Abstract
In general, human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs)1 can be cultured under variable conditions. However, it is not easy to establish an effective system for culturing these cells. Since the culture conditions can influence gene expression that confers pluripotency in hESCs and hiPSCs, the optimization and standardization of the culture method is crucial.
The establishment of hESC lines was first described by using MEFs as feeder cells and fetal bovine serum (FBS)-containing culture medium2. Next, FBS was replaced with knockout serum replacement (KSR) and FGF2, which enhances proliferation of hESCs3. Finally, feeder-free culture systems enable culturing cells on Matrigel-coated plates in KSR-containing conditioned medium (medium conditioned by MEFs)4. Subsequently, hESCs culture conditions have moved towards feeder-free culture in chemically defined conditions5-7. Moreover, to avoid the potential contamination by pathogens and animal proteins culture methods using xeno-free components have been established8.
To obtain improved conditions mouse feeder cells have been replaced with human cell lines (e.g. fetal muscle and skin cells9, adult skin cells10, foreskin fibroblasts11-12, amniotic mesenchymal cells13). However, the efficiency of maintaining undifferentiated hESCs using human foreskin fibroblast-derived feeder layers is not as high as that from mouse feeder cells due to the lower level of secretion of Activin A14. Obviously, there is an evident difference in growth factor production by mouse and human feeder cells.
Analyses of the transcriptomes of mouse and human feeder cells revealed significant differences between supportive and non-supportive cells. Exogenous FGF2 is crucial for maintaining self-renewal of hESCs and hiPSCs, and has been identified as a key factor regulating the expression of Tgfβ1, Activin A and Gremlin (a BMP antagonist) in feeder cells. Activin A has been shown to induce the expression of OCT4, SOX2, and NANOG in hESCs15-16.
For long-term culture, hESCs and hiPSCs can be grown on mitotically inactivated MEFs or under feeder-free conditions in MEF-CM (MEF-Conditioned Medium) on Matrigel-coated plates to maintain their undifferentiated state. Success of both culture conditions fully depends on the quality of the feeder cells, since they directly affect the growth of hESCs.
Here, we present an optimized method for the isolation and culture of mouse embryonic fibroblasts (MEFs), preparation of conditioned medium (CM) and enzyme-linked immunosorbent assay (ELISA) to assess the levels of Activin A within the media.
Keywords: Stem Cell Biology, Issue 64, Molecular Biology, Developmental Biology, mouse embryonic fibroblasts (MEFs), human embryonic stem cells (hESCs), human induced pluripotent stem cells (hiPSCs), Activin A -conditioned medium (CM), cell culture
Protocol
1. Isolation of Mouse Embryonic Fibroblasts (MEFs)
The following two steps are performed under non-aseptic conditions.
Sacrifice a pregnant mouse (CF1, Harlan, USA) at 13 or 14 d.p.c. (day post-coitum) by cervical dislocation.
Dissect out the uterine horns, briefly rinse in 70% (v/v) ethanol and place into a falcon tube containing PBS without Ca2+Mg2+ (Gibco, Invitrogen).
The following steps are carried out in a tissue culture hood under aseptic conditions and using sterile instruments.
Place uterine horns into a Petri dish and separate each embryo from its placenta and embryonic sac.
Dissect head and red organs, wash in PBS and place all embryos in a clean Petri dish. Finely mince the tissue using a sterile razor blade until it becomes possible to pipette.
Add 1 ml of 0.05% trypsin/EDTA (Gibco, Invitrogen), including 100 Kunitz units of DNase I (USB), per embryo.
Transfer the tissue into a 50 ml falcon tube and incubate for 15 min at 37 °C. After each 5 min of incubation, dissociate cells by pipetting up and down thoroughly.
Inactivate the trypsin by adding about 1 volume of freshly prepared MEF medium. MEF culture medium (components to make 500 ml of media, mix all components and filter): 450 ml of DMEM, 50 ml of FBS (10% (v/v)), 5 ml of 200 mM L-glutamine (1/100 (v/v)), 5 ml of Penicillin-streptomycin (1/100 (v/v)).
