Abstract
The enzyme-linked immunosorbent assay (ELISA) has long been the primary tool for detection of analytes of interest in biological samples for both life science research and clinical diagnostics. However, ELISA has limitations. It is typically performed in a 96-well microplate, and the wells are coated with capture antibody, requiring a relatively large amount of sample to capture an antigen of interest . The large surface area of the wells and the hydrophobic binding of capture antibody can also lead to non-specific binding and increased background. Additionally, most ELISAs rely upon enzyme-mediated amplification of signal in order to achieve reasonable sensitivity. Such amplification is not always linear and can thus skew results.
In the past 15 years, a new technology has emerged that offers the benefits of the ELISA, but also enables higher throughput, increased flexibility, reduced sample volume, and lower cost, with a similar workflow 1, 2. Luminex xMAP Technology is a microsphere (bead) array platform enabling both monoplex and multiplex assays that can be applied to both protein and nucleic acid applications 3-5. The beads have the capture antibody covalently immobilized on a smaller surface area, requiring less capture antibody and smaller sample volumes, compared to ELISA, and non-specific binding is significantly reduced. Smaller sample volumes are important when working with limiting samples such as cerebrospinal fluid, synovial fluid, etc. 6. Multiplexing the assay further reduces sample volume requirements, enabling multiple results from a single sample.
Recent improvements by Luminex include: the new MAGPIX system, a smaller, less expensive, easier-to-use analyzer; Low-Concentration Magnetic MagPlex Microspheres which eliminate the need for expensive filter plates and come in a working concentration better suited for assay development and low-throughput applications; and the xMAP Antibody Coupling (AbC) Kit, which includes a protocol, reagents, and consumables necessary for coupling beads to the capture antibody of interest. (See Materials section for a detailed list of kit contents.)
In this experiment, we convert a pre-optimized ELISA assay for TNF-alpha cytokine to the xMAP platform and compare the performance of the two methods 7-11. TNF-alpha is a biomarker used in the measurement of inflammatory responses in patients with autoimmune disorders.
We begin by coupling four candidate capture antibodies to four different microsphere sets or regions. When mixed together, these four sets allow for the simultaneous testing of all four candidates with four separate detection antibodies to determine the best antibody pair, saving reagents, sample and time. Two xMAP assays are then constructed with the two most optimal antibody pairs and their performance is compared to that of the original ELISA assay in regards to signal strength, dynamic range, and sensitivity.
Keywords: Molecular Biology, Issue 65, Luminex, xMAP, Multiplex, MAGPIX, MagPlex Low Concentration Microspheres, xMAP Antibody Coupling Kit, ELISA, Immunoassay, Antibody Screening, Optimization, Conversion
Protocol
I. Reagent Preparation
- Antibody Selection and Preparation
- Identify the antibodies to be used in the experiment.
- Four capture antibodies: specific for human TNF-alpha, either monoclonal or polyclonal; all the same host species.
- Four detection antibodies: specific for human TNF-alpha, either unmodified, biotinylated, or PE-conjugated.
- One confirmation antibody: PE-conjugated and specific for the host species of the capture antibodies.
- Reconstitute all antibodies to the manufacturer-recommended working concentrations.
- Select four vials of Low Concentration MagPlex microsphere (bead) sets or regions, for example, Luminex part numbers MC10012-ID, MC10013-ID, MC10014-ID, and MC10015-ID.
- Coupling Antibodies to MagPlex Microspheres, using the xMAP Antibody (AbC) Coupling Kit Refer to the AbC Kit User's Manual (Part# 89-00002-00-319) for the complete coupling procedure. (Note: Photosensitive microspheres should be protected from light whenever possible.)
- Bring the reagents in the AbC kit to room temperature and label four reaction tubes with the bead region numbers selected for the coupling reaction. Transfer the contents of the four vials of MagPlex beads (1 mL each or 2.5 x 106 beads) into the four labeled reaction tubes.
- Wash each of the bead sets twice in 500 μL of Activation Buffer, as described in the AbC kit manual.
- Activate each bead set with 480 μL of Activation buffer, 10 μL of Sulfo-NHS, and 10 μL of the EDC reagent, according to the procedure in the AbC kit manual, and incubate for 20 minutes. (Note: EDC reagent must be reconstituted in 250 μL of Activation Buffer immediately prior to this step.)
- Repeat the previous wash step with the now "activated" microspheres a total of three times with 500 μL of Activation Buffer, as described in the AbC kit manual.
