 1996;153:1796–1801. doi: 10.1164/ajrccm.153.6.8665037. [DOI] [PubMed] [Google Scholar]
- Berti-Mattera LN, Wilkins PL, Madhun Z, Suchovsky D. P2-purigenic receptors regulate phospholipase C and adenylate cyclase activities in immortalized Schwann cells. Biochem J. 1996;314:555–561. doi: 10.1042/bj3140555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco F, Fumagalli M, Pravettoni E, D’Ambrosi N, Volonté C, Matteoli M, Abbracchio MP, Verderio C. Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Rev. 2005;48:144–156. doi: 10.1016/j.brainresrev.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Bigonnesse F, Levesque SA, Kukulski F, Lecka J, Robson SC, Fernandes MJ, Sevigny J. Cloning and characterization of mouse nucleoside triphosphate diphosphohydrolase-8. Biochemistry. 2004;43:5511–5519. doi: 10.1021/bi0362222. [DOI] [PubMed] [Google Scholar]
- Boarder M, Webb TE. P2Y receptors structure and function, in Purinergic and Pyrimidinergic Signalling. In: Abbracchio MP, Williams M, editors. Handbook of Experimental Pharmacology. part I. Vol. 151. Springer-Verlag; Heidelberg, Germany: 2001. pp. 65–88. [Google Scholar]
- Bodin P, Burnstock G. Synergistic effect of acute hypoxia on flow-induced release of ATP from cultured endothelial cells. Experientia (Basel) 1995;51:256–259. doi: 10.1007/BF01931108. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Purinergic signaling: ATP release. Neurochem Res. 2001a;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001b;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- Bodor ET, Waldo GL, Hooks SB, Corbitt J, Boyer JL, Harden TK. Purification and functional reconstitution of the human P2Y12 receptor. Mol Pharmacol. 2003;64:1210–1216. doi: 10.1124/mol.64.5.1210. [DOI] [PubMed] [Google Scholar]
- Boeynaems JM, Communi D, Gonzalez NS, Robaye B. Overview of the P2 receptors. Semin Thromb Hemost. 2005a;31:139–149. doi: 10.1055/s-2005-869519. [DOI] [PubMed] [Google Scholar]
- Boeynaems JM, Robaye B, Janssens R, Suarez-Huerta N, Communi D. Overview of P2Y receptors as therapeutic targets. Drug Dev Res. 2001;52:187–189. [Google Scholar]
- Boeynaems J-M, van Giezen H, Savi P, Herbert J-M. P2Y receptor antagonists in thrombosis. Curr Opin Invest Drugs. 2005b;6:275–282. [PubMed] [Google Scholar]
- Boeynaems JM, Wilkin F, Marteau F, Duhant X, Savi P, Gonzalez NS, Robaye B, Communi D. P2Y receptors: new subtypes, new functions. Drug Dev Res. 2003;59:30–35. [Google Scholar]
- Bogdanov YD, Dale L, King BF, Whittock N, Burnstock G. Early expression of a novel nucleotide receptor in the neural plate of Xenopus embryos. J Biol Chem. 1997;272:12583–12590. doi: 10.1074/jbc.272.19.12583. [DOI] [PubMed] [Google Scholar]
- Bogdanov Y, Rubino A, Burnstock G. Characterisation of subtypes of the P2X and P2Y families of ATP receptors in the foetal human heart. Life Sci. 1998a;62:697–703. doi: 10.1016/s0024-3205(97)01168-5. [DOI] [PubMed] [Google Scholar]
- Bogdanov YD, Wildman SS, Clements MP, King BF, Burnstock G. Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Br J Pharmacol. 1998b;124:428–430. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolego C, Ceruti S, Brambilla R, Puglisi L, Cattabeni F, Burnstock G, Abbracchio MP. Characterization of the signalling pathways involved in ATP and basic fibroblast growth factor-induced astrogliosis. Br J Pharmacol. 1997;121:1692–1699. doi: 10.1038/sj.bjp.0701294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolego C, Pinna C, Abbracchio MP, Cattabeni F, Puglisi L. The biphasic response of rat vesical smooth muscle to ATP. Br J Pharmacol. 1995;114:1557–1562. doi: 10.1111/j.1476-5381.1995.tb14939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreault F, Grygorczyk R. Cell swelling-induced ATP release and gadolinium-sensitive channels. Am J Physiol. 2002;282:C219–C226. doi: 10.1152/ajpcell.00317.2001. [DOI] [PubMed] [Google Scholar]
- Bouvier M. Oligomerization of G protein-coupled transmitter receptors. Nat Rev Neurosci. 2001;2:274–286. doi: 10.1038/35067575. [DOI] [PubMed] [Google Scholar]
- Bowler WB, Birch MA, Gallagher JA, Bilbe G. Identification and cloning of human P2U purinoceptor present in osteoclastoma, bone, and osteoblasts. J Bone Miner Res. 1995;10:1137–1145. doi: 10.1002/jbmr.5650100720. [DOI] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS. ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J Neurosci. 2004;24:8606–8620. doi: 10.1523/JNEUROSCI.2660-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J, Adams M, Ravi RG, Jacobson KA, Harden TK. 2-Chloro N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate is a selective high affinity P2Y1 receptor antagonist. Br J Pharmacol. 2002;135:2004–2010. doi: 10.1038/sj.bjp.0704673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Delaney SM, Villanueva D, Harden TK. A molecularly identified P2Y receptor simultaneously activates phospholipase C and inhibits adenylyl cyclase and is nonselectively activated by all nucleoside triphosphates. Mol Pharmacol. 2000;57:805–810. doi: 10.1124/mol.57.4.805. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Mohanram A, Camaioni E, Jacobson KA, Harden TK. Competitive and selective antagonism of P2Y1 receptors by N6-methyl 2′-deoxyadenosine 3′-5′-bisposphate. Br J Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Romero-Avila T, Schachter JB, Harden TK. Identification of competitive antagonists of the P2Y1-receptor. Mol Pharmacol. 1996a;50:1323–1329. [PubMed] [Google Scholar]
- Boyer JL, Siddiqi S, Fischer B, Romera-Avila T, Jacobson KA, Harden TK. Identification of potent P2Y-purinoceptor agonists that are derivatives of adenosine 5′-monophosphate. Br J Pharmacol. 1996b;118:1959–1964. doi: 10.1111/j.1476-5381.1996.tb15630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Abbracchio MP. Modulation of cyclooxygenase-2 and brain reactive astrogliosis by purinergic P2 receptors. Ann NY Acad Sci. 2003;939:54–62. doi: 10.1111/j.1749-6632.2001.tb03612.x. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Burnstock G, Bonazzi A, Ceruti S, Cattabeni F, Abbracchio MP. Cyclo-oxygenase-2 mediates P2Y receptor-induced reactive astrogliosis. Br J Pharmacol. 1999;126:563–567. doi: 10.1038/sj.bjp.0702333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Ceruti S, Malorni W, Cattabeni F, Abbracchio MP. A novel gliotic P2Y receptor mediating cyclooxygenase-2 induction in rat and human astrocytes. J Auton Ner Syst. 2000;81:3–9. doi: 10.1016/s0165-1838(00)00152-1. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Neary JT, Cattabeni F, Cottini L, D’ippolito GL, Schiller PC, Abbracchio MP. Induction of COX-2 and reactive astrogliosis by P2Y receptors in rat cortical astrocytes is dependent on ERK1/2 but independent of calcium signaling. J Neurochem. 2002;83:1285–1296. doi: 10.1046/j.1471-4159.2002.01239.x. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Neary JT, Fumagalli M, Cottini L, Cattabeni F, Schiller PR, Abbracchio MP. P2Y receptors in brain astroglial cells: identification of a gliotic P2Y receptor coupled to activation of a calcium-independent Ras/ERK1/2 pathway. Drug Dev Res. 2003;59:161–170. [Google Scholar]
- Braunstein GM, Roman RM, Clancy JP, Kudlow BA, Taylor AL, Shylonsky VG, Jovov B, Peter K, Jilling T, Ismailov II, et al. Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J Biol Chem. 2001;276:6621–6630. doi: 10.1074/jbc.M005893200. [DOI] [PubMed] [Google Scholar]
- Brink C, Dahlen SE, Drazen J, Evans JF, Hay DW, Nicosia S, Serhan CN, Shimizu T, Yokomizo T. International Union of Pharmacology. XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol Rev. 2003;55:195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- Brinson AE, Harden TK. Differential regulation of the uridine nucleotide-activated P2Y4 and P2Y6 receptors: SER-333 and SER-334 in the carboxyl terminus are involved in agonist-dependent phosphorylation desensitization and internalization of the P2Y4 receptor. J Biol Chem. 2001;276:11939–11948. doi: 10.1074/jbc.M009909200. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Dressel D, Herold S, Deitmer JW. Purinergic modulation of synaptic input to Purkinje neurons in rat cerebellar brain slices. Eur J Neurosci. 2004;19:2221–2230. doi: 10.1111/j.0953-816X.2004.03325.x. [DOI] [PubMed] [Google Scholar]
- Brown BS, Yu SP. Modulation and genetic identification of the M channel. Prog Biophys Mol Biol. 2000;73:135–166. doi: 10.1016/s0079-6107(00)00004-3. [DOI] [PubMed] [Google Scholar]
- Brown DA, Filippov AK, Barnard EA. Inhibition of potassium and calcium currents in neurones by molecularly-defined P2Y receptors. J Auton Nerv Syst. 2000a;81:31–36. doi: 10.1016/s0165-1838(00)00150-8. [DOI] [PubMed] [Google Scholar]
- Brown J, Brown CA. Evaluation of reactive blue 2 derivatives as selective antagonists for P2Y receptors. Vasc Pharmacol. 2002;39:309–315. doi: 10.1016/s1537-1891(03)00030-2. [DOI] [PubMed] [Google Scholar]
- Brown SG, King BF, Kim YC, Burnstock G, Jacobson KA. Activity of novel adenine nucleotide derivatives as agonists and antagonists at recombinant rat P2X receptors. Drug Dev Res. 2000b;49:253–259. doi: 10.1002/1098-2299(200004)49:4<253::AID-DDR4>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A, Fischbach GD. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol. 2001;11:287–296. doi: 10.1016/s0959-4388(00)00210-5. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Do some nerve cells release more than one transmitter? Neuroscience. 1976;1:239–248. doi: 10.1016/0306-4522(76)90054-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G. A basis for distinguishing two types of purinergic receptor. In: Straub RW, Bolis L, editors. Cell Membrane Receptors for Drugs and Hormones: a Multidisciplinary Approach. Raven Press; New York: 1978. pp. 107–118. [Google Scholar]
- Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Potential therapeutic targets in the rapidly expanding field of purinergic signalling. Clin Med. 2002;2:45–53. doi: 10.7861/clinmedicine.2-1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Introduction: ATP and its metabolites as potent extracellular Agents. In: Schwiebert EM, editor. Current Topics in Membranes, Purinergic Receptors and Signalling. Vol. 54. Academic Press; San Diego: 2003. pp. 1–27. [Google Scholar]
- Burnstock G. Introduction: P2 receptors. Curr Top Med Chem. 2004;4:793–803. doi: 10.2174/1568026043451014. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Kennedy C. Is there a basis for distinguishing two types of purinoceptor? Gen Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Williams M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J Pharmacol Exp Ther. 2000;295:862–869. [PubMed] [Google Scholar]
- Burrell HE, Bowler WB, Gallagher JA, Sharpe GR. Human keratinocytes express multiple P2Y-receptors: evidence for functional P2Y1, P2Y2, and P2Y4 receptors. J Invest Dermatol. 2003;120:440–447. doi: 10.1046/j.1523-1747.2003.12050.x. [DOI] [PubMed] [Google Scholar]
- Buxton IL, Kaiser RA, Oxhorn BC, Cheek DJ. Evidence supporting the nucleotide axis hypothesis: ATP release and metabolism by coronary endothelium. Am J Physiol. 2001;281:H1657–H1666. doi: 10.1152/ajpheart.2001.281.4.H1657. [DOI] [PubMed] [Google Scholar]
- Buvinic S, Briones R, Huidobro-Toro JP. P2Y1 and P2Y2 receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br J Pharmacol. 2002;136:847–856. doi: 10.1038/sj.bjp.0704789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camaioni E, Boyer JL, Mohanram A, Harden TK, Jacobson KA. Deoxyadenosine-bisphosphate derivatives as potent antagonists at P2Y1 receptors. J Med Chem. 1998;41:183–190. doi: 10.1021/jm970433l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camden JM, Schrader AM, Camden RE, González FA, Erb L, Seye CI, Weisman GA. P2Y2 nucleotide receptors enhance α-secretase-dependent amyloid precursor protein processing. J Biol Chem. 2005;280:18696–18702. doi: 10.1074/jbc.M500219200. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: An unexpected form of cellular plasticity. Nat Rev Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- Capra V, Ravasi S, Accomazzo MR, Citro S, Grimoldi M, Abbracchio MP, Rovati GE. CysLT1 receptor is a target for extracellular nucleotide-induced heterologous desensitization: a possible feedback mechanism in inflammation. J Cell Sci. 2005;118:5625–5636. doi: 10.1242/jcs.02668. [DOI] [PubMed] [Google Scholar]
- Cattaneo M. P2 receptors and congenital platelet function defects. Semin Thromb Hemost. 2005;31:168–173. doi: 10.1055/s-2005-869522. [DOI] [PubMed] [Google Scholar]
- Cattaneo M, Lecchi A, Joshi BV, Ohno M, Besada P, Tchilibon S, Lombardi R, Bischofberger N, Harden TK, Jacobson KA. Antiaggregatory activity in human platelets of potent antagonists of the P2Y1 receptor. Biochem Pharmacol. 2004;68:1995–2002. doi: 10.1016/j.bcp.2004.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo M, Lecchi A, Lombardi R, Gachet C, Zighetti ML. Platelets from a patient heterozygous for the defect of P2CYC receptors for ADP have a secretion defect despite normal thromboxane A2 production and normal granule stores: further evidence that some cases of platelet ‘primary secretion defect’ are heterozygous for a defect of P2CYC receptors. Arterioscler Thromb Vasc Biol. 2000;20:E101–E106. doi: 10.1161/01.atv.20.11.e101. [DOI] [PubMed] [Google Scholar]
- Cattaneo M, Lecchi A, Randi AM, McGregor JL, Mannucci PM. Identification of a new congenital defect of platelet function characterized by severe impairment of platelet responses to adenosine diphosphate. Blood. 1992;80:2787–2796. [PubMed] [Google Scholar]
- Cattaneo M, Lombardi R, Zighetti ML, Gachet C, Ohlmann P, Cazenave JP, Mannucci PM. Deficiency of (33P)2MeS-ADP binding sites on platelets with secretion defect, normal granule stores and normal thromboxane A2 production: evidence that ADP potentiates platelet secretion independently of the formation of large platelet aggregates and thromboxane A2 production. Thromb Haemost. 1997;77:986–990. [PubMed] [Google Scholar]
- Cattaneo M, Zighetti ML, Lombardi R, Martinez C, Lecchi A, Conley PB, Ware J, Ruggeri ZM. Molecular bases of defective signal transduction in the platelet P2Y12 receptor of a patient with congenital bleeding. Proc Natl Acad Sci USA. 2003;100:1978–1983. doi: 10.1073/pnas.0437879100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centemeri C, Bolego C, Abbracchio MP, Cattabeni F, Puglisi L, Burnstock G, Nicosia S. Characterization of the Ca2+ responses evoked by ATP and other nucleotides in mammalian brain astrocytes. Br J Pharmacol. 1997;121:1700–1706. doi: 10.1038/sj.bjp.0701293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha B, Kim JH, Hut H, Hogema BM, Nadarja J, Zizak M, Cavet M, Lee-Kwon W, Lohmann SM, Smolenski A, et al. cGMP inhibition of Na+/H+ antiporter 3 (NHE3) requires PDZ domain adapter NHERF2, a broad specificity protein kinase G-anchoring protein. J Biol Chem. 2005;280:16642–16650. doi: 10.1074/jbc.M500505200. [DOI] [PubMed] [Google Scholar]
- Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, Zhu Y, McLaughlin MM, Murdock P, McMillan L, et al. A G protein-coupled receptor for UDP-glucose. J Biol Chem. 2000;275:10767–10771. doi: 10.1074/jbc.275.15.10767. [DOI] [PubMed] [Google Scholar]
- Chang K, Hanaoka K, Kumada M, Takuwa Y. Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J Biol Chem. 1995;270:26152–26158. doi: 10.1074/jbc.270.44.26152. [DOI] [PubMed] [Google Scholar]
- Charlton ME, Williams AS, Fogliano M, Sweetnam PM, Duman RS. The isolation and characterization of a novel G protein-coupled receptor regulated by immunologic challenge. Brain Res. 1997;764:141–148. doi: 10.1016/s0006-8993(97)00438-1. [DOI] [PubMed] [Google Scholar]
- Charlton SJ, Brown CA, Weisman GA, Turner JT, Erb L, Boarder MR. PPADS and suramin as antagonists at cloned P2Y- and P2U-purinoceptors. Br J Pharmacol. 1996;118:704–710. doi: 10.1111/j.1476-5381.1996.tb15457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BC, Lin WW. PKC- and ERK-dependent activation of IκB kinase by lipopolysaccaride in macrophages: enhancement by P2Y receptor-mediated CaMK activation. Br J Pharmacol. 2001;134:1055–1065. doi: 10.1038/sj.bjp.0704334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TK, Luo G, Ewing AG. Amperometric monitoring of stimulated catecholamine release from rat pheochromocytoma (PC12) cells at the zeptomole level. Anal Chem. 1994;66:3031–3035. doi: 10.1021/ac00091a007. [DOI] [PubMed] [Google Scholar]
- Chen ZP, Krull N, Xu S, Levy A, Lightman SL. Molecular cloning and functional characterization of a rat pituitary G protein-coupled adenosine triphosphate (ATP) receptor. Endocrinology. 1996;137:1833–1840. doi: 10.1210/endo.137.5.8612522. [DOI] [PubMed] [Google Scholar]
- Cheng AWM, Kong LW, Tung EKK, Siow NL, Choi RCY, Zhu SQ, Peng BH, Tsim KWK. cDNA encodes Xenopus P2Y1 nucleotide receptor: expression at the neuromuscular junctions. Neuroreport. 2003;14:351–357. doi: 10.1097/00001756-200303030-00011. [DOI] [PubMed] [Google Scholar]
- Cheung KK, Ryten M, Burnstock G. Abundant and dynamic expression of G protein-coupled P2Y receptors in mammalian development. Dev Dyn. 2003;228:254–266. doi: 10.1002/dvdy.10378. [DOI] [PubMed] [Google Scholar]
- Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analogue. J Pharmacol Exp Ther. 2004;311:1038–1043. doi: 10.1124/jpet.104.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi RC, Man ML, Ling KK, Ip NY, Simon J, Barnard EA, Tsim KW. Expression of P2Y1 receptors in chick muscle: their functional role in the regulation of acetylcholinesterase and acetylcholine receptors. J Neurosci. 2001;21:9224–9234. doi: 10.1523/JNEUROSCI.21-23-09224.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi RC, Siow NL, Cheng AW, Ling KK, Tung EK, Simon J, Barnard EA, Tsim KW. ATP acts via P2Y1 receptors to stimulate acetylcholinesterase and acetylcholine receptor expression: transduction and transcription control. J Neurosi. 2003;23:4445–4456. doi: 10.1523/JNEUROSCI.23-11-04445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi RC, Wong CSS, Simon J, Barnard EA. Agonist-induced homodimerisation of P2Y1 receptors demonstrated by fluorescence resonance energy transfer analysis. Br J Pharmacol. 2005a;146:11P. Abstract online. [Google Scholar]
- Choi RC, Wong CSS, Simon J, Barnard EA. Roles of the C-terminal domain in signal transduction, dimerisation, desensitisation and internalisation of the P2Y1 receptor. Br J Pharmacol. 2005b;146:C005. Abstract online. [Google Scholar]
- Chorna NE, Santiago-Pérez II, Erb L, Seye CI, Neary JT, Sun GY, Weisman GA, Gonzalez FA. P2Y2 receptors activate neuroprotective mechanisms in astrocytic cells. J Neurochem. 2004;91:119–132. doi: 10.1111/j.1471-4159.2004.02699.x. [DOI] [PubMed] [Google Scholar]
- Claeysen S, Govaerts C, Lefort A, Van Sande J, Costagliola S, Pardo L, Vassart G. A conserved Asn in TM7 of the thyrotropin receptor is a common requirement for activation by both mutations and its natural agonist. FEBS Lett. 2002;517:195–200. doi: 10.1016/s0014-5793(02)02620-0. [DOI] [PubMed] [Google Scholar]
- Clarke LL, Boucher RC. Chloride secretory response to extracellular ATP in human normal and cystic fibrosis nasal epithelia. Am J Physiol. 1992;263:C348–C356. doi: 10.1152/ajpcell.1992.263.2.C348. [DOI] [PubMed] [Google Scholar]
- Clarke LL, Harline MC, Gawenis LR, Walker NM, Turner JT, Weisman GA. Extracellular UTP stimulates electrogenic bicarbonate secretion across CFTR knockout gallbladder epithelium. Am J Physiol. 2000;279:G132–G138. doi: 10.1152/ajpgi.2000.279.1.G132. [DOI] [PubMed] [Google Scholar]
- Clarke LL, Harline MC, Otero MA, Glover GG, Garrad RC, Krugh B, Walker NM, Gonzalez FA, Turner JT, Weisman GA. Desensitization of P2Y2 receptor-activated transepithelial anion secretion. Am J Physiol. 1999;276:C777–C787. doi: 10.1152/ajpcell.1999.276.4.C777. [DOI] [PubMed] [Google Scholar]
- Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- Communi D, Gonzalez NS, Detheux M, Brezillon S, Lannoy V, Parmentier M, Boeynaems J-M. Identification of a novel human ADP receptor coupled to Gi. J Biol Chem. 2001a;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- Communi D, Govaerts C, Parmentier M, Boeynaems J-M. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J Biol Chem. 1997;272:31969–31973. doi: 10.1074/jbc.272.51.31969. [DOI] [PubMed] [Google Scholar]
- Communi D, Janssens R, Robaye B, Zeelis N, Boeynaems J-M. Rapid up-regulation of P2Y messengers during granulocytic differentiation of HL-60 cells. FEBS Lett. 2000;475:39–42. doi: 10.1016/s0014-5793(00)01618-5. [DOI] [PubMed] [Google Scholar]
- Communi D, Marteau F, Ben Addi A, Suarez Gonzalez N, Robaye B, Boeynaems J-M. Nucleotide receptor P2Y13. AfCS-Nature Molecule Pages. 2005a ID A000700. [Google Scholar]
- Communi D, Motte S, Boeynaems J-M, Pirotton S. Pharmacological characterization of the human P2Y4 receptor. Eur J Pharmacol. 1996a;317:383–389. doi: 10.1016/s0014-2999(96)00740-6. [DOI] [PubMed] [Google Scholar]
- Communi D, Paindavoine P, Place GA, Parmentier M, Boeynaems J-M. Expression of P2Y receptors in cell lines derived from the human lung. Br J Pharmacol. 1999a;127:562–568. doi: 10.1038/sj.bjp.0702560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communi D, Parmentier M, Boeynaems J-M. Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem Biophys Res Commun. 1996b;222:303–308. doi: 10.1006/bbrc.1996.0739. [DOI] [PubMed] [Google Scholar]
- Communi D, Pirotton S, Parmentier M, Boeynaems JM. Cloning and functional expression of a human uridine nucleotide receptor. J Biol Chem. 1995;270:30849–30852. doi: 10.1074/jbc.270.52.30849. [DOI] [PubMed] [Google Scholar]
- Communi D, Robaye B, Boeynaems JM. Pharmacological characterization of the human P2Y11 receptor. Br J Pharmacol. 1999b;128:1199–1206. doi: 10.1038/sj.bjp.0702909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Communi D, Robaye B, Boeynaems J-M. Nucleotide receptor P2Y4. AfCS-Nature Molecule Pages. 2005b ID A001691. [Google Scholar]
- Communi D, Suarez-Huerta N, Dussossoy D, Savi P, Boeynaems JM. Cotranscription and intergenic splicing of human P2Y11 and SSF1 genes. J Biol Chem. 2001b;276:16561–16566. doi: 10.1074/jbc.M009609200. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Fernando KC, Gu B, Tasevski V, Zhang W, Luttrell BM, Wiley JS. P2Y11 receptor expression by human lymphocytes: evidence for two cAMP-linked purinoceptors. Eur J Pharmacol. 2001;426:157–163. doi: 10.1016/s0014-2999(01)01222-5. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Lee JY, van der Weyden L, Jiang L, Ward P, Tasevski V, Luttrell BM, Morris MB. Pharmacological profile of a novel cyclic AMP-linked P2 receptor on undifferentiated HL-60 leukemia cells. Br J Pharmacol. 1998;124:1580–1585. doi: 10.1038/sj.bjp.0701985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley PB, Delaney SM. Scientific and therapeutic insights into the role of the platelet P2Y12 receptor in thrombosis. Curr Opin Hematol. 2003;10:333–338. doi: 10.1097/00062752-200309000-00002. [DOI] [PubMed] [Google Scholar]
- Costanzi S, Mamedova L, Gao ZG, Jacobson KA. Architecture of P2Y nucleotide receptors: structural comparison based on sequence analysis, mutagenesis, and homology modeling. J Med Chem. 2004;47:5393–5404. doi: 10.1021/jm049914c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho-Silva R, Parsons M, Robson T, Lincoln J, Burnstock G. P2X and P2Y purinoceptor expression in pancreas from streptozotocin-diabetic mice. Mol Cell Endocrinol. 2003;204:141–154. doi: 10.1016/s0303-7207(03)00003-0. [DOI] [PubMed] [Google Scholar]
- Crane JK, Olson RA, Jones HM, Duffey ME. Release of ATP during host cell killing by enteropathogenic E. coli and its role as a secretory mediator. Am J Physiol. 2002;283:G74–G86. doi: 10.1152/ajpgi.00484.2001. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Lazarowski E, Homolya L, Boucher RC, Koller BH, Grubb BR. Effect of loss of P2Y2 receptor gene expression on nucleotide regulation of murine epithelial Cl− transport. J Biol Chem. 1999;274:26461–26468. doi: 10.1074/jbc.274.37.26461. [DOI] [PubMed] [Google Scholar]
- Cristalli G, Podda GM, Costanzi S, Lambertucci C, Lecchi A, Vittori S, Volpini R, Zighetti ML, Cattaneo M. Effects of 5′-phosphate derivatives of 2-hexynyl adenosine and 2-phenylethynyl adenosine on responses of human platelets mediated by P2Y receptors. J Med Chem. 2005;48:2763–2766. doi: 10.1021/jm0493562. [DOI] [PubMed] [Google Scholar]
- Daniel JL, Dangelmaier C, Jin J, Ashby B, Smith JB, Kunapuli SP. Molecular basis for ADP-induced platelet activation. I. Evidence for three distinct ADP receptors on human platelets. J Biol Chem. 1998;273:2024–2029. doi: 10.1074/jbc.273.4.2024. [DOI] [PubMed] [Google Scholar]
- Deissler H, Lottspeich F, Rajewsky MF. Affinity purification and cDNA cloning of rat neural differentiation and tumor cell surface antigen gp130RB13–6 reveals relationship to human and murine PC-1. J Biol Chem. 1995;270:9849–9855. doi: 10.1074/jbc.270.17.9849. [DOI] [PubMed] [Google Scholar]
- Delmas P, Crest M, Brown DA. Functional organization of PLC signaling microdomains in neurons. Trends Neurosci. 2004;27:41–47. doi: 10.1016/j.tins.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Ding Z, Tuluc F, Bandivadekar KR, Zhang L, Jin J, Kunapuli SP. Arg333 and Arg334 in the COOH terminus of the human P2Y1 receptor are crucial for Gq coupling. Am J Physiol. 2005;288:C559–C567. doi: 10.1152/ajpcell.00401.2004. [DOI] [PubMed] [Google Scholar]
- Dixon SJ, Sims SM. P2 purinergic receptors on osteoblasts and osteoclasts: potential targets for drug development. Drug Dev Res. 2000;49:187–200. [Google Scholar]
- Dolphin AC. G-protein modulation of voltage-gated calcium channels. Pharmacol Rev. 2003;65:607–627. doi: 10.1124/pr.55.4.3. [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Lazarowski ER, Picher M, Knowles MR, Stutts MJ, Boucher RC. Basal nucleotide levels, release and metabolism in normal and cystic fibrosis airways. Mol Med. 2000;6:969–982. [PMC free article] [PubMed] [Google Scholar]
- Donaldson SH, Picher M, Boucher RC. Secreted and cell-associated adenylate kinase and nucleoside diphosphokinase contribute to extracellular nucleotide metabolism on human airway surfaces. Am J Respir Cell Mol Biol. 2002;26:209–215. doi: 10.1165/ajrcmb.26.2.4650. [DOI] [PubMed] [Google Scholar]
- Donowitz M, Cha B, Zachos NC, Brett CL, Sharma A, Tse CM, Li X. The NHERF family and NHE3 regulation. J Physiol(Lond) 2005;567:3–11. doi: 10.1113/jphysiol.2005.090399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsam RT, Kim S, Jin J, Kunapuli SP. Coordinated signaling through both G12/13 and Gi pathways is sufficient to activate GPIIb/IIIa in human platelets. J Biol Chem. 2002;277:47588–47595. doi: 10.1074/jbc.M208778200. [DOI] [PubMed] [Google Scholar]
- Douglass J, Patel RI, Redick C, Brubaker K, Jones AC, Shaver SR, Yerxa B, Baurand A, Gachet C, Boyer JL. Ribose and nucleobase modifications to nucleotides that confer antagonist properties against the P2Y12 platelet receptor. Haematologica. 2002;87:S1–S22. [Google Scholar]
- Dranoff JA, Ogawa M, Kruglov EA, Gaca MD, Sevigny J, Robson SC, Wells RG. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol. 2004;287:G417–G424. doi: 10.1152/ajpgi.00294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclert A, Changeux J-P. Acetylcholine receptor gene expression at the developing neuromuscular junction. Physiol Rev. 1995;75:339–368. doi: 10.1152/physrev.1995.75.2.339. [DOI] [PubMed] [Google Scholar]
- Drury AN, Szent-Györgyi A. The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. J Physiol (Lond) 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta AK, Okada Y, Sabirov RZ. Regulation of an ATP-conductive large-conductance anion channel and swelling-induced ATP release by arachidonic acid. J Physiol (Lond) 2002;542:803–816. doi: 10.1113/jphysiol.2002.019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckly A, Gendrault JL, Hechler B, Cazenave JP, Gachet C. Differential involvement of the P2Y1 and P2YT receptors in the morphological changes of platelet aggregation. Thromb Haemost. 2001;85:694–701. [PubMed] [Google Scholar]
- Ennion SJ, Powell AD, Seward EP. Identification of the P2Y12 receptor in nucleotide inhibition of exocytosis from bovine chromaffin cells. Mol Pharmacol. 2004;66:601–611. doi: 10.1124/mol.104.000224. [DOI] [PubMed] [Google Scholar]
- Erb L, Garrad R, Wang Y, Quinn T, Turner JT, Weisman GA. Site-directed mutagenesis of P2U purinoceptors: positively charged amino acids in transmembrane helices 6 and 7 affect agonist potency and specificity. J Biol Chem. 1995;270:4185–4188. doi: 10.1074/jbc.270.9.4185. [DOI] [PubMed] [Google Scholar]
- Erb L, Liu J, Ockerhausen J, Kong QM, Garrad R, Griffin K, Neal C, Krugh B, Santiago-Pérez LI, Gonzalez F, et al. An RGD sequence in the P2Y2 receptor interacts with αvβ3 integrins and is required for Go-mediated signal transduction. J Cell Biol. 2001;153:491–501. doi: 10.1083/jcb.153.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre JE, Nguyen MT, Latour A, Keifer JA, Audóly LP, Coffman TM, Koller BH. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nat Med. 1999;5:1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- Fam SR, Paquet M, Castleberry AM, Oller H, Lee CJ, Traynelis SF, Smith Y, Yun CC, Hall RA. P2Y1 receptor signaling is controlled by interaction with the PDZ scaffold NHERF-2. Proc Natl Acad Sci USA. 2005;102:8042–8047. doi: 10.1073/pnas.0408818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Williams HL, Axelrod J. A transduction pathway associated with receptors coupled to the inhibitory guanine nucleotide binding protein Gi that amplifies ATP-mediated arachidonic acid release. Proc Natl Acad Sci USA. 1991;88:6477–6480. doi: 10.1073/pnas.88.15.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feranchak AP, Roman RM, Doctor RB, Salter KD, Toker A, Fitz JG. The lipid products of phosphoinositide 3-kinase contribute to regulation of cholangiocyte ATP and chloride transport. J Biol Chem. 1999;274:30979–30986. doi: 10.1074/jbc.274.43.30979. [DOI] [PubMed] [Google Scholar]
- Feranchak AP, Roman RM, Schwiebert EM, Fitz JG. Phosphatidylinositol 3-kinase contributes to cell volume regulation through effects on ATP release. J Biol Chem. 1998;273:14906–14911. doi: 10.1074/jbc.273.24.14906. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez JM, Abogadie FC, Milligan G, Delmas P, Brown DA. Multiple pertussis toxin-sensitive G-proteins can couple receptors to GIRK channels in rat sympathetic neurons when expressed heterologously, but only native Gi-proteins do so in situ. Eur J Neurosci. 2001;14:283–292. doi: 10.1046/j.0953-816x.2001.01642.x. [DOI] [PubMed] [Google Scholar]
- Filippov AK, Brown DA, Barnard EA. The P2Y1 receptor closes the N-type Ca2+ channel in neurones, with both adenosine triphosphates and diphosphates as potent agonists. Br J Pharmacol. 2000;129:1063–1066. doi: 10.1038/sj.bjp.0703185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov AK, Fernández-Fernández JM, Marsh SJ, Simon J, Barnard EA, Brown DA. Activation and inhibition of neuronal G protein-gated inwardly rectifying K+ channels by P2Y nucleotide receptors. Mol Pharmacol. 2004;66:468–477. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Filippov AK, Simon J, Barnard EA, Brown DA. Coupling of the nucleotide P2Y4 receptor to neuronal ion channels. Br J Pharmacol. 2003;138:400–406. doi: 10.1038/sj.bjp.0705043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov AK, Webb TE, Barnard EA, Brown DA. Inhibition by heterologously-expressed P2Y2 nucleotide receptors of N-type calcium currents in rat sympathetic neurones. Br J Pharmacol. 1997;121:849–851. doi: 10.1038/sj.bjp.0701270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov AK, Webb TE, Barnard EA, Brown DA. P2Y2 nucleotide receptors expressed heterologously in sympathetic neurons inhibit both N-type Ca2+ and M-type K+ currents. J Neurosci. 1998;18:5170–5179. doi: 10.1523/JNEUROSCI.18-14-05170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov AK, Webb TE, Barnard EA, Brown DA. Dual coupling of heterologously-expressed rat P2Y6 nucleotide receptors to N-type Ca-2+ and M-type K+ currents in rat sympathetic neurones. Br J Pharmacol. 1999;126:1009–1017. doi: 10.1038/sj.bjp.0702356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filtz TM, Li Q, Boyer JL, Nicholas RA, Harden TK. Expression of a cloned P2Y purinergic receptor that couples to phospholipase C. Mol Pharmacol. 1994;46:8–14. [PubMed] [Google Scholar]
- Finta C, Warner SC, Zaphiropoulos PG. Intergenic mRNAs: minor gene products or tools of diversity? Histol Histopathol. 2002;17:677–682. doi: 10.14670/HH-17.677. [DOI] [PubMed] [Google Scholar]
- Fischbach GD, Rosen KM. ARIA: a neuromuscular junction neuregulin. Annu Rev Neurosci. 1997;20:429–458. doi: 10.1146/annurev.neuro.20.1.429. [DOI] [PubMed] [Google Scholar]
- Fischer B, Boyer JL, Hoyle CHV, Ziganshin AU, Brizzolara AL, Knight GE, Zimmet J, Burnstock G, Harden TK, Jacobson KA. Identification of potent, selective P2Y-purinoceptor agonists: structure-activity relationships for 2-thioether derivatives of adenosine 5′-triphosphate. J Med Chem. 1993;36:3937–3946. doi: 10.1021/jm00076a023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Chulkin A, Boyer JL, Harden TK, Gendron FP, Beaudoin AR, Chapal J, Hillaire-Buys D, Petit P. 2-Thioether 5′-O-(1-thiotriphosphate)adenosine derivatives as new insulin secretagogues acting through P2Y-receptors. J Med Chem. 1999;42:3636–3646. doi: 10.1021/jm990158y. [DOI] [PubMed] [Google Scholar]
- Fong AY, Krstew EV, Barden J, Lawrence AJ. Immunoreactive localisation of P2Y1 receptors within the rat and human nodose ganglia and rat brainstem: comparison with [α33P]deoxyadenosine 5′-triphosphate autoradiography. Neuroscience. 2002;113:809–823. doi: 10.1016/s0306-4522(02)00237-3. [DOI] [PubMed] [Google Scholar]
- Ford CP, Stemkowski PL, Light PE, Smith PA. Experiments to test the role of phosphatidylinositol 4,5-bisphosphate in neurotransmitter-induced M-channel closure in bullfrog sympathetic neurons. J Neurosci. 2003;23:4931–4941. doi: 10.1523/JNEUROSCI.23-12-04931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJ, Jr, Wiekowski MT, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Investig. 2001;107:1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester T, Lind AR. Identification of adenosine triphosphate in human plasma and the concentration in the venous effluent of forearm muscles before, during and after sustained contractions. J Physiol (Lond) 1969;204:347–364. doi: 10.1113/jphysiol.1969.sp008917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H, Krügel U, Grosche J, Heine C, Hartig W, Allgaier C, Illes P. P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2004;127:431–441. doi: 10.1016/j.neuroscience.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Franke H, Krügel U, Illes P. P2 receptor-mediated proliferative effects on astrocytes in vivo. Glia. 1999;28:190–200. [PubMed] [Google Scholar]
