Abstract
Background
The past decade has seen much progress in intrauterine surgery. Randomized trials have documented the benefit of some procedures of this type for the unborn child.
Method
Selective literature review
Results
Randomized trials have demonstrated the benefit of fetoscopic laser coagulation of placental anastomoses in twin-to-twin transfusion syndrome (TTTS) and of intrauterine surgery via hysterotomy for the repair of spina bifida. Other fetoscopic procedures have yielded promising initial results but are not yet supported by findings from randomized trials. Some intrauterine surgical procedures must still be considered experimental in view of the lack of randomized trials and the rarity of the conditions they are designed to treat. Fetoscopic laser coagulation for TTTS is by far the most common procedure in fetal surgery; TTTS arises in roughly 1 in 2500 pregnancies. The other procedures discussed in this article are performed much less often and for rarer indications. In general, intrauterine surgery is indicated only to treat conditions that would otherwise lead to intrauterine death or irreversible prenatal damage.
Conclusion
Intrauterine surgery is a rapidly developing field. Prenatal intervention by laser coagulation is indicated to treat severe TTTS, as its benefit has been shown in a randomized trial. Not enough evidence is yet available for the possible benefit of intrauterine surgery to treat myelomeningocele and congenital diaphragmatic hernia. Other indications are experimental. When an indication for intrauterine surgery exists, the parents should be informed and, depending on their wishes, referred to a center where it can be performed.
Intrauterine repair of fetal malformations has become a focus of attention for prenatal physicians and pediatric surgeons. The potential benefit of such interventions consists in an improved prognosis for certain congenital defects. In contrast to postnatal surgery, the mother is also placed at risk. Moreover, intrauterine interventions entail additional risks for the fetus, in particular premature rupture of membranes, premature birth, and surgical injury.
Researchers are therefore concentrating on establishing which fetuses genuinely benefit from intrauterine surgery compared with postnatal correction. Intrauterine surgery is indicated solely in diseases that can lead to intrauterine fetal death or cause damage that cannot be repaired postnatally.
We present the most important disease patterns amenable to treatment by fetal surgery and give an overview of the current state of knowledge. This review is based on our own experience and on an exhaustive search of the literature (search terms ”fetal surgery” and the individual disease entities).
Twin-to-twin transfusion syndrome
With over 10 000 procedures estimated to have been carried out in the past decade (e1), laser coagulation of placental vascular anastomoses in twin-to-twin transfusion syndrome (TTTS) occupies pride of place in intrauterine surgery. Twins affected by TTTS develop a chronic imbalance in blood volume owing to the placental vascular anastomoses that are always present in the case of a monochorionic placenta. The incidence of TTTS is 1 in 2500 pregnancies or 1 in 50 multiple pregnancies, and it usually manifests itself between the 16th and the 26th completed week of gestation (e2). TTTS is diagnosed by sonography and is deemed to be present when the donor fetus (“stuck twin”) shows oligouric oligo- or anhydramnios with a greatest vertical depth of amniotic fluid <2 cm and the recipient fetus displays polyuric polyhydramnios with amniotic fluid over 8 cm deep. The syndrome is characterized by hypovolemia, oliguria, and oligohydramnios in the donor and hypervolemia, polyuria, and polyhydramnios as an expression of the fluid overload in the recipient.
If left untreated, TTTS leads to fetal death in 80% to 90% of cases. Until around 20 years ago, the sole treatment option was repeated amniotic drainage. In 1990, De Lia was the first to describe occlusion of placental anastomoses by means of laser coagulation with combined access via laparotomy and fetoscopy (1). Ville and colleagues accomplished the first completely fetoscopic occlusion in 1992 (Figures 1, 2) (2). The superiority of laser coagulation over serial amniotic drainage was subsequently demonstrated in a number of studies (3, 4), in particular the randomized controlled trial by Senat et al. (5) (Table).
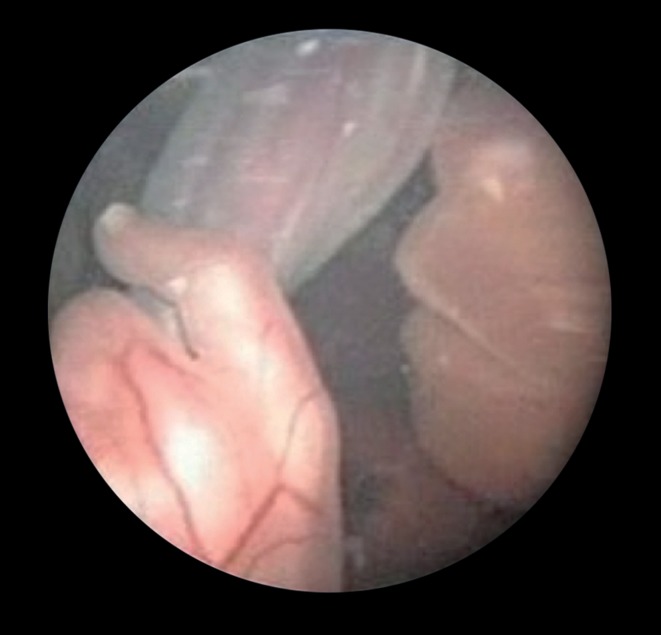
Figure 1.
