Abstract
Purpose
The development of cancer chemopreventative agents with increased safety profile is a major priority of cancer research. The objective of the present study was to delineate the efficacy of tetrathiomolybdate (TM), a novel antiangiogenic anticancer agent, as a chemopreventative agent.
Experimental Design
Nulliparous Her2/neu transgenic mice were treated with water or TM for 180 days and observed for tumor development during treatment and for 180 days post-treatment. Mammary gland composition and architecture were also observed following TM treatment of Her2/neu transgenic and normal FVB mice.
Results
At the 1-year follow-up, 86.7% of control and 40% of TM-treated Her2/neu mice had palpable mammary tumors with a median time to tumor development of 234 days (202, 279; 95% confidence interval) for control and greater than 460 days for TM-treated mice (p<0.0005, n=15). The mammary glands from TM-treated Her2/neu and FVB mice showed a blunted epithelial ductal branching system due to a significant decrease in the number of secondary branches and total number of differentiated mammary epithelial cells. Microvessel density in Her2/neu and FVB mammary glands was lowered by 65.6 ± 6.2% and 50.9 ± 4.5% (p<0.005) following TM therapy, consistent with the antiangiogenic effect of TM. Lastly, TM treatment resulted in a 2-fold increase in the absolute number of ALDH+ mammary stem cells in Her2/neu and FVB mammary glands.
Conclusion
Taken together, these results strongly support that TM is a potent chemopreventative agent as a consequence of hypoplastic remodeling of the mammary gland through modulation of the mammary stem cell compartment.
Keywords: Copper, Angiogenesis, Chemoprevention, Breast, Oncogene
Introduction
Breast cancer affects more than 180,000 women yearly in the United States, and more than 40,000 women each year die of the disease (1). Although the incidence rate appears to have stabilized during the last decade and a decline in the mortality rate has been noted, breast cancer remains a major health problem (2). Recent focus has been on the development of tamoxifen as a chemopreventative strategy against breast cancer. Results from the National Surgical Adjuvant Breast Project (NSABP) and International Breast Cancer Intervention Study (IBIS) trials demonstrated that tamoxifen therapy for five years is associated with a significant reduction in breast cancer incidence in high-risk women (3);(4). However, tamoxifen was found to only reduce the incidence of estrogen receptor positive breast cancers and failed to affect the risk of estrogen receptor negative breast cancer, a generally more aggressive form of breast cancer. Moreover, the deleterious side-effects of tamoxifen, particularly uterine cancer, thromboembolism and menopausal symptoms, limit its use as a beneficial chemopreventative agent to only a subset of high-risk women with a calculated 5-year Gail risk of 1.7% or higher in whom it is estimated that the benefits outweigh the considerable risks (3);(5). These observations indicate that development of novel chemopreventative agents with better safety profiles and activity in ER positive tamoxifen resistant or ER negative breast cancer is a major priority.
Tetrathiomolybdate (TM), a potent copper chelator, was developed for the treatment of Wilson’s disease, a rare autosomal recessive disorder with a defect in copper transport that results in life-threatening accumulation of copper in multiple organs. Research from our laboratory in the past five years, both pre-clinical and clinical, has provided solid evidence that copper deficiency induced by TM is antiangiogenic and an effective modality for the treatment of solid tumors (6–15). Additionally, our laboratory reported that TM prevents Her2/neu-induced breast carcinoma by maintaining these transformed cells as “micro-tumors” in an avascular, dormant-like state (7). In this study, we focused on uncovering the cellular and molecular basis for this novel finding and explored the specific mechanism by which TM protects against Her2/neu-induced breast carcinoma. Mammary glands from TM-treated mice had a decrease in the total number of mammary epithelial cells, a decrease in the complexity of mammary epithelial ductal branching, an increase in the number of ALDH+ mammary stem cells, and lower microvessel density.