- Fredholm BB, Assender JW, Irenius EE, Kodama N, Saito N. Synergistic effects of adenosine A1 and P2Y receptor stimulation on calcium mobilisation and PKC translocation on DDT1 MF-2 cells. Cell Mol Neurobiol. 2003;23:379–400. doi: 10.1023/A:1023644822539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman K, Tsui P, Moore D, Emson PC, Vawter L, Naheed S, Lane P, Bawagan H, Herrity N, Murphy K, et al. Cloning, pharmacology, and tissue distribution of G-protein-coupled receptor GPR105 (KIAA0001) rodent orthologs. Genomics. 2001;78:124–128. doi: 10.1006/geno.2001.6662. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families: Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Fretz H, Houille O, Hilpert K, Peter O, Breu V, Giller T, Valdenaire O, Riederer M. Novel pyrazolidine-3,5-dione derivatives are P2Y12 receptor antagonists and inhibit ADP-triggered blood platelet aggregation. Proceedings of the 229th National Meeting of the American Chemical Society; 2005 March 15–17; San Diego, CA. 2005. [Google Scholar]
- Fumagalli M, Brambilla R, D’Ambrosi N, Volonté C, Matteoli M, Verderio C, Abbracchio MP. Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia. 2003;43:218–230. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Trincavelli L, Lecca D, Martini C, Ciana P, Abbracchio MP. Cloning, pharmacological characterisation and distribution of the rat G-protein-coupled P2Y13 receptor. Biochem Pharmacol. 2004;68:113–124. doi: 10.1016/j.bcp.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Gachet C. ADP receptors of platelets and their inhibition. Thromb Haemost. 2001;86:222–232. [PubMed] [Google Scholar]
- Gachet C. The platelet P2 receptors as molecular targets for old and new antiplatelet drugs. Pharmacol Ther. 2005;108:180–192. doi: 10.1016/j.pharmthera.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Gachet C, Hechler B. The platelet P2 receptors in thrombosis. Semin Thromb Hemost. 2005;31:162–167. doi: 10.1055/s-2005-869521. [DOI] [PubMed] [Google Scholar]
- Gamper N, Reznikov V, Yamada Y, Yang J, Shapiro MS. Phosphatidylinositol 4,5-bisphosphate signals underlie receptor-specific Gq/11-mediated modulation of N-type Ca2+ channels. J Neurosci. 2004;24:10980–10992. doi: 10.1523/JNEUROSCI.3869-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao ZG, Mamedova L, Tchilibon S, Gross AS, Jacobson KA. 2,2′-Pyridylisatogen tosylate antagonizes P2Y1 receptor signaling without affecting nucleotide binding. Biochem Pharmacol. 2004;68:231–237. doi: 10.1016/j.bcp.2004.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrad RC, Otero MA, Erb L, Theiss PM, Clarke LL, Gonzalez FA, Turner JT, Weisman GA. Structural basis of agonist-induced desensitization and sequestration of the P2Y2 nucleotide receptor: consequences of truncation of the C-terminus. J Biol Chem. 1998;273:29437–29444. doi: 10.1074/jbc.273.45.29437. [DOI] [PubMed] [Google Scholar]
- Gendron FP, Newbold NL, Vivas-Mejia PE, Wang M, Neary JT, Sun GY, Gonzalez FA, Weisman GA. Signal transduction pathways for P2Y2 and P2X7 nucleotide receptors that mediate neuroinflammatory responses in astrocytes and microglial cells. Biomed Res. 2003;14:47–61. [Google Scholar]
- Gerasimovskaya EV, Ahmad S, White CW, Jones PL, Carpenter TC, Stenmark KR. Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth. J Biol Chem. 2002;277:44638–44650. doi: 10.1074/jbc.M203012200. [DOI] [PubMed] [Google Scholar]
- Gerevich Z, Borvendeg SJ, Schroder W, Franke H, Wirkner K, Norenberg W, Furst S, Gillen C, Illes P. Inhibition of N-type voltage-activated calcium channels in rat dorsal root ganglion neurons by P2Y receptors is a possible mechanism of ADP-induced analgesia. J Neurosci. 2004;24:797–807. doi: 10.1523/JNEUROSCI.4019-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwins P, Fredholm BB. ATP and its metabolite adenosine act synergistically to mobilize intracellular calcium via the formation of inositol 1,4,5-trisphosphate in a smooth muscle cell line. J Biol Chem. 1992;267:16081–16087. [PubMed] [Google Scholar]
- Ghanem E, Robaye B, Leal T, Leipziger J, Van Driessche W, Beauwens R, Boeynaems J-M. The role of epithelial P2Y2 and P2Y4 receptors in the regulation of intestinal chloride secretion. Br J Pharmacol. 2005;146:364–369. doi: 10.1038/sj.bjp.0706353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godecke S, Decking UK, Godecke A, Schrader J. Cloning of the rat P2u receptor and its potential role in coronary vasodilation. Am J Physiol. 1996;270:C570–C577. doi: 10.1152/ajpcell.1996.270.2.C570. [DOI] [PubMed] [Google Scholar]
- Goding JW, Grobben B, Slegers H. Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim Biophys Acta. 2003;1638:1–19. doi: 10.1016/s0925-4439(03)00058-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- Gorodeski GI. Regulation of transcervical permeability by two distinct P2 purinergic receptor mechanisms. Am J Physiol. 2002;282:C75–C83. doi: 10.1152/ajpcell.2002.282.1.C75. [DOI] [PubMed] [Google Scholar]
- Greig AV, James SE, McGrouther DA, Terenghi G, Burnstock G. Purinergic receptor expression in the regeneration epidermis in a rat model of normal and delayed wound healing. Exp Dermatol. 2003a;12:860–871. doi: 10.1111/j.0906-6705.2003.00110.x. [DOI] [PubMed] [Google Scholar]
- Greig AV, Linge C, Cambrey A, Burnstock G. Purinergic receptors are part of a signaling system for keratinocyte proliferation, differentiation, and apoptosis in human fetal epidermis. J Invest Dermatol. 2003b;121:1145–1149. doi: 10.1046/j.1523-1747.2003.12567.x. [DOI] [PubMed] [Google Scholar]
- Gratacap MP, Herault JP, Viala C, Ragab A, Savi P, Herbert JM, Chap H, Plantavid M, Payrastre B. FcγRIIA requires a Gi-dependent pathway for an efficient stimulation of phosphoinositide 3-kinase, calcium mobilization, and platelet aggregation. Blood. 2000;96:3439–3446. [PubMed] [Google Scholar]
- Grobben B, Anciaux K, Roymans D, Stefan C, Bollen M, Esmans EL, Slegers H. An ecto-nucleotide pyrophosphatase is one of the main enzymes involved in the extracellular metabolism of ATP in rat C6 glioma. J Neurochem. 1999;72:826–834. doi: 10.1046/j.1471-4159.1999.0720826.x. [DOI] [PubMed] [Google Scholar]
- Grygorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol. 1997;272:C1058–C1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- Guerra L, Favia M, Fanelli T, Calamita G, Svelto M, Bagorda A, Jacobson KA, Reshkin SJ, Casavola V. Stimulation of Xenopus P2Y1 receptor activates CFTR in A6 cells. Pflueg Arch Eur J Physiol Eur J Physiol. 2004;449:66–75. doi: 10.1007/s00424-004-1293-2. [DOI] [PubMed] [Google Scholar]
- Guo D, von Kügelgen I, Moro S, Kim Y-C, Jacobson KA. Evidence for the recognition of nonnucleotide antagonists within the transmembrane domains of the human P2Y1 receptor. Drug Dev Res. 2002;57:173–181. doi: 10.1002/ddr.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gür S, Öztürk B. Altered relaxant responses to adenosine and adenosine 5′-triphosphate in the corpus cavernosum from men and rats with diabetes. Pharmacology. 2000;60:105–112. doi: 10.1159/000028354. [DOI] [PubMed] [Google Scholar]
- Haley JE, Delmas P, Offermanns S, Abogadie FC, Simon MI, Buckley NJ, Brown DA. Muscarinic inhibition of calcium current and M current in Gαq-deficient mice. J Neurosci. 2000;20:3973–3979. doi: 10.1523/JNEUROSCI.20-11-03973.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RA, Ostedgaard LS, Premont RT, Blitzer JT, Rahman N, Welsh MJ, Lefkowitz RJ. A C-terminal motif found in the β2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins. Proc Natl Acad Sci USA. 1998;95:8496–8501. doi: 10.1073/pnas.95.15.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa M, Guidotti G. Purification and cloning of a soluble ATP-diphosphohydrolase (apyrase) from potato tubers (Solanum tuberosum) Biochem Biophys Res Commun. 1996;218:916–1023. doi: 10.1006/bbrc.1996.0162. [DOI] [PubMed] [Google Scholar]
- Harden TK. Nucleotide receptor P2Y14. AfCS-Nature Molecule Pages. 2004 ID A002814. [Google Scholar]
- Haslam RJ. Interactions of the pharmacological receptors of blood platelets with adenylate cyclase. Ser Haematol. 1973;6:333–350. [PubMed] [Google Scholar]
- Hayashi S, Hazama A, Dutta AK, Sabirov RZ, Okada Y. Detecting ATP release by a biosensor method. Sci STKE. 2004;258:114. doi: 10.1126/stke.2582004pl14. [DOI] [PubMed] [Google Scholar]
- Hazama A, Fan HT, Abdullaev I, Maeno E, Tanaka S, Ando-Akatsuka Y, Okada Y. Swelling-activated, cystic fibrosis transmembrane conductance regulatoraugmented ATP release and Cl− conductances in murine C127 cells. J Physiol (Lond) 2000;523:1–11. doi: 10.1111/j.1469-7793.2000.t01-6-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature (Lond) 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- Hechler B, Eckly A, Ohlmann P, Cazenave JP, Gachet C. The P2Y1 receptor, necessary but not sufficient to support full ADP- induced platelet aggregation, is not the target of the drug clopidogrel. Br J Haematol. 1998a;103:858–866. doi: 10.1046/j.1365-2141.1998.01056.x. [DOI] [PubMed] [Google Scholar]
- Hechler B, Cattaneo M, Gachet C. The P2 receptors in platelet function. Semin Thromb Haemost. 2005;31:150–161. doi: 10.1055/s-2005-869520. [DOI] [PubMed] [Google Scholar]
- Hechler B, Lenain N, Marchese P, Vial C, Heim V, Freund M, Cazenave JP, Cattaneo M, Ruggeri ZM, Evans R, et al. A role of the fast ATP-gated P2X1 cation channel in thrombosis of small arteries in vivo. J Exp Med. 2003;198:661–667. doi: 10.1084/jem.20030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hechler B, Vigne P, Léon C, Breittmayer JP, Gachet C, Frelin C. ATP derivatives are antagonists of the P2Y1 receptor: similarities to the platelet ADP receptor. Mol Pharmacol. 1998b;53:727–733. [PubMed] [Google Scholar]
- Henderson DJ, Elliot DG, Smith GM, Webb TE, Dainty IA. Cloning and characterisation of a bovine P2Y receptor. Biochem Biophys Res Commun. 1995;212:648–656. doi: 10.1006/bbrc.1995.2018. [DOI] [PubMed] [Google Scholar]
- Herold CL, Li Q, Schachter JB, Harden TK, Nicholas RA. Lack of nucleotide-promoted second messenger signaling responses in 1321N1 cells expressing the proposed P2Y receptor, p2y7. Biochem Biophys Res Commun. 1997;235:717–721. doi: 10.1006/bbrc.1997.6884. [DOI] [PubMed] [Google Scholar]
- Herold CL, Qi AD, Harden TK, Nicholas RA. Agonist versus antagonist action of ATP at the P2Y4 receptor is determined by the second extracellular loop. J Biol Chem. 2004;279:11456–11464. doi: 10.1074/jbc.M301734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Ho C, Hicks J, Salter MW. A novel P2-purinoceptor expressed by a subpopulation of astrocytes from the dorsal spinal cord of the rat. Br J Pharmacol. 1995;116:2909–2918. doi: 10.1111/j.1476-5381.1995.tb15944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebertz A, Mahendran S, Burnstock G, Arnett TR. ATP and UTP at low concentrations strongly inhibit bone formation by osteoblasts: a novel role for the P2Y2 receptor in bone remodeling. J Cell Biochem. 2002;86:413–419. doi: 10.1002/jcb.10236. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Moro S, Nicholas RA, Harden TK, Jacobson KA. The role of amino acids in extracellular loops of the human P2Y1 receptor in surface expression and activation processes. J Biol Chem. 1999;274:14639–14647. doi: 10.1074/jbc.274.21.14639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Soltysiak K, West PL, Jacobson KA. Shift in purine/pyrimidine base recognition upon exchanging extracellular domains in P2Y1/6 chimeric receptors. Biochem Pharmacol. 2004;68:2075–2086. doi: 10.1016/j.bcp.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Conley PB. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature (Lond) 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- Holton FA, Holton P. The capillary dilator substances in dry powders of spinal roots: A possible role of adenosine triphosphate in chemical transmission from nerve endings. J Physiol (Lond) 1954;126:124–140. doi: 10.1113/jphysiol.1954.sp005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol. 2000;150:1349–1360. doi: 10.1083/jcb.150.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou M, Harden TK, Kuhn CM, Baldetorp B, Lazarowski E, Pendergast W, Moller S, Edvinsson L, Erlinge D. UDP acts as a growth factor for vascular smooth muscle cells by activation of P2Y6 receptors. Am J Physiol. 2002;282:H784–H792. doi: 10.1152/ajpheart.00997.2000. [DOI] [PubMed] [Google Scholar]
- Hou M, Moller S, Edvinsson L, Erlinge D. MAPKK-dependent growth factor-induced upregulation of P2Y2 receptors in vascular smooth muscle cells. Biochem Biophys Res Commun. 1999;258:648–652. doi: 10.1006/bbrc.1999.0676. [DOI] [PubMed] [Google Scholar]
- Hou M, Moller S, Edvinsson L, Erlinge D. Cytokines induce upregulation of vascular P2Y2 receptors and increased mitogenic responses to UTP and ATP. Arterioscler Thromb Vasc Biol. 2000;20:2064–2069. doi: 10.1161/01.atv.20.9.2064. [DOI] [PubMed] [Google Scholar]
- Housley GD, Jagger DJ, Greenwood D, Raybould NP, Salih SG, Jarlebark LE, Vlajkovic SM, Kanjhan R, Nikolic P, Munoz DJ, et al. Purinergic regulation of sound transduction and auditory neurotransmission. Audiol Neurootol. 2002;7:55–61. doi: 10.1159/000046865. [DOI] [PubMed] [Google Scholar]
- Houston D, Ohno M, Nicholas RA, Jacobson KA, Harden TK. Quantification of P2Y1 receptors in the adult rat with a novel 32P-labeled radioligand. Proceedings of Experimental Biology 2005; 2005 2–6 April; San Diego, CA. 2005. [Google Scholar]
- Huang PB, Lazarowski ER, Tarran R, Milgram SL, Boucher RC, Stutts MJ. Compartmentalized autocrine signaling to cystic fibrosis transmembrane conductance regulator at the apical membrane of airway epithelial cells. Proc Natl Acad Sci USA. 2001;98:14120–14125. doi: 10.1073/pnas.241318498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur E-M, Kim K-T. G protein-coupled receptor signalling and cross-talk: Achieving rapidity and specificity. Cell Signal. 2002;14:397–405. doi: 10.1016/s0898-6568(01)00258-3. [DOI] [PubMed] [Google Scholar]
- Hutchings H, Ortega N, Plouet J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. FASEB J. 2003;17:1520–1522. doi: 10.1096/fj.02-0691fje. [DOI] [PubMed] [Google Scholar]
- Huwiler A, Wartmann M, van den Bosch H, Pfeilschifter J. Extracellular nucleotides activate the p38-stress-activated protein kinase cascade in glomerular mesangial cells. Br J Pharmacol. 2000;129:612–618. doi: 10.1038/sj.bjp.0703077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko M, Panther E, Sorichter S, Herouy Y, Berod L, Geissler M, Mockenhaupt M, Elsner P, Girolomoni G, Norgauer J. Characterization of the biological activities of uridine diphosphate in human dendritic cells: Influence on chemotaxis and CXCL8 release. J Cell Physiol. 2004;201:286–293. doi: 10.1002/jcp.20070. [DOI] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein subunits. Nature (Lond) 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- Inbe H, Watanabe S, Miyawaki M, Tanabe E, Encinas JA. Identification and characterization of a cell-surface receptor, P2Y15, for AMP and adenosine. J Biol Chem. 2004;279:19790–19799. doi: 10.1074/jbc.M400360200. [DOI] [PubMed] [Google Scholar]
- Ingall AH, Dixon J, Bailey A, Coombs ME, Cox D, McInally JI, Hunt SF, Kindon ND, Teobald BJ, Willis PA, et al. Antagonists of the platelet P2T receptor: a novel approach to antithrombotic therapy. J Med Chem. 1999;42:213–220. doi: 10.1021/jm981072s. [DOI] [PubMed] [Google Scholar]
- Jackson EK. P1 and P2 receptors in the renal system. In: Abbracchio MP, Williams M, editors. Purinergic and Pyrimidinergic Signalling II: Cardiovascular, Respiratory, Immune, Metabolic and Gastrointestinal Tract Function. Springer; Berlin: 2001. pp. 33–71. [Google Scholar]
- Jacobson KA, Jarvis MF, Williams M. Purine and pyrimidine (P2) receptors as drug targets. J Med Chem. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PubMed] [Google Scholar]
- Janssens R, Communi D, Pirotton S, Samson M, Parmentier M, Boeynaems JM. Cloning and dstribution of the human P2Y1 receptor. Biochem Biophys Res Commun. 1996;221:588–593. doi: 10.1006/bbrc.1996.0640. [DOI] [PubMed] [Google Scholar]
- Jantzen HM, Milstone DS, Gousset L, Conley P, Mortensen RM. Impaired activation of murine platelets lacking Gαi2. J Clin Investig. 2001;108:477–483. doi: 10.1172/JCI12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis GE, Humphries RG, Robertson MJ, Leff P. ADP can induce aggregation of human platelets via both P2Y1 and P2T receptors. Br J Pharmacol. 2000;129:275–282. doi: 10.1038/sj.bjp.0703046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Foster FM, Ward P, Tasevski V, Luttrell BM, Conigrave AD. Extracellular ATP triggers cyclic AMP-dependent differentiation of HL-60 cells. Biochem Biophys Res Commun. 1997a;232:626–630. doi: 10.1006/bbrc.1997.6345. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Guo D, Lee BX, Van Rhee AM, Kim YC, Nicholas RA, Schachter JB, Harden TK, Jacobson KA. A mutational analysis of residues essential for ligand recognition at the human P2Y1 receptor. Mol Pharmacol. 1997b;52:499–507. doi: 10.1124/mol.52.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Mak D, Devidas S, Schwiebert EM, Bragin A, Zhang Y, Skach WR, Guggino WB, Foskett JK, Engelhardt JF. Cystic fibrosis transmembrane conductance regulator-associated ATP release is controlled by a chloride sensor. J Cell Biol. 1998;143:645–657. doi: 10.1083/jcb.143.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998a;273:2030–2034. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- Jin J, Dasari VR, Sistare FD, Kunapuli SP. Distribution of P2Y receptor subtypes on haematopoietic cells. Br J Pharmacol. 1998b;123:789–794. doi: 10.1038/sj.bjp.0701665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci USA. 1998;95:8070–8074. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin-Hua P, Goding JW, Nakamura H, Sano K. Molecular cloning and chromosomal localization of PD-Iβ (PDNP3), a new member of the human phosphodiesterase I genes. Genomics. 1997;45:412–415. doi: 10.1006/geno.1997.4949. [DOI] [PubMed] [Google Scholar]
