Abstract
Enucleation is the process of retrieving the ocular globe from a cadaveric donor leaving the rest of the globe undisturbed. Excision refers to the retrieval of ocular tissues, especially cornea, by cutting it separate from the ocular globe. Evisceration is the process of removing the internal organs referred here as retina. The ocular globe consists of the cornea, the sclera, the vitreous body, the lens, the iris, the retina, the choroid, muscles etc (Suppl. Figure 1). When a patient is suffering from corneal damage, the cornea needs to be removed and a healthy one must be transplanted by keratoplastic surgeries. Genetic disorders or defects in retinal function can compromise vision. Human ocular globes can be used for various surgical procedures such as eye banking, transplantation of human cornea or sclera and research on ocular tissues. However, there is little information available on human corneal and retinal excision, probably due to the limited accessibility to human tissues. Most of the studies describing similar procedures are performed on animal models. Research scientists rely on the availability of properly dissected and well-conserved ocular tissues in order to extend the knowledge on human eye development, homeostasis and function. As we receive high amount of ocular globes out of which approximately 40% (Table 1) of them are used for research purposes, we are able to perform huge amount of experiments on these tissues, defining techniques to excise and preserve them regularly.
The cornea is an avascular tissue which enables the transmission of light onto the retina and for this purpose should always maintain a good degree of transparency. Within the cornea, the limbus region, which is a reservoir of the stem cells, helps the reconstruction of epithelial cells and restricts the overgrowth of the conjunctiva maintaining corneal transparency and clarity. The size and thickness of the cornea are critical for clear vision, as changes in either of them could lead to distracted, unclear vision. The cornea comprises of 5 layers; a) epithelium, b) Bowman's layer, c) stroma, d) Descemet's membrane and e) endothelium. All layers should function properly to ensure clear vision4,5,6. The choroid is the intermediate tunic between the sclera and retina, bounded on the interior by the Bruch's membrane and is responsible for blood flow in the eye. The choroid also helps to regulate the temperature and supplies nourishment to the outer layers of the retina5,6. The retina is a layer of nervous tissue that covers the back of the ocular globe (Suppl. Figure 1) and consists of two parts: a photoreceptive part and a non-receptive part. The retina helps to receive the light from the cornea and lens and converts it into the chemical energy eventually transmitted to the brain with help of the optic nerve5,6.
The aim of this paper is to provide a protocol for the dissection of corneal and retinal tissues from human ocular globes. Avoiding cross-contamination with adjacent tissues and preserving RNA integrity is of fundamental importance as such tissues are indispensable for research purposes aimed at (i) characterizing the transcriptome of the ocular tissues, (ii) isolating stem cells for regenerative medicine projects, and (iii) evaluating histological differences between tissues from normal/affected subjects. In this paper we describe the technique we currently use to remove the cornea, the choroid and retinal tissues from an ocular globe. Here we provide a detailed protocol for the dissection of the human ocular globe and the excision of corneal and retinal tissues. The accompanying video will help researchers to learn an appropriate technique for the retrieval of precious human tissues which are difficult to find regularly.
Keywords: Medicine, Issue 64, Physiology, Human cadaver ocular globe, in situ excision, corneal tissue, in situ evisceration, retinal tissue
Protocol
1. In situ Excision of the Cornea from Ocular Globes
Switch on the laminar air flow cabinet approximately 15 minutes before use. Clean the laminar flow hood using 70% isopropyl alcohol. Put on personal protective clothing such as surgical cap and mask. Scrub hands and forearms and dry using a sterile towel. Wear sterile gloves and gown or sleeves using aseptic technique.
Set up the sterile field by placing a sterile instrument tray aseptically. Verify that the instruments are sterile. Open the sterile instruments pack and flip onto the sterile field avoiding any contamination.
Position all sterile materials and instruments (Table 2) along with bottles (eye jars) containing eye globes so that they are adjacent to the edge of the sterile field for ease of use.