Materials and Methods
Chemoprevention experimental protocol
MMTV-Her2/neu transgenic mice were purchased from Jackson Laboratory and a breeding colony was maintained at the University of Michigan Comprehensive Cancer Center. Nulliparous female 100-day old MMTV-Her2/neu mice were randomly assigned to two treatment groups and gavaged with water (control) or 0.75 mg/day TM for 180 days (n=15 for each group). Mice treated with TM were rendered copper deficient (ceruloplasmin levels < 30% of control) after 1 week of therapy and remained so for as long as TM therapy was administered. After 180 days of treatment, control and TM-treated mice that did not develop mammary tumors were released from therapy and observed for the next 180 days. Mice were monitored weekly for palpable tumors and disease-free survival (DFS) was calculated as a function of time. The DFS curves for the two groups in this treatment-release protocol were compared using log rank analysis.
Mammary gland whole mount, histology, and immunohistochemistry
Nulliparous female MMTV-Her2/neu mice (100-day old for both strains) were gavaged with water (control) or 0.75 mg/day TM for 30 days. Mice were euthanized and mammary glands were isolated and processed for whole mount analysis using carmine-red or fixed in 10% buffered formalin. Formalin-fixed glands were embedded in paraffin and processed for hematoxylin and eosin staining or immunohistochemistry with anti-CD31 (Dako), or anti-ALDH1 (Abcam). The complexity of mammary ductal branching was quantified using carmine-red stained mammary glands to count the number of secondary branches in three representative fields (100X). Mean number of mammary epithelial cells per field (100X) was calculated and presented as mean ± SEM.
Quantification of microvessel density
Mammary gland microvessel density was assessed with anti-CD31 staining using the vascular hotspot technique (16). Sections were scanned at low-power to determine areas of highest vascular density. Within this region, individual microvessels were counted in three separate random fields at high power (400X magnification). The mean vessel count from the three fields was used. A single countable microvessel was defined as any endothelial cell or group of cells that was clearly separate from other vessels, stroma, or tumor cells without the necessity of a vessel lumen. Quantification of microvessel density in the mammary glands was performed by a blind observer to eliminate subjectivity of the analysis.
Results and Discussion
Antiangiogenics have demonstrated to be effective and safe anticancer agents in preclinical animal tumor models but have had mixed results in clinical trials with advanced disease. One possible explanation for the lack of efficacy in these clinical trials could be due to the bulky size of tumors in patients with stage III/IV disease. In theory, antiangiogenics would be predicted to be more effective in preventing smaller avascular tumors from activating the angiogenic switch and thus would be an ideal class of compounds for use in a chemopreventative setting. TM is a potent antiangiogenic compound that has completed numerous Phase I/II trials for solid tumors. Results from these clinical trials demonstrated that TM is very well-tolerated with minimal adverse effects (6);(11–15). The attractive safety profile of TM suggests that it may be amendable to long-term use and thus be developed as a chemopreventative agent.
