Abstract
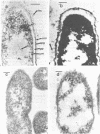
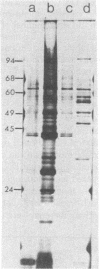
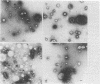
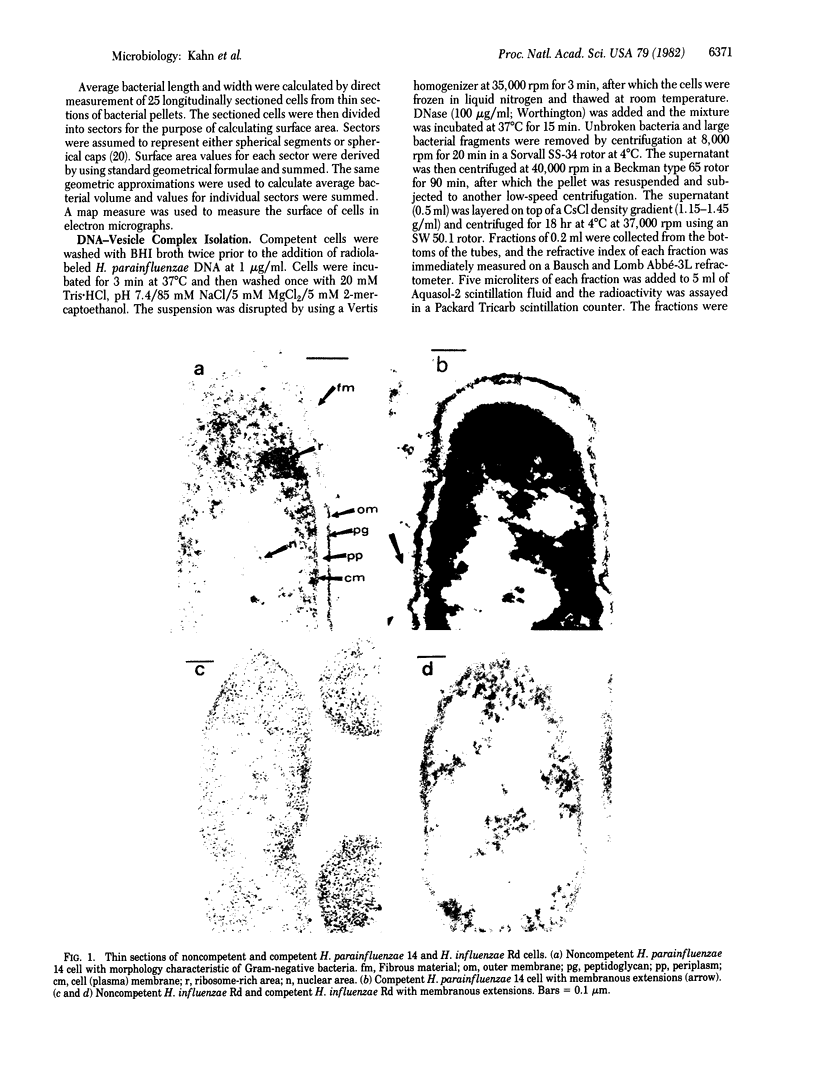
Morphological differences were observed in competent and noncompetent Haemophilus parainfluenzae and Haemophilus influenzae when thin sections of these cells were examined by electron microscopy. The membranous extensions present on the surface of competent H. parainfluenzae cells disappeared on treatment with transforming DNA, while vacuole-like structures appeared in the periplasm. Noncompetent cells had 1/5th as many extensions on their surface as competent cells, and no vacuoles were observed after treatment with homologous DNA. Competent cells treated with radiolabeled DNA were disrupted and the clarified lysate was centrifuged on CsCl density gradients. Material having a density of 1.34 g/ml was found to contain the majority of the DNase-resistant radioactive DNA recovered from the bacteria and was shown by electron microscopy to be composed of membrane vesicles. The polypeptide composition of this dense membrane fraction was similar to that of H. parainfluenzae outer membrane.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingham D. P., Barnhart B. J. Inhibition of transformation by antibodies against competent Haemophilus influenzae. J Gen Microbiol. 1973 Apr;75(2):249–258. doi: 10.1099/00221287-75-2-249. [DOI] [PubMed] [Google Scholar]
- Concino M. F., Goodgal S. H. Haemophilus influenzae polypeptides involved in deoxyribonucleic acid uptake detected by cellular surface protein iodination. J Bacteriol. 1981 Oct;148(1):220–231. doi: 10.1128/jb.148.1.220-231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. B., Deich R. A., Sisco K. L., Smith H. O. An eleven-base-pair sequence determines the specificity of DNA uptake in Haemophilus transformation. Gene. 1980 Nov;11(3-4):311–318. doi: 10.1016/0378-1119(80)90071-2. [DOI] [PubMed] [Google Scholar]
- Deich R. A., Smith H. O. Mechanism of homospecific DNA uptake in Haemophilus influenzae transformation. Mol Gen Genet. 1980 Feb;177(3):369–374. doi: 10.1007/BF00271475. [DOI] [PubMed] [Google Scholar]
- Devoe I. W., Gilchrist J. E. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973 Nov 1;138(5):1156–1167. doi: 10.1084/jem.138.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Every D., Skerman T. M. Ultrastructure of the Bacteroides nodosus cell envelope layers and surface. J Bacteriol. 1980 Feb;141(2):845–857. doi: 10.1128/jb.141.2.845-857.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromkova R., Goodgal S. Transformation by plasmid and chromosomal DNAs in Haemophilus parainfluenzae. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1428–1434. doi: 10.1016/0006-291x(79)91139-2. [DOI] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn M., Concino M., Gromkova R., Goodgal S. DNA binding activity of vesicles produced by competence deficient mutants of Haemophilus. Biochem Biophys Res Commun. 1979 Apr 13;87(3):764–772. doi: 10.1016/0006-291x(79)92024-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K. Mechanism of bacterial transformation and transfection. Prog Nucleic Acid Res Mol Biol. 1974;14(0):39–100. doi: 10.1016/s0079-6603(08)60205-6. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranhand J. M., Lichstein H. C. Effect of selected antibiotics and other inhibitors on competence development in Haemophilus influenzae. J Gen Microbiol. 1969 Jan;55(1):37–43. doi: 10.1099/00221287-55-1-37. [DOI] [PubMed] [Google Scholar]
- SCHAEFFER P., EDGAR R. S., ROLFE R. [On the inhibition of bacterial transformation by desoxyribonucleates having various structures]. C R Seances Soc Biol Fil. 1960;154:1978–1983. [PubMed] [Google Scholar]
- Scocca J. J., Poland R. L., Zoon K. C. Specificity in deoxyribonucleic acid uptake by transformable Haemophilus influenzae. J Bacteriol. 1974 May;118(2):369–373. doi: 10.1128/jb.118.2.369-373.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E. Bacteriophage of Haemophilus influenzae. II. Repair of ultraviolet-irradiated phage DNA and the capacity of irradiated cells to make phage. J Mol Biol. 1972 Feb 14;63(3):349–362. doi: 10.1016/0022-2836(72)90432-9. [DOI] [PubMed] [Google Scholar]
- Seto H., Lopez R., Tomasz A. Cell surface-located deoxyribonucleic acid receptors in transformable pneumococci. J Bacteriol. 1975 Jun;122(3):1339–1350. doi: 10.1128/jb.122.3.1339-1350.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisco K. L., Smith H. O. Sequence-specific DNA uptake in Haemophilus transformation. Proc Natl Acad Sci U S A. 1979 Feb;76(2):972–976. doi: 10.1073/pnas.76.2.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Vermeulen C. A., Venema G. Evidence for the involvement of membranous bodies in the processes leading to genetic transformation in Bacillus subtilis. J Bacteriol. 1966 Oct;92(4):1111–1121. doi: 10.1128/jb.92.4.1111-1121.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoon K. C., Habersat M., Scocca J. J. Synthesis of envelope polypeptides by Haemophilus influenzae during development of competence for genetic transformation. J Bacteriol. 1976 Jul;127(1):545–554. doi: 10.1128/jb.127.1.545-554.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]