Abstract
Humans are potentially exposed to phthalate esters (PEs) through ingestion, inhalation, and dermal contact. Studies quantifying exposure to PEs include “biomarker studies” and “indirect studies.” Biomarker studies use measurements of PE metabolites in urine to back-calculate exposure to the parent diester, while indirect studies use the concentration of the PE in each medium of exposure and the rate of intake of that medium to quantify intake of the PE. In this review, exposure estimates from biomarker and indirect studies are compiled and compared for seven PEs to determine if there are regional differences and if there is a preferred approach. The indirect and biomarker methods generally agree with each other within an order of magnitude and discrepancies are explained by difficulties in accounting for use of consumer products, uncertainty concerning absorption, regional differences, and temporal changes. No single method is preferred for estimating intake of all PEs; it is suggested that biomarker estimates be used for low molecular weight PEs for which it is difficult to quantify all sources of exposure and either indirect or biomarker methods be used for higher molecular weight PEs. The indirect methods are useful in identifying sources of exposure while the biomarker methods quantify exposure.
Keywords: phthalate ester, human exposure, biomarker
INTRODUCTION
Phthalate esters (PEs) are a diverse group of chemicals having a vast range of applications (Stanley et al. 2003). The higher molecular weight PEs are added to vinyl resin to improve its flexibility; di-2-ethylhexyl phthalate (DEHP), di-isononyl phthalate (DiNP), and di-isodecyl phthalate (DiDP) are the predominant PEs used as vinyl plasticizers. The lower molecular weight PEs have a considerable range of applications. Dimethyl phthalate (DMP) is used as a stabilizing diluent for the shipping and storage of organic peroxides. Diethyl phthalate (DEP) is used as a fixative or carrier for perfumes and fragrances and also in time-released pharmaceuticals. Dibutyl phthalate (DBP) and diisobutyl phthalate (DiBP) are used in vinyl acetate emulsion adhesives and in cellulose lacquers. Butyl benzyl phthalate (BBP) is normally used with other general-purpose plasticizers in polyvinyl chloride applications.
PEs have been measured in numerous media, including: surface water, groundwater, landfill leachate, drinking water, sediment, suspended particulate matter, soil, air (outdoor and indoor), dust, precipitation, wastewater, sewage sludge, food, vegetation, and wildlife (Clark et al. 2003a). Weathering of plastics and other PE-containing articles results in the release of PEs to the environment, including air and water (Bauer 1997; Michael et al. 1984; Tabor and Loper 1985). The source of PEs in indoor air, dust, or soil may be from weathering of products containing PEs or directly from household products containing PEs. Humans may be exposed to PEs simultaneously through a variety of exposure pathways, including ingestion of food, drinking water, dust, and soil; and inhalation of air (outdoors and indoors). The use of the lower molecular weight PEs in consumer products such as cosmetics and pharmaceuticals may result in their direct release to air or direct absorption through the skin or gastrointestinal tract. The number of household products containing PEs is not clear, nor is it clear how these products might contribute to overall exposure.
PEs have been measured in human milk, blood, and urine (Zhu et al. 2006; Hogberg et al. 2008; Koo and Lee 2005), and their metabolites have been measured in human urine, blood, amniotic fluid, and milk (Barr et al. 2003; Calafat et al. 2004, 2006; Teitelbaum et al. 2008). The largest database of metabolic concentrations in biological fluids is from the NHANES (National Health and Nutrition Examination Survey) analyses conducted by the Centers for Disease Control and Prevention (CDC 2001, 2003, 2005, 2009) in which the metabolites of the major PEs were measured relative to a unit of volume and in comparison to the amount of creatinine present. Other surveys of limited populations in Europe have also been published. While the attraction of using this information is high, there are limitations and difficulties in applying it to estimating exposure.
Numerous studies have quantified human exposure to PEs. These studies may be grouped into two types, “indirect” and “biomarker” studies. Indirect studies use the concentration of the PE in each medium of exposure (e.g., air, water, food, consumer product, etc.) and the rate of intake of that medium (e.g., inhalation or ingestion rates) to quantify the intake of the PE. Biomarker studies use measurements of PE metabolites in urine to back-calculate exposure to the parent diester.
The indirect studies require quality data concerning the concentration of the PE in every medium to which humans may be exposed and also the intake rate of each medium. These estimates provide information on a population level because individual habits may vary from the intake estimated for each population. The indirect studies may help elucidate the source(s) of exposure and the relative importance of the various exposure pathways. They are, however, plagued by contamination issues and require rigorous sample handling to exclude PE contamination from sources inside and outside the analytical laboratory. In many studies, contamination issues lead to false high values.
The biomarker studies are less subject to concerns about contamination of samples with the diesters compared with the indirect studies because the metabolites are far less likely to arise from sample contamination. Biomarker studies, however, do not provide any information about the source(s) of exposure and are susceptible to physiological variability. The biomarker studies also require an understanding of the metabolism of the parent diester, which may differ for different PEs. Furthermore, normalizing urinary concentrations of metabolites to a constant such as excreted creatinine, which can account for variation in urinary output, is necessary for comparison. However, creatinine excretion can vary with age and gender, and possibly race (Barr et al. 2005). All these factors make using biomonitoring data a challenge. However, biomonitoring data can provide information on an individual basis, which may be useful to evaluate exposure-related effects.
In this article, estimates of exposure to PEs from indirect studies and biomarker studies are compiled for: DMP, DEP, DBP, DiBP, BBP, DEHP, and DiNP. The results are compared to determine if there are regional differences and to determine if one approach is preferred over the other.
METHODOLOGY
Indirect Studies
The general procedure used to estimate intake includes the following steps: description of exposure to the various media containing the PEs; assigning a concentration of the PE in each medium; and assigning an intake rate for that medium. Inclusion of absorption factors for the various media converts the estimated intakes into uptakes, facilitating a more direct comparison with the biomarker studies. Uptake is calculated for each medium and then summed, using the following equation:
where: D = Absorbed dose of PE (μg/kg/d), Ci = Concentration of PE in medium (μg/g), IRi = Intake rate of medium (g/d), Ai = Absorption factor (unitless), BW = Body weight (kg)
The intake rates and concentrations in each medium, used for the indirect exposure estimates, are summarized in Tables 1 and 2 and are discussed below. The source of information for intake rates is primarily Health and Welfare Canada (1993) and Health Canada (1995). Additional details and an explanation of the selected distributions are provided in Clark et al. (2003b). An absorption factor of 100% was used for all calculations; however, it is recognized that this will overestimate uptake.
Table 1.
Intake rates used to calculate indirect exposure.
| Adult (20 to 70 y) |
Teen (12 to 19 7) |
Child (5 to 11 7) |
Toddler (0.5 to 4 7) |
Neonate (0 to 0.5 7) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Input Parameter | Units | Dist | Mean | Std Dev | Dist | Mean | Std Dev | Dist | Mean | Std Dev | Dist | Mean | Std Dev | Dist | Mean | Std Dev |
| General receptor characteristics | ||||||||||||||||
| Body weight | kg | LN | 71 | 14 | LN | 60 | 14 | LN | 27 | 7.3 | LN | 15 | 3.8 | LN | 7.5 | 3.2 |
| Inhalation rate | m3/d | LN | 16 | 3.9 | LN | 16 | 4 | LN | 15 | 3.2 | LN | 9.3 | 2.6 | LN | 2.1 | 0.57 |
| Receptor ingestion rates | ||||||||||||||||
| Tap water | L/d | LN | 0.8 | 0.52 | LN | 1 | 0.67 | LN | 1.1 | 0.7 | LN | 0.7 | 0.46 | LN | 0.8 | 0.52 |
| Beverages | L/d | LN | 0.96 | 0.62 | LN | 0.43 | 0.28 | LN | 0.23 | 0.15 | LN | 0.12 | 0.08 | — | — | — |
| Cereals | g/d | LN | 27 | 16 | LN | 24 | 15 | LN | 34 | 22 | LN | 42 | 27 | — | — | — |
| Dairy products (excl. milk) | g/d | LN | 53 | 34 | LN | 50 | 33 | LN | 45 | 29 | LN | 38 | 25 | — | — | — |
| Eggs | g/d | LN | 32 | 21 | LN | 22 | 14 | LN | 21 | 14 | LN | 24 | 16 | — | — | — |
| Fats and oils | g/d | LN | 25 | 16 | LN | 29 | 19 | LN | 21 | 14 | LN | 11 | 7.1 | — | — | — |
| Fish | g/d | LN | 14 | 9 | LN | 11 | 7.3 | LN | 8.4 | 5.5 | LN | 3.4 | 2.2 | — | — | — |
| Fruits | g/d | LN | 190 | 120 | LN | 160 | 100 | LN | 200 | 130 | LN | 190 | 120 | — | — | — |
| Grains | g/d | LN | 160 | 100 | LN | 210 | 130 | LN | 190 | 120 | LN | 90 | 58 | — | — | — |
| Meats | g/d | LN | 95 | 61 | LN | 93 | 60 | LN | 55 | 36 | LN | 38 | 25 | — | — | — |
| Milk | L/d | LN | 0.23 | 0.15 | LN | 0.523 | 0.34 | LN | 0.564 | 0.37 | LN | 0.632 | 0.41 | — | — | — |
| Nuts and beans | g/d | LN | 28 | 18 | LN | 31 | 20 | LN | 24 | 15 | LN | 15 | 9.7 | — | — | — |
| Other foods | g/d | LN | 220 | 144 | LN | 250 | 160 | LN | 210 | 140 | LN | 270 | 180 | — | — | — |
| Poultry | g/d | LN | 21 | 14 | LN | 20 | 13 | LN | 17 | 11 | LN | 13 | 8.6 | — | — | — |
| Processed meats | g/d | LN | 22 | 14 | LN | 23 | 15 | LN | 19 | 12 | LN | 11 | 7 | — | — | — |
| Vegetables | g/d | LN | 230 | 150 | LN | 240 | 150 | LN | 190 | 120 | LN | 120 | 76 | — | — | — |
| Infant formula (powder) | g/d | — | — | — | — | — | — | — | — | — | — | — | — | LN | 130 | 85 |
| Breast milk | L/d | — | — | — | — | — | — | — | — | — | — | — | — | LN | 0.75 | 0.49 |
| Total food | g/d | LN | 2300 | 1495 | LN | 2100 | 1365 | LN | 1800 | 1170 | LN | 1500 | 975 | LN | 820 | 533 |
| Incidental soil | mg/d | LN | 40 | 100 | LN | 40 | 100 | LN | 40 | 100 | LN | 40 | 100 | LN | 40 | 100 |
| Incidental dust | mg/d | LN | 40 | 100 | LN | 40 | 100 | LN | 40 | 100 | LN | 40 | 100 | LN | 40 | 100 |
| Exposure frequency | ||||||||||||||||
| Time spent indoors | h/d | U | 20 to 24 | U | 20 to 24 | U | 20 to 24 | U | 20 to 24 | U | 20 to 24 | |||||
Dist = Distribution type; LN = log normal; U = uniform; References for information provided in Clark et al. (2003b).
Table 2.
Concentrations used to calculate indirect exposure.