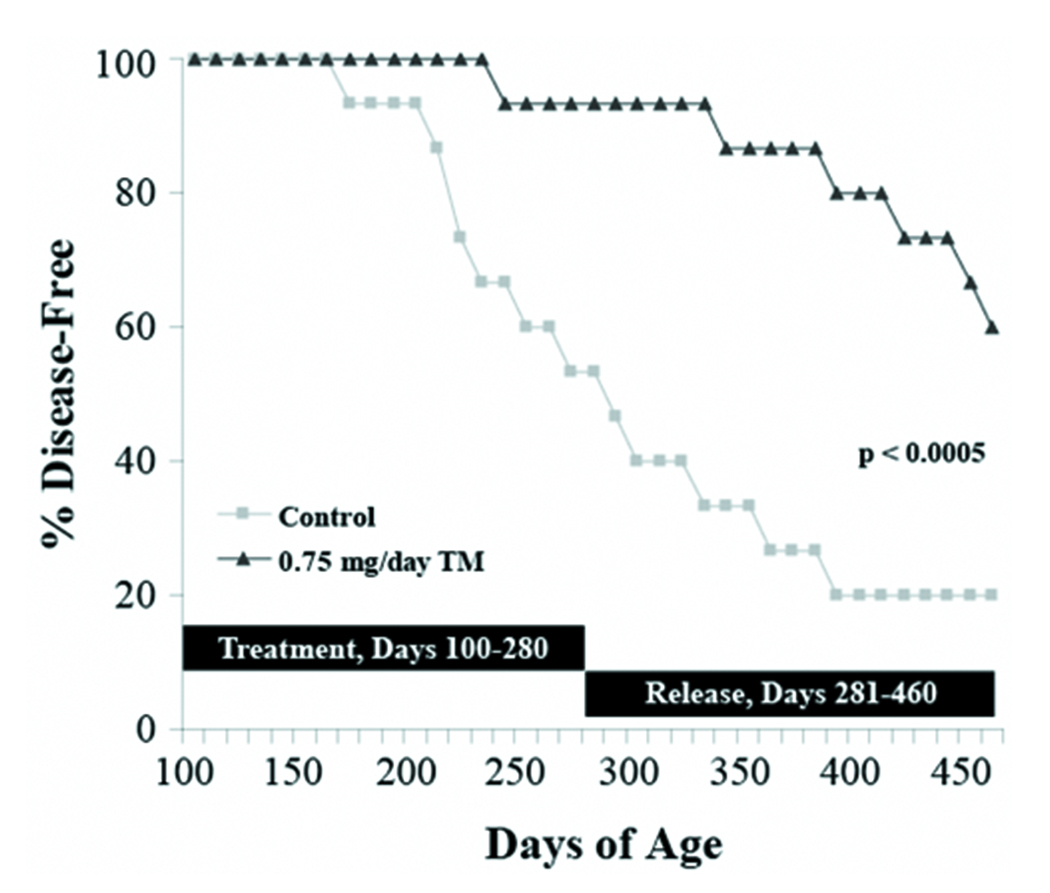
Recent work from our laboratory demonstrated that TM significantly retards the development of Her2/neu-induced breast carcinoma in transgenic mice (7). To confirm and extend on this exciting finding, we were interested in repeating this experimental protocol with a longer follow-up period. Nulliparous Her2/neu transgenic mice (100-day old) were randomly assigned to two different groups and gavaged with water (control) or TM (0.75 mg/day) for 180 days or until palpable mammary tumors developed. At the end of the 180-day treatment protocol, 66.7% (10/15) control Her2/neu transgenic mice but only 13.3% (2/15) TM-treated Her2/neu transgenic mice developed palpable tumors (Figure 1). Control and TM-treated Her2/neu transgenic mice that did not develop mammary tumors were released from therapy and observed for the next 180 days. At the 1-year follow-up (360 days after start of treatment protocol), 3 additional control mice developed tumors to bring the total of control mice with overt clinical disease to 86.7%. Moreover, 4/13 of the TM-treated mice that were released from therapy developed tumors to bring the total of TM-treated mice with palpable disease to only 40%. The median time to tumor development was 234 (202, 279; 95% confidence interval) days for control mice. Since 50% of the TM-treated animals did not develop tumors, the median time to tumor development for mice on systemic TM therapy could not be measured but it is estimated to be greater than 460 days. Using data from the 1-year follow-up, log-rank analysis indicates that the disease-free survival for TM-treated mice was significantly longer than controls (Figure 1; p<0.0005; n=15). These observations unequivocally demonstrate that chemoprevention with TM was not limited to the time when TM was on board as prolonged protection was observed long after releasing TM-treated mice from therapy. These results add to our exciting initial findings and indicate that further understanding of TM as a chemopreventative agent is warranted.
Figure 1.
Systemic TM therapy protects against Her2/neu-induced mammary tumorigenesis. Nulliparous female 100-day old MMTV-Her2/neu mice were randomly assigned to two treatment groups and gavaged with water (control) or 0.75 mg/day TM for 180 days (n=15 for each group). At the end of the treatment protocol, control and TM-treated mice that did not develop mammary tumors were released from therapy and observed for the next 180 days. The disease-free survival curves for the two groups were compared using log rank analysis (p<0.0005).
