Abstract
Over the last decade, we have witnessed an explosion of information on genetic factors underlying common human diseases and disorders. This ‘human genomics’ information revolution has occurred as a backdrop to a rapid increase in the rates of many human disorders and diseases. For example, obesity, Type 2 diabetes, asthma, autism spectrum disorder and attention deficit hyperactivity disorder have increased at rates that cannot be due to changes in the genetic structure of the population, and are difficult to ascribe to changes in diagnostic criteria or ascertainment. A likely cause of the increased incidence of these disorders is increased exposure to environmental factors that modify gene function. Many environmental factors that have epidemiological association with common human disorders are likely to exert their effects through epigenetic alterations. This general mechanism of gene–environment interaction poses special challenges for individuals, educators, scientists and public policy makers in defining, monitoring and mitigating exposures.
Keywords: aging, behavior, DNA methylation, endocrine disruptors, maternal diet, windows of sensitivity
The Human Genome Project promises many new and exciting ways to use human genetic data to understand, prevent, treat and cure human diseases and disorders. Coupled with emerging stem cell technologies, we face tantalizing prospects and unparalleled opportunities for improving health, lifestyle, wellbeing and lifespan, even at the level of individualized approaches. This exciting genome-science revolution is occurring as a backdrop to an alarming increase in the prevalence of a range of human disorders and diseases. Obesity, diabetes, asthma, autism spectrum disorders (ASDs) and attention deficit hyperactivity disorder (ADHD) are all increasing at dramatic rates (Table 1).
Table 1.
Increases in the prevalence of some common human diseases and disorders, and releationship to genotype, epigenetic factors and environmental agents.
| Disorder | GWAS | % variance explained or OR | Increase in prevalence | Epigenetic effect | Environmental agents |
|---|---|---|---|---|---|
| Obesity | Y | <10% [311] | 100% between 1991 and 2011 |
Y | EDCs, obesogens, arsenic, maternal obesity and maternal diabetes |
| Diabetes | Y | Average OR: 1.18 for ten SNPs with strong association [312] |
176% between 1980 and 2010 |
Y | EDCs |
| Asthma | Y | OR: 1.08–2.04 for SNPs associated with non-toluene- induced disease [313] |
75% between 1980 and 1994 |
Y | Second-hand smoke, automobile exhausts and industrial emissions |
| ADHD | Y | OR: 0.45–1.65 for top 25 SNPs [314] |
33% between 1997 and 2008 |
Y | EDCs |
| ASD | Y | OR: 0.56–1.19 for five SNPs with strongest association [315] |
289.5% between 1997 and 2008 |
Y | EDCs |
| Hypertension | Y | OR: 0.85–0.9, few SNPs confirmed except in extreme case–control designs [316] |
60% between 1995 and 2005 |
Y | EDCs and maternal diet |
| Low birthweight |
N | Not available | Increased use of assisted reproduction, advanced maternal age |
Y | PFOS, PFOA and covariates |
| Cancer | Y | OR: 1.1–2.5, depending on disease [317] |
Overall cancer rates are in decline since 2008, but specific cancers (e.g., lung cancer in women) are increasing and some are linked to environmental factors |
Y | BPA, cadmium, arsenic and dioxins |
ADHD: Attention deficit hyperactivity disorder; ASD: Autism spectrum disorder; BPA: Bisphenol A; EDC: Endocrine-disrupting compound; GWAS: Genome-wide association study; N: No; OR: Odds ratio; PFOA: Perfluorooctanesulfonic acid; PFOS: Perfluorooctanoic acid; Y: Yes.
Obesity affects over 33% of Americans. Although obesity is not a disease phenotype, in and of itself, it contributes to the development of multiple adverse health effects [1]. The WHO reports that 44% of Type 2 diabetes cases, 23% of ischemic heart disease and 7–14% of all cancers are obesity related [401]. Globally, obesity-related diseases are the fifth leading cause of death [401]. No US state has an obesity rate of less than 20%, whereas 10 years ago, no state had an obesity rate above 20% [2,3,402,403], and the overall incidence of obesity has more than doubled in the past 20 years. Obesity rates increased disproportionately among men, children, individuals over 65 years of age, and the non-Hispanic black population. The increase is particularly alarming among children; obesity in children aged 6–18 years rose from 6 to 15% between 1980 and 2000 [2,3,402,403].
Data for developmental disorders and cognitive disorders are more difficult to evaluate, but also point to apparent increases in occurrence (Table 1). The number of children diagnosed with ADHD increased by 33% from 1997 to 2008 [4], and by more than 21% between 2003 and 2007 among all socioeconomic groups. A range of factors (language, socioeconomic status and method of diagnosis) may affect the ascertainment of ADHD, and further studies are needed to conclude the extent of the increase in risk of ADHD. ASDs increased by 289.5% from 1997 to 2008 in one study group [4], and by 57% in ten reporting sites from 2002 to 2006 [5,404]. The increase ranged from 27 to 95% among different sites. Diagnosed ASD was more prevalent among boys, but increased in all major sex, racial, ethnic and cognitive function categories (Table 1). As with ADHD, a range of factors may affect ASD ascertainment.
These observations (Table 1) indicate that significant changes have occurred in the incidence of obesity, diabetes and asthma. Developmental and cognitive disorders also appear to be increasing. While the magnitude of the effect of ascertain ment bias is unclear for ADHD, ASD and developmental delays, it is unlikely that all or most of the observed increase can be attributed solely to disparate access to healthcare and generalized changes in screening practices.
If we accept the argument that these increases are not explicable on the basis of a broad-based change in the genetic make up of the human population and/or ascertainment bias, then an environmental effect is a plausible explanation. Environmental factors may have direct effects on physiological process within cells, tissues and organs. Alternatively, effects could be mediated by changes in gene regulation, arising through molecular and cellular processes that culminate in chemical modifications of the DNA or chromatin (epigenetic changes). Epigenetic changes can arise at any time during the life of the individual, exerting effects on health through immediate effects on development and function, or through a prolonged accumulation of changes that collectively modify individual phenotype. Furthermore, epigenetic changes may be retained during gametogenesis and transmissible to the next generation [6], creating a potential for familial inheritance that is not strictly genetic. This review addresses the potential actions of environmental factors on the epigenome throughout the life of the individual, the nature of such environmental factors, when they might exert their effects, the nature of their actions, and the implications for education and public policy to minimize their impact.
Our changing epigenome
Owing to the high fidelity of DNA replication, DNA repair and mitotic chromosome segregation, our genomes remain largely genetically intact, with point mutations, deletions, recombination and aneuploidies arising at a very low rate in our somatic cells throughout our lifespans. By contrast, our epigenomes may be altered throughout our lifespans, owing to the demands of developmental processes or physiological adaptations to the environment. Such continuous chemical modification of our genomes provides us with a high degree of plasticity but also creates the potential for unfavorable changes.
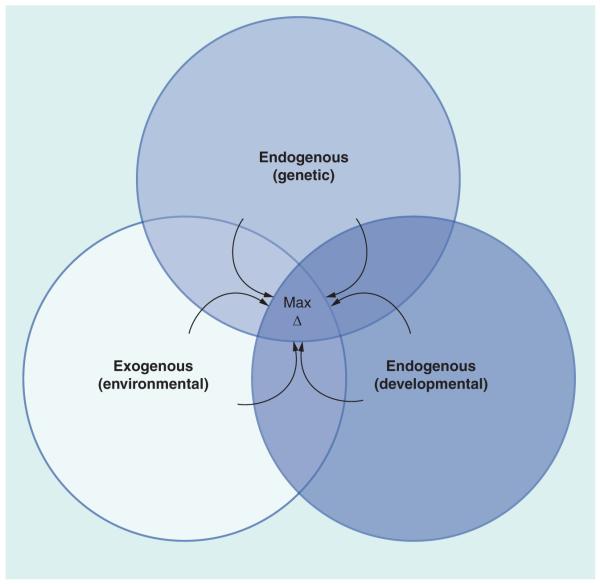
Epigenetic changes can be grouped into two basic categories: endogenously programmed changes and responses to exogenous factors. Endogenously programmed changes encompass genetic variation and developmental processes. Genetic variation affects genes related to epigenetic modification of the genome and cellular and organismal sensitivity to exogenous influences. Epigenetic changes during early development, during elaboration of diverse cell lineages and tissue-specific stem cell lineages, and age-related changes comprise other endogenous factors. Exogenous factors include a wide range of physical and chemical agents and a range of other stressors. Physical factors such as radiation, hypoxia, nutrient restriction and excessive cold or heat present obvious challenges to cells, leading to genetic, molecular and cellular damage that must be repaired, but that may compromise the cell’s ability to mediate such repairs. Endogenously programmed changes and responses to the environment intersect with each other, and each may provide an increased opportunity for the other to occur, as well as affecting the outcome of the other (Figure 1).
Figure 1. Interaction of endogenous (genetic and developmental) and exogenous (environmental) factors in generating changes in the epigenome during an individual’s lifespan.
Environmental factors include chemicals, toxins, physical agents and behavioral, dietary, social and other lifestyle factors. These exert effects preferentially at different times during development. Genetic variation affects developmental processes, responses to environmental factors and the molecular mechanisms that mediate epigenetic change. The arrows indicate that these three forces continue to interact throughout life to modify the epigenome. Maximum epigenetic change occurs when genotype, developmental stage and environmental exposure operate additively (exposure at sensitive stage in individual genetically predisposed to effect).
Chemical factors affecting the epigenome are many and diverse, and some of these are reviewed below. Dietary constituents interact with, or modify the effects of these agents, and genetic variation in the molecular targets of these compounds modulate effects. These relationships create an interesting situation in which the outcome of chemical exposures can be context dependent, thereby complicating our ability to assess environmental impacts in natural populations. Other exogenous factors include psychological, behavioral and social stress.
The effects of exogenous factors are developmental stage dependent. A key aspect of the concept of developmental or fetal origins of adult disease is that the effects observed in progeny depend upon when during gestation the exposure occurs [7]. Developmental ‘windows of sensitivity’ exist, such that particular organs or functions will be most adversely affected following fetal or embryonic exposure at the time when that organ or function is being generated [8]. Endogenous factors intersect with exogenous factors to define windows of sensitivity.
Some environmental causes of epigenetic changes
The interactions between genotype, developmental processes and the environment are complex. This review begins by considering the relevant environmental factors, how those factors interact with developing systems and how genotype might modify these interactions.
Toxins & environmental epigenome disruptors
Endocrine-disrupting compounds (EDCs; chemicals that affect endocrine glands, their function, hormone, receptors and signaling pathways) are chemically diverse and include both naturally occurring compounds (e.g., genistein), and a large array of synthetic compounds including the plasticizing agent bisphenol A (BPA), fluorosurfactants (e.g., perfluoro octanesulfonic acid and perfluorooctanoic acid), herbicides (e.g., atrazine) and phthalates (e.g., bis-[2-eth-ylhexyl] phthalate or di-2-ethylhexyl] phthalate DEHP]), used as emulsifiers and surfactants. An additional class of compounds, organotins (organic moieties coupled to tin atoms), have become known as ‘obesogens’ for their potent effects at promoting obesity and adipogenesis [9–17]. Approximately 800 distinct chemicals have been classified as EDCs [405]. The list includes compounds that are intentionally consumed (caffeine) and others of obvious toxicity (e.g., lead, arsenic, dioxins, benzene, toluene, pesticides, plasticizing agents and so on). We will focus here on a subset of compounds that have received a great deal of attention in research studies. These have well-documented prevalence in the environment and in human tissues, known effects on animal or human health and potential or documented effects on the epigenome.
EDCs and obesogens exist widely in our environment (e.g., drinking water, household dust, numerous consumer products, thermal printer paper and food and beverage containers) [18–40,406,407]. Human exposure is an unavoidable everyday occurrence not limited to occupational sources. EDCs are found in the serum of the general population, in maternal serum and cord blood, and contribute to adverse health effects among humans and animals [9–14,27,28,32,33,35,39,41–98]. Concerns related to these compounds cannot be dismissed as merely ‘alarmist’, but reflect the growing awareness of their serious impact on public health.
In both humans and animal models, EDCs cause phenotypic abnormalities and diseases when administered postnatally. Through anti-androgenic, estrogenic or other endocrine effects, these compounds can contribute to ovarian dysfunction, gynecomastia, miscarriage, obesity, cancer, prostate hyperplasia, immuno toxicity, preeclampsia, neonatal mortality, ADHD, abnormal chromosome segregation, altered metabolism and sterility. They cause defects in progeny when given to pregnant female rodents (see below). Chronic exposure to low concentrations that approximate observed environ mental exposures are sufficient to produce adverse effects. Epidemiological and other studies in humans indicate possible effects in children [99].
BPA, an estrogen mimic, has received considerable media attention and scientific scrutiny. BPA is found in polycarbonate plastics, food and drink containers, thermal paper and the household environment, with estimated intake ranging from 1.6 μg/kg to 100 mg/kg per day [100]. BPA exposure is associated with prostate hyperplasia, prostate and breast cancer, reproductive system effects, sterility, miscarriage, obesity, effects on oocyte meiosis, changes in fetal karyotype and polycystic ovarian syndrome [101].
DEHP is an example of the phthalate class of chemicals, which are implicated in developmental effects [39,44,102–108]. Maternal serum and cord blood concentrations are related to the incidence of ADHD and other childhood conditions [39,95,105,108–114]. Exposure is through inhalation at the workplace, oral ingestion or injection during medical procedures. DEHP is an antiandrogen and can lead to reproductive effects (follicular atresia, inhibition of follicle-stimulating hormone activity, pre eclampsia, miscarriage, reduced estrogen, prolonged estrus and premature thelarche in various species [39,44,102,104,109,115–123]) and other effects in various species [44,90,116,124–127]. No detailed data exist on epigenetic effects of phthalates, but effects on imprinting have been reported.
Fluorosurfactants like perfluoro octanesulfonic acid and perfluorooctanoic acid are implicated in immune system toxicity, cancer, neonatal mortality, preeclampsia, ADHD, endocrine system and reproduction effects, thyroid function effects, hyperinsulinemia and metabolic effects [128]. Inconsistencies exist between studies in conclusions concerning effects on birthweight, which may reflect differences in study design and data analysis, as well as impact of environmental and physiological factors that require further study [99].
Many pesticides are implicated in possible health effects. Atrazine, for example, is wide-spread in the environment and associated with CNS, endocrine and immune system effects, obesity, mitochondrial dysfunction, insulin resistance and cancer [61,62,65,67,69,73–79,129–133]. The widespread herbicide glyphosphate can affect the placenta and the endocrine system [134]. Other pesticides and herbicides can also function as EDCs.
Low arsenic concentrations alter glucocorticoid signaling [135], function in oxidative stress [136], contribute to hypertension [137] and increase the risk of birth defects [138]. Arsenic also can induce cancer [137] and contributes to increased cardiovascular disease [139], skin lesions, diabetes, reproductive toxicity, neurologic disease and neurobehavioral disorders.
