Abstract
An immunology-based in vivo screening regime was used to assess the potential pathogenicity of biotechnology-related microbes. Strains of Bacillus cereus (Bc), Bacillus subtilis (Bs), Bacillus thuringiensis (Bt), and Bt commercial products (CPs) were tested. Balb/c mice were endotracheally instilled with purified spores, diluted CP, or vegetative cells (VC) (live or dead). Exposed mice were evaluated for changes in behavioral and physical symptoms, bacterial clearance, pulmonary granulocytes, and pulmonary and circulatory pyrogenic cytokines (interleukins (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α), as well as acute phase biomarkers (fibrinogen and serum amyloid A). Except for some differences in clearance rates, no marked effects were observed in mice exposed to any spore at 106 or 107 colony forming units (cfu). In contrast, live Bc or Bt VCs (105 or 106 cfu) produced shock-like symptoms (lethargy, hunched appearance, ruffled fur, and respiratory distress), and 11–200-fold elevations in pyrogenic cytokines at 2-h post-exposure. In the study, 4-h effects included increased lethargy, ocular discharge, and 1.5–4-fold rise in circulatory acute phase markers, but no indications of recovery. Bs VC did not produce any changes in symptoms or biomarkers. After 2 or 4 h of exposure to dead VC, increases of only plasma IL-1? and TNF-α (4.6- and 12.4-fold, respectively) were observed. These findings demonstrate that purified spores produced no marked effects in mice compared to that of metabolically active bacteria. This early screening regime was successful in distinguishing the pathogenicity of the different Bacillus species, and might be useful for assessing the relative hazard potential of other biotechnology-related candidate strains.
Keywords: acute phase, gram-positive, inflammation, pulmonary, spore, vegetative cell
Introduction
The Bacillus cereus (Bc) group encompasses genetically related species of clinical and industrial interest. Within this group, a number of strains typed as B. cereus (Bc) sensu stricto were implicated in poisoning from contaminated foods, bacteraemia, endocarditis, endophthalmitis, neonatal meningoencephalitis, and soft tissue infections (Abusin, Bhimaraj, and Khadra 2008; Castedo et al. 1999; David, Kirkby, and Noble 1994; McIntyre et al. 2008; Stenfors Arnesen, Fagerlund, and Granum 2008). In contrast, many strains of the Bc group member Bacillus thuringiensis (Bt) are known to be entomopathic, and some of them have been developed for large-scale commercial use in forestry (e.g., against spruce budworm, gypsy moth), agriculture (e.g., against corn borer, tobacco budworm) and prevention of disease dissemination (e.g., against mosquitos, blackflies). However, there are reports that some Bt strains are infectious to humans, and similar in properties to Bc strains examined (Damgaard et al. 1997; Hernandez et al. 1998, 1999, 2000; Jackson et al. 1995; McIntyre et al. 2008; Samples and Buettner 1983).
Genomic and proteomic comparisons showed that members of the Bc group are related and possess similar virulence factors, including enterotoxins, exotoxins, hemolysins, phospholipases, and proteases, many of which are controlled by a common regulator, PlcR (Gohar et al. 2005; Rasko et al. 2005; Sergeev et al. 2006, Stenfors Arnesen, Fagerlund, and Granum 2008; Vilas-Bôas, Peruca, and Arantes 2007). The molecular similarity (sequence and organization) of Bc group chromosomes strongly supports claims that existing species classification need to be re-evaluated (Helgason et al. 2000). Given the close relationship between Bc and Bt, there is a necessity for strain-specific data on their functional properties, especially as they relate to harmful attributes.
There are only a few reports of Bt infections in humans and animals (Hernandez et al. 1998, 1999, Salamitou et al. 2000; Siegel 2001; Siegel and Shadduck 1990; Siegel, Shadduck, and Szabo 1987). This lack of data creates a need for clarification about specific strain differences in relation to molecular identity, pathogenic capacity, and overlapping environmental niches. Furthermore, experimental exposures demonstrate conflicting information about the capacity of Bc and Bt to infect non-target animals. Hernandez et al. (1998, 1999) found that mice instilled intranasally with high doses (107–108 cfu per mouse) of either dormant spores or actively growing vegetative cells (VC) of Bc or Bt strains, showed hemorrhagic symptoms, neutrophil infiltration and death. Siegel and colleagues argued that death of mice does not occur with inhalation exposure, and that only intracerebral or intraperitoneal routes result in murine death (Siegel 2001; Siegel and Shadduck 1990; Siegel, Shadduck, and Szabo 1987).
Another Bacillus species, whose strains are often used in industrial applications is Bacillus subtilis (Bs). This species is generally recognized as safe (GRAS). However, like Bt, it is taxonomically complex (Rooney et al. 2009), and there is no published information on its inhalational safety. However, in the analysis of probiotic activity of various Bacillus strains and products, mice exposed intragastrically to spores of a Bs lab strain (PY79) showed increased tissue (spleen, liver, mesenteric lymph nodes, and submandibular glands) cytokine levels (TNF-α and IFN-γ) and plasma immunoglobulin G (Duc et al. 2004). In addition, it was suggested that Bs persists and possibly replicates in the gut, but is not disseminated beyond the gastrointestinal tract (Hoa et al. 2001).
Further successful safe exploitation of Bacillus species would benefit considerably from research which clarifies the species/strain differences of the types and potencies of various virulence factors. Towards this goal, the present study describes a screening methodology using in vivo experiments with indicators of early murine immune responses to evaluate the pathogenicity potential of Bacillus biotechnology strains relevant to the Canadian Environmental Protection Act (CEPA 1999). The methodology exploits immune markers indicative of acute lung inflammation, fever induction and acute phase response (APR) (Conti et al. 2004; Cray, Zaias, and Altman 2009; Kabir et al. 2002). Comparisons were made between strains of Bc and Bt, commercially used Bt subspecies kurstaki and israelensis biopesticides (referred to as CP1 and CP2, respectively), and a biotechnology-relevant Bs strain. The effects of purified spores, as well as spore-derived live and dead VC were also compared.