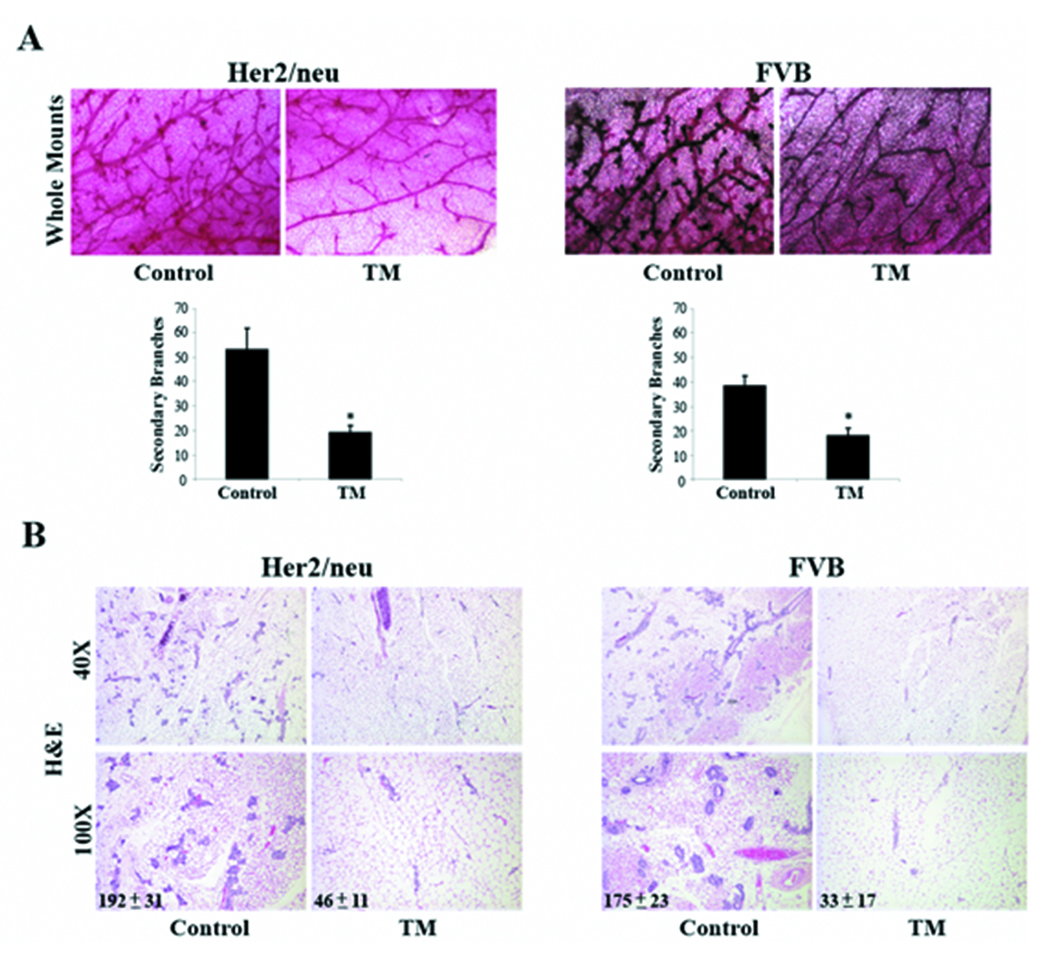
To determine the effects of TM on adult mammary gland architecture, mammary glands were isolated from control and TM-treated (30 day treatment) Her2/neu and its normal background strain FVB mice for histological examination. Carmine-red stained whole mounts revealed that the epithelial ductal branching network of mammary glands from TM-treated Her2/neu mice was strikingly less complex as compared to glands from untreated Her2/neu mice (Figure 2A). The difference in the ductal branching network primarily is due to a significant decrease in the number of secondary branches. Moreover, terminal end-buds of TM-treated glands were less robust in number and size. It appears that TM is capable of remodeling the adult virgin mammary gland to a state that resembles a pre-pubertal or early pubertal mammary gland with one notable exception, the lack of actively proliferating terminal end-buds. In Figure 2B, histologic analyses of mammary glands showed that the TM-treated glands had a dramatic decrease (76 ± 6% reduction, p<0.05) in the total number of mammary epithelial cells suggesting that TM may be modulating the turnover of mammary epithelial cells, either promoting apoptosis or inhibiting proliferation. The inhibitory effect of TM on mammary epithelial cell number and branching morphogenesis was not limited to Her2/neu transformed mammary glands as systemic TM therapy had the same effect on normal mammary glands from FVB mice. This suggests that TM is able to remodel the mammary glands regardless of the stage of oncogenic transformation and therefore be an effective chemopreventative strategy against pre-neoplastic and neoplasic lesions.