Fetoscopic view of the face of a fetus. Umbilical cord, hand, and face are shown
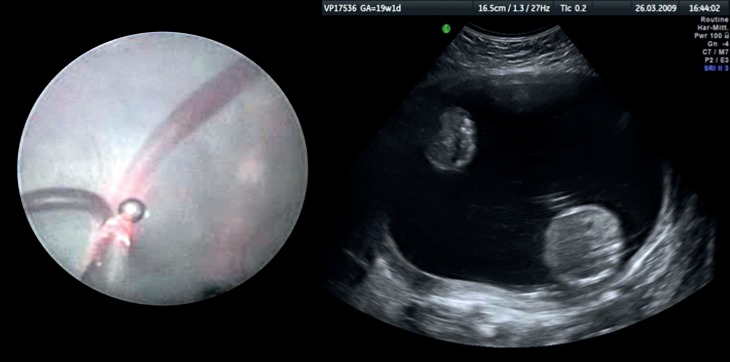
Figure 2: Twin-to-twin transfusion syndrome.
Left) Laser coagulation of a placental anastomosis; Right) typical ultrasound appearance: Below right) larger recipient with polyhydramnios (abdominal cross section); Above left) smaller donor with anhydramnios (stuck twin)
Table. The most important fetal surgery procedures.
| Indication | Treatment | Level of evidence | References |
| Twin-to-twin transfusion syndrome | Fetoscopic laser coagulation | I II | Senat et al. 2004 (5) |
| Hecher et al. 1999 (3) | |||
| Twin reversed arterial perfusion (TRAP) syndrome | Fetoscopic laser coagulation | II | Hecher et al. 2006 (10) |
| Myelomeningocele | Prenatal repair via hysterotomy | I | Adzick et al. 2011 (16) |
| Congenital diaphragmatic hernia | Fetoscopic tracheal occlusion | I | Harrison et al. 2003 (12) |
| I | Ruano et al. 2012 (15) | ||
| II | Jani et al. 2009 (13) | ||
| Aortic stenosis, pulmonary stenosis | Percutaneous valvuloplasty of the fetal aortic/pulmonary valve | III | Kohl et al. 2000 (20) |
| Tulzer et al. 2002 (21) | |||
| Tworetzky et al. 2009 (22) | |||
| Arzt et al. 2011 (19) | |||
| Sacrococcygeal teratomas | Fetoscopic laser obliteration of tumor vessels,high-frequency ablation | III | Hecher und Hackelöer 1996 (23) Makin et al. 2006 (OR) (e27) Paek al. 2001 (OR) (e25) Lee et al. 2011 (OR (e26) |
| Bilateral lower urinary tract obstruction | Percutaneous vesicoamniotic shunt Percutaneous cystoscopy (lasering of valves, bladder marsupialization, cystostomy) | III | Morris und Kilby 2009 (OR) (e33) |
| III | Morris et al. 2011 (24) | ||
| Congenital cystic adenomatoid malformation, pulmonary sequestration | Percutaneous sclerotherapy Fetoscopic laser coagulation | III | Bermudez et al. 2008 (OR) (e30) |
| III | Oepkes et al. 2007 (e34) |
Laser coagulation yielded significantly better results as assessed by the endpoints of fetal survival, gestational age at birth, and neurological outcome. At least one twin survived after 76% of laser procedures, compared with 56% after amniotic drainage (relative risk [RR] for death of both twins 0.63, 95% confidence interval [CI] 0.25–0.93, p = 0.009). The rates of neurological complications (31% vs. 52%, p = 0.003) and periventricular leukomalacia (6% vs. 14%, p = 0.02) were lower after laser coagulation (5).
Salomon and coworkers examined 120 children up to 6 years of age following laser coagulation and showed that the outcome was determined above all by the severity of the TTTS and by the type of treatment. Children who had received laser therapy displayed a significantly better development score than those treated by amniotic drainage (6).
It should be emphasized that laser coagulation of placental anastomoses directly treats the cause of the disease, while amniotic drainage merely represents symptomatic treatment of the polyhydramnios.
Specialized centers are now in the position to achieve survival rates of 90% for at least one twin and 70% for both twins (7, e3, e4). Similar results have been attained for triplets (survival of at least one fetus in 83% of cases, two in 72%, and all three in 39%) (8).
The rate of neurological late complications after 2 years was only 6% for severe and 7% for mild deficits in a study published in 2006 (4). Lopriore and colleagues found an incidence of 14% for distinct cerebral lesions on ultrasound in a group of neonates who had been treated by laser coagulation for TTTS, compared with 6% in a control group of monochorionic twins without TTTS (9). Another publication by Lopriore et al. stated an incidence of 7% for cerebral paresis and 17% for all neurological late complications (e5).