Materials and methods
Preparation of bacterial spores
Bacillus spp. were either obtained from the American Type Culture Collection (ATCC) or as commercial products (CP) from Valent Biosciences, Libertyville, IL (formerly Abbot Laboratories Inc.). The ATCC strains were B. cereus (Bc14579TM), B. subtilis (Bs6051a™), and B. thuringiensis (Bt13367™). The spore-containing CP, referred to as CP1 (Foray 48B, Bt spp. kurstaki) and CP2 (Vectobac 12AS, Bt spp. israelensis) were diluted in physiological saline, based on spore content calculated as colony forming units (cfu). To prepare pure ATCC Bt or Bs(a) spores, approximately 107cfu were incubated at 28°C for 10 days in 50 mL of either Luria Bertani (LB) broth (10 gL−1 Tryptone, 5 gL−1 Yeast Extract, 10 gL−1 NaCl) or 2X SG medium (16 gL−1 Difco Nutrient broth, 0.5 gL−1 MgSO4 · 7H2O, 2 gL−1 KCl, pH 7) supplemented with 1 mmol Ca(NO3)2 · 4H2O, 0.1 mmol MnCl2 · 4H2O, 1 ?mol FeSO4 · 7H2O, 0.1% (w/v) glucose. Bc spores were prepared according to the procedure of Gaillard et al. (2005). Sporulation plates (40 mgL−1 MnSO4, 100 mgL−1 CaCl2, 100 mgL−1 KCL absorbed into solidified nutrient broth-agar) were inoculated with 200 ?L of Bc liquid culture, and incubated at 37°C for 1 week. Bacterial cells were scraped from the plates and suspended in ddH2O. The amount of spores, VC and other culture components was monitored daily using a modification of the Schaeffer-Fulton method (Conn 1977). Briefly, preparations were stained with 5% (w/v) malachite green oxalate and counterstained with 3% (w/v) safranin (Sigma, Oakville, ON), and slide smears were visualized by light microscopy. When spore concentrations were >90%, the cell fraction was washed with saline, and any live VC were killed by heating at 60°C for 30 min. Spores were further purified from killed VC and other culture components with an aqueous-polymer two phase separation procedure (Sacks and Alderton 1961). This procedure yielded 99–100% viable Bacillus spores, which were concentrated to 108 cfu mL−1 and frozen at —80°C in PBS with 10% (v/v) glycerol until use. Spore viability and concentration were validated regularly.
Preparation of live and dead VC
Washed VC were generated by incubating spores for 72 h at 37°C in LB to yield cultures containing primarily live and dead VC, but also spores (<1%) and insoluble culture debris. These cultures were washed of culture supernatant and debris with three centrifugations (5000 × g, 10 min) and re-suspension steps using 5–10 volumes of sterile PBS. Both CP were efficient sporulators, and 72-h cultures contained 99% spores. Therefore, VC for CP-1 and CP-2 were derived from 18-h cultures grown at 37°C. For some experiments, VC were killed by heating at 100°C for 30 min or incubating with 50 ?g mL−1 gentamicin for 2 h, followed by three washes with PBS. To track dose delivery, killed Bc VC were stained with Alexa-Fluor555TM Succinimydl Ester (Molecular Probes, Eugene, OR) using a modification of the supplier's protocol. Briefly, bacteria were centrifuged at 5000 × g for 10 min, resuspended in 100 mmol sodium bicarbonate (pH 8.3) and mixed with Alexa dye. After constant agitation for 1 h, the stained bacteria were washed 3 × 10 min at 5000 × g to remove unbound dye and resuspended in fresh saline.
Animals and exposures
All procedures involving animals were approved by the Health Canada Animal Care Committee. Female Balb/c mice were purchased from Charles River Laboratories Inc. (Saint-Constant, Quebec) or Taconic Inc. (Germantown, NY), and were maintained and treated in a pathogen-free biosafety level 2 containment facility. Only animals between 18 and 23 g (8–10 weeks) that had been acclimated for at least 2 weeks were used for experiments. Mice had access to food and water ad libitum. For endotracheal instillation, mice were anesthetized with isoflurane and the 25 μL dose of bacteria or saline alone was aerosolized through the trachea into the lungs with a syringe-style nebulizer (MicrosprayerTM, Penn Century, Philadelphia, PA). Mice typically recovered from anaesthesia and dosing within 2 min. Either 3 or 6 mice were used for each treatment group.
The selection of the first time point for routine screening was done following pilot experiments that tracked tissue bacterial content and cytokine levels at 20 min, 2 and 4 h. These experiments demonstrated that the 2-h time point allowed for measurements of both cytokine and bacterial content of target organs. Even though the numbers of bacteria in tissue were lower compared to 20 min exposures, the 2-h interval permitted confirmation of dose delivery. Furthermore, the 2-h point was the earliest time that might accommodate dosing, blood collection and necropsy. The 4-h point was most useful for detecting protein expression changes in the lungs, blood, and liver, but bacterial counts were substantially reduced, preventing reliable estimates of target organ delivery. Some experiments were extended to 4 h to determine if recovery was possible, but the resultant severe shock-like symptoms observed at 4 h after Bc VC exposure deterred routine use of this time point.
Blood and tissue analysis
Treated mice were anesthetized and blood (approximately 500–1000 mL) was immediately collected by cardiac puncture and transferred to blood collection tubes containing ethylenediaminetetraacetic acid (EDTA). After mixing to prevent clots, the blood was centrifuged at 1000 × g for 10 min to collect plasma. The latter was stored at —80°C until use. Tissues (liver, lung, and trachea) were collected and also stored at —80°C. Plasma samples were analyzed for levels of circulating, pyrogenic cytokines, as well as Th1 and Th2 cytokines. These were measured with pre-conjugated bead kits (BioRad, Mississauga, ON) using a high throughput multiplexed bead array system (Bioplex, BioRad). Cytokines are listed here with their limit of detection in parentheses (pgmL−1): IL-1β (9.4), IL-2 (0.6), IL-4 (2.1), IL-5 (0.3), IL-6 (0.2), IL-12(p70) (2.3), TNF-α (1.4), granulocyte macrophagecolony stimulating factor (GM-CSF; 2.3), interferon-gamma (IFN-γ; 1.2). ELISA kits were used for validation of cytokine levels (Peprotech, Rocky Hill, NJ; R&D Systems, Minneapolis, MN), and also for measurement of fibrinogen (Innovative Research Inc., Southfield, MI; detection limit 30 ng mL−1) and serum amyloid A (SAA; Biosource International Inc., Camarillo, CA; detection limit 3 ng mL−1). The multiplex and ELISA assays were also used for measurement of cytokine and acute phase protein levels from lung and liver homogenized (PowerGen 125, Fisher Scientific) in the presence of a protease inhibitor cocktail (Roche, Laval, QC).
In order to measure the clearance of bacteria from tissues, animals were sacrificed by cervical dislocation at various times following exposure. The lung, trachea, and esophagus were excised and homogenized in 1 mL saline with a hand-held homogenizer (PowerGen 125, Fisher Scientific). Serial dilutions were plated on LB-agar plates, and cfu content was determined after incubation at 28°C for 18 h.
Granulocyte infiltration into tissues was monitored by immunofluorescence microscopy using antibodies to the surface glycoprotein Ly-6G of granulocytes. Tissue pieces were placed into 4% (w/v) paraformaldehyde for 18–24 h, followed by storage in 70% ethanol at 4°C until further processing. Tissues were sectioned with a vibrating microtome (Vibratome Inc., St. Louis, MO). Sections (25–60 ?m) were incubated for 60 min in blocking/incubation buffer (0.05% Tween-20), 90 min at 37°C in 1:50 rat anti-LY-6G (eBioscience, San Diego, CA) and 60 min with 1: 200 TRITC-conjugated anti-rat IgG. Two PBS rinses were conducted between each step. Sections were mounted with Prolong™ antifade solution (Molecular Probes, Eugene, OR) and viewed with a Nikon TE2000 inverted microscope with epifluorescence optics, connected to a Nikon C1 Laser Scanning Confocal unit with three lasers (488, 543, and 633 nm). Three random fields for each mouse (nine fields total for each group) were enumerated for LY-6G-positive cells.