Figure 2.
Systemic TM therapy remodels mammary gland ductal morphogenesis in Her2/neu transgenic and FVB mice. A, mammary gland ductal network. Mammary glands from control and TM-treated Her2/neu and FVB mice were isolated and processed for whole mount analysis using carmine-red staining. The complexity of mammary ductal branching was quantified using carmine-red stained mammary glands to count the number of secondary branches in three representative fields (100X). Mean number of secondary branches per field was calculated and presented as mean ± SEM. *, p-value <0.05. B, mammary gland histology. Mammary glands from control and TM-treated Her2/neu and FVB mice were isolated, fixed, and processed for hematoxylin and eosin staining. Mean number of mammary epithelial cells per field (100X) was calculated and presented as mean ± SEM.
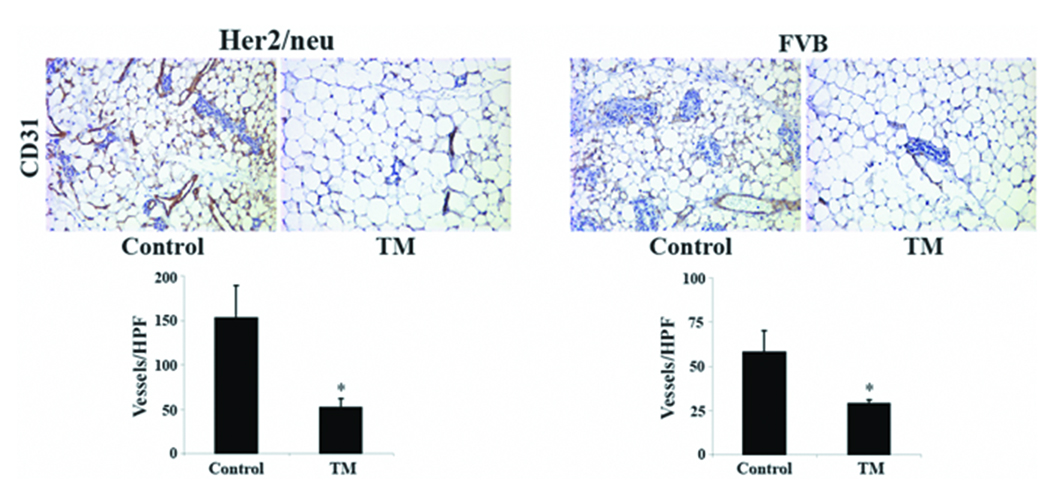
An extensive literature supports that overexpression of Her2/neu in breast cancer patients correlates with poor prognoses and decreased overall survival (17);(18). A study demonstrated that high intratumoral microvessel density is an independent marker of early relapse in node-negative breast cancer and intratumoral microvessel density is highest in breast cancer patients positive for Her2/neu overexpression (19). Indeed, we found that mammary glands from control Her2/neu mice had higher microvessel density than control FVB mice (153 ± 36 vs. 58 ± 12 vessels/high power field) supporting the notion that the Her2/neu oncogene is proangiogenic and perhaps a reason why Her2/neu is a predicator of a more aggressive and metastatic form of breast cancer. Importantly, mean microvessel density in the mammary glands of Her2/neu and FVB mice was significantly lower following systemic TM therapy; 65.6 ± 6.2% and 50.9 ± 4.5% inhibition (p<0.005) for Her2/neu and FVB mice, respectively (Figure 3). It is likely that a less vascularized mammary gland environment will make it more challenging for avascular, incipient tumors to activate the angiogenic switch and grow into bulky tumors with metastatic potential. This line of reasoning is supported by our previous finding that TM retarded the development of mammary tumors in Her2/neu transgenics by keeping the transformed “microtumors” in stasis largely devoid of neovascularization (7). However, the results of the present study extend much further and indicate that the chemopreventative action of TM is more complex than simply through attenuating the angiogenic switch of avascular tumors as TM was found to induce a profound remodeling of the mammary gland resulting in a severe deficit in the total number of ductal epithelial cells. A critical question that remains is whether hypoplastic remodeling of the mammary gland is a consequence of a decrease in mammary gland vascularity. Although our study was not designed to strictly address this question, the observation that the mean microvessel density of TM-treated Her2/neu mammary glands is similar to control FVB mammary glands that have normal ductal morphology (52 ± 10 vs. 58 ± 12 vessels/high power field) argues against a direct link between mammary gland vascularization and morphology. Additional work with specific molecularly-targeted antiangiogenics, such as VEGF-KDR inhibitors, will be necessary to adequately address whether the change in mammary gland architecture, namely a lower number of epithelial cells and secondary branches, is a direct consequence of inhibiting angiogenesis in the mammary gland.