Twin reversed arterial perfusion syndrome
Another indication for fetoscopic laser ablation of placental anastomoses or coagulation of the umbilical cord by means of a bipolar forceps is twin reversed arterial perfusion (TRAP) syndrome. In this rare syndrome (incidence ca. 1 : 35 000 [e6]) affecting monochorionic twins, there is retrograde perfusion of one twin via an arterio-arterial and a veno-venous anastomosis between the two umbilical cord insertions. This frequently causes heart failure in the “pump” twin, leading to intrauterine fetal death or extreme prematurity. The other twin exhibits hydrops fetalis and fails to develop a heart (acardius) and, usually also, a head (acranius). Fetoscopic treatment comprises coagulation of the anastomoses or the umbilical cord of the acardiac twin. In a prospective study, this led to survival of the pump twin in 80% of cases, and two thirds of the survivors were born at the 37th week of gestation or later (10).
Congenital diaphragmatic hernia
Congenital diaphragmatic hernia occurs in between 1 in 2500 and 1 in 5000 births (e7, e8).The overwhelming majority of the affected fetuses show a left-sided diaphragmatic defect. The prognosis is limited above all by intrauterine pulmonary hypoplasia and the resulting pulmonary hypertonia. Nowadays more than two thirds of cases are diagnosed in utero. The classic sign of congenital diaphragmatic hernia is the presence of abdominal organs in the thorax, with rightward displacement of the mediastinum in fetuses that have a left-sided hernia.
Assessment of the individual prognosis is crucial in the planning of a possible intervention in the prenatal period. Besides ultrasonography (e9, e10), a major role is played by magnetic resonance imaging (MRI) of the fetus (e11). The most important parameter estimated by MRI is the residual lung volume, assessed with the aid of the sonographically measured observed to expected lung-to-head ratio (O/E L/H ratio).
Specialized centers offer fetoscopic endoluminal tracheal occlusion (FETO) to selected patients (Figure 3) (11). The accumulation of pulmonary fluid caused by the tracheal occlusion leads to an increase in intra-alveolar pressure, inducing proliferation of lung tissue (e12). One serious problem is the potential necessity of emergency removal of the balloon in the event of premature birth. For this reason, FETO patients have to stay in the vicinity of a specialized center.
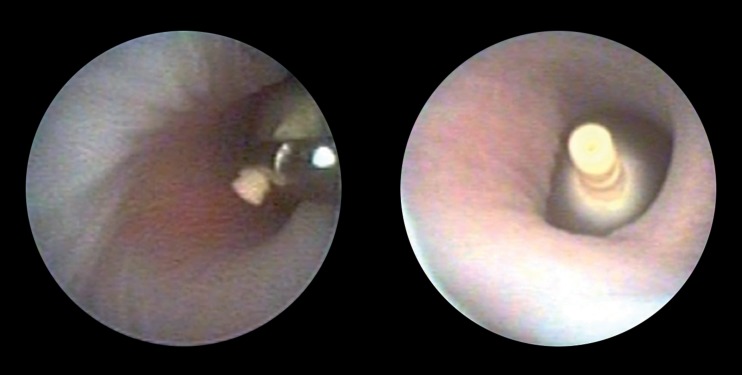
Figure 3.
Congenital diaphragmatic hernia: fetoscopic endoluminal tracheal occlusion (FETO)
Left) Fetoscopic balloon placement; Right) inflated balloon in the fatal trachea
In 2003 Harrison et al. published a small randomized trial of 24 patients in which they compared endoscopic balloon occlusion with purely postnatal treatment. They found no advantage of fetal treatment with regard to either 90-day survival (73% vs. 77%) or morbidity (12). It was shown, however, that particularly fetuses with an L/H ratio below 0.90 have a poor prognosis; these are the fetuses that are supposed to benefit most from prenatal intervention (12, 13).
Jani and coworkers reported their experience with FETO in a series of 210 patients treated in the period 2000 to 2008 (14). In comparison with registry data on the mortality of non-prenatally treated congenital diaphragmatic hernia, FETO achieved a much higher survival rate. After FETO, 49% of newborns treated for left-sided congenital diaphragmatic hernia left the hospital alive, compared to 24% without prenatal treatment. For right-sided defects the survival rate improved from 0% to 35%. Alternatively, a survival rate of 50% to 60% has been described for extracorporeal membrane oxygenation (ECMO) (e13). It remains questionable, however, whether the groups are comparable. Prematurity is a serious problem in FETO patients, one third of whom are born before the 34th week of gestation. To date, there are no evidence-based data on the long-term prognosis following FETO. Randomized trials are being conducted to compare FETO with conservative management. In one study fetuses with pronounced pulmonary hypoplasia (O/E L/H ratio ≤ 25%) are being treated by FETO in the 27th to 30th week of gestation, and in another trial fetuses with milder pulmonary hypoplasia are receiving FETO in the 30th to 32nd gestational week (www.totaltrial.eu). A recently published randomized trial with a small number of cases essentially confirmed the findings of Jani et al. (survival rate after FETO 52%, without FETO 5%) (15).