Centrifuge the cells with low-speed (300 x g), 5 min, carefully remove the supernatant and resuspend cell pellet in warm MEF medium.
Plate approximately a number of cells which is equivalent to 3-4 embryos in each T150 (TPP) flask coated with 0.2% gelatine (Gelatine from bovine skin, Type B, Sigma) for 2 hr. The fibroblasts (P0, passage 0) are the only cells that have the ability to attach to the gelatine-coated flasks.
Ideally, cells are 80-90% confluent after 24 hr and at this stage a major part of P0 cells is frozen for future usage.
Expand the remaining T150 flask(s) of P0 cells till P3 or P4, then inactivate and use as feeders to replate hESCs or to produce conditioned medium (CM).
2. Inactivation and Plating MEFs (Feeder Cells Preparation)
All steps are carried out in a tissue culture hood under aseptic conditions.
Coat T150 flasks with 0.2% gelatine and incubate at RT for at least 2 hr.
Dilute mitomycin C in PBS (1 mg/ml) and filter.
Aspirate media from MEFs and wash with PBS without Ca2+Mg2+.
Place 20 ml of medium containing 10 μg/ml of mitomycin C on MEFs.
Incubate for 2 hr at 37 °C with mitomycin C containing medium then wash twice with PBS, trypsinize, centrifuge (for 5 min at 300 x g) and resuspend cells in warm medium.
Count cells and plate at a density of 56.000 cells/cm2 in T150 flasks and use for CM production for the following 6 days.
3. Conditioned Medium (CM) Preparation
All steps are carried out in a tissue culture hood under aseptic conditions.
The day after plating inactivated MEFs at a density of 56.000 cells/cm2 replace the MEF medium with hESC medium (UM, unconditioned medium) (0.5 ml/cm2) supplemented freshly with 4 ng/ml of FGF2.
Collect CM from feeder flasks after 24 h incubation and add fresh hESC medium containing 4 ng/ml of FGF2 to the feeders.
Repeat this procedure for the next 6 days. Each day store collected CM at -20 °C.
After 6 days mix all aliquots of medium and filter (Corning, 0.22 μm, PAS). Make 50 ml aliquots and store at -80 °C.
Supplement CM with additional 4 ng/ml of FGF2 before adding to hESCs grown on Matrigel.
4. Measurement of Activin A in Conditioned Media (ELISA)15
Bring all samples and reagents to room temperature.
Dilute capture antibody (Human\Mouse\Rat Activin A MAb, R&D Systems) in PBS with 1% BSA, add to microplate (100 μl/well) and incubate overnight at RT.
After 24 hr wash the wells three times with PBST (PBS with 0.05% Tween 20) (300 μl/well) then block (1% BSA/PBS, 300 μl/well) for 1 hr at RT.
In this time prepare an Activin A (R&D Systems) standard curve, including 7 dilutions (concentration less than 30 ng/ml) and blank sample. The linear working range of the Activin A is between 0.25 and 32 ng/ml.
Add duplicates of standards and samples to the wells (100 μl/well) and incubate for 2 hr at RT.
Wash wells three times (300 μl of PBST).
Add the secondary (biotinylated) antibody (Human/Mouse/Rat Biotinylated Activin A MAb, R&D Systems) (0.25 μg/ml in 1% BSA/PBS) and incubate for 2 hr at RT.
Wash wells three times (300 μl of PBST), add Streptavidin-HRP (diluted in 1%BSA/PBS, R&D Systems) and incubate for 20 min at RT.
Wash wells three times (300 μl of PBST), add 100 μl of substrate solution (Quantikine, R&D Systems) and incubate for 30 min at RT in the dark.
Add 100 μl of stop solution (Quantikine, R&D Systems) to each well and mix gently.