Figure 3.
Effects of systemic TM therapy on microvessel density in Her2/neu transgenic and FVB mice. Mammary glands from control and TM-treated Her2/neu and FVB were isolated, fixed, and processed for immunohistochemistry with an anti-CD31 antibody for microvessel density. Mean vessel count per high power field (400X) was calculated and presented as mean ± SEM. *, p-value <0.05.
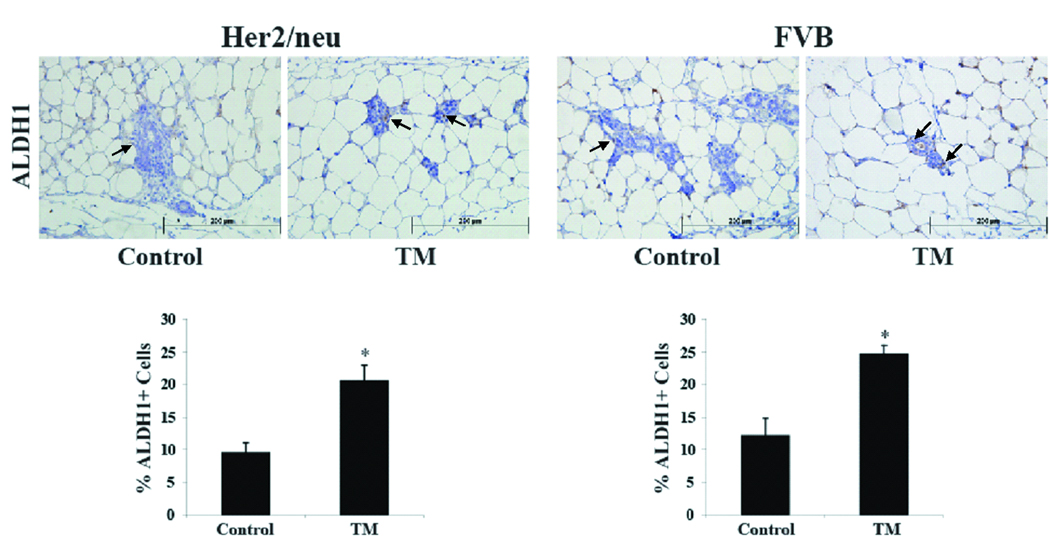
Human normal and tumor mammary epithelial cells with high aldehyde dehydrogenase (ALDH) activity possesses stem cell characteristics (20). Xeno-transplantation of ALDH+ mammary epithelial cells isolated from human breast tumors into the cleared mammary glands of recipient NOD/scid mice was sufficient to induce tumor formation (20). Tumors formed in recipient mice consisted of ALDH− and ALDH+ tumor cells and thus, recapitulated the heterogeneity of the parental human breast tumors (20). Moreover, immunohistochemical analysis with ALDH1 is able to identify tumor initiating cells in fixed paraffin-embedded tumor sections (20). A recent report showed ALDH+ as a marker for the identification of mouse tumor mammary stem cells (21). Results from these two groups provide evidence that ALDH can be used as a robust marker to identify human and mouse tumor mammary epithelial stem cells. As shown in Figure 4, mammary glands from TM-treated Her2/neu transgenic mice had a significant 2-fold increase in the percentage of ALDH+ mammary epithelial cells compared to control-treated Her2/neu transgenic mice; 9.5 ± 1.6% vs. 20.5 ± 2.4% for ALDH+ respectively (p<0.004). Similarly, in normal FVB mice, systemic TM treatment resulted in 24.6 ± 1.4% ALDH+ mammary epithelial cells compared to 12.2 ± 1.4% ALDH+ mammary epithelial cells for the control treatment (p<0.004).
