Abstract
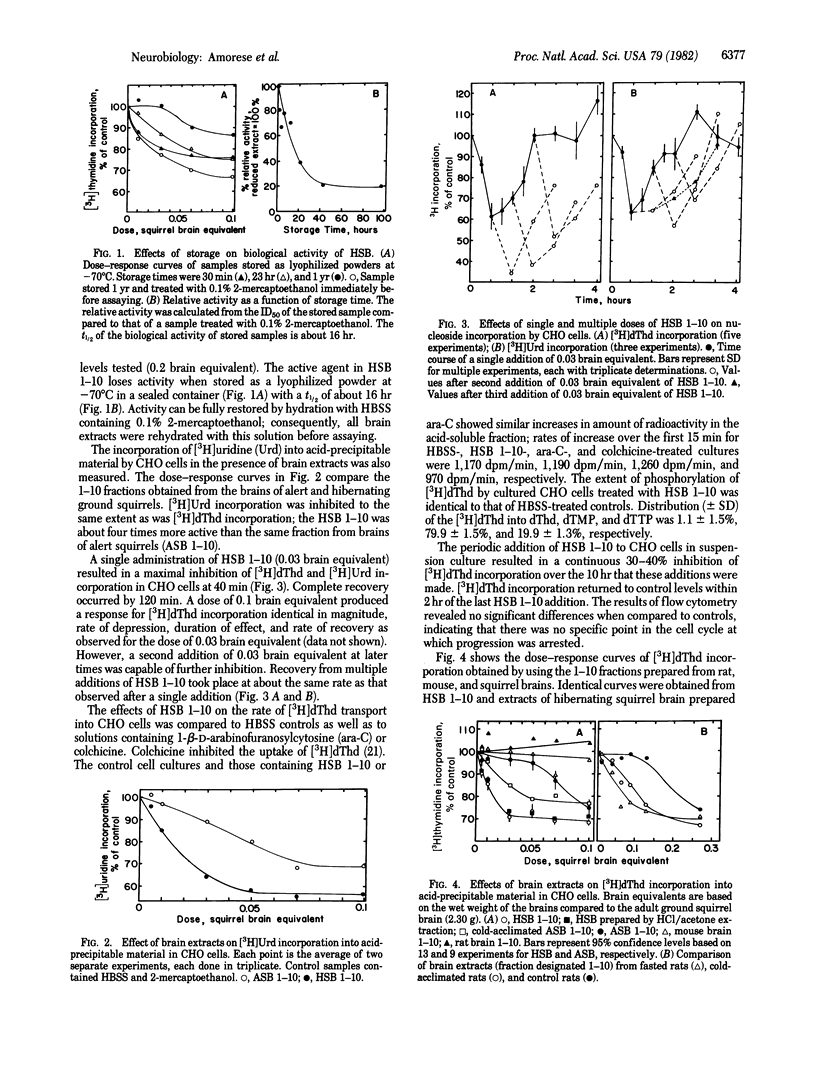
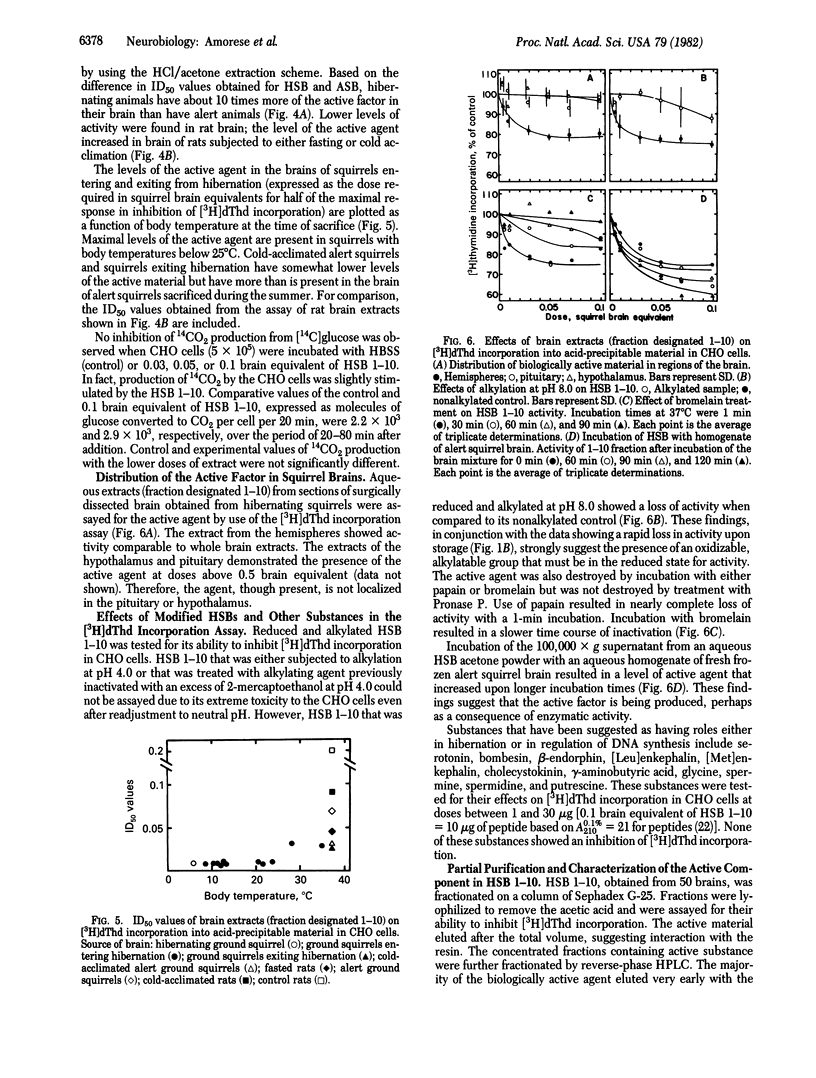
Aqueous extracts were prepared from pulverized, acetone-dehydrated brains of hibernating and alert ground squirrels. Addition of these extracts to Chinese hamster ovary cells in suspension culture resulted in a decrease in the amount of [3H]thymidine incorporated into acid-precipitable material without affecting the transport or phosphorylation of the nucleoside. The inhibition was time- and dose-dependent and full recovery occurred about 2 hr after exposure of the cells to the active extract. The active factor is readily oxidized during storage at -70 degrees C but full activity can be restored by treatment with 2-mercaptoethanol. The peptide nature of the active material is indicated by its susceptibility to proteases and by loss of activity after alkylation. Fasted or cold-acclimated rats also develop increased levels of active substance in their brains; however, brains of hibernating squirrels contain 10- to 50-fold more of the active substance than brains from either alert squirrels or rats. A time-dependent increase in activity of extracts from hibernating brain incubated with a homogenate from alert brain suggests that the peptide is activated or generated in the mixture.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELSTEIN S. J., LYMAN C. P., O'BRIEN R. C. VARIATIONS IN THE INCORPORATION OF THYMIDINE INTO THE DNA OF SOME RODENT SPECIES. Comp Biochem Physiol. 1964 Jun;12:223–231. doi: 10.1016/0010-406x(64)90176-8. [DOI] [PubMed] [Google Scholar]
- Abbotts B., Wang L. C., Glass J. D. Absence of evidence for a hibernation "trigger" in blood dialyzate of Richardson's ground squirrel. Cryobiology. 1979 Apr;16(2):179–183. doi: 10.1016/0011-2240(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Dawe A. R., Spurrier W. A., Armour J. A. Summer hibernation induced by cryogenically preserved blood "trigger". Science. 1970 Apr 24;168(3930):497–498. doi: 10.1126/science.168.3930.497. [DOI] [PubMed] [Google Scholar]