- Prepare four separate solutions, each containing 7.5 μg (i.e., 3 μg/million microspheres) of capture antibody in Activation buffer.
- Add these four capture antibody solutions to their respective reaction tubes, vortex each tube immediately, and incubate for two hours on a rotator.
- Repeat the previous wash step with the now "coupled" microspheres a total of three times with 500 μL of the Wash Buffer included with the AbC kit.
- After the final wash step, add 500 μL Wash Buffer to each reaction tube to provide a final stock concentration of 5 million antibody-coupled beads per milliliter. Vortex and sonicate the reaction tubes to disperse the microspheres. NOTE: Partially submerging the closed microsphere vial in an ultrasonic cleaner filled with DI water provides effective sonication for all wash steps. (See Materials table for equipment details.)
- Store the coupled beads at 2-8 °C and protected from light until needed.
- Enumeration of Coupled Microspheres
- Count the number of microspheres recovered after the coupling reaction using a cell counter or hemacytometer. Refer to the counting instrument's user's manual for appropriate instructions for doing so. The recovery from the coupling reaction is typically over 90%.
- Coupling Confirmation
- Confirm the coupling reaction was successful by preparing test solutions of the coupled bead stocks for each set, with the final concentration of 100 beads/μL in Assay Buffer (PBS with 1% BSA). Prepare dilutions of the phycoerythrin- (PE-) labeled anti-species IgG confirmation antibody at 4 μg/mL in Assay Buffer.
- Aliquot 50 μL of each test solution into four wells of a round-bottom, 96-well plate, for a total of 16 wells. Then add 50 μL of Assay Buffer into eight of the wells, to measure background, and 50 μL of diluted confirmation antibody into the eight remaining wells.
- Mix the reactions gently by pipetting up and down several times with a multi-channel pipettor. Cover the plate, and incubate for 30 minutes at room temperature on a plate shaker.
- Place the plate on a magnetic plate separator for 1-2 minutes to draw the beads out of solution. Then, remove the liquid by forcefully inverting the plate, while on the separator, over a waste receptacle.
- Wash each well twice by adding 100 μL of Assay Buffer and removing the supernatant from the plate in a similar fashion using the magnetic plate separator.
- Resuspend the beads in 100 μL of Assay Buffer by gently pipetting up and down five times with a multi-channel pipettor.
- Analyze on a Luminex xMAP instrument, such as the MAGPIX Instrument. The intensity of the fluorescent signal of this reaction is directly proportional to the amount of protein on the surface of the beads, providing a rapid assessment of the relative amount of protein coupled to the beads.
- Coupling Biotin to Unmodified Antibodies for Detection
- If using unmodified detection antibodies, biotinylate these antibodies with the Thermo Fisher EZ-Link Sulfo-NHS-LC-Biotin Reagent (Cat. No. PI-21335) and the procedure described in the package insert. Once biotinylated, the detection antibodies can later be labeled with streptavidin phycoerythrin (SA-PE) in the assay (Step III.6.) so that they can be detected with the xMAP analyzer.
II. Assay Setup
Prepare an initial mixture of all four bead sets by adding 10 μL of each to 0.96 mL of PBS with 1% BSA (Assay Buffer. see Materials table) to determine which is most effective with the xMAP Assay.
Prepare the detection antibody solutions by diluting each to 1 μg/mL in Assay Buffer.
Prepare the R&D Systems TNF-α protein standard at 2000 pg/mL in Assay Buffer.
Dilute the Streptavidin-rPhycoerythrin (SA-PE) (provided @ 1 mg/mL) to 8 μg/mL in Assay Buffer.
III. Antibody Screening
Add 50 μL of the antibody-coupled microsphere mixture to each of 16 wells of a Costar round-bottom 96-well plate for the screening assay.
Add 50 μL of Assay Buffer to 8 of the 16 wells, to measure background.
Add 50 μL of R&D Systems TNF-α standard (@ 2,000 pg/mL) to the other 8 wells, to measure response.
Incubate for one hour at room temperature, protected from light, while shaking on an assay plate shaker.
Add 50 μL of each of the four detection antibodies to four wells (two background and two response) and incubate for 30 minutes at room temperature, protected from light, while shaking on an assay plate shaker.
Add 50 μL of the SA-PE reagent to all wells and incubate 15 minutes at room temperature, protected from light, while shaking on an assay plate shaker.