Set Microplate reader (Molecular Devices Spectra Max 250, Global Medical Instrumentation, Inc., Minnesota) to 450 nm, with wavelength correction at 540 or 570 nm, to determine the optical density of each well.
5. Representative Results
The overall scheme of the isolation procedure is presented in Figure 1. The typical morphology of hESCs and hiPSCs cultured under different conditions is presented in Figure 2. The morphology of MEFs and inactivated feeder cells used to prepare CM is presented in Figure 3. In general, cells should be confluent 24 hours post-isolation and ready to be frozen or expanded. However, sometimes it might take 2-3 days before obtaining confluent cultures. The CM should be prepared from cells at passage 4 and not later. This is crucial because primary cells can only be expanded for 4-5 passages before the onset of senescence.
The cytokine, Activin A, is considered as the most critical factor secreted by feeder cells for the support of undifferentiated growth of pluripotent cells14. The measurement of the level of Activin A in CM (Figure 4) is a very convenient quantitative assay to monitor the quality of MEFs.
 Figure 1. A schematic representation of the MEFs isolation procedure.
Figure 1. A schematic representation of the MEFs isolation procedure.
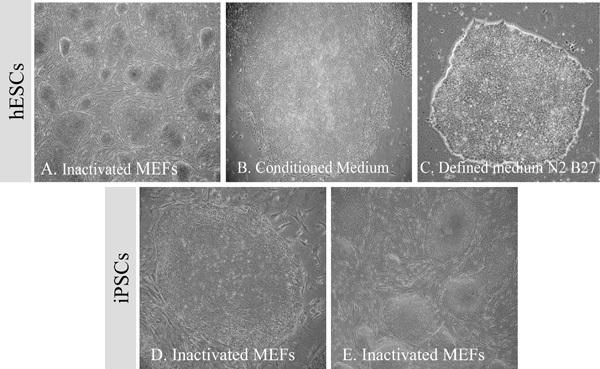 Figure 2. The typical morphology of undifferentiated hESCs cultured in the presence of (A) feeder cells, (B) conditioned medium and (C) defined medium. The typical morphology of hiPSCs cultured in the presence of (D, E) feeder cells.
Figure 2. The typical morphology of undifferentiated hESCs cultured in the presence of (A) feeder cells, (B) conditioned medium and (C) defined medium. The typical morphology of hiPSCs cultured in the presence of (D, E) feeder cells.
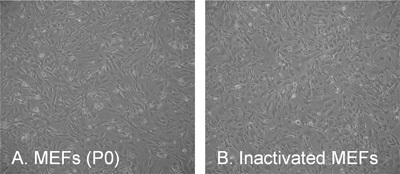 Figure 3. The typical morphology of mouse embryonic fibroblasts (MEFs). (A) Passage 0 (P0) two days after plating/isolation, (B) inactivated feeder layer at a density of 56.000 cells/cm.2
Figure 3. The typical morphology of mouse embryonic fibroblasts (MEFs). (A) Passage 0 (P0) two days after plating/isolation, (B) inactivated feeder layer at a density of 56.000 cells/cm.2
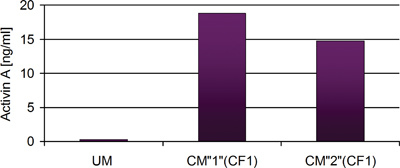 Figure 4. Enzyme-linked immunosorbent assay (ELISA)-based measurements of the concentration of Activin A in conditioned medium (CM) prepared with mouse embryonic fibroblasts derived from the CF1 mouse strain. CM was collected for 6 days and then pooled. CM"1" and CM"2" refer to different batches of media and UM to unconditioned media. As the function of the conditioning process is Activin A secretion into the medium by MEFs, Activin A is almost undetectable in UM.