Figure 4.
Systemic TM therapy increases the number of epithelial stem cells in the mammary glands of Her2/neu transgenic and FVB mice. Mammary glands from control and TM-treated Her2/neu and FVB mice were isolated, fixed, and processed for immunohistochemistry with an anti-ALDH1 antibody (arrows indicate representative stained cells). The percent of ALDH1+ mammary epithelial cells per high power field (400X) was calculated and presented as mean ± SEM. *, p-value <0.05.
It is intriguing that systemic TM therapy results in a clear increase in the absolute number of ALDH+ mammary stem cells. The current stem cell paradigm indicates that symmetric cell division where one stem cell self-renews into two stem cells is the postulated mechanism to produce a net increase in stem cell number. Thus, our results argue that the mammary gland microenvironment of TM-treated Her2/neu transgenic mice favors the mammary stem cells toward symmetric rather than asymmetric cell division. The mammary gland is composed of a network of branching ducts. Ductal branches are comprised of terminally differentiated mammary epithelial cells consisting of an inner layer of luminal cells surrounded by an outer layer of myoepithelial cells. Therefore, the mammary gland would be expected to be nascent or less complex in an environment where there is a relative paucity of asymmetric cell division to yield daughter cells capable of differentiating to luminal or myoepithelial cells. This is entirely consistent with our observation that TM-treated mammary glands are less complex with a lower number of secondary branches. It is unclear how systemic TM therapy leads mammary stem cells toward symmetric cell division. Does TM directly regulate mammary stem cell fate, or does TM alter the stem cell niche in the mammary gland to indirectly drive symmetric cell division? It is critical to address these key questions to better understand the mechanism of action of TM as a chemopreventative agent.
It is of interest and further supports the explanation above that the effects of TM on mammary gland development were reversible as a few of the 30 day post-release and a majority of the 60 day post-release mammary glands isolated from TM-treated/released mice had normal breast morphology, in terms of the ductal epithelial branching network (data not shown). Moreover, female mice on continuous long-term TM therapy were able to conceive, carry to term and nurse their pups without apparent difficulty. These results indicate that the effects of TM on the mammary gland are not permanent and that biological signals during pregnancy can override the TM effects by allowing ductal epithelial cells to rapidly proliferate and differentiate to form lactating mammary glands. This observation has important clinical implications in the future development of TM as a chemopreventative agent as it suggests that TM, if used in women of reproductive age, may not permanently affect the lactating potential of the adult mammary gland in humans.
In conclusion, TM is preventing the development of breast carcinoma in Her2/neu transgenic mice by hypoplastic remodeling of the mammary gland. This study demonstrated, for the first time, that alterations in the mammary gland ductal morphology and tissue architecture can result in an environment less conducive for carcinogenesis.
Translational Relevance.
The development of cancer chemopreventative agents with increased safety profile is a major priority of cancer research. Tetrathiomolybdate (TM) is a novel anticancer agent that has exhibited antiangiogenic properties in preclinical and clinical studies with minimal adverse events. Here we demonstrate that TM retards the development of Her2/neu mammary tumors by hypoplastic remodeling of the mammary gland. In addition to being efficacious, we found that TM’s effects on the normal mammary gland are reversible, and TM-treated mice can still conceive, carry to term and nurse their pups. This study demonstrates, for the first time, that alterations in the mammary gland ductal morphology and tissue architecture can result in an environment less conducive for carcinogenesis, a principle that can be applied to future design of prevention trials.
Acknowledgements
We thank Deborah Key for her secretarial assistance and Kent A. Griffith for statistical analysis.
Work supported in part by NIH Grants R01CA77612, P30CA46592, M01-RR00042, Head and Neck SPORE P50CA97248, Susan G. Komen Breast Cancer Foundation, NIH Cancer Biology Postdoctoral Fellowship T32CA09676, Department of Defense Breast Cancer Research Program Postdoctoral Fellowship), the Burroughs Wellcome Fund, the Breast Cancer Research Foundation, Department of Defense Grant DAMD17-02-1-0490, the Tempting Tables Organization, Muskegon, MI and Attenuon, LLC.