Place the plate on a magnetic plate separator for one minute and then remove the liquid by forcefully inverting the plate.
Add 100 μL Assay Buffer to each of the 16 wells, place the plate on the magnetic plate separator for one minute and then remove the liquid by forcefully inverting the plate while on the separator.
Add 100 μL of Assay Buffer to each of the 16 wells and read the plate with the Luminex MAGPIX instrument; referring to the user's manual for proper operation.
Select an antibody pair that meets your desired signal strength.
IV. xMAP Functional Assay
After selecting the best capture antibody, dilute 100 μL of that antibody-coupled bead stock (from Step I.B.8) to 10 mL with Assay Buffer.
Add 50 μL of the diluted beads to 78 wells of two Costar round-bottom 96-well plates. (78 wells x 2 plates = 156 wells) Each plate will be used to assess the performance of a different detection antibody.
Prepare a 12-point standard curve, beginning at 8000 pg/mL and ending at 4 pg/mL, with the R&D Systems TNF-α standard. Add six 50 μL replicates of each dilution to each of the plates, plus six wells with 50 μL of Assay Buffer each, as a background, for a total of 78 wells/plate.
Incubate the plates for one hour at room temperature, protected from light, while shaking on an assay plate shaker.
Add 50 μL of the first detection antibody to all 78 wells of the first plate. Repeat for the second detection antibody on the second plate.
Incubate the plates for 30 minutes, at room temperature, protected from light while shaking on an assay plate shaker.
Add the 50 μL of the SA-PE reagent to all wells of each plate.
Incubate the two plates for 15 minutes, at room temperature, protected from light while shaking on an assay plate shaker.
Place the plates on magnetic plate separators for one minute, then remove the liquid by forcefully inverting the plate while on the separator.
Add 100 μL of Assay Buffer to each of the 78 wells on the plates; place the plates on magnetic plate separators for one minute, then remove the liquid by forcefully inverting the plate.
Add 100 μL of Assay Buffer to each of the 78 wells on the plates and analyze on the MAGPIX instrument, referring to the user's manual for proper operation.
V. ELISA Assay
Following the instructions included with the R&D Systems Human TNF-α/TNFSF1A DuoSet ELISA kit (R&D Part# DY210), measure the response generated by the standard provided with the R&D kit. Repeat the ELISA assay three more times, substituting the R&D Systems capture and detection antibodies with the antibodies of the other vendors. For simplicity, pair the antibodies by vendor (e.g., Millipore capture antibody with Millipore detection antibody, Abcam capture antibody with Abcam detection antibody, etc.).
Evaluate each pair independently, as required by the ELISA format, with each of the three TNF-α protein standards.
VI. Representative Results
This protocol demonstrates how a typical ELISA can be converted to the xMAP platform while utilizing the multiplex capability of the technology to rapidly optimize the assay. The ELISA used in this example was the Human Tumor Necrosis Factor-alpha (TNF-α) DuoSet ELISA kit from R&D Systems (R&D Part# DY210).
In addition to the antibody pair provided in the kit, three other antibody pairs from different sources (see the Materials table) were evaluated simultaneously using the xMAP platform. Four of the antibodies were designated as capture antibodies and were coupled to MagPlex Low Concentration Microspheres. The other four antibodies were designated as detection antibodies; three of which were purchased as biotin-coupled and the fourth was biotinylated as described in the Protocol.
The antibodies for this study were chosen based on availability and vendor. However, in practical setting, antibodies should be chosen based on the individual user's preference and past performance experience with that antibody. Although, this experiment does not test the suitability of an antibody as a capture antibody versus detection antibody, this protocol can be easily modified for that purpose.
The Luminex xMAP assays were performed as a multiplex to evaluate all four capture antibodies as a mixture, by combining four sets of TNF-α antibody coupled MagPlex microspheres. The capture antibodies were evaluated with each of the four biotinylated detection antibodies individually, such that the interaction of one detection antibody with each of the four capture antibodies could be determined simultaneously. Four such assays, performed in parallel, determined the interactions of all four detection antibodies with all four capture antibodies. Figure 1 shows the comparative data from these screening assays.
The results indicated that the antibody pair from the R&D Systems DuoSet performed best with a resulting response at 6183 Median Fluorescence Intensity (MFI) units. It was also observed that the detection antibodies from Millipore (86% of the R&D antibody pair response) and Abcam (67%) provided a reasonable response in the xMAP assay, when combined with the R&D Systems capture antibody. The capture antibodies from Abcam, Millipore and Novus produced a less desirable response in the xMAP assay.