- Joost P, Methner A. Phylogenetic analysis of 277 human G-protein-coupled receptors as a tool for the prediction of orphan receptor ligands. Genome Biol. 2002;3:RESEARCH0063.1–RESEARCH0063.16. doi: 10.1186/gb-2002-3-11-research0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem. 2003;278:23331–23342. doi: 10.1074/jbc.M302680200. [DOI] [PubMed] [Google Scholar]
- Joseph SM, Pifer MA, Przybylski RJ, Dudyak GR. Methylene ATP analogs as modulators of extracellular ATP metabolism and accumulation. Br J Pharmacol. 2004;142:1002–1014. doi: 10.1038/sj.bjp.0705865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek E, Erb L, Koziak K, Jarzyna R, Wink MR, Guckelberger O, Blusztajn KB, Trinkaus-Randall V, Weisman GA, Robson SC. Modulation of endothelial cell migration by extracellular nucleotides: involvement of focal adhesion kinase and phosphatidylinositol 3-kinase-mediated pathways. Thromb Haemost. 2005;93:735–742. doi: 10.1267/THRO05040735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RA, Buxton IL. Nucleotide-mediated relaxation in guinea-pig aorta: selective inhibition by MRS2179. Br J Pharmacol. 2002;135:537–545. doi: 10.1038/sj.bjp.0704476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol. 2004;173:1503–1510. doi: 10.4049/jimmunol.173.3.1503. [DOI] [PubMed] [Google Scholar]
- Kannan S. Neutrophil chemotaxis: potential role of chemokine receptors in extracellular nucleotide induced Mac-1 expression. Med Hypotheses. 2003;61:577–579. doi: 10.1016/s0306-9877(03)00234-2. [DOI] [PubMed] [Google Scholar]
- Kauffenstein G, Bergmeier W, Eckly A, Ohlmann P, Leon C, Cazenave JP, Nieswandt B, Gachet C. The P2Y12 receptor induces platelet aggregation through weak activation of the αIIbβ3 integrin—a phosphoinositide 3-kinase-dependent mechanism. FEBS Lett. 2001;505:281–290. doi: 10.1016/s0014-5793(01)02824-1. [DOI] [PubMed] [Google Scholar]
- Kauffenstein G, Hechler B, Cazenave JP, Gachet C. Adenine triphosphate nucleotides are antagonists at the P2Y12 receptor. J Thromb Haemost. 2004;2:1980–1988. doi: 10.1111/j.1538-7836.2004.00926.x. [DOI] [PubMed] [Google Scholar]
- Kaulich M, Streicher F, Mayer R, Müller I, Müller C. Flavonoids—novel lead compounds for the development of P2Y2 receptor antagonists. Drug Dev Res. 2003;59:72–81. [Google Scholar]
- Kawagoe H, Soma O, Goji J, Nishimura N, Narita M, Inazawa J, Nakamura H, Sano K. Molecular cloning and chromosomal assignment of the humans brain-type phosphodiesterase I/nucleotide pyrophosphatase gene (PDNP2) Genomics. 1995;30:380–384. doi: 10.1006/geno.1995.0036. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Gachet C, Inoue K, Kato F. Direct excitation of inhibitory interneurons by extracellular ATP mediated by P2Y1 receptors in the hippocampal slice. J Neurosci. 2004;24:10835–10845. doi: 10.1523/JNEUROSCI.3028-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellerman D, Evans R, Mathews D, Shaffer C. Inhaled P2Y2 receptor agonists as a treatment for patients with cystic fibrosis lung disease. Adv Drug Deliv Rev. 2002;54:1463–1474. doi: 10.1016/s0169-409x(02)00154-0. [DOI] [PubMed] [Google Scholar]
- Kennedy C, Assis TS, Currie A, Rowan EG. Crossing the pain barrier: P2 receptors as targets for novel analgesics. J Physiol (Lond) 2003;553:683–694. doi: 10.1113/jphysiol.2003.049114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Qi AD, Herold CL, Harden TK, Nicholas RA. ATP, an agonist at the rat P2Y4 receptor, is an antagonist at the human P2Y4 receptor. Mol Pharmacol. 2000;57:926–931. [PubMed] [Google Scholar]
- Khakh B, Gittermann D, Cockayne DA, Jones A. ATP modulation of excitatory synapses onto interneurons. J Neurosci. 2003;23:7426–7437. doi: 10.1523/JNEUROSCI.23-19-07426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Kim SS, Choi JY, Shin JH, Kim JY, Namkung W, Lee JG, Lee MG, Yoon JH. Membrane-specific expression of functional purinergic receptors in normal human nasal epithelial cells. Am J Physiol. 2004;287:L835–L842. doi: 10.1152/ajplung.00285.2003. [DOI] [PubMed] [Google Scholar]
- Kim HF, Ohno M, Xu B, Kim HO, Choi Y, Ji XD, Maddileti S, Marquez VE, Harden TK, Jacobson KA. 2-Substitution of adenine nucleotide analogues containing a bicyclo[3.1.0]hexane ring system locked in a Northern conformation: enhanced potency as P2Y1 antagonists. J Med Chem. 2003a;46:4974–4987. doi: 10.1021/jm030127+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Ravi RG, Marquez VE, Maddileti S, Wihlborg AK, Erlinge D, Malmsjö M, Boyer JL, Harden TK, Jacobson KA. Methanocarba modification of uracil and adenine nucleotides: high potency of northern ring conformation at P2Y1, P2Y2, or P2Y4 and P2Y11, but not P2Y6 receptors. J Med Chem. 2002;45:208–218. doi: 10.1021/jm010369e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KC, Park HR, Shin CY, Akiyama T, Ko KH. Nucleotide-induced mucin release from primary hamster tracheal surface epithelial cells involves the P2U purinoceptor. Eur Respir J. 1996;9:1579. [PubMed] [Google Scholar]
- Kim SG, Soltysiak KA, Gao Z-G, Chang T-S, Chung E, Jacobson KA. Tumor necrosis factor α-induced apoptosis in astrocytes is prevented by the activation of P2Y6, but not P2Y4 nucleotide receptors. Biochem Pharmacol. 2003b;65:923–931. doi: 10.1016/s0006-2952(02)01614-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-C, Lee J-S, Sak K, Marteau F, Mamedova L, Boeynaems J-M, Jacobson KA. Synthesis of pyridoxal phosphate derivatives with antagonist activity at the P2Y13 receptor. Biochem Pharmacol. 2005;70:266–274. doi: 10.1016/j.bcp.2005.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF. Molecular biology of P2X purinoreceptors. In: Burnstock G, Dobson JG, Liang BT, Linden J, editors. Cardiovascular Biology of Purines. Kluwer Academic Publishers; Norwell, MA: 1998. pp. 159–186. [Google Scholar]
- King BF, Dacquet C, Ziganshin AU, Weetman DF, Burnstock G, Vanhoutte PM, Spedding M. Potentiation by 2,2′-pyridylisatogen tosylate of ATP-responses at a recombinant P2Y1 purinoceptor. Br J Pharmacol. 1996;117:1111–1118. doi: 10.1111/j.1476-5381.1996.tb16704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Townsend-Nicholson A. Recombinant P2Y receptors: the UCL experience. J Auton Nerv Syst. 2000;81:164–170. doi: 10.1016/s0165-1838(00)00134-x. [DOI] [PubMed] [Google Scholar]
- King BF, Townsend-Nicholson A. Tocris Rev No 23. Tocris Cookson; Bristol, UK: 2003. Nucleotide and nucleoside receptors. [Google Scholar]
- Kirischuk S, Scherer J, Kettenmann H, Verkhratsky A. Activation of P2-purinoreceptors triggered Ca2+ release from InsP3-sensitive internal stores in mammalian oligodendrocytes. J Physiol (Lond) 1995;483:41–57. doi: 10.1113/jphysiol.1995.sp020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittner H, Franke H, Fischer W, Schultheis N, Krugel U, Illes P. Stimulation of P2Y1 receptors causes anxiolytic-like effects in the rat elevated plusmaze: implications for the involvement of P2Y1 receptor-mediated nitric oxide production. Neuropsychopharmacology. 2003;28:435–444. doi: 10.1038/sj.npp.1300043. [DOI] [PubMed] [Google Scholar]
- Klepeis VE, Weinger I, Kaczmarek E, Trinkaus-Randall V. P2Y receptors play a critical role in epithelial cell communication and migration. J Cell Biochem. 2004;93:1115–1133. doi: 10.1002/jcb.20258. [DOI] [PubMed] [Google Scholar]
- Knight GE, Bodin P, De Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol. 2002;282:F281–F288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Fujishita K, Tsuda M, Shigemoto-Mogami Y, Inoue K. Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc Natl Acad Sci USA. 2003;100:11023–11028. doi: 10.1073/pnas.1834448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korcok J, Raimundo LN, Du X, Sims SM, Dixon SJ. P2Y6 nucleotide receptors activate NF-κB and increase survival of osteoclasts. J Biol Chem. 2005;280:16909–16915. doi: 10.1074/jbc.M410764200. [DOI] [PubMed] [Google Scholar]
- Koshiba M, Apasov S, Sverdlov V, Chen P, Erb L, Turner JT, Weisman GA, Sitkovsky MV. Transient up-regulation of P2Y2 nucleotide receptor mRNA expression is an immediate early gene response in activated thymocytes. Proc Natl Acad Sci USA. 1997;94:831–836. doi: 10.1073/pnas.94.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotevic I, Kirschner KM, Porzig H, Baltensperger K. Constitutive interaction of the P2Y2 receptor with the hematopoietic cell-specific G protein Gα16 and evidence for receptor oligomers. Cell Signal. 2005;17:869–880. doi: 10.1016/j.cellsig.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Köttgen M, Löffler T, Jacobi C, Nitschke R, Pavenstädt H, Schreiber R, Frische S, Nielsen S, Leipziger J. P2Y6 receptor mediates colonic NaCl secretion via differential activation of cAMP-mediated transport. J Clin Investig. 2003;111:371–379. doi: 10.1172/JCI16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, Oike M, Ito Y. Involvement of Rho-kinase and tyrosine kinase in hypotonic stress-induced ATP release in bovine aortic endothelial cells. J Physiol (Lond) 2001;532:759–769. doi: 10.1111/j.1469-7793.2001.0759e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krügel U, Kittner H, Franke H, Illes P. Stimulation of P2 receptors in the ventral tegmental area enhances dopaminergic mechanisms in vivo. Neuropharmacology. 2001;40:1084–1093. doi: 10.1016/s0028-3908(01)00033-8. [DOI] [PubMed] [Google Scholar]
- Kumari R, Goh G, Ng LL, Boarder MR. ATP and UTP responses of cultured rat aortic smooth muscle cells revisited: dominance of P2Y2 receptors. Br J Pharmacol. 2003;140:1169–1176. doi: 10.1038/sj.bjp.0705526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunapuli SP, Daniel JL. P2 receptor subtypes in the cardiovascular system. Biochem J. 1998;336:513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrecht G, Braun K, Damer M, Ganso M, Hildebrandt C, Ullmann H, Kassack MU, Nickel P. Structure-activity relationships of suramin and pyridoxal-5′-phosphate derivatives as P2 receptor antagonists. Curr Pharm Des. 2002;8:2371–2399. doi: 10.2174/1381612023392973. [DOI] [PubMed] [Google Scholar]
- Larson MK, Chen H, Kahn ML, Taylor AM, Fabre JE, Mortensen RM, Conley PB, Parise LV. Identification of P2Y12-dependent and independent mechanisms of glycoprotein VI-mediated Rap1 activation in platelets. Blood. 2003;101:1409–1415. doi: 10.1182/blood-2002-05-1533. [DOI] [PubMed] [Google Scholar]
- La Sala A, Sebastiani S, Ferrari D, Di Virgilio F, Idzko M, Norgauer J, Girolomoni G. Dendritic cells exposed to extracellular adenosine triphosphate acquire the migratory properties of mature cells and show a reduced capacity to attract type 1 T lymphocytes. Blood. 2002;99:1715–1722. doi: 10.1182/blood.v99.5.1715. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Interplay of constitutively released nucleotides, nucleotide metabolism and activity of P2Y receptors. Drug Dev Res. 2001a;53:66–71. [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003a;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Harden TK. Quantitation of extracellular UTP using a sensitive enzymatic assay. Br J Pharmacol. 1999;127:1272–1278. doi: 10.1038/sj.bjp.0702654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Homolya L, Boucher RC, Harden TK. Identification of an ecto-nucleoside diphosphokinase and its contribution to interconversion of P2 receptor agonists. J Biol Chem. 1997a;272:20402–20407. doi: 10.1074/jbc.272.33.20402. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Homolya L, Boucher RC, Harden TK. Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J Biol Chem. 1997b;272:24348–24354. doi: 10.1074/jbc.272.39.24348. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Paradiso AM, Watt WC, Harden TK, Boucher RC. UDP activates a mucosal-restricted receptor on human nasal epithelial cells that is distinct from the P2Y2 receptor. Proc Natl Acad Sci USA. 1997c;94:2599–2603. doi: 10.1073/pnas.94.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Rochelle LG, O’Neal WK, Ribeiro CM, Grubb BR, Zhang V, Harden TK, Boucher RC. Cloning and functional characterization of two murine uridine nucleotide receptors reveal a potential target for correcting ion transport deficiency in cystic fibrosis gallbladder. J Pharmacol Exp Ther. 2001b;297:43–49. [PubMed] [Google Scholar]
- Lazarowski ER, Shea DA, Boucher RC, Harden TK. Release of cellular UDP-glucose as a potential exracellular signaling molecule. Mol Pharmacol. 2003b;63:1190–1197. doi: 10.1124/mol.63.5.1190. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Tarran R, Grubb BR, van Heusden CA, Okada S, Boucher RC. Nucleotide release provides a mechanism for airway surface liquid homeostasis. J Biol Chem. 2004;279:36855–36864. doi: 10.1074/jbc.M405367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Watt WC, Stutts MJ, Boucher RC, Harden TK. Pharmacological selectivity of the cloned human P2U-purinoceptor: potent activation by diadenosine tetraphosphate. Br J Pharmacol. 1995a;116:1619–1627. doi: 10.1111/j.1476-5381.1995.tb16382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Watt WC, Stutts MJ, Brown HA, Boucher RC, Harden TK. Enzymatic synthesis of UTPγS, a potent hydrolysis resistant agonist of P2U-purinergic receptors. Br J Pharmacol. 1995b;117:203–209. doi: 10.1111/j.1476-5381.1996.tb15175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner SG, Dorostkar MD, Mayer M, Edelbauer H, Pankevych H, Boehm S. Autoinhibition of transmitter release from PC12 cells and sympathetic neurons through a P2Y12 receptor-mediated inhibition of voltage-gated Ca2+ channels. Eur J Neurosci. 2004;20:2917–2928. doi: 10.1111/j.1460-9568.2004.03760.x. [DOI] [PubMed] [Google Scholar]
- Lee BC, Cheng T, Adams G, Attar C, Miura N, Lee SB, Saito Y, Olszak I, Dombkowski D, Olson DP, et al. P2Y-like receptor, GPR105 (P2Y14) identifies and mediates chemotaxis of bone-marrow haematopoietic stem cells. Genes Dev. 2003a;17:1592–1604. doi: 10.1101/gad.1071503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Nguyen T, Lynch KR, Cheng R, Vanti WB, Arkhitko O, Lewis T, Evans JF, George SR, O’Dowd BF. Discovery and mapping of ten novel G protein-coupled receptor genes. Gene. 2001;275:83–91. doi: 10.1016/s0378-1119(01)00651-5. [DOI] [PubMed] [Google Scholar]
- Lee SY, Wolff SC, Nicholas RA, O’Grady SM. P2Y receptors modulate ion channel function through interactions involving the C-terminal domain. Mol Pharmacol. 2003b;63:878–885. doi: 10.1124/mol.63.4.878. [DOI] [PubMed] [Google Scholar]
- Leipziger J. Control of epithelial transport via luminal P2 receptors. Am J Physiol. 2003;284:F419–F432. doi: 10.1152/ajprenal.00075.2002. [DOI] [PubMed] [Google Scholar]
- Lenain N, Freund M, Leon C, Cazenave JP, Gachet C. Inhibition of localized thrombosis in P2Y1-deficient mice and rodents treated with MRS2179, a P2Y1 receptor antagonist. J Thromb Haemost. 2003;1:1144–1149. doi: 10.1046/j.1538-7836.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- Léon C, Freund M, Latchoumanin O, Farret A, Petit P, Cazenave JP, Gachet C. The P2Y1 receptor is involved in the maintenance of glucose homeostasis and in insulin secretion in mice. Purinergic Signal. 2005;1:1–7. doi: 10.1007/s11302-005-6209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léon C, Freund M, Ravanat C, Baurand A, Cazenave JP, Gachet C. Key role of the P2Y1 receptor in tissue factor-induced thrombin-dependent acute thromboembolism: studies in P2Y1-knockout mice and mice treated with a P2Y1 antagonist. Circulation. 2001;103:718–723. doi: 10.1161/01.cir.103.5.718. [DOI] [PubMed] [Google Scholar]
- Léon C, Hechler B, Freund M, Eckly A, Vial C, Ohlmann P, Dierich A, LeMeur M, Cazenave JP, Gachet C. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y1 receptor-null mice. J Clin Investig. 1999a;104:1731–1737. doi: 10.1172/JCI8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léon C, Hechler B, Vial C, Leray C, Cazenave JP, Gachet C. The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakariyoblastic cells. FEBS Lett. 1997;403:26–30. doi: 10.1016/s0014-5793(97)00022-7. [DOI] [PubMed] [Google Scholar]
- Léon C, Ravanat C, Freund M, Cazenave JP, Gachet C. Differential involvement of the P2Y1 and P2Y12 receptors in platelet procoagulant activity. Arterioscler Thromb Vasc Biol. 2003;23:1941–1947. doi: 10.1161/01.ATV.0000092127.16125.E6. [DOI] [PubMed] [Google Scholar]
- Léon C, Vial C, Cazenave J-P, Gachet C. Cloning and sequencing of a human cDNA encoding endothelial P2Y1 purinoceptor. Gene. 1996;171:295–297. doi: 10.1016/0378-1119(96)00027-3. [DOI] [PubMed] [Google Scholar]
- Léon C, Vial C, Gachet C, Ohlmann P, Hechler B, Cazenave JP, Lecchi A, Cattaneo M. The P2Y1 receptor is normal in a patient presenting a severe deficiency of ADP-induced platelet aggregation. Thromb Haemost. 1999b;81:775–781. [PubMed] [Google Scholar]
- Levitt B, Head RJ, Westfall DP. High-pressure liquid chromatographic-fluorometric detection of adenosine and adenine nucleotides: application to endogenous content and electrically induced release of adenyl purines in guinea pig vas deferens. Anal Biochem. 1984;137:93–100. doi: 10.1016/0003-2697(84)90352-x. [DOI] [PubMed] [Google Scholar]
- Li JM, Fan LM, Shah A, Brooks G. Targeting αvβ3 and α5β1 for gene delivery to proliferating VSMCs: synergistic effect of TGF-α1. Am J Physiol. 2003;285:H1123–H1131. doi: 10.1152/ajpheart.00103.2003. [DOI] [PubMed] [Google Scholar]