| DMP |
DEP |
DBP |
DiBP |
BBP |
DEHP |
DiNP |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | Units | Dist | Mean | Std Dev | Dist | Mean | Std Dev | Dist | Mean | Std Dev | Dist | Mean | Std Dev | Dist | Mean | Std Dev | Dist | Mean | Std Dev | Dist | Mean | Std Dev |
| MEDIUM | ||||||||||||||||||||||
| Outdoor air | μg/m3 | LN | 0.0033 | 0.0021 | LN | 0.013 | 0.0085 | LN | 0.012 | 0.008 | LN | 0.015 | 0.01 | LN | 0.002 | 0.001 | LN | 0.018 | 0.01 | LN | 0.01 | 0.007 |
| Indoor air | μg/m3 | LN | 0.923 | 0.60 | LN | 0.91 | 0.59 | LN | 1.06 | 0.69 | LN | 0.5 | 0.3 | LN | 0.042 | 0.027 | LN | 0.274 | 0.18 | LN | 0.011 | 0.007 |
| Drinking water | μg/L | LN | 0.027 | 0.018 | LN | 0.12 | 0.08 | LN | 0.19 | 0.12 | LN | 0.26 | 0.17 | LN | 0.06 | 0.04 | LN | 1.8 | 1.2 | C | 0 | — |
| Ingested soil | μg/g | LN | 0.0002 | 0.00013 | LN | 0.0023 | 0.0015 | LN | 0.011 | 0.007 | LN | 0.017 | 0.011 | LN | 0.0036 | 0.0023 | LN | 0.025 | 0.016 | LN | 0.011 | 0.007 |
| Ingested dust | μg/g | LN | 2.0 | 1.3 | LN | 25 | 16 | LN | 132 | 86 | LN | 86 | 56 | LN | 236 | 153 | LN | 901 | 586 | LN | 420 | 273 |
| Food | ||||||||||||||||||||||
| Beverages excl. water | μg/L | C | 0 | — | — | — | — | — | — | — | LN | 6 | 3.9 | — | — | — | — | — | — | — | — | — |
| Cereals | μg/g | C | 0 | — | — | — | — | — | — | — | C | 0 | — | — | — | — | — | — | — | — | — | — |
| Dairy products | μg/g | C | 0 | — | — | — | — | — | — | — | C | 0 | — | — | — | — | — | — | — | — | — | — |
| Eggs | μg/g | C | 0 | — | — | — | — | — | — | — | LN | 0.1 | 0.07 | — | — | — | — | — | — | — | — | — |
| Fats and oils | μg/g | C | 0 | — | — | — | — | — | — | — | C | 0 | — | — | — | — | — | — | — | — | — | — |
| Fish | μg/g | LN | 0.0012 | 0.0008 | — | — | — | — | — | — | LN | 0.011 | 0.008 | — | — | — | — | — | — | — | — | — |
| Fruit products | μg/g | C | 0 | — | — | — | — | — | — | — | LN | 0.03 | 0.02 | — | — | — | — | — | — | — | — | — |
| Grains | μg/g | C | 0 | — | — | — | — | — | — | — | LN | 0.13 | 0.08 | — | — | — | — | — | — | — | — | — |
| Meats | μg/g | C | 0 | — | — | — | — | — | — | — | LN | 0.05 | 0.03 | — | — | — | — | — | — | — | — | — |
| Milk | μg/L | LN | 0.7 | 0.5 | — | — | — | — | — | — | LN | 17 | 11 | — | — | — | — | — | — | — | — | — |
| Nuts and beans | μg/g | C | 0 | — | — | — | — | — | — | — | C | 0 | — | — | — | — | — | — | — | — | — | — |
| Other foods | μg/g | C | 0 | — | — | — | — | — | — | — | C | 0 | — | — | — | — | — | — | — | — | — | — |
| Poultry | μg/g | C | 0 | — | — | — | — | — | — | — | LN | 0.06 | 0.04 | — | — | — | — | — | — | — | — | — |
| Processed meats | μg/g | C | 0 | — | — | — | — | — | — | — | LN | 0.03 | 0.02 | — | — | — | — | — | — | — | — | — |
| Vegetable products | μg/g | C | 0 | — | — | — | — | — | — | — | LN | 0.005 | 0.003 | — | — | — | — | — | — | — | — | — |
| Infant formula — powder | μg/g | C | 0 | — | C | 0 | — | LN | 0.048 | 0.031 | LN | 0.06 | 0.04 | LN | 0.003 | 0.002 | LN | 0.15 | 0.10 | C | 0 | — |
| Breast milk | μg/g | C | 0 | — | LN | 0.00031 | 0.0002 | LN | 0.0015 | 0.001 | C | 0 | — | LN | 0.0008 | 0.0005 | LN | 0.148 | 0.096 | C | 0 | — |
| Composite diet samples | μg/g | — | — | — | T | 0.0001,0.0002, | 0.026 | LN | 0.033 | 0.021 | — | — | — | LN | 0.014 | 0.009 | LN | 0.39 | 0.25 | LN | 0.018 | 0.012 |
Dist. = Distribution type; LN = log normal; C = constant, T = triangular.
Measured concentrations obtained from numerous references, contained in ACC database (Clark 2008).
Exposure via food may be evaluated by determining concentrations in a wide variety of foods (often called market basket surveys) and then quantifying typical consumption of each of those foods; however, the market basket survey data for the PEs were collected 20 years ago (e.g., Page and Lacroix 1995). Recent measurements of PEs in foods tend to be for composite diets (e.g., Fromme et al. 2007b; Tsumura et al. 2001a,b and 2003; Wilson et al. 2001 and 2003; Petersen and Breindahl 2000) or for a few selected foods and not for a wide range of foods typical of the diets of most individuals.
Some of the indirect studies evaluate only selected exposure pathways (e.g., ingestion of food and exposure to environmental media), whereas the Wormuth et al. (2006) study also includes exposure to consumer products via ingestion, inhalation, and dermal contact. Inclusion of consumer products provides a more comprehensive evaluation of potential exposures to the users of those products; however, it will overestimate exposures for individuals who are not product users. In addition, the estimates of exposure due to use of consumer products are confounded by very limited information concerning the concentrations of PEs in the products, the scenarios of use including intake rates, and absorption factors.
Human exposure to five PEs: DMP, DEP, DBP, BBP, and DEHP found in food, air, drinking water, soil, and dust was evaluated using information in the American Chemistry Council (ACC) database (Clark et al. 2003b). The exposure assessments have been updated using concentrations in the most recent version of the ACC database, as summarized in Table 2, and assessments are added for two additional PEs: DiBP and DiNP. The ACC database is comprised of more than 500 references reporting measurements of PEs in various media. The references have been reviewed and categorized in terms of data quality, on the basis of analytical and sampling methodologies and reporting of quality assurance and quality control measures; data categorized as “not reliable” are not included in the summary in Table 2.
As an example of the indirect study calculation, the mean daily uptake of DEHP for an adult, assuming 100% absorption for all exposure pathways, is:
Total absorbed dose = food + indoor air + outdoor air + drinking water + soil + dust = (0.39 μg/g × 2300 g/d × 1 + 0.274 μg/m3 × 16 m3/d × 22 h/24 h × 1 + 0.018 μg/m3 × 16 m3/d × 2 h/24 h × 1 + 1.8 μg/L × 0.8 L/d × 1 + 0.025 μg/g × 0.040 g/d × 1 + 901 μg/g × 0.040 g/d × 1)/71 kg = 13 μg/kg/d
The above calculation uses the mean values of the distributions, presented in Tables 1 and 2, whereas the results of the calculations presented in Tables 3 to 10 were performed using the distributions of values with the software Crystal BallTM (Oracle Corporation). Use of the distributions allows calculation of the median and 95th percentile values, which are the values presented in Tables 3 to 10. The preponderance of lognormal distributions as inputs results in median values that are less than the mean.
Table 3.
DMP exposure estimates.
| DMP Intake (μgkg −1d−1) |
|||||
|---|---|---|---|---|---|
| Study | Adult | Teen | Child | Toddler | Infant |
| Present evaluation | |||||
| Update to Clark et al. (2003b), using concentrations in Table 2. Median intake, based on ingestion of food, drinking water, dust/soil, and inhalation of air using data compiled from various countries and various years. Data format: median (95th percentile) | Age 20 to 70 y: 0.16 (0.48) | Age 12 to 19 y: 0.19 (0.60) | Age 5 to 11 y: 0.40 (1.2) | Age 0.5 to 4y: 0.47 (1.4) | Age 0 to 0.5 y: 0.22 (0.8) |
| Clark et al. (2003b) | |||||
| Median intake, based on ingestion of food, drinking water, dust/soil, and inhalation of air using data compiled from various countries and various years | Age 20 to 70 y: 0.7 | Age 12 to 19 y: 0.7 | Age 5 to 11 y: 1.4 | Age 0.5 to 4y: 1.6 | Age 0 to 0.5 y: 0.05 |
| Wormuth et al. (2006) + suppl data | |||||
| Europe: based on oral, inhalation, and dermal exposure pathways, including consumer products; data format = low, intermediate, high estimate; F = female; M = male Fromme et al. (2007b) | Age 18 to 80 y:F: 0.06; 0.94; 7.31 M: 0.08; 0.85; 5.99 | Age 11 to 18 y:F: 0.07; 0.59; 3.50 M: 0.05; 0.53; 3.55 | Age 4 to 10 y: 0.05; 0.46; 5.92 | Age 1 to 3 y: 0.08; 0.76; 9.78 | Age 0 to 1 y: 0.2; 1.27; 16.97 |
| Germany (2005): intake estimated from composite dietary samples collected over 7 days; N = 50 (27 female + 23 male) | Age 14 to 60 y: 0.11 (median); 0.18 (95th percentile); 0.05–0.26 (range) | — | — | — | |
| Itoh et al. (2007) | |||||
| Japan: | |||||
| Based on ingestion of food and inhalation of indoor air; data compiled from various sources | 0.38 (mean) | — | — | — | — |
| Calculated from urinary metabolite data for MMP (2004); N = 35 | 1.4 to 2.0 (range of means); 0.60 to 0.87 (range of geo means) | — | — | — | — |
| CDC (2005)a | |||||
| USA (NHANES 2001–2002): Calculated from urinary metabolite data for MMP; data format: geo mean (95th percentile) | |||||
| N = 2772 | Age 6+ y: Total: 0.034 (0.25); Male: 0.034 (0.23); Female: 0.034 (0.28) | — | — | ||
| N = 1638 | Age 20+ y: 0.031 (0.24) | — | — | — | — |
| N = 742 | — | Age 12 to 19 y: 0.021 (0.12) | — | — | — |
| N = 392 | — | — | Age 6 to 11 y: 0.028 (0.21) | — | — |
| Huang et al. (2006) | |||||
| Taiwan (undated): Calculated from urinary metabolite data for MMP; pregnant women; N = 28 | 0.3 (median) | — | — | — | — |
aDaily intake calculated from reported urinary metabolite data, as described in text.
Table 10.
Summary comparison of indirect and biomarker methods.
| PE Intake (μgkg−1d−1)a |
||
|---|---|---|
| PE | Indirect Studies | Biomarker Studiesb |
| DMP | Diet only: 0.11 Diet, air, dust: 0.16 to 0.38 Diet, air, dust, consumer products: 0.90 |
0.031 to 0.87 [0.38] |
| DEP | Diet only: 0.007 to 0.13 Diet, air, dust: 0.051 to 0.46 Diet, air, dust, consumer products: 4.27 |
0.77 to 12.3 [5.5] |
| DBP | Diet only: 0.26 to 0.29 Diet, air, dust: 0.44 to 2.7 Diet, air, dust, consumer products: 4.82 |
0.58 to 5.3 [1.7] |
| DiBP | Diet only: 0.57 Diet, air, dust: 0.76 Diet, air, dust, consumer products: 0.48 |
0.08 to 1.7 [1.45] |
| BBP | Diet only: 0.068 to 0.23 Diet, air, dust: 0.062 to 0.50 Diet, air, dust, consumer products: 0.25 |
0.093 to 0.88 [0.3] |
| DEHP | Diet only: 2.43 to 10.4 Diet, air, dust: 2.1 to 11 Diet, air, dust, consumer products: 2.16 |
0.60 to 33.9 [2.7] |
| DiNP | Diet only: 0.094 to 1.3 Diet, air, dust: 0.67 Diet, air, dust, consumer products: 0.01 |
0.21 to 0.7 [0.45] |
Biomarker Studies
Many of the papers reporting measurements of PE metabolites in urine also present estimates of the daily intake of the diesters and those estimates are presented herein. For studies reporting only measurements of PE metabolites in urine, the following equation, from David (2000) as expressed by Koch et al. (2003b), was used to estimate the daily intake:
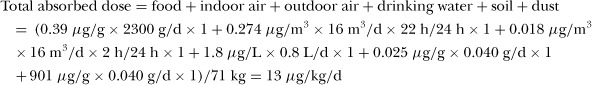 |
where: DI = daily intake of diester (μg/kg/d), UE = creatinine-corrected urinary metabolite concentration (μg/g), CE = creatinine clearance rate normalized by body weight (mg/kg/d), FUE = molar conversion factor that relates urinary excretion of metabolite to diester, MWd = molecular weight of diester (g/mol), MWm = molecular weight of monoester (g/mol).
For short chain PEs (e.g., DBP and BBP), the simple monoesters appear to be the major metabolites (Wittassek and Angerer 2008). Thus, for DMP, DEP, DBP, BBP, and DiBP, the estimates of intake are based on measurements of the following metabolites in urine: monomethyl phthalate (MMP), monoethyl phthalate (MEP), monobutyl phthalate (MBP), monobenzyl phthalate (MBzP), and monoisobutyl phthalate (MiBP), respectively.
For DEHP and DiNP, the oxidized (secondary) metabolites have been found to be more suitable biomarkers of exposure because they are produced in greater quantity compared with the primary metabolites and they are not susceptible to external contamination, as are the primary metabolites (Wittassek and Angerer 2008). For DEHP, intake estimates are based on measurements of mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono-(2-carboxymethylhexyl) phthalate (MCMHP), and mono-2-ethylhexyl phthalate (MEHP). For DiNP, intake estimates are based on measurements of mono(hydroxyisononyl) phthalate (MHiNP), mono(oxoisononyl) phthalate (MOiNP), mono(carboxyisononyl) phthalate (MCiNP), and monoisononyl phthalate (MiNP). It should be noted that some DiNP is produced from a mixed isomeric alcohol unlike the other PEs, which are esters of single structures of alcohols. Therefore, DiNP is a blend of chromatographic peaks and this has limited the ability to accurately measure metabolites in the urine.
The values for FUE are critical to the calculation of exposure. For example, a value of 0.059 for MEHP was derived by Koch et al. (2004) based on a single individual (as are the values for the oxidative metabolites of MEHP), while a value of 0.12 was derived by Anderson et al. (2001) using eight subjects (oxidative metabolites were not analyzed). Clearly, the value selected has an impact on the exposure calculated; additional volunteer studies are necessary to determine more accurate values. The following values were used in the above equation: 0.69 for MMP (Itoh et al. 2007); 0.69 for MEP (Calafat and McKee 2006); 0.69 for MBP and MiBP (Anderson et al. 2001); 0.73 for MBzP (Anderson et al. 2001); 0.12 for MEHP (Anderson et al. 2001); 0.233 for MEHHP, 0.15 for MEOHP, 0.042 for MCMHP, and 0.185 for MECPP (Koch et al. 2005); 0.02 for MiNP, 0.106 for MOiNP and 0.202 for MHiNP (Koch and Angerer 2007).