It is important to note, that the purpose of this study is not necessarily to highlight differences between particular antibodies or vendors but merely to illustrate that there are observable differences in their performance when used under similar conditions, and that the xMAP platform can offer an efficient means of assessing these differences.
The R&D Systems DuoSet protocol was used to compare the four antibody pairs in an ELISA format. The R&D Systems protocol was used with all antibody pairs because it is reflective of typical ELISA protocols widely used today and it is analogous to protocols used with xMAP technology. The ELISA tests showed that the antibody pair from R&D Systems again gave the best results (Figure 2). The antibody pair from Abcam produced no response and the antibody pairs from Millipore and Novus produced modest responses.
In order to assess any variation in antibody reactivity with the standard, all four antibody pairs were tested with three different recombinant TNF-α protein standards, from three different vendors (see the Materials table). The data in Figure 2 show that the recombinant TNF-α protein standards from the three vendors gave equivalent results.
The TNF-α protein from R&D Systems was used to generate standard curves with the ELISA (Table 1) and the xMAP assays (Tables 2 & 3). While the ELISA assay was done with the R&D Systems antibody pair, the xMAP assays utilized the R&D Systems capture antibody and either the detection antibody from R&D Systems or Millipore. The TNF-α protein from R&D Systems was diluted to produce a range of concentrations from 8000 to 4 pg/mL. Only the antibody pair from R&D Systems produced the expected outcome in the xMAP assay, with a response >20,000 MFI, as shown in Table 2 and Figure 3. When the detection antibody from Millipore was used with the xMAP Assay in place of the R&D Systems detection antibody (Table 3), the response (6,000 MFI) was about 30% of the response obtained with the detection antibody from R&D Systems; as shown in Figure 3.
The data in Table 1 represents the standard curve of the ELISA, which had a manufacturer's recommended TNF-α range of 16 to 1000 pg/mL. This range was very limited because the OD at 1000 pg/mL was slightly greater than 2 OD units and the spectrophotometer is unable to measure above 3 OD. Because of the limit with the spectrophotometer, it was not possible to increase the range of the ELISA assay further. Additionally, the data in Table 1 indicate that the R&D Systems DuoSet ELISA is not capable of detecting TNF-α at concentrations much less than 16 pg/mL. On the other hand, the xMAP assay is capable of measuring TNF-α at a concentration of less than 7.8 pg/mL with the capture antibody from R&D Systems combined with the detection antibody from either R&D Systems or Millipore.
The dynamic range and sensitivity of both methods is better illustrated when plotted in a log-log scale (Figure 4). A clear distinction between the slope of the ELISA responses and the responses from the xMAP assays can be seen that further indicates a more limiting capability for detection of TNF-α with the ELISA, at both higher and lower concentrations.
The limits of detection (LOD) for the two functional TNF-α xMAP assays were approximated by identifying the lowest TNF-α concentration with an observed response level (MFI) greater than background, plus three times its standard deviation (SD). To achieve statistical significance, six replicates were used to determine the SD for both the xMAP and ELISA methods. These estimates of LOD are optimistic and intended to provide a "best-case" scenario, with the understanding that in normal operating conditions only two or three replicates will be used. In Table 2, it can be observed that, when using the R&D Systems pair, the lowest TNF-α concentration at 3.91 pg/mL produced a response of 66 MFI, which is greater than the response of the background + 3SD, meeting this criteria. When the Millipore detection antibody was used with the R&D Systems Capture antibody (Table 3), the limit of detection was less than 7.81 pg/mL. In this case, the 2nd lowest TNF-α concentration produced an acceptable response of 17 MFI; greater than the response of the lowest TNF-α concentration plus three times its standard deviation. (10 MFI + 3(2.4) = 16.29 MFI) Similarly, the limit of detection for the R&D Systems DuoSet ELISA was estimated to be between 63 pg/mL and 31 pg/mL (Table 1).