Label the bottles with previously prepared preservation/storage medium as indicated in Table 3 and place them on the surface of the laminar air flow hood along with I-PVP (iodine-polyvinylpyrrolidone), sodium thiosulphate and a sterile saline solution PBS (phosphate buffer saline).
Allow the ocular tissues and solutions to reach normal room temperature as they are preserved in refrigerators at 4 °C. Avoid repeated warming/cooling cycles. Keep the lids of the solution and the eye jars with inner side facing upwards next to their respective jars.
Remove gauzes and sponges from the eye jar using sterile forceps. Immerse the globe in sterile I-PVP 0.5% for 2 minutes to decontaminate the ocular globes. Place the two forceps on the lid of the sterile tray, outside the sterile field. Transfer the globe in sodium thiosulphate 0.1% for 1 minute using another pair of sterile forceps. Using the same forceps, transfer the globe to the bottle containing sterile saline solution (PBS) and leave it till it is operated. Repeat the same procedure for the other ocular globe from the same donor.
Working underneath the laminar air flow hood, wrap the globe using sterile gauze (bandages) leaving the cornea and approximately 5 mm sclera from the cornea uncovered. The eye bulb could be simply held in the hand maintaining the adequate pressure. The tissue should be kept moist during the entire surgery.
Forceps are used along with scissors to remove all eventual remains of the conjunctiva. Keep the instruments used for the above operation separate from other instruments. Use a scalpel blade to perform a scleral incision of 3-4 mm from the limbus region, then extend the incision by 360°, avoiding to perforate the underlying uveal tissue or cause any deformation of the cornea's normal curvature. Cut four big incisions leaving four small gaps avoiding the secretion of the vitreous body. All four gaps are cut ensuring no removal of any other tissue.
Presence of small scleral plaques may hamper the cutting, so make sure to complete the scleral excision with microsurgery scissors. This operation should be performed without damaging the choroid, retina and vitreous body.
Inspect the incision to ensure it is complete. If the incision has been performed correctly the corneo-scleral rim adheres to the ciliary bodies only at the point in correspondence with the cornea.
Set the wrapped ocular globe down near the center of the sterile field. Complete the corneal removal using forceps to hold the scleral rim stationary and the hand used for excision to pull the ciliary body-choroid downward and away from the corneo-scleral button.
Gently separate any remaining adhesions from the corneo-scleral button. Neither pull the corneo-scleral rim in a way that could cause cross-corneal tension nor allow it to drop back down onto the anterior chamber.
The endothelial cell density of the cornea is checked using a hypotonic solution and the cell mortality is checked using a trypan blue staining for around 1 min and the cells are then counted under an optical microscope and measured as cells/mm2. The anterior chamber should face the lid while the posterior should face the bottom of the Petri plate. The endothelial cell density could be counted manually using a grid in the objective under 100X magnification. The minimum accepted cell count for transplantation in Italy is 2200 endothelial cells/mm2.
Transfer the corneo-scleral rim using a corneal claw to previously prepared corneal storage medium preserved at RT (room temperature) as shown in Table 3.
Examine the posterior chamber for a natural crystalline lens. If the sclera is prepared, remove all the residues of uvea and vitreous humor from the globe. Carefully unwrap and return the remaining posterior segment to its respective eye jar, using aseptic technique. Repeat the procedure for the other globe using new instruments. Fix the cornea with corneal claw and preserve under the storage media (organ culture) at 31 °C. The excised cornea, as shown in Figure 1a, can then be used for keratoplasty or research.
Dispose the unnecessary materials and bio-hazardous waste into appropriate waste bins. Clean the working area once the work is completed using 70% isopropyl alcohol spray.
If, after 28 days the cornea maintains all the evaluation parameters [such as morphology, cell count, transparency, thickness and microbiology tests - bacterial and fungal using Bactec 9240 instrument (Becton Dickinson, Milan, Italy)], it can then be transported to the hospitals using the transport medium shown in Table 4. The cornea should be removed and placed from the storage to transport medium under laminar air flow cabinet.