The values for creatinine clearance rate were: 23 and 18 mg/kg/d for male and female adults, respectively (Kohn et al. 2000); and 20, 11, and 9.8 mg/kg/d for all adults combined, children, and infants, respectively (Calafat and McKee 2006). Normalization to creatinine excretion per kg body weight is thought to reduce the diurnal variability in urinary output and the inter-individual variability in urinary output (David 2000).
As an example of the biomarker study calculation, the geometric mean daily intake for age 20+ years, for DMP, based on a creatinine-corrected urinary metabolite concentration of 1.00 μg/g, is:
RESULTS
Presented in Tables 3 through 9 are the results of the comparison of the estimated daily intake for each diester via indirect and biomarker methods. For each study, where available, the location of the study population, the date, the scope of the study, and the number of individuals tested are presented. To facilitate comparison between studies, a central estimate of exposure (median or geometric mean) and a reasonable upper limit (usually the 95th percentile) are presented, if possible. Due to changes in patterns of use of the diesters and changes in analytical methods, the indirect exposure estimates are limited to those published from year 2000 to the present. Note, however, some of the recently published indirect studies include some pre-2000 measurements due to a lack of more recent measurements.
Table 9.
DiNP exposure estimates.
| DiNP Intake (μgkg−1d−1 |
|||||
|---|---|---|---|---|---|
| Study | Adult | Teen | Child | Toddler | Infant |
| Present evaluation | |||||
| Using method described in Clark et al. (2003b) and concentrations in Table 2. Median intake, based on ingestion of food, drinking water, dust/soil, and inhalation of air using data compiled from various countries and various years. Data format: median (95th percentile) | Age 20 to 70 y: 0.67 (2.0) | Age 12 to 19 y: 0.67 (2.6) | Age 5 to 11 y: 1.3 (5.5) | Age 0.5 to 4 y: 2.1 (8.7) | Age 0 to 0.5 y: 0.76 (9.9) |
| Wormuth et al. (2006) + suppl data | |||||
| Europe: based on oral, inhalation, and dermal exposure pathways, including consumer products; data format = low, intermediate, high estimate; F = female; M = male | Age 18 to 80 y: F: 0.01; 0.01; 0.26 M: 0.01; 0.01; 0.28 | Age 11 to 18 y: F: 0.01; 0.01; 0.24 M: 0.01; 0.01; 0.29 | Age 4 to 10 y: 0.00; 0.14; 6.22 | Age 1 to 3 y: 0.01; 5.16; 75.34 | Age 0 to 1 y: 0.02; 16.03; 152.40 |
| Tsumura et al. (2003) | |||||
| Japan (2001): Based on total diet study of hospital food; calculated using body weight of 50 kg | 0.094 | — | — | — | — |
| Tsumura et al. (2001a) | |||||
| Japan (1999): Based on total diet study of hospital food; calculated using body weight of 50 kg | 1.3 | — | — | — | — |
| Gill et al. (2001) | |||||
| Estimates compiled from various sourcesExposure due to mouthing of children's products | — | — | — | Age 1 to 3 y: 39 (average); 5–228 (range) | Age 0.3 to 0.5 y: 73.9 (95th percentile) |
| All sources other than mouthing children's products | — | — | — | Age 0.3 to 3 y: 50 (average) | — |
| Fromme et al. (2007b) | |||||
| Germany (2005): N = 50 (27 female + 23 male) | Age 14 to 60 y: | — | — | — | |
| Calculated from urinary metabolite data: MHiNP | Total: 0.7 (median); 3.5 (95th percentile) |
— | — | — | |
| Female: 0.6 (3.5); Male: 0.8 (3.5) | — | — | — | ||
| Wittassek et al. (2007b) | |||||
| Germany: Calculated from sum of urinary metabolite data for MOiNP + MHiNP; male and female adults; data format: median; 95th percentile; (range) | Age 20 to 29 y: | — | — | — | — |
| 1988 (N = 60) | 0.20; 1.4; (0.04–2.2) | — | — | — | — |
| 1989 (N = 60) | 0.24; 2.2; (0.03–12.9) | — | — | — | — |
| 1991 (N = 60) | 0.22; 4.5; (0.05–20.2) | — | — | — | — |
| 1993 (N = 60) | 0.27; 1.7; (0.04–2.6) | — | — | — | — |
| 1996 (N = 145) | 0.33; 1.6; (0.02–3.4) | — | — | — | — |
| 1998 (N = 68) | 0.30; 7.8; (0.06–11.7) | — | — | — | — |
| 1999 (N–60) | 0.32; 1.9; (0.05–3.1) | — | — | — | — |
| 2001 (N = 60) | 0.34; 2.3; (0.10–4.4) | — | — | — | — |
| 2003 (N = 59) | 0.40; 1.5; (0.12–3.2) | — | — | — | — |
| Total male (N = 325) | 0.27; 1.7; (NA) | — | — | — | — |
| Total female (N = 307) | 0.32; 1.7; (NA) | — | — | — | — |
| Overall total (N = 632) | 0.29; 1.7; (0.03–20.2) | — | — | — | — |
| Wittassek and Angerer (2008) | |||||
| Germany: Calculated from urinary metabolite data for MiNP + MOiNP + MHiNP + MCiNP; N = 102 | Age 6 to 80 y:0.6 (median); 36.8 (maximum) | — | — | ||
| CDC (2003)a | |||||
| USA (NHANES 1999–2000): Calculated | Age 6 + y (N = 2,541): Total: 6.1; Male: 7.0; Female: 5.5 | — | — | ||
| from urinary metabolite data for MiNP; 95th percentile | Age 20 + y (N = 1461): 6.6 | Age 12 to 19 y (N = 752): 1.5 | Age 6 to 11 y(N = 328): 4.7 | — | — |
| David (2000) | |||||
| USA (1988–1994; NHANES III). Intake calculated from urinary metabolite data for MiNP (Blount et al. 2000); N = 289 | Age 20 to 60 y: 0.21 (geo mean); 1.08 (95th percentile) | — | — | — | — |
| Kohn et al. (2000) | |||||
| USA (1988–1994; NHANES III). Intake calculated from urinary metabolite data for MiNP (Blount et al. 2000); N = 289 | Age 20 to 60 y: 1.7 (95th percentile) | — | — | — | — |
Daily intake calculated from reported urinary metabolite data, as described in text.
NA = Not available
Dimethyl Phthalate
When compared with the other diesters, fewer measurements of DMP in environmental media or of its metabolite, MMP, in human urine, are available. The frequency with which DMP is detected is also less than other PEs. This may reflect the overall use pattern of DMP as an industrial solvent, with little use in products. DMP has been evaluated in only a few foods (beverages, fish, milk, and infant formula and breast milk). In the Clark et al. (2003b) study, for foods in which DMP had not been detected, the concentration in that food group was assigned a value equal to one half the detection limit. This likely resulted in an overestimate of the intake of DMP. The concentrations used in the present evaluation are shown in Table 2. DMP has only been detected in fish and milk; for the remainder of the foods, a concentration of zero was used in the calculations.
As shown in Table 3, the highest intake in the present evaluation was estimated to be for the toddler, followed by the child, the infant, the teen, and the adult. For DMP, for all age groups, inhalation of indoor air represents the dominant exposure pathway, accounting for 95% or more of total exposure. Wormuth et al. (2006) estimated that infants had the highest intake of DMP, followed by female and male adults, toddlers, teens, and children (trend based on the intermediate estimates of uptake). For all age groups, inhalation of indoor air was the dominant exposure pathway; dermal contact and ingestion of personal care products represented 10 to 20% of exposure in teens and adults. The indirect study, based on only dietary exposure (Fromme et al. 2007b), produced daily intake estimates somewhat lower than those in the present evaluation and much lower than the Wormuth et al. estimates.
The highest estimated intake of DMP was found in the biomarker study in Japan (Itoh et al. 2007), while the estimated intake of DMP in Taiwan is somewhat less. The results of the biomarker study for the USA suggest a much lower intake of DMP (CDC 2005). In the USA study, adults had the highest intake, followed by children and teens. It is not known whether exposures are truly higher in Japan compared with other countries, as the available measurements of DMP in Japan are quite limited. Another possible explanation for the higher estimated intakes in Japan is that the results are based on a relatively small dataset. Variation between the indirect estimates and the biomarker estimates for DMP may be largely due to variability in the concentration of DMP in indoor air, due to varying patterns of use of products containing DMP.
Diethyl Phthalate
DEP has been measured in a wide variety of environmental media and foods in Europe, North America, and Japan/Asia; however, most of the data for individual foods are more than 20 years old. As shown in Table 4, the lowest estimates of daily intake of DEP are those based on diet (e.g., Fromme et al. 2007b) or diet and inhalation of air (Itoh et al. 2007) or the present evaluation (diet, drinking water, air, soil, and dust). In the present evaluation, ingestion of food accounts for 54% to 60% of the total intake for the adult, teen, child, and toddler with inhalation of indoor air accounting for most of the remainder. For the infant, food accounted for 7% of exposure, inhalation of indoor air 60%, and ingestion of dust 33%.
Table 4.
DEP exposure estimates.
| DEP Intake (μgkg−1d−1) |
|||||
|---|---|---|---|---|---|
| Study | Adult | Teen | Child | Toddler | Infant |
| Present evaluation | |||||
| Update to Clark et al. (2003b), using concentrations in Table 2. Median intake, based on ingestion of food, drinking water, dust/soil, and inhalation of air using data compiled from various countries and various years. Data format: median (95th percentile) | Age 20 to 70 y: 0.46 (1.0) | Age 12 to 19 y: 0.46 (1.3) | Age 5 to 11 y: 0.93 (2.8) | Age 0.5 to 4 y: 1.2 (3.8) | Age 0 to 0.5 y: 0.34 (1.2) |
| Clark et al. (2003b) | |||||
| Based on ingestion of food, drinking water, dust/soil, and inhalation of air using data compiled from various countries and various years | Age 20 to 70 y: 2.5 (median) | Age 12 to 19 y: 3.0 (median) | Age 5 to 11 y: 5.7 (median) | Age 0.5 to 4 y: 10.6 (median) | Age 0 to 0.5 y: 0.2 (median) |
| Wormuth et al. (2006) + suppl data | |||||
| Europe: based on oral, inhalation, and dermal exposure pathways, including consumer products; data format = low, intermediate, high estimate; F = female; M = maleFromme et al. (2007b) | Age 18 to 80 y:F: 0.01, 4.06, 84.11 M: 0.02; 4.47; 49.27 | Age 11 to 18 y:F: 0.01; 1.76; 20.94 M: 0.03; 1.53; 12.12 | Age 4 to 10 y: 0.27; 1.23; 7.12 | Age 1 to 3 y: 0.56; 2.46; 13.89 | Age 0 to 1 y: 1.25; 4.37; 23.86 |
| Germany (2005): intake estimated from composite dietary samples collected over 7 days; N = 50 (27 female + 23 male) | Age 14 to 60 y: 0.13 (median); 0.34 —(95th percentile); 0.06–0.49 (range) | ||||
| Fromme et al. (2007b) | |||||
| Germany (2002: Koch et al. 2003a): N = 85 Calculated from urinary metabolite data for MEP; data format: median (95th percentile) | Age 7 to 63 y: Female: 4.6 (38.5); Male: 2.0 (42.4) | ||||
| Tsurmura et al. (2001a) | |||||
| Japan (1999): Based on total diet study of hospital food; calculated using body weight of 50 kg. | 0.007 (mean) | — | — | — | — |
| Itoh et al. (2007) | |||||
| Japan: | |||||
| Based on ingestion of food and inhalation of indoor air; data compiled from various sources | 0.051 to 0.065 (range of means) | — | — | — | — |
| Calculated from urinary metabolite data for MEP (2004); N = 35 | 0.77 to 1.2 (range of means) | — | — | — | — |
| Calafat and McKee (2006) | |||||
| USA (NHANES 2001–2002; CDC, 2005): Calculated from urinary metabolite data for MEP; data format: geo mean (95th percentile) | |||||
| N = 2772 | Age 6 to > 20 y: 5.5 (61.7); Male: 4.9 (69.0); Female: 6.2 (47.4) | — | — | ||
| N = 742 | — | Age 12 to 19 y: 5.0 (44.1) | — | — | — |
| N = 392 | Age 6 to 11 y: 1.8 (15.3) | — | — | ||
| Marsee et al. (2006) | |||||
| USA (2000–2003): pregnant women (N = 214): Calculated from urinary metabolite data for MEP | 6.64 (median); 112.3 (95th percentile) | — | — | — | — |
| CDC (2003)a | |||||
| USA (NHANES 1999–2000): Calculated from urinary metabolite data for MEP; data format: geo mean (95th percentile) | |||||
| N = 2536 | Age 6+ y: Total: 5.4 (64.7); Male: 5.4 (74.1); Female: 5.6 (57.4) | — | — | ||
| N = 1456 | Age 20+ y: 5.9 (72.0) | — | — | — | — |
| N = 752 | — | Age 12 to 19 y 2.6 (28.3) | — | — | — |
| N = 328 | — | — | Age 6 to 11 y: 1.7 (11.4) | — | — |
| Brock et al. (2002)a | — | — | — | Age 11.8 to 16.5 months: | — |
| USA (2000): Intake calculated from urinarymetabolite data for MEP; 19 children; 30 samples | — | — | — | 6.3 (geo mean); 37 (95th percentile) | — |
| David (2000) | |||||
| USA (1988–1994; NHANES III). Intake calculated from urinary metabolite data for MEP (Blount et al. 2000); N = 289 | Age 20 to 60 y: 12.34 (geo mean); 93.33 (95th percentile) | — | — | — | — |
| Kohn et al. (2000) | |||||
| USA (1988–1994; NHANES III). Intake calculated from urinary metabolite data for MEP (Blount et al. 2000); N = 289 | Age 20 to 60 y:12 (median);110 (95th percentile) | — | — | — | — |
| Huang et al. (2006) | |||||
| Taiwan (undated): Calculated from urinary metabolite data for MEP; pregnant women; N = 28 | 3.01 (median) | — | — | — | — |
| Chen et al. (2008) | |||||
| Taiwan (undated): Calculated from urinary metabolite data for MEP; N = 60 (41 female, 18 male) | Age 21 to 67 y: nd (median); nd to 27.9 (range) | — | — | — | — |
aDaily intake calculated from reported urinary metabolite data, as described in text. nd = Not detected.