Statistical analysis
The significance of the differences in Ly-6G, cytokines, and APR markers between bacteria-exposed mice and saline-treated mice was done by conducting an analysis of variance (ANOVA) followed by a post-hoc Dunnett's Multiple Comparison Test (Dunnett 1980). Statistical analyses were done in “R” version 2.11.1 (R Development Core Team 2010).
Results
Bacteria used for murine exposures
Purity and consistency of the bacterial preparations was ensured with quality control monitoring using a staining protocol in which spores, VC and debris were differentially stained. The micrographs in Figure 1 show smears of spores, washed VC and CP stained with Malachite Green (blue-green spores) and counterstained with safranin (red-orange VC and debris). Staining showed that the preparations of purified spores and CP contained no VC, but did contain some pinkish-red stained, amorphous, extracellular debris. Washed VC were predominantly composed of VC (< 1% spores). Based on cfu counts and hemocytometer measurements of total VC, the ratio of live to dead cells was approximately 1:10 for Bt VC, and 1: 1 for VC derived from Bs(a), Bt and both CP. As expected from previous studies (Seligy et al. 1997, 2000) CP contained large numbers of spores, as well as relatively large safranin-stained amorphous material and smaller regularly shaped structures, resembling parasporal inclusion bodies.
Figure 1.
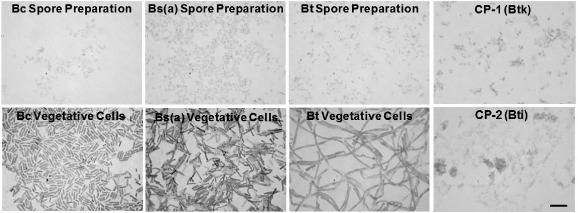
(Color online). Preparations of Bacillus strains used for murine exposures. Micrographs of Bacillus spores and washed VC were prepared as described in the section “Materials and methods”. Samples were dried onto slides and stained using a modification of the Schaeffer-Fulton Method, which results in blue-green spores (Malachite Green) and red-orange VC debris (Safranin). Bc, B. cereus 14579; Bs(a), B. subtilis 6051a; Bt, B. thuringiensis 13367; CP-1 (Btk), commercial product from Bt subsp. kurstaki; CP-2 (Bti), commercial product from Bt subsp. israelensis. Bar in lower right of CP-2 represents 5 ?m, which is the same scale for all micrographs.
Tissue clearance and persistence of Bacillus cells
To localize dose delivery within the airway, washed VC of Bc were labeled with a fluorescent dye before exposure. Spores were not used because they did not stain with sufficient intensity for microscopic detection. At 2-h after exposure with 106cfu per mouse, tissue was excised, fixed, and sectioned. Micrographs in Figure 2 show that fluorescently-labeled VC (orange) were localized around the bronchioles and alveoli (Figure 2a, inset). Other tissues had trace numbers of bacteria in the trachea (arrows in Figure 2b) and esophagus (not shown).
Figure 2.
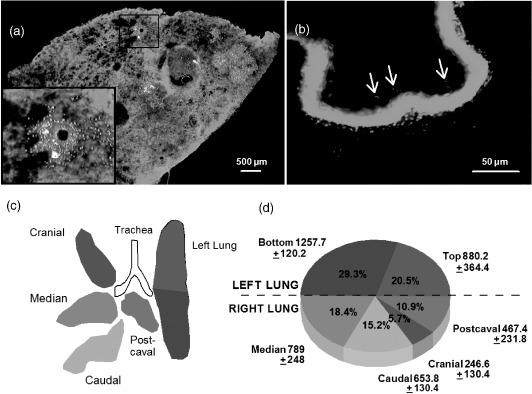
(Color online). Bacillus dose distribution in tissues. VC of Bc (106cfu per mouse) were labeled with Alexa Fluor 555 succinimydl ester and used for endotracheal exposures. Two hours after exposure, airway tissues were excised and either fixed and sectioned for microscopy, or homogenized and plated on LB-agar for bacterial enumerations. Micrographs show immunofluorescence of (a) lung sections with bacteria (orange) around the bronchioles and within alveoli, and (b) traces of bacteria in the trachea as indicated with arrows. Sytox™ Green (nuclei) was used as a counterstain for both (a) and (b). The pulmonary bacterial distribution at 2 h is shown in (c) and (d). The colors of lung segments in (c) correspond to the colors in the pie chart in (d). Numbers adjacent to pie segments in d are cfu mg−1 of tissue obtained from homogenized lung segments. Percentages in d represent the proportion of total cfu's recovered. Data are mean values from three mice ± standard deviation.
To determine the dose distribution within lungs following delivery by the endotracheal method, lung lobes were excised and homogenized, and serial dilutions were plated on LB-agar. Colonies were counted after incubation at 37°C for 18 h. Measurements in different areas of the lungs (Figure 2c, d) indicated bacteria were equally distributed between left and right lungs. For the right lung, the greatest proportion of bacteria was found in the median and caudal lobes, and fewest bacteria were in the cranial lobe.
Figure 3 summarizes bacterial counts in the lungs and trachea following exposure. At 2h for all strains, 1.3–341 cfu mg−1 (total 260–68,000 cfu lung−1) were recovered, which equates to 0.03–6.8% of the applied dose. Since 2 h was the earliest that could be done to accommodate all testing activities, including dosing and blood collection, an experiment for 20 min following exposure was done. Data revealed that almost the entire original dose could be retrieved from the trachea and lungs, which indicates that as much as 99% of pulmonary clearance occurs between 20 and 120 min. To determine if there was a difference in the rate of elimination of bacteria from the lung depending on the species, bacterial content was measured over a 1-week period. Bs(a) was cleared from the lungs within 48 h, at least 2 days before the other bacteria. The commercial Bt preparations showed delayed clearance from both the trachea and lungs since bacteria could still be recovered 3–7 days post-exposure. A difference between CP-1 and CP-2 was that mice exposed to CP-2 showed an apparent transitory fall in pulmonary and tracheal bacteria at 24 h post-exposure, followed by an elevation in bacterial counts in both tissues, which may represent transit from another tissue.
Figure 3.
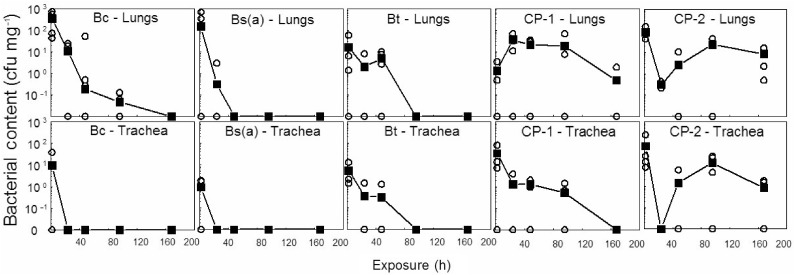
Tissue clearance and persistence of Bacillus cells. Mice inoculated endotracheally with 106cfu of spores or VC per mouse were sacrificed at various time intervals, and the lungs and trachea were homogenized and spread-plated for estimation of bacterial content. Means (filled squares) of 4 control and 3 exposed mice (empty circles) are shown.