Meningomyelocele
Meningomyelocele is a congenital malformation involving herniation of the spinal medulla into a sac filled with cerebrospinal fluid. The incidence of myelomeningocele is around 1 in 800 pregnancies (e14). The damage to the spinal cord and peripheral nerves is generally not amenable to repair by postnatal surgery (e15).
Fetal surgery for correction of myelomeningocele was carried out for the first time in 1997, and by 2003 around 200 fetuses had been treated by open intrauterine surgery via hysterotomy. Early reports showed that the functional outcome was improved by regression of the herniation of the hindbrain, but that both mother and fetus were at much greater risk of complications (e16, e17).
A randomized trial on the repair of myelomeningocele was published in 2011 (16). The Management of Myelomeningocele Study (MOMS) compared the results of fetal surgery with the prevailing standard of postnatal correction. Fetuses in the 19th to 26th week of gestation with a myelomeningocele between spinal segments T1 and S1 were included in the study.
The intention-to-treat analysis revealed a significant reduction in the primary endpoint, i.e., the need for a shunt operation, in the fetal surgery group. Compared with 82% of the patients in the conventional treatment arm (postnatal repair), implantation of a CSF shunt was necessary in only 40% of those who had received prenatal surgery (RR 0.48, 95% CI 0.36–0.64, p = 0.001). Furthermore, prenatal surgery led to significantly better cognitive and motor development 30 months after birth. This difference persisted with increasing age: The proportion of children who could walk unaided at the age of 3 years was 42% after prenatal surgery and 21% after conventional postnatal surgery (RR 2.01, 95% CI 1.16–3.48, p = 0.01).
Notwithstanding these positive results, however, there were significantly higher rates of complications in the fetal surgery group with regard to prematurity: a 46% rate of premature rupture of membranes compared with 8% in the conventionally treated group, and a premature birth rate of 79% versus 15%. One third of the mothers in the fetal surgery group showed uterine dehiscence or an extremely thin uterine wall in the area of the hysterotomy.
In the MOMS, all procedures for prenatal repair of myelomeningocele were carried out at only three locations worldwide over a period of several years. A steep learning curve has to be assumed for these complex interventions, and extension to a high number of centers could have had a negative influence on the results. Moreover, it must be underlined that the patients in the MOMS were a highly selected group. Contraindications such as accompanying fetal malformations or illness of the mother prevented operation in 83% of the patients screened, and some women did not consent to randomization. Furthermore, the optimal time for prenatal repair of myelomeningocele remains controversial. Exchange of amniotic fluid to prevent neural toxicity has been successfully performed as an alternative treatment for myelomeningocele (e18), but there are no comparative data.
In summary, the MOMS shows that the outcome is improved by intrauterine surgery, but that attention needs to be concentrated on the development of minimally invasive endoscopic techniques. Fetoscopic intrauterine treatment of spina bifida has already been described in a small case series (17). The results were sobering, however; improved neurological function, but a high rate of complications such as intrauterine death, intraoperative hemorrhage, and pronounced prematurity (e19).
Rare/experimental indications
Congenital aortic stenosis
Congenital aortic stenosis (incidence ca. 1 in 5000 births) (e20) can lead to prenatal overexpansion of the left ventricle. This leads to deficient contractility and endocardial fibroelastosis of the left ventricle and secondarily to hypoplastic left heart syndrome.
Various groups have carried out prenatal balloon dilatation of the aortic valve to prevent hypoplastic left heart syndrome in small numbers of patients (18– 20, e21, e22). In one series of 23 fetuses, 70% of interventions were performed successfully and in 67% of these cases (10 of 23 fetuses = 43%) hypoplasia of the left ventricle could be prevented and thus biventricular circulation preserved (19). The intervention was carried out after the 20th week of gestation. The candidates are, above all, fetuses in which the echocardiographic findings point to a high risk for the development of hypoplastic left heart syndrome (e23). Given the small numbers of cases and the absence of randomized studies, fetal valvuloplasty still has to be regarded with caution.
Congenital pulmonary stenosis
Pulmonary stenosis with hypoplasia of the right ventricle (incidence ca. 1 in 6000 births) (e20) is another complex malformation of the heart with a poor prognosis. Percutaneous treatment by means of in-utero balloon valvuloplasty has been carried out successfully in small series of patients (21, 22). The hypoplasia of the right ventricle and the pulmonary valve was corrected by in-utero valvuloplasty in 6 of 10 cases (22).
Sacrococcygeal teratoma
Another indication for fetal surgery is the obliteration of tumor vessels in sacrococcygeal teratomas (23). These tumors (incidence ca. 1 in 27 000 births) (e24) are highly vascularized and contain numerous arteriovenous shunts. In a few cases tumor growth was halted or even reversed by fetoscopic laser coagulation of the tumor vessels. Another method that can be used is fetoscopic radiofrequency ablation (e25– e27).