- Li JY, Jahn R, Dahlstrom A. Axonal transport and targeting of the t-SNAREs SNAP-25 and syntaxin 1 in the peripheral nervous system. Eur J Cell Biol. 1996;70:12–22. [PubMed] [Google Scholar]
- Li Q, Olesky M, Palmer RK, Harden TK, Nicholas RA. Evidence that the p2y3 receptor is the avian homologue of the mammalian P2Y6 receptor. Mol Pharmacol. 1998;54:541–546. doi: 10.1124/mol.54.3.541. [DOI] [PubMed] [Google Scholar]
- Li Q, Schachter JB, Harden TK, Nicholas RA. The 6H1 orphan receptor, claimed to be the p2y5 receptor, does not mediate nucleotide-promoted second messenger responses. Biochem Biophys Res Commun. 1997;236:455–460. doi: 10.1006/bbrc.1997.6984. [DOI] [PubMed] [Google Scholar]
- Liu J, Liao Z, Camden J, Griffin KD, Garrad RC, Santiago-Perez LI, Gonzalez FA, Seye CI, Weisman GA, Erb L. SH3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, Pyk2, and growth factor receptors. J Biol Chem. 2004;279:8212–8218. doi: 10.1074/jbc.M312230200. [DOI] [PubMed] [Google Scholar]
- Lova P, Paganini S, Hirsch E, Barberis L, Wymann M, Sinigaglia F, Balduini C, Torti M. A selective role for phosphatidylinositol 3,4,5-trisphosphate in the Gi-dependent activation of platelet Rap1B. J Biol Chem. 2003;278:131–138. doi: 10.1074/jbc.M204821200. [DOI] [PubMed] [Google Scholar]
- Lova P, Paganini S, Sinigaglia F, Balduini C, Torti M. A Gi-dependent pathway is required for activation of the small GTPase Rap1B in human platelets. J Biol Chem. 2002;277:12009–12015. doi: 10.1074/jbc.M111803200. [DOI] [PubMed] [Google Scholar]
- Lustig KD, Landis DM, Hicks-Taylor CS, Zhang X, Erb L, Sportiello M, Weisman GA. Mechanisms by which extracellular ATP and UTP stimulate the release of prostacyclin from bovine pulmonary artery endothelial cells. Biochim Biophys Acta. 1992;1134:61–72. doi: 10.1016/0167-4889(92)90028-a. [DOI] [PubMed] [Google Scholar]
- Lustig KD, Shiau AK, Brake AJ, Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci USA. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthardt J, Borvendeg SJ, Sperlagh B, Poelchen W, Wirkner K, Illes P. P2Y1 receptor activation inhibits NMDA receptor-channels in layer V pyramidal neurons of the rat prefrontal and parietal cortex. Neurochem Int. 2003;42:161–172. doi: 10.1016/s0197-0186(02)00069-4. [DOI] [PubMed] [Google Scholar]
- Lüthje J, Ogilvie A. Catabolism of Ap4A and Ap3A in whole blood: the dinucleotides are long-lived signal molecules in the blood ending up as intracellular ATP in the erythrocytes. Eur J Biochem. 1988;173:241–245. doi: 10.1111/j.1432-1033.1988.tb13990.x. [DOI] [PubMed] [Google Scholar]
- Luttikhuizen DT, Harmsen MC, de Leij LF, van Luyn MJ. Expression of P2 receptors at sites of chronic inflammation. Cell Tissue Res. 2004;317:289–298. doi: 10.1007/s00441-004-0939-x. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Della Rocca GJ, Van Biesen T, Luttrell DK, Lefkowitz RJ. Gβγ subunits mediate Src-dependent phosphorylation of the epidermal growth factor receptor: a scaffold for G protein-coupled receptor-mediated Ras activation. J Biol Chem. 1997;272:4637–4644. doi: 10.1074/jbc.272.7.4637. [DOI] [PubMed] [Google Scholar]
- Malam-Souley R, Seye C, Gadeau AP, Loirand G, Pillois X, Campan M, Pacaud P, Desgranges C. Nucleotide receptor P2u partially mediates ATP-induced cell cycle progression of aortic smooth muscle cells. J Cell Physiol. 1996;166:57–65. doi: 10.1002/(SICI)1097-4652(199601)166:1<57::AID-JCP7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith MP, Tolhurst G, Evans RJ. Emerging roles for P2X1 receptors in platelet activation. Platelets. 2004;15:131–144. doi: 10.1080/09537100410001682788. [DOI] [PubMed] [Google Scholar]
- Malmsjö M, Adner M, Harden TK, Pendergast W, Edvinsson L, Erlinge D. The stable pyrimidines UDPβS and UTPγS discriminate between the P2 receptors that mediate vascular contraction and relaxation of the rat mesenteric artery. Br J Pharmacol. 2000;131:51–56. doi: 10.1038/sj.bjp.0703536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmsjö M, Hou M, Pendergast W, Erlinge D, Edvinsson L. The stable pyrimidines UDPβS and UTPγS discriminate between contractile cerebrovascular P2 receptors. Eur J Pharmacol. 2003a;458:305–311. doi: 10.1016/s0014-2999(02)02787-5. [DOI] [PubMed] [Google Scholar]
- Malmsjö M, Hou M, Pendergast W, Erlinge D, Edvinsson L. Potent P2Y6 receptor mediated contractions in human cerebral arteries. BMC Pharmacol. 2003b;3:4. doi: 10.1186/1471-2210-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamedova L, Capra V, Accomazzo MR, Gao ZG, Ferrario S, Fumagalli M, Abbracchio MP, Rovati GE, Jacobson KA. CysLT1 leukotriene receptor antagonists inhibit the effects of nucleotides acting at P2Y receptors. Biochem Pharmacol. 2005;71:115–125. doi: 10.1016/j.bcp.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamedova LK, Joshi BV, Gao ZG, von Kügelgen I, Jacobson KA. Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol. 2004;67:1763–1770. doi: 10.1016/j.bcp.2004.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin P, Ohlmann P, Eckly A, Cazenave JP, Lanza F, Gachet C. The P2Y receptor plays an essential role in the platelet shape change induced by collagen when TxA2 formation is prevented. J Thromb Haemost. 2004;2:969–977. doi: 10.1111/j.1538-7836.2004.00722.x. [DOI] [PubMed] [Google Scholar]
- Mantegazza M, Yu FH, Powell AJ, Clare JJ, Catterall WA, Scheuer T. Molecular determinants for modulation of persistent sodium current by G-protein subunits βγ. J Neurosci. 2005;25:3341–3349. doi: 10.1523/JNEUROSCI.0104-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DC, Scofield MA. Apical P2Y4 purinergic receptor controls K+ secretion by vestibular dark cell epithelium. Am J Physiol. 2001;281:C282–C289. doi: 10.1152/ajpcell.2001.281.1.C282. [DOI] [PubMed] [Google Scholar]
- Mark MD, Ruppersberg JP, Herlitze S. Regulation of GIRK channel deactivation by Gαq and Gαi/o pathways. Neuropharmacology. 2000;39:2360–2373. doi: 10.1016/s0028-3908(00)00080-0. [DOI] [PubMed] [Google Scholar]
- Marriott I, Inscho EW, Bost KL. Extracellular uridine nucleotides initiate cytokine production by murine dendritic cells. Cell Immunol. 1999;195:147–156. doi: 10.1006/cimm.1999.1531. [DOI] [PubMed] [Google Scholar]
- Marteau F, Communi D, Boeynaems JM, Suarez Gonzalez N. Involvement of multiple P2Y receptors and signaling pathways in the action of adenine nucleotides diphosphates on human monocyte-derived dendritic cells. J Leukoc Biol. 2004;76:796–803. doi: 10.1189/jlb.0104032. [DOI] [PubMed] [Google Scholar]
- Marteau F, Gonzalez NS, Communi D, Goldman M, Boeynaems JM, Communi D. Thrombospondin-1 and indoleamine 2,3-dioxygenase are major targets of extracellular ATP in human dendritic cells. Blood. 2005;106:3860–3866. doi: 10.1182/blood-2005-05-1843. [DOI] [PubMed] [Google Scholar]
- Marteau F, Le Poul E, Communi D, Labouret C, Savi P, Boeynaems JM, Gonzalez NS. Pharmacological characterization of the human P2Y13 receptor. Mol Pharmacol. 2003;64:104–112. doi: 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- Martin KA, Kertesy SB, Dubyak GR. Down-regulation of P2U-purinergic nucleotide receptor messenger RNA expression during in vitro differentiation of human myeloid leukocytes by phorbol esters or inflammatory activators. Mol Pharmacol. 1997;51:97–108. doi: 10.1124/mol.51.1.97. [DOI] [PubMed] [Google Scholar]
- Masino SA, Diao L, Illes P, Zahniser NR, Larson GA, Johansson B, Fredholm BB, Dunwiddie TV. Modulation of hippocampal glutamatergic transmission by ATP is dependent on adenosine A1 receptors. J Pharmacol Exp Ther. 2002;303:356–363. doi: 10.1124/jpet.102.036731. [DOI] [PubMed] [Google Scholar]
- Mateo J, Miras-Portugal MT, Rotllan P. Ecto-enzymatic hydrolysis of diadenosine polyphosphates by cultured adrenomedullary vascular endothelial cells. Am J Physiol. 1997a;273:C918–C927. doi: 10.1152/ajpcell.1997.273.3.C918. [DOI] [PubMed] [Google Scholar]
- Mateo J, Rotllan P, Marti E, Gomez De Aranda I, Solsona C, Miras-Portugal MT. Diadenosine polyphosphate hydrolase from presynaptic plasma membranes of Torpedo electric organ. Biochem J. 1997b;323:677–684. doi: 10.1042/bj3230677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu R, Baurand A, Schmitt M, Gachet C, Bourguignon JJ. Synthesis and biological activity of 2-alkylated deoxyadenosine bisphosphate derivatives as P2Y1 receptor antagonists. Bioorg Med Chem. 2004;12:1769–1779. doi: 10.1016/j.bmc.2003.12.041. [DOI] [PubMed] [Google Scholar]
- Matos JE, Robaye B, Boeynaems JM, Beauwens R, Leipziger J. Colonic K+-secretion activated by luminal P2Y2 and P2Y4 receptors. J Physiol (Lond) 2005;564:269–279. doi: 10.1113/jphysiol.2004.080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ. D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent sodium currents in prefrontal cortex pyramidal neurons. J Neurosci. 2001;21:2268–2277. doi: 10.1523/JNEUROSCI.21-07-02268.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara N, Khong A, McKemy D, Caterina M, Boyer J, Julius D, Basbaum C. ATP transduces signals from ASGM1, a glycolipid that functions as a bacterial receptor. Proc Natl Acad Sci USA. 2001;98:9086–9091. doi: 10.1073/pnas.161290898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghani P. The design of P2Y2 antagonists for the treatment of inflammatory diseases. Proceedings of the 224th ACS National Meeting and Exposition; 2002 Aug 18–22; Boston. 2002. [Google Scholar]
- Megson AC, Dickenson JM, Townsend-Nicholson A, Hill SJ. Synergy between the inositol phosphate responses to transfected human adenosine A1-receptors and constitutive P2-purinoceptors on CHO-K1 cells. Br J Pharmacol. 1995;115:1415–1424. doi: 10.1111/j.1476-5381.1995.tb16632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor EA, Austen KF, Boyce JA. Cysteinyl leukotrienes and uridine diphosphate induce cytokine generation by human mast cells through an interleukin 4-regulated pathway that is inhibited by leukotriene receptor antagonists. J Exp Med. 2002;195:583–592. doi: 10.1084/jem.20020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor EA, Frank N, Soler D, Hodge MR, Lora JM, Austen KF, Boyce JA. Expression of the type 2 receptor for cysteinyl leukotrienes (CysLT2R) by human mast cells: functional distinction from CysLT1R. Proc Natl Acad Sci USA. 2003;100:11589–11593. doi: 10.1073/pnas.2034927100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor EA, Maekawa A, Austen KF, Boyce JA. Cysteinyl leukotriene receptor 1 is also a pyrimidinergic receptor and is expressed by human mast cells. Proc Natl Acad Sci USA. 2001;98:7964–7969. doi: 10.1073/pnas.141221498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshki J, Tuluc F, Bredetean O, Ding Z, Kunapuli SP. Molecular mechanism of nucleotide-induced primary granule release in human neutrophils: role for the P2Y2 receptor. Am J Physiol. 2004;286:C264–C271. doi: 10.1152/ajpcell.00287.2003. [DOI] [PubMed] [Google Scholar]
- Meyer CH, Hotta K, Peterson WM, Toth CA, Jaffe GJ. Effect of INS37217, a P2Y2 receptor agonist, on experimental retinal detachment and electroretinogram in adult rabbits. Invest Ophthalmol Vis Sci. 2002;43:3567–3574. [PubMed] [Google Scholar]
- Meyer MP, Clarke JDW, Patel K, Townsend-Nicholson A, Burnstock G. Selective expression of purinoceptor cP2Y1 suggests a role for nucleotide signalling in development of the chick embryo. Dev Dyn. 1999;214:152–158. doi: 10.1002/(SICI)1097-0177(199902)214:2<152::AID-AJA5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Milligan G. G protein-coupled receptor dimerization: function and ligand pharmacology. Mol Pharmacol. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- Miras-Portugal MT, Gualix J, Mateo J, Diaz-Hernandez M, Gomez-Villafuertes R, Castro E, Pintor J. Diadenosine polyphosphates, extracellular function and catabolism. Prog Brain Res. 1999;120:397–409. doi: 10.1016/s0079-6123(08)63572-4. [DOI] [PubMed] [Google Scholar]
- Miras-Portugal MT, Pintor J, Gualix J. Ca2+ signalling in brain synaptosomes activated by dinucleotides. J Membr Biol. 2003;194:1–10. doi: 10.1007/s00232-003-2024-x. [DOI] [PubMed] [Google Scholar]
- Mitchell CH, Carre DA, McGlinn AM, Stone RA, Civan MM. A release mechanism for stored ATP in ocular ciliary epithelial cells. Proc Natl Acad Sci USA. 1998;95:7174–7178. doi: 10.1073/pnas.95.12.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi Y, Kobayashi S, Ahmed A, Nishimura J, Fukui M, Kanaide H. P2U purinergic activation leads to the cell cycle progression from the G1 to the S and M phases but not from the G0 to G1 phase in vascular smooth muscle cells in primary culture. Biochem Biophys Res Commun. 1996;222:652–658. doi: 10.1006/bbrc.1996.0750. [DOI] [PubMed] [Google Scholar]
- Moers A, Nieswandt B, Massberg S, Wettschureck N, Gruner S, Konrad I, Schultz V, Aktas B, Gratacap MP, Simon MI, et al. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat Med. 2003;9:1418–1422. doi: 10.1038/nm943. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Chambers JK, Wahlin JP, Tan KB, Moore GB, Jenkins O, Emson PC, Murdock PR. Expression pattern of human P2Y receptor subtypes: a quantitative reverse transcription-polymerase chain reaction study. Biochim Biophys Acta. 2001;1521:107–119. doi: 10.1016/s0167-4781(01)00291-3. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Chambers JK, Waldvogel H, Faull R, Emson PC. Regional and cellular distribution of the P2Y1 purinergic receptor in the human brain: striking neuronal localisation. J Comp Neurol. 2000a;421:374–384. doi: 10.1002/(sici)1096-9861(20000605)421:3<374::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Iritani S, Chambers J, Emson P. Immunohistochemical localisation of the P2Y1 purinergic receptor in Alzheimer’s disease. Neuroreport. 2000b;11:3799–3803. doi: 10.1097/00001756-200011270-00041. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Murdock PR, Watson JM, Faull RLM, Waldvogel HJ, Szekeres PG, Wilson S, Freeman KB, Emson PC. GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunological challenge: possible role in neuroimmune function. Mol Brain Res. 2003;118:10–23. doi: 10.1016/s0169-328x(03)00330-9. [DOI] [PubMed] [Google Scholar]
- Moran-Jiminez MJ, Matute C. Immunohistochemical localisation of the P2Y1 purinergic receptor in neurons and glial cells of the central nervous system. Mol Brain Res. 2000;78:50–58. doi: 10.1016/s0169-328x(00)00067-x. [DOI] [PubMed] [Google Scholar]
- Moro S, Guo D, Camaioni E, Boyer JL, Harden TK, Jacobson KA. Human P2Y1 receptor: molecular modelling and site-directed mutagenesis as tools to identify agonist and antagonist recognition sites. J Med Chem. 1998;41:1456–1466. doi: 10.1021/jm970684u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro S, Jacobson KA. Molecular modeling as a tool to investigate molecular recognition in P2Y receptors. Curr Pharmaceut Des. 2002;8:2401–2413. doi: 10.2174/1381612023392892. [DOI] [PubMed] [Google Scholar]
- Moro O, Lameh J, Hogger P, Sadee W. Hydrophobic amino acid in the i2 loop plays a key role in receptor-G protein coupling. J Biol Chem. 1993;268:22273–22276. [PubMed] [Google Scholar]
- Mosbacher J, Maier R, Fakler B, Glatz A, Crespo J, Bilbe G. P2Y receptor subtypes differentially couple to inwardly-rectifying potassium channels. FEBS Lett. 1998;436:104–110. doi: 10.1016/s0014-5793(98)01066-7. [DOI] [PubMed] [Google Scholar]
- Müller C. P2-pyrimidinergic receptors and their ligands. Curr Pharm Des. 2002;8:2353–2369. doi: 10.2174/1381612023392937. [DOI] [PubMed] [Google Scholar]
- Murata J, Lee HJ, Clair T, Krutzsch HC, Arestad AA, Sobel ME, Liotta LA, Stracke ML. cDNA cloning of the human motility-stimulating protein, autotaxin, reveals a homology with phosphodiesterases. J Biol Chem. 1994;269:30479–30484. [PubMed] [Google Scholar]
- Murthy KS, Makhlouf GM. Coexpression of ligand-gated P2X and G protein-coupled P2Y receptors in smooth muscle: preferential activation of P2Y receptors coupled to phospholipase C (PLC)-βl via Gαq/11 and to PLC-β3 via Gβγi3. J Biol Chem. 1998;273:4695–4704. doi: 10.1074/jbc.273.8.4695. [DOI] [PubMed] [Google Scholar]
- Muscella A, Elia MG, Greco S, Storelli C, Marsigliante S. Activation of P2Y2 receptor induces c-FOS protein through a pathway involving mitogen-activated protein kinases and phosphoinositide 3-kinases in HeLa cells. J Cell Physiol. 2003;195:234–240. doi: 10.1002/jcp.10242. [DOI] [PubMed] [Google Scholar]
- Nagy AK, Shuster TA, Delgado-Escueta V. Rat brain synaptosomal ATP:AMP-phosphotransferase activity. J Neurochem. 1989;53:1166–1172. doi: 10.1111/j.1471-4159.1989.tb07410.x. [DOI] [PubMed] [Google Scholar]
- Nahum V, Zundorf G, Levesque SA, Beaudoin AR, Reiser G, Fischer B. Adenosine 5′-O-(1-boranotriphosphate) derivatives as novel P2Y1 receptor agonists. J Med Chem. 2002;45:5384–5396. doi: 10.1021/jm020251d. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Strittmatter SM. P2Y1 receptors in sensory neurones: contribution to touch-induced impulse generation. Proc Natl Acad Sci USA. 1996;93:10465–10470. doi: 10.1073/pnas.93.19.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata H, Yoshioka K, Saitoh O. Hetero-oligomerization between adenosine A1 and P2Y1 receptors in living cells: formation of ATP-sensitive adenosine receptors. Drug Dev Res. 2003;58:340–349. [Google Scholar]