Physical/behavioral symptoms of Bacillus exposure
The behavior and appearance of mice were monitored throughout every exposure for symptoms of discomfort or stress. Mice treated with 106 purified spores or CP showed no apparent symptoms over a 1-week period. Mice treated with 107 spores had noticeable ruffled fur at 24 h, but otherwise resembled saline-treated controls. In contrast, experiments involving washed Bc and Bt VC were prematurely terminated at 2h in accordance with animal care guidelines, because animals showed obvious signs of stress, including hypoactivity, hunched-over appearance, ruffled fur and respiratory distress. The proportion of treated animals that displayed such symptoms differed for Bc (all symptomatic), Bt (two of four symptomatic) and Bs(a) (asymptomatic).
Immune effects of purified spores, CP, and washed VC
Several biomarkers were monitored following endotracheal instillaton with 106cfu of either purified spores or diluted CPs. Enumeration of LY-6G-positive granulocytes, and pulmonary and blood inflammatory cytokines IL-1β, IL-6, and TNF-α), and Th1 and Th2 cytokines (IL-2, IL-4, IL-5, IL-12(p70), GM-CSF, IFN-γ) showed that there were no significant changes compared to saline-treated controls when monitored over a 1-week period, or even during weekly Bc spore exposures for 1 month. In contrast, confocal micrographs of exposures to washed Bc, Bt, and CP-1 VC showed a significant elevation in the number of pulmonary granulocytes (Figure 4b) compared to those treated with saline (Figure 4a). The box plots in Figure 4c illustrate that individual responses were variable, as indicated by the large area within the boxes. Yet the means were statistically different compared to controls. In contrast, the Bs(a) or CP-2 strains did not induce a significant elevation in granulocytes.
Figure 4.
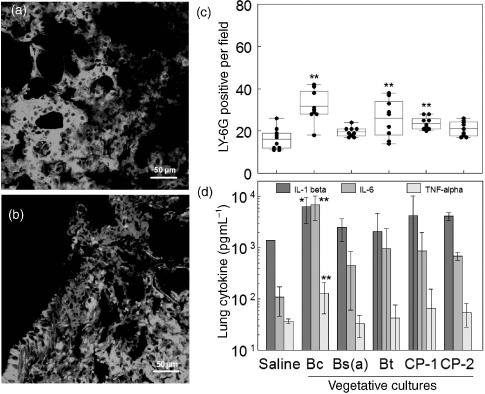
(Color online). Immune effects of washed VC. Three mice were instilled endotracheally with either saline or 106cfu per mouse of VC, and were sacrificed at 2-h post-exposure and lung sections were stained with rat anti-mouse-LY-6G and anti-rat-TRITC antibodies. Examples of confocal micrographs shown in (a) (saline) and (b) (Bc VC) were used to enumerate three random fields from each lung section of three mice (nine fields total per group) of LY-6G-positive cells (red-orange), and are summarized with box-whisker plots in (c). Box limits represent the 25th and 75th percentiles and whiskers represent the 5th and 95th percentiles. Means (horizontal line within boxes) are for 10 individual counts (solid circles). Pulmonary pyrogenic cytokine levels (d) were derived from homogenized lung tissue using a multiplex bead array system against antigenic standards supplied by the manufacturer. Error bars represent the standard deviation. Asterisks indicates significantly different values from pooled saline exposures, as determined using ANOVA and Dunnett's Multiple Comparison Test (∗p < 0.1; ∗∗p < 0.05).
The increased numbers of pulmonary granulocytes suggested that several cytokines may have been elicited by lung cells. To test this hypothesis, excised lung tissues and plasma of mice treated for 2 h with washed VC were screened for the pyrogenic cytokines IL-1β, IL-6, and TNF-α. None of the pyrogenic cytokines levels for any of the strains were significantly altered in plasma. In lung tissue, Bc showed increases in the mean levels of IL-1β, IL-6, and TNF-α by 4.5, 63, and 3.5-fold, respectively (Figure 4d) compared to controls.
Inactivated vegetative cell exposures
Washed VC contain variable amounts of dead, inactive VC. To test whether they could contribute to immunostimulation, mice were dosed with VC of each bacterial strain killed with either heat or gentamicin. Both inactivation methods resulted in no bacterial colonies after plating or bacterial metabolic activity after staining with MTT, which is a viability indicator that detects the presence of even a single metabolically active bacterium (Seligy et al. 1997). At a dose of 106 or 108 Bc inactivated VC (cfu-equivalents), exposed mice showed no symptoms at 2 h. There was also no significant elevation of granulocytes compared to controls. The only effects observed were an increase in the means of plasma IL-1? (4.6-fold) and TNF-α (12.4-fold). Mean levels of IL-6 did not change during any exposure to dead VC. Data demonstrate that live bacteria are necessary to generate the majority of effects observed during exposures to washed VC.
Dose effects of washed VC
To further investigate the effects of washed VC and determine the no observed effect level (NOEL) for immune responses, exposures were conducted with log10 serial dilutions of Bc, Bs(a), and Bt washed VC (106-102 cfu per mouse). These experiments showed that some animals (usually one of three) did not respond to the exposures and produced high standard deviations (Table 1). For those that did respond, pyrogenic cytokines were significantly elevated in blood and lungs for 105 and 106cfu doses of washed Bc VC but not significantly for the same doses of Bs(a) or Bt. The NOEL for cytokine changes was 104 cfu for both lung and blood.
Table 1.
Cytokine levels (pg mL−1) 2h after Bc14579 vegetative cell exposure.
| Dose (cfu per 25 μL) | IL-1β | IL-6 | TNF-α |
|---|---|---|---|
| Lung cytokines | |||
| 0 (vehicle) | 614.6±545.5 | 47.6±26.8 | 15.0±2.4 |
| 520.5±74.6 | 60.8±24.5 | 20.4±1.8 | |
| 103 | 322.07±91.0 | 42.4±26.5 | 12.2±3.0 |
| 104 | 592.6±387.6 | 43.0±24.1 | 9.8±3.8 |
| 105 | 6515.4±4571.1 | 6336.3±5226.7 | 181.5±169.8 |
| 106 | 6873.0±6321.9 | 9520.2±9478.3 | 271.6±379.6 |
| Plasma cytokines | |||
| 0 (vehicle) | Not detected | 4.6±1.4 | 0.0±0.0 |
| 102 | Not detected | 5.5±4.5 | 0.7±0.0 |
| 103 | Not detected | 3.5±0.9 | 0.7±0.0 |
| 104 | Not detected | 5.9±0.9 | 0.7±0.4 |
| 105 | Not detected | 133.6±149.6 | 3.3±3.6 |
| 106 | Not detected | 122.2±137.6 | 4.3±0.0 |
Notes: Mice (three per group) instilled endotracheally with either saline or various doses of VCs were sacrificed at 2-h post-exposure. Pyrogenic cytokines were measured in homogenized portions of lung tissue and plasma using a multiplex bead array system. The high standard deviation in some exposures resulted from usually one non-responsive animal per group.