- Dawe A. R., Spurrier W. A. Hibernation induced in ground squirrels by blood transfusion. Science. 1969 Jan 17;163(3864):298–299. doi: 10.1126/science.163.3864.298. [DOI] [PubMed] [Google Scholar]
- Dawe A. R., Spurrier W. A. Summer hibernation of infant (six week old) 13-lined ground squirrels, Citellus tridecemlineatus. Cryobiology. 1974 Feb;11(1):33–43. doi: 10.1016/0011-2240(74)90036-4. [DOI] [PubMed] [Google Scholar]
- Dawe A. R., Spurrier W. A. The blood-borne "trigger" for natural mammalian hibernation in the 13-lined ground squirrel and the woodchuck. Cryobiology. 1972 Jun;9(3):163–172. doi: 10.1016/0011-2240(72)90028-4. [DOI] [PubMed] [Google Scholar]
- Fox M. H., Coulter J. R. Enhanced light collection in a flow cytometer. Cytometry. 1980 Jul;1(1):21–25. doi: 10.1002/cyto.990010106. [DOI] [PubMed] [Google Scholar]
- Jensen R. H. Chromomycin A3 as a fluorescent probe for flow cytometry of human gynecologic samples. J Histochem Cytochem. 1977 Jul;25(7):573–579. doi: 10.1177/25.7.70448. [DOI] [PubMed] [Google Scholar]
- Kimura S., Lewis R. V., Stern A. S., Rossier J., Stein S., Udenfriend S. Probable precursors of [Leu]enkephalin and [Met]enkephalin in adrenal medulla: peptides of 3-5 kilodaltons. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1681–1685. doi: 10.1073/pnas.77.3.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Minor J. G., Bishop D. A., Badger C. R., Jr The golden hamster and the blood-borne hibernation trigger. Cryobiology. 1978 Oct;15(5):557–562. doi: 10.1016/0011-2240(78)90078-0. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Wilson L. Nucleoside transport in mammalian cells. Inhibition by colchicine. Biochemistry. 1972 Jul 4;11(14):2573–2578. doi: 10.1021/bi00764a003. [DOI] [PubMed] [Google Scholar]