As, during storage, the cornea gets thicker than its usual size required for transplantation, eye banks use de-swelling agents to reduce thickness. We use 6% dextran T500 to get the cornea back to its normal size in transport medium. Though 4-8% dextran T500 could be used, 6% has served best for us. This makes it easier for the surgeons to transplant the cornea as soon as they receive it.
2. Evisceration of Retina from the Ocular Globe after Excising the Cornea
Following excision, the cornea is selected for transplantation or research based on its morphology and endothelial cell density and is placed in the storage or preservation medium at 31 °C. The remaining of the ocular globe is then processed for further use such as sclera isolation for surgical purposes, retina for research etc.
Prior to retinal excision, make sure that the ocular globe is intact and only the cornea with a part of sclera is removed. If the gelatinous vitreous body is secreting out it becomes difficult to remove a retinal tissue.
If the donor is below 65 years of age, the sclera is preserved under sterile PBS for surgical purposes after serological testing. In this case, the sclera is not cut, only the vitreous body is removed and the entire sclera is preserved.
For retinal excision, start with a small cut or incision from the corneal side of the sclera moving towards the optic nerve using sterile forceps and scissors. Hold the part of the sclera using forceps and cut with the scissors straight towards the optic nerve cutting part by part avoiding damage to the vitreous body.
Choroidal plaques should be separated from the sclera as they are stuck together. Cut the entire sclera to expose the choroid tissue. Once the sclera is completely cut, separate the sclera with the remaining of the vitreous body by cutting it near the optic nerve. An entire ball of vitreous body covered with choroid will be visible as shown in Figure 2a.
Using two sterile forceps remove the choroid layer gently by picking it up with one pair of forceps and removing it with the other. Alternatively, the choroid layer can be removed using scissors but this should ideally be avoided as it may cause damages to the retina. Remove the choroid layer partially and cut it to facilitate the removal.
Once the choroid layer is removed, a transparent layer of retina will be seen clearly as shown in Figure 2b. The choroid layer has an interior Bruch's membrane which could also be removed but it is difficult to identify it with a naked eye.
Similarly, use two forceps to remove the retinal layer as shown in Figure 2c. It is easy to isolate high amount of retina if excised near the optic nerve. All the layers of the eye could be now seen as shown in Figure 2d.
Once the retinal tissue has been removed, it could be used for different purposes and therefore the subsequent steps and conditions used for preservation may differ accordingly. For instance, the retinal tissue can be placed in tubes containing RNA stabilization reagents (such as RNAlater) or lysis buffer (for RNA or DNA extraction), dispase/trypsin (for isolation of cells), fixing solutions (prior to embed tissues in paraffin or OCT for immunohistochemistry).
Clear all the working area and clean the area using 70% isopropyl alcohol spray.
3. Representative Results
The corneo-scleral rim as seen in Figure 1a is a properly excised cornea with required amount of scleral rim and endothelial morphology, this kind of cornea is generally used for penetrating keratoplasty. If the anterior stroma of the excised cornea is damaged with presence of opacity, the cornea could be used for endothelial keratoplasty / posterior lamellar keratoplasty (endothelium with a part of stroma). Whereas, if the endothelial layer is damaged or if the endothelial cell density is < 2200 cells /mm2 (Italian standard), the cornea could be used for anterior lamellar keratoplasty (epithelium with a part of anterior stroma). If the endothelial cell count is less than the required, the cornea could be used for anterior lamellar keratoplasty or research whereas, if the cornea is damaged completely, it could be used for research as shown in Figure 1b, 3b and 3c or discarded. The damage could be a cause of mishandling the tissue, prior cataract surgeries leading to stromal or descemet endothelial scars, development of physical injury during the patient's life etc. Therefore, it becomes necessary to check every tissue with slit lamp and then under optical microscope for fine scars / folds / detachments. The cornea is usually preserved under two different conditions such as organ culture (approximately 31 °C) usually used in European countries or hypothermic conditions (approximately 4 °C) usually used in the USA. We use organ culture as it helps to regenerate the damaged epithelial cells, to re-vitalize endothelial cell check with a high post mortem time, a storage period of 4 weeks which gives sufficient window for planning a surgery, gives sufficient time for microbiological (bacterial and fungal) and serological testing etc. The cornea can be well preserved in the transport medium for 7 days.