Obesogens modulate adipogenesis in cultured cell lines, and can bias stem cell fate along the adipogenic pathway in vivo [9–17]. Tributyltin (TBT) is an obesogenic organotin, a chemical class containing compounds that combine organic moieties with tin atoms, and that are widely prevalent in our environment [19]. Organotins are known for their effects on stem cells and DNA methylation states [13–16,140,141]. Organotins may contribute to the escalating incidence of obesity [142,143], with far-reaching effects on health, and a large associated annual expense in healthcare. Organotins affect other endocrine tissues such as pituitary, gonad and thyroid, and large doses can affect the bone, CNS and GI tract. Organotins are immunotoxic, and they lead to implantation failure and fetal demise, along with uterine and placenta defects [144–146]. Children may have intakes of organotins up to eight-times higher than adults [19].
The mechanisms by which EDCs may exert their effects are diverse, with a potential to contribute to human disease [147]. EDCs may exert indirect, long-term effects on progeny health via effects on the placenta. The placenta is a target of EDCs [44,116,126,148–153], and may be especially sensitive [153,154]. Placental function can be altered by epigenetic changes, such as loss of imprinting [155–160]. Placental insufficiency can lead to intrauterine growth restriction and low birthweight (LBW), a leading cause of perinatal morbidity and mortality. LBW increases the risk of neonatal death, and this increased risk continues after birth [161]. LBW is associated with cognitive impairment [162], and increased risk of childhood and adult diseases, including cardiovascular disease, hypertension, Type 2 diabetes and obesity [163,164].
EDCs exert epigenetic effects in animals and in cultured cells via changes in DNA methylation. Offspring of female mice carrying the A vy allele, in which coat coloration is affected by methylation of an element near the Agouti locus [98], display changes in coat coloration when the mothers are given BPA during gestation, indicative of DNA hypomethylation. Dietary folate reverses this effect [42]. TBT can induce hypomethylation at the Fabp4 locus in cultured 3T3L1 adipogenic cells [13]. Increased adiposity with TBT or other obesogens occurs as a result of DNA hypomethylation combined with activation of retinoid X receptor and PPARγ signaling, which can convert cells to adipogenic fates [13,14,16,140,141]. Other effects include altering hypothalamic function [165], revealing a strikingly broad capacity for these compounds to interfere with diverse developmental processes, potentially contributing to a broad spectrum of diseases and developmental defects. In addition, effects on DNA methylation are seen in the gametes of exposed individuals, and can persist across generations (see below) [147,166–168].
Arsenic increases or decreases DNA methylation [169–173]. Such changes arise gradually and progressively and persist, indicating a complex series of events leading to overall epigenetic change. How are such effects mediated? DNA hypomethylation may arise following chemical modification of arsenic. Arsenic is bio transformed by addition of methyl groups. This creates adducts that can damage DNA, but in addition can deplete the available pool of S-adenosyl methionine. DNA hypermethylation may arise secondarily via physiological adjustments and/or cell selection. Genetic polymorphisms in glutathione S-transferase are a factor with high exposure [174]. Sexual dimorphism may exist in the interaction between arsenic and dietary methyl donors [175]. Arsenic, other heavy metals and organic toxins may affect the epigenome via cellular responses to oxidative stress involving insulin signaling PPARγ-dependent pathways [136,139].
Lifestyle factors as epigenome disruptors
How we live our lives affects our environment, and those around us. These effects can result in epigenome changes through a variety of means. Lifestyle is not often considered in the same context as, for example, environmental toxins, but the two are likely to interact to affect our epigenomes.
Substance abuse
Cocaine increases DNMT3A expression in the mouse nucleus accumbens, and this increase persists after drug withdrawal, possibly contributing to addiction [176–178]. Administering methionine as a methyl donor or a non-nucleoside inhibitor of DNA methylation to that region of the brain decreases and increases cocaine reward response, respectively. Chronic social defeat stress also increased DNMT3A expression in this region. These results illustrate the ability of environmental agents, including substances of abuse, to modify the epigenome in responsive tissues.
Delayed reproduction & assisted reproduction technology
As females age, fertility and reproductive success decline. The uterus becomes less receptive and abortion rates increase [179–181]. Reduced oocyte quality exerts the largest restriction on fertility with aging [180–182]. The number of oocytes with spindle abnormalities and aneuploidies increases [183,184], and mitochondrial function declines [185]. Delaying reproduction may increase potential exposures of oocytes to environmental agents, and because meiotic competence and oocyte genetic quality are affected by environ mental factors [186], age and environment may work together to affect reproductive aging. Maternal exposure to BPA can increase meiotic errors in mice [27,43]. Nutrition affects age of menarche as well as oocyte quality. A decline in age of menarche from 13.2 to 12.5 years of age was seen for women born during the period from 1919 to 1952. Menarchal age was positively correlated with height and negatively correlated with body weight and BMI [187]. Maternal caloric restriction, or mimicking caloric restriction in mice by deletion of Pgc1a can prevent the age-related increase in oocyte aneuploidy, spindle abnormalities, chromosome m isalignments and mitochondrial dysfunction [188].
These observations indicate that a range of environmental factors influence reproductive age and duration in women. Increased caloric intake throughout life may accelerate menarche, degradation of oocyte quality, and eventually reproductive senescence. Immediate effects of the environment on oocyte quality are likely, but additional effects on other reproductive and endocrine organs are likely as well. Genetic variation in genes related to metabolism, food intake, energy expenditure, endocrine function, and epigenome modifying processes could interact with these environmental variables to affect reproductive aging relative to chronological age. The degree to which the epigenome is modified in oocytes, ovarian follicular cells and the uterus needs further scrutiny.
Parenting behavior
Stress affects epigenetic processes during develop ment. Rhesus monkeys subjected to early-life stress in the form of peer rearing as opposed to maternal rearing display behavioral changes, and changes related to behavioral stress are seen in histology, gene expression and DNA methylation in the brain and other cell types in monkeys and other mammalian species, including humans [189–201]. Genetic variation modulates these and other effects [202–204]. Epigenetic alterations are associated with suicidal behavior [205], schizophrenia [206], antisocial behavior [207], substance abuse [208] and smoking [209,210], and this is sensitive to genotype and sex [211]. Thus, potent epigenetic responses in the brain and other organs arise in response to parenting behavior. This effect is sensitive to genotype, sex and presence of other environmental agents.
Our aging epigenome
Like other aspects of biological systems, the passage of time affects the epigenome in ways that are likely to be degenerative. Both longitudinal [212–215] and cross-sectional [216–218] studies of human populations reveal age-associated increases and decreases in site-specific DNA methylation, and comparison of young and old inbred animals reveal similar changes [219]. Absolute rates of change appear to be small [212–215], but epigenetic differences between individuals who differ in the presence of age-related diseases, such as colon cancer [220] and diabetic nephropathy [221], suggest that rates of progression toward a disease state may vary between individuals as a result of genetics or environmental exposures.
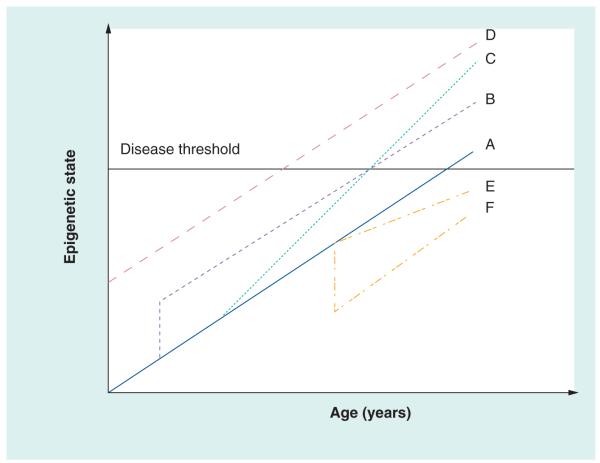
If genetic and environmental factor-related changes occur on a background of constant age-related change, it is possible to envision dramatic effects on the onset of age-related diseases (Figure 2). If, for example, two individuals are born with different levels of DNA methylation at a collection of disease-associated loci (individuals A and D in Figure 2), then one individual will cross the disease threshold at an earlier age than the other, all things being equal. Similarly, an acute exposure to an environmental epigenetic disruptor (individual B in Figure 2) might also decrease the age at which that individual becomes symptomatic. Chronic exposures to an epigenetic disruptor (individual C in Figure 2) might increase the rate at which degenerative epigenetic changes occur, but it is also possible to imagine treatments or other environmental factors that would ‘de-age’ the epigenome (individual F in Figure 2) or decrease the rate of progression (individual E in Figure 2) towards the disease state. Given the strong and consistent association between birthweight, and the decades later onset of several diseases, including diabetes and hypertension [221–227], epigenetic mechanisms that invoke modifications early in development and continued modification with aging (individuals B or D) are one way of explaining how early developmental phenotypes can be associated with disease later in life.
Figure 2. Relationship between epigenetic state, disease threshold and environment.
Individual A represents a reference pattern, wherein the epigenome changes at a given rate throughout life and reaches a threshold to elicit a disease or disorder. Individual B experienced an acute early-life exposure that resulted in a parallel path of change, but reaching the threshold earlier than individual A. Individual C experienced a change in environment that increased the rate of change. Individual D began life at a different epigenetic state (this could be higher or lower) and then progressed in parallel with individual A. Individual E experienced a change in environment that lessened the pace of acquiring epigenetic change, such as an improvement in environment. Individual F experienced an acute drop in epigenetic change, perhaps as a result of a sudden improvement of environment, and then progressed in parallel with individual A.
The interactions between endogenous and exogenous factors affecting the epigenome (Figure 1) reveal a dynamic pattern of variable risk for diseases and disorders that can arise through unfortunate and unforeseen confluence of developmental process, genetic predispositions and environmental exposures. It is impossible to evaluate the entire spectrum of risk factors and all possible interactions here. The authors focus instead on a range of endogenous and exogenous factors that may be to varying degrees within our ability to modulate, and hence reduce risks, and consider possible ways to approach such a reduction.
Developmental origins & windows of sensitivity
A founding principle of the Barker hypothesis of developmental origins of adult disease is that different organ systems have different windows of sensitivity during gestation related to critical periods of specific organ development [228]. An interactive timeline that displays particular windows of sensitivity for certain EDCs has been developed [408]. In this section, details of effects during different windows of sensitivity will be considered.
Windows of epigenome sensitivity probably begin with parental gametogenesis, and then extend to early preimplantation development, fetal development and postnatal exposures. Effects may be exerted directly upon the gametes, embryos, fetuses or children, or indirectly via effects on the reproductive tract.
Oogenesis
During oogenesis three major periods of sensitivity have been proposed, corresponding to meiotic prophase arrest in the early fetal ovary when synapsis and recombination occur, second trimester fetal ovarian development when follicle formation occurs, and oocyte growth, which occurs throughout adult life [183]. EDCs, maternal diet, maternal health and other factors can affect these processes. BPA can disrupt synapsis, increase the rates of meiotic errors and change gene expression in the fetal ovary [229]. This effect is sensitive to maternal diet [230].
Folliculogenesis entails the orderly production of follicles with single oocytes surrounded by supporting somatic cells. Natural and manmade environmental estrogens alter this process, leading to the formation of multi-oocyte follicles, which may generate oocytes of lower quality and affect progeny phenotype, or which may be eliminated, shortening reproductive lifespan [183].
The initiation of oocyte growth in adults is poorly understood. Oocyte growth is carefully coordinated with activities in the supporting somatic cells of the follicle, establishing a complex dialog that ensures a high quality oocyte is produced [231]. BPA can disrupt chromosome assembly in the oocytes in adult ovaries, and can also alter the DNA methylation state of imprinted genes during oocyte growth [232]. Availability of methyl donors during in vitro oocyte growth can also affect imprinted gene methylation [233]. Insufficient dietary folate and follicular fluid homocysteine level, and genetic variants limiting MTHFR correlate with reduced oocytre quality [234,235].
Maternal obesity exerts strong negative effects on oocyte and embryo quality [236,237], and leads to intrauterine growth restriction and obesity amongst progeny [238] and impaired pregnancy outcome [236,239,240]. Elevated nonesterified fatty acids during oocyte maturation lead to reduced oocyte quality, altered embryonic physiology, increased apoptosis and reduced cell numbers [241,242]. A high-fat diet can cause lipotoxicity in oocyte–cumulus complexes, and decreased fertilization rates [243], and other studies indicate effects of dietary lipids on oocyte quality [244–246]. These negative effects of fatty acids could ultimately lead to epigenetic alterations through the activation of signaling pathways that modulate gene activity.
Spermatogenesis
Resetting of DNA methylation at imprinted genes and at gene promoters creates an opportunity for epigenetic changes in response to the environment, particularly in the testis. This occurs during early spermatogenesis, with paternal-specific patterns established by prophase of meiosis I (14.5 days post coitum to after birth) [247–250]. Proper establishment of imprints and promoter methylation patterns is vital for embryogenesis and long-term progeny health. Spermatogenesis is characterized by the progressive acquisition of the highly specialized chromatin structure of the sperm. Transition events during this process are still poorly understood, but include replacement of histones with sperm-specific histone variants and ultimately with protamines, resulting in the extreme condensation of the genome typical of sperm. Central to the developmental potential of the sperm is the retention of histones at genes involved in early embryogenesis [251–253]. These genes are generally key transcription factors and signaling proteins. Thus, toxic stressors can affect not only the gametogenic process, but also the development of the progeny embryos.
Spermatogenesis is coordinated by hormones. EDCs disrupt this coordination, with effects varying by developmental stage. EDCs act on estrogen receptors, which are expressed during fetal gonad development in both males and females. Exposure to antiandrogenic compounds, such as vinclozolin, during embryogenesis and postnatally, causes abnormalities in gonad development [249,254]. By disrupting this coordination, EDCs may alter other processes including imprinting and chromatin modifications.
Sperm quality affects embryonic development, including cleavage speed, morphology and rate of blastocyst formation. Perturbations of preimplantation development in embryos derived from poor quality sperm are attributed to effects occurring before the major activation of the embryonic genome [255]. Although toxicity studies have examined effects on sperm count and quality in humans [408], screening for epigenetic modifications in response to environmental effects is needed for both adult and prenatal germ cell development in animal models.
Preimplantation development & implantation
The early embryo is exquisitely sensitive to perturbations, in part due to a proclivity of the early embryonic genome to undergo epigenetic alterations. A low protein maternal diet in rats and sheep, limited to the preimplantation period, leads to hypertensive progeny and changes in gene imprinting [256]. A low-protein diet may cause maternal hyperglycemia and subsequent stress responses in the embryo to elevated glucose levels [257]. Maternal hyperglycemia, hypoinsulinemia and a low protein diet can diminish oocyte quality when applied before ovulation, and compromise embryo quality when applied during preimplantation development [257–266]. A low protein diet can also lead to epigenetic changes in the progeny. Detailed studies of epigenetic effects of EDCs during preimplantation development have been limited. Preimplantation high-dose BPA exposure negatively affects outcome [267] and low-dose exposures lead to heavier progeny [268]. BPA also negatively affects the development of trophoblast cells and uterine physiology, which together may impair embryo implantation [149,269–271]. Phthalates also can alter trophoblast cell and placenta function, including increased expression of genes related to fatty acid transport, including PPARγ [272].