The estimates of Wormuth et al. (2006), which include exposure to personal care products, are very similar to the German study in which intake was estimated based on the biomarker MEP (Fromme et al. 2007b). The indirect exposure estimates, which do not include exposure to personal care products, underestimate the daily intake of DEP. These results are supported by the use pattern of DEP. DEP is commonly used in perfumes and fragrances (Shen et al. 2007).
Based on the biomarker data, intake of DEP is highest in the USA, followed by Germany, Taiwan, and Japan. This difference between regions is also apparent in the measured concentrations of DEP in indoor air; in the USA, the average concentration is approximately two times the average concentration in Europe and six times the average concentration in Japan. However, the average concentration of DEP in dust in the USA is less than that in Europe (by a factor of three or more); no data for DEP in dust are available for Japan. The concentration of DEP in composite diet samples is less in Japan compared with Germany. Although indoor air and diet may not represent the primary sources of exposure to DEP, regional differences in the concentrations in these media may reflect different use patterns of products containing DEP.
Dibutyl Phthalate
DBP is one of the most extensively evaluated PEs; concentration data are available for Europe, North America, and Japan/Asia for most media. However, as for most of the other PEs, recent data for a wide variety of foods are not available and the results of composite diet samples were used in the present evaluation. The lowest estimates of daily intake of DBP (see Table 5) are those based on diet only (e.g., Tsumura et al. 2001a, 2003) or diet and inhalation of air (Franco et al. 2007; Itoh et al. 2007). In the present evaluation, ingestion of food accounts for approximately 75% of total exposure for the adult, teen, child, and toddler, with the remainder due to inhalation of indoor air and incidental ingestion of dust. For the formula-fed infant, ingestion of food accounts for 46% of exposure, followed by ingestion of dust (38%) and inhalation of indoor air (15%). For the breast-fed infant, ingestion of dust is the dominant exposure pathway (62% of total exposure), followed by inhalation of indoor air (25%) and ingestion of food (13%).
Table 5.
DBP exposure estimates.
| DBP Intake (μgkg−1d−1) |
|||||
|---|---|---|---|---|---|
| Study | Adult | Teen | Child | Toddler | Infant |
| Present evaluation | |||||
| Update to Clark et al. (2003b), using concentrations in Table 2. Median intake, based on ingestion of food, drinking water, dust/soil, and inhalation of air using data compiled from various countries and various years. Data format: median (95th percentile) | Age 20 to 70 y: 1.2 (3.0) | Age 12 to 19 y: 1.2 (4.0) | Age 5 to 11 y: 2.4 (8.1) | Age 0.5 to 4 y: 3.4 (12) | Age 0 to 0.5 y: 1.5 (5.7) formula-fed; 0.78 (4.0) breast-fed |
| Clark et al. (2003b) | |||||
| Based on ingestion of food, drinking water, dust/soil, and inhalation of air using data compiled from various countries and various years | Age 20 to 70 y: 5.6 (median) | Age 12 to 19 y: 6.4 (median) | Age 5 to 11 y: 11 (median) | Age 0.5 to 4 y: 14 (median) | Age 0 to 0.5 y: 1.5 (formula-fed); 2.9 (breast-fed) (median) |
| Wormuth et al. (2006) + suppl data | |||||
| Europe: based on oral, inhalation, and dermal exposure pathways, including consumer products; data format = low, intermediate, high estimate; F = female; M = male | Age 18 to 80 y: F: 1.42; 5.33; 54.20 M: 1.63; 4.31; 25.82 |
Age 11 to 18 y: F: 0.19; 1.74; 17.35 M: 0.17; 1.37; 17.02 |
Age 4 to 10 y: 0.83; 2.41; 19.60 |
Age 1 to 3 y: 0.35; 2.62; 26.74 |
Age 0 to 1 y: 1.02; 7.37; 45.63 |
| Franco et al. (2007) | |||||
| Based on ingestion of food, drinking water, dust/soil, and inhalation of air using data compiled from various countries and various years | 2.7 (median) | — | — | — | — |
| Based on ingestion of leaf and root crops, fish, beef, dairy, drinking water, and inhalation of outdoor air using the EUSES model and data from the Netherlands | 0.21 (median) | — | — | — | — |
| Wilson et al. (2003) | |||||
| USA (1997): Based on ingestion of food, dust, and soil and inhalation of indoor and outdoor air | — | — | — | Age 2 to 5 y: 1.4 (mean); 0.745 to 2.85 (range) | — |
| Tsumura et al. (2003) | |||||
| Japan (2001): Based on total diet study of hospital food; calculated using body weight of 50 kg. | 0.26 | — | — | — | — |
| Tsumura et al. (2001a) | |||||
| Japan (1999): Based on total diet study of hospital food; calculated using body weight of 50 kg | 0.29 | — | — | — | — |
| Itoh et al. (2007) | |||||
| Japan: Based on ingestion of food and inhalation of indoor air; data compiled from various sources Calculated from urinary metabolite data for MBP (2004); N = 35 |
0.44 to 0.75 — (range of means) 1.7 (mean) | — | — | — | — |
| — | — | — | — | ||
| Fromme et al. (2007a and b) | |||||
| Germany (2005): N = 50 (27 female + 23 male) Calculated from composite dietary samples collected over 7 days |
Age 14 to 60 y: 0.26 (median); 1.35 (95th percentile); 0.12–1.63 (range) |
— | — | — | |
| Calculated from urinary metabolite data for MBP; data format: median (95th percentile) | Total: 1.7 (4.2)Female: 1.7 (4.4); Male: 1.8 (3.9) | — | — | — | |
| — | — | — | |||
| Fromme et al. (2007b) | |||||
| Germany (2002: Koch et al 2003a): N = 85Calculated from urinary metabolite data for MBP; data format: median (95th percentile) | Age 7 to 63 y: Female: 6.0 (17.5); Male: 4.6 (15.9) | — | — | ||
| Wittassek et al. (2007b) | |||||
| Germany: Calculated from urinary metabolite data for MBP; male and female adults; data format: median; 95th percentile; (range) | Age 20 to 29 y: | — | — | — | — |
| 1988 (N = 60) | 7.0; 24.2; (0.72–27.8) | — | — | — | — |
| 1989 (N = 60) | 7.5; 21.7; (1.5–70.1) | — | — | — | — |
| 1991 (N = 60) | 6.4; 14.3; (2.1–28.7) | — | — | — | — |
| 1993 (N = 60) | 6.6; 44.4; (1.5–56.3) | — | — | — | — |
| 1996 (N = 145) | 3.7; 15.5; (1.1–90.2) | — | — | — | — |
| 1998 (N = 68) | 3.1; 11.9; (0.22–20.3) | — | — | — | — |
| 1999 (N = 60) | 2.8; 16.2; (0.83–32.8) | — | — | — | — |
| 2001 (N = 60) | 2.5; 19.4; (0.81–116) | — | — | — | — |
| 2003 (N = 59) | 1.9; 5.3; (0.49–71.8) | — | — | — | — |
| Total male (N = 325) | 3.7; 16.2; (NA) | — | — | — | — |
| Total female (N = 307) | 4.6; 20.3; (NA) | — | — | — | — |
| Overall total (N = 632) | 4.1; 19.1; (0.22–116) | — | — | — | — |
| Wittassek and Angerer (2008) | |||||
| Germany: Calculated from urinary metabolite data for MBP; N = 102 | Age 6 to 80 y:2.1 (median); 230 (maximum) | — | — | ||
| Children: N = 239 | Age 2 to 14 y: | ||||
| Creatinine-based model | — | 4.1 (median); 76.4 (maximum) | — | ||
| Volume-based model | — | 7.6 (median); 110 (maximum) | — | ||
| CDC (2005)a | |||||
| USA (NHANES 2001–2002): Calculated from urinary metabolite data for MBP; data format: geo mean (95th percentile) | |||||
| N = 2,772 | Age 6+ y: Total: 0.65 (3.0); Male: 0.60 (2.5); Female: 0.71 (3.0) | — | — | ||
| N = 1638 | Age 20+ y: 0.58 (2.6) | — | — | — | — |
| N = 742 | — | Age 12 to 19 y: 0.39 (1.8) | — | — | — |
| N = 392 | — | — | Age 6 to 11 y: 0.71 (2.9) | — | — |
| Marsee et al. (2006) | |||||
| USA (2000–2003): pregnant women (N = 214): Calculated from urinary metabolite data for MBP | 0.84 (median); 2.33 (95th percentile) | — | — | — | — |
| CDC (2003)a | |||||
| USA (NHANES 1999–2000): Calculated from urinary metabolite data for MBP + MiBP; data format: geo mean (95th percentile) | |||||
| N = 2,541 | Age 6+ y: Total: 0.81 (3.5); Male: 0.72 (2.7); Female: 0.93 (4.3) | — | — | ||
| N = 1,461 | Age 20+ y: 0.74 (3.3) | — | — | — | — |
| N = 752 | — | Age 12 to 19 y: 0.49 (1.8) | — | — | — |
| N = 328 | — | — | Age 6 to 11 y: 0.84 (3.2) | — | — |
| Brock et al. (2002)a | |||||
| USA (2000): Intake calculated from urinary metabolite data for MBP; 19 children; 30 samples | — | — | — | Age 11.8 to 16.5 months: 2.45 (geo mean); 16.6 (95th percentile) | — |
| David (2000) | |||||
| USA (1988–1994; NHANES III). Intake calculated from urinary metabolite data for MBP (Blount et al. 2000); N = 289 | Age 20 to 60 y: 1.56 (geo mean); 6.87 (95th percentile) | — | — | — | — |
| Kohn et al. (2000) | |||||
| USA (1988–1994; NHANES III). Intake calculated from urinary metabolite data for MBP (Blount et al. 2000); N = 289 | Age 20 to 60 y: 1.5 (median); 7.2 (95th percentile) | — | — | — | — |
| Huang et al. (2006) | |||||
| Taiwan (undated): Calculated from urinary metabolite data for MBP; pregnant women; N = 28 | 9.28 (median) | — | — | — | — |
| Chen et al. (2008) | |||||
| Taiwan (undated): Calculated from urinary metabolite data for MBP; N = 60 (41 female, 18 male) | Age 21 to 67 y: 2.2 (median); nd to 23.5 (range) | — | — | — | — |
aDaily intake calculated from reported urinary metabolite data, as described in text.
NA = Not available
nd = Not detected
For the indirect estimates by Wormuth et al. (2006), ingestion of food is the dominant exposure pathway for adults, while for teens (especially female teens), dermal contact and ingestion of personal care products and inhalation of air are important exposure pathways, in addition to ingestion of food. For the three youngest age groups (children, toddlers, and infants), ingestion of food is the most important pathway, followed by inhalation of air, and ingestion of dust (toddlers and infants). The indirect estimates of Wilson et al. (2003) for the toddler, based on ingestion of food, dust, and soil and inhalation of air, are slightly lower than the estimates in the present evaluation and those of Wormuth et al. for the same age group.
The biomarker-based estimates vary by region; some biomarker estimates are higher than the indirect estimates and some are lower (see Tables 5 and 10). Using measurements of the metabolite MBP, the highest estimated intake of DBP is for Germany, followed by Taiwan, Japan, and the USA. This is supported by higher measured concentrations of DBP in environmental media in Europe compared with the USA (the concentration of DBP is five times higher in indoor air and more than six times higher in dust), suggesting greater use of DBP in Germany. The biomarker-based estimate of intake for Japan is also larger than the estimated intake for the USA and this is supported by the concentration of DBP in indoor air in Japan, which is approximately 50% higher than the concentration in the USA (no data for dust are available for Japan). Both the German and USA data show a decrease in DBP intake with time and both indicate that intake is higher for female adults compared with males. The gender difference may be due to the use of DBP in consumer products, including nail polish (Shen et al. 2007).