- Nandanan E, Camaioni E, Jang SY, Kim YC, Cristalli G, Herdewijn P, Secrist JA, Tiwari KN, Mohanram A, Harden TK, et al. Structure activity relationships of bisphosphate nucleotide derivatives as P2Y1 receptor antagonists and partial agonists. J Med Chem. 1999;42:1625–1638. doi: 10.1021/jm980657j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandanan E, Jang SY, Moro S, Kim HO, Siddiqui MA, Russ P, Marquez VE, Busson R, Herdewijn P, Harden TK, et al. Synthesis, biological activity, and molecular modeling of ribose-modified deoxyadenosine bisphosphate analogues as P2Y1 receptor ligands. J Med Chem. 2000;43:829–842. doi: 10.1021/jm990249v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Goji J, Nakamura H, Sano K. Molecular cloning, expression, and localization of a brain-specific phosphodiesterase I/nucleotide pyrophosphatase (PD-I α) from rat brain. J Biol Chem. 1994;269:28235–28242. [PubMed] [Google Scholar]
- Neary JT, Kang Y, Bu Y, Yu E, Akong K, Peters CM. Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/calcium pathway. J Neurosci. 1999;19:4211–4220. doi: 10.1523/JNEUROSCI.19-11-04211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Willoughby KA, Ellis EF. Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J Neurosci. 2003;23:2348–2356. doi: 10.1523/JNEUROSCI.23-06-02348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- Neary JT, Whittemore SR, Zhu Q, Norenberg MD. Synergistic activation of DNA synthesis in astrocytes by fibroblast growth factors and extracellular ATP. J Neurochem. 1994;63:490–494. doi: 10.1046/j.1471-4159.1994.63020490.x. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Erb L, Weisman GA, Marchese A, Heng HH, Garrad RC, George SR, Turner JT, O’Dowd BF. Cloning, expression, and chromosomal localization of the human uridine nucleotide receptor gene. J Biol Chem. 1995;270:30845–30848. doi: 10.1074/jbc.270.52.30845. [DOI] [PubMed] [Google Scholar]
- Nguyen TD, Meichle S, Kim US, Wong T, Moody MW. P2Y11, a purinergic receptor acting via cAMP, mediates secretion by pancreatic duct epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G795–G804. doi: 10.1152/ajpgi.2001.280.5.G795. [DOI] [PubMed] [Google Scholar]
- Nicholas RA, Watt WC, Lazarowski ER, Li Q, Harden K. Uridine nucleotide selectivity of three phospholipase C-activating P2 receptors: identification of a UDP-selective, a UTP-selective, and an ATP- and UTP-specific receptor. Mol Pharmacol. 1996;50:224–229. [PubMed] [Google Scholar]
- Nichols KK, Yerxa B, Kellerman DJ. Diquafosol tetrasodium : a novel dry eye therapy. Expert Opin Investig Drugs. 2004;13:47–54. doi: 10.1517/13543784.13.1.47. [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Bergmeier W, Eckly A, Schulte V, Ohlmann P, Cazenave JP, Zirngibl H, Offermanns S, Gachet C. Evidence for cross-talk between glycoprotein VI and Gi-coupled receptors during collagen-induced platelet aggregation. Blood. 2001;97:3829–3835. doi: 10.1182/blood.v97.12.3829. [DOI] [PubMed] [Google Scholar]
- Niitsu Y, Jakubowski JA, Sugidachi A, Asai F. Pharmacology of CS-747 (prasugrel, LY640315), a novel, potent antiplatelet agent with in vivo P2Y12 receptor antagonist activity. Semin Thromb Hemost. 2005;31:184–194. doi: 10.1055/s-2005-869524. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- North A. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Nurden P, Savi P, Heilmann E, Bihour C, Herbert JM, Maffrand JP, Nurden A. An inherited bleeding disorder linked to a defective interaction between ADP and its receptor on platelets: its influence on glycoprotein IIb-IIIa complex function. J Clin Investig. 1995;95:1612–1622. doi: 10.1172/JCI117835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlmann P, Laugwitz KL, Nurnberg B, Spicher K, Schultz G, Cazenave JP, Gachet C. The human platelet ADP receptor activates Gi2 proteins. Biochem J. 1995;312:775–779. doi: 10.1042/bj3120775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Costanzi S, Kim HS, Kempeneers V, Vastmans K, Herdewijn P, Maddileti S, Gao ZG, Harden TK, Jacobson KA. Nucleotide analogues containing 2-oxa-bicyclo[2.2.1]heptane and L-α-threofuranosyl ring systems: interactions with P2Y receptors. Bioorg Med Chem. 2004;12:5619–5630. doi: 10.1016/j.bmc.2004.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Nakagawa T, Minami M, Satoh M. Analgesic effects of intrathecal administration of P2Y nucleotide receptor agonists UTP and UDP in normal and neuropathic pain model rats. J Pharmacol Exp Ther. 2002;303:66–73. doi: 10.1124/jpet.102.036079. [DOI] [PubMed] [Google Scholar]
- Okada SF, O’Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC. Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J Gen Physiol. 2004;124:513–526. doi: 10.1085/jgp.200409154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier KN, Bennett WD, Hohneker KW, Zeman KL, Edwards LJ, Boucher RC, Knowles MR. Acute safety and effects on mucociliary clearance of aerosolized uridine 5′-triphosphate +/− amiloride in normal human adults. Am J Respir Crit Care Med. 1996;154:217–223. doi: 10.1164/ajrccm.154.1.8680683. [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Gregorian C, Insel PA. Cellular release of and response to ATP as key determinants of the set-point of signal transduction pathways. J Biol Chem. 2000;275:11735–11739. doi: 10.1074/jbc.275.16.11735. [DOI] [PubMed] [Google Scholar]
- Otero M, Garrad RC, Velázquez B, Hernández-Pérez MG, Camden JM, Erb L, Clarke LL, Turner JT, Weisman GA, González FA. Mechanisms of agonist-dependent and -independent desensitization of a recombinant P2Y2 nucleotide receptor. Mol Cell Biochem. 2000;205:115–123. doi: 10.1023/a:1007018001735. [DOI] [PubMed] [Google Scholar]
- Palea S, Artibani W, Ostardo E, Trist DG, Pietra C. Evidence for purinergic neurotransmission in human urinary bladder affected by interstitial cystitis. J Urol. 1993;50:2007–2012. doi: 10.1016/s0022-5347(17)35955-4. [DOI] [PubMed] [Google Scholar]
- Palmer RK, Boyer JL, Schacter JB, Nicolas RA, Harden TK. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol Pharmacol. 1998;54:1118–1123. [PubMed] [Google Scholar]
- Parker MS, Onyenekwu NN, Bobbin RP. Localization of the P2Y4 receptor in the guinea pig organ of Corti. J Am Acad Audiol. 2003;14:286–295. [PubMed] [Google Scholar]
- Parr CE, Sullivan DM, Paradiso AM, Lazarowski ER, Burch LH, Olsen JC, Erb L, Weisman GA, Boucher RC, Turner JT. Cloning and expression of a human P2U nucleotide receptor, a target for cystic-fibrosis pharmacotherapy. Proc Natl Acad Sci USA. 1994;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JD, Gordon JL. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature (Lond) 1979;281:384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- Pearson PJ, Evora PR, Schaff HV. Bioassay of EDRF from internal mammary arteries: implications for early and late bypass graft patency. Ann Thor Surg. 1992a;54:1078–1084. doi: 10.1016/0003-4975(92)90073-d. [DOI] [PubMed] [Google Scholar]
- Pearson PJ, Lin PJ, Schaff HV. Global myocardial ischemia and reperfusion impair endothelium-dependent relaxations to aggregating platelets in the canine coronary artery: a possible cause of vasospasm after cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1992b;103:1147–1154. [PubMed] [Google Scholar]
- Pendergast W, Yerxa BR, Douglass JG, 3rd, Shaver SR, Dougherty RW, Redick CC, Sims IF, Rideout JL. Synthesis and P2Y receptor activity of a series of uridine dinucleoside 5′-polyphosphates. Bioorg Med Chem Lett. 2001;11:157–160. doi: 10.1016/s0960-894x(00)00612-0. [DOI] [PubMed] [Google Scholar]
- Peters G, Robbie G. Single dose pharmacokinetics and pharmacodynamics of AZD6140—an oral reversible ADP receptor antagonist. Haematologica. 2004;989:14. Abstract. [Google Scholar]
- Picher M, Boucher RC. Biochemical evidence for an ecto alkaline phosphodiesterase I in human airways. Am J Respir Cell Mol Biol. 2000;23:255–261. doi: 10.1165/ajrcmb.23.2.4088. [DOI] [PubMed] [Google Scholar]
- Picher M, Boucher RC. Human airway ecto-adenylate kinase: a mechanismto propagate ATP signaling on airway surfaces. J Biol Chem. 2003;278:11256–11264. doi: 10.1074/jbc.M208071200. [DOI] [PubMed] [Google Scholar]
- Picher M, Burch LH, Boucher RC. Metabolism of P2 receptor agonists in human airways: implications for mucociliary clearance and cystic fibrosis. J Biol Chem. 2004;279:20234–20241. doi: 10.1074/jbc.M400305200. [DOI] [PubMed] [Google Scholar]
- Pidgeon GP, Tang K, Cai YL, Piasentin E, Honn KV. Overexpression of platelet-type 12-lipoxygenase promotes tumor cell survival by enhancing αvβ3 and αvβ5 integrin expression. Cancer Res. 2003;63:4258–4267. [PubMed] [Google Scholar]
- Pillois X, Chaulet H, Belloc I, Dupuch F, Desgranges C, Gadeau AP. Nucleotide receptors involved in UTP-induced rat arterial smooth muscle cell migration. Circ Res. 2002;90:678–681. doi: 10.1161/01.res.0000013700.98464.8e. [DOI] [PubMed] [Google Scholar]
- Pintor J, Pelaez T, Peral A. Adenosine tetraphosphate, Ap4, a physiological regulator of intraocular pressure in normotensive rabbit eyes. J Pharmacol Exp Ther. 2004;308:468–473. doi: 10.1124/jpet.103.058669. [DOI] [PubMed] [Google Scholar]
- Pintor J, Peral A, Pelaez T, Carracedo G, Bautista A, Hoyle CH. Nucleotides and dinucleotides in ocular physiology: new possibilities of nucleotides as therapeutic agents in the eye. Drug Dev Res. 2003;59:136–145. [Google Scholar]
- Qi AD, Harden TK, Nicholas RA. GPR80/99, proposed to be the P2Y15 receptor activated by adenosine and AMP, is not a P2Y receptor. Purinergic Signal. 2004;1:67–74. doi: 10.1007/s11302-004-5069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi AD, Kennedy C, Harden TK, Nicholas RA. Differential coupling of the human P2Y11 receptor to phospholipase C and adenylyl cyclase. Br J Pharmacol. 2001a;132:318–326. doi: 10.1038/sj.bjp.0703788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi AD, Wolff SC, Nicholas RA. The apical targeting signal of the P2Y2 receptor is located in its first extracellular loop. J Biol Chem. 2005;280:29169–29175. doi: 10.1074/jbc.M501301200. [DOI] [PubMed] [Google Scholar]
- Qi AD, Zambon AC, Insel PA, Nicholas RA. An arginine/glutamine difference at the juxtaposition of transmembrane domain 6 and the third extracellular loop contributes to the markedly different nucleotide selectivities of human and canine P2Y11 receptors. Mol Pharmacol. 2001b;60:1375–1382. doi: 10.1124/mol.60.6.1375. [DOI] [PubMed] [Google Scholar]
- Raboisson P, Baurand A, Cazenave JP, Gachet C, Retat M, Spiess B, Bourguignon JJ. Novel antagonists acting at the P2Y1 purinergic receptor: synthesis and conformational analysis using potentiometric and nuclear magnetic resonance titration techniques. J Med Chem. 2002a;45:962–972. doi: 10.1021/jm0104062. [DOI] [PubMed] [Google Scholar]
- Raboisson P, Baurand A, Cazenave JP, Gachet C, Schultz D, Spiess B, Bourguignon JJ. A general approach toward the synthesis of C-nucleoside pyrazolo[1,5-a]-1,3,5-triazines and their 3′,5′-bisphosphate C-nucleotide analogues as the first reported in vivo stable P2Y1-receptor antagonists. J Org Chem. 2002b;67:8063–8071. doi: 10.1021/jo026268l. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Involvement of purinergic signalling in cardiovascular diseases. Drug News Perspect. 2003;16:133–140. doi: 10.1358/dnp.2003.16.3.876886. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Jankowski J, Schluter H. Structure-activity relationships of diadenosine polyphosphates (ApnAs), adenosine polyphospho guanosines (ApnGs) and guanosine polyphospho guanosines (GpnGs) at P2 receptors in the rat mesenteric arterial bed. Br J Pharmacol. 2001;134:1073–1083. doi: 10.1038/sj.bjp.0704341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Rotllan P. Specific dinucleoside polyphosphate cleaving enzymes from chromaffin cells: a fluorimetric study. Biochim Biophys Acta. 1995;1253:103–111. doi: 10.1016/0167-4838(95)00154-m. [DOI] [PubMed] [Google Scholar]
- Rao S, Garrett-Sinha LA, Yoon J, Simon MC. The Ets factors PU.1 and Spi-B regulate the transcription in vivo of P2Y10, a lymphoid restricted heptahelical receptor. J Biol Chem. 1999;274:34245–34252. doi: 10.1074/jbc.274.48.34245. [DOI] [PubMed] [Google Scholar]
- Ravi RG, Kim HS, Servos J, Zimmermann H, Lee K, Maddileti S, Boyer JL, Harden TK, Jacobson KA. Adenine nucleotide analogues locked in a northern methanocarba conformation: enhanced stability and potency as P2Y1 receptor agonists. J Med Chem. 2002;45:2090–2100. doi: 10.1021/jm010538v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM, Haws C, Wine JJ, Grygorczyk R, Tabcharani JA, Hanrahan JW, Gunderson KL, Kopito RR. Failure of the cystic fibrosis transmembrane conductance regulator to conduct ATP. Science (Wash DC) 1996;271:1876–1879. doi: 10.1126/science.271.5257.1876. [DOI] [PubMed] [Google Scholar]
- Reisin IL, Prat AG, Abraham EH, Amara JF, Gregory RJ, Ausiello DA, Cantiello HF. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J Biol Chem. 1994;269:20584–20591. [PubMed] [Google Scholar]
- Rice WR, Burton FM, Fiedeldey DT. Cloning and expression of the alveolar type II cell P2u-purinergic receptor. Am J Resp Cell Mol Biol. 1995;12:27–32. doi: 10.1165/ajrcmb.12.1.7811468. [DOI] [PubMed] [Google Scholar]
- Robaye B, Boeynaems JM, Communi D. Slow desensitization of the human P2Y6 receptor. Eur J Pharmacol. 1997;329:231–236. [PubMed] [Google Scholar]
- Robaye B, Ghanem E, Wilkin F, Fokan D, Van Driessche W, Schurmans S, Boeynaems JM, Beauwens R. Loss of nucleotide regulation of epithelial chloride transport in the jejunum of P2Y4-null mice. Mol Pharmacol. 2003;63:777–783. doi: 10.1124/mol.63.4.777. [DOI] [PubMed] [Google Scholar]
- Roman RM, Lomri N, Braunstein G, Feranchak AP, Simeoni LA, Davison AK, Mechetner E, Schwiebert EM, Fitz JG. Evidence for multidrug resistance-1 P-glycoprotein-dependent regulation of cellular ATP permeability. J Membr Biol. 2001;183:165–173. doi: 10.1007/s00232-001-0064-7. [DOI] [PubMed] [Google Scholar]
- Ruan HZ, Burnstock G. Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochem Cell Biol. 2003;120:415–426. doi: 10.1007/s00418-003-0579-3. [DOI] [PubMed] [Google Scholar]
- Sabirov RZ, Dutta AK, Okada Y. Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J Gen Physiol. 2001;118:251–266. doi: 10.1085/jgp.118.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirov RZ, Okada Y. ATP-conducting maxi-anion channel: a new player in stress-sensory transduction. Jpn J Physiol. 2004;54:7–14. doi: 10.2170/jjphysiol.54.7. [DOI] [PubMed] [Google Scholar]
- Sage CL, Marcus DC. Immunolocalization of P2Y4 and P2Y2 purinergic receptors in strial marginal cells and vestibular dark cells. J Membr Biol. 2002;185:103–115. doi: 10.1007/s00232-001-0116-z. [DOI] [PubMed] [Google Scholar]
- Sak K, Uri A, Enkvist E, Raidaru G, Subbi J, Kelve M, Jarv J. Adenosine-derived non-phosphate antagonists for P2Y1 purinoceptors. Biochem Biophys Res Commun. 2000;272:327–331. doi: 10.1006/bbrc.2000.2775. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Santiago-Perez LI, Flores RV, Santos-Berrios C, Chorna NE, Krugh B, Garrad RC, Erb L, Weisman GA, González FA. P2Y2 nucleotide receptor signaling in human monocytic cells: activation, desensitization and coupling to mitogen-activated protein kinases. J Cell Physiol. 2001;187:196–208. doi: 10.1002/jcp.1063. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Hoshi M, Akazawa C, Nakamura Y, Tsuzuki H, Inoue K, Kohsaka S. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia. 2003;44:242–250. doi: 10.1002/glia.10293. [DOI] [PubMed] [Google Scholar]
- Savi P, Beauverger P, Labouret C, Delfaud M, Salel V, Kaghad M, Herbert JM. Role of P2Y1 purinoceptor in ADP-induced platelet activation. FEBS Lett. 1998;422:291–295. doi: 10.1016/s0014-5793(98)00025-8. [DOI] [PubMed] [Google Scholar]
- Savi P, Herbert JM. Clopidogrel and ticlopidine: P2Y12 ADP-receptor antagonists for the prevention of atherothrombosis. Semin Thromb Haemost. 2005;6:755–764. doi: 10.1055/s-2005-869523. [DOI] [PubMed] [Google Scholar]
- Savi P, Labouret C, Delesque N, Guette F, Lupker J, Herbert JM. P2Y12, a new platelet ADP receptor, target of clopidogrel. Biochem Biophys Res Commun. 2001;283:379–383. doi: 10.1006/bbrc.2001.4816. [DOI] [PubMed] [Google Scholar]
- Savi P, Pereillo JM, Uzabiaga MF, Combalbert J, Picard C, Maffrand JP, Pascal M, Herbert JM. Identification and biological activity of the active metabolite of clopidogrel. Thromb Haemost. 2000;84:891–896. [PubMed] [Google Scholar]
- Scarborough RM, Laibelman AM, Clizbe LA, Fretto LJ, Conley PB, Reynolds EE, Sedlock DM, Jantzen HM. Novel tricyclic benzothiazolo[2,3-c]thiadiazine antagonists of the platelet ADP receptor (P2Y12) Bioorg Med Chem Lett. 2001;11:1805–1808. doi: 10.1016/s0960-894x(01)00313-4. [DOI] [PubMed] [Google Scholar]
- Schacter JB, Li Q, Boyer JL, Nicholas RA, Harden TK. Second messenger cascade specificity and pharmacological selectivity of the human P2Y1 purinoceptor. Br J Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer L, de Kerchove d’Exaerde A, Changeux J-P. Targeting transcription to the neuromuscular synapse. Neuron. 2001;31:5–22. doi: 10.1016/s0896-6273(01)00353-1. [DOI] [PubMed] [Google Scholar]
- Schafer R, Sedehizade F, Welte T, Reiser G. ATP- and UTP-activated P2Y receptors differently regulate proliferation of human lung epithelial tumor cells. Am J Physiol. 2003;285:L376–L385. doi: 10.1152/ajplung.00447.2002. [DOI] [PubMed] [Google Scholar]
- Schnurr M, Toy T, Stoitzner P, Cameron P, Shin A, Beecroft T, Davis ID, Cebon J, Maraskovsky E. ATP gradients inhibit the migratory capacity of specific human dendritic cell types: implications for P2Y11 receptor signaling. Blood. 2003;102:613–620. doi: 10.1182/blood-2002-12-3745. [DOI] [PubMed] [Google Scholar]
- Schöneberg T, Schulz A, Grosse R, Schade R, Henhlein P, Schultz G, Gudermann T. A novel subgroup of class I G-protein-coupled receptors. Biochim Biophys Acta. 1999;1446:57–70. doi: 10.1016/s0167-4781(99)00081-0. [DOI] [PubMed] [Google Scholar]