Congenital cystic adenomatoid malformation of the lung and pulmonary sequestration
These rare malformations (incidence ca. 1 in 8000 births) (e28) belong to the hamartomas. Large pulmonary cysts have been successfully treated by means of open hysterotomy (e29). Recently, percutaneous sclerotherapy of the cysts has also been performed successfully in a few cases (e30). Given the small numbers of cases and the absence of randomized studies, these treatments still have to be regarded with caution. A non-invasive treatment option for the microcystic form of congenital cystic adenomatoid malformation (CCAM) is administration of steroids that cross the placenta, with success rates of 60% to 70% (e31).
Infravesical obstruction
Early bilateral lower urinary tract obstruction (incidence ca. 1 in 1500 births) (e32) leads to anhydramnios with subsequent pulmonary hypoplasia and kidney failure. Case series of insertion of a vesicoamniotic shunt via a pigtail catheter have been reported. An ongoing randomized trial (PLUTO) is investigating the long-term effect in selected patients (24, e33), particularly with regard to the potential avoidance of kidney failure.
The new fetal surgery procedures must be compared with the established postnatal treatments, ideally in randomized prospective studies.
Key Messages.
Fetal surgery is indicated solely for diseases that can lead to intrauterine fetal death or irreversible damage to the fetus.
Fetal surgery interventions are divided into minimally invasive fetoscopic procedures and open surgery.
In twin-to-twin transfusion syndrome, fetoscopic laser coagulation of placental anastomoses is the gold standard.
Randomized studies have demonstrated that fetoscopic laser coagulation in twin-to-twin transfusion syndrome and intrauterine surgery of spina bifida via hysterotomy improve the prognosis of the affected fetuses.
Given the absence of randomized trials, no other fetoscopic treatments are yet universally indicated; however, the parents should be informed about the possibility of intrauterine surgery and referred to an appropriate center.
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.De Lia JE, Cruikshank DP, Keye WR. Fetoscopic neodymium:YAG laser occlusion of placental vessels in severe twin-twin transfusion syndrome. Obstet Gynecol. 1990;75:1046–1053. [PubMed] [Google Scholar]
- 2.Ville Y, Hecher K, Ogg D, Warren R, Nicolaides K. Successful outcome after Nd: YAG laser separation of chorioangiopagus-twins under sonoendoscopic control. Ultrasound Obstet Gynecol. 1992;2:429–431. doi: 10.1046/j.1469-0705.1992.02060429.x. [DOI] [PubMed] [Google Scholar]
- 3.Hecher K, Plath H, Bregenzer T, Hansmann M, Hackelöer BJ. Endoscopic laser surgery versus serial amniocenteses in the treatment of severe twin-twin transfusion syndrome. Am J Obstet Gynecol. 1999;180:717–724. doi: 10.1016/s0002-9378(99)70278-4. [DOI] [PubMed] [Google Scholar]
- 4.Graef C, Ellenrieder B, Hecher K, Hackeloer BJ, Huber A, Bartmann P. Long-term neurodevelopmental outcome of 167 children after intrauterine laser treatment for severe twin-twin transfusion syndrome. Am J Obstet Gynecol. 2006;194:303–308. doi: 10.1016/j.ajog.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 5.Senat M-V, Deprest J, Boulvain M, Paupe A, Winer N, Ville Y. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl J Med. 2004;351:136–144. doi: 10.1056/NEJMoa032597. [DOI] [PubMed] [Google Scholar]
- 6.Salomon LJ, Ortqvist L, Aegerter P, et al. Long-term developmental follow-up of infants who participated in a randomized clinical trial of amniocentesis vs laser photocoagulation for the treatment of twin-to-twin transfusion syndrome. Am J Obstet Gynecol. 2010;203-444:e1–e7. doi: 10.1016/j.ajog.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 7.Chalouhi GE, Essaoui M, Stirnemann J, et al. Laser therapy for twin-to-twin transfusion syndrome (TTTS) Prenat Diagn. 2011;31:637–646. doi: 10.1002/pd.2803. [DOI] [PubMed] [Google Scholar]
- 8.Diemert A, Diehl W, Huber A, Glosemeyer P, Hecher K. Laser therapy of twin-to-twin transfusion syndrome in triplet pregnancies. Ultrasound Obstet Gynecol. 2010;35:71–74. doi: 10.1002/uog.7328. [DOI] [PubMed] [Google Scholar]