Di-isobutyl Phthalate
DiBP has been measured in a variety of environmental media and foods. In the present evaluation, for all age groups, food is the dominant source of exposure (especially grains, fruit, milk, and beverages). Inhalation of indoor air is also an important exposure pathway. As shown in Table 6, for the adult and teen, the estimated intake in the present evaluation is 50% to 2.5 times higher than the indirect estimates of Wormuth et al. (2006) and Fromme et al. (2007a,b). For the child and toddler, the estimated intakes in the present evaluation are three to five times higher than the estimates of Wormuth et al. (2006), while, for the infant, the estimates are similar. In the present evaluation, higher concentrations in several foods were used compared to those of Wormuth et al. (2006). Food was the predominant exposure pathway for all age groups in the Wormuth et al. estimates. For the youngest age groups (child and infant) ingestion of dust was also important.
Table 6.
DiBP exposure estimates.
| DiBP Intake (μgkg−1d−1) |
|||||
|---|---|---|---|---|---|
| Study | Adult | Teen | Child | Toddler | Infant |
| Present evaluation | |||||
| Using method described in Clark et al. (2003b) and concentrations in Table 2. Median intake, based on ingestion of food, drinking water, dust/soil, and inhalation of air using data compiled from various countries and various years. Data format: median (95th percentile) | Age 20 to 70 y: 0.76 (1.6) | Age 12 to 19 y: 0.98 (2.2) | Age 5 to 11 y: 2.1 (4.8) | Age 0.5 to 4 y: 2.6 (6.2) | Age 0 to 0.5 y: 1.3 (5.5) |
| Wormuth et al. (2006) + suppl data | |||||
| Europe: based on oral, inhalation, and dermal exposure pathways, including consumer products; data format = low, intermediate, high estimate; F = female; M = male | Age 18 to 80 y:F: 0.03; 0.45; 1.61 M: 0.03; 0.50; 1.82 | Age 11 to 18 y:F: 0.05; 0.30; 0.98 M: 0.06; 0.40; 1.27 | Age 4 to 10 y: 0.04; 0.39; 1.55 | Age 1 to 3 y: 0.07; 0.69; 2.44 | Age 0 to 1 y: 0.16; 1.53; 4.73 |
| Fromme et al. (2007a and b) | |||||
| Germany (2005): N = 50 (27 female + 23 male) | Age 14 to 60 y: | — | — | — | |
| Calculated from composite dietary samples collected over 7 days | 0.57 (median); 2.14 (95th percentile); 0.23–3.47 (range) | — | — | — | |
| Calculated from urinary metabolite data for MiBP; data format: median (95th percentile) | Total: 1.7 (5.2)Female: 1.6 (4.7); Male: 1.8 (5.3) | — | — | — | |
| Wittassek et al. (2007b) | |||||
| Germany: Calculated from urinary metabolite data for MiBP; male and female adults; data format: median; 95th percentile; (range) | Age 20 to 29 y: | ||||
| 1988 (N = 60) | 1.1; 3.6; (0.27–6.2) | — | — | — | — |
| 1989 (N = 60) | 1.0; 4.2; (0.30–12.9) | — | — | — | — |
| 1991 (N = 60) | 1.2; 8.7; (0.36–20.2) | — | — | — | — |
| 1993 (N = 60) | 1.2; 2.8; (0.39–4.8) | — | — | — | — |
| 1996 (N = 145) | 1.6; 8.4; (0.45–29.0) | — | — | — | — |
| 1998 (N = 68) | 1.4; 5.8; (0.10–12.2) | — | — | — | — |
| 1999 (N = 60) | 1.5; 4.4; (0.41–15.1) | — | — | — | — |
| 2001 (N = 60) | 1.6; 4.6; (0.29–12.6) | — | — | — | — |
| 2003 (N = 59) | 1.4; 3.9; (0.46–5.2) | — | — | — | — |
| Total male (N = 325) | 1.3; 4.8; (NA) | — | — | — | — |
| Total female (N = 307) | 1.4; 6.6; (NA) | — | — | — | — |
| Overall total (N = 632) | 1.4; 5.7; (0.10–29.0) | — | — | — | — |
| Wittassek and Angerer (2008) | |||||
| Germany: Calculated from urinary metabolite data for MiBP; N = 102 | Age 6 to 80 y: 1.5 (median); 27.3 (maximum) | — | — | ||
| CDC (2005)a | |||||
| USA (NHANES 2001–2002): Calculated from urinary metabolite data for MiBP; data format: geo mean (95th percentile) | |||||
| N = 2,772 | Age 6+ y: Total: 0.09 (0.44); Male: 0.09 (0.46); Female: 0.09 (0.44) | — | — | ||
| N = 1,638 | Age 20+ y: 0.08 (0.38) | — | — | — | — |
| N = 742 | — | Age 12 to 19 y: 0.05 (0.26) | — | — | — |
| N = 392 | — | — | Age 6 to 11 y: 0.10 (0.49) | — | — |
| Marsee et al. (2006) | |||||
| USA (2000–2003): pregnant women (N = 214): Calculated from urinary metabolite data for MiBP | 0.12 (median); 0.41 (95th percentile) | — | — | — | — |
aDaily intake calculated from reported urinary metabolite data, as described in text.
NA = Not available
The estimated intake of DiBP in Germany based on diet is approximately one third of the total estimated using the biomarker approach (Fromme et al. 2007a,b). Wittassek et al. (2007b) found that the intake of DiBP increased slightly between 1988 and 1996, and then remained relatively constant. They also found that female adults had significantly higher intakes of DiBP compared to male adults. The results of the biomarker studies indicate that the estimated intake of DiBP is more than an order of magnitude larger in Germany compared with the USA. This may be due to the use of larger quantities of DiBP in Germany compared with the USA and is supported by measurements of DiBP in dust and indoor air. No gender difference is apparent in the USA data. For the adult, the indirect estimates in the present evaluation are lower than the biomarker-based estimates for Germany and higher than the estimates for the USA.
Butyl Benzyl Phthalate
BBP has been measured in a variety of environmental media and foods. For most environmental media, BBP measurements are available for Europe and North America. Fewer data are available for Japan. In a recent dietary study in Germany, BBP was detected in only 35 of 350 composite samples (detection limit of 0.01 μg/g) (Fromme et al. 2007b). Despite this low frequency of detection of BBP in composite foods, in the present evaluation, ingestion of food accounts for 68% to 77% of total exposure for the adult, teen, child, and toddler, with the remainder primarily due to incidental ingestion of dust and a minor contribution due to inhalation of indoor air. For both the formula-fed and breast-fed infants, ingestion of dust accounts for approximately 94% of exposure, with ingestion of food comprising most of the remainder. Ingestion of food represents approximately 60% of total exposure for the adult and inhalation of spray paints comprises most of the remainder in the estimates by Wormuth et al. (2006). For the teen, these two pathways are reversed in importance. For children, ingestion of food is the dominant exposure pathway, while for toddlers and infants, ingestion of dust is the most important pathway.
As shown in Table 7, for the present evaluation, the estimated intake to the toddler is equal to the biomarker-based estimate for toddlers in the USA using the data of Brock et al. (2002). It is also similar to the indirect estimates of Wilson et al. (2003) for toddlers in the USA. The indirect estimates of Wormuth et al. (2006), for the adult, are comparable to the biomarker-based estimates for German adults (e.g., Fromme et al. 2007a,b; Wittassek et al. 2007b).
Table 7.
BBP exposure estimates.
| BBP Intake (μgkg−1d−1) |
|||||
|---|---|---|---|---|---|
| Study | Adult | Teen | Child | Toddler | Infant |
| Present evaluation | |||||
| Update to Clark et al. (2003b), using concentrations in Table 2. Median intake, based on ingestion of food, drinking water, dust/soil, and inhalation of air using data compiled from various countries and various years. Data format: median (95th percentile) | Age 20 to 70 y: 0.50 (1.4) | Age 12 to 19 y: 0.49 (1.9) | Age 5 to 11 y: 0.97 (4.0) | Age 0.5 to 4 y: 1.5 (6.1) | Age 0 to 0.5 y: 0.51 (6.1) formula-fed; 0.53 (6.1) breast-fed |
| Clark et al. (2003b) | |||||
| Based on ingestion of food, drinking water, dust/soil, and inhalation of air using data compiled from various countries and various years | Age 20 to 70 y: 3.7 (median) | Age 12 to 19 y: 5.7 (median) | Age 5 to 11 y: 7.9 (median) | Age 0.5 to 4 y: 9.3 (median) | Age 0 to 0.5 y: 1.5 (median) |
| Wormuth et al. (2006) + suppl data | |||||
| Europe: based on oral, inhalation, and dermal exposure pathways, including consumer products; data format = low, intermediate, high estimate; F = female; M = male | Age 18 to 80 y:F: 0.02; 0.24; 2.62 M: 0.02; 0.26; 2.97 | Age 11 to 18 y:F: 0.02; 0.11; 2.24 M: 0.02; 0.13; 2.76 | Age 4 to 10 y: 0.01; 0.13; 1.68 | Age 1 to 3 y: 0.02; 0.44; 5.89 | Age 0 to 1 y: 0.06; 1.16; 11.70 |
| Wilson et al. (2003) | |||||
| USA (1997): Based on ingestion of food (composite samples), dust, and soil and inhalation of indoor and outdoor air | — | — | — | Age 2 to 5 y:1.9 (mean); 0.744 to 2.88 (range) | — |
| Tsumura et al. (2003) | |||||
| Japan (2001): Based on total diet study of hospital food; calculated using body weight of 50 kg. | 0.068 | — | — | — | — |
| Tsumura et al. (2001a) | |||||
| Japan (1999): Based on total diet study of hospital food; calculated using body weight of 50 kg | 0.094 | — | — | — | — |
| Itoh et al. (2007) | |||||
| Japan: | |||||
| Based on ingestion of food and inhalation of indoor air; data compiled from various sources | 0.062 to 0.083 (range of means) | — | — | — | — |
| Calculated from urinary metabolite data for MBzP (2004); N = 35 | 0.093 (mean) | — | — | — | — |
| Fromme et al. (2007a and b) | |||||
| Germany (2005): N = 50 (27 female + 23 male) | Age 14 to 60 y: | ||||
| Calculated from composite dietary samples collected over 7 days | 0.23 (median); 0.38 (95th percentile); 0.11–0.50 (range) | — | — | — | |
| Calculated from urinary metabolite data for MBzP; data format: median (95th percentile) | Total: 0.2 (1.2) | — | — | — | |
| Female: 0.2 (1.5); Male: 0.2 (1.0) | — | — | — | ||
| Fromme et al. (2007b) | |||||
| Germany (2002: Koch et al 2003a): N = 85 | Age 7 to 63 y: | ||||
| Calculated from urinary metabolite data for MBzP; data format: median (95th percentile) | Female: 0.6 (2.5); Male: 0.5 (2.4) | — | — | ||
| Wittassek and Angerer (2008) | |||||
| Germany: Calculated from urinary metabolite data for MBzP; N = 102 | Age 6 to 80 y: 0.3 (median); 2.2 (maximum) | — | — | ||
| Children: N = 239 | Age 2 to 14 y: | ||||
| Creatinine-based model | — | — | 0.42 (median); 13.9 (maximum) | — | — |
| Volume-based model | — | — | 0.77 (median); 31.3 (maximum) | — | — |
| Wittassek et al. (2007b) | |||||
| Germany: Calculated from urinary metabolite data for MBzP; male and female adults; data format: median; 95th percentile; (range) | Age 20 to 29 y: | ||||
| 1988 (N = 60) | 0.25; 0.77; (0.02–6.6) | — | — | — | — |
| 1989 (N = 60) | 0.30; 2.2; (0.07–2.8) | — | — | — | — |
| 1991 (N = 60) | 0.43; 1.6; (0.11–2.8) | — | — | — | — |
| 1993 (N = 60) | 0.27; 1.9; (0.07–2.2) | — | — | — | — |
| 1996 (N = 145) | 0.29; 5.5; (0.04–27.3) | — | — | — | — |
| 1998 (N = 68) | 0.22; 1.4; (0.01–4.0) | — | — | — | — |
| 1999 (N–60) | 0.21; 3.7; (0.03–10.9) | — | — | — | — |
| 2001 (N = 60) | 0.22; 0.75; (0.02–0.99) | — | — | — | — |
| 2003 (N = 59) | 0.22; 0.91; (0.05–1.74) | — | — | — | — |
| Total male (N = 325) | 0.25; 1.9; (NA) | — | — | — | — |
| Total female (N = 307) | 0.28; 1.5; (NA) | — | — | — | — |
| Overall total (N = 632) | 0.26; 1.6; (0.01–27.3) | — | — | — | — |
| CDC (2005)a | |||||
| USA (NHANES 2001–2002): Calculated from urinary metabolite data for MBzP; data format: geo mean (95th percentile) | |||||
| N = 2,772 | Age 6+ y: Total: 0.47 (3.0); Male: 0.49 (3.1); Female: 0.47 (2.9) | — | — | ||
| N = 1,638 | Age 20+ y: 0.40 (2.2) | — | — | — | — |
| N = 742 | — | Age 12 to 19 y: 0.33 (1.8) | — | — | — |
| N = 392 | — | — | Age 6 to 11 y: 0.70 (3.6) | — | — |
| Marsee et al. (2006) | |||||
| USA (2000–2003): pregnant women (N = 214): Calculated from urinary metabolite data for MBzP | 0.50 (median); 2.47 (95th percentile) | — | — | — | — |
| Brock et al. (2002)a | |||||
| USA (2000): Intake calculated from urinary metabolite data for MBzP; 19 children; 30 samples | — | — | — | Age 11.8 to 16.5 months: 1.5 (geo mean); 6.4 (95th percentile) | — |
| CDC (2003)a | |||||
| USA (NHANES 1999–2000): Calculated from urinary metabolite data for MBzP; data format: geo mean (95th percentile) | |||||
| N = 2,541 | Age 6+ y: Total: 0.47 (2.6); Male: 0.49 (2.8); Female: 0.46 (2.4) | — | — | ||
| N = 1,461 | Age 20 + y: 0.39 (1.9) | — | — | — | — |
| N = 752 | — | Age 12 to 19 y: 0.32 (1.3) | — | — | — |
| N = 328 | — | — | Age 6 to 11 y: 0.73 (2.6) | — | — |
| David (2000) | |||||
| USA (1988–1994; NHANES III). Intake calculated from urinary metabolite data for MBzP (Blount, et al. 2000); N = 289 | Age 20 to 60 y: 0.73 (geo mean); 3.34 (95th percentile) | — | — | — | — |
| Kohn et al. (2000) | |||||
| USA (1988–1994; NHANES III). Intake calculated from urinary metabolite data for MBzP (Blount, et al. 2000); N = 289 | Age 20 to 60 y: 0.88 (median); 4.0 (95th percentile) | — | — | — | — |
| Huang et al. (2006) | |||||
| Taiwan (undated): Calculated from urinary metabolite data for MBzP; pregnant women; N = 28 | <0.1 (median) | — | — | — | — |
| Chen et al. (2008) | |||||
| Taiwan (undated): Calculated from urinary metabolite data for MBzP; N = 60 (41 female, 18 male) | Age 21 to 67 y: 0.2 (median); nd to 1.6 (range) | — | — | — | — |
aDaily intake calculated from reported urinary metabolite data, as described in text.