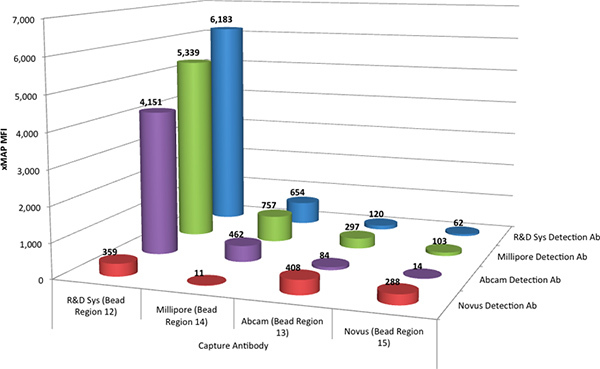 Figure 1. The median fluorescence intensity (MFI) of the R&D Systems standard (@ 2,000 pg/mL) for every possible combination of the four capture antibodies (coupled to four different microsphere regions) and the four detection antibodies. Click here to view larger figure.
Figure 1. The median fluorescence intensity (MFI) of the R&D Systems standard (@ 2,000 pg/mL) for every possible combination of the four capture antibodies (coupled to four different microsphere regions) and the four detection antibodies. Click here to view larger figure.
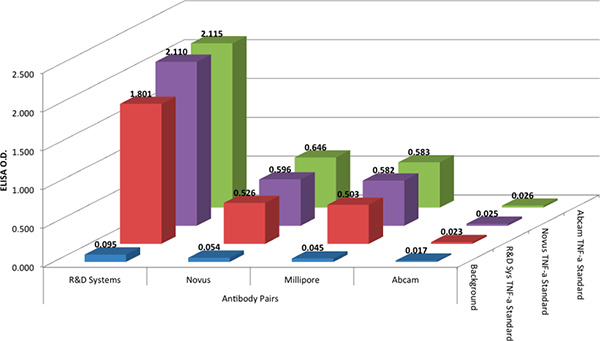 Figure 2. The optical density (O.D.) of three different recombinant standards (@ 1,000 pg/mL) for four capture and detection antibody pair combinations. Capture and detection antibodies were arbitrarily paired by vendor, for simplicity. Click here to view larger figure.
Figure 2. The optical density (O.D.) of three different recombinant standards (@ 1,000 pg/mL) for four capture and detection antibody pair combinations. Capture and detection antibodies were arbitrarily paired by vendor, for simplicity. Click here to view larger figure.
| R&D Systems DuoSet | |||
| pg/mL | O.D. | Std Dev | +3 SD |
| 1000 | 2.084 | 0.035 | 2.187 |
| 500 | 1.328 | 0.038 | 1.441 |
| 250 | 0.787 | 0.025 | 0.863 |
| 125 | 0.476 | 0.026 | 0.554 |
| 63 | 0.304 | 0.023 | 0.374 |
| 31.3 | 0.212 | 0.025 | 0.287 |
| 15.6 | 0.167 | 0.026 | 0.244 |
| 0 | 0.118 | 0.021 | 0.182 |
Table 1. The optical density (O.D.) of the 2-fold dilution series specified by the R&D Systems DuoSet package insert, for use as a standard curve; including standard deviation (SD) and the estimated limit of detection (L.O.D.), between 31.3 pg/mL and 63 pg/mL.
| R&D Systems Capture and Detection Antibodies | |||
| pg/mL | MFI | Std Dev | +3 SD |
| 8000 | 20,320 | 463 | 21,707 |
| 4000 | 15,594 | 223 | 16,263 |
| 2000 | 11,098 | 79 | 11,336 |
| 1000 | 6,985 | 160 | 7,465 |
| 500 | 4,149 | 80 | 4,390 |
| 250 | 2,233 | 30.0 | 2,323 |
| 125 | 1,199 | 43.8 | 1,330 |
| 63 | 636 | 14.0 | 678 |
| 31.3 | 340 | 12.9 | 379 |
| 15.6 | 183 | 5.9 | 201 |
| 7.8 | 103 | 2.2 | 109 |
| 3.9 | 66 | 2.4 | 73 |
| 0 | 11 | 0.8 | 13.8 |
Table 2. The median fluorescence intensity (MFI) of a standard dilution series measured by xMAP technology, using the antibody pair included with the R&D Systems DuoSet; including standard deviation (SD) and the estimated limit of detection (L.O.D.), less than 3.91 pg/mL.