The sclera could be cut part by part ensuring gentle removal of all choroidal plaques. The vitreous liquid can excrete when the optic nerve, choroid or retina is cut sharply as shown in Figure 4. Retinas if excised for RNA analysis should be preserved in RNAlater as soon as the tissues are excised. Usually 1X1 cm of the choroid and retinal layer is excised and after keeping it in RNAlater for 24 hours at 4 °C is preserved under -80 °C in fresh Eppendorf tubes (safe-lock tubes, 2 ml) until used or shipped with dry ice. The retinal tissue should be excised using two forceps near optic nerve away from iris or ciliary margin to avoid contamination with other pigmented structures and should be transferred to the preserving container immediately as RNA gets unstable while retrieving the retina.
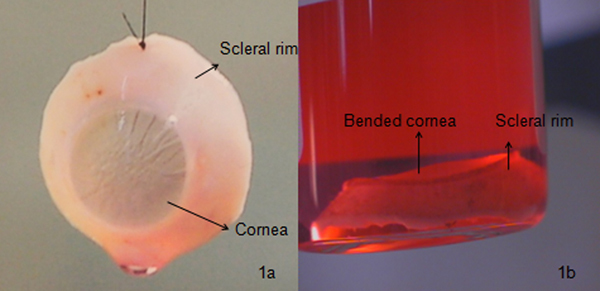 Figure 1. Comparison between a good and a poor quality cornea: a) A properly excised cornea. Such a cornea is usually preserved under organ culture for 4 weeks. Microbiological (using Bactec 9240 instrument) and morphological (staining the cells with trypan blue for cell mortality determination and observing cells under an optical microscope with 100X magnification) examination is performed before transporting it to the hospital (Figure 3a); b) a bent cornea, which eventually leads to graft rejection due to high descemet's folds and high endothelial cell damage examined using a slit lamp microscope. Such a cornea is usually used for research or is discarded.
Figure 1. Comparison between a good and a poor quality cornea: a) A properly excised cornea. Such a cornea is usually preserved under organ culture for 4 weeks. Microbiological (using Bactec 9240 instrument) and morphological (staining the cells with trypan blue for cell mortality determination and observing cells under an optical microscope with 100X magnification) examination is performed before transporting it to the hospital (Figure 3a); b) a bent cornea, which eventually leads to graft rejection due to high descemet's folds and high endothelial cell damage examined using a slit lamp microscope. Such a cornea is usually used for research or is discarded.
 Figure 2. Method used to ensure an accurate excision of the retinal tissue. a) Dissecting the sclera from the ocular globe using 24mm blade with 95 mm curved or blunt end sterile scissors and 100 mm 11X2 ruled by 0.70 mm teeth sterile forceps. The sclera should be cut by holding one side with the forceps cutting it straight towards the optic nerve using the scissors. The choroid layer will now be easily seen; b) Using two 100 mm 11X2 ruled by 0.70 mm teeth sterile forceps, choroid is removed by tearing it apart, which reveals the underlying transparent retinal layer; c) Transparent retinal layer is excised very carefully near the optic nerve without excretion of vitreous fluid using the same forceps. Both the forceps should hold each side of the retina and the tissue should gently be removed starting near the optic nerve moving towards the cornea, to obtain a good amount of retinal tissue. The tissue could be cut into different sizes and used for analysis experiments; d) Different layers of the eye following corneal removal can be viewed in this figure. The choroid body is removed first followed by retina without damaging the other parts of the eye.