- Nelles L. P., Bamburg J. R. Thin-layer mapping of peptides labeled with 3H or 14C via reductive methylation. Anal Biochem. 1979 Apr 1;94(1):150–159. doi: 10.1016/0003-2697(79)90804-2. [DOI] [PubMed] [Google Scholar]
- Randerath K. An evaluation of film detection methods for weak beta-emitters, particularly tritium. Anal Biochem. 1970 Mar;34:188–205. doi: 10.1016/0003-2697(70)90100-4. [DOI] [PubMed] [Google Scholar]
- Spurrier W. A., Folk G. E., Jr, Dawe A. R. Induction of summer hibernation in the 13-lined ground squirrel shown by comparative serum transfusions from arctic mammals. Cryobiology. 1976 Jun;13(3):368–374. doi: 10.1016/0011-2240(76)90120-6. [DOI] [PubMed] [Google Scholar]
- Swan H., Schätte C. Antimetabolic extract from the brain of the hibernating ground squirrel Citellus tridecemlineatus. Science. 1977 Jan 7;195(4273):84–85. doi: 10.1126/science.831261. [DOI] [PubMed] [Google Scholar]
- TOMBS M. P., SOUTER F., MACLAGAN N. F. The spectrophotometric determination of protein at 210 millimicrons. Biochem J. 1959 Sep;73:167–171. doi: 10.1042/bj0730167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenius L. Endogenous peptides and analgesia. Annu Rev Pharmacol Toxicol. 1978;18:189–204. doi: 10.1146/annurev.pa.18.040178.001201. [DOI] [PubMed] [Google Scholar]
- Vosbeck K. D., Greenberg B. D., Ochoa M. S., Whitney P. L., Awad W. M., Jr Proteolytic enzymes of the K-1 strain of Streptomyces griseus obtained from a commercial preparation (Pronase). Effect of pH, metal ions, and amino acids on aminopeptidase activity. J Biol Chem. 1978 Jan 10;253(1):257–260. [PubMed] [Google Scholar]
- Zingg H. H., Patel Y. C. Somatostatin precursors: evidence for presence in and release from rat median eminence and neurohypophysis. Biochem Biophys Res Commun. 1979 Sep 27;90(2):466–472. doi: 10.1016/0006-291x(79)91258-0. [DOI] [PubMed] [Google Scholar]