Modeling early exposures using stem cell systems
One key concept related to windows of sensitivity is the relationship between ‘stemness’, the timing of epigenetic changes, and sensitivity to exogenous factors. There are specific stages of the life cycle during which long-term epigenetic marks can be erased and re-established. Largescale demethylation and remethylation occur during development of primordial germ cells and during preimplantation development, leading to massive reprogramming of the patterns of epigenetic marks. However, cell differentiation and organ development depend on selective epigenetic modifications for the tissue-specific activation or repression of genes, which in turn trigger the regulatory network that leads to establishment of a pool of adult stem/ progenitor cells and more differentiated cells [273]. Terminally differentiated cells are not static entities, and different lineages retain the capacity to respond epigenetically to cues such as hormones and growth factors.
The status of the epigenome determines the expression signature of each cell type in a sustainable and heritable fashion. Thus, early-life exposure to environmental agents may compromise both tissue-specific stem cells and differentiated cells, and the former will transmit alterations to progeny cells [274]. It is especially challenging to determine if cell type-specific changes in DNA methylation or chromatin structure arise as a consequence of exposures using whole animal models. The fact that embryonic stem (ES) cells can be induced to differentiate into a variety of cell types in vitro [275] provides the opportunity to create a reliable and reproducible model to assay the effects of toxin exposure on downstream developmental pathways.
ES cells are derived from the inner cell mass of blastocyst stage embryos and are pluripotent, with the capacity to contribute to all tissues of the adult organism. Detailed epigenomic studies on murine ES cells show that unique chromatin properties distinguish them from differentiated cells [276,277]. Pluripotency depends on a dynamic open chromatin state characterized by specifically expressed subunits of chromatin remodeling complexes. Many genes critical for development exhibit bivalent histone marks in their promoter regions that render them poised for induction upon appearance of the proper cues. Absence of DNA methylation is vital to the responsiveness of these genes.
ES cells provide a defined system to test toxicity of individual or combined agents during early development. ES models may also be useful to test antidotes for toxic compounds. Mouse ES cells have a higher throughput and robustness than human ES cells for developmental toxicology, with less variability in vitro and in vivo, and greater ease of differentiation [278,279]. The ES cell test incorporating a range of differentiation protocols is being improved continuously to synchronize the differentiation steps, to refine quant itative methods of assessing toxicity, to improve sensitivity, to accelerate the differentiation process and to diversify the cell types that can be assayed [280,281]. Tools for genetic modification of mouse ES cells are also more advanced. More research is needed to achieve a comparable status with human ES protocols.
Morphological analysis is giving way to cell sorting and genome-wide studies in toxicology screens. These screens may also provide basic insights into normal differentiation mechanisms. Key to these advances will be mapping the epigenomic features that accompany cell fate and identification of epigenetic biomarkers, preferably at several critical genomic regions or candidate genes. Genome-, transcriptome- and proteome-based technologies are accelerating the ability to meet the demand of understanding plasticity and development at the molecular level [282–284]. More detailed study of the impact of environmental factors on the epigenetic status of ES cells and other stem cells along differentiation pathways will help determine whether certain epigenetic states are selected, which is an attractive hypothesis, but for which there are insufficient data [285].
Adult progenitor/stem cells are also vital to the maintenance of mature tissues and organs and have been identified and enriched from some adult tissues, including the heart, skin, bone marrow, liver and brain [286–289]. Studies to character ize epigenetic features of adult progenitor/ stem cells are underway. The epigenetic basis of plasticity and how environ mental factors affect cell differentiation is an expanding area of research. However, enrichment for stem cells has not yet been successful for many tissues.
Outstanding questions related to environmental effects on stem cells include: how do stem cells and later lineage cells differ in responses to environmental factors at the epigenetic level; and, are there epigenetic states that are more sensitive to, or selected for in the response of stem cells to environmental insults? These questions are key in understanding the epigenetic effects of environmental factors on stem cells.
Fetal life
A diet deficient in methyl donors alters the epigenome and gene imprinting in fetal mice [290], and elevated dietary methyl donors increases DNA methylation and alters phenotype [291,292]. In both mice and humans, these effects can arise even before gastrulation and persist into later life [293]. Maternal BPA exposure during fetal life in mice can alter DNA methylation and pheno type, and supplementation of dietary methyl donors counters this effect [42]. Dietary folate supplementation in pregnant women reduces DNA hypermethylation at the imprinted H19 and IGF2 genes leading to favorable shifts in methylation profile, an effect that is more pronounced in male progeny [294]. Importantly, folate supplementation during pregnancy has a favorable epigenetic effect, even for women who are not folate deficient [291].
Studies on the effects of EDCs and organotins have been limited in some respects. Many studies have described effects of moderate to large doses administered pre- and postnatally on fetuses and progeny, on oocytes or embryos, on reproductive organs, and effects on cell or organ cultures [64–67,70,71,73–75,77,78,95,109,116,129,294–302]. More recent studies reported effects in progeny following chronic and/or low dose exposure during pregnancy or in cultured cells [13,53,56,57,59,82,116,144–146,165,303–309]. Other studies report associations between human diseases or adverse conditions and maternal exposure levels of EDCs. Fetal exposure to the endocrine disruptor methyoxychlor can lead to life-long changes in neuroendocrine gene expression and DNA methyl ation in progeny [310], and exposures to BPA and phthalates can lead to loss of imprinting [44].
Transgenerational effects: ‘you are what you eat’ (or what your mother, father or grandparents ate)
One of the more disturbing aspects of environmental effects on the epigenome is the potential for transgenerational effects. The term ‘transgenerational effects’ is often used in two contexts. One context of usage relates exposures of gametes or fetuses that lead to aberrant qualities in the organs of individuals developing from those exposed gametes or fetuses, an effect that is not truly transgenerational. True transgenerational epigenetic inheritance refers to effects in generations that come after the exposed individuals or gametes. In this case, developmental effects are seen in the progeny of the exposed individual independently of exposure of that second generation individual (i.e., neither that individual nor its gametes of origin were exposed). One mechanism of this involves DNA modifications arising in gametes following some form of environmental exposure, which are retained during gametogenesis and transmitted through multiple generations in the absence of continuing exposure [166,167,254]. A prevailing view is that much of the epigenetic information that exists in the fertilized embryo is eventually erased in the germ line, leaving a blank slate upon which to reimpose a new epigenetic profile. In the case of imprinted genes this allows erasure and reestablishment of the sex-appropriate imprint, and in the case of nonimprinted genes, this process would allow reprogramming of the epigenome to a ground state that is amenable to the normal progression of developmentally acquired epigenetic changes that drive cellular specification and differentiation. Failure to erase acquired epi genetic alterations in the gamete would interrupt this normal progression, the result being abnormal epigenomes being passaged transgenerationally.
But epigenetic changes that modify cellular or organismal characteristics could set the stage for future transgenerational effects in other ways. For example, an environmental agent that alters gonad function and compromises gamete quality can affect the subsequent generation. One example would be a maternal exposure that alters fetal oogenesis in the unborn daughter. That child could become an adult with compromised oocyte quality. Those compromised oocytes could in turn give rise to females that go on to display an altered intrauterine environment, perpetuating effects via additional rounds of altered ovarian function and oocyte quality in the subsequent generations. This cycle could continue. A second scenario would involve environmental alterations of behavior that affect subsequent rearing practices, with behavioral effects. In this case, environmental alterations in the brain could compromise parenting behavior, and then be recapitulated via postnatal epigenetic programming of the brain in subsequent progeny. In such cases, an epigenetic state at the level of DNA modification may not be present and passaged in the gametes, but instead becomes re-established as a result of recurring physiological or behavioral changes that direct epigenetic change in each generation
Conclusion & future perspective: evaluating & managing our epigenetic risks
The foregoing discussion reveals that we are at significant risk of epigenetic changes induced by our environment. While the importance of genetics in inheritance is obvious, our genes nevertheless interact with the environment, and the environment can exert profound effects on the penetrance and expressivity of many genetic traits. Two parameters will need to be addressed in future evaluations of environmental effects on our genomes. One relates to how diseases and developmental changes in prevalence over time. The second relates to understanding how our environment is changing, which is crucial for establishing causation and eventually preventative or remedial measures.
The foregoing discussion also shows that environmental effects arise at many different stages during the life cycle. The potential for transgenerational effects attests to the importance of understanding life-long exposures, how those exposures affect the long-term health of the exposed individual and later generations and whether such effects can be minimized.
One key component to managing our epigenomes will be to understand our total environmental exposure profiles. This will mean understanding what toxins are prevalent in our environments (e.g., home, school, work and outdoors), food, water and air, and how combinations of different environmental factors affect our epigenomes. The range of factors includes chemicals like EDCs [147] and lifestyle factors such as diet and social and psychological variables. This is a daunting proposition, as the number of EDCs alone is quite large, their prevalence and abundances are not well understood, and other factors are difficult to quantify.
Combining increased knowledge of our exposures with increased understanding of how our epigenomes respond to the environment may lead us to important new strategies for reducing our environmental epigenetic burdens. These may include enhanced education to minimize risky behaviors, remediation of exposures, nutritional changes, preconception counseling, new genetic screens to identify at-risk individuals early in life, improved diagnostic approaches based on improved ascertainment of disease and disorders or novel biomarkers of past or ongoing exposure, and new therapies targeting epigenetically damaged cells. In addition, such strategies may be effective in abrogating the risks of transgenerational epigenetic effects. In this age of hoped-for personalized medicine based on individual genetic information, it will be critical to define how environmental and epigenetic states influence how an individual’s genotype relates to clinical outcome. Finally, a better understanding of environmental risks and genome–environment interactions may lead to a greater public awareness, and transformative changes in public policy that will reverse the recent increases in common human diseases and disorders.
Executive summary.
Background
▪ Recent increases in the incidence of common human diseases and disorders are not explicable on the basis of changes in the population genetic landscape.
▪ A likely cause is an increasing effect of our environment on our epigenomes.
Our changing epigenome
▪ Epigenetic changes during our lifespan are caused by endogenous or exogenous factors.
▪ Endogenous factors include effects of genetic variation and normal developmental processes.
▪ Exogenous factors include a wide range of environmental factors.
Some environmental causes of epigenetic changes
▪ Toxins and endocrine disruptors can modify the epigenome.
▪ Lifestyle factors can modify the epigenome, including substance abuse, delayed reproduction and assisted reproduction technology, and parenting behavior.
▪ Our epigenome displays changes with age.
Developmental origins & windows of sensitivity
▪ Environmental factors exert their effects at particular times during development.
▪ Periods of sensitivity include oogenesis, spermatogenesis, preimplantation development and implantation, modeling early exposures using stem cell systems and fetal life.
▪ Transgenerational effects may also arise: ‘you are what you eat’ (or what your mother, father or grandparents ate).
Conclusion & future perspective: evaluating & managing our epigenetic risks
▪ There is a need to account for changes in ascertainment over time in order to monitor changes in prevalence.
▪ There is a need to define our life-long environmental exposures.
▪ There is a need to define how our genomes respond to all environmental factors at different times during life, with respect to epigenetic change.
▪ Exploiting individual genetic information, for example, in personalized medicine, will require knowledge of past and current environmental exposures, and current epigenetic states for maximum effect.
Acknowledgments
Research in the authors’ laboratories is supported in part by grants from the NIH National Institute of Child Health and Development (HD43092; KE Latham), the NIH Office of the Director, Comparative Medicine Branch, Office of Research Infrastructure Program, R24OD012221-12 (KE Latham), U54HD068157 (C Sapienza), and RO1GM093066 and K22CA140361-3 (N Engel).
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle CA, Boulet S, Schieve LA, et al. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011;127:1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 5.Rice CE. The changing prevalence of the autism spectrum disorders. Am. Fam. Phys. 2011;83:515–520. [PubMed] [Google Scholar]
- 6.Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat. Rev. Genet. 2012;13(3):153–162. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- 7.Hochberg Z, Feil R, Constancia M, et al. Child health, developmental plasticity, and epigenetic programming. Endocr. Rev. 2011;32:159–224. doi: 10.1210/er.2009-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards TM, Myers JP. Environmental exposures and gene regulation in disease etiology. Environ. Health Perspect. 2007;115:1264–1270. doi: 10.1289/ehp.9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gogvadze V, Stridh H, Orrenius S, Cotgreave I. Tributyltin causes cytochrome C release from isolated mitochondria by two discrete mechanisms. Biochem. Biophys. Res. Commun. 2002;292(4):904–908. doi: 10.1006/bbrc.2002.6679. [DOI] [PubMed] [Google Scholar]
- 10.Grote K, Hobler C, Andrade AJ, et al. Effects of in utero and lactational exposure to triphenyltin chloride on pregnancy outcome and postnatal development in rat offspring. Toxicology. 2007;238:177–185. doi: 10.1016/j.tox.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 11.Grun F, Watanabe H, Zamanian Z, et al. Endocrine-disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol. Endocrinol. 2006;20(9):2141–2155. doi: 10.1210/me.2005-0367. [DOI] [PubMed] [Google Scholar]
- 12.Inadera H, Shimomura A. Environmental chemical tributyltin augments adipocyte differentiation. Toxicol. Lett. 2005;159(3):226–234. doi: 10.1016/j.toxlet.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Kirchner S, Kieu T, Chow C, Casey S, Blumberg B. Prenatal exposure to the environmental obesogen tributyltin predisposes multipotent stem cells to become adipocytes. Mol. Endocrinol. 2010;24(3):526–539. doi: 10.1210/me.2009-0261. ▪ Illustrates how endocrine-disrupting compounds (EDCs) can affect epigenetic states and stem cell fates related to phenotype, such as adiposity.