Footnotes
S.D.M. was a consultant and has a financial interest in Attenuon, LLC, which has licensed TM as an anticancer compound from the University of Michigan.
Reference List
- 1.The American Cancer Society. Cancer Facts & Figures 2001. 2001. [Google Scholar]
- 2.Chu KC, Tarone RE, Kessler LG, et al. Recent trends in U.S. breast cancer incidence, survival, and mortality rates. J Natl Cancer Inst. 1996;88:1571–1579. doi: 10.1093/jnci/88.21.1571. [DOI] [PubMed] [Google Scholar]
- 3.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 4.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 5.Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91:1829–1846. doi: 10.1093/jnci/91.21.1829. [DOI] [PubMed] [Google Scholar]
- 6.Brewer GJ, Dick RD, Grover DK, et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin Cancer Res. 2000;6:1–10. [PubMed] [Google Scholar]
- 7.Pan Q, Kleer CG, van Golen KL, et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002;62:4854–4859. [PubMed] [Google Scholar]
- 8.Cox C, Teknos TN, Barrios M, Brewer GJ, Dick RD, Merajver SD. The role of copper suppression as an antiangiogenic strategy in head and neck squamous cell carcinoma. Laryngoscope. 2001 Apr;111:696–701. doi: 10.1097/00005537-200104000-00024. [DOI] [PubMed] [Google Scholar]
- 9.van Golen KL, Bao L, Brewer GJ, et al. Suppression of tumor recurrence and metastasis by a combination of the PHSCN sequence and the antiangiogenic compound tetrathiomolybdate in prostate carcinoma. Neoplasia. 2002;4:373–379. doi: 10.1038/sj.neo.7900258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan Q, Bao LW, Kleer CG, Brewer GJ, Merajver SD. Anti-angiogenic tetrathiomolybdate potentiates doxorubicin therapy against refractory breast carcinoma. Mol. Cancer Ther. 2003 Jul;:617–622. [PubMed] [Google Scholar]
- 11.Redman BG, Esper P, Pan Q, et al. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin Cancer Res. 2003;9:1666–1672. [PubMed] [Google Scholar]
- 12.Henry NL, Dunn R, Merajver S, et al. Phase II trial of copper depletion with tetrathiomolybdate as an antiangiogenesis strategy in patients with hormone-refractory prostate cancer. Oncology. 2006;71:168–175. doi: 10.1159/000106066. [DOI] [PubMed] [Google Scholar]
- 13.Pass HI, Brewer GJ, Dick R, Carbone M, Merajver S. A phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: final results. Ann Thorac Surg. 2008;86:383–390. doi: 10.1016/j.athoracsur.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Lowndes SA, Adams A, Timms A, et al. Phase I study of copper-binding agent ATN-224 in patients with advanced solid tumors. Clin Cancer Res. 2008;14:7526–7534. doi: 10.1158/1078-0432.CCR-08-0315. [DOI] [PubMed] [Google Scholar]
- 15.Gartner EM, Griffith KA, Pan Q, et al. A pilot trial of the anti-angiogenic copper lowering agent tetrathiomolybdate in combination with irinotecan, 5-flurouracil, and leucovorin for metastatic colorectal cancer. Invest New Drugs. 2009;27:159–165. doi: 10.1007/s10637-008-9165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am. J Pathol. 1995;147:9–19. [PMC free article] [PubMed] [Google Scholar]
- 17.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 18.Paterson MC, Dietrich KD, Danyluk J, et al. Correlation between c-erbB-2 amplification and risk of recurrent disease in node-negative breast cancer. Cancer Res. 1991;51:556–567. [PubMed] [Google Scholar]
- 19.Koukourakis MI, Manolas C, Minopoulos G, Giatromanolaki A, Sivridis E. Angiogenesis relates to estrogen receptor negativity, c-erbB-2 overexpression and early relapse in node-negative ductal carcinoma of the breast. Int J Surg Pathol. 2003;11:29–34. doi: 10.1177/106689690301100107. [DOI] [PubMed] [Google Scholar]
- 20.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lue M, Fan H, Nagy T, et al. Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res. 2009;69:466–474. doi: 10.1158/0008-5472.CAN-08-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]