- Schrader AM, Camden JM, Weisman GA. P2Y2 nucleotide receptor up-regulation in submandibular gland cells from the NOD.B10 mouse model of Sjögren’s syndrome. Arch Oral Biol. 2004;50:533–540. doi: 10.1016/j.archoralbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Schulz A, Schöneberg T. The structural evolution of a P2Y-like G-protein-coupled receptor. J Biol Chem. 2003;278:35531–35541. doi: 10.1074/jbc.M303346200. [DOI] [PubMed] [Google Scholar]
- Schwarz UR, Geiger J, Walter U, Eigenthaler M. Flow cytometry analysis of intracellular VASP phosphorylation for the assessment of activating and inhibitory signal transduction pathways in human platelets-definition and detection of ticlopidine/clopidogrel effects. Thromb Haemost. 1999;82:1145–1152. [PubMed] [Google Scholar]
- Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, Guggino WB. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- Schwiebert EM, Kishore BK. Extracellular nucleotide signaling along the renal epithelium. Am J Physiol. 2001;280:F945–F963. doi: 10.1152/ajprenal.2001.280.6.F945. [DOI] [PubMed] [Google Scholar]
- Schwiebert LM, Rice WC, Kudlow BA, Taylor AL, Schwiebert EM. Extracellular ATP signaling and P2X nucleotide receptors in monolayers of primary human vascular endothelial cells. Am J Physiol. 2002;282:C289–C301. doi: 10.1152/ajpcell.01387.2000. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Delautier D, Meerson NR, Trugnan G, Goding JW, Maurice M. Biochemical and molecular identification of distinct forms of alkaline phosphodiesteraseI expressed on the apical and basolateral plasma membrane surfaces of rat hepatocytes. Hepatology. 1997;25:995–1002. doi: 10.1002/hep.510250434. [DOI] [PubMed] [Google Scholar]
- Seifert R, Schultz G. Involvement of pyrimidinoceptors in the regulation of cell functions by uridine and by uracil nucleotides. Trends Pharmacol Sci. 1989;10:365–369. doi: 10.1016/0165-6147(89)90009-6. [DOI] [PubMed] [Google Scholar]
- Selbie LA, Hill SJ. G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signalling pathways. Trends Pharmacol Sci. 1998;19:87–93. doi: 10.1016/s0165-6147(97)01166-8. [DOI] [PubMed] [Google Scholar]
- Selbie LA, King NV, Dickenson JM, Hill SJ. Role of G protein βγ subunits in the augmentation of P2Y2 (P2U) receptor-stimulated responses by neuropeptide Y Y1 Gi/o-coupled receptors. Biochem J. 1997;328:153–158. doi: 10.1042/bj3280153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers LA, Simon J, Lundahl TS, Cousens DJ, Humphrey PP, Barnard EA. Adenosine nucleotides acting at the human P2Y1 receptor stimulate mitogen-activated protein kinases and induce apoptosis. J Biol Chem. 2001;276:16379–16390. doi: 10.1074/jbc.M006617200. [DOI] [PubMed] [Google Scholar]
- Selyanko AA, Hadley JK, Brown DA. Properties of single M-type KCNQ2/KCNQ3 potassium channels expressed in mammalian cells. J Physiol. 2001;534:15–24. doi: 10.1111/j.1469-7793.2001.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seye CI, Gadeau AP, Daret D, Dupuch F, Alzieu P, Capron L, Desgranges C. Overexpression of P2Y2 purinoceptor in intimal lesions of the rat aorta. Arterioscl Thromb Vasc Biol. 1997;17:3602–3610. doi: 10.1161/01.atv.17.12.3602. [DOI] [PubMed] [Google Scholar]
- Seye CI, Kong Q, Erb L, Garrad RC, Krugh B, Wang M, Turner JT, Sturek M, Gonzalez FA, Weisman GA. Functional P2Y2 nucleotide receptors mediate uridine 5′-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation. 2002;106:2720–2726. doi: 10.1161/01.cir.0000038111.00518.35. [DOI] [PubMed] [Google Scholar]
- Seye CI, Yu N, Gonzalez FA, Erb L, Weisman GA. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1) J Biol Chem. 2004;279:35679–35686. doi: 10.1074/jbc.M401799200. [DOI] [PubMed] [Google Scholar]
- Seye CI, Yu N, Jain R, Kong Q, Minor T, Newton J, Erb L, Gonzalez FA, Weisman GA. The P2Y2 nucleotide receptor mediates UTP-induced vascular cell adhesion molecule-1 expression in coronary artery endothelial cells. J Biol Chem. 2003;278:24960–24965. doi: 10.1074/jbc.M301439200. [DOI] [PubMed] [Google Scholar]
- Sharon E, Zundorf G, Levesque SA, Beaudoin AR, Reiser G, Fischer B. Fluorescent epsilon-ATP analogues for probing physicochemical properties of proteins. Synthesis, biochemical evaluation, and sensitivity to properties of the medium. Bioorg Med Chem. 2004;12:6119–6135. doi: 10.1016/j.bmc.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Shaver SR, Rideout J, Pendergast W, Douglass JG, Brown EG, Boyer JL, Patel RI, Redick CC, Jones AC, Picher M, et al. Structure–activity relationships of dinucleotides: potent and selective agonists of P2Y receptors. Purinergic Signal. 2005;1:183–191. doi: 10.1007/s11302-005-0648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Seye CI, Wang M, Weisman GA, Wilden PA, Sturek M. Cloning, upregulation, and mitogenic role of porcine P2Y2 receptor in coronary artery smooth muscle cells. Mol Pharmacol. 2004;66:1265–1274. doi: 10.1124/mol.104.002642. [DOI] [PubMed] [Google Scholar]
- Silinsky EM, Redman RS. Synchronous release of ATP and neurotransmitter within milliseconds of a motor nerve impulse in the frog. J Physiol (Lond) 1996;492:815–822. doi: 10.1113/jphysiol.1996.sp021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Barnard EA. The P2Y nucleotide receptors in the human genome. Acta Biol Hung. 2003;54:191–201. doi: 10.1556/ABiol.54.2003.2.8. [DOI] [PubMed] [Google Scholar]
- Simon J, Filippov AK, Goransson S, Wong YH, Frelin C, Michel AD, Brown DA, Barnard EA. Characterization and channel coupling of the P2Y12 nucleotide receptor of brain capillary endothelial cells. J Biol Chem. 2002;277:31390–31400. doi: 10.1074/jbc.M110714200. [DOI] [PubMed] [Google Scholar]
- Simon J, Vigne P, Eklund KM, Michel AD, Carruthers AM, Humphrey PP, Frelin C, Barnard EA. Activity of adenosine diphosphates and triphosphates on a P2Y(T)-type receptor in brain capillary endothelial cells. Br J Pharmacol. 2001;132:173–182. doi: 10.1038/sj.bjp.0703816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton L, Cooper M, Murphy M, Platt A. Human immature monocyte-derived dendritic cells express the G-protein-coupled receptor GPR105 (KIAA0001, P2Y14) and increase intracellular calcium in response to its agonist, uridine diphosphoglucose. J Immunol. 2003;171:1941–1949. doi: 10.4049/jimmunol.171.4.1941. [DOI] [PubMed] [Google Scholar]
- Slater M, Murphy CR, Barden JA. Purinergic receptor expression in the apical plasma membrane of rat uterine epithelial cells during implantation. Cell Calcium. 2002;31:201–207. doi: 10.1016/S0143-4160(02)00033-7. [DOI] [PubMed] [Google Scholar]
- Solini A, Chiozzi P, Morelli A, Passaro A, Fellin R, Di Virgilio F. Defective P2Y purinergic receptor function: a possible novel mechanism for impaired glucose transport. J Cell Physiol. 2003;197:435–444. doi: 10.1002/jcp.10379. [DOI] [PubMed] [Google Scholar]
- Soltoff SP. Related adhesion focal tyrosine kinase and the epidermal growth factor receptor mediate the stimulation of mitogen-activated protein kinase by the G-protein-coupled P2Y2 receptor. Phorbol ester or [Ca2+]i elevation can substitute for receptor activation. J Biol Chem. 1998;273:23110–23117. doi: 10.1074/jbc.273.36.23110. [DOI] [PubMed] [Google Scholar]
- Soltoff SP, Avraham H, Avraham S, Cantley LC. Activation of P2Y2 receptors by UTP and ATP stimulates mitogen-activated kinase activity through a pathway that involves related adhesion focal tyrosine kinase and protein kinase C. J Biol Chem. 1998;273:2653–2660. doi: 10.1074/jbc.273.5.2653. [DOI] [PubMed] [Google Scholar]
- Somers GR, Bradbury R, Trute L, Conigrave A, Venter DJ. Expression of the human P2Y6 nucleotide receptor in normal placenta and gestational trophoblastic disease. Lab Invest. 1999;79:131–139. [PubMed] [Google Scholar]
- Somers GR, Hammet F, Woollatt E, Richards RI, Southey MC, Venter DJ. Chromosomal localization of the human P2y6 purinoceptor gene and phylogenetic analysis of the P2y purinoceptor family. Genomics. 1997;44:127–130. doi: 10.1006/geno.1997.4841. [DOI] [PubMed] [Google Scholar]
- Somers GR, Hammet FM, Trute L, Southey MC, Venter DJ. Expression of the P2Y6 purinergic receptor in human T cells infiltrating inflammatory bowel disease. Lab Invest. 1998;78:1375–1383. [PubMed] [Google Scholar]
- Sorensen CE, Novak I. Visualization of ATP release in pancreatic acini in response to cholinergic stimulus: use of fluorescent probes and confocal microscopy. J Biol Chem. 2001;276:32925–32932. doi: 10.1074/jbc.M103313200. [DOI] [PubMed] [Google Scholar]
- Soulet C, Sauzeau V, Plantavid M, Herbert JM, Pacaud P, Payrastre B, Savi P. Gi-dependent and –independent mechanisms downstream of the P2Y12 ADP-receptor. J Thromb Haemost. 2004;2:135–146. doi: 10.1111/j.1538-7836.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- Spedding M, Weetman DF. Identification of separate receptors for adenosine and adenosine 5′-triphosphate in causing relaxation of the isolated taenia of the guinea-pig caecum. Br J Pharmacol. 1976;57:305–310. doi: 10.1111/j.1476-5381.1976.tb07480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperlágh B, Vizi ES. Neuronal synthesis, storage and release of ATP. Semin Neurosci. 1996;8:175–186. [Google Scholar]
- Sprague RS, Stephenson AH, Ellsworth ML, Keller C, Lonigro AJ. Impaired release of ATP from red blood cells of humans with primary pulmonary hypertension. Exp Biol Med. 2001;226:434–439. doi: 10.1177/153537020122600507. [DOI] [PubMed] [Google Scholar]
- Springthorpe B. From ATP to AZD6140: design of an orally active P2Y12 (P2T) receptor antagonist for the treatment of thrombosis. Proceedings of the 25th ACS National Meeting; 2003 March23–27; New Orleans, LA. 2003. [Google Scholar]
- Stracke ML, Krutzsch HC, Unsworth EJ, Arestad AA, Cioce V, Schiffmann E, Liotta LA. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267:2524–2529. [PubMed] [Google Scholar]
- Stam NJ, Klomp J, Van de Heuvel N, Olijve W. Molecular cloning and characterization of a novel orphan receptor (P2P) expressed in human pancreas that shows high structural homology to the P2U purinoceptor. FEBS Lett. 1996;384:260–264. doi: 10.1016/0014-5793(96)00321-3. [DOI] [PubMed] [Google Scholar]
- Stanfield PR, Nakajima S, Nakajima Y. Constitutively active and G-protein coupled inward rectifier K+ channels: Kir2.0 and Kir3.0. Rev Physiol Biochem Pharmacol. 2002;145:47–179. doi: 10.1007/BFb0116431. [DOI] [PubMed] [Google Scholar]
- Suarez Gonzalez N, Communi D, Hannedouche S, Boeynaems JM. The fate of P2Y-related orphan receptors: GPR80/99 and GPR91 are receptors of dicarboxylic acids. Purinergic Signal. 2004;1:17–20. doi: 10.1007/s11302-004-5071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Huerta N, Pouillon V, Boeynaems J, Robaye B. Molecular cloning and characterization of the mouse P2Y4 nucleotide receptor. Eur J Pharmacol. 2001;416:197–202. doi: 10.1016/s0014-2999(01)00875-5. [DOI] [PubMed] [Google Scholar]
- Sueta T, Paki B, Everett AW, Robertson D. Purinergic receptors in auditory neurotransmission. Hear Res. 2003;183:97–108. doi: 10.1016/s0378-5955(03)00221-1. [DOI] [PubMed] [Google Scholar]
- Sugidachi A, Asai F, Yoneda K, Iwamura R, Ogawa T, Otsuguro K, Koike H. Antiplatelet action of R-99224, an active metabolite of a novel thienopyridine-type Gi-linked P2T antagonist, CS-747. Br J Pharmacol. 2001;132:47–54. doi: 10.1038/sj.bjp.0703761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita M, Yue Y, Foskett JK. CFTR Cl− channel and CFTR-associated ATP channel: distinct pores regulated by common gates. EMBO J. 1998;17:898–908. doi: 10.1093/emboj/17.4.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugo T, Tachimoto H, Chikatsu T, Murakami Y, Kikukawa Y, Sato S, Kikuchi K, Nagi T, Harada M, Ogi K, et al. Identification of a lysophosphatidylserine receptor on mast cells. Biochem Biophys Res Commun. 2006;341:1078–1087. doi: 10.1016/j.bbrc.2006.01.069. [DOI] [PubMed] [Google Scholar]
- Suh BC, Horowitz LF, Hirdes W, Mackie K, Hille B. Regulation of KCNQ2/KCNQ3 current by G protein cycling: the kinetics of receptor-mediated signaling by Gq. J Gen Physiol. 2004;123:657–662. doi: 10.1085/jgp.200409029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Kim TD, Lee IS, Kim KT. Differential regulation of P2Y11 receptor-mediated signalling to phospholipase C and adenylyl cyclase by protein kinase C in HL-60 promyelocytes. Br J Pharmacol. 2000;131:489–497. doi: 10.1038/sj.bjp.0703581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki J, Kamohara M, Saito T, Matsumoto M, Matsumoto S, Ohishi T, Soga T, Matsushime H, Furuichi K. Molecular cloning of the platelet P2TAC ADP receptor: pharmacological comparison with another ADP receptor, the P2Y1 receptor. Mol Pharmacol. 2001;60:432–439. [PubMed] [Google Scholar]
- Taylor AL, Kudlow BA, Marrs KL, Gruenert DC, Guggino WB, Schwiebert EM. Bioluminescence detection of ATP release mechanisms in epithelia. Am J Physiol. 1998;275:C1391–C1406. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- Teixeira M, Butlen D, Ferrary E, Sterkers O, Escoubet B. Identification of uridine 5′-triphosphate receptor mRNA in rat cochlear tissues. Acta Otolaryngol. 2000;120:156–159. doi: 10.1080/000164800750000810. [DOI] [PubMed] [Google Scholar]
- Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem. 2002;277:39436–39442. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- Tokuyama Y, Hara M, Jones EMC, Fan Z, Bell GI. Cloning of rat and mouse P2Y purinoceptors. Biochem Biophys Res Commun. 1995;211:211–218. doi: 10.1006/bbrc.1995.1798. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci USA. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Van Nhieu G, Clair C, Bruzzone R, Mesnil M, Sansonetti P, Combettes L. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat Cell Biol. 2003;5:720–726. doi: 10.1038/ncb1021. [DOI] [PubMed] [Google Scholar]
- Trumel C, Payrastre B, Plantavid M, Hechler B, Viala C, Presek P, Martinson EA, Cazenave JP, Chap H, Gachet C. A key role of adenosine diphosphate in the irreversible platelet aggregation induced by the PAR1-activating peptide through the late activation of phosphoinositide 3-kinase. Blood. 1999;94:4156–4165. [PubMed] [Google Scholar]
- Tsim KW, Choi RC, Siow NL, Cheng AW, Ling KK, Jiang JX, Tung EK, Lee HH, Xie QH, Simon J, et al. ATP induces post-synaptic gene expressions in vertebrate skeletal neuromuscular junctions. J Neurocytol. 2003;32:603–617. doi: 10.1023/B:NEUR.0000020613.25367.78. [DOI] [PubMed] [Google Scholar]
- Tu MT, Luo SF, Wang CC, Chien CS, Chiu CT, Lin CC, Yang CM. P2Y2 receptor-mediated proliferation of C6 glioma cells via activation of Ras/Raf/MEK/MAPK pathway. Br J Pharmacol. 2000;129:1481–1489. doi: 10.1038/sj.bjp.0703182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung EKK, Choi RCY, Siow NL, Jiang JXS, Karen KY, Ling KKY, Simon J, Barnard EA, Tsim KWK. P2Y2 receptor activation regulates the expression of acetylcholinesterase and acetylcholine receptor genes at vertebrate neuromuscular junctions. Mol Pharmacol. 2004;66:794–806. doi: 10.1124/mol.104.003269. [DOI] [PubMed] [Google Scholar]
- Turner C, Tam FWK, Lai P-C, Tarzi RM, Burnstock G, Pusey CD, Cook HT, Unwin R. Glomerular expression of the ATP-sensitive P2X7 receptor is increased in proliferative glomerulonephritis. Proceedings of the Annual Meeting of the American Society for Microbiology; 2004 May 23–27; New Orleans, LA. 2004. [Google Scholar]
- Turner CM, Vonend O, Chan C, Burnstock G, Unwin RJ. The pattern of distribution of selected ATP-sensitive P2 receptor subtypes in normal rat kidney: an immunohistological study. Cells Tissues Organs. 2003;175:105–117. doi: 10.1159/000073754. [DOI] [PubMed] [Google Scholar]
- Turner JT, Park M, Camden JM, Weisman GA. Salivary gland nucleotide receptors. Changes in expression and activity related to development and tissue damage. Ann NY Acad Sci. 1998;842:70–75. doi: 10.1111/j.1749-6632.1998.tb09633.x. [DOI] [PubMed] [Google Scholar]
- Turner JT, Weisman GA, Camden JM. Upregulation of P2Y2 nucleotide receptors in rat salivary gland cells during short-term culture. Am J Physiol. 1997;273:C1100–C1107. doi: 10.1152/ajpcell.1997.273.3.C1100. [DOI] [PubMed] [Google Scholar]
- Uemura T, Takamatsu H, Kawasaki T, Taniguchi M, Yamamoto E, Tomura Y, Uchida W, Miyata K. Effect of YM-254890, a specific Gαq/11 inhibitor, on experimental peripheral arterial disease in rats. Eur J Pharmacol. 2006;536:154–161. doi: 10.1016/j.ejphar.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Ullmann H, Meis S, Hongwiset D, Marzian C, Wiese M, Nickel P, Communi D, Boeynaems J-M, Wolf C, Hausmann R, et al. Synthesis and structure-activity relationships of suramin-derived P2Y11 receptor antagonists with nanomolar potency. J Med Chem. 2005;48:7040–7048. doi: 10.1021/jm050301p. [DOI] [PubMed] [Google Scholar]
- Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterberger U, Moskvina E, Scholze T, Freissmuth M, Boehm S. Inhibition of adenylyl cyclase by neuronal P2Y receptors. Br J Pharmacol. 2002;135:673–684. doi: 10.1038/sj.bjp.0704514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weyden L, Adams DJ, Morris BJ. Capacity for purinergic control of renin promoter via P2Y11 receptor and cAMP pathways. Hypertension. 2000a;36:1093–1098. doi: 10.1161/01.hyp.36.6.1093. [DOI] [PubMed] [Google Scholar]
- van der Weyden L, Rakyan V, Luttrell BM, Morris MB, Conigrave AD. Extracellular ATP couples to cAMP generation and granulocytic differentiation in human NB4 promyelocytic leukaemia cells. Immunol Cell Biol. 2000b;78:467–473. doi: 10.1111/j.1440-1711.2000.t01-4-.x. [DOI] [PubMed] [Google Scholar]