Extended exposures with washed VC
To investigate whether longer exposures may result in recovery or worsened effects, 106 cfu of washed VC of each Bacillus strain was administered to mice, and observations were made 4-h post-exposure. All Bc-exposed animals had ruffled fur at 2 h, and 4 of 6 showed additional symptoms beginning at 2.5 h, which included hypoactivity and ocular discharge resulting in a ring of damp fur around the eyes. Effects did not diminish at 4h when the experiments were terminated. Almost equivalent effects were observed with mice treated with washed VC of Bt (2 of 3 positive), CP-1 (1 of 3 positive) or CP-2 (2 of 3 positive). However, still no marked effects were observed with Bs(a) exposure.
Plating of homogenized lung, trachea, and esophagus showed that the number of bacteria recovered was essentially equivalent at 2 and 4 h post-exposure. At 4 h, the mean number of pulmonary granulocytes was 6–9-fold greater compared to controls (Figure 5) and up to 2-fold greater than those at 2-h post-exposure. Levels of plasma and pulmonary cytokine markers, IL-1β, IL-6, and TNF-α, were also elevated (Figure 6a, b), but not substantially greater than those at 2 h. Compared to saline controls, Bc exposures produced mean levels of IL-1β, IL-6, and TNF-α to increase by 25, 15, and 16-fold, respectively in lung tissue, and 4.7, 11, and 27-fold, respectively in plasma. No statistical significance was observed with these data, suggesting that the elevation in cytokines levels previously observed at 2 h post-exposure was transient and probably diminishing by 4 h post-exposure.
Figure 5.
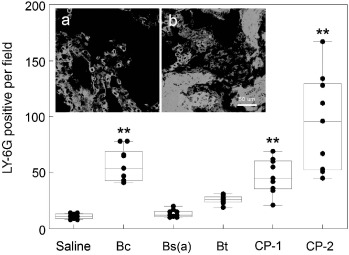
(Color online). Granulocyte infiltration 4 h after exposure. Three mice were instilled endotracheally with either saline or 106 cfu per mouse of VC, and were sacrificed at 4-h post-exposure. LY-6G-positive granulocytes were enumerated from three random fields of each lung section from three mice (nine fields total per group). Insets show lung sections from mice treated with either saline (a) or Bc (b). Asterisks indicates significantly different values from pooled saline exposures, as determined using ANOVA and Dunnett's Multiple Comparison Test (∗p < 0.1; ∗∗p < 0.05).
Figure 6.
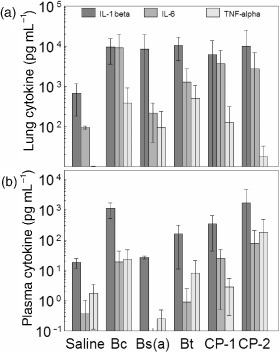
Pyrogenic cytokine levels 4 h after exposure. Mice instilled endotracheally with either saline or 106cfu per mouse of VC were sacrificed at 4-h post-exposure. Pyrogenic cytokine levels were measured using a multiplex bead array system in portions of lung tissue (a) and plasma (b). Data are mean values from three mice ± standard deviation.
The presence of pyrogenic cytokines in the circulation suggested that APR proteins may be expressed by the liver, since hepatic cells are known to possess receptors to these cytokines (Gruys et al. 2005). Therefore, two APR proteins, fibrinogen and serum amyloid A (SAA), known to be elevated in the liver and blood during APR response (Gruys et al. 2005) were tested. Measurement 2-h post-exposure did not show increases in either of these markers, but at 4-h post-Bc exposure both markers were elevated in the blood, and post-Bt exposure SAA was significantly elevated (Figure 7).
Figure 7.
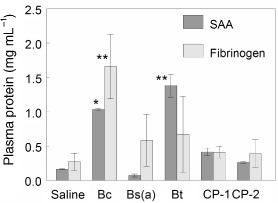
Levels of APR Indicators. Mice instilled endotracheally with either saline or 106cfu per mouse of VC were sacrificed at 4-h post-exposure. APR markers, fibrinogen and SAA were measured by ELISA. The graph summarizes plasma fibrinogen and SAA levels from 4-h after exposure to all sources of washed VC. Data are mean values from three mice ± standard deviation. Asterisks indicates significantly different values from pooled saline exposures, as determined using ANOVA and Dunnett's Multiple Comparison Test (∗p < 0.1; ∗∗p < 0.05).
Discussion
This study describes an animal screening methodology which builds on in vitro virulence tests in order to assist in differentiating between pathogenic and non-pathogenic Bacillus organisms proposed for use in biotechnology. One of the tenets of the ethical use of experimental animals is the reduction in the numbers used, so experiments were intentionally designed with a minimal number of animals per exposure group. The confidence in observations was enhanced with preliminary testing using in vitro cytotoxicity assays (Tayabali and Seligy 2000), employing toxicity indicators from various sampling sites (lung, liver, and blood) of the same animal, presentation of data in formats such as box plots to highlight where inter-animal variation is significant, as well as dose-response experiments and time courses to demonstrate reproducibility and trends. In some exposure groups, a proportion of animals were unresponsive, while the majority showed clear and large changes compared to saline-treated animals. Individual animals and their tissues were tracked throughout the analysis, and no significant correlation could be made with the cfu's recovered from the lungs, and extent of response observed. The reason for the inter-animal variability is currently unknown, but was observed to the same extent when the sample size was increased to 6 instead of 3 for 4-h post-exposure measurement of cytokines and APR markers. The larger sample sizes elevated the confidence in the data (lower p values), but did not alter the overall result, which still clearly demonstrated the potential effects of the exposure in affected animals.
The murine exposure data presented here show that for all strains, washed VC containing live VC are most immunoreactive and toxic (physical symptoms, local and systemic effects), and that the potency of spp. can be ranked as Bc > Bt > Bs(a). In comparison, dilute commercial Bt products (mainly CP-spores), purified spores, or dead VC elicited little or no inflammatory response. Since the spores were known to be viable (∼99%) up to the point of instillation, and there was low recovery of cfu from the lung at 2 h, this lack of inflammatory response may have been due to early clearance, inhibition of spore germination, or that early germinants were inactivated prior to replication. However, for both dead and live VC, there was an increase in expression of IL-1? and TNF-α, which is similar to the production of these cytokines by monocytes during lipopolysaccharide exposure (Schindler et al. 1990; Werling et al. 1996). The lack of IL-6 expression with killed VC may be due to the early stage in the cytokine expression cascade, since IL-6 is known to be a secondary inflammatory mediator (Uhlar and Whitehead 1999).
The immunostimulatory capacity of the three different Bacillus species was not the same, and may relate to findings recently reported. Ryu et al. (2009) showed that different species of gram-positive bacteria have different TNF-α and nitric oxide inducing capacities in RAW 264.7 macrophage, which were related to their respective lipotechoic acid (LTA) content. Further, Ryu et al. (2009) suggested that the greater D-alanine content of LTA from pathogenic species results in enhanced immunological properties. Elevated LTA D-alanine levels may function to increase the net negative charge of the bacterial cell wall, influencing interactions with mammalian cellular components.