Figure 2. Method used to ensure an accurate excision of the retinal tissue. a) Dissecting the sclera from the ocular globe using 24mm blade with 95 mm curved or blunt end sterile scissors and 100 mm 11X2 ruled by 0.70 mm teeth sterile forceps. The sclera should be cut by holding one side with the forceps cutting it straight towards the optic nerve using the scissors. The choroid layer will now be easily seen; b) Using two 100 mm 11X2 ruled by 0.70 mm teeth sterile forceps, choroid is removed by tearing it apart, which reveals the underlying transparent retinal layer; c) Transparent retinal layer is excised very carefully near the optic nerve without excretion of vitreous fluid using the same forceps. Both the forceps should hold each side of the retina and the tissue should gently be removed starting near the optic nerve moving towards the cornea, to obtain a good amount of retinal tissue. The tissue could be cut into different sizes and used for analysis experiments; d) Different layers of the eye following corneal removal can be viewed in this figure. The choroid body is removed first followed by retina without damaging the other parts of the eye.
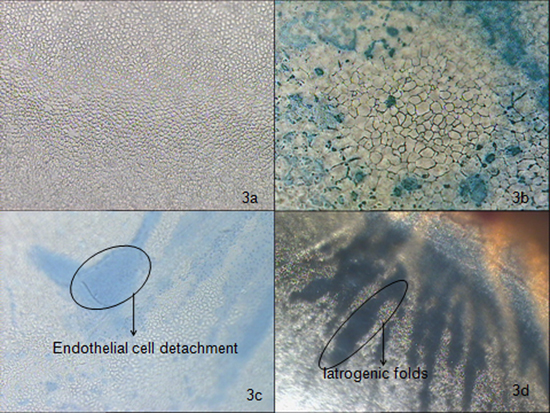 Figure 3. Comparison of the different types of cell density and morphology viewed under 100X magnification of an optical microscope. a) Good endothelial cell density (>2200 cells / mm2) and morphology (less polymorphism, low degeneration, no dystrophy and 0% mortality) in a cornea which is suitable for transplantation; b) Poor endothelial cell density (approximately 1200 – 1400 cells / mm2) and morphology (very high polymorphism and degeneration of cells) along with approximately 30-40% overall mortality are observed after trypan blue staining; c) A part of endothelial cells are damaged due to detachment of an endothelial sheet while retrieving the cornea from ocular globe. This is confirmed by observing the mortality of the cells in the region stained by trypan blue; d) The descemet's folds are formed due to mishandling of cornea by bending, creating cross corneal tension, using the instruments intensely etc at the time of excision. The figure shows the thick developed iatrogenic folds found between the periphery and optic zone of the cornea.
Figure 3. Comparison of the different types of cell density and morphology viewed under 100X magnification of an optical microscope. a) Good endothelial cell density (>2200 cells / mm2) and morphology (less polymorphism, low degeneration, no dystrophy and 0% mortality) in a cornea which is suitable for transplantation; b) Poor endothelial cell density (approximately 1200 – 1400 cells / mm2) and morphology (very high polymorphism and degeneration of cells) along with approximately 30-40% overall mortality are observed after trypan blue staining; c) A part of endothelial cells are damaged due to detachment of an endothelial sheet while retrieving the cornea from ocular globe. This is confirmed by observing the mortality of the cells in the region stained by trypan blue; d) The descemet's folds are formed due to mishandling of cornea by bending, creating cross corneal tension, using the instruments intensely etc at the time of excision. The figure shows the thick developed iatrogenic folds found between the periphery and optic zone of the cornea.
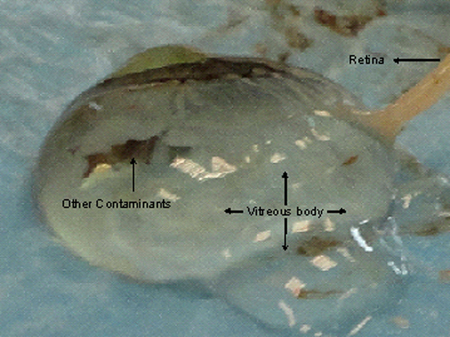 Figure 4. Damages to the choroid and retinal tissues while excising the cornea eventually leads to excretion of vitreous fluid. The retina gets implicated in the vitreous body making it difficult to excise a good quality tissue without contamination.