NA = Not available
nd = Not detected
The biomarker-based estimates for the USA are higher than the German estimates and decrease by approximately 50% from the 1988–1994 study (NHANES III) to the studies in 1999–2000 and 2001–2002 (CDC 2003, 2005). Wittassek et al. (2007b) report only a slight decrease in the estimated intake of BBP over the period of 1988 to 2003 in German adults. The higher biomarker-based estimates for the USA compared with Germany are supported by differences in concentrations in indoor and outdoor air, drinking water, and soil. However, the average concentration in dust in the USA is approximately one half the average concentration in Europe. The lowest estimated intakes of BBP are reported for Japan (both indirect and biomarker-based). The data available for Japan indicate that the measured concentrations of BBP in indoor and outdoor air and composite diet samples are less than in the USA or Europe.
Di-2-ethylhexyl Phthalate
DEHP is the most widely studied PE and measured concentrations are available for all environmental media and food groups. However, as for the other PEs, few recent measurements of food are available. In the present evaluation, the highest estimated intake of DEHP is for the toddler, followed by the child. For the adult, teen, child, and toddler, ingestion of food is the predominant exposure pathway, accounting for approximately 95% of total exposure. Most of the remainder is due to incidental ingestion of dust. For the formula-fed infant, incidental ingestion of dust accounts for 63% of total exposure, ingestion of food 34%, and ingestion of drinking water 2%. For the breast-fed infant, ingestion of food accounts for 76% of total exposure and incidental ingestion of dust 24%. In the indirect estimates by Wormuth et al. (2006), ingestion of food accounts for more than 95% of total exposure to the adult, teen, and child. For the toddler and infant, ingestion of food and ingestion of dust are the predominant exposure pathways, having approximately equal importance.
As shown in Table 8, for all age groups except the infant, the intermediate estimates of Wormuth et al. (2006) are much less than those in the present evaluation. Wormuth et al. used minimum, mean, and maximum absorption fractions of 0.153, 0.552, and 0.95, respectively, whereas 100% absorption was assumed in the present evaluation. Thus, uptake of DEHP is likely overestimated in the present evaluation; if an oral absorption factor of 0.153 were used, the estimated intake for the adult would be lowered from 11 μg/kg/d to approximately 2 μg/kg/d. In addition to the difference in absorption factors, the concentration of DEHP in some of the individual foods in Wormuth et al. is also less than the concentration in the composite samples used in the present evaluation. The indirect estimates of Fromme et al. (2007a,b), based on diet only, are also considerably less than the estimates in the present evaluation.
Table 8.
DEHP exposure estimates.
| DEHP Intake (μgkg−1 d−1) |
|||||
|---|---|---|---|---|---|
| Study | Adult | Teen | Child | Toddler | Infant |
| Present evaluation | |||||
| Update to Clark et al. (2003b), using concentrations in Table 2. Median intake, based on ingestion of food, drinking water, dust/soil, and inhalation of air using data compiled from various countries and various years. Data format: median (95th percentile) | Age 20 to 70 y: 11 (31) | Age 12 to 19 y: 11 (42) | Age 5 to 11 y: 20 (81) | Age 0.5 to 4 y: 30 (124) | Age 0 to 0.5 y: 5.0 (27) formula— fed; 16 (66) breast—fed |
| Clark et al. (2003b) | |||||
| Based on ingestion of food, drinking water, dust/soil, and inhalation of air using data compiled from various countries and various years | Age 20 to 70 y: 8.2 (median) | Age 12 to 19 y: 10 (median) | Age 5 to 11 y: 19 (median) | Age 0.5 to 4 y: 26 (median) | Age 0 to 0.5 y:5.0 (formula—fed); 7.3 (breast—fed) (median) |
| Wormuth et al. (2006) + suppl data | |||||
| Europe: based on oral, inhalation, and dermal exposure pathways, including consumer products; data format = low, intermediate, high estimate; F = female; M = male | Age 18 to 80 y:F: 0.23; 2.06; 11.39 M: 0.26; 2.25; 12.93 | Age 11 to 18 y:F: 0.10; 1.25; 10.40 M: 0.14; 1.68; 14.25 | Age 4 to 10 y: 0.17; 2.00; 14.51 | Age 1 to 3 y: 0.24; 4.91; 47.23 | Age 0 to 1 y 0.54; 12.33; 106.67 |
| Franco et al. (2007) | |||||
| Based on ingestion of food, drinking water, dust/soil, and inhalation of air using data compiled from various countries and various years | 5.6 (median) | — | — | — | — |
| Based on ingestion of leaf and root crops, fish, beef, dairy, drinking water, and inhalation of outdoor air using the EUSES model and data from the NetherlandsJensen and Knudsen (2006) | 0.68 (median) | — | — | — | — |
| Denmark: estimated intake due to exposure to consumer products and dust indoors | — | — | — | 10–20 (typical); 50–250 (worst case) | — |
| Tsumura et al. (2003) | |||||
| Japan (2001): Based on total diet study of hospital food; calculated using body weight of 50 kg. | 3.2 | — | — | — | — |
| Tsumura et al. (2001a) | |||||
| Japan (1999): Based on total diet study of hospital food; calculated using body weight of 50 kg | 10.4 | — | — | — | — |
| Itoh et al. (2007) | |||||
| Japan: | |||||
| Based on ingestion of food and inhalation of indoor air; data compiled from various sources | 2.1 to 2.8 (range of means) | — | — | — | — |
| Calculated from urinary metabolite data for MEHP (2004); N = 35 | 2.7 (mean) | — | — | — | — |
| Fujimaki et al. (2006) | |||||
| Japan (2003): pregnant women; N = 40 | median (range) | ||||
| Calculated from urinary metabolite data: MEHP | 10.4 (3.45–41.6) | — | — | — | — |
| Calculated from urinary metabolite data: MEOHP | 4.55 (0.66–17.9) | — | — | — | — |
| Calculated from urinary metabolite data: MEHHP | 3.51 (1.47–8.57) | — | — | — | — |
| Fromme et al. (2007a and b) | |||||
| Germany (2005): N = 50 (27 female + 23 male) Calculated from composite dietary samples collected over 7 days |
Age 14 to 60 y: 2.43 (median); 3.95 (95th percentile); 1.0–4.80 (range) |
— | — | — | |
| Calculated from urinary metabolite data: MEHP | Total: 2.2 (median); 7.2 (95th percentile)Female: 1.9 (7.1); Male: 2.4 (7.6) | — | — | — | |
| Calculated from urinary metabolite data: MEOHP | Total: 2.3 (median); 7.2 (95th percentile)Female: 2.3 (8.2); Male: 2.5 (6.5) | — | — | — | |
| Calculated from urinary metabolite data: MEHHP | Total: 2.0 (median); 6.5 (95th percentile) | — | — | — | |
| Female: 1.7 (7.0); Male: 2.3 (6.0) | — | — | — | ||
| Fromme et al. (2007b) | |||||
| Germany (2002: Koch et al. 2003a): Age 7 to 63 y; N = 85 | median (95th percentile): | ||||
| Calculated from urinary metabolite data: MEHP | Female: 4.0 (14.8); Male: 4.5 (20.5) | — | — | ||
| Calculated from urinary metabolite data: MEOHP | Female: 4.8 (16.2); Male: 6.3 (23.3) | — | — | ||
| Calculated from urinary metabolite data: MEHHP | Female: 3.7 (14.2); Male: 5.9 (23.6) | — | — | ||
| Wittassek et al. (2007b) | |||||
| Germany: Calculated from sum of urinary metabolite data for MEHHP + MEOHP + MECPP + MCMHP + MEHP; male and female adults 20 to 29 y | median; 95th percentile; (range) | ||||
| 1988 (N = 60) | 3.9; 9.9; (0.78–39.8) | — | — | — | — |
| 1989 (N = 60) | 4.2; 10.0; (0.84–33.6) | — | — | — | — |
| 1991 (N = 60) | 4.0; 18.8; (1.2–23.6) | — | — | — | — |
| 1993 (N = 60) | 4.2; 12.9; (1.4–14.1) | — | — | — | — |
| 1996 (N = 145) | 3.7; 13.4; (0.76–30.4) | — | — | — | — |
| 1998 (N = 68) | 3.1; 8.1; (0.19–10.9) | — | — | — | — |
| 1999 (N–60) | 2.7; 9.6; (1.0–13.9) | — | — | — | — |
| 2001 (N = 60) | 3.1; 7.4; (1.1–20.1) | — | — | — | — |
| 2003 (N = 59) | 2.4; 5.7; (0.82–7.1) | — | — | — | — |
| Total male (N = 325) | 3.4; 10.2; (NA) | — | — | — | — |
| Total female (N = 307) | 3.5; 10.5; (NA) | — | — | — | — |
| Overall total (N = 632) | 3.5; 10.1; (0.19–39.8) | — | — | — | — |
| Wittassek and Angerer (2008) | |||||
| Germany: Calculated from urinary metabolite data for MEHHP + MEOHP + MECPP + MCMHP + MEHP; N = 102 | Age 6 to 80 y: 2.7 (median); 42.2 (maximum) |
— | — | ||
| Wittassek et al. (2007a) | |||||
| Germany: Calculated from urinary metabolite data for MEHHP + MEOHP + MECPP + MCMHP + MEHP; Children: N = 239 (paper contains additional breakdown of data by age and gender) | Age 2 to 14 y: | ||||
| Creatinine-based model | — | 4.3 (median); 15.2 (95th percentile); 0.6–140 (range) | — | ||
| Volume-based model | — | 7.8 (median); 25.2 (95th percentile); 0.4–409 (range) | — | ||
| Calafat and McKee (2006) | |||||
| USA (NHANES 2001–2002: CDC, 2005); data format: geo mean (95th percentile) | Age 6 to > 20 y: (N = 2,772) | ||||
| Calculated from urinary metabolite data: MEHP | 0.9 (7.1) | — | — | ||
| Calculated from urinary metabolite data: MEHHP | 2.1 (16.8) | — | — | ||
| Calculated from urinary metabolite data: MEOHP | 2.2 (15.6) | — | — | ||
| Age 12 to 19 y:(N = 742) | Age 6 to 11 y:(N = 392) | ||||
| Calculated from urinary metabolite data: MEHP | — | 0.8 (5.5) | 0.6 (3.7) | — | — |
| Calculated from urinary metabolite data: MEHHP | — | 2.2 (11.6) | 2.4 (13.2) | — | — |
| Calculated from urinary metabolite data: MEOHP | — | 2.4 (12.6) 2.6 (12.8) | — | — | |
| Germany (2001–2002: Becker et al., 2004); data format: geo mean (95th percentile) | Age 3 to 14 y: (N = 254) | ||||
| Calculated from urinary metabolite data: MEHP | — | 0.7 (2.8) | — | ||
| Calculated from urinary metabolite data: MEHHP | — | 2.6 (10.7) | — | ||
| Calculated from urinary metabolite data: MEOHP | — | 3.1 (11.7) | — | ||
| Marsee et al. (2006) | |||||
| USA (2000–2003): pregnant women (N = 214) | median (95th percentile): | — | — | — | — |
| Calculated from urinary metabolite data: MEHP | 2.37; (16.8) | — | — | — | — |
| Calculated from urinary metabolite data: MEHHP | 1.33; (9.11) | — | — | — | — |
| Calculated from urinary metabolite data: MEOHP | 2.00; (12.8) | ||||
| Brock et al. (2002)a | |||||
| USA (2000): Intake calculated from urinary metabolite data for MEHP; 19 children; 30 samples | — | — | — | Age 11.8 to 16.5 months: 1.8 (geo mean); 7.0 (95th percentile) |
— |
| CDC (2003)a | |||||
| USA (NHANES 1999–2000): Calculated from urinary metabolite data for MEHP; data format: geo mean (95th percentile) | |||||
| N = 2,541 | Age 6+ y: Total: 0.73 (4.3); Male: 0.78 (5.8): Female: 0.71 (3.4) | — | — | ||
| N = 1,461 | Age 20+ y: 0.71 (4.1) | — | — | — | — |
| N = 752 | — | Age 12 to 19 y: 0.33 (1.6) | — | — | — |
| N = 328 | — | — | Age 6 to 11 y: 0.67 (5.4) | — | — |
| David (2000) | |||||
| USA (1988–1994; NHANES III). Intake calculated from urinary metabolite data for MEHP (Blount, et al. 2000); N = 289 | Age 20 to 60 y: 0.60 (geo mean); 3.05 (95th percentile) | — | — | — | — |
| Kohn et al. (2000) | |||||
| USA (1988–1994; NHANES III). Intake calculated from urinary metabolite data for MEHP (Blount, et al. 2000); N = 289 | Age 20 to 60 y: 0.71 (median); 3.6 (95th percentile) | — | — | — | — |
| Huang et al. (2006) | |||||
| Taiwan (undated): Calculated from urinary metabolite data for MEHP; pregnant women; N = 28Chen et al. (2008) | 5.17 (median) | — | — | — | — |
| Taiwan (undated): Calculated from urinary metabolite data for MEHP; N = 60 (41 female, 18 male) | Age 21 to 67 y: 33.9 (median); 0.1 to 309.6 (ranee) | — | — | — | — |
aDaily intake calculated from reported urinary metabolite data, as described in text. One half detection limit used for non-detect results.