The findings in the present study support investigations previously done in vitro with Bacillus spp. for rapid throughput screening of strains from public repositories and CP (Tayabali and Seligy 1995, 1997, 2000). These studies demonstrated that a number of animal cell lines were useful for predicting toxicity and the potential for eliciting innate immune responses. Spores from commercial sources, and type-strains of Bc and Bt, but not Bs(a), were not toxic if inactivated with antibiotic. However, if spores were permitted to germinate and replicate in the presence of HT29 epithelial cells, rapid and severe necrotic cell death was observed, which resulted from exoproteins produced during vegetative growth. The present experiments showed that VC exerted their effects beginning at about 1.5 h, and that equivalent numbers of killed VC did not produce similar pathogenesis as live VC. Our previous in vitro findings for effects of washed VC also reflected these observations and showed that the cytolytic effects resulting from toxin accumulation occurred at about 1.5 h, which is approximately equivalent in duration to one doubling of VC number (Tayabali and Seligy 2000). Data presented suggest that VC toxin production and replication probably also occurs in vivo.
Washed VC produced shock-like symptoms that corresponded to sensitive local and systemic cellular responses, and elevations in molecular indicators such as local inflammation, pyrogenic cytokines, and APR proteins. Biomarker elevations were dependent on the bacterial species: animals exposed to live Bc elicited the most inflammation, pyrogenicity, and early APR, whereas VC of Bt or Bs(a) resulted in relatively attenuated immune stimulations. One possible explanation for lowered APR from commercial Bt strains is their propensity to rapidly re-sporulate. Significant elevations of the APR proteins were detected (means were 2–3-fold compared to controls). These markers have been recommended for the diagnosis of bacterial infections (Gruys et al. 2005; Urbach et al. 2004). SAA levels increase 5–1000-fold in the blood beginning at 4–6 h (Uhlar and Whitehead 1999), making it a sensitive indicator of APR. The multiple roles of SAA in recovery from bacterial infections is well-documented, and was reviewed in depth by Uhlar and Whitehead (1999). During the APR, fibrinogen levels rise 2–3-fold, as observed in our study, and its role in coagulation is well known.
Some of our findings are consistent to those of Hernandez et al. (1999) and Salamitou et al. (2000) who reported that intranasal instillation of Bt subsp. Konkukian or Bc14579 (107–108 spores) and 108 Bt serotype 407, 3a3b or H34 induced shock-like symptoms such as nasal bleeding pulmonary hemorrhaging, neutrophil infiltration, and lesions from acute bronchitis, followed by mortality at 24 h. Their control soil Bt serotype H12 only produced limited pulmonary inflammation. These effects were observed with either spores or VC. However, our 24 h exposures with 107 spores of Bc (same strain they used), or spores of our other test strains, failed to induce any immune effects, but rather showed that bacteria were rapidly cleared from the lungs. Differences in methodologies could account for the difference in observations. First, our pilot experiments with saline alone showed that a 50 ?L volume, such as that used in studies by Hernandez et al. (1999) and Salamitou et al. (2000) was too large for the small Balb/c mice, and often resulted in death from asphyxiation, not produced by bacteria. Furthermore, significant precautions were taken to minimize the amount of VC material in our spore preparations (Materials and methods). Data presented demonstrate that killing VC by heat, which was used by Hernandez et al. (1999) in preparation of spores, results in dead VC that still produce low level inflammation. Another difference between experimental observations is that their spores persisted for at least 10 days, whereas our spore preparations were cleared by 99.9% within 4 days. This discrepancy may be due to the heat-killed VC debris present in their spore preparations, adding to the particulate burden in the lungs. This is reminiscent to the slow clearance observed with Bt CPs which contain large amounts of acellular material.
Siegel (2001) argued that the pathogenic effects seen by Hernandez et al. (1999) and Salamitou et al. (2000) are due to doses that were unrealistic by > 1000-fold based on human exposures from Bt spray operations (Noble, Riben, and Cook 1992). According to Burges (1980), a microbial control agent can be considered toxic if a dose of 106cfu per mouse produces mortality or clinical/pathological changes similar to the symptoms observed here. Our routine exposures were at, or below this level and all were sublethal for the duration of the study.
A recent study examined the lethal dose of VC and spores of clinical Bt strains using Balb/c mice exposed intratracheally by surgery (Ghelardi et al. 2007). The study revealed that VC produced 50% lethality with doses of 1.8 × 106cfu at 48 h after inoculation.
Similar to our study, no marked effects were observed at or below 7.2 × 104cfu, and all animals showed effects with 1.7 × 107cfu. Although our study was not designed to intentionally measure lethal effects, similar death rates with VC-treated animals may have been observed based on our observations at 4 h. Moribund animals in Ghelardi et al. (2007) study noted identical physical symptoms to those described here. Interestingly, spores from the same strains could also kill the mice, albeit with 20-fold greater cfu. The exaggerated effects observed by Ghelardi et al. (2007) may be invariably due to the virulence of their strains, and the relatively more invasive intratracheal route of exposure.
In our findings, none of the Bs(a)-treated mice showed physical symptoms, but high VC doses still resulted in increased pulmonary granulocytes. However, there were no increases in any cytokine, which is in contrast to Duc et al. (2004), who showed that Bs strains PY79 and probiotic Biosubtyl™ were able to induce IFN-γ and TNF-α from spleens, livers, submandibular glands, and mesentery lymph nodes of intragastrically infected Balb/c mice. The discrepancy in observations may be accounted for by considering their intragastric route of exposure, Bs strain-specific characteristics, and high dose level (109cfu).
Given the findings reported here, a general model for early immune responses resulting from exposure to these Bacillus strains can be summarized as follows. Spores at concentrations up to 107cfu per mouse and live VC up to 104cfu per mouse, were cleared by professional macrophages without intervention of granulocytes. No marked changes were observed in the levels of granulocyte infiltration (LY-6G) or tissue and blood cytokines, and yet almost all test bacteria were completely cleared from the lungs 1-week after exposure. In exposures containing >104 live VC, at least Bc was able to produce toxins, as observed in vitro (Tayabali and Seligy 2000). Macrophage-induced clearance was likely inadequate and necessitated augmentation with granulocyte action. This same mechanism of macrophage-assisted bacterial elimination at low doses, and impaired clearance and granulocyte recruitment at higher doses, was reported previously (Delclaux and Azoulay 2003; Marriott and Dockrell 2007). The recruitment of granulocytes is brought on by expression of pulmonary and possibly circulatory chemokines, which are expressed by macrophage and lung epithelial cells (Delclaux and Azoulay 2003). These granulocytes have potent microbicidal properties against Bacillus, as demonstrated by their ability to kill B. anthracis (Mayer-Scholl et al. 2005) and their involvement in the defence against Bt (Ghelardi et al. 2007). The more severe effects that were observed at 4 h after exposure to Bc VC resemble those of early APR, including elevated systemic levels of liver APR markers, fibrinogen and SAA (Cray, Zaias, and Altman 2009). In this model of Bacillus infection, it is not known whether our VC-exposed animals are capable of recovery without therapeutic intervention.
The testing regime used here was successful in comparing the toxicity of prioritized biotechnology-related Bacillus species, and may be applied to any microbe whose pathogenicity potential is uncertain. This study revealed that for clearance and early immune effects, pure spores, as well as other substances and additives in diluted CP, exhibit no observable effects compared to those elicited by live, metabolically active bacteria (VC). As such, the commercial preparations tested here are not expected to be toxic following inhalational exposure to non-target mammals, including humans, and should be safe when used as intended. Furthermore, data underscore the need for scrutiny of organisms beyond the genus and species levels, especially if proposed for large scale use. Specific strains and their purity need to be evaluated through their life cycle in product preparations, as well as various phases of proposed product use.