Figure 4. Damages to the choroid and retinal tissues while excising the cornea eventually leads to excretion of vitreous fluid. The retina gets implicated in the vitreous body making it difficult to excise a good quality tissue without contamination.
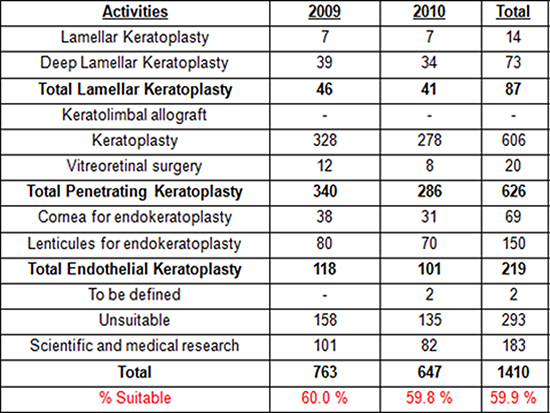 Table 1. Summary of the range of activities performed using human ocular globes during 2009 and 2010 at Fondazione Banca Degli Occhi (Venezia, Italy). Approximately 60% of tissues were used for transplantation, thus leaving a high number of ocular globes (around 40%) for scientific and medical research.
Table 1. Summary of the range of activities performed using human ocular globes during 2009 and 2010 at Fondazione Banca Degli Occhi (Venezia, Italy). Approximately 60% of tissues were used for transplantation, thus leaving a high number of ocular globes (around 40%) for scientific and medical research.
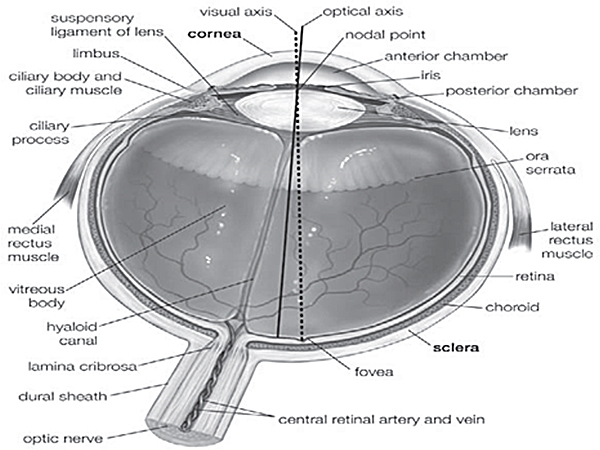 Supplementary Figure 1. Anatomy of the human eye5. Different parts of human eye are indicated in this figure, used to follow the exact position of the tissues.
Supplementary Figure 1. Anatomy of the human eye5. Different parts of human eye are indicated in this figure, used to follow the exact position of the tissues.
Discussion
Both, correct excision and proper preservation of corneal and retinal tissues are critical as minor defects such as endothelial damage or high number of descemet's folds can lead to graft failure of cornea whereas temperature alterations or mishandling can compromise the integrity of retinal tissues. The aim of this paper is to show how corneal and retinal tissues can be isolated optimally, without inducing damages or alterations that could compromise their use for transplantation or research purposes. The required minimum cell density (>2200 cells / mm2), good morphology such as cell size, structure, low degeneration of intercellular borders and absence of dystrophy along with good transparency and proper thickness of the cornea are hallmarks which make a tissue suitable for transplantation. If the scalpel blade is used roughly or other instruments are used intensely, it does not only damage the cornea, but also the vitreous body which makes further excision of other tissues more difficult (Figure 4). Retina is a transparent tissue and therefore difficult to identify if the vitreous liquid is released with it, this normally makes excision of the retinal tissue more complicated. If excised with vitreous body, the quality of the retinal tissue or RNA extracted will be very poor due to contamination with other ocular parts. Thus, an appropriate technique to excise a cornea or retina is very important to retrieve a superior quality tissue.