- 14.Li X, Ycaza J, Blumberg B. The environmental obesogen tributyltin chloride acts via peroxisome proliferator activated receptor γ to induce adipogenesis in murine 3T3-L1 preadipocytes. J. Steroid Biochem. Mol. Biol. 2011;127(1–2):9–15. doi: 10.1016/j.jsbmb.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grun F, Blumberg B. Minireview: the case for obesogens. Mol. Endocrinol. 2009;23(8):1127–1134. doi: 10.1210/me.2008-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grun F, Blumberg B. Environmental obesogens: organotins and endocrine disruption via nuclear receptor signaling. Endocrinology. 2006;147(6 Suppl.):S50–S55. doi: 10.1210/en.2005-1129. [DOI] [PubMed] [Google Scholar]
- 17.Liu G, Cunningham C, Downs SM, Fineberg N. A spatial ana lysis of obesogenic environments for children. Proc. AMIA Symp. 2002;2002:459–463. [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Y, Kannan K. Comparative assessment of human exposure to phthalate esters from house dust in China and the United States. Environ. Sci. Technol. 2011;45(8):3788–3794. doi: 10.1021/es2002106. [DOI] [PubMed] [Google Scholar]
- 19.Kannan K, Takahashi S, Fujiwara N, Mizukawa H, Tanabe S. Organotin compounds, including butyltins and octyltins, in house dust from Albany, New York, USA. Arch. Environ. Contam. Toxicol. 2010;58(4):901–907. doi: 10.1007/s00244-010-9513-6. ▪ Illustrates common presence of EDCs in the environment.
- 20.Kannan K, Tanabe S, Tatsukawa R. Occurrence of butyltin residues in certain foodstuffs. Bull. Environ. Contam. Toxicol. 1995;55(4):510–516. doi: 10.1007/BF00196029. [DOI] [PubMed] [Google Scholar]
- 21.Liao C, Kannan K. High levels of bisphenol a in paper currencies from several countries, and implications for dermal exposure. Environ. Sci. Technol. 2011;45:6761–6768. doi: 10.1021/es200977t. [DOI] [PubMed] [Google Scholar]
- 22.Loganathan BG, Kannan K, Senthilkumar K, Sickel J, Owen DA. Occurrence of butyltin residues in sediment and mussel tissues from the lower-most Tennessee River and Kentucky Lake, U.S.A. Chemosphere. 1999;39(14):2401–2408. doi: 10.1016/s0045-6535(99)00167-8. [DOI] [PubMed] [Google Scholar]
- 23.Loganathan SN, Kannan K. Occurrence of bisphenol a in indoor dust from two locations in the eastern United States and implications for human exposures. Arch. Environ. Contam. Toxicol. 2011;61(1):68–73. doi: 10.1007/s00244-010-9634-y. [DOI] [PubMed] [Google Scholar]
- 24.Ma J, Addink R, Yun S, et al. Polybrominated dibenzo-p-dioxins/ dibenzofurans and polybrominated diphenyl ethers in soil, vegetation, workshop-floor dust, and electronic shredder residue from an electronic waste recycling facility and in soils from a chemical industrial complex in eastern China. Environ. Sci. Technol. 2009;43(19):7350–7356. doi: 10.1021/es901713u. [DOI] [PubMed] [Google Scholar]
- 25.Murata S, Takahashi S, Agusa T, et al. Contamination status and accumulation profiles of organotins in sea otters (Enhydra lutris) found dead along the coasts of California, Washington, Alaska (USA), and Kamchatka (Russia) Mar. Pollut. Bull. 2008;56(4):641–649. doi: 10.1016/j.marpolbul.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 26.U.S. Environmental Protection Agency Office of Pesticide Programs Health Effects Division and Environmental Fate and Effects Division in collaboration with the Office of Research and Development Re-Evaluation of Human Health Effects of Atrazine. Review of non-cancer epidemiology, experimental animal and in vitro studies and drinking water monitoring frequency. Presented to: The Federal Insecticide, Fungicide, and Rodenticide Act Scientific Advisory; Washington DC, USA. Apr, 2010. [Google Scholar]
- 27.Fujimoto VY, Kim D, vom Saal FS, et al. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil. Steril. 2011;95(5):1816–1819. doi: 10.1016/j.fertnstert.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Kandaraki E, Chatzigeorgiou A, Livadas S, et al. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J. Clin. Endocrinol. Metab. 2011;96(3):E480–E484. doi: 10.1210/jc.2010-1658. [DOI] [PubMed] [Google Scholar]
- 29.Vandenberg LN, Chahoud I, Heindel JJ, et al. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandenberg LN, Chahoud I, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Biomonitoring studies should be used by regulatory agencies to assess human exposure levels and safety of bisphenol A. Environ. Health Perspect. 2010;118(8):1051–1054. doi: 10.1289/ehp.0901717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod. Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Vandenberg LN, Maffini MV, Wadia PR, et al. Exposure to environmentally relevant doses of the xenoestrogen bisphenol-A alters development of the fetal mouse mammary gland. Endocrinology. 2007;148:116–127. doi: 10.1210/en.2006-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein CR, Savitz DA, Dougan M. Serum levels of perfluorooctanoic acid and perfluorooctane sulfonate and pregnancy outcome. Am. J. Epidemiol. 2009;170:837–846. doi: 10.1093/aje/kwp212. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman K, Webster TF, Bartell SM, et al. Private drinking water wells as a source of exposure to perfluorooctanoic acid (PFOA) in communities surrounding a fluoropolymer production facility. Environ. Health Perspect. 2011;119(1):92–97. doi: 10.1289/ehp.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in U.S. children 12–15 years of age. Environ. Health Perspect. 2010;118(12):1762–1767. doi: 10.1289/ehp.1001898. ▪ Discusses increased odds of attention deficit hyperactivity disorder in children with higher serum polyfluoroalkyl chemical levels.
- 36.Zhang T, Sun HW, Wu Q, et al. Perfluorochemicals in meat, eggs and indoor dust in China: assessment of sources and pathways of human exposure to perfluorochemicals. Environ. Sci. Technol. 2010;44(9):3572–3579. doi: 10.1021/es1000159. [DOI] [PubMed] [Google Scholar]
- 37.Lu Y, Yuan T, Yun SH, et al. Occurrence of cyclic and linear siloxanes in indoor dust from China, and implications for human exposures. Environ. Sci. Technol. 2010;44(16):6081–6087. doi: 10.1021/es101368n. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Alomirah H, Cho HS, et al. Urinary bisphenol a concentrations and their implications for human exposure in several asian countries. Environ. Sci. Technol. 2011;45(16):7044–7050. doi: 10.1021/es200976k. [DOI] [PubMed] [Google Scholar]
- 39.Durmaz E, Ozmert EN, Erkekoglu P, et al. Plasma phthalate levels in pubertal gynecomastia. Pediatrics. 2010;125(1):E122–E129. doi: 10.1542/peds.2009-0724. [DOI] [PubMed] [Google Scholar]
- 40.Rudel RA, Camann DE, Spengler JD, Korn LR, Broady JG. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ. Sci. Technol. 2003;37(20):4543–4553. doi: 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- 41.Arase S, Ishii K, Igarashi K, et al. Endocrine disrupter bisphenol A increases in situ estrogen production in the mouse urogenital sinus. Biol. Reprod. 2010;84(4):734–742. doi: 10.1095/biolreprod.110.087502. [DOI] [PubMed] [Google Scholar]
- 42.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl Acad. Sci. USA. 2007;104(32):13056–13061. doi: 10.1073/pnas.0703739104. ▪ Illustrates how maternal nutrients can modify EDC effects in progeny.
- 43.Hunt PA, Koehler KE, Susiarjo M, et al. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr. Biol. 2003;13:546–553. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 44.Kang ER, Iqbal K, Tran DA, et al. Effects of endocrine disruptors on imprinted gene expression in the mouse embryo. Epigenetics. 2011;6(7):937–950. doi: 10.4161/epi.6.7.16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lenie S, Cortvrindt R, Eichenlaub-Ritter U, Smitz J. Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Mutat. Res. 2008;651(1–2):71–81. doi: 10.1016/j.mrgentox.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 46.Nishizawa H, Morita M, Sugimoto M, Imanishi S, Manabe N. Effects of in utero exposure to bisphenol A on mRNA expression of arylhydrocarbon and retinoid receptors in murine embryos. J. Reprod. Dev. 2005;51(3):315–324. doi: 10.1262/jrd.16008. [DOI] [PubMed] [Google Scholar]
- 47.Richter CA, Birnbaum LS, Farabollini F, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez HA, Santambrosio N, Santamaria CG, Munoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod. Toxicol. 2010;30(4):550–557. doi: 10.1016/j.reprotox.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Rubin BS, Lenkowski JR, Schaeberle CM, et al. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147(8):3681–3691. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- 50.Vandenberg LN, Maffini MV, Schaeberle CM, et al. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod. Toxicol. 2008;26(3–4):210–219. doi: 10.1016/j.reprotox.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wadia PR, Vandenberg LN, Schaeberle CM, et al. Perinatal bisphenol A exposure increases estrogen sensitivity of the mammary gland in diverse mouse strains. Environ. Health Perspect. 2007;115(4):592–598. doi: 10.1289/ehp.9640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weng YI, Hsu PY, Liyanarachchi S, et al. Epigenetic influences of low-dose bisphenol A in primary human breast epithelial cells. Toxicol. Appl. Pharmacol. 2010;248(2):111–121. doi: 10.1016/j.taap.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cooney CM. PFOS alters immune response at very low exposure levels. Environ. Sci. Technol. 2008;42:3486–3487. doi: 10.1021/es0871098. [DOI] [PubMed] [Google Scholar]
- 54.Peden-Adams MM, Keller JM, Eudaly JG, et al. Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicol. Sci. 2008;104:144–154. doi: 10.1093/toxsci/kfn059. [DOI] [PubMed] [Google Scholar]
- 55.Fenton SE, Reiner JL, Nakayama SF, et al. Analysis of PFOA in dosed CD-1 mice. Part 2. Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod. Toxicol. 2009;27(3–4):365–372. doi: 10.1016/j.reprotox.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White SS, Calafat AM, Kuklenyik Z, et al. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol. Sci. 2007;96(1):133–144. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- 57.White SS, Fenton SE, Hines EP. Endocrine disrupting properties of perfluorooctanoic acid. J. Steroid Biochem. Mol. Biol. 2011;127:16–26. doi: 10.1016/j.jsbmb.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White SS, Kato K, Jia LT, et al. Effects of perfluorooctanoic acid on mouse mammary gland development and differentiation resulting from cross-foster and restricted gestational exposures. Reprod. Toxicol. 2009;27(3–4):289–298. doi: 10.1016/j.reprotox.2008.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White SS, Stanko JP, Kato K, et al. Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice. Environ. Health Perspect. 2011;119(8):1070–1076. doi: 10.1289/ehp.1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolf CJ, Fenton SE, Schmid JE, et al. Developmental toxicity of perfluorooctanoic acid in the CD-1 mouse after cross-foster and restricted gestational exposures. Toxicol. Sci. 2007;95(2):462–473. doi: 10.1093/toxsci/kfl159. [DOI] [PubMed] [Google Scholar]
- 61.Birnbaum LS, Fenton SE. Cancer and developmental exposure to endocrine disruptors. Environ. Health Perspect. 2003;111:389–394. doi: 10.1289/ehp.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Enoch RR, Stanko JP, Greiner SN, et al. Mammary gland development as a sensitive end point after acute prenatal exposure to an atrazine metabolite mixture in female Long-Evans rats. Environ. Health Perspect. 2007;115(4):541–547. doi: 10.1289/ehp.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Foradori CD, Hinds LR, Hanneman WH, Handa RJ. Effects of atrazine and its withdrawal on gonadotropin-releasing hormone neuroendocrine function in the adult female Wistar rat. Biol. Reprod. 2009;81(6):1099–1105. doi: 10.1095/biolreprod.109.077453. [DOI] [PubMed] [Google Scholar]
- 64.Foradori CD, Hinds LR, Hanneman WH, et al. Atrazine inhibits pulsatile luteinizing hormone release without altering pituitary sensitivity to a gonadotropin-releasing hormone receptor agonist in female Wistar rats. Biol. Reprod. 2009;81(1):40–45. doi: 10.1095/biolreprod.108.075713. [DOI] [PubMed] [Google Scholar]
- 65.Foradori CD, Hinds LR, Quihuis AM, et al. The differential effect of atrazine on luteinizing hormone release in adrenalectomized adult female Wistar rats. Biol. Reprod. 2011;85(4):684–689. doi: 10.1095/biolreprod.111.092452. [DOI] [PubMed] [Google Scholar]
- 66.Gojmerac T, Kartal B, Curic S, et al. Serum biochemical changes associated with cystic ovarian degeneration in pigs after atrazine treatment. Toxicol. Lett. 1996;85(1):9–15. doi: 10.1016/0378-4274(96)03631-4. [DOI] [PubMed] [Google Scholar]
- 67.Graves JE, Richardson ME, Bernard RS, Camper ND, Bridges WC. Atrazine effects on in vitro maturation and in vitro fertilization in the bovine oocyte. J. Environ. Sci. Health B. 2002;37(2):103–112. doi: 10.1081/PFC-120002982. [DOI] [PubMed] [Google Scholar]
- 68.Gunderson MP, Veldhoen N, Skirrow RC, et al. Effect of low dose exposure to the herbicide atrazine and its metabolite on cytochrome P450 aromatase and steroidogenic factor-1 mRNA levels in the brain of premetamorphic bullfrog tadpoles (Rana catesbeiana) Aquat. Toxicol. 2011;102(1–2):31–38. doi: 10.1016/j.aquatox.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayes TB, Anderson LL, Beasley VR, et al. Demasculinization and feminization of male gonads by atrazine: consistent effects across vertebrate classes. J. Steroid Biochem. Mol. Biol. 2011;127(1–2):64–73. doi: 10.1016/j.jsbmb.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Juliani CC, Silva-Zacarin EC, Santos DC, Boer PA. Effects of atrazine on female Wistar rats: morphological alterations in ovarian follicles and immunocytochemical labeling of 90 kDa heat shock protein. Micron. 2008;39(5):607–616. doi: 10.1016/j.micron.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 71.Moon HJ, Han SY, Shin JH, et al. Gestational exposure to nonylphenol causes precocious mammary gland development in female rat offspring. J. Reprod. Dev. 2007;53(2):333–344. doi: 10.1262/jrd.18055. [DOI] [PubMed] [Google Scholar]