| R&D Systems Capture Ab with Millipore Detection Ab | |||
| pg/mL | MFI | Std Dev | +3 SD |
| 8000 | 5,800 | 143 | 6,229 |
| 4000 | 3,881 | 120 | 4,242 |
| 2000 | 2,176 | 73 | 2,396 |
| 1000 | 1,138 | 32.1 | 1,234 |
| 500 | 578 | 31.3 | 671 |
| 250 | 289 | 6.2 | 307 |
| 125 | 142 | 3.1 | 151 |
| 63 | 75 | 5.3 | 91 |
| 31.3 | 44 | 3.3 | 54 |
| 15.6 | 28 | 2.6 | 35.5 |
| 7.8 | 17 | 1.5 | 21.2 |
| 3.9 | 10 | 2.0 | 16.3 |
| 0 | 7 | 1.4 | 11.4 |
Table 3. The median fluorescence intensity (MFI) of a standard dilution series measured by xMAP technology, using the R&D Systems capture antibody and the EMD Millipore detection antibody; including standard deviation (SD) and the estimated limit of detection (L.O.D.), less than 7.81 pg/mL.
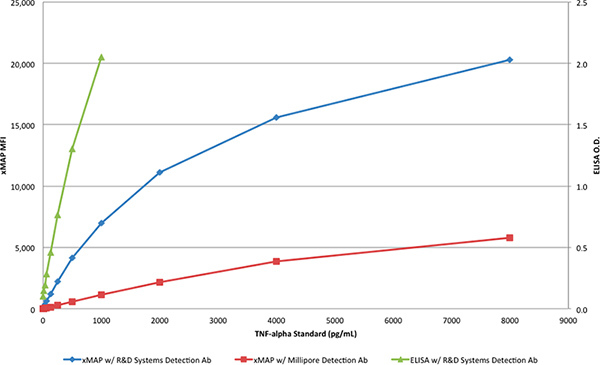 Figure 3. The standard curves of the two xMAP assays and the R&D Systems DuoSet ELISA. Click here to view larger figure.
Figure 3. The standard curves of the two xMAP assays and the R&D Systems DuoSet ELISA. Click here to view larger figure.
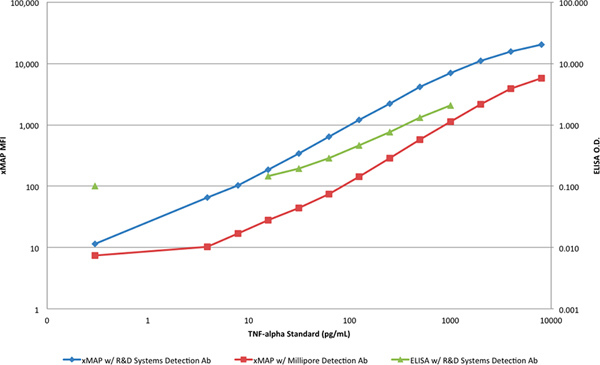 Figure 4. A comparison of the xMAP standard curves and the ELISA standard curve in a log-log scale. Click here to view larger figure.
Figure 4. A comparison of the xMAP standard curves and the ELISA standard curve in a log-log scale. Click here to view larger figure.
Discussion
The conversion of an ELISA assay to the Luminex xMAP platform can be as simple as substituting streptavidin horseradish peroxidase (SA-HRP) in a typical ELISA kit with streptavidin phycoerythrin (SA-PE), and optimizing for performance. For those who want to create an xMAP immunoassay from the ground up, this can be accomplished with a straightforward protocol that also enables the fast, multiplex evaluation of the antibody pairs. The reagents for the xMAP assay were easily prepared using the xMAP Antibody Coupling kit to couple the designated capture antibodies to MagPlex low concentration microspheres. The use of low concentration microspheres reduces the cost of assay development while providing the same assay performance of higher concentration microspheres. The amount of time required to prepare coupled MagPlex microspheres is approximately 3 hours, which is much faster than the 22 - 24 hours required to coat the well of an ELISA plate, followed by treatment of the coated wells. The performance of the xMAP assay is also superior to the ELISA in terms of limit of detection (<4 pg/mL vs. >31 pg/mL) and dynamic range (<4 pg/mL to >8000 pg/mL vs. 16 pg/mL to 1000 pg/mL). Plate readers have a limited OD range which is either 3 or 4 OD, restricting the upper limit of the dynamic range for an assay.
Undoubtedly, not all antibodies will work in an ELISA format and not all antibodies that work well in an ELISA are readily transferable to the xMAP assay format. However, since xMAP assays can be multiplexed (i.e., run simultaneously), it is possible to evaluate several capture and detection antibody combinations concurrently to identify the best pair to use for an assay. This process saves significant time and reagents compared to the ELISA development procedure, which is limited to the evaluation of one pair at a time. Should two or more antibody pairs perform equivalently; other parameters of the assay can be considered to determine suitability of the pair (e.g., availability, cost, etc.).