- 9.Lopriore E, Sueters M, Middeldorp JM, Oepkes D, Vandenbussche FP, Walther FJ. Neonatal outcome in twin-to-twin transfusion syndrome treated with fetoscopic laser occlusion of vascular anastomoses. J Pediatr. 2005;147:597–602. doi: 10.1016/j.jpeds.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Hecher K, Lewi L, Gratacos E, Huber A, Ville Y, Deprest J. Twin reversed arterial perfusion: fetoscopic laser coagulation of placental anastomoses or the umbilical cord. Ultrasound Obstet Gynecol. 2006;28:688–691. doi: 10.1002/uog.3816. [DOI] [PubMed] [Google Scholar]
- 11.FETO Task Group. Deprest J, Gratacos E, Nicolaides KH. Fetoscopic tracheal occlusion (FETO) for severe congenital diaphragmatic hernia: evolution of a technique and preliminary results. Ultrasound Obstet Gynecol. 2004;24:121–126. doi: 10.1002/uog.1711. [DOI] [PubMed] [Google Scholar]
- 12.Harrison MR, Keller RL, Hawgood SB, Kitterman JA, Sandberg PL, Farmer DL, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med. 2003;349:1916–1924. doi: 10.1056/NEJMoa035005. [DOI] [PubMed] [Google Scholar]
- 13.Jani JC, Benachi A, Nicolaides KH, et al. Prenatal prediction of neonatal morbidity in survivors with congenital diaphragmatic hernia: a multicenter study. Ultrasound Obstet Gynecol. 2009;33:64–69. doi: 10.1002/uog.6141. [DOI] [PubMed] [Google Scholar]
- 14.Jani JC, Nicolaides KH, Gratacos E, et al. Severe diaphragmatic hernia treated by fetal endoscopic tracheal occlusion. Ultrasound Obstet Gynecol. 2009;34:304–310. doi: 10.1002/uog.6450. [DOI] [PubMed] [Google Scholar]
- 15.Ruano R, Yoshisaki CT, da Silva MM, et al. A randomized controlled trial of fetal endoscopic tracheal occlusion versus postnatal management of severe isolated congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. 2012;39:20–27. doi: 10.1002/uog.10142. [DOI] [PubMed] [Google Scholar]
- 16.Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364:993–1004. doi: 10.1056/NEJMoa1014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohl T, Hering R, Heep A, et al. Percutaneous fetoscopic patch coverage of spina bifida aperta in the human—early clinical experience and potential. Fetal Diagn Ther. 2006;21:185–193. doi: 10.1159/000089301. [DOI] [PubMed] [Google Scholar]
- 18.Tworetzky W, Wilkins-Haug L, Jennings RW, et al. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Circulation. 2004;110:2125–2131. doi: 10.1161/01.CIR.0000144357.29279.54. [DOI] [PubMed] [Google Scholar]
- 19.Arzt W, Wertaschnigg D, Veit I, Klement F, Gitter R, Tulzer G. Intrauterine aortic valvuloplasty in fetuses with critical aortic stenosis: experience and results of 24 procedures. Ultrasound Obstet Gynecol. 2011;37:689–695. doi: 10.1002/uog.8927. [DOI] [PubMed] [Google Scholar]
- 20.Kohl T, Sharland G, Allan LD, et al. World experience of percutaneous ultrasound-guided balloon valvuloplasty in human fetuses with severe aortic valve obstruction. Am J Cardiol. 2000;85:1230–1233. doi: 10.1016/s0002-9149(00)00733-5. [DOI] [PubMed] [Google Scholar]
- 21.Tulzer G, Arzt W, Franklin RCG, Loughna PV, Mair R, Gardiner HM. Fetal pulmonary valvuloplasty for critical pulmonary stenosis or atresia with intact septum. Lancet. 2002;360:1567–1568. doi: 10.1016/S0140-6736(02)11531-5. [DOI] [PubMed] [Google Scholar]
- 22.Tworetzky W, McElhinney DB, Marx GR, et al. In utero valvuloplasty for pulmonary atresia with hypoplastic right ventricle: techniques and outcomes. Pediatrics. 2009;124:e510–e518. doi: 10.1542/peds.2008-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hecher K, Hackelöer BJ. Intrauterine endoscopic laser surgery for fetal sacrococcygeal teratoma. Lancet. 1996;347 doi: 10.1016/s0140-6736(96)90045-8. [DOI] [PubMed] [Google Scholar]
- 24.Morris RK, Ruano R, Kilby MD. Effectiveness of fetal cystoscopy as a diagnostic and therapeutic intervention for lower urinary tract obstruction: a systematic review. Ultrasound Obstet Gynecol. 2011;37:629–637. doi: 10.1002/uog.8981. [DOI] [PubMed] [Google Scholar]
- e1.Luks FI. New and/or improved aspects of fetal surgery. Prenatal Diagnosis. 2011;31:252–258. doi: 10.1002/pd.2706. [DOI] [PubMed] [Google Scholar]