Acknowledgments
We thank Drs Phil Shwed, Hari Vijay and Ivan Curran of Health Canada for reviewing the manuscript and Dr Martha Navarro of the Health Canada Animal Resources Division (HC-ARD) for veterinary advice, technical help, and hematology analysis. We also thank Kevin Kittle, Douglas Parks, and Svetlana Popovic of HC-ARD for technical help, animal maintenance, dose administration and blood collection, as well as Remi Gagne and Andrew Williams for help with the “R”-based statistical analysis. This research was supported by the Canadian Regulatory System for Biotechnology (CRSB HC3.3/4.4) to VLS.
References
- Abusin S. Bhimaraj A., Khadra S. Bacillus cereus endocarditis in a permanent pacemaker: A case report. Cases Journal. 2008;1:95. doi: 10.1186/1757-1626-1-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burges H.D. Safety, testing and quality control of microbial pesticides. In: Burges H.D., editor. Microbial control of pests and plant diseases 1970–1980. New York: Academic Press Inc.; 1980. p. 738. 767. [Google Scholar]
- Canadian Environmental Protection Act (CEPA) 1999. Available at: http://laws.justice.gc.ca/en/C-15.31/text.html (accessed March 31, 2010)
- Castedo E. Castro A. Martin P. Roda J., Montero C.G. Bacillus cereus prosthetic valve endocarditis. Annals of Thoracic Surgery. 1999;68:2351–2. doi: 10.1016/s0003-4975(99)01163-7. [DOI] [PubMed] [Google Scholar]
- Conn H.J. Biological stains. 9th ed. Baltimore: The Williams & Wilkins Co.; 1977. [Google Scholar]
- Conti B. Tabarean I. Andrei C., Bartfai T. Cytokines and fever. Frontiers in Bioscience. 2004;9:1433–49. doi: 10.2741/1341. [DOI] [PubMed] [Google Scholar]
- Cray C. Zaias J., Altman N.H. Acute phase response in animals: A review. Comparative Medicine. 2009;59:517–26. [PMC free article] [PubMed] [Google Scholar]
- Damgaard P.H. Granum P.E. Bresciani J. Torregrossa M.V. Eilenberg J., Valentino L. Characterization of Bacillus thuringiensis isolated from infections in burn wounds. FEMS Immunoology and Medical Microbiology. 1997;18:47–53. doi: 10.1111/j.1574-695X.1997.tb01026.x. [DOI] [PubMed] [Google Scholar]
- David D.B. Kirkby G.R., Noble B.A. Bacillus cereus endophthalmitis. British Journal of Ophthalmology. 1994;78:577–80. doi: 10.1136/bjo.78.7.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclaux C., Azoulay E. Inflammatory response to infectious pulmonary injury. European Respiratory Journal. 2003;22(Suppl. 42):10s–4s. doi: 10.1183/09031936.03.00420203. [DOI] [PubMed] [Google Scholar]
- Duc L.H. Hong H.A. Barbosa T.M. Henriques A.O., Cutting S.M. Characterization of Bacillus probiotics available for human use. Applied and Environmental Microbiology. 2004;70:2161–71. doi: 10.1128/AEM.70.4.2161-2171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett C.W. Pairwise multiple comparisons in the unequal variance case. Journal of the American Statistical Association. 1980;75:796–800. [Google Scholar]
- Gaillard S. Leguerinel I. Savy N., Mafart P. Quantifying the combined effects of the heating time, the temperature and the recovery medium pH on the regrowth lag time of Bacillus cereus spores after a heat treatment. International Journal of Food Microbiology. 2005;105:53–8. doi: 10.1016/j.ijfoodmicro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Ghelardi E. Celandroni F. Salvetti S. Fiscarelli E., Senesi S. Bacillus thuringiensis pulmonary infection: Critical role for bacterial membrane-damaging toxins and host neutrophils. Microbes and Infection. 2007;9:591–8. doi: 10.1016/j.micinf.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Gohar M. Gilois N. Graveline R. Garreau C. Sanchis V., Lereclus D. A comparative study of Bacillus cereusBacillus thuringiensis and Bacillus anthracis extracellular proteomes. Proteomics. 2005;5:3696–711. doi: 10.1002/pmic.200401225. [DOI] [PubMed] [Google Scholar]
- Gruys E. Toussanint M.J.M. Niewold T.A., Koopmans S.J. Acute phase reaction and acute phase proteins. Journal of Zhejiang. University Science. 2005;6B:1045–56. doi: 10.1631/jzus.2005.B1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgason E. Okstad O.A. Caugant D.A. Johansen H.A. Fouet A. Mock M. Hegna I., Kolsto A. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis: One species on the basis of genetic evidence. Applied and Environmental Microbiology. 2000;66:2627–30. doi: 10.1128/aem.66.6.2627-2630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez E. Ramisse F. Cruel T. le Vangueresse R., Cavallo J.-D. Bacillus thuringiensis serotype H34 isolated from human and insecticidal strains serotypes 3a3b and H14 can lead to death of immunocompetent mice after pulmonary infection. FEMS Immunology and Medical Microbiology. 1999;24:43–7. doi: 10.1111/j.1574-695X.1999.tb01263.x. [DOI] [PubMed] [Google Scholar]
- Hernandez E. Ramisse F. Ducoureau J.P. Cruel T., Cavallo J.-D. Bacillus thuringiensis subsp. konkukian (serotype H34) superinfection: Case report and experimental evidence of pathogenicity in immunosuppressed mice. Journal of Clinical Microbiology. 1998;36:2138–9. doi: 10.1128/jcm.36.7.2138-2139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez E. Ramisse F. Gros P., Cavallo J. Super-infection by Bacillus thuringiensis H34 or 3a3b can lead to death in mice infected with the influenza A virus. FEMS Immunology and Medical Microbiology. 2000;29:177–81. doi: 10.1111/j.1574-695X.2000.tb01520.x. [DOI] [PubMed] [Google Scholar]
- Hoa T.T. Duc L.H. Isticato R. Baccigalupi L. Ricca E. Van P.H., Cutting S.M. Fate and dissemination of Bacillus subtilis spores in a murine model. Appllied and Environmental Microbiology. 2001;67:3819–23. doi: 10.1128/AEM.67.9.3819-3823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S.G. Goodbrand R.B. Ahmed R., Kasatiya S. Bacillus cereus and Bacillus thuringiensis isolated in a gastroenteritis outbreak investigation. Letters in Applied Microbiology. 1995;21:103–5. doi: 10.1111/j.1472-765x.1995.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Kabir K. Gelinas J.P. Chen M. Chen D. Zhang D. Luo X. Yang J.H. Carter D., Rabinovici R. Characterization of a murine model of endotoxin-induced acute lung injury. Shock. 2002;17:300–3. doi: 10.1097/00024382-200204000-00010. [DOI] [PubMed] [Google Scholar]
- Marriott H.M., Dockrell D.H. The role of the macrophage in lung disease mediated by bacteria. Experimental Lung Research. 2007;33:493–505. doi: 10.1080/01902140701756562. [DOI] [PubMed] [Google Scholar]
- Mayer-Scholl A. Hurwitz R. Brinkmann V. Schmid M. Jungblut P. Weinrauch Y., Zychlinsky A. Human neutrophils kill. Bacillus anthracis PloS Pathogens. 2005;1:179–86. doi: 10.1371/journal.ppat.0010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre L. Bernard K. Beniac D. Isaac-Renton J.L., Naseby D.C. Identification of Bacillus cereus group species, associated with food poisoning outbreaks in British Columbia, Canada. Applied and Environmental Microbiology. 2008;74:7451–3. doi: 10.1128/AEM.01284-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M.A. Riben P.D., Cook G.J. Microbiological and epidemiological surveillance programme to monitor the health effects of Foray 48B Btk spray. 1992. Report to the British Columbia Ministry of Forests, September 30 1992.