- 72.Orton F, Lutz I, Kloas W, Routledge EJ. Endocrine disrupting effects of herbicides and pentachlorophenol: in vitro and in vivo evidence. Environ. Sci. Technol. 2009;43(6):2144–2150. doi: 10.1021/es8028928. [DOI] [PubMed] [Google Scholar]
- 73.Rayner JL, Enoch RR, Fenton SE. Adverse effects of prenatal exposure to atrazine during a critical period of mammary gland growth. Toxicol. Sci. 2005;87:255–266. doi: 10.1093/toxsci/kfi213. [DOI] [PubMed] [Google Scholar]
- 74.Rayner JL, Enoch RR, Wolf DC, Fenton SE. Atrazine-induced reproductive tract alterations after transplacental and/or lactational exposure in male Long-Evans rats. Toxicol. Appl. Pharmacol. 2007;218:238–248. doi: 10.1016/j.taap.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 75.Rowe AM, Brundage KM, Barnett JB. Developmental immunotoxicity of atrazine in rodents. Basic Clin. Pharmacol. Toxicol. 2008;102:139–145. doi: 10.1111/j.1742-7843.2007.00175.x. [DOI] [PubMed] [Google Scholar]
- 76.Rowe AM, Brundage KM, Barnett JB. In vitro atrazine-exposure inhibits human natural killer cell lytic granule release. Toxicol. Appl. Pharmacol. 2007;221(2):179–188. doi: 10.1016/j.taap.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rowe AM, Brundage KM, Schafer R, Barnett JB. Immunomodulatory effects of maternal atrazine exposure on male Balb/c mice. Toxicol. Appl. Pharmacol. 2006;214(1):69–77. doi: 10.1016/j.taap.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stanko JP, Enoch RR, Rayner JL, et al. Effects of prenatal exposure to a low dose atrazine metabolite mixture on pubertal timing and prostate development of male Long-Evans rats. Reprod. Toxicol. 2010;30(4):540–549. doi: 10.1016/j.reprotox.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suzawa M, Ingraham HA. The herbicide atrazine activates endocrine gene networks via non-steroidal NR5A nuclear receptors in fish and mammalian cells. PLoS ONE. 2008;3(5):e2117. doi: 10.1371/journal.pone.0002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bo E, Viglietti-Panzica C, Panzica GC. Acute exposure to tributyltin induces c-fos activation in the hypothalamic arcuate nucleus of adult male mice. Neurotoxicology. 2011;32(2):277–280. doi: 10.1016/j.neuro.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 81.Chen Y, Zuo Z, Chen S, et al. Reduction of spermatogenesis in mice after tributyltin administration. Toxicology. 2008;251(1–3):21–27. doi: 10.1016/j.tox.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 82.Kishta O, Adeeko A, Li D, et al. In utero exposure to tributyltin chloride differentially alters male and female fetal gonad morphology and gene expression profiles in the Sprague-Dawley rat. Reprod. Toxicol. 2007;23(1):1–11. doi: 10.1016/j.reprotox.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 83.Ohno S, Nakajima Y, Nakajin S. Triphenyltin and Tributyltin inhibit pig testicular 17β-hydroxysteroid dehydrogenase activity and suppress testicular testosterone biosynthesis. Steroids. 2005;70(9):645–651. doi: 10.1016/j.steroids.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 84.Padros J, Pelletier E, Ribeiro CO. Metabolic interactions between low doses of benzo a] pyrene and tributyltin in arctic charr (Salvelinus alpinus): a long-term in vivo study. Toxicol. Appl. Pharmacol. 2003;192(1):45–55. doi: 10.1016/s0041-008x(02)00042-x. [DOI] [PubMed] [Google Scholar]
- 85.Pavlikova N, Kortner TM, Arukwe A. Peroxisome proliferator-activated receptors, estrogenic responses and biotransformation system in the liver of salmon exposed to tributyltin and second messenger activator. Aquat. Toxicol. 2010;99(2):176–185. doi: 10.1016/j.aquatox.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 86.Roepke TA, Snyder MJ, Cherr GN. Estradiol and endocrine disrupting compounds adversely affect development of sea urchin embryos at environmentally relevant concentrations. Aquat. Toxicol. 2005;71(2):155–173. doi: 10.1016/j.aquatox.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 87.Wilson S, Dzon L, Reed A, Pruitt M, Whalen MM. Effects of in vitro exposure to low levels of organotin and carbamate pesticides on human natural killer cell cytotoxic function. Environ. Toxicol. 2004;19(6):554–563. doi: 10.1002/tox.20061. [DOI] [PubMed] [Google Scholar]
- 88.Yamada J, Inoue K, Furukawa T, Fukuda A. Low-concentration tributyltin perturbs inhibitory synaptogenesis and induces neuronal death in immature but not mature neurons. Toxicol. Lett. 2010;198(2):282–288. doi: 10.1016/j.toxlet.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 89.Zuo Z, Chen S, Wu T, et al. Tributyltin causes obesity and hepatic steatosis in male mice. Environ. Toxicol. 2011;26(1):79–85. doi: 10.1002/tox.20531. [DOI] [PubMed] [Google Scholar]
- 90.Lind PM, Lind L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis. 2011;218(1):207–213. doi: 10.1016/j.atherosclerosis.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 91.Chang SC, Noker PE, Gorman GS, et al. Comparative pharmacokinetics of perfluorooctanesulfonate (PFOS) in rats, mice, and monkeys. Reprod. Toxicol. 2011;33(4):428–440. doi: 10.1016/j.reprotox.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 92.Florentin A, Deblonde T, Diguio N, Hautemaniere A, Hartemann P. Impacts of two perfluorinated compounds (PFOS and PFOA) on human hepatoma cells: cytotoxicity but no genotoxicity? Int. J. Hyg. Environ. Health. 2011;214(6):493–499. doi: 10.1016/j.ijheh.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 93.Bogdanska J, Borg D, Sundstrom M, et al. Tissue distribution of (3)S-labelled perfluorooctane sulfonate in adult mice after oral exposure to a low environmentally relevant dose or a high experimental dose. Toxicology. 2011;284:54–62. doi: 10.1016/j.tox.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 94.Zheng L, Dong GH, Zhang YH, et al. Type 1 and Type 2 cytokines imbalance in adult male C57BL/6 mice following a 7-day oral exposure to perfluorooctanesulfonate (PFOS) J. Immunotoxicol. 2011;8(1):30–38. doi: 10.3109/1547691X.2010.537287. [DOI] [PubMed] [Google Scholar]
- 95.van Dartel DA, Pennings JL, Robinson JF, Kleinjans JC, Piersma AH. Discriminating classes of developmental toxicants using gene expression profiling in the embryonic stem cell test. Toxicol. Lett. 2011;201(2):143–151. doi: 10.1016/j.toxlet.2010.12.019. ▪ Describes development of embryonic stem cell-based screens for toxicity.
- 96.Lin Z, Fisher JW, Ross MK, Filipov NM. A physiologically based pharmacokinetic model for atrazine and its main metabolites in the adult male C57BL/6 mouse. Toxicol. Appl. Pharmacol. 2011;251(1):16–31. doi: 10.1016/j.taap.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 97.Chauvigne F, Menuet A, Lesne L, et al. Time- and dose-related effects of di-(2-ethylhexyl) phthalate and its main metabolites on the function of the rat fetal testis in vitro. Environ. Health Perspect. 2009;117(4):515–521. doi: 10.1289/ehp.11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bernal AJ, Jirtle RL. Epigenomic disruption: the effects of early developmental exposures. Birth Defects Res. A. Clin. Mol. Teratol. 2010;88(10):938–944. doi: 10.1002/bdra.20685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Olsen GW, Butenhoff JL, Zobel LR. Perfluoroalkyl chemicals and human fetal development: an epidemiologic review with clinical and toxicological perspectives. Reprod. Toxicol. 2009;27(3–4):212–230. doi: 10.1016/j.reprotox.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 100.Hengstler JG, Foth H, Gebel T, et al. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit. Rev. Toxicol. 2011;41(4):263–291. doi: 10.3109/10408444.2011.558487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 102.Gupta RK, Singh JM, Leslie TC, et al. Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol. Appl. Pharmacol. 2010;242(2):224–230. doi: 10.1016/j.taap.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee J, Park J, Jang B, Knudsen TB. Altered expression of genes related to zinc homeostasis in early mouse embryos exposed to di-2-ethylhexyl phthalate. Toxicol. Lett. 2004;152(1):1–10. doi: 10.1016/j.toxlet.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 104.Anas MK, Suzuki C, Yoshioka K, Iwamura S. Effect of mono-(2-ethylhexyl) phthalate on bovine oocyte maturation in vitro. Reprod. Toxicol. 2003;17(3):305–310. doi: 10.1016/s0890-6238(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 105.Yolton K, Xu Y, Strauss D, et al. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol. Teratol. 2011;33(5):558–566. doi: 10.1016/j.ntt.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environ. Health Perspect. 2011;119(10):1396–1402. doi: 10.1289/ehp.1103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Janer G, Verhoef A, Gilsing HD, Piersma AH. Use of the rat postimplantation embryo culture to assess the embryotoxic potency within a chemical category and to identify toxic metabolites. Toxicol. In vitro. 2008;22(7):1797–1805. doi: 10.1016/j.tiv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 108.Maranghi F, Lorenzetti S, Tassinari R, et al. In utero exposure to di-(2-ethylhexyl) phthalate affects liver morphology and metabolism in post-natal CD-1 mice. Reprod. Toxicol. 2010;29(4):427–432. doi: 10.1016/j.reprotox.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 109.Jones HB, Garside DA, Liu R, Roberts JC. The influence of phthalate esters on Leydig cell structure and function in vitro and in vivo. Exp. Mol. Pathol. 1993;58(3):179–193. doi: 10.1006/exmp.1993.1016. [DOI] [PubMed] [Google Scholar]
- 110.Kim BN, Cho SC, Kim Y, et al. Phthalates exposure and attention-deficit/hyperactivity disorder in school-age children. Biol. Psychiatry. 2009;66(10):958–963. doi: 10.1016/j.biopsych.2009.07.034. ▪ Discusses relationship between EDCs and attention deficit hyperactivity disorder in children.
- 111.Latini G, De Felice C, Presta G, et al. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ. Health Perspect. 2003;111:1783–1785. doi: 10.1289/ehp.6202. ▪ Raises interesting possibility of maternal diet to counteract effects of phthalates.
- 112.Lin LC, Wang SL, Chang YC, et al. Associations between maternal phthalate exposure and cord sex hormones in human infants. Chemosphere. 2011;83(8):1192–1199. doi: 10.1016/j.chemosphere.2010.12.079. [DOI] [PubMed] [Google Scholar]
- 113.Swan SH, Liu F, Hines M, et al. Prenatal phthalate exposure and reduced masculine play in boys. Int. J. Androl. 2010;33(2):259–269. doi: 10.1111/j.1365-2605.2009.01019.x. ▪ Establishes possible connection between maternal phthalate exposure and altered brain development in children.
- 114.Latini G, Del Vecchio A, Massaro M, Verrotti A, DE Felice C. In utero exposure to phthalates and fetal development. Curr. Med. Chem. 2006;13:2527–2534. doi: 10.2174/092986706778201666. [DOI] [PubMed] [Google Scholar]
- 115.Agarwal DK, Lawrence WH, Autian J. Antifertility and mutagenic effects in mice from parenteral administration of di-2-ethylhexyl phthalate (DEHP) J. Toxicol. Environ. Health. 1985;16(1):71–84. doi: 10.1080/15287398509530720. [DOI] [PubMed] [Google Scholar]
- 116.Xu Y, Agrawal S, Cook TJ, Knipp GT. Maternal di-(2-ethylhexyl)-phthalate exposure influences essential fatty acid homeostasis in rat placenta. Placenta. 2008;29(11):962–969. doi: 10.1016/j.placenta.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Balabanic D, Rupnik M, Klemencic AK. Negative impact of endocrine-disrupting compounds on human reproductive health. Reprod. Fertil. Dev. 2011;23(3):403–416. doi: 10.1071/RD09300. [DOI] [PubMed] [Google Scholar]
- 118.Jurewicz J, Hanke W. Exposure to phthalates: reproductive outcome and children health. A review of epidemiological studies. Int. J. Occup. Med. Environ. Health. 2011;24(2):115–141. doi: 10.2478/s13382-011-0022-2. [DOI] [PubMed] [Google Scholar]
- 119.Seidlova-Wuttke D, Jarry H, Wuttke W. Pure estrogenic effect of benzophenone-2 (BP2) but not of bisphenol A (BPA) and dibutylphtalate (DBP) in uterus, vagina and bone. Toxicology. 2004;205(1–2):103–112. doi: 10.1016/j.tox.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 120.Struve MF, Gaido KW, Hensley JB, et al. Reproductive toxicity and pharmacokinetics of di-n-butyl phthalate (DBP) following dietary exposure of pregnant rats. Birth Defects Res. B Dev. Reprod. Toxicol. 2009;86(4):345–354. doi: 10.1002/bdrb.20199. [DOI] [PubMed] [Google Scholar]
- 121.Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ. Health Perspect. 2003;111:139–145. doi: 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Davis BJ, Maronpot RR, Heindel JJ. Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol. Appl. Pharmacol. 1994;128(2):216–223. doi: 10.1006/taap.1994.1200. [DOI] [PubMed] [Google Scholar]
- 123.McKee RH. Phthalate exposure and early thelarche. Environ. Health Perspect. 2004;112:A541–A543. doi: 10.1289/ehp.112-a541b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bell FP. Effects of phthalate esters on lipid metabolism in various tissues, cells and organelles in mammals. Environ. Health Perspect. 1982;45:41–50. doi: 10.1289/ehp.824541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen SQ, Chen JN, Cai XH, et al. Perinatal exposure to di-(2-ethylhexyl) phthalate leads to restricted growth and delayed lung maturation in newborn rats. J. Perinat. Med. 2010;38(5):515–521. doi: 10.1515/jpm.2010.083. [DOI] [PubMed] [Google Scholar]
- 126.Xu Y, Knipp GT, Cook TJ. Effects of di-(2-ethylhexyl)-phthalate and its metabolites on the lipid profiling in rat HRP-1 trophoblast cells. Arch. Toxicol. 2006;80(5):293–298. doi: 10.1007/s00204-005-0047-z. [DOI] [PubMed] [Google Scholar]