Figure 4. Enzyme-linked immunosorbent assay (ELISA)-based measurements of the concentration of Activin A in conditioned medium (CM) prepared with mouse embryonic fibroblasts derived from the CF1 mouse strain. CM was collected for 6 days and then pooled. CM"1" and CM"2" refer to different batches of media and UM to unconditioned media. As the function of the conditioning process is Activin A secretion into the medium by MEFs, Activin A is almost undetectable in UM.
Discussion
The MEFs isolation procedure presented here enables the establishment of a standardized culture condition for hESCs and hiPSCs. Moreover the ELISA-based system used to evaluate cytokine production by feeder cells is a useful indicator of the quality of the MEF-derived conditioned media. The routine maintenance of the mouse strain (CF1) providing supporting fibroblasts is necessary to avoid batch-to-batch variation of culture media. Moreover, it is recommended to isolate embryos from several mice simultaneously to obtain consistent quality of cells. Certainly freshly isolated MEFs can be kept frozen at P0 and P1. Also inactivated MEFs can be kept frozen in aliquots of appropriate amounts depending on the requirements for cell culture. Usually approximately 250.000 cells should be plated in a single well of a 6-well plate to culture hESCs and iPS cells. The established and optimized method can be routinely used to minimize the variability between different experiments.
Disclosures
No conflicts of interest declared.
Acknowledgments
Special thanks to Dr. Boris Greber for setting up the Activin A ELISA protocol as published in Greber et al. 2007. We are particularly grateful to Mrs. Monica Shevack for preparing the graphical overview. We are very grateful to Dr Heiko Fuchs for all help and valuable suggestions before and during the filming. We would like to thank all members of the Adjaye laboratory, especially Elisabeth Socha for maintaining a constant supply of MEFs and CM. We also acknowledge our colleagues at the animal facility of the MPIMG for their permanent support. This work was partly funded by The Max Planck Society and the [BMBF; grant number 0315717A], partners of the ERASysBio+ initiative supported under the EU ERA-NET Plus scheme in FP7.
References
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Thomson JA. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Amit M. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Xu C. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- Ludwig TE. Derivation of human embryonic stem cells in defined conditions. Nat. Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- Yao S. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc. Natl. Acad. Sci. U.S.A. 2006;103:6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Hou R, Booth CJ, Yang SH, Snyder M. Defined culture conditions of human embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5688–5693. doi: 10.1073/pnas.0601383103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skottman H, Hovatta O. Culture conditions for human embryonic stem cells. Reproduction. 2006;132:691–698. doi: 10.1530/rep.1.01079. [DOI] [PubMed] [Google Scholar]
- Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- Richards M. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21:546–556. doi: 10.1634/stemcells.21-5-546. [DOI] [PubMed] [Google Scholar]
- Amit M. Human feeder layers for human embryonic stem cells. Biol. Reprod. 2003;68:2150–2156. doi: 10.1095/biolreprod.102.012583. [DOI] [PubMed] [Google Scholar]
- Inzunza J. Derivation of human embryonic stem cell lines in serum replacement medium using postnatal human fibroblasts as feeder cells. Stem Cells. 2005;23:544–549. doi: 10.1634/stemcells.2004-0201. [DOI] [PubMed] [Google Scholar]
- Zhang K. Utilization of Human Amniotic Mesenchymal Cells as Feeder Layers to Sustain Propagation of Human Embryonic Stem Cells in the Undifferentiated State. Cell Reprogram. 2011. [DOI] [PubMed]
- Eiselleova L. Comparative study of mouse and human feeder cells for human embryonic stem cells. Int. J. Dev. Biol. 2008;52:353–363. doi: 10.1387/ijdb.082590le. [DOI] [PubMed] [Google Scholar]
- Greber B, Lehrach H, Adjaye J. Fibroblast growth factor 2 modulates transforming growth factor beta signaling in mouse embryonic fibroblasts and human ESCs (hESCs) to support hESC self-renewal. Stem Cells. 2007;25:455–464. doi: 10.1634/stemcells.2006-0476. [DOI] [PubMed] [Google Scholar]
- Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J. Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]