- e2.Lewi L, Gucciardo L, Huber A. Clinical outcome and placental characteristics of monochorionic diamniotic twin pairs with early- and late-onset discordant growth. American Journal of Obstetrics & Gynecology. 2008;199:511.11–511.17. doi: 10.1016/j.ajog.2008.04.022. [DOI] [PubMed] [Google Scholar]
- e3.Sago H, Hayashi S, Saito M, et al. The outcome and prognostic factors of twin-twin transfusion syndrome following fetoscopic laser surgery. Prenatal Diagnosis. 2010;30:1185–1191. doi: 10.1002/pd.2647. [DOI] [PubMed] [Google Scholar]
- e4.Papanna R, Biau DJ, Mann LK, et al. Use of the Learning Curve-Cumulative Summation test for quantitative and individualized assessment of competency of a surgical procedure in obstetrics and gynecology: fetoscopic laser ablation as a model. American Journal of Obstetrics and Gynecology. 2011;204(218):e1–e9. doi: 10.1016/j.ajog.2010.10.910. [DOI] [PubMed] [Google Scholar]
- e5.Lopriore E, Middeldorp JM, Sueters M, et al. Long-term neurodevelopmental outcome in twin-to-twin transfusion syndrome treated with fetoscopic laser surgery. American Journal of Obstetrics and Gynecology. 2007;196(231):e1–e4. doi: 10.1016/j.ajog.2006.10.906. [DOI] [PubMed] [Google Scholar]
- e6.Gillim DL, Hendricks CH. Holoacardius; review of the literature and case report. Obstetrics and Gynecology. 1953;2(6):647–653. [PubMed] [Google Scholar]
- e7.Harrison MR, Bjordal RI, Langmark F, Knutrud O. Congenital diaphragmatic hernia: the hidden mortality. Journal of Pediatric Surgery. 1978;13:227–230. doi: 10.1016/s0022-3468(78)80391-1. [DOI] [PubMed] [Google Scholar]
- e8.Yang W, Carmichael SL, Harris JA, Shaw GM. Epidemiologic characteristics of congenital diaphragmatic hernia among 25. million California births, 1989-1997. Birth defects research. Part A, Clinical and molecular teratology. 2006;76:170–174. doi: 10.1002/bdra.20230. [DOI] [PubMed] [Google Scholar]
- e9.Beaudoin S, Bargy F, Mahieu D, Barbet P. Anatomic study of the umbilical vein and ductus venosus in human fetuses: ultrasound application in prenatal examination of left congenital diaphragmatic hernia. Surgical and Radiologic Anatomy. 1998;20:99–103. doi: 10.1007/BF01628909. [DOI] [PubMed] [Google Scholar]
- e10.Doné E, Allegaert K, Lewi P, et al. Maternal hyperoxygenation test in fetuses undergoing FETO for severe isolated congenital diaphragmatic hernia. Ultrasound in Obstetrics & Gynecology. 2011;37:264–271. doi: 10.1002/uog.7753. [DOI] [PubMed] [Google Scholar]
- e11.Jani JC, Benachi A, Nicolaides KH, et al. Prenatal prediction of neonatal morbidity in survivors with congenital diaphragmatic hernia: a multicenter study. Ultrasound Obstet Gynecol. 2009;33:64–69. doi: 10.1002/uog.6141. [DOI] [PubMed] [Google Scholar]
- e12.Deprest J, Nicolaides K, Doné E, et al. Technical aspects of fetal endoscopic tracheal occlusion for congenital diaphragmatic hernia. Journal of Pediatric Surgery. 2011;46:22–32. doi: 10.1016/j.jpedsurg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- e13.Schaible T, Hermle D, Loersch F, Demirakca S, Reinshagen K, Varnholt V. A 20-year experience on neonatal extracorporeal membrane oxygenation in a referral center. Intensive Care Medicine. 2010;36:1229–1234. doi: 10.1007/s00134-010-1886-5. [DOI] [PubMed] [Google Scholar]
- e14.Shin M, Besser LM, Siffel C, et al. Prevalence of spina bifida among children and adolescents in 10 regions in the United States. Pediatrics. 2010;126:274–279. doi: 10.1542/peds.2009-2084. [DOI] [PubMed] [Google Scholar]
- e15.Mitchell LE, Adzick NS, Melchionne J, Pasquariello PS, Sutton LN, Whitehead AS. Spina bifida. Lancet. 2004;364:1885–1895. doi: 10.1016/S0140-6736(04)17445-X. [DOI] [PubMed] [Google Scholar]
- e16.Tulipan N, Bruner JP, Hernanz-Schulman M, et al. Effect of intrauterine myelomeningocele repair on central nervous system structure and function. Pediatr Neurosurg. 1999;31(4):183–188. doi: 10.1159/000028859. [DOI] [PubMed] [Google Scholar]
- e17.Bouchard S, Davey MG, Rintoul NE, Walsh DS, Rorke LB, Adzick NS. Correction of hindbrain herniation and anatomy of the vermis after in utero repair of myelomeningocele in sheep. Journal of Pediatric Surgery. 2003;38:451–458. doi: 10.1053/jpsu.2003.50078. [DOI] [PubMed] [Google Scholar]