- 127.Zhang Y, Lin L, Cao Y, et al. Phthalate levels and low birth weight: a nested case-control study of Chinese newborns. J. Pediatr. 2009;155(4):500–504. doi: 10.1016/j.jpeds.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 128.Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environ. Sci. Technol. 2011;45:7954–7961. doi: 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- 129.Greenlee AR, Ellis TM, Berg RL. Low-dose agrochemicals and lawn-care pesticides induce developmental toxicity in murine preimplantation embryos. Environ. Health Perspect. 2004;112(6):703–709. doi: 10.1289/ehp.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Munger R, Isacson P, Hu S, et al. Intrauterine growth retardation in Iowa communities with herbicide-contaminated drinking water supplies. Environ. Health Perspect. 1997;105(3):308–314. doi: 10.1289/ehp.97105308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ochoa-Acuna H, Frankenberger J, Hahn L, Carbajo C. Drinking-water herbicide exposure in Indiana and prevalence of small-for-gestational-age and preterm delivery. Environ. Health Perspect. 2009;117(10):1619–1624. doi: 10.1289/ehp.0900784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pinchuk LM, Lee SR, Filipov NM. In vitro atrazine exposure affects the phenotypic and functional maturation of dendritic cells. Toxicol. Appl. Pharmacol. 2007;223(3):206–217. doi: 10.1016/j.taap.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Villanueva CM, Durand G, Coutte MB, Chevrier C, Cordier S. Atrazine in municipal drinking water and risk of low birth weight, preterm delivery, and small-for-gestationalage status. Occup. Environ. Med. 2005;62(6):400–405. doi: 10.1136/oem.2004.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Benachour N, Seralini GE. Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem. Res. Toxicol. 2009;22(1):97–105. doi: 10.1021/tx800218n. [DOI] [PubMed] [Google Scholar]
- 135.Martinez-Finley EJ, Goggin SL, Labrecque MT, Allan AM. Reduced expression of MAPK/ERK genes in perinatal arsenic-exposed offspring induced by glucocorticoid receptor deficits. Neurotoxicol. Teratol. 2011;33(5):530–537. doi: 10.1016/j.ntt.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Martin SA, Emilio R, Mahara V. Role of oxidative stress in transformation induced by metal mixture. Oxid. Med. Cell Longev. 2011;2011:935160. doi: 10.1155/2011/935160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jomova K, Jenisova Z, Feszterova M, et al. Arsenic: toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 138.Wu J, Chen G, Liao Y, et al. Arsenic levels in the soil and risk of birth defects: a population-based case-control study using GIS technology. J. Environ. Health. 2011;74(4):20–25. [PubMed] [Google Scholar]
- 139.Alissa EM, Ferns GA. Heavy metal poisoning and cardiovascular disease. J. Toxicol. 2011;2011:870125. doi: 10.1155/2011/870125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grun F, Blumberg B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev. Endocr. Metab. Disord. 2007;8(2):161–171. doi: 10.1007/s11154-007-9049-x. [DOI] [PubMed] [Google Scholar]
- 141.Janesick A, Blumberg B. Obesogens, stem cells and the developmental programming of obesity. Int. J. Androl. 2012;35(3):437–448. doi: 10.1111/j.1365-2605.2012.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Flegal KM, Carroll MD, Ogden CL, Curti LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 143.Wolf AM, Woodworth KA. Obesity prevention: recommended strategies and challenges. Am. J. Med. 2009;122:S19–S23. doi: 10.1016/j.amjmed.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 144.Harazono A, Ema M. Suppression of decidual cell response induced by tributyltin chloride in pseudopregnant rats: a cause of early embryonic loss. Arch. Toxicol. 2000;74(10):632–637. doi: 10.1007/s002040000163. [DOI] [PubMed] [Google Scholar]
- 145.Ema M, Fujii S, Ikka T, et al. Early pregnancy failure induced by dibutyltin dichloride in mice. Environ. Toxicol. 2007;22(1):44–52. doi: 10.1002/tox.20232. [DOI] [PubMed] [Google Scholar]
- 146.Ema M, Miyawaki E, Kawashima K. Adverse effects of diphenyltin dichloride on initiation and maintenance of pregnancy in rats. Toxicol. Lett. 1999;108(1):17–25. doi: 10.1016/s0378-4274(99)00074-0. [DOI] [PubMed] [Google Scholar]
- 147.Schug TT, Janesick A, Blumberg B, Heindel JJ. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011;127:204–215. doi: 10.1016/j.jsbmb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Avissar-Whiting M, Veiga KR, Uhl KM, et al. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod. Toxicol. 2010;29(4):401–406. doi: 10.1016/j.reprotox.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Benachour N, Aris A. Toxic effects of low doses of Bisphenol-A on human placental cells. Toxicol. Appl. Pharmacol. 2009;241:322–328. doi: 10.1016/j.taap.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 150.Tachibana T, Wakimoto Y, Nakamuta N, et al. Effects of bisphenol A (BPA) on placentation and survival of the neonates in mice. J. Reprod. Dev. 2007;53(3):509–514. doi: 10.1262/jrd.18171. [DOI] [PubMed] [Google Scholar]
- 151.Jin H, Audus KL. Effect of bisphenol A on drug efflux in BeWo, a human trophoblast-like cell line. Placenta. 2005;26(Suppl. A):S96–S103. doi: 10.1016/j.placenta.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 152.Imanishi S, Manabe N, Nishizawa H, et al. Effects of oral exposure of bisphenol A on mRNA expression of nuclear receptors in murine placentae assessed by DNA microarray. J. Reprod. Dev. 2003;49(4):329–336. doi: 10.1262/jrd.49.329. [DOI] [PubMed] [Google Scholar]
- 153.Cooke GM, Forsyth DS, Bondy GS, et al. Organotin speciation and tissue distribution in rat dams, fetuses, and neonates following oral administration of tributyltin chloride. J. Toxicol. Environ. Health A. 2008;71(6):384–395. doi: 10.1080/15287390701801653. [DOI] [PubMed] [Google Scholar]
- 154.Takeda Y, Liu X, Sumiyoshi M, et al. Placenta expressing the greatest quantity of bisphenol A receptor ERRγ among the human reproductive tissues: Predominant expression of type-1 ERRγ isoform. J. Biochem. 2009;146(1):113–122. doi: 10.1093/jb/mvp049. [DOI] [PubMed] [Google Scholar]
- 155.Fowden AL, Sibley C, Reik W, Constancia M. Imprinted genes, placental development and fetal growth. Horm. Res. 2006;65(Suppl. 3):50–58. doi: 10.1159/000091506. [DOI] [PubMed] [Google Scholar]
- 156.Angiolini E, Fowden A, Coan P, et al. Regulation of placental efficiency for nutrient transport by imprinted genes. Placenta. 2006;27(Suppl. A):S98–S102. doi: 10.1016/j.placenta.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 157.Constancia M, Angiolini E, Sandovici I, et al. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc. Natl Acad. Sci. USA. 2005;102(52):19219–19224. doi: 10.1073/pnas.0504468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Reik W, Constancia M, Fowden A, et al. Regulation of supply and demand for maternal nutrients in mammals by imprinted genes. J. Physiol. 2003;547:35–44. doi: 10.1113/jphysiol.2002.033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Constancia M, Hemberger M, Hughes J, et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417(6892):945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- 160.Reik W, Davies K, Dean W, Kelsey G, Constancia M. Imprinted genes and the coordination of fetal and postnatal growth in mammals. Novartis Found. Symp. 2001;237:19–31. doi: 10.1002/0470846666.ch3. Discussion 31–42. [DOI] [PubMed] [Google Scholar]
- 161.Ashworth A. Effects of intrauterine growth retardation on mortality and morbidity in infants and young children. Eur. J. Clin. Nutr. 1998;52(Suppl. 1):S34–S41. Discussion S41–S42. [PubMed] [Google Scholar]
- 162.Ortiz-Mantilla S, Choudhury N, Leevers H, Benasich AA. Understanding language and cognitive deficits in very low birth weight children. Dev. Psychobiol. 2008;50:107–126. doi: 10.1002/dev.20278. [DOI] [PubMed] [Google Scholar]
- 163.Barker DJ. Human growth and cardiovascular disease. Nestle Nutr. Workshop Ser. Pediatr. Program. 2008;61:21–38. doi: 10.1159/000113163. [DOI] [PubMed] [Google Scholar]
- 164.Shapira N. Prenatal nutrition: a critical window of opportunity for mother and child. Womens Health. 2008;4(6):639–656. doi: 10.2217/17455057.4.6.639. [DOI] [PubMed] [Google Scholar]
- 165.Decherf S, Demeneix BA. The obesogen hypothesis: a shift of focus from the periphery to the hypothalamus. J. Toxicol. Environ. Health B Crit. Rev. 2011;14(5–7):423–448. doi: 10.1080/10937404.2011.578561. [DOI] [PubMed] [Google Scholar]
- 166.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of endocrine disruptors. Reprod. Toxicol. 2011;31:337–343. doi: 10.1016/j.reprotox.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Anway MD, Skinner MK. Epigenetic programming of the germ line: effects of endocrine disruptors on the development of transgenerational disease. Reprod. Biomed. Online. 2008;16:23–25. doi: 10.1016/s1472-6483(10)60553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Martinez VD, Vucic EA, Adonis M, Gil L, Lam WL. Arsenic biotransformation as a cancer promoting factor by inducing DNA damage and disruption of repair mechanisms. Mol. Biol. Int. 2011;2011:718974. doi: 10.4061/2011/718974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang W, Wang L, Fan Q, et al. Arsenic trioxide re-sensitizes ERα-negative breast cancer cells to endocrine therapy by restoring ERα expression in vitro and in vivo. Oncol. Rep. 2011;26(3):621–628. doi: 10.3892/or.2011.1352. [DOI] [PubMed] [Google Scholar]
- 171.Fu HY, Shen JZ, Wu Y, et al. Arsenic trioxide inhibits DNA methyltransferase and restores expression of methylation-silenced CDKN2B/ CDKN2A genes in human hematologic malignant cells. Oncol. Rep. 2010;24(2):335–343. doi: 10.3892/or_00000864. [DOI] [PubMed] [Google Scholar]
- 172.Smeester L, Rager JE, Bailey KA, et al. Epigenetic changes in individuals with arsenicosis. Chem. Res. Toxicol. 2011;24(2):165–167. doi: 10.1021/tx1004419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jensen TJ, Novak P, Wnek SM, Gandolfi AJ, Futscher BW. Arsenicals produce stable progressive changes in DNA methylation patterns that are linked to malignant transformation of immortalized urothelial cells. Toxicol. Appl. Pharmacol. 2009;241(2):221–229. doi: 10.1016/j.taap.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hsu LI, Chen WP, Yang TY, et al. Genetic polymorphisms in glutathione S-transferase (GST) superfamily and risk of arsenic-induced urothelial carcinoma in residents of southwestern Taiwan. J. Biomed. Sci. 2011;18:51. doi: 10.1186/1423-0127-18-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nohara K, Baba T, Murai H, et al. Global DNA methylation in the mouse liver is affected by methyl deficiency and arsenic in a sex-dependent manner. Arch. Toxicol. 2011;85(6):653–661. doi: 10.1007/s00204-010-0611-z. [DOI] [PubMed] [Google Scholar]
- 176.LaPlant Q, Vialou V, Covington HE, 3rd, et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat. Neurosci. 2010;13(9):1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Maze I, Feng J, Wilkinson MB, et al. Cocaine dynamically regulates heterochromatin and repetitive element unsilencing in nucleus accumbens. Proc. Natl Acad. Sci. USA. 2011;108(7):3035–3040. doi: 10.1073/pnas.1015483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Anier K, Malinovskaja K, Aonurm-Helm A, Zharkovsky A, Kalda A. DNA methylation regulates cocaine-induced behavioral sensitization in mice. Neuropsychopharmacology. 2010;35(12):2450–2461. doi: 10.1038/npp.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Speroff L. The effect of aging on fertility. Curr. Opin. Obstet. Gynecol. 1994;6:115–120. [PubMed] [Google Scholar]
- 180.Sauer MV. The impact of age on reproductive potential: lessons learned from oocyte donation. Maturitas. 1998;30(2):221–225. doi: 10.1016/s0378-5122(98)00077-2. [DOI] [PubMed] [Google Scholar]
- 181.Navot D, Drews MR, Bergh PA, et al. Age-related decline in female fertility is not due to diminished capacity of the uterus to sustain embryo implantation. Fertil. Steril. 1994;61:97–101. doi: 10.1016/s0015-0282(16)56459-0. [DOI] [PubMed] [Google Scholar]
- 182.Sherins RJ, Thorsell LP, Dorfmann A, et al. Intracytoplasmic sperm injection facilitates fertilization even in the most severe forms of male infertility: pregnancy outcome correlates with maternal age and number of eggs available. Fertil. Steril. 1995;64(2):369–375. doi: 10.1016/s0015-0282(16)57737-1. [DOI] [PubMed] [Google Scholar]
- 183.Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24(2):86–93. doi: 10.1016/j.tig.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 184.Volarcik K, Sheean L, Goldfarb J, et al. The meiotic competence of in-vitro matured human oocytes is influenced by donor age: evidence that folliculogenesis is compromised in the reproductively aged ovary. Hum. Reprod. 1998;13(1):154–160. doi: 10.1093/humrep/13.1.154. [DOI] [PubMed] [Google Scholar]
- 185.Bentov Y, Yavorska T, Esfandiari N, Jurisicova A, Casper RF. The contribution of mitochondrial function to reproductive aging. J. Assist. Reprod. Genet. 2011;28:773–783. doi: 10.1007/s10815-011-9588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wise DD, Shear JB. Quantitation of nicotinamide and serotonin derivatives and detection of flavins in neuronal extracts using capillary electrophoresis with multiphoton-excited fluorescence. J. Chromatogr. A. 2006;1111(2):153–158. doi: 10.1016/j.chroma.2005.07.067. [DOI] [PubMed] [Google Scholar]
- 187.Okasha M, McCarron P, McEwen J, Smith GD. Age at menarche: secular trends and association with adult anthropometric measures. Ann. Hum. Biol. 2001;28:68–78. doi: 10.1080/03014460150201896. [DOI] [PubMed] [Google Scholar]
- 188.Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc. Natl Acad. Sci. USA. 2011;108:12319–12324. doi: 10.1073/pnas.1018793108. ▪ Illustrates the effect of diet on reproductive aging.