In addition to improved assay performance and flexibility with the xMAP assay, there are also significant cost savings. The recommended quantity of antibody required to coat a single well of an ELISA plate is 400ng, whereas the quantity required for the beads used in one well of an xMAP assay is approximately 7.5 ng. Thus, the amount of antibody required for one ELISA well will provide more than 50 test results, if used in an xMAP assay. For applications involving precious samples, xMAP also has a significant advantage. The volume of sample recommended for the ELISA is 100 μL whereas the volume required for the xMAP assay can be half of that, or less.
In summary, conversion of an ELISA assay to the Luminex xMAP platform is uncomplicated, efficient and cost-saving, while producing an assay with superior dynamic range and sensitivity.
Disclosures
This work was done at Luminex Corporation with equipment manufactured at Luminex Corporation.
R&D Systems & EMD Millipore are strategic partners of Luminex Corporation; licensed to develop and commercialize multiplex xMAP based assays.
Acknowledgments
This work was funded by Luminex Corporation.
References
- Fulton JR, McDade RL, Smith PL, Kienker LJ, Kettman JR. Advanced multiplexed analysis with the FlowMetrix system. Clinical Chemistry. 1997;43:1749–1756. [PubMed] [Google Scholar]
- Carson RT, Vignali AA. Simultaneous quantation of 15 cytokines using a multiplexed flow cytometric assay. J. Immunol. Methods. 1999;227:41–52. doi: 10.1016/s0022-1759(99)00069-1. [DOI] [PubMed] [Google Scholar]
- Peck D, Crawford ED, Ross KN, Stegmaier K, Golub TR, Lamb J. A method for high-throughput gene expression signature analysis. Genome Biol. 2006;7:61. doi: 10.1186/gb-2006-7-7-r61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDerMeid KR, Su SP, Krenzer KL, Ward KW, Zhang JZ. A method to extract cytokines and matrix metalloproteinases from Schirmer strips and analyze using Luminex. Mol Vis. 2011;17:1056–1063. [PMC free article] [PubMed] [Google Scholar]
- Liu J, Kibiki G, Maro V, Maro A, Kumburu H, Swai N, Taniuchi M, Gratz J, Toney D, Kang G, Houpt E. Multiplex reverse transcription PCR Luminex assay for detection and quantitation of viral agents of gastroenteritis. J. Clin. Virol. 2011;50:308–313. doi: 10.1016/j.jcv.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jager W, Prakken BJ, Bijlsma J, WJ Kuis W, Rijkers GT. Improved multiplex immunoassay performance in human plasma and synovial fluid following removal of interfering heterophilic antibodies. J. Immunol. Methods. 2005;300:124–135. doi: 10.1016/j.jim.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Codorean E, Nichita C, Albulescu L, Raducan E, Popescu ID, Lonita AC, Albulescu R. Correlation of xMAP and ELISA cytokine profiles; development and validation for immunotoxicological studies in vitro. Roum. Arch. Microbiol. Immunol. 2010;69:3–19. [PubMed] [Google Scholar]
- de Jager W, te Velthuis H, Prakken BJ, Kuis W, Rijkers GT. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin. Diagn. Lab. Immunol. 2003;10:133–139. doi: 10.1128/CDLI.10.1.133-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont NC, Wang KH, Wadhwa PD, Culhane JF, Nelson EL. Validation and comparison of Luminex multiplex cytokine analysis kits with ELISA: Determinations of a panel of nine cytokines in clinical sample culture supernatants. J. Reprod. Immunol. 2005;66:175–191. doi: 10.1016/j.jri.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richens JL, Urbanowicz RA, Metcalf R, Corne J, O'Shea P, Fairclough L. Quantitative validation and comparison of multiplex cytokine kits. Journal of Biomolecular Screening. 2010;15:562–568. doi: 10.1177/1087057110362099. [DOI] [PubMed] [Google Scholar]
- Rizzi G, Zhang YJ, Latek R, Weiner R, Rhyne PW. Characterization and development of a Luminex-based assay for the detection of human IL-23. Bioanalysis. 2010;2:1561–1572. doi: 10.4155/bio.10.68. [DOI] [PubMed] [Google Scholar]