- e18.Olguner M, Akgür FM, Ozdemir T, Aktuð T, Ozer E. Amniotic fluid exchange for the prevention of neural tissue damage in myelomeningocele: an alternative minimally invasive method to open in utero surgery. Pediatric Neurosurgery. 2000;33:252–256. doi: 10.1159/000055964. [DOI] [PubMed] [Google Scholar]
- e19.Verbeek RJ, Heep A, Maurits NM, et al. Fetal endoscopic myelomeningocele closure preserves segmental neurological function. Developmental Medicine and Child Neurology. 2012;54:15–22. doi: 10.1111/j.1469-8749.2011.04148.x. [DOI] [PubMed] [Google Scholar]
- e20.Hoffman JIE, Kaplan S. The incidence of congenital heart disease. Journal of the American College of Cardiology. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- e21.Wilkins-Haug LE, Tworetzky W, Benson CB, et al. Factors affecting technical success of fetal aortic valve dilation. Ultrasound in Obstetrics & Gynecology. 2006;28:47–52. doi: 10.1002/uog.2732. [DOI] [PubMed] [Google Scholar]
- e22.McElhinney DB, Marshall AC, Wilkins-Haug LE, et al. Predictors of technical success and postnatal biventricular outcome after in utero aortic valvuloplasty for aortic stenosis with evolving hypoplastic left heart syndrome. Circulation. 2009;120(15):1482–1490. doi: 10.1161/CIRCULATIONAHA.109.848994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e23.Mäkikallio K, McElhinney DB, Levine JC, et al. Fetal aortic valve stenosis and the evolution of hypoplastic left heart syndrome: patient selection for fetal intervention. Circulation. 2006;113:1401–1405. doi: 10.1161/CIRCULATIONAHA.105.588194. [DOI] [PubMed] [Google Scholar]
- e24.Swamy R, Embleton N, Hale J. Sacrococcygeal teratoma over two decades: birth prevalence, prenatal diagnosis and clinical outcomes. Prenatal Diagnosis. 2008;28:1048–1051. doi: 10.1002/pd.2122. [DOI] [PubMed] [Google Scholar]
- e25.Paek BW, Jennings RW, Harrison MR, et al. Radiofrequency ablation of human fetal sacrococcygeal teratoma. American Journal of Obstetrics and Gynecology. 2001;184:503–507. doi: 10.1067/mob.2001.110446. [DOI] [PubMed] [Google Scholar]
- e26.Lee M-Y, Won H-S, Hyun M-K, et al. Perinatal outcome of sacrococcygeal teratoma. Prenatal Diagnosis. 2011;31:1217–1221. doi: 10.1002/pd.2865. [DOI] [PubMed] [Google Scholar]
- e27.Makin EC, Hyett J, Ade-Ajayi N, et al. Outcome of antenatally diagnosed sacrococcygeal teratomas: single-center experience (1993-2004) Journal of Pediatric Surgery. 2006;41:388–393. doi: 10.1016/j.jpedsurg.2005.11.017. [DOI] [PubMed] [Google Scholar]
- e28.Shanti CM, Klein MD. Cystic lung disease. Seminars in Pediatric Surgery. 2008;17:2–8. doi: 10.1053/j.sempedsurg.2007.10.002. [DOI] [PubMed] [Google Scholar]
- e29.Harrison MR, Adzick NS, Jennings RW, et al. Antenatal intervention for congenital cystic adenomatoid malformation. Lancet. 1990;336(8721):965–967. doi: 10.1016/0140-6736(90)92420-m. [DOI] [PubMed] [Google Scholar]
- e30.Bermúdez C, Pérez-Wulff J, Arcadipane M, et al. Percutaneous fetal sclerotherapy for congenital cystic adenomatoid malformation of the lung. Fetal Diagnosis and Therapy. 2008;24:237–240. doi: 10.1159/000151345. [DOI] [PubMed] [Google Scholar]
- e31.Curran PF, Jelin EB, Rand L, et al. Prenatal steroids for microcystic congenital cystic adenomatoid malformations. Journal of Pediatric Surgery. 2010;45:145–150. doi: 10.1016/j.jpedsurg.2009.10.025. [DOI] [PubMed] [Google Scholar]
- e32.Morin L, Cendron M, Crombleholme TM, et al. Minimal hydronephrosis in the fetus: clinical significance and implications for management. The Journal of Urology. 1996;155:2047–2049. doi: 10.1016/s0022-5347(01)66102-0. [DOI] [PubMed] [Google Scholar]
- e33.Morris RK, Kilby MD. An overview of the literature on congenital lower urinary tract obstruction and introduction to the PLUTO trial: percutaneous shunting in lower urinary tract obstruction. The Australian & New Zealand Journal of Obstetrics & Gynaecology, 2009;49:6–10. doi: 10.1111/j.1479-828X.2008.00940.x. [DOI] [PubMed] [Google Scholar]
- e34.Oepkes D, Devlieger R, Lopriore E, Klumper FJCM. Successful ultrasound-guided laser treatment of fetal hydrops caused by pulmonary sequestration. Ultrasound Obstet Gynecol. 2007;29:457–459. doi: 10.1002/uog.3984. [DOI] [PubMed] [Google Scholar]