NA = Not available
The biomarker studies differ in the metabolites that were measured. The older studies (Brock et al. 2002; CDC 2003; David 2000; Kohn et al. 2000) evaluated only MEHP. The estimates of DEHP intake from those studies are generally the lowest. The exceptions are the studies of Huang et al. (2006) and Chen et al. (2008), which evaluated MEHP in the urine of pregnant women and male and female adults, respectively, in Taiwan. The intakes of DEHP, estimated from the MEHP concentrations in the Taiwanese studies, are larger than other studies with estimates based on MEHP. Using measurements of five metabolites of DEHP, Wittassek et al. (2007b) found that between 1988 and 1993, the intake of DEHP was nearly constant, but decreased after 1996. The estimated intakes in Wittassek et al. (2007a,b) are similar to other studies of the German population, but somewhat higher than the estimates for the U.S. population (CDC 2005).
Di-isononyl Phthalate
DiNP has been measured in water, soil, and air. It has been evaluated in a variety of foods, but is not often detected. Numerous studies have documented the presence of DiNP in indoor dust, at concentrations equal to approximately 50% of the level of DEHP. For the indirect studies, as shown in Table 9, the lowest median intake of DiNP for the adult is 0.01 ßg/kg/d (Wormuth et al. 2006) due to ingestion of dust, inhalation of air, inhalation of spray paints, and dermal contact with gloves. Wormuth et al. (2006) used a value of zero as the concentration of DiNP in all foods except fish in their intermediate calculations; thus, food represents only a very small fraction of the total DiNP intake. Wormuth et al. (2006) estimated higher intakes of DiNP with decreasing age, with the highest intake estimated to be for the infant. For the infant, toddler, and child, the estimated intake is predominantly due to mouthing of toys.
In the present evaluation, the estimated median intake of DiNP to the adult is 0.67 μg/kg/d. The estimated intake for the teen and infant are comparable to the adult, but are higher for the child and toddler. For the adult, teen, child, and toddler, ingestion of food accounts for 61% to 71% of intake, depending on the age group. The remainder of the exposure for these age groups (and all of the exposure to the infant) is due to ingestion of dust. The estimated intakes of Tsumura et al. (2003, 2001a), based on dietary exposure in Japan decrease from 1999 to 2001 due to a decrease in the measured concentrations of DiNP in total diet samples. Gill et al. (2001) estimated intakes of DiNP to the toddler and infant as follows: an average of 39 μg/kg/d for the toddler due to mouthing children's products and 50 μg/kg/d due to other sources. For the infant, Gill et al. estimated the 95th percentile intake to be 73.9 μg/kg/d, due to mouthing children's products. These estimates are in the range of the upper estimates by Wormuth et al. (2006).
The biomarker studies differ in the metabolites that have been measured. The older studies (CDC 2003; David 2000; Kohn et al. 2000) evaluated only MiNP. MiNP is reported to be only a minor urinary metabolite of DiNP, while the oxidative metabolites: mono(carboxyisooctyl) phthalate (MCiOP), MOiNP, and MHiNP are the major urinary metabolites in rats. Silva et al. (2006) analysed all four metabolites in the urine of adults and confirmed that the oxidative metabolites were found in higher concentrations compared to MiNP (which was not detected). Silva et al. concluded that human exposure to DiNP is underestimated by using MiNP as the only urinary biomarker of DiNP. This conclusion is supported by the biomarker data for the USA, where MiNP was rarely detected and only the 95th percentile concentrations are reported.
Over the period of 1988 to 2003, the median intake of DiNP to German adults, based on the sum of MOiNP and MHiNP, ranges from 0.20 to 0.40 μg/kg/d, with the intakes increasing with time (Wittassek et al. 2007b). Intakes are estimated to be higher for female adults than males. Based on measurements of only MHiNP, the estimated median intake of DiNP in adults in 2005 is 0.7 μg/kg/d, with males having a greater intake compared with females (Fromme et al. 2007b). Wittassek and Angerer (2007) estimated the median intake of DiNP, based on the sum of MOiNP, MHiNP, and MCiNP, to be 0.6 μg/kg/d.
The estimated median intake of DiNP to the adult in the present indirect evaluation (0.67 μg/kg/d) is comparable to the biomarker-based estimates for Germany using MHiNP (0.7 μg/kg/d in Fromme et al. 2007b) and using the sum of MiNP, MOiNP, MHiNP, and MCiNP (0.6 μg/kg/d in Wittassek and Angerer 2008). The above indirect and biomarker estimates are higher than those of Wittassek et al. (2007b).
DISCUSSION AND CONCLUSIONS
In Table 10 is presented an overall comparison of the estimated daily intake for each diester via indirect and biomarker methods. Selected results for three of the PEs (DEP, BBP, and DEHP) are presented in Figures 1 to 3.
Figure 1.
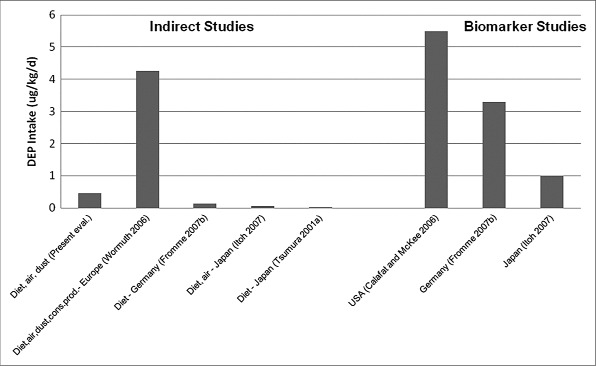
Estimates of median DEP intake to adults.
Figure 3.
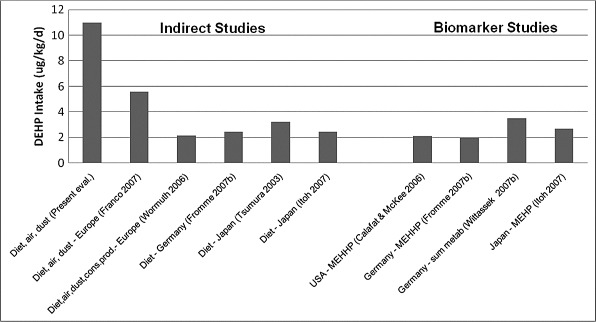
Estimates of median DEHP intake to adults.
In Figure 1 is presented the estimated intake of DEP from eight of the studies presented in Table 4. To facilitate comparison, the values presented in Figure 1 are the central (usually median) estimates for male and female adults. As shown in Figure 1 and Table 10, the intakes estimated in the indirect studies, which did not include exposure to personal care products (present evaluation; Fromme et al. 2007b; Itoh et al. 2007; Tsumura et al. 2001a), are much less than the indirect study that included such exposures (Wormuth et al. 2006) and less than the biomarker studies (Calafat and McKee 2006; Fromme et al. 2007b; Itoh et al. 2007).
In Figure 2 is presented the estimated intake of BBP for nine of the studies presented in Table 7. A comparison of Figures 1 and 2 shows that, for BBP, the indirect estimates are more similar to the biomarker-based estimates than was evident for DEP. The indirect estimates of Wormuth et al. (2006) and Fromme et al. (2007b) are similar to the biomarker-based estimates for Germany (Fromme et al. 2007b; Wittassek et al. 2007b) and the indirect estimates for Japan (Tsumura et al. 2003; Itoh et al. 2007) are similar to the biomarker-based estimates (Itoh et al. 2007). The biomarker-based estimates suggest a higher intake in the USA, followed by Germany and then Japan.
Figure 2.
Estimates of median BBP intake to adults.
The estimated intake of DEHP for 10 of the studies presented in Table 8 is presented in Figure 3. As shown in Figure 3, the present indirect estimates are higher than the other indirect estimates and higher than the biomarker-based estimates. This is due to the assumption of complete absorption following ingestion and/or elevated concentrations of DEHP in the composite food samples used in the calculations compared with the other indirect studies. That the intakes estimated by the other indirect studies and the biomarker studies are similar is shown in Figure 3. Although some regional differences were noted in the concentration data for DEHP, the biomarker estimates suggest similar intake in different regions. These regional differences in environmental concentrations may suggest greater use of DEHP in Europe versus North America and may support the slightly higher biomarker-based estimated intakes of DEHP for Germany (Wittassek et al. 2007b) versus the USA (Calafat and McKee 2006).
Based on both the indirect and biomarker methods, the volume and pattern of use of each PE vary with time and by region. As discussed, the biomarker-based estimates for several PEs (e.g., DEP, DBP, DiBP, BBP) indicate that there are regional differences in exposure. In most cases, these differences are supported by regional differences in the concentrations of PEs in environmental media; however, there is generally insufficient data for all media (especially food) to generate region-specific indirect estimates of exposure. Therefore, biomarker studies may have more value in assessing regional or temporal variations in exposure.
The importance of temporal changes in the use of PEs is shown in the work of Wittassek et al. (2007b) who analysed primary and/or secondary metabolites of DBP, DiBP, BBP, DEHP, and DiNP in the urine of adults in Germany. Archived samples, available for nine years in the period of 1988 to 2003, were analysed and the measurements were used to estimate the daily intake of the phthalate diesters. They found that between 1988 and 1993, the intake of DBP and DEHP was nearly constant, but decreased markedly after 1996. The intake of DiBP increased slightly over the period of study, while the intake of BBP decreased slightly. The intake of DiNP increased over the period of study. Female adults had significantly higher intakes of DBP and DiBP compared to male adults. Helm (2007) compared the estimated intake of DEHP from Wittassek et al. (2007b) for the years 1988 to 2003, with DEHP production data for Germany for the same time period and found a very high correlation between estimated intake and production. This suggests that changes in production volume should be considered when comparing intakes for different time periods.
The indirect estimates of Wormuth et al. (2006) incorporate absorption factors for ingestion, inhalation, and dermal contact with PEs. Minimum, mean, and maximum absorption factors are used for the ingestion pathways and, for some PEs, this range is very broad (ranging from 0.153 to 0.95 for DEHP). In contrast, the present evaluation has assumed 100% absorption for the ingestion and inhalation pathways (dermal contact is not included) and it is recognized that this will overestimate the intake for some PEs. This may also affect the relative importance of the various exposure pathways as the assumption of complete absorption may overestimate the relative contribution of food and dust ingestion compared to other pathways.
In summary, numerous estimates of the daily intake of PEs are available, using both indirect and biomarker methods. In many cases, these two methods agree with each other within an order of magnitude. Discrepancies between the two approaches are generally explained by one or more of the following factors: difficulties in accounting for use of consumer products in the indirect estimates, a lack of information concerning human absorption of PEs following ingestion, regional differences in the use of the PEs, and temporal changes in the use of PEs. Similarly, discrepancies when comparing the biomarker estimates with each other are generally explained by regional differences in concentrations of the parent diesters in the environment, suggesting different patterns of use, and temporal changes in use of PEs. No single method is identified as the preferred approach for estimating intake of all PEs; rather it is suggested that biomarker estimates be used for low molecular weight PEs for which it is difficult to quantify all sources of exposure and either indirect or biomarker methods be used for higher molecular weight PEs. The indirect methods are useful in identifying the sources of exposure while the biomarker methods can be used to quantify the amount of exposure. The indirect estimates would be improved by better characterization of the absorption factors and with current region-specific measurements of PEs in all media to which humans may be exposed.
ACKNOWLEDGMENT
This study was funded by the Phthalate Esters Panel of the American Chemistry Council, Washington, DC, USA.