- Van Driel IR, Goding JW. Plasma cell membrane glycoprotein PC-1: primary structure deduced from cDNA clones. J Biol Chem. 1987;262:4882–4887. [PubMed] [Google Scholar]
- Van Driel IR, Wilks AF, Pietersz GA, Goding JW. Murine plasma cell membrane antigen PC-1: molecular cloning of cDNA and analysis of expression. Proc Natl Acad Sci USA. 1985;82:8619–8623. doi: 10.1073/pnas.82.24.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Giezen JJ, Humphries RG. Preclinical and clinical studies with selective reversible direct P2Y12 antagonists. Semin Thromb Hemost. 2005;31:195–204. doi: 10.1055/s-2005-869525. [DOI] [PubMed] [Google Scholar]
- Van Kolen K, Slegers H. P2Y12 receptor stimulation inhibits β-adrenergic receptor-induced differentiation by reversing the cyclic AMP-dependent inhibition of protein kinase B. J Neurochem. 2004;89:442–453. doi: 10.1111/j.1471-4159.2004.02339.x. [DOI] [PubMed] [Google Scholar]
- Vasconcelos EG, Ferreira ST, Carvalho TM, Souza W, Kettlun AM, Mancilla M, Valenzuela MA, Verjovski-Almeida S. Partial purification and immunohistochemical localization of ATP diphosphohydrolase from Schistosoma mansoni: Immunological cross-reactivities with potato apyrase and Toxoplasma gondii nucleoside triphosphate hydrolase. J Biol Chem. 1996;271:22139–22145. doi: 10.1074/jbc.271.36.22139. [DOI] [PubMed] [Google Scholar]
- Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE, Mortrud MT, Brown A, Rodriguez SS, Weller JR, Wright AC, et al. The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci USA. 2003;100:4903–4908. doi: 10.1073/pnas.0230374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassort G. ATP: a P2 purinergic agonist in the myocardium. Physiol Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- Velázquez B, Garrad RC, Weisman GA, González FA. Differential agonist-induced desensitization of P2Y2 nucleotide receptors by ATP and UTP. Mol Cell Biochem. 2000;206:75–89. doi: 10.1023/a:1007091127392. [DOI] [PubMed] [Google Scholar]
- Vial C, Evans RJ. P2X1 receptor-deficient mice establish the native P2X receptor and a P2Y6-like receptor in arteries. Mol Pharmacol. 2002;62:1438–1445. doi: 10.1124/mol.62.6.1438. [DOI] [PubMed] [Google Scholar]
- Vial C, Rolf MG, Mahaut-Smith MP, Evans RJ. A study of P2X1 receptor function in murine megakaryocytes and human platelets reveals synergy with P2Y receptors. Br J Pharmacol. 2002;135:363–372. doi: 10.1038/sj.bjp.0704486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial C, Tobin AB, Evans RJ. G protein-coupled receptor regulation of P2X1 receptors does not involve direct channel phosphorylation. Biochem J. 2004;382:101–110. doi: 10.1042/BJ20031910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmayer P, Clair T, Goding JW, Sano K, Servos J, Zimmermann H. Hydrolysis of diadenosine polyphosphates by nucleotide pyrophosphatases/phosphodiesterases. Eur J Biochem. 2003;270:2971–2978. doi: 10.1046/j.1432-1033.2003.03674.x. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I, Wetter A. Molecular pharmacology of P2Y receptors. Naunyn-Schmiedeberg’s Arch Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji HD, Lacy J, Jacobson KA, Harden TK. Quantification of the P2Y1 receptor with a high affinity radiolabeled antagonist. Mol Pharmacol. 2002;62:1249–1257. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo GL, Harden TK. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol Pharmacol. 2004;65:426–436. doi: 10.1124/mol.65.2.426. [DOI] [PubMed] [Google Scholar]
- Wang L, Jacobsen SE, Bengtsson A, Erlinge D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol. 2004a;5:16. doi: 10.1186/1471-2172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Karlsson L, Moses S, Hultgardh-Nilsson A, Andersson M, Borna C, Gudbjartsson T, Jern S, Erlinge D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2002;40:841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- Wang L, Yao H, Yang YE, Song I, Owyang C. Enhanced purinergic pathway occurs in postoperative ileus: reversal by orphanin FQ. Gastroenterology. 2004b;126:75. [Google Scholar]
- Warny M, Aboudola S, Robson SC, Sevigny J, Communi D, Soltoff SP, Kelly CP. P2Y6 nucleotide receptor mediates monocyte interleukin-8 production in response to UDP or lipopolysaccharide. J Biol Chem. 2001;276:26051–26056. doi: 10.1074/jbc.M102568200. [DOI] [PubMed] [Google Scholar]
- Watt WC, Lazarowski ER, Boucher RC. Cystic fibrosis transmembrane regulator-independent release of ATP: its implications for the regulation of P2Y2 receptors in airway epithelia. J Biol Chem. 1998;273:14053–14058. doi: 10.1074/jbc.273.22.14053. [DOI] [PubMed] [Google Scholar]
- Webb TE, Henderson D, King BF, Wang S, Simon J, Bateson AN, Burnstock G, Barnard EA. A novel G protein-coupled P2 purinoceptor (P2Y3) activated preferentially by nucleoside diphosphates. Mol Pharmacol. 1996a;50:258–265. [PubMed] [Google Scholar]
- Webb TE, Henderson DJ, Roberts JA, Barnard EA. Molecular cloning and characterization of the rat P2Y4 receptor. J Neurochem. 1998;71:1348–1357. doi: 10.1046/j.1471-4159.1998.71041348.x. [DOI] [PubMed] [Google Scholar]
- Webb TE, Kaplan MG, Barnard EA. Identification of 6H1 as a P2Y purinoceptor: P2Y5. Biochem Biophys Res Commun. 1996b;219:105–110. doi: 10.1006/bbrc.1996.0189. [DOI] [PubMed] [Google Scholar]
- Webb TE, Simon J, Krishek BJ, Bateson AN, Smart TG, King BF, Burnstock G, Barnard EA. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993;324:219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- Weick M, Cherkas PS, Hartig W, Pannicke T, Uckermann O, Bringmann A, Tal M, Reichenbach A, Hanani M. P2 receptors in satellite glial cells in trigeminal ganglia of mice. Neuroscience. 2003;120:969–977. doi: 10.1016/s0306-4522(03)00388-9. [DOI] [PubMed] [Google Scholar]
- Weisman GA, Garrad RC, Erb LJ, Otero M, Gonzalez FA, Clarke LL. Structure and function of P2Y2 nucleotide receptors in cystic fibrosis (CF) epithelium. Adv Exp Med Biol. 1998;431:417–424. doi: 10.1007/978-1-4615-5381-6_82. [DOI] [PubMed] [Google Scholar]
- Weisman GA, Garrad RC, Erb LJ, Santos-Berrios C, Gonzalez FA. P2Y receptors in the nervous system: molecular studies of a P2Y2 receptor subtype from NG108–15 neuroblastoma × glioma hybrid cells. Prog Brain Res. 1999;120:33–43. doi: 10.1016/s0079-6123(08)63544-x. [DOI] [PubMed] [Google Scholar]
- Welch BD, Carlson NG, Shi H, Myatt L, Kishore BK. P2Y2 receptor-stimulated release of prostaglandin E2 by rat inner medullary collecting duct preparations. Am J Physiol. 2003;285:F711–F721. doi: 10.1152/ajprenal.00096.2003. [DOI] [PubMed] [Google Scholar]
- Werry TD, Christie MI, Dainty IA, Wilkinson GF, Willars GB. Ca2+ signalling by recombinant human CXCR2 chemokine receptors is potentiated by P2Y nucleotide receptors in HEK cells. Br J Pharmacol. 2002;135:1199–1208. doi: 10.1038/sj.bjp.0704566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werry TD, Wilkinson GF, Willars GB. Cross talk between P2Y2 nucleotide receptors and CXC chemokine receptor 2 resulting in enhanced Ca2+ signalling involves enhancement of phospholipase C activity and is enabled by incremental Ca2+ release in human embryonic kidney cells. J Pharmacol Exp Ther. 2003;307:661–669. doi: 10.1124/jpet.103.055632. [DOI] [PubMed] [Google Scholar]
- White PJ, Kumari R, Porter KE, London NJM, Ng LL, Boarder MR. Anti-proliferative effect of UTP on human arterial and venous smooth muscle. Am J Physiol. 2000;279:H2735–H2742. doi: 10.1152/ajpheart.2000.279.6.H2735. [DOI] [PubMed] [Google Scholar]
- White PJ, Webb TE, Boarder MR. Characterization of a Ca2+ response to both UTP and ATP at human P2Y11 receptors: evidence for agonist-specific signaling. Mol Pharmacol. 2003;63:1356–1363. doi: 10.1124/mol.63.6.1356. [DOI] [PubMed] [Google Scholar]
- Wihlborg AK, Wang L, Braun OO, Eyjolfsson A, Gustafsson R, Gudbjartsson T, Erlinge D. ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler Thromb Vasc Biol. 2004;24:1810–1815. doi: 10.1161/01.ATV.0000142376.30582.ed. [DOI] [PubMed] [Google Scholar]
- Wilden PA, Agazie YM, Kaufman R, Halenda SP. ATP-stimulated smooth muscle cell proliferation requires independent ERK and PI3K signaling pathways. Am J Physiol. 1998;275:H1209–H1215. doi: 10.1152/ajpheart.1998.275.4.H1209. [DOI] [PubMed] [Google Scholar]
- Wildman SS, Unwin RJ, King BF. Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br J Pharmacol. 2003;140:1177–1186. doi: 10.1038/sj.bjp.0705544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, Robaye B. The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol. 2001;166:7172–7177. doi: 10.4049/jimmunol.166.12.7172. [DOI] [PubMed] [Google Scholar]
- Wilkin F, Stordeur P, Goldman M, Boeynaems JM, Robaye B. Extracellular adenine nucleotides modulate cytokine production by human monocyte-derived dendritic cells: dual effect on IL-12 and stimulation of IL-10. Eur J Immunol. 2002;32:2409–2417. doi: 10.1002/1521-4141(200209)32:9<2409::AID-IMMU2409>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Wilkinson GF, Purkiss JR, Boarder MR. Differential heterologous and homologous desensitization of two receptors for ATP (P2Y pruinoceptors and nucleotide receptors) coexisting on endothelial cells. Mol Pharmacol. 1994;45:731–736. [PubMed] [Google Scholar]
- Winks JS, Hughes S, Filippov AK, Tatulian L, Abogadie FC, Brown DA, Marsh SJ. Relationship between membrane phosphatidylinositol-4,5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J Neurosci. 2005;25:3400–3413. doi: 10.1523/JNEUROSCI.3231-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirkner KL, Köles S, Thümmler J, Luthardt W, Poelchen H, Franke H, Fürst S, Illes P. Interaction between P2Y and NMDA receptors in layer V pyramidal neurons of the rat prefrontal cortex. Neuropharmacology. 2002;42:476–488. doi: 10.1016/s0028-3908(01)00199-x. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Schweigel J, Gerevich Z, Franke H, Allgaier C, Barsoumian EL, Draheim H, Illes P. Adenine nucleotides inhibit recombinant N-type calcium channels via G protein-coupled mechanisms in HEK 293 cells: involvement of the P2Y13 receptor-type. Br J Pharmacol. 2004;141:141–151. doi: 10.1038/sj.bjp.0705588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberger T, Schaller HC, Hellebrand S. An expressed sequence tag (EST) data mining strategy succeeding in the discovery of new G-protein coupled receptors. J Mol Biol. 2001;307:799–813. doi: 10.1006/jmbi.2001.4520. [DOI] [PubMed] [Google Scholar]
- Woulfe D, Jiang H, Mortensen R, Yang J, Brass LF. Activation of Rap1B by Gi family members in platelets. J Biol Chem. 2002;277:23382–23390. doi: 10.1074/jbc.M202212200. [DOI] [PubMed] [Google Scholar]
- Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, et al. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc Natl Acad Sci USA. 2002;99:8360–8365. doi: 10.1073/pnas.122231899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin C, Ren S, Pfeilschifter J, Huwiler A. Heterologous desensitisation of the sphingosine-1-phosphate receptors by purinoceptor activation in renal mesangial cells. Br J Pharmacol. 2004;143:581–589. doi: 10.1038/sj.bjp.0705980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing M, Post S, Ostrom RS, Samardzija M, Insel PA. Inhibition of phospholipase A2-mediated arachidonic acid release by cyclic AMP defines a negative feedback loop for P2Y receptor activation in Madin-Darby canine kidney D1 cells. J Biol Chem. 1999;274:10035–10038. doi: 10.1074/jbc.274.15.10035. [DOI] [PubMed] [Google Scholar]
- Xu B, Stephens A, Kirschenheuter G, Greslin AF, Cheng X, Sennelo J, Cattaneo M, Zighetti ML, Chen A, Kim SA, et al. Acyclic analogues of adenosine bisphosphates as P2Y receptor antagonists: phosphate substitution leads to multiple pathways of inhibition of platelet aggregation. J Med Chem. 2002a;45:5694–5709. doi: 10.1021/jm020173u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Chalimoniuk M, Shu Y, Simonyi A, Sun AY, Gonzalez FA, Weisman GA, Wood WG, Sun GY. Prostaglandin E2 production in astrocytes: regulation by cytokines, extracellular ATP, and oxidative agents. Prostaglandins Leukotrienes Essent Fatty Acids. 2003;69:437–448. doi: 10.1016/j.plefa.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Xu J, Weng YI, Simonyi A, Krugh BW, Liao Z, Weisman GA, Sun GY, Simonyi A. Role of PKC and MAPK in cytosolic PLA2 phosphorylation and arachidonic acid release in primary murine astrocytes. J Neurochem. 2002b;83:259–270. doi: 10.1046/j.1471-4159.2002.01145.x. [DOI] [PubMed] [Google Scholar]
- Yang S, Cheek DJ, Westfall DP, Buxton IL. Purinergic axis in cardiac blood vessels: agonist-mediated release of ATP from cardiac endothelial cells. Circ Res. 1994;74:401–407. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- Yegutkin G, Bodin P, Burnstock G. Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5-nucleotidase along with endogenous ATP from vascular endothelial cells. Br J Pharmacol. 2000;129:921–926. doi: 10.1038/sj.bjp.0703136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegutkin GG, Henttinen T, Jalkanen S. Extracellular ATP formation on vascular endothelial cells is mediated by ecto-nucleotide kinase activities via phosphotransfer reactions. FASEB J. 2001;15:251–260. doi: 10.1096/fj.00-0268com. [DOI] [PubMed] [Google Scholar]
- Yegutkin GG, Henttinen T, Samburski SS, Spychala J, Jalkanen S. The evidence for two opposite, ATP-generating and ATP-consuming, extracellular pathways on endothelial and lymphoid cells. Biochem J. 2002;367:121–128. doi: 10.1042/BJ20020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerxa BR. Therapeutic use of nucleotides in respiratory and ophthalmic diseases. Drug Dev Res. 2001;52:196–201. [Google Scholar]
- Yerxa BR, Sabater JR, Davis CW, Stutts MJ, Lang-Furr M, Picher M, Jones AC, Cowlen M, Dougherty R, Boyer J, et al. Pharmacology of INS37217 [P1-(uridine 5′)-P4-(2′-deoxycytidine 5′)tetraphosphate, tetrasodium salt], a next-generation P2Y2 receptor agonist for the treatment of cystic fibrosis. J Pharmacol Exp Ther. 2002;302:871–880. doi: 10.1124/jpet.102.035485. [DOI] [PubMed] [Google Scholar]
- Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature (Lond) 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Hosada R, Kuroda Y, Nakata H. Hetero-oligomerization of adenosine A1 receptors with P2Y1 receptors in rat brains. FEBS Lett. 2002a;531:299–303. doi: 10.1016/s0014-5793(02)03540-8. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Saitoh O, Nakata H. Heteromeric association creates a P2Y-like adenosine receptor. Proc Natl Acad Sci USA. 2001;98:7617–7622. doi: 10.1073/pnas.121587098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K, Saitoh O, Nakata H. Agonist-promoted heteromeric oligomerization between adenosine A1 and P2Y1 receptors in living cells. FEBS Lett. 2002b;523:147–151. doi: 10.1016/s0014-5793(02)02965-4. [DOI] [PubMed] [Google Scholar]
- Zambon AC, Brunton LL, Barrett KE, Hughes RJ, Torres B, Insel PA. Cloning, expression, signaling mechanisms, and membrane targeting of P2Y11 receptors in Madin Darby canine kidney cells. Mol Pharmacol. 2001;60:26–35. doi: 10.1124/mol.60.1.26. [DOI] [PubMed] [Google Scholar]
- Zambon AC, Hughes RJ, Meszaros JG, Wu JJ, Torres B, Brunton LL, Insel PA. P2Y2 receptor of MDCK cells: Cloning, expression, and cell-specific signaling. Am J Physiol. 2000;279:F1045–F1052. doi: 10.1152/ajprenal.2000.279.6.F1045. [DOI] [PubMed] [Google Scholar]
- Zamecnik PC, Kim B, Gao MJ, Taylor G, Blackburn GM. Analogues of diadenosine 5′,5′″-P1,P4-tetraphosphate (Ap4A) as potential anti-platelet-aggregation agents. Proc Natl Acad Sci USA. 1992;89:2370–2373. doi: 10.1073/pnas.89.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FL, Luo L, Gustafson E, Lachowicz J, Smith M, Qiao X, Liu YH, Chen G, Pramanik B, Laz TM, et al. ADP is the cognate ligand for the orphan G protein-coupled receptor SP1999. J Biol Chem. 2001;276:8608–8615. doi: 10.1074/jbc.M009718200. [DOI] [PubMed] [Google Scholar]
- Zhang FL, Luo L, Gustafson E, Palmer K, Qiao X, Fan X, Yang S, Laz TM, Bayne M, Monsma F., Jr P2Y13: identification and characterization of a novel Gαi-coupled ADP receptor from human and mouse. J Pharmacol Exp Ther. 2002a;301:705–713. doi: 10.1124/jpet.301.2.705. [DOI] [PubMed] [Google Scholar]
- Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, Jin T, Logothetis DE. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M-currents. Neuron. 2003a;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li Z, Viklund EK, Stromblad S. P21-activated kinase 4 interacts with integrin αvβ5 and regulates αvβ5-mediated cell migration. J Cell Biol. 2002b;158:1287–1297. doi: 10.1083/jcb.200207008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003b;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. 5′-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992;285:345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. Signalling via ATP in the nervous system. Trends Neurosci. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular purine metabolism. Drug Dev Res. 1996a;39:337–352. [Google Scholar]
- Zimmermann H. Biochemistry, localization and functional roles of ecto-nucleotidases in the nervous system. Prog Neurobiol. 1996b;49:589–618. doi: 10.1016/0301-0082(96)00026-3. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg’s Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- Zona C, Marchetti C, Volonté C, Mercuri NB, Bernardi G. Effect of P2 purinoceptor antagonists on kainate-induced currents in rat cultured neurons. Brain Res. 2000;882:26–35. doi: 10.1016/s0006-8993(00)02781-5. [DOI] [PubMed] [Google Scholar]