- 189.Spinelli S, Chefer S, Carson RE, et al. Effects of early-life stress on serotonin(1A) receptors in juvenile Rhesus monkeys measured by positron emission tomography. Biol. Psychiatry. 2010;67(12):1146–1153. doi: 10.1016/j.biopsych.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ichise M, Vines DC, Gura T, et al. Effects of early life stress on 11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. J. Neurosci. 2006;26(17):4638–4643. doi: 10.1523/JNEUROSCI.5199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cirulli F, Reif A, Herterich S, et al. A novel BDNF polymorphism affects plasma protein levels in interaction with early adversity in rhesus macaques. Psychoneuroendocrinology. 2011;36(3):372–379. doi: 10.1016/j.psyneuen.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lindell SG, Schwandt ML, Sun H, et al. Functional NPY variation as a factor in stress resilience and alcohol consumption in rhesus macaques. Arch. Gen. Psychiatry. 2010;67(4):423–431. doi: 10.1001/archgenpsychiatry.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kinnally EL, Capitanio JP, Leibel R, et al. Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes. Brain Behav. 2010;9(6):575–582. doi: 10.1111/j.1601-183X.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Falkenberg VR, Gurbaxani BM, Unger ER, Rajeevan MS. Functional genomics of serotonin receptor 2A (HTR2A): interaction of polymorphism, methylation, expression and disease association. Neuromol. Med. 2011;13(1):66–76. doi: 10.1007/s12017-010-8138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Roth TL, Zoladz PR, Sweatt JD, Diamond DM. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J. Psychiatry Res. 2011;45(7):919–926. doi: 10.1016/j.jpsychires.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Champagne FA, Curley JP. Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci. Biobehav. Rev. 2009;33(4):593–600. doi: 10.1016/j.neubiorev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 197.Szyf M. The early life social environment and DNA methylation: DNA methylation mediating the long-term impact of social environments early in life. Epigenetics. 2011;6:971–978. doi: 10.4161/epi.6.8.16793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Szyf M. The early life environment and the epigenome. Biochim. Biophys. Acta. 2009;1790:878–885. doi: 10.1016/j.bbagen.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 199.Szyf M, McGowan P, Meaney MJ. The social environment and the epigenome. Environ. Mol. Mutagen. 2008;49:46–60. doi: 10.1002/em.20357. [DOI] [PubMed] [Google Scholar]
- 200.Lee RS, Tamashiro KL, Yang X, et al. A measure of glucocorticoid load provided by DNA methylation of Fkbp5 in mice. Psychopharmacol. (Berl.) 2011;218(1):303–312. doi: 10.1007/s00213-011-2307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Oberlander TF, Weinberg J, Papsdorf M, et al. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 202.Chen GL, Novak MA, Meyer JS, et al. The effect of rearing experience and TPH2 genotype on HPA axis function and aggression in rhesus monkeys: a retrospective ana lysis. Horm. Behav. 2010;57(2):184–191. doi: 10.1016/j.yhbeh.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schwandt ML, Lindell SG, Higley JD, et al. OPRM1 gene variation influences hypothalamic–pituitary–adrenal axis function in response to a variety of stressors in rhesus macaques. Psychoneuroendocrinology. 2011;36(9):1303–1311. doi: 10.1016/j.psyneuen.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Newman TK, Syagailo YV, Barr CS, et al. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol. Psychiatry. 2005;57(2):167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 205.Labonte B, Turecki G. The epigenetics of suicide: explaining the biological effects of early life environmental adversity. Arch. Suicide Res. 2010;14(4):291–310. doi: 10.1080/13811118.2010.524025. [DOI] [PubMed] [Google Scholar]
- 206.Chen Y, Zhang J, Zhang L, Shen Y, Xu Q. Effects of MAOA promoter methylation on susceptibility to paranoid schizophrenia. Hum. Genet. 2011;131(7):1081–1087. doi: 10.1007/s00439-011-1131-5. [DOI] [PubMed] [Google Scholar]
- 207.Philibert RA, Wernett P, Plume J, et al. Gene environment interactions with a novel variable monoamine oxidase A transcriptional enhancer are associated with antisocial personality disorder. Biol. Psychol. 2011;87(3):366–371. doi: 10.1016/j.biopsycho.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Philibert RA, Gunter TD, Beach SR, Brody GH, Madan A. MAOA methylation is associated with nicotine and alcohol dependence in women. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147B(5):565–570. doi: 10.1002/ajmg.b.30778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Philibert RA, Beach SR, Gunter TD, et al. The effect of smoking on MAOA promoter methylation in DNA prepared from lymphoblasts and whole blood. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2010;153B(2):619–628. doi: 10.1002/ajmg.b.31031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Launay JM, Del Pino M, Chironi G, et al. Smoking induces long-lasting effects through a monoamine-oxidase epigenetic regulation. PLoS ONE. 2009;4(11):e7959. doi: 10.1371/journal.pone.0007959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shumay E, Fowler JS. Identification and characterization of putative methylation targets in the MAOA locus using bioinformatic approaches. Epigenetics. 2010;5(4):325–342. doi: 10.4161/epi.5.4.11719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sandovici I, Leppert M, Hawk PR, et al. Familial aggregation of abnormal methylation of parental alleles at the IGF2/H19 and IGF2R differentially methylated regions. Hum. Mol. Genet. 2004;13:781. doi: 10.1093/hmg/ddg167. [DOI] [PubMed] [Google Scholar]
- 213.Sandovici I, Naumova AK, Leppert M, Linares Y, Sapienza C. A longitudinal study of X-inactivation ratio in human females. Hum. Genet. 2004;115:387–392. doi: 10.1007/s00439-004-1177-8. [DOI] [PubMed] [Google Scholar]
- 214.Bjornsson HT, Sigurdsson MI, Fallin MD, et al. Intra-individual change over time in DNA methylation with familial clustering. JAMA. 2008;299(24):2877–2883. doi: 10.1001/jama.299.24.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Madrigano J, Baccarelli A, Mittleman MA, et al. Aging and epigenetics: longitudinal changes in gene-specific DNA methylation. Epigenetics. 2012;7(1):63–70. doi: 10.4161/epi.7.1.18749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl Acad. Sci. USA. 2005;102(30):10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Poulsen P, Esteller M, Vaag A, Fraga MF. The epigenetic basis of twin discordance in age-related diseases. Pediatr. Res. 2007;61(5 Pt 2):38R–42R. doi: 10.1203/pdr.0b013e31803c7b98. [DOI] [PubMed] [Google Scholar]
- 218.Bollati V, Schwartz J, Wright R, et al. Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mech. Ageing Dev. 2009;130(4):234–239. doi: 10.1016/j.mad.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Maegawa S, Hinkal G, Kim HS, et al. Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res. 2010;20(3):332–340. doi: 10.1101/gr.096826.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Silviera ML, Smith BP, Powell J, Sapienza C. Epigenetic differences in normal colon mucosa of cancer patients suggest altered dietary metabolic pathways. Cancer Prev. Res. (Phila.) 2012;5(3):374–384. doi: 10.1158/1940-6207.CAPR-11-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sapienza C, Lee J, Powell J, et al. DNA methylation profiling identifies epigenetic differences between diabetes patients with ESRD and diabetes patients without nephropathy. Epigenetics. 2011;6(1):20–28. doi: 10.4161/epi.6.1.13362. [DOI] [PubMed] [Google Scholar]
- 222.Barker DJ. The fetal origins of adult hypertension. J. Hypertens. 1992;(Suppl. 10):S39–S44. ▪ Landmark study on the fetal origins of disease.
- 223.Barker DJ, Gluckman PD, Godfrey KM, et al. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 224.Barker DJ, Hales CN, Fall CH, et al. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36(1):62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 225.Eriksson JG, Forsen TJ, Kajantie E, Osmond C, Barker DJ. Childhood growth and hypertension in later life. Hypertension. 2007;49:1415–1421. doi: 10.1161/HYPERTENSIONAHA.106.085597. [DOI] [PubMed] [Google Scholar]
- 226.Barker DJ, Osmond C, Kajantie E, Eriksson JG. Growth and chronic disease: findings in the Helsinki Birth Cohort. Ann. Hum. Biol. 2009;36:445–458. doi: 10.1080/03014460902980295. [DOI] [PubMed] [Google Scholar]
- 227.Sachdev HP, Osmond C, Fall CH, et al. Predicting adult metabolic syndrome from childhood body mass index: follow-up of the New Delhi birth cohort. Arch. Dis. Child. 2009;94(10):768–774. doi: 10.1136/adc.2008.140905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Barker DJ. The developmental origins of adult disease. J. Am. Coll. Nutr. 2004;23:S588–S595. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 229.Lawson C, Gieske M, Murdoch B, et al. Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A. Biol. Reprod. 2011;84(1):79–86. doi: 10.1095/biolreprod.110.084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Muhlhauser A, Susiarjo M, Rubio C, et al. Bisphenol A effects on the growing mouse oocyte are influenced by diet. Biol. Reprod. 2009;80(5):1066–1071. doi: 10.1095/biolreprod.108.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Su YQ, Sugiura K, Wigglesworth K, et al. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development. 2008;135(1):111–121. doi: 10.1242/dev.009068. [DOI] [PubMed] [Google Scholar]
- 232.Chao HH, Zhang XF, Chen B, et al. Bisphenol A exposure modifies methylation of imprinted genes in mouse oocytes via the estrogen receptor signaling pathway. Histochem. Cell Biol. 2012;137(2):249–259. doi: 10.1007/s00418-011-0894-z. [DOI] [PubMed] [Google Scholar]
- 233.Anckaert E, Romero S, Adriaenssens T, Smitz J. Effects of low methyl donor levels in culture medium during mouse follicle culture on oocyte imprinting establishment. Biol. Reprod. 2010;83(3):377–386. doi: 10.1095/biolreprod.109.082164. [DOI] [PubMed] [Google Scholar]
- 234.Laanpere M, Altmae S, Stavreus-Evers A, et al. Folate-mediated one-carbon metabolism and its effect on female fertility and pregnancy viability. Nutr. Rev. 2010;68(2):99–113. doi: 10.1111/j.1753-4887.2009.00266.x. [DOI] [PubMed] [Google Scholar]
- 235.Jongbloet PH, Verbeek AL, den Heijer M, Roeleveld N. Methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms resulting in suboptimal oocyte maturation: a discussion of folate status, neural tube defects, schizophrenia, and vasculopathy. J. Exp. Clin. Assist. Reprod. 2008;5:5. doi: 10.1186/1743-1050-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Purcell SH, Moley KH. The impact of obesity on egg quality. J. Assist. Reprod. Genet. 2011;28:517–524. doi: 10.1007/s10815-011-9592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Marquard KL, Stephens SM, Jungheim ES, et al. Polycystic ovary syndrome and maternal obesity affect oocyte size in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil. Steril. 2011;95(6):2146–2149. 2149.e1. doi: 10.1016/j.fertnstert.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jungheim ES, Schoeller EL, Marquard KL, et al. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151(8):4039–4046. doi: 10.1210/en.2010-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jungheim ES, Moley KH. Current knowledge of obesity’s effects in the pre- and periconceptional periods and avenues for future research. Am. J. Obstet. Gynecol. 2010;203(6):525–530. doi: 10.1016/j.ajog.2010.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Jungheim ES, Lanzendorf SE, Odem RR, et al. Morbid obesity is associated with lower clinical pregnancy rates after in vitro fertilization in women with polycystic ovary syndrome. Fertil. Steril. 2009;92(1):256–261. doi: 10.1016/j.fertnstert.2008.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Van Hoeck V, Sturmey RG, Bermejo-Alvarez P, et al. Elevated non-esterified fatty acid concentrations during bovine oocyte maturation compromise early embryo physiology. PLoS ONE. 2011;6(8):e23183. doi: 10.1371/journal.pone.0023183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jungheim ES, Macones GA, Odem RR, et al. Associations between free fatty acids, cumulus oocyte complex morphology and ovarian function during in vitro fertilization. Fertil. Steril. 2011;95(6):1970–1974. doi: 10.1016/j.fertnstert.2011.01.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wu LL, Dunning KR, Yang X, et al. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology. 2010;151(11):5438–5445. doi: 10.1210/en.2010-0551. [DOI] [PubMed] [Google Scholar]
- 244.Zachut M, Dekel I, Lehrer H, et al. Effects of dietary fats differing in n-6:n-3 ratio fed to high-yielding dairy cows on fatty acid composition of ovarian compartments, follicular status, and oocyte quality. J. Dairy Sci. 2010;93(2):529–545. doi: 10.3168/jds.2009-2167. [DOI] [PubMed] [Google Scholar]
- 245.Fouladi-Nashta AA, Wonnacott KE, Gutierrez CG, et al. Oocyte quality in lactating dairy cows fed on high levels of n-3 and n-6 fatty acids. Reproduction. 2009;138(5):771–781. doi: 10.1530/REP-08-0391. [DOI] [PubMed] [Google Scholar]
- 246.Chen SE, McMurtry JP, Walzem RL. Overfeeding-induced ovarian dysfunction in broiler breeder hens is associated with lipotoxicity. Poult. Sci. 2006;85(1):70–81. doi: 10.1093/ps/85.1.70. [DOI] [PubMed] [Google Scholar]
- 247.Davis TL, Yang GJ, McCarrey JR, Bartolomei MS. The H19 methylation imprint is erased and re-established differentially on the parental alleles during male germ cell development. Hum. Mol. Genet. 2000;9(19):2885–2894. doi: 10.1093/hmg/9.19.2885. [DOI] [PubMed] [Google Scholar]
- 248.Ueda T, Abe K, Miura A, et al. The paternal methylation imprint of the mouse H19 locus is acquired in the gonocyte stage during foetal testis development. Genes. Cells. 2000;5(8):649–659. doi: 10.1046/j.1365-2443.2000.00351.x. [DOI] [PubMed] [Google Scholar]
- 249.Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE. 2010;5(9):e13100. doi: 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lees-Murdock DJ, Walsh CP. DNA methylation reprogramming in the germ line. Adv. Exp. Med. Biol. 2008;626:1–15. doi: 10.1007/978-0-387-77576-0_1. [DOI] [PubMed] [Google Scholar]
- 251.Hammoud SS, Nix DA, Zhang H, et al. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460(7254):473–478. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Brykczynska U, Hisano M, Erkek S, et al. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat. Struct. Mol. Biol. 2010;17(6):679–687. doi: 10.1038/nsmb.1821. [DOI] [PubMed] [Google Scholar]
- 253.Carrell DT. Epigenetics of the male gamete. Fertil. Steril. 2011;97:267–274. doi: 10.1016/j.fertnstert.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 254.Anway MD, Rekow SS, Skinner MK. Comparative anti-androgenic actions of vinclozolin and flutamide on transgenerational adult onset disease and spermatogenesis. Reprod. Toxicol. 2008;26(2):100–106. doi: 10.1016/j.reprotox.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tesarik J. Paternal effects on cell division in the human preimplantation embryo. Reprod. Biomed. Online. 2005;10:370–375. doi: 10.1016/s1472-6483(10)61798-1. [DOI] [PubMed] [Google Scholar]
- 256.Metges CC. Early nutrition and later obesity: animal models provide insights into mechanisms. Adv. Exp. Med. Biol. 2009;646:105–112. doi: 10.1007/978-1-4020-9173-5_11. [DOI] [PubMed] [Google Scholar]
- 257.Jungheim ES, Moley KH. The impact of Type 1 and Type 2 diabetes mellitus on the oocyte and the preimplantation embryo. Semin. Reprod. Med. 2008;26(2):186–195. doi: 10.1055/s-2008-1042957. [DOI] [PubMed] [Google Scholar]
- 258.Wang Q, Moley KH. Maternal diabetes and oocyte quality. Mitochondrion. 2010;10:403–410. doi: 10.1016/j.mito.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wang Q, Ratchford AM, Chi MM, et al. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol. Endocrinol. 2009;23(10):1603–1612. doi: 10.1210/me.2009-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Jungheim ES, Macones GA, Odem RR, Patterson BW, Moley KH. Elevated serum α-linolenic acid levels are associated with decreased chance of pregnancy after in vitro fertilization. Fertil. Steril. 2011;96(4):880–883. doi: 10.1016/j.fertnstert.2011.07.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Watkins AJ, Ursell E, Panton R, et al. Adaptive responses by mouse early embryos to maternal diet protect fetal growth but predispose to adult onset disease. Biol. Reprod. 2008;78(2):299–306. doi: 10.1095/biolreprod.107.064220. [DOI] [PubMed] [Google Scholar]
- 262.Watkins AJ, Wilkins A, Cunningham C, et al. Low protein diet fed exclusively during mouse oocyte maturation leads to behavioural and cardiovascular abnormalities in offspring. J. Physiol. 2008;586(8):2231–2244. doi: 10.1113/jphysiol.2007.149229. ▪▪ Illustrates the effect of maternal diet on preimplantation programming of adult phenotype.