REFERENCES
- Anderson WAC. Castle L. Scotter MJ, et al. A biomarker approach to measuring human dietary exposure to certain phthalate diesters. Food Addit Contam. 2001;18:1068–74. doi: 10.1080/02652030110050113. [DOI] [PubMed] [Google Scholar]
- Barr DB. Silva MJ. Kato K, et al. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ Health Perspect. 2003;111:1164–9. doi: 10.1289/ehp.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB. Wilder LC. Caudill SP, et al. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer MJ. Estimation of the environmental contamination by phthalic acid esters leaching from household wastes. Sci Total Environ. 1997;208:49–57. doi: 10.1016/s0048-9697(97)00272-6. [DOI] [PubMed] [Google Scholar]
- Becker K. Seiwert M. Angerer J, et al. DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Environ Health. 2004;207:409–17. doi: 10.1078/1438-4639-00309. [DOI] [PubMed] [Google Scholar]
- Blount BC. Silva MJ. Caudill SP, et al. Levels of seven urinary phthalate metabolites in a human reference population. Environ Health Perspect. 2000;108(10):979–82. doi: 10.1289/ehp.00108979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JW. Caudill SP. Silva MJ, et al. Phthalate monoesters levels in the urine of young children. Bull Environ Contam Toxicol. 2002;68:309–14. doi: 10.1007/s001280255. [DOI] [PubMed] [Google Scholar]
- Calafat AM, McKee RH. Integrating biomonitoring exposure data into the risk assessment process: Phthalates (diethyl phthalate and di[2-ethylhexyl] phthalate) as a case study. Environ Health Perspect. 2006;114(11):1783–9. doi: 10.1289/ehp.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM. Slakman AR. Silva MJ, et al. Automated solid phase extraction and quantitative analysis of human milk for 13 phthalate metabolites. J Chromatogr B. 2004;805:49–56. doi: 10.1016/j.jchromb.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Calafat AM. Brock JW. Silva MJ, et al. Urinary and amniotic fluid levels of phthalate monoesters in rats after the oral administration of di(2-ethylhexyl) phthalate and di-n-butyl phthalate. Toxicology. 2006;27:22–30. doi: 10.1016/j.tox.2005.08.013. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) National Report on Human Exposure to Environmental Chemicals. Atlanta, GA, USA: Department of Health and Human Services, National Center for Environmental Health, Division of Laboratory Sciences; 2001. [Google Scholar]
- CDC. Second National Report on Human Exposure to Environmental Chemicals. Atlanta, GA, USA: Department of Health and Human Services, National Center for Environmental Health, Division of Laboratory Sciences; 2003. January, revised March. [Google Scholar]
- CDC. Third National Report on Human Exposure to Environmental Chemicals. Atlanta, GA, USA: Department of Health and Human Services, National Center for Environmental Health, Division of Laboratory Sciences; 2005. July. [Google Scholar]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA, USA: Department of Health and Human Services, National Center for Environmental Health, Division of Laboratory Sciences; 2009. December. [Google Scholar]
- Chen M-L. Chen J-S. Tang C-L, et al. The internal exposure of Taiwanese to phthalate—An evidence of intensive use of plastic materials. Environ Internat. 2008;34:79–85. doi: 10.1016/j.envint.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Clark K. Report on Update to the Phthalate Ester Concentration Database—2007. 2008. Prepared for American Chemistry Council, Arlington, VA, USA. June.
- Clark K. Cousins IT, Mackay D. Observed concentrations in the environment. In: Staples CA, editor. Phthalate Esters: The Handbook of Environmental Chemistry, Vol 3 Anthropogenic Compounds, Part Q. Heidelberg, Germany: Springer-Verlag; 2003a. pp. 125–77. [Google Scholar]
- Clark K. Cousins IT, Mackay D. Assessment of critical exposure pathways. In: Staples CA, editor. Phthalate Esters: The Handbook of Environmental Chemistry, Vol 3 Anthropogenic Compounds, Part Q. Heidelberg, Germany: Springer-Verlag; 2003b. pp. 227–62. [Google Scholar]
- David RM. Exposure to phthalate esters. Environ Health Perspect. 2000;108(10):A440. doi: 10.1289/ehp.108-a440a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A. Prevedouros K. Alli R, et al. Comparison and analysis of different approaches for estimating the human exposure to phthalate esters. Environ Internat. 2007;33:283–91. doi: 10.1016/j.envint.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Fromme H. Bolte G. Koch HM, et al. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. Int J Hyg Environ Health. 2007a;210:21–33. doi: 10.1016/j.ijheh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Fromme H. Gruber L. Schlummer M, et al. Intake of phthalates and di(2-ethylhexyl)adipate: Results of the Integrated Exposure Assessment Survey based on duplicate diet samples and biomonitoring data. Environ Internat. 2007b;33:1012–20. doi: 10.1016/j.envint.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Fujimaki K. Yoshinaga J. Watanabe C, et al. Estimation of intake level of di (2-ethyhexyl) phthalate (DEHP) in Japanese pregnant women based on measurement of concentrations of three urinary metabolites. Nippon Eiseigaku Zasshi (Japanese Journal of Hygiene) 2006;61(3):340–7. doi: 10.1265/jjh.61.340. [DOI] [PubMed] [Google Scholar]
- Gill US. Craan AG. Subramanian KS, et al. Ware GW, editor. Diisononyl phthalate: Chemistry, environmental path, and toxicology. 2001. Reviews of Environmental Contamination and Toxicology, vol 172, pp 87–127. Springer-Verlag, New York, NY, USA.
- Health and Welfare Canada. Reference Values for Canadian Populations. 1993. Prepared by the Environmental Health Directorate Working Group on Reference Values, Ottawa, ON, Canada. July 1988; updated May 1993.
- Health Canada. Probabilistic Assessment of 24-hour Breathing Rates. 1995. Prepared by Cornerstone Engineering and Consulting Inc. for the Health Protection Branch, Ottawa, ON, Canada. October.
- Helm D. Correlation between production amounts of DEHP and daily intake. Sci Total Environ. 2007;388:389–91. doi: 10.1016/j.scitotenv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Hogberg J. Hanberg A. Berglund M, et al. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect. 2008;116:334–9. doi: 10.1289/ehp.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PC. Kuo PL, Lee CC. The association between urinary monoester phthalates and thyroid hormone in pregnant women. Epidemiology. 2006;17(6):S195. November 2006 Suppl: [Google Scholar]
- Itoh H. Yoshida K, Masunaga S. Quantitative identification of unknown exposure pathways of phthalates based on measuring their metabolites in human urine. Environ Sci Technol. 2007;41(13):4542–7. doi: 10.1021/es062926y. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Knudsen HK. Total Health Assessment of Chemicals in Indoor Climate from Various Consumer Products. 2006. Danish Environmental Protection Agency: Survey of Chemical Substances in Consumer Products No. 75. Available at http://www.mst.dk/Udgivelser/Publications/2006/12/87-7052-214-6.htm.
- Koch HM. Rossbach B. Drexler H, et al. Internal exposure of the general population to DEHP and other phthalates—Determination of secondary and primary phthalate monoester metabolites in urine. Environ Res. 2003a;93(2):177–85. doi: 10.1016/s0013-9351(03)00083-5. [DOI] [PubMed] [Google Scholar]
- Koch HM. Drexler H, Angerer J. An estimation of the daily intake of di(2-ethylhexyl)phthalate (DEHP) and other phthalates in the general population. Int J Hyg Environ Health. 2003b;206:77–83. doi: 10.1078/1438-4639-00205. [DOI] [PubMed] [Google Scholar]
- Koch HM. Bolt HM, Angerer J. Di(2-ethylhexyl) phthalate (DEHP) metabolites in human urine and serum after a single oral dose of deuterium labelled DEHP. Arch Toxicol. 2004;78:123–30. doi: 10.1007/s00204-003-0522-3. [DOI] [PubMed] [Google Scholar]
- Koch HM. Bolt HM. Preuss R, et al. New metabolites of di(2-ethylhexyl) phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol. 2005;79:367–76. doi: 10.1007/s00204-004-0642-4. [DOI] [PubMed] [Google Scholar]
- Koch HM, Angerer J. Di-iso-nonyl phthalate (DINP) metabolites in human urine after single oral dose of deuterium-labelled DINP. Int J Hygiene Environ Health. 2007;210:9–19. doi: 10.1016/j.ijheh.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Kohn MC. Parham F. Masten SA, et al. Human exposure estimates for phthalates. Environ Health Perspect. 2000;108(10):A440–2. doi: 10.1289/ehp.108-a440b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo HJ, Lee BM. Human monitoring of phthalates and risk assessment. J Toxicol Environ Health A. 2005;68:1379–92. doi: 10.1080/15287390590956506. [DOI] [PubMed] [Google Scholar]
- Marsee K. Woodruff TJ. Axelrad DA, et al. Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ Health Perspect. 2006;114(6):805–9. doi: 10.1289/ehp.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael PR. Adams WJ. Werner AF, et al. Surveillance of phthalate esters on surface waters and sediments in the United States. Environ Toxicol Chem. 1984;3:377–89. [Google Scholar]
- Page BD, Lacroix GM. The occurrence of phthalate ester and di-2-ethylhexyl adipate plasticizers in Canadian packaging and food sampled in 1985–1989: A survey. Food Addit Contam. 1995;12:129–51. doi: 10.1080/02652039509374287. [DOI] [PubMed] [Google Scholar]
- Petersen JH, Breindahl T. Placticizers in total diet samples, baby food and infant formulae. Food Addit Contam. 2000;17(2):133–41. doi: 10.1080/026520300283487. [DOI] [PubMed] [Google Scholar]
- Shen H-Y. Jiang H-L. Mao H-L, et al. Simultaneous determination of seven phthalates and four parabens in cosmetic products using HPLC-DAD and GC-MS methods. J Sep Sci. 2007;30(1):48–54. doi: 10.1002/jssc.200600215. [DOI] [PubMed] [Google Scholar]
- Silva MJ. Reidy JA. Preau JL, Jr., et al. Oxidative metabolites of diisononyl phthalate as biomarkers for human exposure assessment. Environ Health Perspect. 2006;114(8):1158–61. doi: 10.1289/ehp.8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley MK. Robillard KA, Staples CA. Introduction. In: Staples CA, editor. Phthalate Esters: The Handbook of Environmental Chemistry, vol 3 Anthropogenic Compounds, Part Q. Heidelberg, Germany: Springer-Verlag; 2003. pp. 1–7. [Google Scholar]
- Tabor MW, Loper JC. Analytical isolation, separation and identification of mutagens from nonvolatile organics of drinking water. Internat J Environ Anal Chem. 1985;19:281–317. doi: 10.1080/03067318508077039. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. Briutton JA. Calafat AM, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106(2):257–69. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Tsumura Y. Ishimitsu S. Saito I, et al. Eleven phthalate esters and di(2-ethylhexyl) adipate in one-week duplicate diet samples obtained from hospitals and their estimated daily intake. Food Addit Contam. 2001a;18(5):449–60. doi: 10.1080/02652030117484. [DOI] [PubMed] [Google Scholar]
- Tsumura Y. Ishimitsu S. Kaihara A, et al. Di(2-ethylhexyl) phthalate contamination of retail packed lunches caused by PVC gloves used in the preparation of foods. Food Addit Contam. 2001b;18(6):569–79. doi: 10.1080/02652030120071. [DOI] [PubMed] [Google Scholar]
- Tsumura Y. Ishimitsu S. Saito I, et al. Estimated daily intake of plasticizers in 1-week duplicate diet samples following regulation of DEHP-containing PVC gloves in Japan. Food Addit Contam. 2003;20(4):317–24. doi: 10.1080/0265203031000122021. [DOI] [PubMed] [Google Scholar]
- Wilson NK. Chuang JC, Lyu C. Levels of persistent organic pollutants in several child day care centers. J Expos Anal Environ Epidemiol. 2001;11:449–58. doi: 10.1038/sj.jea.7500190. [DOI] [PubMed] [Google Scholar]
- Wilson NK. Chuang JC. Lyu C, et al. Aggregate exposures of nine preschool children to persistent organic pollutants at day care and at home. J Expos Anal Environ Epidemiol. 2003;13:187–202. doi: 10.1038/sj.jea.7500270. [DOI] [PubMed] [Google Scholar]
- Wittassek M, Angerer J. Phthalates: metabolism and exposure. Int J Andrology. 2008;31(2):131–8. doi: 10.1111/j.1365-2605.2007.00837.x. [DOI] [PubMed] [Google Scholar]
- Wittassek M. Heger W. Koch HM, et al. Daily intake of di(2-ethylhexyl)phthalate (DEHP) by German children — A comparison of two estimation models based on urinary DEHP metabolite levels. Int J Hyg Environ Health. 2007a;210(1):35–42. doi: 10.1016/j.ijheh.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Wittassek M. Wiesmuller GA. Koch HM, et al. Internal phthalate exposure over the last two decades—A. retrospective human biomonitoring study. Int J Hyg Environ Health. 2007b;210(3–4):319–33. doi: 10.1016/j.ijheh.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Wormuth M. Scheringer M. Vollenweider M, et al. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006;26(3):803–24. doi: 10.1111/j.1539-6924.2006.00770.x. Supplemental calculations provided by Matthias Wormuth to Kathryn Clark, November 15, 2007. [DOI] [PubMed] [Google Scholar]
- Zhu J. Phillips SP. Feng YL, et al. Phthalate esters in human milk: Concentration variations over a 6-month postpartum time. Environ Sci Technol. 2006;40:5276–81. doi: 10.1021/es060356w. [DOI] [PubMed] [Google Scholar]