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. ISBN 3-900051-07-0, URL http://www.R-project.org. [Google Scholar]
- Rasko D.A. Altherr M.R. Han C.S., Ravel J. Genomics of the Bacillus cereus group of organisms. FEMS Microbiology Reviews. 2005;29:303–29. doi: 10.1016/j.femsre.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Rooney A.P. Price N.P. Ehrhardt C. Swezey J.L., Bannan J.D. Phylogeny and molecular taxonomy of the Bacillus subtilis species complex and description of Bacillus subtilis subsp. inaquosorum subsp. nov. International Journal of Systematic Evolutionary Microbiology. 2009;59:2429–36. doi: 10.1099/ijs.0.009126-0. [DOI] [PubMed] [Google Scholar]
- Ryu Y.E. Baik J.E. Yang J.S. Kang S. Im J. Yun C. Kim D.W., et al. Differential immunostimulatory effects of gram-positive bacteria due to their lipotechoic acids. International Immunophamacology. 2009;9:127–33. doi: 10.1016/j.intimp.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Sacks L.E., Alderton G. Behavior of bacterial spores in aqueous polymer two-phase systems. Journal of Bacteriology. 1961;82:331–41. doi: 10.1128/jb.82.3.331-341.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamitou S. Ramisse F. Brehélin M. Bourguet D. Gilois N. Gominet M. Hernandez E., Lereclus D. The plcR regulon is involved in the opportunistic properties of Bacillus thuringiensis and Bacillus cereus in mice and insects. Microbiology. 2000;146:2825–32. doi: 10.1099/00221287-146-11-2825. [DOI] [PubMed] [Google Scholar]
- Samples J.R., Buettner H. Ocular infection caused by a biological insecticide. Journal of Infectious Diseases. 1983;148:614. doi: 10.1093/infdis/148.3.614. [DOI] [PubMed] [Google Scholar]
- Schindler R. Macilla J. Endres S. Ghorbani R. Clark S.C., Dinarello C.A. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990;75:40–7. [PubMed] [Google Scholar]
- Seligy V.L. Beggs R.W. Rancourt J.M., Tayabali A.F. Quantitative bioreduction assays for calibrating spore content and viability of commercial Bacillus thuringiensis insecticides. Journal of Industrial Microbiology and Biotechnology. 1997;18:370–8. [Google Scholar]
- Seligy V.L. Douglas G.R. Rancourt J.M. Tayabali A.F. Otvos I. van Frankenhuyzen K. Dugal J. Rousseau G., Szabo A.G. Comparative performance of conventional and molecular dosimetry methods in environmental biomonitoring: Assessment using Bacillus-based commercial biopesticides as models. In: Stopa P.J., editor. Rapid methods for the analysis of biological materials in the environment, NATO ASI Series. Netherlands: Kluwer Academic Publishers; 2000. pp. 279–97. [Google Scholar]
- Sergeev N. Distler M. Vargas M. Chizhikov V. Herold K.E., Rasooly A. Microarray analysis of Bacillus cereus group virulence factors. Journal of Microbiology Methods. 2006;65:488–502. doi: 10.1016/j.mimet.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Siegel J.P. The mammalian safety of Bacillus thuringiensis-based insecticides. Journal of Invertebrate Pathology. 2001;77:13–21. doi: 10.1006/jipa.2000.5000. [DOI] [PubMed] [Google Scholar]
- Siegel J.P., Shadduck J.A. Clearance of Bacillus sphaericus and Bacillus thuringiensis ssp. israelensis from mammals. Journal of Economic Entomology. 1990;83:347–55. doi: 10.1093/jee/83.2.347. [DOI] [PubMed] [Google Scholar]
- Siegel J.P. Shadduck J.A., Szabo J. Safety of the entomopathogen Bacillus thuringiensis var. israelensis for mammals. Journal of Economic Entomology. 1987;80:717–23. doi: 10.1093/jee/80.4.717. [DOI] [PubMed] [Google Scholar]
- Stenfors Arnesen L.P. Fagerlund A., Granum P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiology Review. 2008;32:579–606. doi: 10.1111/j.1574-6976.2008.00112.x. [DOI] [PubMed] [Google Scholar]
- Tayabali A.F., Seligy V.L. Semiautomated quantification of cytotoxic damage in cultured insect cells exposed to commercial Bacillus thuringiensis biopesticides. Journal of Applied Toxicology. 1995;15:365–73. doi: 10.1002/jat.2550150505. [DOI] [PubMed] [Google Scholar]
- Tayabali A.F., Seligy V.L. Cell integrity biomarkers and evaluation of cytotoxic responses of Bacillus thuringiensis-containing commercial bioinsecticides. Ecotoxicology and Environmental Safety. 1997;37:152–62. doi: 10.1006/eesa.1997.1525. [DOI] [PubMed] [Google Scholar]
- Tayabali A.F., Seligy V.L. Human cell exposure assays of Bacillus thuringiensis commercial insecticides: Production of Bacillus cereus-like cytolytic effects from outgrowth of spores. Environmental Health Perspectives. 2000;108:919–30. doi: 10.1289/ehp.00108919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlar C.M., Whitehead A.S. Serum amyloid A, the major vertebrate acute-phase reactant. European Journal of Biochemistry. 1999;265:501–23. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- Urbach J. Shapira I. Branski D., Berliner S. Acute phase response in the diagnosis of bacterial infections in children. Pediatric. Infectious Disease Journal. 2004;23:159–60. doi: 10.1097/01.inf.0000115735.78960.a4. [DOI] [PubMed] [Google Scholar]
- Vilas-Bôas G.T. Peruca A.P., Arantes O.M. Biology and taxonomy of Bacillus cereus, Bacillus anthracis, and Bacillus thuringiensis. Canadian Journal of Microbiology. 2007;53:673–87. doi: 10.1139/W07-029. [DOI] [PubMed] [Google Scholar]
- Werling D. Sutter F. Arnold M. Kun G. Tooten P.C.J., Gruys E. Characterization of the acute phase response of heifers to a prolonged low dose infusion of lipopolysaccharide. Research in Veterinary Science. 1996;61:252–7. doi: 10.1016/s0034-5288(96)90073-9. [DOI] [PubMed] [Google Scholar]