- 263.Watkins AJ, Platt D, Papenbrock T, et al. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc. Natl Acad. Sci. USA. 2007;104(13):5449–5454. doi: 10.1073/pnas.0610317104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kwong WY, Miller DJ, Wilkins AP, et al. Maternal low protein diet restricted to the preimplantation period induces a gender-specific change on hepatic gene expression in rat fetuses. Mol. Reprod. Dev. 2007;74(1):48–56. doi: 10.1002/mrd.20606. [DOI] [PubMed] [Google Scholar]
- 265.Kwong WY, Miller DJ, Ursell E, et al. Imprinted gene expression in the rat embryo-fetal axis is altered in response to periconceptional maternal low protein diet. Reproduction. 2006;132(2):265–277. doi: 10.1530/rep.1.01038. ▪▪ Illustrates the effect of maternal diet on preimplantation epigenome programming and adult phenotype.
- 266.Fleming TP, Kwong WY, Porter R, et al. The embryo and its future. Biol. Reprod. 2004;71:1046–1054. doi: 10.1095/biolreprod.104.030957. [DOI] [PubMed] [Google Scholar]
- 267.Xiao S, Diao H, Smith MA, Song X, Ye X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and uterine receptivity in mice. Reprod. Toxicol. 2011;32(4):434–441. doi: 10.1016/j.reprotox.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Takai Y, Tsutsumi O, Ikezuki Y, et al. Preimplantation exposure to bisphenol A advances postnatal development. Reprod. Toxicol. 2001;15(1):71–74. doi: 10.1016/s0890-6238(00)00119-2. [DOI] [PubMed] [Google Scholar]
- 269.Varayoud J, Ramos JG, Bosquiazzo VL, et al. Neonatal exposure to bisphenol A alters rat uterine implantation-associated gene expression and reduces the number of implantation sites. Endocrinology. 2011;152(3):1101–1111. doi: 10.1210/en.2009-1037. [DOI] [PubMed] [Google Scholar]
- 270.Morice L, Benaitreau D, Dieudonne MN, et al. Antiproliferative and proapoptotic effects of bisphenol A on human trophoblastic JEG-3 cells. Reprod. Toxicol. 2011;32(1):69–76. doi: 10.1016/j.reprotox.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 271.Berger RG, Foster WG, deCatanzaro D. Bisphenol-A exposure during the period of blastocyst implantation alters uterine morphology and perturbs measures of estrogen and progesterone receptor expression in mice. Reprod. Toxicol. 2010;30(3):393–400. doi: 10.1016/j.reprotox.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 272.Xu Y, Cook TJ, Knipp GT. Effects of di-(2-ethylhexyl)-phthalate (DEHP) and its metabolites on fatty acid homeostasis regulating proteins in rat placental HRP-1 trophoblast cells. Toxicol. Sci. 2005;84(2):287–300. doi: 10.1093/toxsci/kfi083. [DOI] [PubMed] [Google Scholar]
- 273.Barrero MJ, Boue S, Izpisua Belmonte JC. Epigenetic mechanisms that regulate cell identity. Cell. Stem Cell. 2010;7:565–570. doi: 10.1016/j.stem.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 274.Heijmans BT, Tobi EW, Lumey LH, Slagboom PE. The epigenome: archive of the prenatal environment. Epigenetics. 2009;4(8):526–531. doi: 10.4161/epi.4.8.10265. [DOI] [PubMed] [Google Scholar]
- 275.Trounson A. The production and directed differentiation of human embryonic stem cells. Endocr. Rev. 2006;27:208–219. doi: 10.1210/er.2005-0016. [DOI] [PubMed] [Google Scholar]
- 276.Mikkelsen TS, Ku M, Jaffe DB, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448(7153):553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Barrera LO, Li Z, Smith AD, et al. Genome-wide mapping and ana lysis of active promoters in mouse embryonic stem cells and adult organs. Genome Res. 2008;18(1):46–59. doi: 10.1101/gr.6654808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Osafune K, Caron L, Borowiak M, et al. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat. Biotechnol. 2008;26(3):313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- 279.Abeyta MJ, Clark AT, Rodriguez RT, et al. Unique gene expression signatures of independently-derived human embryonic stem cell lines. Hum. Mol. Genet. 2004;13(6):601–608. doi: 10.1093/hmg/ddh068. [DOI] [PubMed] [Google Scholar]
- 280.Kuegler PB, Zimmer B, Waldmann T, et al. Markers of murine embryonic and neural stem cells, neurons and astrocytes: reference points for developmental neurotoxicity testing. ALTEX. 2010;27(1):17–42. doi: 10.14573/altex.2010.1.16. [DOI] [PubMed] [Google Scholar]
- 281.Seiler A, Visan A, Buesen R, Genschow E, Spielmann H. Improvement of an in vitro stem cell assay for developmental toxicity: the use of molecular endpoints in the embryonic stem cell test. Reprod. Toxicol. 2004;18(2):231–240. doi: 10.1016/j.reprotox.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 282.Suzuki N, Ando S, Sumida K, Horie N, Saito K. Analysis of altered gene expression specific to embryotoxic chemical treatment during embryonic stem cell differentiation into myocardiac and neural cells. J. Toxicol. Sci. 2011;36(5):569–585. doi: 10.2131/jts.36.569. [DOI] [PubMed] [Google Scholar]
- 283.van Dartel DA, Pennings JL, de la Fonteyne LJ, et al. Evaluation of developmental toxicant identification using gene expression profiling in embryonic stem cell differentiation cultures. Toxicol. Sci. 2011;119(1):126–134. doi: 10.1093/toxsci/kfq291. [DOI] [PubMed] [Google Scholar]
- 284.Groebe K, Hayess K, Klemm-Manns M, et al. Protein biomarkers for in vitro testing of embryotoxicity. J. Proteome Res. 2010;9:5727–5738. doi: 10.1021/pr100514e. [DOI] [PubMed] [Google Scholar]
- 285.Rappolee DA, Xie Y, Slater JA, Zhou S, Puscheck EE. Toxic stress prioritizes and imbalances stem cell differentiation: implications for new biomarkers and in vitro toxicology tests. Syst. Biol. Reprod. Med. 2012;58(1):33–40. doi: 10.3109/19396368.2011.647381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 287.Bryder D, Rossi DJ, Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am. J. Pathol. 2006;169(2):338–346. doi: 10.2353/ajpath.2006.060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Alvarez-Buylla A, Seri B, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Res. Bull. 2002;57:751–758. doi: 10.1016/s0361-9230(01)00770-5. [DOI] [PubMed] [Google Scholar]
- 289.Ourednik J, Ourednik V, Lynch WP, Schachner M, Snyder EY. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat. Biotechnol. 2002;20(11):1103–1110. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- 290.Waterland RA, Lin JR, Smith CA, Jirtle RL. Post-weaning diet affects genomic imprinting at the insulin-like growth factor 2 (Igf2) locus. Hum. Mol. Genet. 2006;15(5):705–716. doi: 10.1093/hmg/ddi484. [DOI] [PubMed] [Google Scholar]
- 291.Hoyo C, Murtha AP, Schildkraut JM, et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics. 2011;6(7):928–936. doi: 10.4161/epi.6.7.16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Waterland RA, Dolinoy DC, Lin JR, et al. Maternal methyl supplements increase offspring DNA methylation at Axin Fused. Genesis. 2006;44(9):401–406. doi: 10.1002/dvg.20230.. n Illustrates the effect of maternal diet on offspring epigenome.
- 293.Waterland RA, Kellermayer R, Laritsky E, et al. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet. 2010;6(12):e1001252. doi: 10.1371/journal.pgen.1001252. ▪▪ Illustrates the effect of maternal diet on offspring epigenome in humans.
- 294.Kmetic I, Gaurina Srcek V, Slivac I, et al. Atrazine exposure decreases cell proliferation in Chinese hamster ovary (CHO-K1) cell line. Bull. Environ. Contam. Toxicol. 2008;81(2):205–209. doi: 10.1007/s00128-008-9425-6. [DOI] [PubMed] [Google Scholar]
- 295.Rayner JL, Wood C, Fenton SE. Exposure parameters necessary for delayed puberty and mammary gland development in Long-Evans rats exposed in utero to atrazine. Toxicol. Appl. Pharmacol. 2004;195(1):23–34. doi: 10.1016/j.taap.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 296.Solari M, Paquin J, Ducharme P, Boily M. P19 neuronal differentiation and retinoic acid metabolism as criteria to investigate atrazine, nitrite, and nitrate developmental toxicity. Toxicol. Sci. 2010;113(1):116–126. doi: 10.1093/toxsci/kfp243. [DOI] [PubMed] [Google Scholar]
- 297.Thomas P, Sweatman J. Interference by atrazine and bisphenol-A with progestin binding to the ovarian progestin membrane receptor and induction of oocyte maturation in Atlantic croaker. Mar. Environ. Res. 2008;66(1):1–2. doi: 10.1016/j.marenvres.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 298.Aloisi AM, Della Seta D, Ceccarelli I, Farabollini F. Bisphenol-A differently affects estrogen receptors-α in estrous-cycling and lactating female rats. Neurosci. Lett. 2001;310(1):49–52. doi: 10.1016/s0304-3940(01)02092-4. [DOI] [PubMed] [Google Scholar]
- 299.Ashby J, Tinwell H. Uterotrophic activity of bisphenol A in the immature rat. Environ. Health Perspect. 1998;106:719–720. doi: 10.1289/ehp.98106719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Dalman A, Eimani H, Sepehri H, et al. Effect of mono-(2-ethylhexyl) phthalate (MEHP) on resumption of meiosis, in vitro maturation and embryo development of immature mouse oocytes. Biofactors. 2008;33(2):149–155. doi: 10.1002/biof.5520330207. [DOI] [PubMed] [Google Scholar]
- 301.Gao L, Li Y, Pei X, Chen X. Effects of Di(2-ethylhexyl) phthalate(DEHP) on mouse embryos development in vitro] Wei Sheng Yan Jiu. 2003;32:198–200. [PubMed] [Google Scholar]
- 302.Yoshino S, Yamaki K, Li X, et al. Prenatal exposure to bisphenol A up-regulates immune responses, including T helper 1 and T helper 2 responses, in mice. Immunology. 2004;112(3):489–495. doi: 10.1111/j.1365-2567.2004.01900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Zhou W, Liu J, Liao L, Han S, Liu J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol. Cell Endocrinol. 2008;283:12–18. doi: 10.1016/j.mce.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 304.Liu Y, Balaraman Y, Wang G, Nephew KP, Zhou FC. Alcohol exposure alters DNA methylation profiles in mouse embryos at early neurulation. Epigenetics. 2009;4(7):500–511. doi: 10.4161/epi.4.7.9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Zhou R, Zhang Z, Zhu Y, et al. Deficits in development of synaptic plasticity in rat dorsal striatum following prenatal and neonatal exposure to low-dose bisphenol A. Neuroscience. 2009;159(1):161–171. doi: 10.1016/j.neuroscience.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 306.Yu C, Tai F, Song Z, et al. Pubertal exposure to bisphenol A disrupts behavior in adult C57BL/6J mice. Environ. Toxicol. Pharmacol. 2011;31(1):88–99. doi: 10.1016/j.etap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 307.Yoshida M, Shimomoto T, Katashima S, et al. Maternal exposure to low doses of bisphenol a has no effects on development of female reproductive tract and uterine carcinogenesis in Donryu rats. J. Reprod. Dev. 2004;50(3):349–360. doi: 10.1262/jrd.50.349. [DOI] [PubMed] [Google Scholar]
- 308.Yaoi T, Itoh K, Nakamura K, et al. Genome-wide ana lysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem. Biophys. Res. Commun. 2008;376(3):563–567. doi: 10.1016/j.bbrc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 309.Ye L, Zhao B, Hu G, Chu Y, Ge RS. Inhibition of human and rat testicular steroidogenic enzyme activities by bisphenol A. Toxicol. Lett. 2011;207(2):137–142. doi: 10.1016/j.toxlet.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 310.Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M. Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Mol. Endocrinol. 2011;25(12):2157–2168. doi: 10.1210/me.2011-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of Type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316(5829):1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Todd JL, Goldstein DB, Ge D, Christie J, Palmer SM. The state of genome-wide association studies in pulmonary disease: a new perspective. Am. J. Respir. Crit. Care Med. 2011;184(8):873–880. doi: 10.1164/rccm.201106-0971PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Franke B, Neale BM, Faraone SV. Genome-wide association studies in ADHD. Hum. Genet. 2009;126:13–50. doi: 10.1007/s00439-009-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Devlin B, Melhem N, Roeder K. Do common variants play a role in risk for autism? Evidence and theoretical musings. Brain Res. 2011;1380:78–84. doi: 10.1016/j.brainres.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Wang X, Prins BP, Sõber S, Laan M, Snieder H. Beyond genome-wide association studies: new strategies for identifying genetic determinants of hypertension. Curr. Hypertens. Rep. 2011;13(6):442–451. doi: 10.1007/s11906-011-0230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Chung CC, Chanock SJ. Current status of genome-wide association studies in cancer. Hum. Genet. 2011;130:59–78. doi: 10.1007/s00439-011-1030-9. [DOI] [PubMed] [Google Scholar]
Websites
- 401.WHO obesity and overweight. www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 402.Ogden CL, Carroll MD, National Center for Health Statistics . Prevalence of obesity among children and adolescents: United States, trends 1963–1965 through 2007–2008. MCHS health e-stat: National Center for Health Statistics; 2010. www.cdc.gov/nchs/data/hestat/obesity_child_07_08/obesity_child_07_08.pdf. [Google Scholar]
- 403.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. National Center for Health Statistics; Hyattsville, MD: 2012. www.cdc.gov/nchs/data/databriefs/db82.pdf. [PubMed] [Google Scholar]
- 404.Rice CD. Prevalence of autism spectrum disorders: Autism and developmental disabilities monitoring network. CDC; Atlanta, GA, USA: 2009. pp. 1–20. Morbid. Mortal. Weekly Rep. www.cdc.gov/mmwr/preview/mmwrhtml/ss5810a1.htm. [PubMed] [Google Scholar]
- 405.TEDX: the endocrine distruption exchange. TEDX list of potential endocrine disruptors. www.endocrinedisruption.com/endocrine. TEDXList.overview.php.
- 406.Office of Environmental Health Hazard Assessment California Environmental Protection Agency Public health goal for atrazine in drinking water. 1999 http://oehha.ca.gov/water/phg/pdf/atraz_f.pdf.
- 407.Determination of perfluorooctanoic acid (PFOA) in aqueous samples. New Jersey Department of Environmental Protection Division of Water Supply Bureau of Safe Drinking Water. 2007 http://slic.njstatelib.org/slic_files/digidocs/w329/w3292007.pdf.
- 408.TEDX: the endocrine distruption exchange. Critical windows of development. www.criticalwindows.com/go_display.php.