Our previous data from the last two years (Table 1) indicate that approximately 60% of corneas that we collect from eye globes are suitable for various types of transplantation. This is a further demonstration that the corneal excision technique described in this paper is a good method for collecting high quality corneal tissues from ocular globes. The corneas may, of course, be unsuitable for transplantation due to other parameters such as stromal abnormalities, contamination, endothelial density/morphology, donor suitability and mechanical/physical trauma caused while maintaining the cornea. However, if the above parameters are fine, the technique we have described here allows retrieval of good quality tissues to be used for transplantation in patients with ocular surface diseases.
While information on evisceration of retinal tissues from animal models is widely available11,12, to our knowledge this is the first time that such a technique is described for isolation of retinal tissues from human eye globes. Indeed, research on the literature, did not yield any result on papers discussing this topic. We believe that if human ocular globe dissection is carried out as described, research scientists would be able to have tissues of good quality to be used for their research projects. Many eye banks around the world are likely to receive eye bulbs (but also corneas) that are not suitable for transplantation. In our case, nearly 40% of corneas (and eye bulbs) that we collect and process cannot be transplanted (due to a wide range of causes) but could still be used for research purposes. The technique described in this paper does allow the collection of high quality tissues. This was recently demonstrated by our group when analyzing RNA with T<24hrs extracted from retinal tissues which were harvested using the technique described in this paper10. We believe the same technique could be used to isolate the retinal tissues for many other different purposes such as (i) for retinal transcriptome studies, (ii) to study and advance the use of human induced pluripotent stem cells in personalized medicine8, (iii) to develop RNA expression atlas of retinitis pigmentosa genes in human retinas9, (iv) to optimize the RNA extraction method, (v) stem cell studies to regenerate retinal tissues, etc.
This technique will be beneficial for those who want to perform such excisions or experiments but have limited information on the method to use human corneas or retinas.
Disclosures
The authors have no conflict of interest.
Acknowledgments
This work was supported partly through grants of the Regione Veneto (Ricerca Sanitaria Finalizzata n.292/2008 and Ricerca Sanitaria Finalizzata 2009). The authors thank Dr C. Griffoni for the 2009/2010 summary data on the use of human ocular globes.
References
- Fay A. Diseases of the visual system. In: Goldman L, Ausiello D, editors. Cecil Medicine. 23rd ed. Philadelphia, Pa: Saunders Elsevier; 2007. [Google Scholar]
- Parekh M, Megaw R, Ray-Chaudhuri A, Ahmad S. Patents in Limbal Stem Cell Biology. Recent patents on Regenerative Medicine. 2011;1:207–212. [Google Scholar]
- Pharm US. The Eye: The physiology of human perception. Britannica educational publishing. 2011;36:19–27. [Google Scholar]
- Lang G. Ophthalmology: A pocket textbook atlas. 2nd Edition. pp. 115–117.
- Liesbeth Pels. Organ Culture: The method of choice for preservation of human donor corneas. Br. J. Ophthalmol. 1997;81:523–525. doi: 10.1136/bjo.81.7.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, Pinilla I, Martin JM, Tian S, Stewart R, Pattnaik B, Thomson J, Gamm DM. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011. [DOI] [PMC free article] [PubMed]
- Trifunovic D, Karali M, Camposampiero D, Ponzin D, Banfi S, Marigo V. A high-resolution RNA expression atlas of retinitis pigmentosa genes in human and mouse retinas. Invest. Ophthalmol. Vis. Sci. 2008;49:2330–2336. doi: 10.1167/iovs.07-1513. [DOI] [PubMed] [Google Scholar]
- Mora P, Montanini L, Ferrari S. Retina. 2010;30:1555. doi: 10.1097/IAE.0b013e3181f04a23. [DOI] [PubMed] [Google Scholar]
- Claybon A, Bishop AJR. Dissection of a Mouse Eye for a Whole Mount of the Retinal Pigment Epithelium. J. Vis. Exp. 2011. p. e2563. [DOI] [PMC free article] [PubMed]
- Skeie JM, Tsang SH, Mahajan VB. Evisceration of Mouse Vitreous and Retina for Proteomic Analyses. J. Vis. Exp. 2011. p. e2795. [DOI] [PMC free article] [PubMed]


